1. Introduction
Human milk (HM) is universally recognized the gold standard of infants nutrition, insomuch as breastfeeding should be considered a health issue and not only a lifestyle choice [
1,
2]. Besides the nutritional importance of its macronutrients, its well known that its numerous benefits are mediated by several bioactive substances that can regulate newborns growth and development [
3,
4]. These components can perform multiple functions playing a protective role from neonatal infections, other than ensuring a long-term benefit on healthy and neurodevelopment. Being HM a dynamic fluid, its constituents constantly change during lactation phases, time of the day and single feeding [
5]. In addition to this, its composition is heavily influenced also by other conditions such as gestational age (GA), maternal diet, ethnicity and various diseases [
5,
6]. The two main gestational pathologies, Gestational diabetes mellitus (GDM) and Preeclampsia (PE), also can affect not only lactation but also milk composition.
GDM is a pathological condition defined as any degree of glucose intolerance first recognized during pregnancy [
7]. Its incidence depends on diagnostic criteria which are not universally recognized but its frequency is unanimously increasing in recent years, depending on women entering pregnancy at an older age and more frequently obese or overweight [
8]. It can determine several delivery complications and constitutes a significant risk factor for newborn morbidity, mainly that the offspring of women with GDM are at increased long-term risk for type 2 diabetes [
9,
10]. GDM also holds the ability of delaying lactogenesis' onset and affecting the composition of human milk throughout its various phases [
11].
PE affect about 2-8% of all pregnant women and can be associated with various important maternal and fetal complications [
12,
13]. In PE there is an abnormal interaction between placental and maternal tissues with production of oxidant substances and proinflammatory cytokines, which would be able to damage the vascular endothelium of the mother and generalizing the damage [
14]. In the light of the pathogenetic mechanisms, PE can interfere with the physiological functioning of the mammary gland and consequently altering the production and transfer of nutrients and other components in milk [
15].
Bearing in mind these considerations, it appears important evaluate the impact that these gestational diseases have on HM composition but, until now, previous studies focus mainly on specific biomarker associated with GDM or PE, often evaluating a single lactating phase or only term delivery [
15,
16]. It therefore seems clinical to assess if their presence, as source of oxidative stress in the mother, can influence milk’s redox homeostasis. Notably Glutathione (GSH) and Lipid hydroperoxides (LOOHs) appear to have been the target of extensive research, which however fails to deliver useful information around their presence both in HM of itself and in HM of pathological mothers. More specifically, GSH has often been used as one of the indicators of total antioxidant capacity in HM, as a substrate of the main antioxidant enzymes [
17]. On the other hand, LOOHs are important intermediates of peroxidative reactions induced by reactive species that disrupt membrane structure and function and can be deleterious to cells [
18].
In order to assess and evaluate PE and GDM’s burden on milk’s total oxidative load, we chose to investigate both oxidative and antioxidant molecules, such as LOOHs and GSH.
2. Methods
2.1. Setting and Population
The study has been conducted in the Neonatal Unit, Department of Public Health and Pediatrics, University of Turin. The study protocol was approved by the local Ethic Committee of the Italian Association of Human Milk Donor Banks (AIBLUD). Mothers admitted into the study gave signed and informed consent. Newborns’ mothers were recruited after delivery at Neonatal Unit.
The women recruited in the study were divided in three different study groups:
Preeclamptic group: according to the PE definition (Artery blood pressure >140/90 after 20 weeks of gestational age and proteinuria >290 mg/l, possibly associated with headache, edema, scotomas and epigastric pain) [
19].
Gestational diabetes mellitus group: according to GDM definition (any degree of glucose intolerance first recognized during pregnancy) [
7]
Healthy women: the control group was formed at the same time and made up of normotensive and euglycemic women who also met the same exclusion criteria.
Criteria exclusion: Women with seizure, psychiatric syndromes, history of alcohol or drug abuse, smoking, autoimmune pathologies, chorioamnionitis, mastitis and a newborn with congenital anomalies or infection.
A minimum of 30 women for each group will be recruited.
2.2. Collection Human Milk Samples
According to standard criteria, we classified as “colostrum” the milk collected in the first seven days after delivery; “transition milk” the milk collected from the 8th day to 14th day after delivery and “mature milk” the milk collected after the 15th day [
20]. All the breast milk samples were collected using the same procedure outlined below. Fresh milk samples were collected in the morning (between 9 a.m. and 12 a.m.) into sterile disposable. Milk expression was obtained by emptying one breast completely by means of an electric breast pump (Medela Simphony). Milk expression by the other breast was performed only in cases in which it was not possible to obtain 10 ml from a single breast.
2.3. Biochemical Analysis
Whole milk samples were divided into aliquots and stored at -80°C until analysis. They were then defrosted in a 37 ° C bath and immediately analyzed. Regarding GSH levels, the concentrations of non-protein thiol groups (RSH), approximately 90% of the GSH content, in 200 μL of milk were measured using a spectrophotometric test based on the reaction of thiol groups with acid 2,2-dithio-bis-nitrobenzoic (DTNB) at λ = 412 nm (εM = 13,600). LOOHs levels in milk samples were assessed following oxidation from Fe2 + to Fe3 + in presence of orange xylenol at λ = 560 nm. One milliliter of the mixture contained: 100 μl of milk sample, 100 μM orange xylenol, 250 μM ferrous ammonium sulfate, 90% methanol, 4 mM butylated hydroxytoluene and 25 mM H2SO4. After 30 minutes of incubation at room temperature, the absorbance at λ = 560 nm was measured with a Hitachi U2000 spectrophotometer. Calibration was achieved using hydrogen peroxide (0.2-20 μM). The results are expressed in nanomoles.
2.4. Statistical Methods
Birth weight was transformed in z-score according to the country-specific Italian Neonatal Study (INeS) charts [
21]. Newborns with a birth weight lower than the 10th or higher than the 90th centile were classified as Small for GA (SGA) or Large for GA (LGA) respectively. Regarding GSH, to investigate the distribution by HM phase and pathology specific box plot was performed. Then GSH concentration were normalized with the more appropriate Box-Cox transformation. To investigate the effect of pathology in the GSH concentration in the 3 different phases, mixed linear model with unstructured covariance structure, mother as random effect, and fixed effects were performed. The fixed effects were HM phase, pathology, smoker, delivery mode, newborn GA and mother age (continuous), and the interaction between phase hm×pathology. Distributions of continuous variables were assessed for normality, and transformations of skewed variables were used in statistical analyses as appropriate. A P value <0,05 was used to define statistical significance.
3. Results
3.1. Demographic Characteristics
A total of 120 mothers were recruited for our study, divided as follow: 35 in the GDM group, 39 in the PE group and 46 in the healthy group.
Table 1 shows demographic characteristics of mothers and newborns in all three groups. The fraction of primigravida in diabetic mothers is lower than in the healthy women group. As expected, diabetic women have a higher percentage of LGA while PE women have a higher percentage of IUGR and SGA.
3.2. GSH
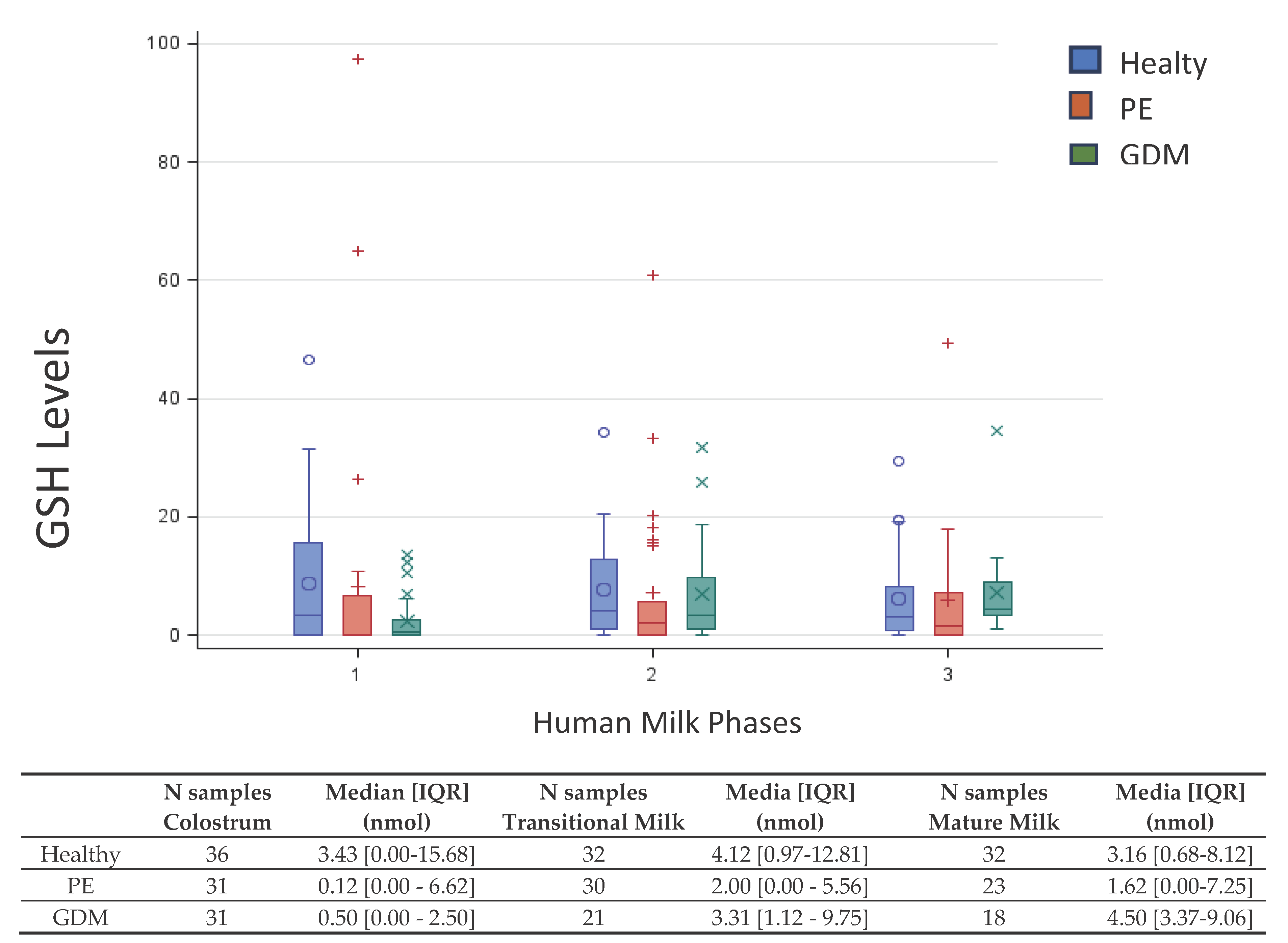
Figure 1 shows the box-plot GSH distribution by HM phase and pathology (PE, GDM and Healthy). The box-plot by HM phase and pathology (GDM vs Healthy) shows a positively skewed GSH distribution at each HM phase. In colostrum of GDM group that the 25% of the measures results equal to 0, λ=0.25 is the more appropriate Box-Cox transformation. The GSH concentration in GDM group is significant lower in colostrum than in transitional milk (p=0.02) and mature milk (p<0.01), while not significant difference was detected between transitional and mature milk. About the interaction between groups, the GSH concentration results significant lower in GDM women than in healthy only in colostrum (p<0.01). No other differences emerged.
3.3. LOOHs
LOOHs did not detectable in most samples (223 of 242 samples).
Table 2 shows in detail the description of the samples in which LOOHs was found and its relative concentrations.
4. Discussion
There is evidence that newborns are already more vulnerable to oxidative stress in the first days of life, due to the inefficiency of their antioxidant defense system and the increase in free radical production outside the womb. Neonates need then more antioxidants from the first phase of breast milk to protect their health [
22,
23]. Exposure to maternal pathology can influence the antioxidant burden of colostrum and mature milk and can adversely affect the infant's health, playing a role in the pathogenesis of various conditions such as necrotizing enterocolitis, bronchopulmonary dysplasia and retinopathy of prematurity among others [
24].
GSH is considered one of the main water-soluble antioxidants in biological fluids [
22]. Previous research has already investigated GSH levels specifically in HM of healthy women [
23]. More precisely, available data provide indications about the total antioxidant capacity of HM and the enzymes that use glutathione as a substrate, which are increased in colostrum of healthy full-term mothers, probably to address the greater vulnerability of newborns to oxidative stress and relative immaturity of their endogenous antioxidant means [
23].
While the increase in GSH concentration appears to be a universal cellular response to oxidative stress, some diseases like GDM seem to be linked to a decrease of its levels in the body [
25]. In the presence of metabolic deregulation and uncontrolled hyperglycemia, oxidative stress and formation of ROS rise significantly. This ultimately leads to an increase in lipid peroxidation and its byproducts and depletion of antioxidants such as GSH [
25,
26]. It also has been certified that in diabetic subjects the increase in lipid peroxidation and the depletion of antioxidants, in addition to the insulin-dependent expression of enzymes degrading GSH, contribute to decrease its concentration in the body [
26]. As far as concerned GSH and PE, numerous previous studies have investigated the overall maternal redox status related to gestational hypertension and the authors generally agree in stating that there is a significant correlation between oxidative stress and pregnancy pathology. Especially in PE, the levels of oxidative stress markers increase and the concentration of GSH and other antioxidant molecules in plasma decreases [
27]. Some studies have demonstrated this association even before the diagnosis of PE, demonstrating that high blood levels of oxidative stress markers (e.g., malondialdehyde) and low levels of glutathione and other antioxidants before the 20th week of gestation correlate with a higher probability of subsequently diagnose the presence of preeclampsia [
28,
29,
30,
31].
Our study is the first that evaluate the GSH concentrations during all lactation phases of mothers with GDM and PE. Regarding data about GSH values of diabetic mother, our findings seem to match previous studies highlighting diabetes higher oxidative burden: the GSH concentration results significant lower in colostrum in GDM group than in healthy. Concerning the HM of PE group, our study demonstrated that there are no differences in the GSH concentrations compared to healthy group, highlighting how the oxidative maternal status, in this case, does not directly affected the milk concentrations.
In our study, LOOHs is not detectable in most sample regardless of the lactation phase and the gestational pathology. We observed that in the HM of GDM mothers compared to healthy mothers LOOHs was detectable in more samples and the concentrations were higher. Moreover, LOOHs concentration was detectable only in one of the 72 samples of the hypertensive group. There are few studies that have evaluated the presence of these molecules in HM and most of them have focused on the evaluation of the concentration of lipid hydroperoxides after the various processing methods that are used in HM Banks [
32]. There are no studies in the literature that have directly investigated the presence of lipid hydroperoxides in the human milk of PE or GDM women, therefore our study is the first that evaluate the presence of LOOHs in the milk of pathological and healthy women. However, various research groups have evaluated the association between preeclampsia and the concentration of lipid hydroperoxides (as a marker of oxidative stress), analyzing samples of different types. In general, researchers agree that the plasma LOOHs levels of hypertensive and preeclamptic women are higher than the levels of healthy women [
33,
34,
35]. The results of our study are in partial disagreement with the results of the previous data literature in the field of oxidative stress in the milk of hypertensive women. In fact, previous studies report, in the milk of women with hypertension, lower values of total antioxidant capacity and vitamins A and E and higher values of oxidative stress index and total peroxides [
36,
37,
38], malondialdehyde (MDA), conjugated dienes, bases of Schiff (some lipid peroxidation products): these results are in agreement with the pathogenetic mechanism of the disease and with the data concerning the levels of these molecules in the plasma of affected women. Only one instead reports higher levels of polyphenols in the hypertensive group, while lower levels of MDA: in this case the authors hypothesize a mechanism of adaptation of the maternal organism to protect the newborn from stress oxidative [
39].
5. Conclusions
In conclusion, our study is the first to extensively evaluate the concentration of these two molecules in the milk of women with gestational pathologies. The main observation is that GDM can alter the antioxidant composition of human milk mainly in colostrum, more than throughout other lactating phases. On the contrary, in case of PE, the composition of HM milk is preserved: this is probably possible thanks to an active compensatory mechanism of the maternal organism, which tends to adapt to protect the infant from the harmful effects of the pathology itself. Our results, therefore, once again confirm the importance of breastfeeding for all infants.
Author Contributions
Conceptualization, C.P. and D.G.; methodology, E.S. I.B., G.L. and F.G.; formal analysis I.B., G.L. and F.G.; data curation, E.S.; writing—original draft preparation, C.P., L.R., E.S., A.C. and D.G.; writing—review and editing, C.P., L.R., A.C. and D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of the Italian Association of Human Milk Donor Banks (AIBLUD).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. 2012. [CrossRef]
- Horta BL, Victora CG. Long-Term Health Effects of Breastfeeding: A Systematic Review.; WHO 2013.
- Hamosh, M. Bioactive factors in human milk. Pediatr Clin North Am. 2001. [CrossRef] [PubMed]
- Ballard O, Morrow AL. Human Milk Composition. Nutrients and Bioactive Factors. Pediatr Clin North Am. 2013. [CrossRef]
- Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early Hum Dev. 2015. [CrossRef]
- Dritsakou K, Liosis G, Valsami G, Polychronopoulos E, Skouroliakou M. The impact of maternal- and neonatal-associated factors on human milk’s macronutrients and energy. J Matern Neonatal Med. 2017. [CrossRef]
- ACOG Practice Bulletin, No. 190: Gestational Diabetes Mellitus. Obstetrics & Gynecology 131(2):p e49-e64, 2018. |. February. [CrossRef]
- Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016. [CrossRef]
- Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006. [CrossRef]
- Arenz S, Rückerl R, Koletzko B, Von Kries R. Breast-feeding and childhood obesity - A systematic review. Int J Obes. 2004. [CrossRef]
- Hartmann P, Cregan M. Lactogenesis and the effects of insulin-dependent diabetes mellitus and prematurity. J Nutr. 2001. [CrossRef]
- Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. [CrossRef]
- Leitner Y, Harel S, Geva R, Eshel R, Yaffo A, Many A. The neurocognitive outcome of IUGR children born to mothers with and without preeclampsia. J Matern Fetal Neonatal Med. 2012 Nov;25(11):2206-8. [CrossRef] [PubMed]
- Hod T, Cerdeira AS, Karumanchi SA. Molecular Mechanisms of Preeclampsia. Cold Spring Harb Perspect Med. 2015 Aug 20;5(10):a023473. [CrossRef]
- Peila C, Bertino E, Cresi F, Coscia A. Interactions between preeclampsia and composition of the human milk: what do we know? J Matern Fetal Neonatal Med. 2022 Dec;35(25):6219-6225. [CrossRef]
- Peila C, Gazzolo D, Bertino E, Cresi F, Coscia A. Influence of diabetes during pregnancy on human milk composition. Nutrients. 2020;12(1). [CrossRef]
- Castillo-Castañeda PC, Gaxiola-Robles R, Labrada-Martagón V, Acosta Vargas B, Méndez-Rodríguez LC, Zenteno-Savín T. Oxidative damage to proteins related to metals and antioxidant defenses in breastmilk. Nutr Hosp. 2017. [CrossRef]
- Girotti, AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998.
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstetrics & Gynecology 135(6):p e237-e260, 2020. |. June. [CrossRef]
- Playford RJ, Macdonald CE, Johnson WS. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am J Clin Nutr. 2000 Jul;72(1):5-14. [CrossRef] [PubMed]
- Bertino E, Di Nicola P, Varalda A, Occhi L, Giuliani F, Coscia A. Neonatal growth charts. J Matern Fetal Neonatal Med. 2012 Apr;25 Suppl 1:67-9. [CrossRef] [PubMed]
- Teskey G, Abrahem R, Cao R, et al. Glutathione as a Marker for Human Disease. Adv Clin Chem. 2018. [CrossRef]
- Ma L, Shi H, Lian K, Diao Y, Chen Y, Ma C, Kang W. Highly selective and sensitive determination of several antioxidants in human breast milk using high-performance liquid chromatography based on Ag(III) complex chemiluminescence detection. Food Chem. 2017 Mar 1;218:422-426. [CrossRef]
- Graziosi A, Perrotta M, Russo D, et al. Oxidative Stress Markers and the Retinopathy of Prematurity. J Clin Med. 2020. [CrossRef]
- Whiting PH, Kalansooriya A, Holbrook I, Haddad F, Jennings PE. The relationship between chronic glycaemic control and oxidative stress in type 2 diabetes mellitus. Br J Biomed Sci. 2008. [CrossRef]
- López-Tinoco C, Roca M, García-Valero A, et al. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. In: Acta Diabetologica. ; 2013. [CrossRef]
- Madazli R, Benian A, Aydin S, Uzun H, Tolun N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J Obstet Gynaecol. 2002 Sep;22(5):477-80. [CrossRef]
- D'Souza V, Rani A, Patil V, Pisal H, Randhir K, Mehendale S, Wagh G, Gupte S, Joshi S. Increased oxidative stress from early pregnancy in women who develop preeclampsia. Clin Exp Hypertens. 2016;38(2):225-32. [CrossRef]
- Siddiqui IA, Jaleel A, Al'Kadri HM, Akram S, Tamimi W. Biomarkers of oxidative stress in women with pre-eclampsia. Biomark Med. 2013 Apr;7(2):229-34. [CrossRef]
- Ahmad IM, Zimmerman MC, Moore TA. Oxidative stress in early pregnancy and the risk of preeclampsia. Pregnancy Hypertens. 2019 Oct;18:99-102. [CrossRef]
- Llurba E, Gratacós E, Martín-Gallán P, Cabero L, Dominguez C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med. 2004 Aug 15;37(4):557-70. [CrossRef]
- Ankrah NA, Appiah-Opong R, Dzokoto C. Human breastmilk storage and the glutathione content. J Trop Pediatr. 2000 Apr;46(2):111-3. [CrossRef]
- Loverro G, Greco P, Capuano F, Carone D, Cormio G, Selvaggi L. Lipoperoxidation and antioxidant enzymes activity in pregnancy complicated with hypertension. Eur J Obstet Gynecol Reprod Biol. 1996 Dec 27;70(2):123-7. [CrossRef]
- Gupta S, Aziz N, Sekhon L, Agarwal R, Mansour G, Li J, Agarwal A. Lipid peroxidation and antioxidant status in preeclampsia: a systematic review. Obstet Gynecol Surv. 2009 Nov;64(11):750-9. [CrossRef]
- Erdem M, Harma M, Harma IM, Arikan I, Barut A. Comparative study of oxidative stress in maternal blood with that of cord blood and maternal milk. Arch Gynecol Obstet. 2012 Feb;285(2):371-5. [CrossRef]
- Gutikova, LV. [Chemical composition of milk of puerperas suffered from gestosis of different degree of severity]. Biomed Khim. 2007 May-Jun;53(3):332-7.
- Fares S, Sethom MM, Kacem S, Ksibi I, Feki M, Jebnoun S, Kaabachi N. Retinol and Alpha-tocopherol in the Colostrum of Lactating Tunisian Women Delivering Prematurely: Associations with Maternal Characteristics. Pediatr Neonatol. 2016 Apr;57(2):120-6. [CrossRef]
- van Zoeren-Grobben D, Moison R, Ester W, Berger H. Lipid peroxidation in human milk and infant formula: effect of storage, tube feeding and exposure to phototherapy. Acta Pædiatrica. 1993. [CrossRef]
- Silberstein T, Hamou B, Cervil S, Barak T, Burg A, Saphier O. Colostrum of Preeclamptic Women Has a High Level of Polyphenols and Better Resistance to Oxidative Stress in Comparison to That of Healthy Women. Oxid Med Cell Longev. 2019 Feb 21;2019:1380605. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).