1. Introduction
Children living with HIV (CHIV) currently require life-long antiretroviral therapy (ART) and continue to have more limited medication options than adolescents and adults living with HIV.[
1,
2] Additionally, children are frequently exposed to antiretrovirals as part of perinatal transmission prevention efforts. Rates of virologic suppression in CHIV on ART are lower than for adults on treatment, exposing children to consequent risks of poor growth, suboptimal neurodevelopment, clinical progression including opportunistic infections, and mortality.[
3]
The World Health Organization (WHO) and others have expressed concerns that HIV DR to antiretrovirals will undermine the attainment of the global targets for HIV, and CHIV face unique challenges for DR.[
4,
5,
6] Pre-treatment DR in ART naïve infants living with HIV is alarmingly high with a pooled estimate of 45.5% from recent multi-country reports and is associated with increased risk of virologic failure (VF).[
6] Acquired DR while on ART is also increasingly recognized as a concern among CHIV but less data are available. Among children with VF, small surveillance reports in east and southern Africa indicate between 48-71% may have DR mutations to non-nucleos(t)ide-reverse transcriptase inhibitor (NNRTI)-containing ART.[
6,
7,
8] This problem is likely to be worsening over time, with more ART exposure through earlier initiation and combination ART now used for prevention of perinatal transmission.[
9,
10] Despite recent rollout of integrase inhibitor regimens, primarily dolutegravir (DTG)-containing regimens, for CHIV, DR is likely to be a continued threat to the sustainable use of these regimens and the impact of common NRTI DR in CHIV is not fully understood.[
11,
12,
13]
Kenya is heavily burdened by HIV with an estimated 83,000 CHIV with around 60% on ART in 2021.[
14] A national pediatric HIV DR surveillance study conducted in 2013, revealed that at least a third of children had VF to first-line ART and over 90% of those had multiple DR.[
15] This survey, however, was conducted prior to the use of more efficacious ART regimens among CHIV, including protease inhibitors (PI)-containing ART, and universal ART for all pregnant women.[
15] The current levels and pattern of DR among CHIV with VF in Kenya are not known.
Understanding DR patterns and associated factors among children on ART in Kenya may inform algorithms used to manage children with VF. Here, we present DR testing (DRT) results from a randomized trial evaluating targeted DRT for children with VF accompanied by a collaborative, multidisciplinary case review to inform ART recommendations. We describe drug resistance patterns, characteristics associated with major drug resistance, and clinical outcomes of CHIV undergoing DRT from this trial.
2. Materials and Methods
The Optimizing Viral Suppression in Children on ART in Kenya (Opt4Kids) study protocol and primary findings have been described previously.[
16,
17] In brief, 704 CHIV ages 1-14 years were enrolled from five public facilities in Kisumu County, Kenya between March and December 2019 and followed for 12 months. Children were individually randomized 1:1 stratified by site and age groups (ages 1-9 years and 10-14 years) to the control (standard-of-care) or intervention groups (point-of-care viral load testing every three months with DRT for those with VF (HIV RNA
> 1000 copies/ml) and followed for 12 months. Participants in both groups underwent point of care viral load testing and targeted DRT, if indicated, at 12 months post-enrollment.
Study Procedures: Whole blood was collected from study participants for POC VL testing and separated into plasma for testing using GeneXpert system on site at study facilities or via daily transport to a facility less than 2 kilometers away.[
18] The study facilities participated in a quarterly external quality assurance program for HIV POC VL testing using GeneXpert and each facility passed each check. HIV DR testing was performed on plasma samples using Sanger sequencing with Applied Biosystems 3130xl Genetic Analyzers at the KEMRI-CDC HIV Research and Sanger 3730xl at the Kenya National HIV Reference Laboratories. These laboratories utilize validated, WHO-certified, optimized in-house assays to detect reverse transcriptase and PI mutations.[
19,
20] Integrase strand transfer inhibitor (INSTI) mutations were not routinely evaluated during the study period. The DRT results reports contained a list of the DR genotypes as well as phenotypic interpretations, based on the scoring systems generated by the Stanford Genotypic Resistance Interpretation Algorithm versions available during the study period.[
21]
Children in the intervention group underwent DRT at episodes of VF detection. A multidisciplinary committee, called the Clinical Management Committee (CMC), which included facility providers and peer leaders, HIV implementing partner technical advisors, chairperson of the local HIV Technical Working Group, and study principal investigators reviewed the DRT results to provide interpretation and clinical management recommendations. The CMC utilized a standardized Kenya Ministry of Health (MoH) case summary form, prepared by facility providers and research staff in advance of CMC review, to discuss the cases. CMC recommendations were summarized orally during virtual meetings and shared in writing along with DRT results to facility providers within one week.
For children in the control group, providers were instructed to follow current Kenya MoH guidelines for the management of any child with VF, which recommended enhanced adherence counseling for children with VF and repeat viral load (VL) testing after three months of provider-determined “good” adherence. DRT was limited to patients approved by the regional HIV Technical Working Group and generally included those failing a PI-containing regimen or with persistent VF despite good adherence. The Technical Working Group reviewed case summaries and DRT results, when available, and provided guidance to facility staff on patient management, though facility staff did not participate in working group meetings. At study end, participants in the control group underwent intervention procedures, including CMC review for those with DRT results.
Study Setting: First-line ART regimens in Kenya during the study period for children included lamivudine with either abacavir (preferred) or zidovudine (alternative) and lopinavir/ritonavir for those less than three years of age and lamivudine with either abacavir (preferred) or zidovudine (alternative) and efavirenz for those three years of age and older.[
22] Second-line ART regimens included change of NRTI medication from abacavir to zidovudine or vice versa depending on which the child was on as first-line with maintenance of lamivudine. Those on lopinavir/ritonavir as first-line required review by the HIV Technical Working Group to recommend a second-line regimen while those on efavirenz were recommended to switch to lopinavir/ritonavir. In 2020, the guidelines were updated to recommend dolutegravir (DTG) for those weighing at least 20 kilograms for treatment initiation and optimization (switch to DTG-containing regimen regardless of viral load), which was rolled out during the study period.[
23,
24]
Study Population: Children ages 1-14 years of age, enrolled at a study site, on or initiating ART, and with consenting caregiver were enrolled to the study. HIV care and treatment was provided by government staff per national guidelines.
Data Collection and Management: We abstracted routinely collected data from standardized MoH forms in medical files and registers using direct, electronic data entry via tablets into a REDCap database. Similarly, we entered study-collected data, including DRT results, in this REDCap database.
Primary analytic outcome: A participant was considered to have clinically significant DR if they had any mutation listed for NRTI and NNRTI drugs with a penalty score or if listed as “major” for PIs by Stanford’s Genotypic Resistance Interpretation Algorithm (i.e., Stanford HIVdb) on any DRT.21
Exposures and covariates: We selected potential risk factors
a priori, based on existing literature and content knowledge, which included age, sex, duration on ART, prior antiretroviral exposure, prior history of VF.[
25,
26] History of VF was defined as any VL result
> 1000 copies/ml within in two years prior to study enrollment. Given the exploratory nature of this analysis, we also included the following covariates: WHO stage, self-reported HIV status of primary caregiver at enrollment, clinic location urban, peri-urban, or rural and clinic volume defined as high, medium, and low based number of HIV patient visits per month at each facility.
Statistical Analysis: First, we describe the proportion of participants from either group who underwent DRT as part of the study intervention or at study end, per protocol, and the proportion with DR mutations detected by HIV drug classes, e.g., NRTIs, NNRTIs, and PIs. We report on prevalence of HIV DR by antiretroviral (ARV) medication using WHO’s definition of a penalty score of
> 15 using the Standford HIVdb algorithm.[
6,
21] We further estimate prevalence of the seven most common DR mutations (K65R, L74V/I, Y115F, M184V, K103N, Y181C, G190A) with 95% confidence intervals (CI).
Second, to evaluate which CHIV are most likely to have clinically relevant DR, we used logistic regression models to identify factors associated with clinically significant DR. Variables with p-values <0.20 in univariate analyses were included in the multivariate model.
Third, to explore any potential impact of DRT on clinical outcomes, we report descriptive statistics for outcomes including viral suppression, loss to follow up, and death by study group among those with DRT results, categorized by whether an ART regimen change occurred or not.
3. Results
A total of 704 children were enrolled in the study with a median age 9 years (interquartile range (IQR) 7,12), 344 (49%) were female, and median time on ART was 5 years (IQR 3, 8). A total of 349 (49.5%) and 355 (50.4%) of the CHIV were randomized to the intervention and control group, respectively. Overall, 382 (54.3%) of participants were on an NNRTI-containing regimen at study enrollment, 294 (41.8%) on a PI-containing regimen, 27 (3.8%) on an INSTI-containing regimen, and 1 (0.1%) on a PI and INSTI regimen. (
Table 1)
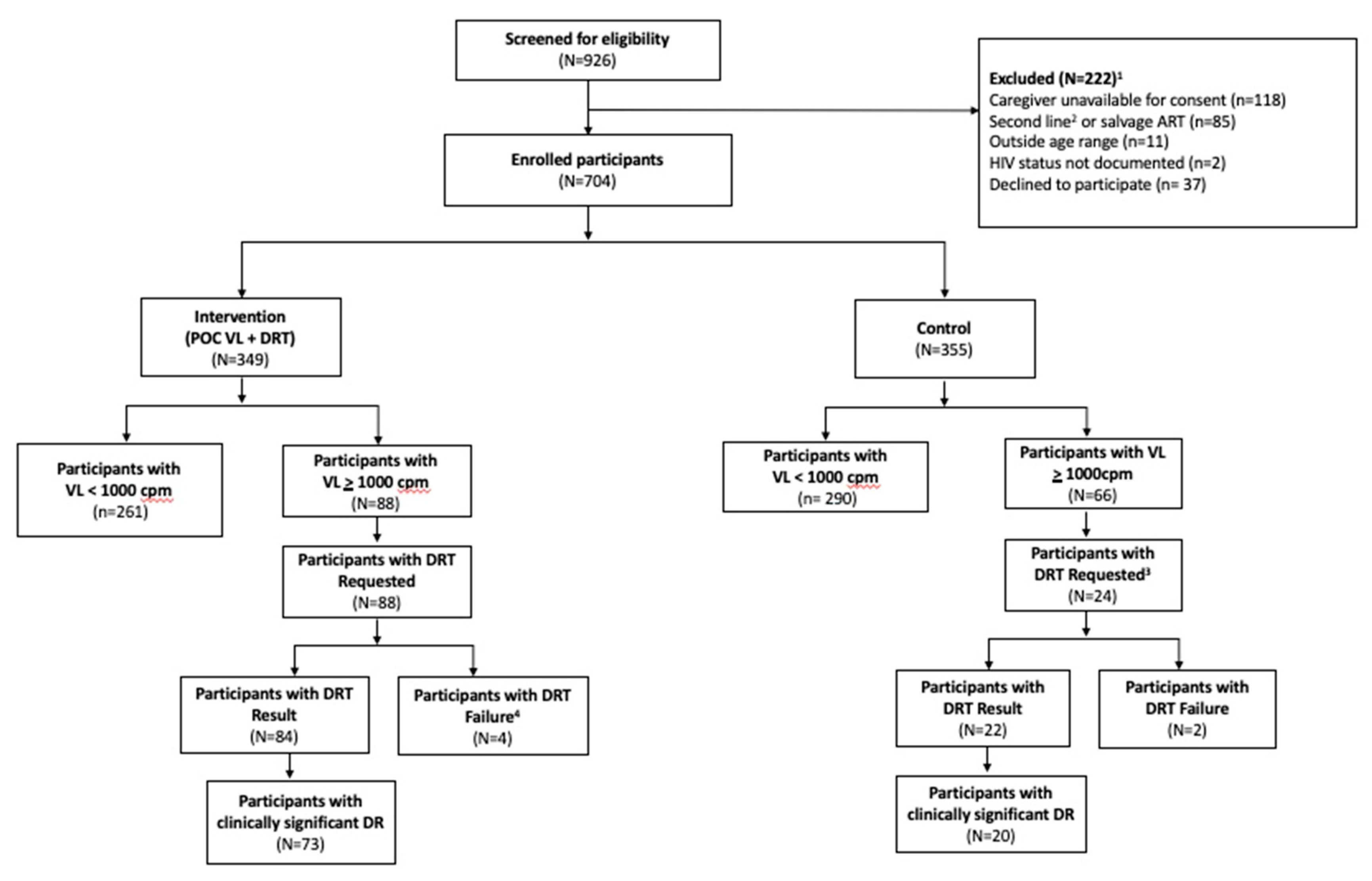
During the 12-month study period, 190 study DRTs were requested for 106 (15%) participants across the two study groups. Among intervention participants, 88 (25%) children experienced at least one episode of VF, and all had a least one DRT requested for a total of 166 DRT requests and 152 (92%) with results. (
Figure 1) A total of 66 (19%) children in the control group had a least one episode of viremia, including 24 (7.6%) control group participants with VF identified at the 12-month study visit, all of whom had a study DRT requested of which 22 (92%) with results. Prior to the final study visit in the control group, 57 (16%) were identified with VF; but not included in our DRT analyses; 5 (9%) of these had a DRT requested through the standard of care procedures with 2 (40%) having results. Of the 24 control group participants with VF at 12-month study visit, 15 also had earlier VF during the study.
Of the total 190 DRT requested by the study, 16 (9%) total samples from 16 participants did not yield a result, with 14 (87%) samples failing to amplify and 2 (13%) having insufficient volume to be tested. Fourteen participants in the intervention group had a “failed” result, of whom, 10 (71%) had successful subsequent repeat DRT. An additional 3 (21%) intervention participants re-suppressed to VL <1000/ml and had no further indication for DR testing. Two children in the control group who had DRT performed at 12-month visit per protocol that failed to amplify were referred for repeat of DRT by facility staff via routine care.
3.1. Drug resistance among children on ART with virologic failure
Among 106 participants with at least one DRT result, all demonstrated at least one major or minor DR mutation, as defined by Stanford HIV Database. A total of 93 (88.0%) had major mutations and 13 (12.1%) had minor mutations only (
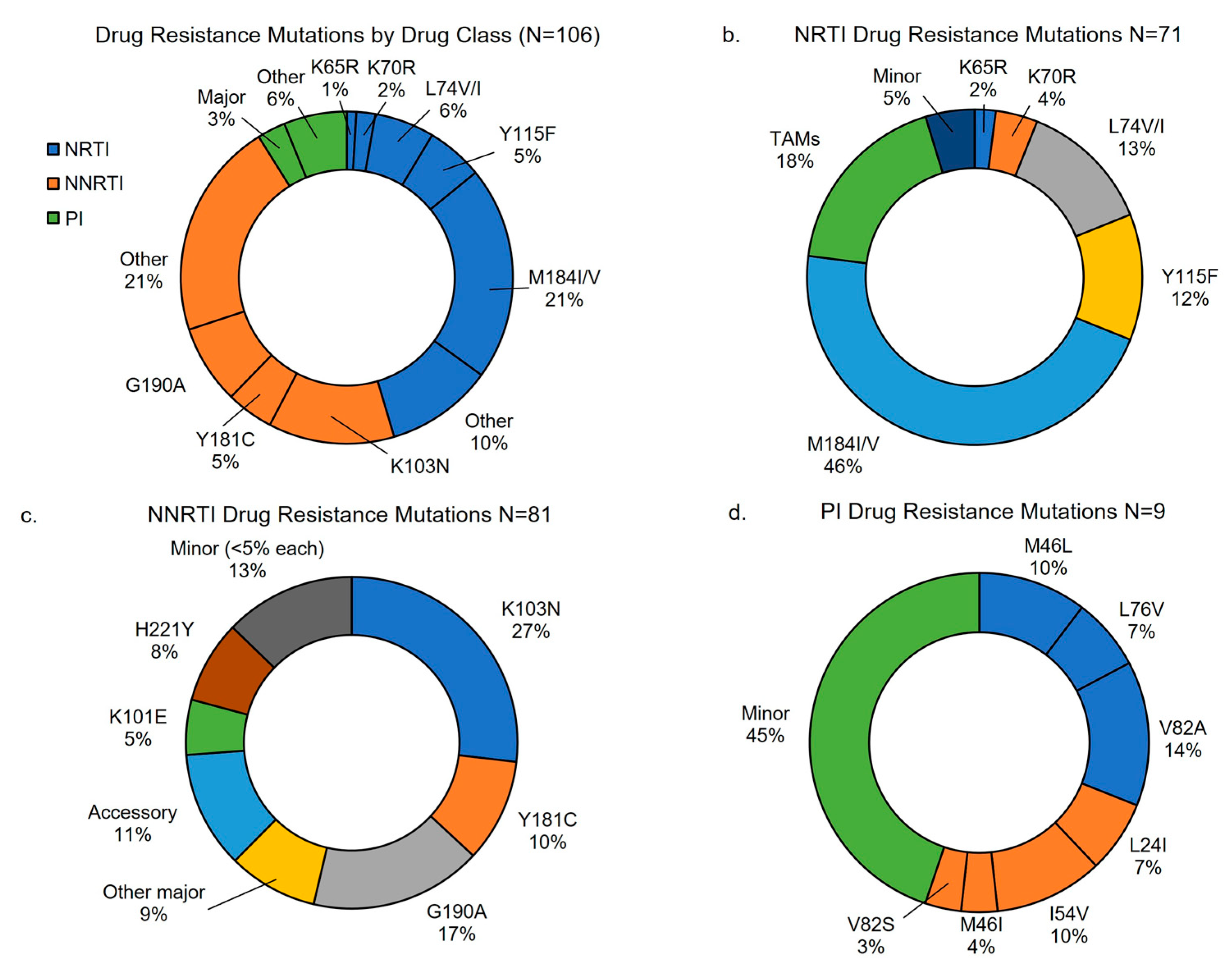
Table 1). In children with major mutations, 87 (92.6%) had NNRTI resistance, 70 (74.5%) had NRTI, and 10 (10.9%) had PI resistance. Additionally, more than half of children with major resistance had dual class resistance to NRTI and NNRTIs (n=51, 54.3%), two (2.1%) had dual class with either NRTI or NNRTI and PIs, and eight (8.5%) had triple class to NRTI, NNRTI, and PIs. The most common DR mutations identified were among NRTI and NNRTIs, with most common being M184V (57.0%, 95% CI 48%,67%) followed by K103N (35.8%, 95% CI 27%,45%). (
Table 2) Additional description of DR found by drug class are shown in
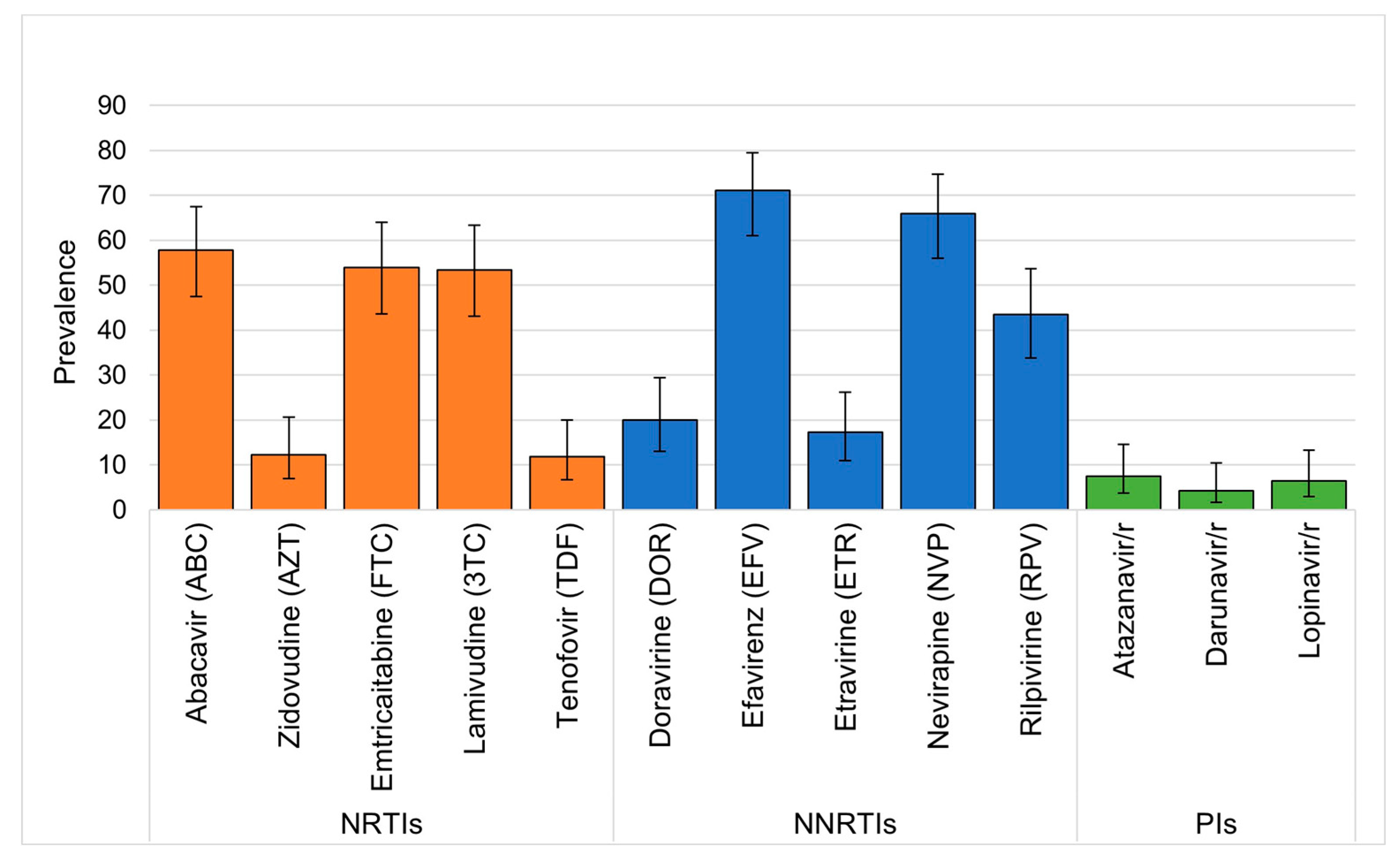
Figure 2. Over half of those with DRT results demonstrated DR to abacavir, emtricitabine, lamivudine, efavirenz, and nevirapine, while over 10% had resistance to zidovudine and tenofovir. (
Figure 3) DR to newer generation NNRTIs were also high, with nearly half having DR to rilpivirine and fewer having DR to etravirine and doravirine. Among the 88 CHIV in the intervention group of the study who had DRT requested, 47 (53%) had more than one DRT requested for repeat viremia during the study, whereas no CHIV in the control group had more than on DRT. Only three participants initially had minor resistance and later had major resistance on repeat DRT. Similarly, only six children had significant change in class of DR such as going from a single class with resistance to two or more drug classes. No children with change in resistance on follow up DRT had ART regimen changes between DRT.
Among the 88 CHIV in the intervention group of the study who had DRT requested, 47 (53%) had more than one DRT requested for repeat viremia during the study, whereas no CHIV in the control group had more than on DRT. Only three participants initially had minor resistance and later had clinically significant resistance on repeat DRT. Similarly, only six children had significant change in class of DR such as going from a single class with resistance to two or more drug classes. No children with change in resistance on follow up DRT had ART regimen changes between DRT.
3.2. Characteristics associated with major drug resistance
Association between participant characteristics and clinically significant DR are shown in
Table 1 and
Table 3. Median age and sex were similar between the overall cohort and DR groups (
Table 1). However, younger children 1-4 years of age had over 3-fold odds of major DR compared to older children aged 5-10 years or 11-14 years demonstrated (odds ratio (OR) 3.2, 95% CI 1.6, 6.1). Similarly, those on ART for < 2 years had and children on a PI-containing regimen at enrollment or first DRT had higher odds of major DR (OR 2.9, 95%CI 1.7-4.9, OR 1.9, 95%CI 1.2, 2.0 and OR 1.9, 95% CI 1.2,3.0 respectively ). (
Table 3) Further, a history of virologic failure in the 2 years prior to study enrollment was associated with a nearly 11-fold increased odds of major DR compared to no prior VF (95% CI 6.6, 18.5). In adjusted analysis, time on ART and history of virologic failure remained significantly associated with major DR. There were no associations with type of primary caregiver (e.g., mother, father, other family, or non-family member), HIV status of primary caregiver, household socioeconomic status, or facility volume or location between groups.
3.3. Clinical management and outcomes of children with DRT
The CMC carried out case reviews for all participants with DRT results and recommended an ART regimen change for 46 (43%) of 106 participants with a DRT. In the control group, 22 participants had a DRT after the 12-month study visit and 100% had any DR with 19 (86%) with major DR. Eight of those with results (36.4%) had recommendation for ART regimen change after clinical review. Control group participants were not followed beyond this final study visit.
In the intervention group, 38/88 (42.7%) were recommended to change ART after case review while 34 (42.5%) were advised to continue the current regimen. An additional 12 (13.5%) intervention participants underwent an ART change due to national transition to dolutegravir that was initiated by clinic providers. Of those with an ART change recommendation in the intervention group, 35 (92%) had changed to the recommended ART by study end. (
Table 4) All intervention participants with an ART change recommendation had major DR. Nearly a third (N=11, 28.9%) with an ART change recommendation were able to preserve their current NRTI medications based on DRT results, which would have otherwise been switched per in-country guidelines. A total of 31/34 (91.2%) children who did not have an ART change recommendation had major DR but their current regimen was still evaluated to be effective. Most children without an ART regimen change recommendation were on a PI-containing regimen 27/34 (79.4%) without major PI-resistance and no age-appropriate available medication alternatives (e.g., dolutegravir formulation not available or recommended for age at time of study). An additional three older participants were on an INSTI-containing regimen (specifically dolutegravir) and four were on an NNRTI (specifically efavirenz) without DR impacting the current regimen.
For intervention participants with DRT prior to final study visit (N=77), 42 (55%) were virally suppressed at study end. Viral suppression (VS) at the 12-month visit was observed in 21/36 (58%) with recommendation to change ART and 12/31 (39%) without recommendation to change ART (p=0.40)(
Table 4) Additionally, VS was observed in 9/10 (90%) with programmatic switch to DTG. Loss to follow up was higher in those without ART change recommendation (N=6, 19%) compared to those with an ART change recommendation (N=3, 8%) (p=0.28). One child died in the study who had VF and a DRT result that did not result in ART change recommendation.
In this cohort of over 700 children on ART in Kenya, detected high levels of DR in CHIV with VF. All children with VF had some DR, the majority of whom had major DR. Children on ART for > 2 years and those with a history of VF were significantly more likely to have major DR. We observed half of the CHIV with DR re-suppressed by study end following use of a multidisciplinary care team approach for case review and clinical management recommendations. Our study provides data on evolving DR patterns in CHIV and can inform prioritization approaches of DRT for vulnerable groups of CHIV as new ARV options become available.
4. Discussion
Our study identified major DR in most CHIV with VF. The last published comprehensive DR surveillance for children in Kenya was in 2013 before changes to recommend PI-containing ART for children less than three years of age.[
15] Over 90% of children in this national assessment were prescribed an NNRTI-containing regimen compared to less than 50% in our contemporary cohort. However, a similarly high proportion were found to have any DR (89%). More recent surveillance studies carried out in children in Lesotho, Uganda, and Zambia demonstrate high rates of NRTI (50-80%) and NNRTI (84-97%) resistance and low rates of PI resistance (4-6%) in children with VF. Related adult HIV DR surveillance studies carried out in 8 African countries between 2015 and 2019 also show high rates of NRTI and NNRTI DR among those with VF on an NNRTI-containing regimen. Low rates of PI resistance in children with VF show that despite transition to PI-containing regimens for a significant proportion of children, PI medications (specifically lopinavir/ritonavir among our cohort) maintained a high barrier to resistance. However, PI-containing regimens remain less attractive than alternatives; adult surveillance studies and one study in children from Zambia consistently identify lower viral suppression among people on PI-containing regimens compared to INSTI- or NNTRI- containing regimens, likely due to non-adherence.[
6] Additionally, the need to dose twice daily and the side effect profile of lopinavir/ritonavir support transition away from this PI-drug in children when other options are available.
Developing strategies to optimize cost-effective use for targeted DRT among CHIV with VF is key to achieving higher rates of viral suppression in CHIV. A prior history of VF was a strong predictor of major DR. This finding is important as many country guidelines do not take this into account in algorithms to guide management of children with VF. Studies in both adults and children have demonstrated accumulation of new drug resistance with continued virologic failure.[
27,
28] Additionally, children on ART for less than 2 years with VF had higher odds of major DR compared to CHIV on ART for longer periods. It is possible that children on ART for shorter periods were younger children who received antiretroviral prophylaxis during breastfeeding selecting for pretreatment DR.[
29] Pre-treatment DR is a growing concern noted by WHO and others and NNRTI resistance has increased to a pooled estimate of 46% in infants whereas it is less than 15% in newly initiating adults.[
6] We had very few children newly initiating ART and so are unable to estimate pre-treatment DR in this cohort.
Our findings support broader use of DRT in CHIV which is lacking in many settings.[
30] While many HIV providers in LMIC lack experience interpreting DRT, our multidisciplinary virtual approach to case reviews was feasible and highly acceptable. Given that nearly 60% of children with prior VF in the last two years demonstrated major DR in our study, guidelines should consider if DRT should be utilized sooner in children with persistent VF since adherence interventions alone may be insufficient. DRT may also allow for preservation of NRTIs in children with VF which is important given limited ARV options for CHIV and the potential side effects of less preferred NRTIs such as zidovudine. Even when major DR is not identified by DRT, these findings can inform clinic staff of the need to re-focus on psychosocial and structural factors contributing to non-adherence.
The rapid transition to DTG-containing regimens in children provides hope for improved VS in all CHIV including for those with VF. Our findings showed high viral resuppression in a small number of children with VF who were switched to a DTG-containing regimen. How DRT should be used in the setting of wide-spread DTG use in children remains uncertain. NRTI mutations were highly prevalent in CHIV with VF in this cohort similar to other reports in children.[
6] Over half of children in our cohort were resistant to abacavir and lamivudine and over 10% to tenofovir. This is concerning given these drugs are part of current 1
st line regimens co-formulated with DTG for CHIV in Kenya and other LMIC. While some adult studies show DTG-containing regimens may remain suppressive even in the setting of NRTI DR, this remains to be demonstrated in CHIV.[
31,
32] Kenya’s current treatment guidelines recommend switching to PI-containing regimens for children who fail on DTG, but as noted in our study and others,[
6]those on PI-containing regimens as second line were less likely to resuppress.[
6] Kenya has incorporated recommendations for DRT in CHIV who experience VF on DTG, but subsequent regimen sequencing is uncertain especially in light of frequent NRTI DR. Our results also show a concerningly high level of DR to rilpvirine, threatening the use of highly anticipated long-acting injectable caobtegravir/rilpivrine for CHIV. Similar results in adults in South Africa prompted investigators to suggest that DRT may be required before long-acting injectable ART can be used in LMIC.[
33,
34] Thus, there is likely an important role for targeted DRT in determining how best to manage CHIV with VF while on DTG.
Limitations of this study include the inability to determine pre-treatment DR due to few children who were initiating ART in this cohort and lack of data on maternal ART and DR history which is likely to correlate with DR identified in participants. Additionally, our study utilized DRT laboratories in Kenya to optimize generalizability of DRT use in CHIV but, INSTI DR testing was limited during the study period. While we observed a rapid transition to DTG-containing regimens, we were not able to perform INSTI DR on CHIV with VF on DTG. We plan to use stored samples to further explore these issues.
5. Conclusions
Findings from this study demonstrated high levels of major DR in children living with VF. Providers and policy makers should consider identified factors associated with major DR when considering which children may benefit most from DRT while it remains a limited resource. Further research is needed to understand how DRT may be optimally used among children who are now mostly using DTG-containing regimens.
Author Contributions
Conceptualization, L.A., R.P., P.O., I.M., and K.T..; methodology, L.A., R.P., P.O., B.C., and K.T.; formal analysis, G.W., K.T., L.A., R.P..; investigation, L.A., R.P., P.O., I.M., J.W., L.K., E.K., and K.T.; software: N.Y., K.T., B.O., and L. K.; resources, P.O., L.A., R.P., J.W., E.K., F.O., F.O., B.O., L.K., E.K., N.Y., L.O.,I.M., and K.T.; data curation, F.W., K.T., A. S., N.O., and B.A.; writing—original draft preparation, L.A., R. P., P. O., K.T., and G.W.; writing—review and editing, all; visualization, A.S., K.T., and S.H.; supervision, E.B., P.O., K.T.< J.W.< E.K., F.O., F.O., B.O., L.K., E.K., N.Y., L.O., R.P., and L.A.; project administration, all.; funding acquisition, R.P., L.A., and P. O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Mental Health of the U.S. National Institutes of Health (NIH, R34MH115769) and the Thrasher Research Fund. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington Institute of Translational Health Sciences and supported by the National Center for Advancing Translational Sciences of the NIH (UL1 TR002319). The funding sources or study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Boards of (protocol code XXX and date of approval) the African Medical and Research Foundation (AMREF) (Protocol P545, approved November 27, 2018) and Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) (Protocol GEN/21A/V94, approved September 19, 2019) in Kenya, as well as the University of Washington (Protocol 00004861, approved June 1, 2018) and the University of Colorado Denver (Protocol 18-1702, approved October 2, 2018) in the United States. .
Informed Consent Statement
Informed consent was obtained from legal guardians for all subjects involved in the study and assent for those 13 years of age and older.
Data Availability Statement
The de-identified data presented in this study are available on request from the corresponding author. The data are not publicly available due to the sensitive nature of the disease being studied.
Acknowledgments
We recognize the study participants, caregivers, and facility staff participating in and supporting the Opt4Kids study. We acknowledge the support of the Kisumu County Health Management Team, Ministry of Health, and Family AIDS Care and Education Services. We also thank members of our Scientific Advisory Committee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Penazzato M, Irvine C, Vicari M, Essajee SM, Sharma A, Puthanakit T, et al. A Global Research Agenda for Pediatric HIV. J Acquir Immune Defic Syndr. 2018;78 Suppl 1:S10-S5. [CrossRef]
- Abrams EJ, Strasser S. 90-90-90--Charting a steady course to end the paediatric HIV epidemic. J Int AIDS Soc. 2015;18(Suppl 6):20296. Epub 2015/12/08.
- Suboptimal Viral Suppression Rates Among HIV-Infected Children in Low- and Middle-Income Countries: A Meta-analysis. . Clin Infect Dis 2016;63(12):1645-1654.
- Organization, WH. Global report on early warning indicators of HIV drug resistance.. Geneva, Switzerland: 2016.
- World Health Organization (WHO). Global Action Plan on HIV Drug Resistance 2017-2021. 2021.
- World Health Organization. HIV Drug Resistance Report 2021. Geneva, Switzerland: WHO; 2021.
- Wamalwa DC, Lehman DA, Benki-Nugent S, Gasper MA, Gichohi R, Maleche-Obimbo E, et al. Long-term virologic response and genotypic resistance mutations in HIV-1 infected Kenyan children on combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;62(3):267-74. [CrossRef]
- Hackett S, Teasdale CA, Pals S, Muttiti A, Mogashoa M, Chang J, et al. Drug Resistance Mutations Among South African Children Living With HIV on WHO-recommended ART Regimens. Clin Infect Dis. 2021;73(7):e2217-e25. Epub 2020/08/01. [CrossRef]
- Crowell CS, Maiga AI, Sylla M, Taiwo B, Kone N, Oron AP, et al. High Rates of Baseline Drug Resistance and Virologic Failure Among ART Naive HIV-Infected Children in Mali. The Pediatric infectious disease journal. 2017. [CrossRef]
- Soeria-Atmadja S, Amuge P, Nanzigu S, Bbuye D, Rubin J, Eriksen J, et al. Pretreatment HIV drug resistance predicts accumulation of new mutations in ART-naïve Ugandan children. Acta Paediatr. 2020;109(12):2706-16. Epub 2020/04/19. [CrossRef]
- World Health Organization. Priorities for antiretroviral drug optimization in adults and children: report of a CADO, PADO and HIVResNet joint meeting. Geneva, Switzerland: WHO; 2021. Available from: https://www.who.int/publications/i/item/9789240053038.
- Turkova A, White E, Mujuru HA, Kekitiinwa AR, Kityo CM, Violari A, et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N Engl J Med. 2021;385(27):2531-43. Epub 2021/12/30. [CrossRef]
- Turkova A and Namusoke Magongo E, editor Debate: DTG Transition: A Switch to DTG Must be Accompanied by Change in NRTI Backbone in Children. International Workshop on HIV and Pediatrics,; 2022.
- Joint United Nations Programme on HIV/AIDS. UNAIDS Kenya-HIV and AIDS Estimates 2020.
- Health KMo. Report of the 2013 cross-sectional survey of acquired HIV drug resistance among adults and children on antiretroviral therapy at sentinel sites in Kenya.. In: Program NAaSC, editor. Nairobi, Kenya2016.
- Patel, RC. Impact of point-of-care HIV viral load and targeted drug resistance mutation testing on viral suppression among Kenyan children on antiretroviral therapy: Results from an open-label, randomized controlled trial (Opt4Kids) Unpublished manuscript. 2022.
- Patel RC, Oyaro P, Odeny B, Mukui I, Thomas KK, Sharma M, et al. Optimizing viral load suppression in Kenyan children on antiretroviral therapy (Opt4Kids). Contemp Clin Trials Commun. 2020;20:100673. Epub 2020/11/17. [CrossRef]
- Bwana P AoJ, Mwau M.. Performance and usability of Cepheid GeneXpert HIV-1 qualitative and quantitative assay in Kenya.. PLoS One 2019; 14(3): e0213865. 2019.
- Organization, WH. Organization WH. List of WHO-designated laboratories for HIV drug resistance surveillance, November 2022. 2022.
- Laboratories KNPH. National HIV Research Lab 2022 [cited 2023 ]. Available from: https://nphl.go.ke/national-hiv-reference-laboratory-nhrl.
- University, S. HIV Drug Resistance Database 2021. Available from: https://hivdb.stanford.edu.
- NASCOP. Ministry of Health, National AIDS & STI Control Program. New guidance on ART transition for children and adolescents less than 15 years of age living with HIV (CALHIV) in Kenya. 2019. Print. Nairobi, Kenya: August 2018.
- Ministry of Health NASCPN. DTG Use of Dolutegravir based Regimen in Adolescent Girls & Women Living with HIV. Nairobi, Kenya: NASCOP, 2020 Print. 2020 Edition.
- Ministry of Health NASCPN. Use of Dolutegravir based regimen in Adolescent Girls and Women Living with HIV Nairobi, Kenya:. Nairobi, Kenya.
- Muri L, Gamell A, Ntamatungiro AJ, Glass TR, Luwanda LB, Battegay M, et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS. 2017;31(1):61-70. Epub 2016/09/28. [CrossRef]
- Nyandiko W, Holland S, Vreeman R, DeLong AK, Manne A, Novitsky V, et al. HIV-1 Treatment Failure, Drug Resistance, and Clinical Outcomes in Perinatally Infected Children and Adolescents Failing First-Line Antiretroviral Therapy in Western Kenya. J Acquir Immune Defic Syndr. 2022;89(2):231-9. Epub 2021/11/02. [CrossRef]
- Boender TS, Kityo CM, Boerma RS, Hamers RL, Ondoa P, Wellington M, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother. 2016;71(10):2918-27. Epub 2016/06/28. [CrossRef]
- Kityo C, Boerma RS, Sigaloff KCE, Kaudha E, Calis JCJ, Musiime V, et al. Pretreatment HIV drug resistance results in virological failure and accumulation of additional resistance mutations in Ugandan children. J Antimicrob Chemother. 2017;72(9):2587-95. [CrossRef]
- Boerma RS, Sigaloff KC, Akanmu AS, Inzaule S, Boele van Hensbroek M, Rinke de Wit TF, et al. Alarming increase in pretreatment HIV drug resistance in children living in sub-Saharan Africa: a systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(2):365-71. Epub 2016/12/22. [CrossRef]
- Koay WLA, Kose-Otieno J, Rakhmanina N. HIV Drug Resistance in Children and Adolescents: Always a Challenge? Curr Epidemiol Rep. 2021;8(3):97-107. Epub 2021/03/25. [CrossRef]
- Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700-8. Epub 2013/07/09. [CrossRef]
- Aboud M, Kaplan R, Lombaard J, Zhang F, Hidalgo JA, Mamedova E, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253-64. Epub 2019/02/09. [CrossRef]
- Steegen K, Chandiwana N, Sokhela S, Venter WDF, Hans L. Impact of rilpivirine cross-resistance on long-acting cabotegravir-rilpivirine in low and middle-income countries. Aids. 2023;37(6):1009-11. Epub 2023/02/14. https://doi.org/10.1097/qad.0000000000003505. PubMed PMID: 36779485; PubMed Central PMCID: PMCPMC10090297 drug donations from ViiV, Merck, Gilead, and J&J, for treatment and PrEP, on long-acting injectables. W.D.F.V. has accepted honoraria for ad boards and talks for ViiV, Merck, Gilead, and J&J. N.C. has accepted honoraria from Cipla, Johnson and Johnson, Frontiers Biotech, outside the submitted work. K.S. and L.H. have no conflicts of interest to declare.
- Cervo A, Russo A, Di Carlo D, De Vito A, Fabeni L, D'Anna S, et al. Long-acting combination of cabotegravir plus rilpivirine: A picture of potential eligible and ineligible HIV-positive individuals from the Italian ARCA cohort. J Glob Antimicrob Resist. 2023;34:141-4. Epub 2023/07/16. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).