Submitted:
15 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. INTRODUCTION
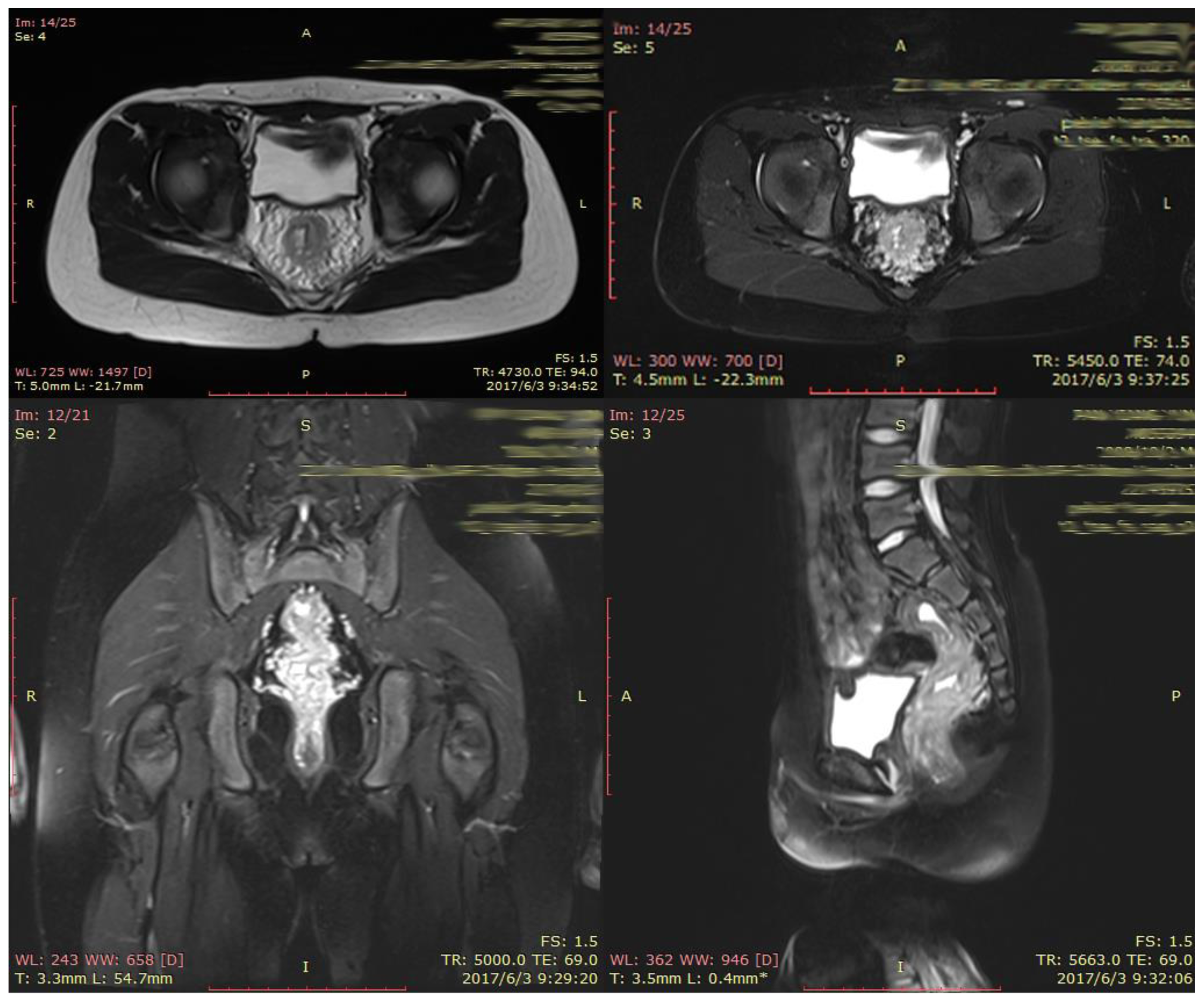
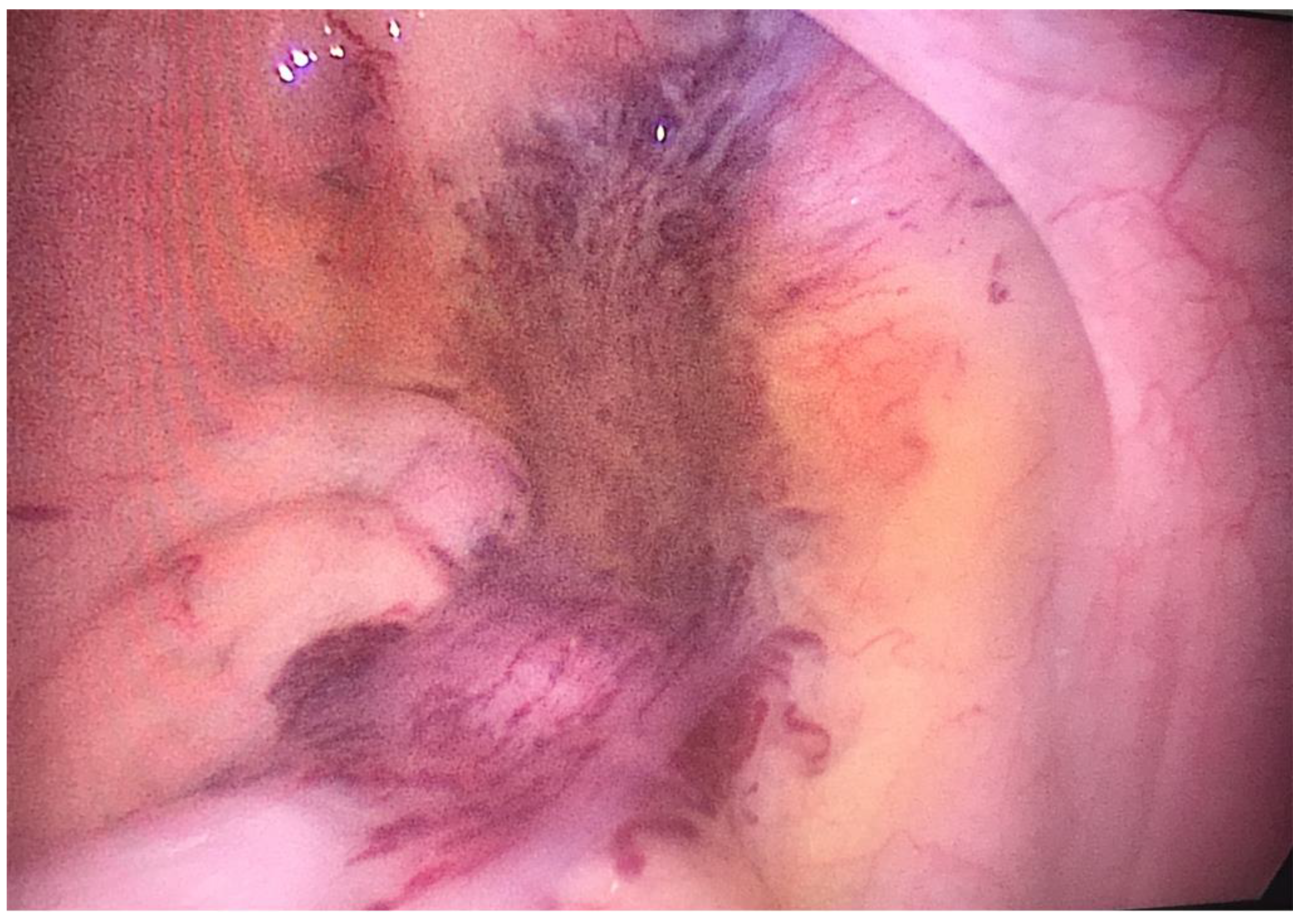
2. CASE PRESENTATION
3. DISCUSSION
References
- Wang, H.T. et al. (2010). Diagnosis and treatment of diffuse cavernous hemangioma of the rectum: Report of 17 cases. World Journal of Surgery. [CrossRef]
- Leal, R.F. et al. (2011). Laparoscopic-assisted bowel resection with construction of a colonic reservoir for cavernous hemangioma of the rectum: Report of two cases. Techniques in Coloproctology. [CrossRef]
- Sood, R. et al. (2013). Chronic haematochezia caused by diffuse cavernous haemangioma of the rectum. Journal of Gastrointestinal and Liver Diseases.
- Andrade, P. et al. (2015). Diffuse cavernous hemangioma of the rectum: case report and literature review. International Journal of Colorectal Disease. [CrossRef]
- Kimura, S. et al. (2007). Cavernous hemangioma in the ascending colon treated by endoscopic mucosal resection. Journal of Gastroenterology and Hepatology (Australia). [CrossRef]
- Fujikawa, H. et al. (2014). Sphincter-saving resection of rectal hemangioma based on Doppler transrectal ultrasonography findings: report of a case. International surgery. [CrossRef]
- Amarapurkar, D.N. et al. (1998). Cavernous hemangiomas of the rectum: Report of three cases. American Journal of Gastroenterology. [CrossRef]
- Wang, H.T. et al. (2005). Diffuse cavernous hemangioma of the rectosigmoid colon. Techniques in Coloproctology. [CrossRef]
- Yang, G.Z. et al. (2013). Giant mesenteric hemangioma of cavernous and venous mixed type: A rare case report. BMC Surgery. [CrossRef]
- Amati, A.L. et al. (2014). A hemangioma of the sigmoid colon mesentery presenting as a retroperitonealtumor: A case report and review. World Journal of Surgical Oncology. [CrossRef]
- Lyon, D.T. and Mantia, A.G. (1984). Large-bowel hemangiomas. Diseases of the Colon & Rectum. [CrossRef]
- Cunningham, J.A. et al. (1989). Diffuse cavernous rectal hemangioma-Sphincter-sparing approach to therapy - Report of a case. Diseases of the Colon & Rectum. [CrossRef]
- Dachman, H.; et al. Colorectal Hemangloma : Radlologic Findings. Pathology 1987. [Google Scholar]
- Wang, A.Y. and Ahmad, N.A. (2007). Diffuse Cavernous Hemangioma of the Colon and Rectum. Clinical Gastroenterology and Hepatology. [CrossRef]
- Londono-Schimmer, E.E. et al. (1994). Coloanal sleeve anastomosis in the treatment of diffuse cavernous haemangioma of the rectum: Long-term results. British Journal of Surgery. [CrossRef]
- Hervías, D. et al. (2004). Diffuse cavernous hemangioma of the rectum: an atypical cause of rectal bleeding. Revista Española de Enfermedades Digestivas. [CrossRef]
- Gottlieb, K.; et al. Massive hemorrhage in pregnancy caused by a diffuse cavernous hemangioma of the rectum - EUS as imaging modality of choice. MedGenMed Medscape General Medicine 2008. [Google Scholar]
- Chen, K. et al. (2019). Successful endoscopic submucosal dissection of a large cavernous hemangioma in the colon. Endoscopy. [CrossRef]
- Liu, W. et al. (2023). Successful resection of a cavernous hemangioma involving the rectal muscularis propria layer by endoscopic full-thickness resection. Endoscopy. [CrossRef]
- Deijen, C.L. et al. (2016). COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surgical Endoscopy. [CrossRef]
- Fujii, Y. et al. (2020). Robotic surgical procedure for diffuse cavernous hemangioma of the rectum: A case report. Asian journal of endoscopic surgery. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



