Submitted:
23 August 2023
Posted:
28 August 2023
You are already at the latest version
Abstract
Keywords:
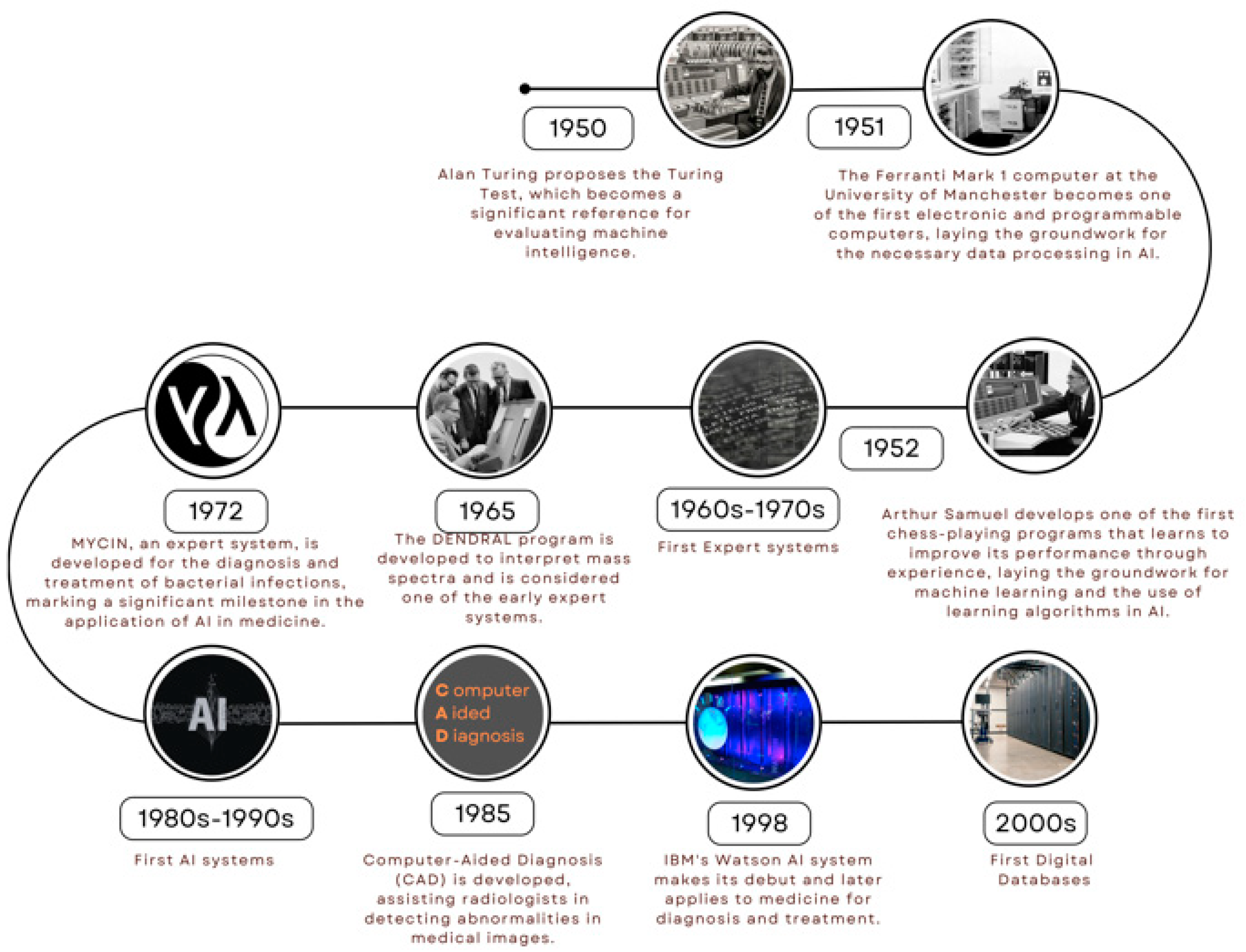
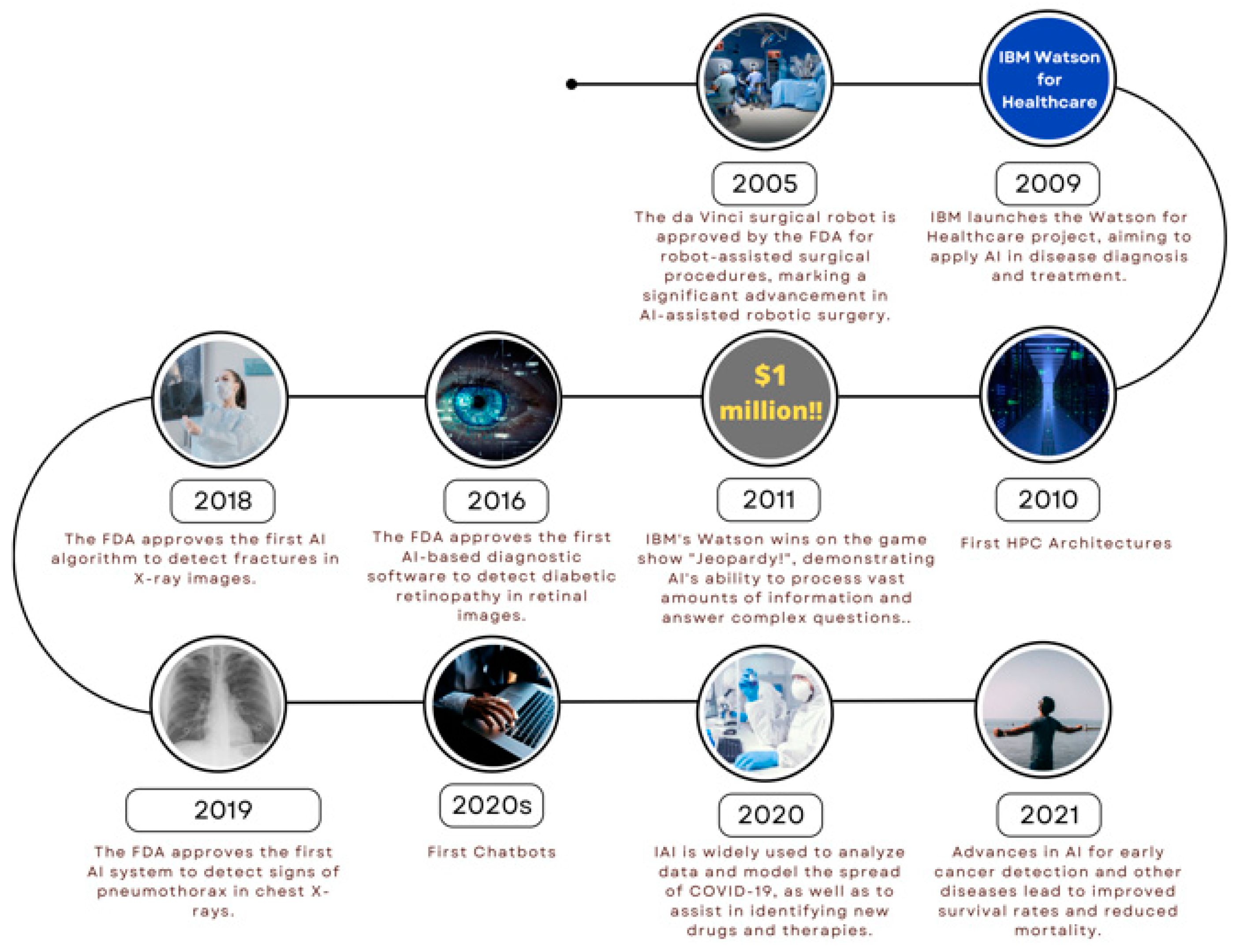
1. Introduction
2. Neural network
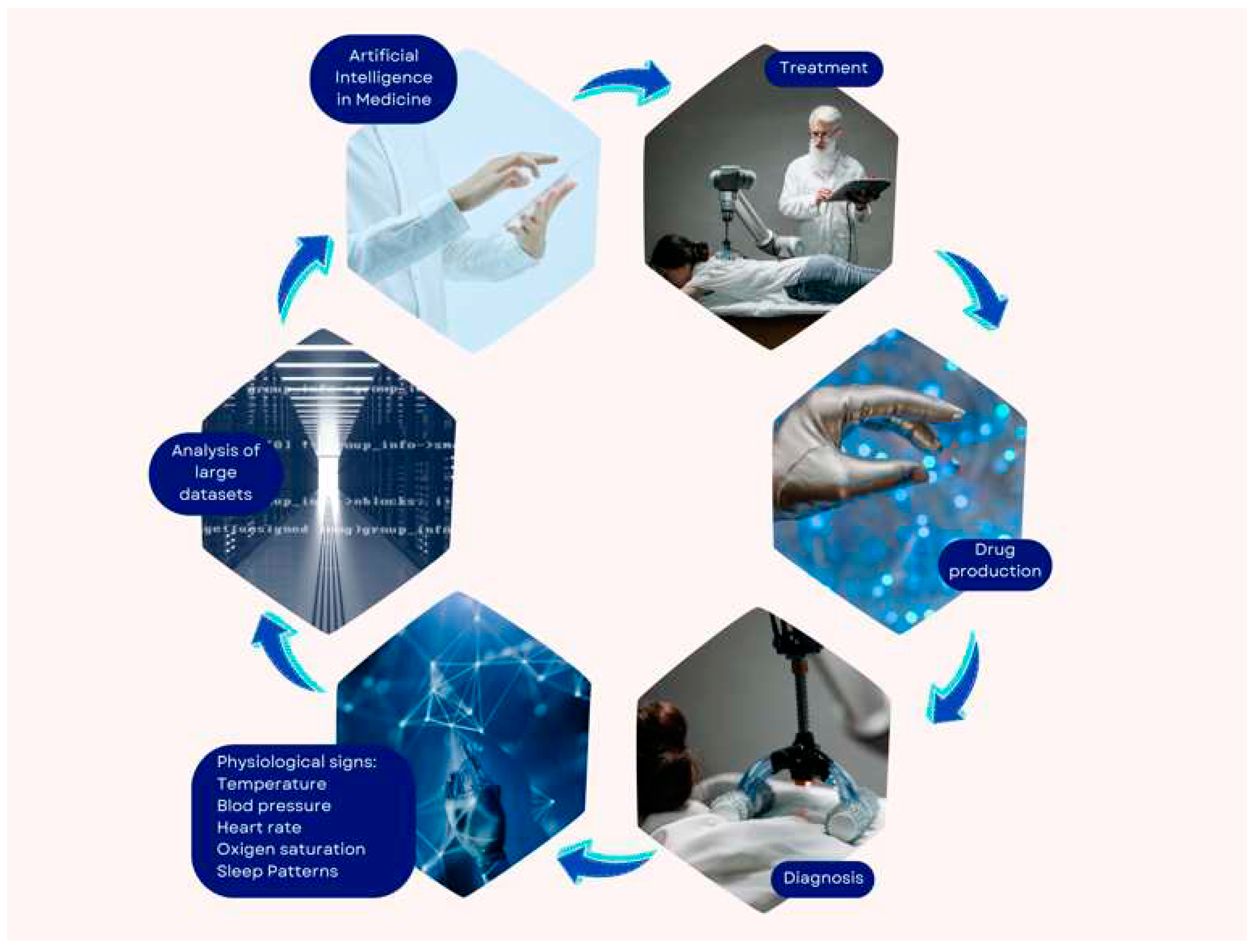
3. Recent advances in artificial intelligence in the field of therapeutics
3.1. Medical diagnosis
3.2. Medical treatment
3.3. Drug design
4. AI Applications in Cardiac Diagnosis
4.1. Machine Learning
4.2. Deep Learning
4.3. Neural Networks
5. AI in cardiac diagnosis based on physiological signs
6. Advantages and Challenges of New Programmatic AI Applications in Cardiac Diagnosis
6.1. Improved accuracy and speed of diagnosis
6.2. Early disease detection
6.3. Supplying personalized recommendations
7. Significant challenges and constraints
7.1. Need for vast data sets:
7.2. Interpretability of results
7.3. Clinical practice integration
7.4. Data privacy and security
7.5. Clinical validation
8. Future Applications of the AI
9. Relevance of the ongoing research as well as the development of AI approaches
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amisha; Malik, P.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Razavian, N.; Knoll, F.; Geras, K.J. Artificial Intelligence Explained for Nonexperts. Semin. Musculoskelet. Radiol. 2020, 24, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial intelligence in healthcare: past, present and future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Bogojeski, M.; Vogt-Maranto, L.; Tuckerman, M.E.; Müller, K.-R.; Burke, K. Quantum chemical accuracy from density functional approximations via machine learning. Nat. Commun. 2020, 11, 5223. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Soto, J.T.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Haq, I.U.; Chhatwal, K.; Sanaka, K.; Xu, B. Artificial Intelligence in Cardiovascular Medicine: Current Insights and Future Prospects. Vasc. Heal. Risk Manag. 2022, ume 18, 517–528. [Google Scholar] [CrossRef]
- Namikawa, K.; Hirasawa, T.; Yoshio, T.; Fujisaki, J.; Ozawa, T.; Ishihara, S.; Aoki, T.; Yamada, A.; Koike, K.; Suzuki, H.; et al. Utilizing artificial intelligence in endoscopy: a clinician’s guide. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 689–706. [Google Scholar] [CrossRef]
- Liang, G.; Fan, W.; Luo, H.; Zhu, X. The emerging roles of artificial intelligence in cancer drug development and precision therapy. BioMedicine 2020, 128, 110255. [Google Scholar] [CrossRef]
- Turing, A.M. Computing machinery and intelligence. Mind 1950, 49, 433–460. [Google Scholar] [CrossRef]
- Wang, S.; Yang, D.M.; Rong, R.; Zhan, X.; Xiao, G. Pathology Image Analysis Using Segmentation Deep Learning Algorithms. Am. J. Pathol. 2019, 189, 1686–1698. [Google Scholar] [CrossRef]
- Attia, Z.I.; A Noseworthy, P.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; E Carter, R.; Yao, X.; A Rabinstein, A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, M.; Cui, T.; Hauskrecht, M. Monitoring ICU Mortality Risk with A Long Short-Term Memory Recurrent Neural Network. Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing 2020, 25, 103–114. [Google Scholar] [PubMed]
- Whittington JC, R.; Bogacz, R. Theories of Error Back-Propagation in the Brain. Trends in cognitive sciences 2019, 23, 235–250. [Google Scholar] [CrossRef] [PubMed]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H.; Back, T.; Chesus, M.; Corrado, G.S.; Darzi, A.; et al. International evaluation of an AI system for breast cancer screening. Nature 2020, 577, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Kim, K.W.; Kim, S.; Youn, Y.C. Artificial Neural Network: Understanding the Basic Concepts without Mathematics. Dement. Neurocognitive Disord. 2018, 17, 83–89. [Google Scholar] [CrossRef]
- McCulloch, W.S.; Pitts, W. A logical calculus of the ideas immanent in nervous activity. 1943. Bulletin of mathematical biology 1990, 52, 97–99. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. Machine learning can predict survival of patients with heart failure from serum creatinine and ejection fraction alone. BMC Med Informatics Decis. Mak. 2020, 20, 16. [Google Scholar] [CrossRef]
- Mortazavi, B.J.; Downing, N.S.; Bucholz, E.M.; Dharmarajan, K.; Manhapra, A.; Li, S.-X.; Negahban, S.N.; Krumholz, H.M.; D, H.; K, K.; et al. Analysis of Machine Learning Techniques for Heart Failure Readmissions. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B.J.; Downing, N.S.; Bucholz, E.M.; Dharmarajan, K.; Manhapra, A.; Li, S.-X.; Negahban, S.N.; Krumholz, H.M.; D, H.; K, K.; et al. Analysis of Machine Learning Techniques for Heart Failure Readmissions. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Petmezas, G.; Stefanopoulos, L.; Kilintzis, V.; Tzavelis, A.; A Rogers, J.; Katsaggelos, A.K.; Maglaveras, N. State-of-the-Art Deep Learning Methods on Electrocardiogram Data: Systematic Review. Psychopharmacol. 2022, 10, e38454. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Ghassemi, M.M.; Clifford, G.D. Optimal medication dosing from suboptimal clinical examples: a deep reinforcement learning approach. Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference 2016, 2016, 2978–2981. [Google Scholar] [PubMed]
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep Learning: A Primer for Radiologists. Radiographics 2017, 37, 2113–2131. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, K.; Balla, S.; Bianco, C.; Cheung, J.; Pachulski, R.; Asti, D.; Nalluri, N.; Tejpal, A.; Mir, P.; Shah, J.; et al. Applications of Machine Learning in Cardiology. Cardiol. Ther. 2022, 11, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Iniesta, R.; Stahl, D.; McGuffin, P. Machine learning, statistical learning and the future of biological research in psychiatry. Psychol. Med. 2016, 46, 2455–2465. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep learning. MIT Press. LeCun, Y., Bengio, Y., Hinton, G. (2015). Deep learning. Nature 2016, 521, 436–444. [Google Scholar]
- Seetharam, K.; Balla, S.; Bianco, C.; Cheung, J.; Pachulski, R.; Asti, D.; Nalluri, N.; Tejpal, A.; Mir, P.; Shah, J.; et al. Applications of Machine Learning in Cardiology. Cardiol. Ther. 2022, 11, 355–368. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, R.; Stahl, D.; McGuffin, P. Machine learning, statistical learning and the future of biological research in psychiatry. Psychol. Med. 2016, 46, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep learning. MIT Press. LeCun, Y., Bengio, Y., Hinton, G. (2015). Deep learning. Nature 2016, 521, 43–444. [Google Scholar]
- Remedios, S.M.; Armstrong, D.P.; Graham, R.B.; Fischer, S.L. Exploring the Application of Pattern Recognition and Machine Learning for Identifying Movement Phenotypes During Deep Squat and Hurdle Step Movements. Front. Bioeng. Biotechnol. 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Ouyang, D.; Abid, A.; He, B.; Chen, J.H.; Harrington, R.A.; Liang, D.H.; Asley, E.A.; Zou, J.Y. Deep learning interpretation of echocardiograms. NPJ. Digit. Med. 2020, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Qin, T.; Xi, Z.; Sun, L.; Wu, H.; Li, D. 3D printing in adult cardiovascular surgery and interventions: a systematic review. J. Thorac. Dis. 2020, 12, 3227–3237. [Google Scholar] [CrossRef]
- Holzinger, A.; Langs, G.; Denk, H.; Zatloukal, K.; Müller, H. Causability and explainability of artificial intelligence in medicine. WIREs Data Min. Knowl. Discov. 2019, 9. [Google Scholar] [CrossRef]
- Ozdemir, M.A.; Ozdemir, G.D.; Guren, O. Classification of COVID-19 electrocardiograms by using hexaxial feature mapping and deep learning. BMC Med Informatics Decis. Mak. 2021, 21, 170. [Google Scholar] [CrossRef]
- Roshanov, P.S.; You, J.J.; Dhaliwal, J.; Koff, D.; Mackay, J.A.; Weise-Kelly, L.; Navarro, T.; Wilczynski, N.L.; Haynes, R.B.; CCDSS Systematic Review Team. Can computerized clinical decision support systems improve practitioners’ diagnostic test ordering behavior? A decision-maker-researcher partnership systematic review. Implement. Sci. 2011, 6, 88. [Google Scholar] [CrossRef]
- Davis, A.; Billick, K.; Horton, K.; Jankowski, M.; Knoll, P.; Marshall, J.E.; Paloma, A.; Palma, R.; Adams, D.B. Artificial Intelligence and Echocardiography: A Primer for Cardiac Sonographers. Journal of the American Society of Echocardiography official publication of the American Society of Echocardiography 2020, 33, 1061–106. [Google Scholar] [CrossRef]
| Bibliography |
| da Silva, H. P., Lourenço, A., Fred, A., & Martins, R. (2014). BIT: Biosignal Igniter Toolkit. Computer methods and programs in biomedicine, 115(1), 20–32. |
| Choi, A., Chung, K., Chung, S. P., Lee, K., Hyun, H., & Kim, J. H. (2022). Advantage of Vital Sign Monitoring Using a Wireless Wearable Device for Predicting Septic Shock in Febrile Patients in the Emergency Department: A Machine Learning-Based Analysis. Sensors (Basel, Switzerland), 22(18), 7054. |
| Alqaraawi, A., Alwosheel, A., & Alasaad, A. (2016). Heart rate variability estimation in photoplethysmography signals using Bayesian learning approach. Healthcare technology letters, 3(2), 136–142. |
| Tzevelekakis, K., Stefanidi, Z., & Margetis, G. (2021). Real-Time Stress Level Feedback from Raw Ecg Signals for Personalised, Context-Aware Applications Using Lightweight Convolutional Neural Network Architectures. Sensors (Basel, Switzerland), 21(23), 7802. |
| Tzevelekakis, K., Stefanidi, Z., & Margetis, G. (2021). Real-Time Stress Level Feedback from Raw Ecg Signals for Personalised, Context-Aware Applications Using Lightweight Convolutional Neural Network Architectures. Sensors (Basel, Switzerland), 21(23), 7802. |
| Sabry, F., Eltaras, T., Labda, W., Alzoubi, K., & Malluhi, Q. (2022). Machine Learning for Healthcare Wearable Devices: The Big Picture. Journal of healthcare engineering, 2022, 4653923. |
| Kwon, K., Kwon, S., & Yeo, W. H. (2022). Automatic and Accurate Sleep Stage Classification via a Convolutional Deep Neural Network and Nanomembrane Electrodes. Biosensors, 12(3), 155. |
| Wang, T., Lu, C., & Shen, G. (2019). Detection of Sleep Apnea from Single-Lead ECG Signal Using a Time Window Artificial Neural Network. BioMed research international, 2019, 9768072. |
| Song, J., Li, J., Zhao, R., & Chu, X. (2023). Developing predictive models for surgical outcomes in patients with degenerative cervical myelopathy: a comparison of statistical and machine learning approaches. The spine journal: official journal of the North American Spine Society, S1529-9430(23)03315-6. |
| Wijsman, J., Grundlehner, B., Liu, H., Hermens, H., & Penders, J. (2011). Towards mental stress detection using wearable physiological sensors. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2011, 1798–1801. |
| Frey, L., Menon, C., & Elgendi, M. (2022). Blood pressure measurement using only a smartphone. NPJ digital medicine, 5(1), 86. |
| Baig, M. M., GholamHosseini, H., Moqeem, A. A., Mirza, F., & Lindén, M. (2017). A Systematic Review of Wearable Patient Monitoring Systems - Current Challenges and Opportunities for Clinical Adoption. Journal of medical systems, 41(7), 115. |
| Chowdhury, M. H., Shuzan, M. N. I., Chowdhury, M. E. H., Mahbub, Z. B., Uddin, M. M., Khandakar, A., & Reaz, M. B. I. (2020). Estimating Blood Pressure from the Photoplethysmogram Signal and Demographic Features Using Machine Learning Techniques. Sensors (Basel, Switzerland), 20(11), 3127. |
| Yin, J., Xu, J., & Ren, T. L. (2023). Recent Progress in Long-Term Sleep Monitoring Technology. Biosensors, 13(3), 395. |
| Parak, J., & Korhonen, I. (2014). Evaluation of wearable consumer heart rate monitors based on photopletysmography. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2014, 3670–3673. |
| Matsubara, T., Sato, K., Hama, K., Tachibana, R., & Uehara, K. (2022). Deep Generative Model Using Unregularized Score for Anomaly Detection with Heterogeneous Complexity. IEEE transactions on cybernetics, 52(6), 5161–5173. |
| Iqbal, T., Elahi, A., Wijns, W., & Shahzad, A. (2022). Exploring Unsupervised Machine Learning Classification Methods for Physiological Stress Detection. Frontiers in medical technology, 4, 782756. |
| Gonzalez-Abreu, A. D., Osornio-Rios, R. A., Elvira-Ortiz, D. A., Jaen-Cuellar, A. Y., Delgado-Prieto, M., & Antonino-Daviu, J. A. (2023). Power Disturbance Monitoring through Techniques for Novelty Detection on Wind Power and Photovoltaic Generation. Sensors (Basel, Switzerland), 23(6), 2908. |
| Sathyanarayana, A., Joty, S., Fernandez-Luque, L., Ofli, F., Srivastava, J., Elmagarmid, A., Arora, T., & Taheri, S. (2016). Sleep Quality Prediction from Wearable Data Using Deep Learning. JMIR mHealth and uHealth, 4(4), e125. |
| Verkruysse, W., Svaasand, L. O., & Nelson, J. S. (2008). Remote plethysmographic imaging using ambient light. Optics express, 16(26), 21434–21445. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




