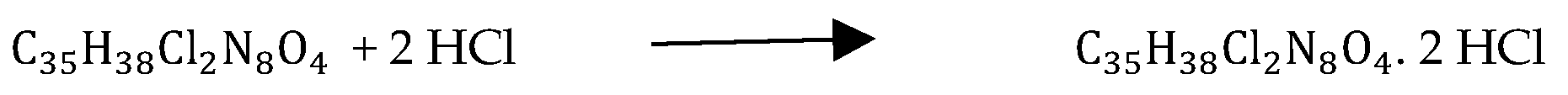1. Introduction
Nails, once considered a necessary evil, have become a fashion statement today. The need to maintain nail-hygiene is paramount to one’s overall health and well-being. Besides problems of etiquette, unkempt and unwashed nails can lead to a host of medical problems and adversely impact an individual’s health. Aesthetic need for healthy-looking nails has made the consumer aware and conscious of nail hygiene and the harmful effects of numerous nail disorders. Corpus unguis, commonly known as the nail plate is considered to be a hydrophilic gel matrix3. It can be macroscopically segmented into three layers: dorsal, intermediate and ventral in the ratio 3: 5: 2, respectively3. The nail is comprised of 9-35% water, 0.1-1% lipid, and 10.60% disulfide linkage3. It has a maximum swelling capacity of 25% and a water loss rate of 1.94 mg/cm2 /h3
The fungal infection, Onychomycosis, is a superficial filamentous infection which falls under the category of dermatophytosis. Dermatophytes account for causing around 90-95% infections, and 50-80% of incidences of onychomycosis are attributed to dermatophytes.4,5,6. The prevalence of onychomycosis is 50% in a population of patients suffering from nail disorders8 The destructive invasive mode of action adopted by fungi, target the inhibition of the human biosynthetic path way9Abnormal nail thickening and a substantial nail plate discoloration are manifestations of Onychomycosis.10 Age is an inevitable predisposing factor for Onychomycosis, however conditions such as diabetes, HIV infection, immunosuppression, presence of athlete’s foot, obesity, smoking, outdoor recreational activities, and tight clothing also prevail6,9. The lion's share of the Onychomycosis is caused by Trichophyton. Rubrum6,9. This specific strain requires a concentration of 0.19μg/mL of Itraconazole to inhibit 90% of the fungal organisms.11
Itraconazole is known to be an efficient broad-spectrum antifungal agent whose mechanism of action involves impeding CYP450 enzymes that play a critical role in the production of ergosterol, a principle component of a fungal cell wall.9,12.
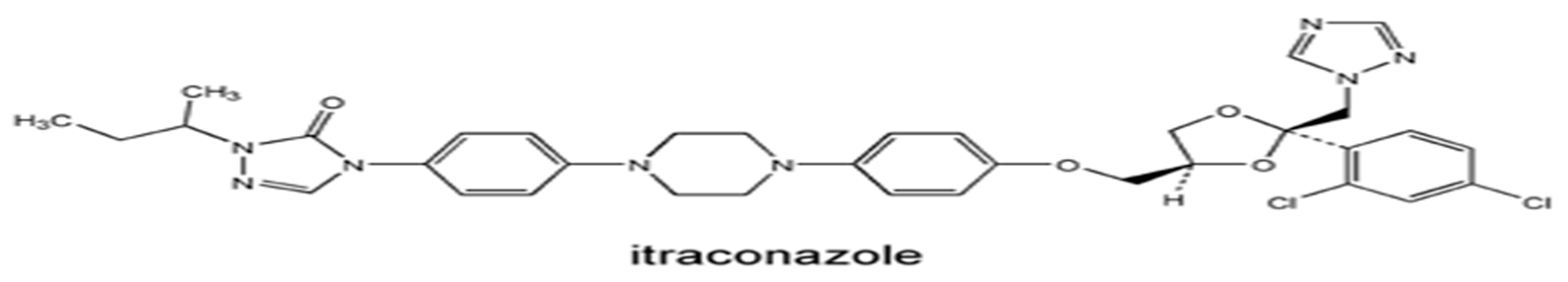
Picture A: Itraconazole chemical structure13
Penetration enhancers, namely, Papain, salicylic acid, and urea are keratolytic agents that are utilized as chemical methods to enhance the transungual diffusion of an antifungal agent through the intimidating nail barrier 4,6,14. Nail lacquers are an upcoming drug delivery system intended for transungual mode of action. They not only have the potential to effectively treat nail disorders but also conceal the repulsive appearance of the affected nail plate.
Previous studies conducted suggested that Sally Hansen Ultimate Shield Base & Topcoat (reference) was a compatible vehicle to accommodate salicylic acid, urea, and papain in combination with Itraconazole. The drying time was set at 5 minutes by the text, and the pH criteria were targeted to be within a range of 5-6 5,8,15,16。
Problem: Systemic therapies for onychomycosis have shown to have significant harmful effects on the liver.6 Associated drawbacks are large dose requirements, high recurrence rates, drug interactions, osmotic diarrhea and expense 17
Hypothesis: Inclusion of Salicylic acid, Papain, and Urea in an Itraconazole (1%w/w) nail lacquer will have the highest transungual penetration on application to the nail plate.
2. Materials and Methods
Chemicals: Acetonitrile (Omni Solv/ AX0142-1), Dichloromethane (Spectrum/2DJ0112), Dimethyl sulfoxide (Fisher Chemical/173578), Ethanol (EMD Chemical/201021524), Glycerin (J.T Baker/ G18592), Hydrochloric acid 36% (Lot.no:18H0356473), Isopropyl alcohol (VWR Analytical/031317B), Itraconazole ( Ark Pharm Inc, USA/ WG0022830), Papain (Ward’s Science/470301-924), Salicylic acid (Alfa Aesar/10154219), Sally Hansen Ultimate Base & Top Coat (Sally Hansen/45111), Trifluoroacetic acid (VWR Chemicals/18050306), Urea (Alfa Aesar, Massachusetts, USA/10198865)
Equipment: Analytical weighing balance (Mettler Toledo/1125442832), Analytical weighing balance (Mettler PM4000/000500), Custom-made Franz cell apparatus (Campbell University School of Engineering/Mr. Paul Johnson), Digital Heat block (VWR/000536), Gas Chromatography system (CTC Analytics, leap technologies/000686), High-Performance Liquid Chromatography (Agilent/1100 Series), Thermogravimetric Analyzer (Universal TA Instruments /TGA Q500V20.13), Microscope and accessories (Mc CRONE Olympus/000523), Rotavapor (BUCHI 011/186012128), Vacuum oven (Precision Scientific, NAPCO/395024-0025), Refrigeration chamber (Climate Technology. Inc/347-1), Spin centrifuge (VWR Galaxy)
Chemicals: Acetonitrile (Omni Solv/ AX0142-1), Dichloromethane (Spectrum/2DJ0112), Dimethyl sulfoxide (Fisher Chemical/173578), Ethanol (EMD Chemical/201021524), Glycerin (J.T Baker/ G18592), Hydrochloric acid 36% (Lot.no:18H0356473), Isopropyl alcohol (VWR Analytical/031317B), Itraconazole ( Ark Pharm Inc, USA/ WG0022830), Papain (Ward’s Science/470301-924), Salicylic acid (Alfa Aesar/10154219), Sally Hansen Ultimate Base & Top Coat (Sally Hansen/45111), Trifluoroacetic acid (VWR Chemicals/18050306), Urea (Alfa Aesar, Massachusetts, USA/10198865)
Equipment: Analytical weighing balance (Mettler Toledo/1125442832), Analytical weighing balance (Mettler PM4000/000500), Custom-made Franz cell apparatus (Campbell University School of Engineering/Mr. Paul Johnson), Digital Heat block (VWR/000536), Gas Chromatography system (CTC Analytics, leap technologies/000686), High-Performance Liquid Chromatography (Agilent/1100 Series), Thermogravimetric Analyzer (Universal TA Instruments /TGA Q500V20.13), Microscope and accessories (Mc CRONE Olympus/000523), Rotavapor (BUCHI 011/186012128), Vacuum oven (Precision Scientific, NAPCO/395024-0025), Refrigeration chamber (Climate Technology. Inc/347-1), Spin centrifuge (VWR Galaxy)
Chemicals: Acetonitrile (Omni Solv/ AX0142-1), Dichloromethane (Spectrum/2DJ0112), Dimethyl sulfoxide (Fisher Chemical/173578), Ethanol (EMD Chemical/201021524), Glycerin (J.T Baker/ G18592), Hydrochloric acid 36% (Lot.no:18H0356473), Isopropyl alcohol (VWR Analytical/031317B), Itraconazole ( Ark Pharm Inc, USA/ WG0022830), Papain (Ward’s Science/470301-924), Salicylic acid (Alfa Aesar/10154219), Sally Hansen Ultimate Base & Top Coat (Sally Hansen/45111), Trifluoroacetic acid (VWR Chemicals/18050306), Urea (Alfa Aesar, Massachusetts, USA/10198865)
Itraconazole Hydrochloride Salt preparation18: The salt of Itraconazole was chemically synthesized by an acid addition reaction to enhance the physicochemical properties of the antifungal agent18. The first step involved in the formulation of the hydrochloride salt of Itraconazole was the addition of 10.8mL of Dichloromethane to a measured amount of 1.20898g (1.71331mmol) Itraconazole (MW: 705.641g/mol) in an amber-colored rotary evaporator flask. The flask was stoppered and swirled to allow solubilization of Itraconazole in the solvent18. The second step included the addition of 0.33mL (13.70648mmol) concentrated hydrochloric acid (36%) (MW: 36.47g/mol) to the solution18. The amber-colored rotatory flask containing the suspension was clamped onto a stand with its bottom touching the surface of the water bath. The third step involved heating the suspension under reflux at 50 degrees Celsius for 10minutes at a speed of 100rpm. On completion of the time duration, a vacuum was applied, and the rotating speed of the flask was reduced to 85rpm. Finally, the precipitate of the salt was formed, and the rotatory evaporator flask was dried in a vacuum oven at 60 degrees Celsius for 1 hour. The Itraconazole hydrochloride salt chemically synthesized was transferred into an amber-colored glass bottle, sealed and labeled well.
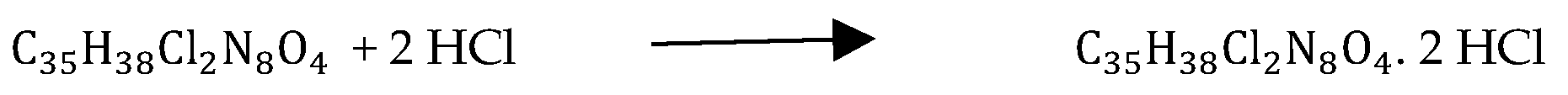
Quantification of Dichloromethane20,23: A gas chromatographic system was used for the quantification of Dichloromethane. The column flow was set at 1.6 mL/min with a front inlet pressure of 16.72 and signal valve of 9.6. The parameters for the front inlet and outlet temperature were set at 140 and 250 degrees Celsius, respectively. The oven temperature was 40 degrees Celsius, and the overall run time was 40 minutes. Standards were prepared of concentrations, 6ppm, 60ppm, and 600ppm.
Quantification of Itraconazole Hydrochloride synthesized (19 A high-performance liquid chromatography system was used. An isocratic method was used for which column, column temperature, and flow rate were C18 (150 x 4.6mm, 3µ), 40 degrees Celsius, and 1mL/min, respectively. The mobile phase composition employed was 0.05% Trifluoroacetic acid in water for mobile phase A and 0.05% Trifluoroacetic acid in acetonitrile for mobile phase B (50% mobile phase A and 50% mobile phase B) Standards of concentrations 0.05mg/mL, 0.1mg/mL and 0.5mg/mL were prepared and samples of itraconazole and itraconazole hydrochloride were prepared by diluting 1mg in 10mL of diluent (0.05% TFA in 50:50 ACN: Water) individually. The standards and samples had an injection volume of 10µL.
Quantification of water content in drug synthesized: A mass of itraconazole hydrochloride (35.4720mg) was measured for water content over a temperature ramp of 45 to 75 degrees Celsius.
Formulation protocol
Design of experiment
| Run |
Urea |
Papain |
Salicylic acid |
Drug extracted |
| 1 |
2.8 |
5 |
0 |
. |
| 2 |
2.8 |
0 |
5 |
. |
| 3 |
0 |
5 |
5 |
. |
| 4 |
2.8 |
5 |
5 |
. |
| 5 |
2.8 |
0 |
5 |
. |
| 6 |
0 |
5 |
0 |
. |
| 7 |
2.8 |
5 |
0 |
. |
| 8 |
0 |
0 |
0 |
. |
| 9 |
0 |
0 |
0 |
. |
| 10 |
2.8 |
0 |
0 |
. |
| 11 |
0 |
0 |
5 |
. |
| 12 |
0 |
5 |
5 |
. |
Formulation protocol of optimized nail lacquer formulation
| Ingredient |
Theoretical Quantity |
| Itraconazole hydrochloride |
1 %w/w |
| Urea |
2.8 %w/w |
| Papain |
5 %w/w |
| Salicylic acid |
5 %w/w |
| Isopropyl alcohol: water (50:50) |
10 % v/w |
| Glycerin |
13.2% v/w |
| Ethanol pure |
13% v/w |
| Water |
14% v/w |
| Sally Hansen Ultimate Shield Top Coat |
Qs. 100 %w/w |
To formulate the optimized 1 % w/w Itraconazole lacquer, a measured amount of glycerin was added to water in a glass vial (Vial 1) and vortexed. A weighed quantity of Urea was added to the water-glycerin solution and mixed well. Papain was accurately weighed and added to the solution and vortexed gently to ensure the solution was homogenous. In another glass vial (Vial 2), a weighed quantity of Salicylic acid was solubilized in pure ethanol. To this solution, previously solubilized Itraconazole hydrochloride hydroalcoholic solution (50:50, Isopropanol: Water) was added and sonicated until homogenous. The theoretical amount of Sally Hansen Ultimate Shield Top Coat (vehicle) was divided equally and incorporated into each of the two glass vials. The final step of formulation involved incorporating Vial 2 into Vial 1, followed by a gentle vortex.
Diffusion study protocol19: The procured nail clippings were altered to the dimensions of the custom-made diffusion cell, which was used as a support. The segment of the nail clipping to be coated with the nail lacquer was marked, and the nails were swiped clean, three times using distilled water. The nail clippings were then patted dry and weighed individually on the analytical weighing balance. The cleaned nails were aligned (dorsal side upwards) onto to the custom-made diffusion cell, following which three coats of the formulated lacquers were applied. The nails treated with lacquer were set aside for five days following which the nails were swiped clean, patted dry, and weighed. The drug extraction procedure involved trituration of the nail clippings using a mortar/pestle and transference into 1mL of diluent (0.05% TFA in 50:50 Acetonitrile: Water. The extracts were agitated for 24 hours in a light protected environment. The supernatant was sampled out and analyzed using the HPLC method elaborated in the Quantification of transungual drug penetration section. The cleaning procedure was checked by conducting a drug extraction study at 0th hour on the procured nail clipping.
Quantification of transungual drug penetration ( The column, column temperature, and flow rate were C18 (150 x 4.6mm, 3µ), 40 degrees Celsius, and 1mL/min, respectively. The mobile phase composition employed was 0.05% TFA in water for mobile phase A and 0.05% TFA in ACN for mobile phase B (50% mobile phase A and 50% mobile phase B). Standards of concentrations 0.06µg/mL, 0.1µg/mL and 1µg/mL had an injection volume of 200µL, whereas 100µg/mL had 50µL and 500 µg/mL had 10µL injection volume. The nail extract samples had an injection volume of 200µL.
Quantification of drug content in lacquer formulation: The drug content assay was performed to determine the reasonable amount of drug present in the optimized lacquer formulation. The stipulated acceptable range of Itraconazole hydrochloride contained in the lacquer should not be less than 90% and not exceed 110% of the labeled quantity of the Itraconazole hydrochloride21. A standard calibration curve was constructed using Itraconazole hydrochloride standard solutions of concentrations, 0.01mg/mL(20µL), 0.5mg/mL(10µL), 1mg/mL(5µL) and 5mg/mL(5µL). The diluent used was 0.05% Trifluoroacetic acid in Acetonitrile: Water (50:50). The blank for standard solution was solely the diluent. The sample solution was prepared by adding 0.35mL of sample in a centrifugal vial containing a 0.45µm membrane filter and spin centrifuging it at a speed of 10,000 rpm for 6 minutes. A second blank, excluding the drug and containing all the three penetration enhancers were prepared and analyzed for comparison to the sample solutions. In the case of the accelerated stability study, a sample aliquot of 0.5mL was taken and centrifuged before analysis. The sample and standard solution were analyzed using an isocratic HPLC method with column, column flow rate, and temperature set at C18 (150 x 4.6mm, 3µ), 1mL/min, and 40 degrees Celsius respectively. The mobile phase composition employed was 0.05% TFA in water for mobile phase A and 0.05% TFA in ACN for mobile phase B (45% mobile phase A and 55% mobile phase B). The second blank and sample had an injection volume of 0.5µL
Spreadability evaluation22: A drop of the reference, Sally Hansen's ultimate shield topcoat, and sample optimize formulations were poured onto Different glass slides. The vertical distance traveled by the drops of the optimized a lacquer formulation was measured and compared to the reference lacquer.
Precipitation evaluation: The experiment was conducted to ascertain the absence of any little precipitation in the optimized formulations that might hinder the therapeutic effect. A drop of the optimized lacquers was applied onto different glass slides, and coverslips were placed on it. The glass slides were observed under the microscope at three different magnification powers, 5X, 10X, and 20X.
pH evaluation: The formulated nail lacquers under investigation were tested for their natural pH using a litmus paper previously wetted using distilled water.
Study for accelerated stability: The six optimized nail lacquers subjected to refrigeration temperature (4 degrees Celsius) for three weeks. Quality control tests, namely, drug content, spreadability, check for precipitation, and pH, were conducted to account for the formulations’ stability, shelf life, and consistency when subjected to extreme temperature.
3. Results
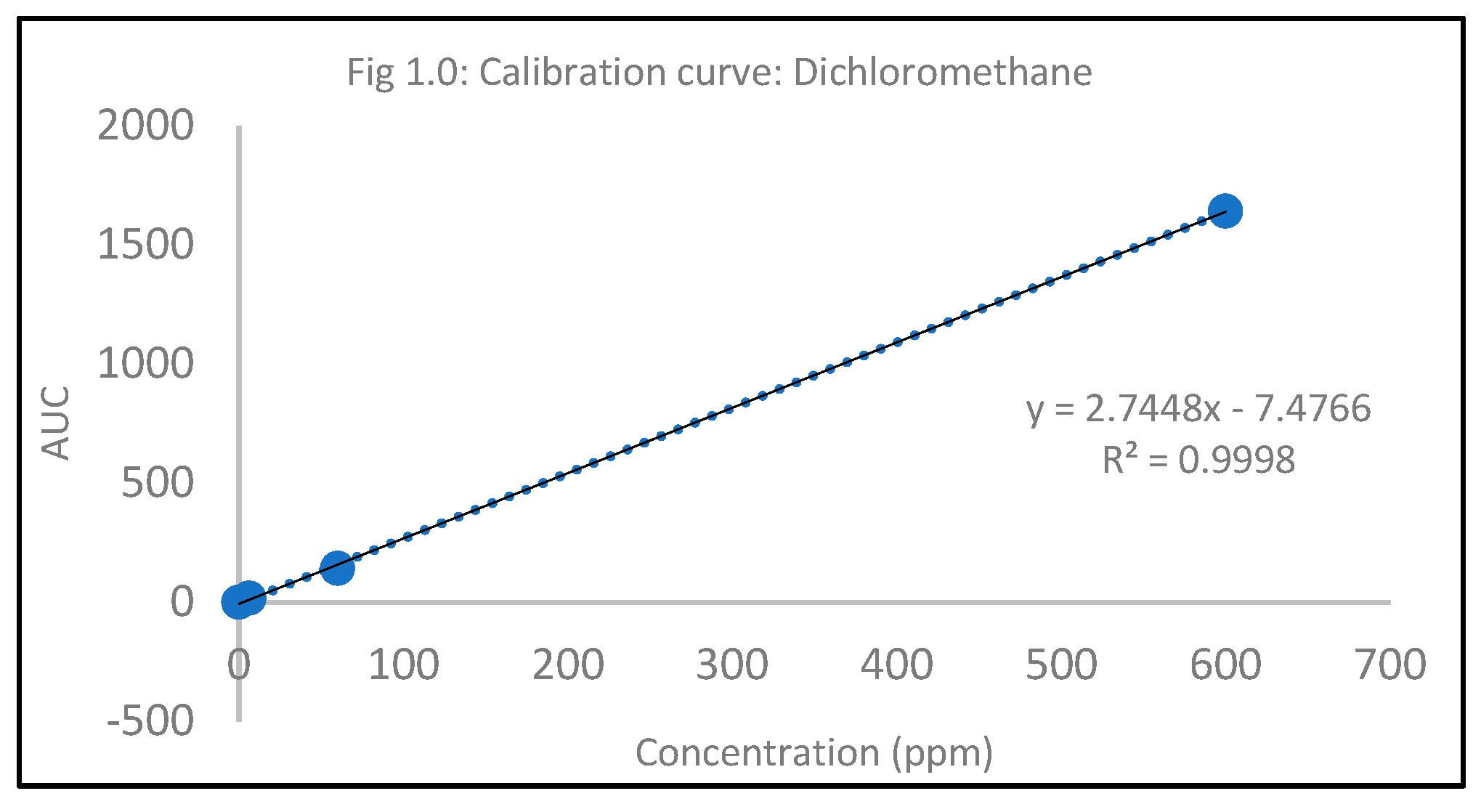
Quantification of residual solvent, Dichloromethane
Table 1.0.
Standard Calibration Curve.
Table 1.0.
Standard Calibration Curve.
| Standard Concentration (ppm) |
AUC |
| 0 |
0 |
| 6 |
15.93905 |
| 60 |
141.264 |
| 600 |
1640.941 |
Determination of percent recovery of itraconazole hydrochloride salt synthesized
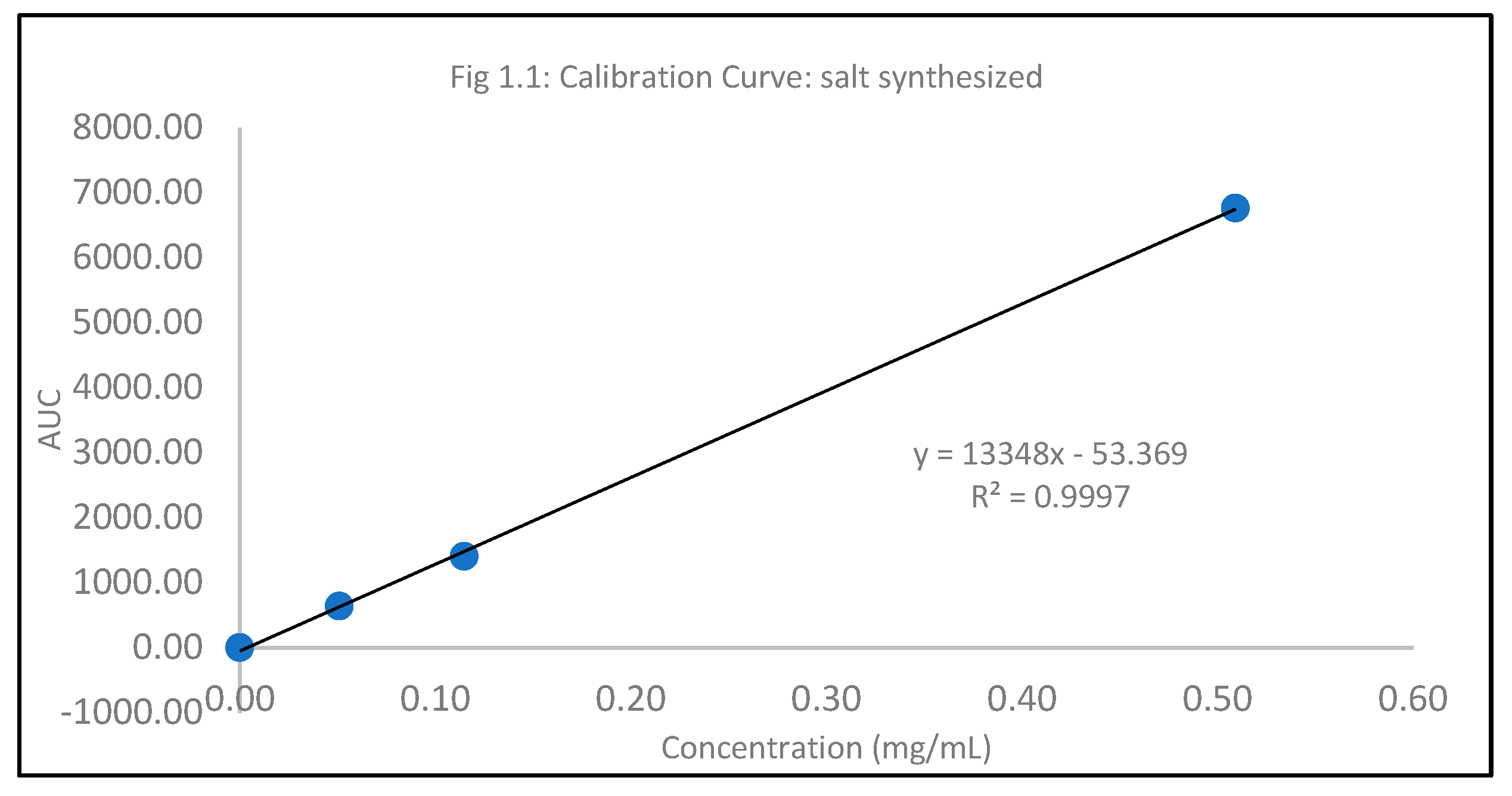
Table 1.1.
Standard Calibration curve.
Table 1.1.
Standard Calibration curve.
| Concentration(mg/mL) |
AUC |
| 0.051 |
634.10 |
| |
633.78 |
| |
632.46 |
| 0.115 |
1397.40 |
| |
1406.34 |
| |
1401.75 |
| 0.509 |
6743.40 |
| |
6735.34 |
| |
6802.04 |
Calculation for ITR.HCl content (salt)
Table 1.2.
ITR.HCl content (salt).
Table 1.2.
ITR.HCl content (salt).
| ITR std |
AUC |
| |
1955.12 |
| |
1945.08 |
| |
1952.13 |
| ITR average |
1950.78 |
|
|
| ITR.HCl sample |
1549.98 |
| |
1558.47 |
| |
1550.08 |
| ITR.HCL average |
1552.84 |
| yield mg |
9.07 |
| % yield |
90.60% |
Salt factor (2 HCl) = 1.10, Weight of Itraconazole = 1.21 g, Weight of Itraconazole 2HCl = 1.51 g. Therefore, the theoretical amount of Itraconazole = 1.33 g. The obtained percent yield was calculated to be 113%.
Optimization of itraconazole hydrochloride nail lacquer (DOE 1-12)
Table 2.0.
Standard Calibration curve DOE 1-6 .
Table 2.0.
Standard Calibration curve DOE 1-6 .
| Standard Conc (mcg/mL) |
AUC |
| 0.00 |
0.00 |
| 0.06 |
20.85 |
| 0.10 |
1.75 |
| 1.01 |
17.71 |
| 101.40 |
1862.99 |
| 501.70 |
9581.25 |
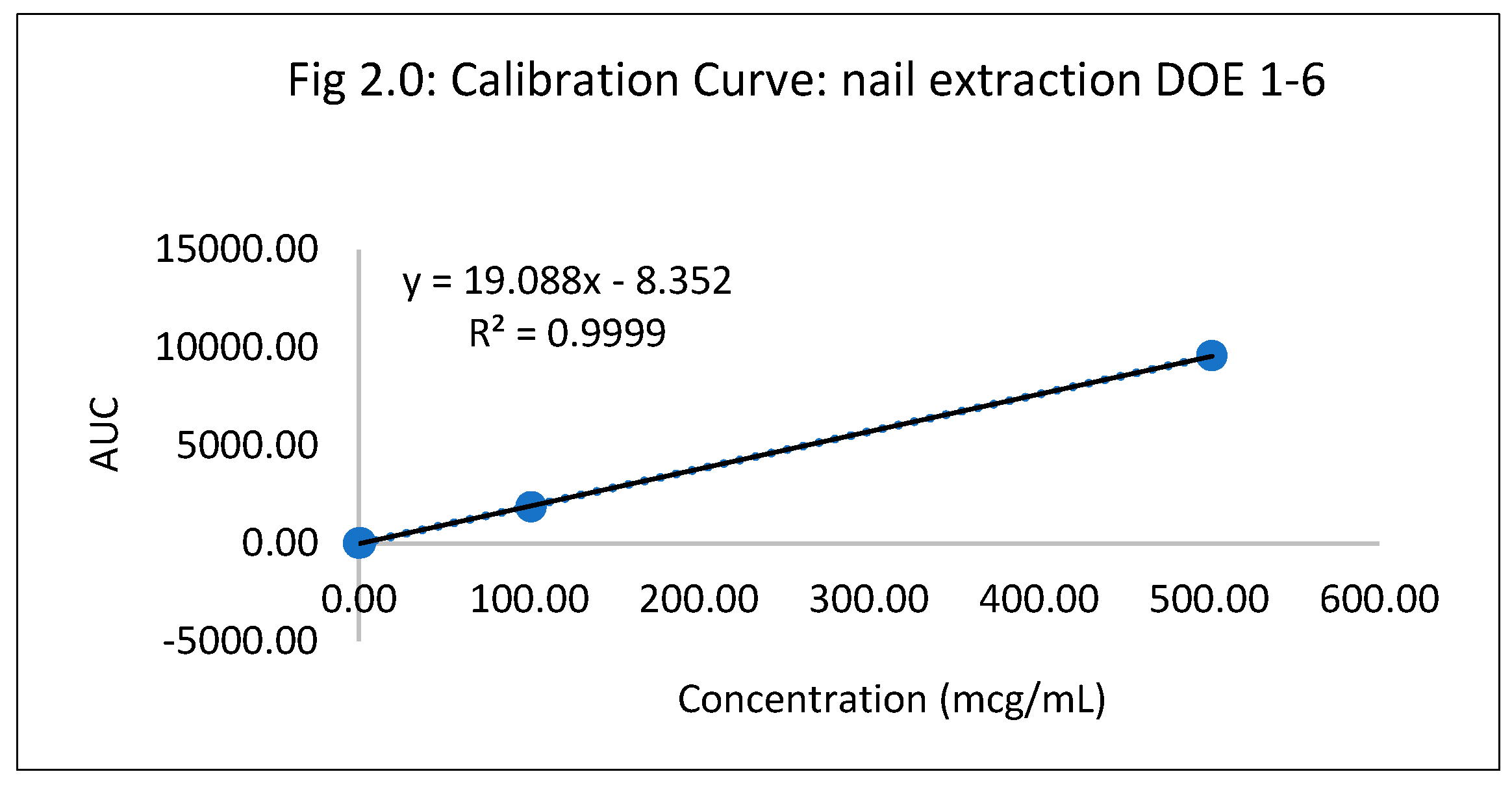
Table 2.1.
Nail extraction of DOE 1-6.
Table 2.1.
Nail extraction of DOE 1-6.
| Nail |
AUC |
Conc (x) |
| 1.00 |
0.00 |
0.00 |
| 2.00 |
0.00 |
0.00 |
| 3.00 |
1.12 |
0.50 |
| 4.00 |
7.84 |
0.85 |
| 5.00 |
0.00 |
0.00 |
| 6.00 |
0.00 |
0.00 |
Table 2.3.
Standard Calibration curve DOE 6-12.
Table 2.3.
Standard Calibration curve DOE 6-12.
| Standard conc (mcg/mL) |
AUC |
| 0.0 |
0.0 |
| 0.06 |
19.31 |
| 0.10 |
1.71 |
| 1.01 |
17.80 |
| 100.62 |
1836.06 |
| 503.10 |
9612.49 |
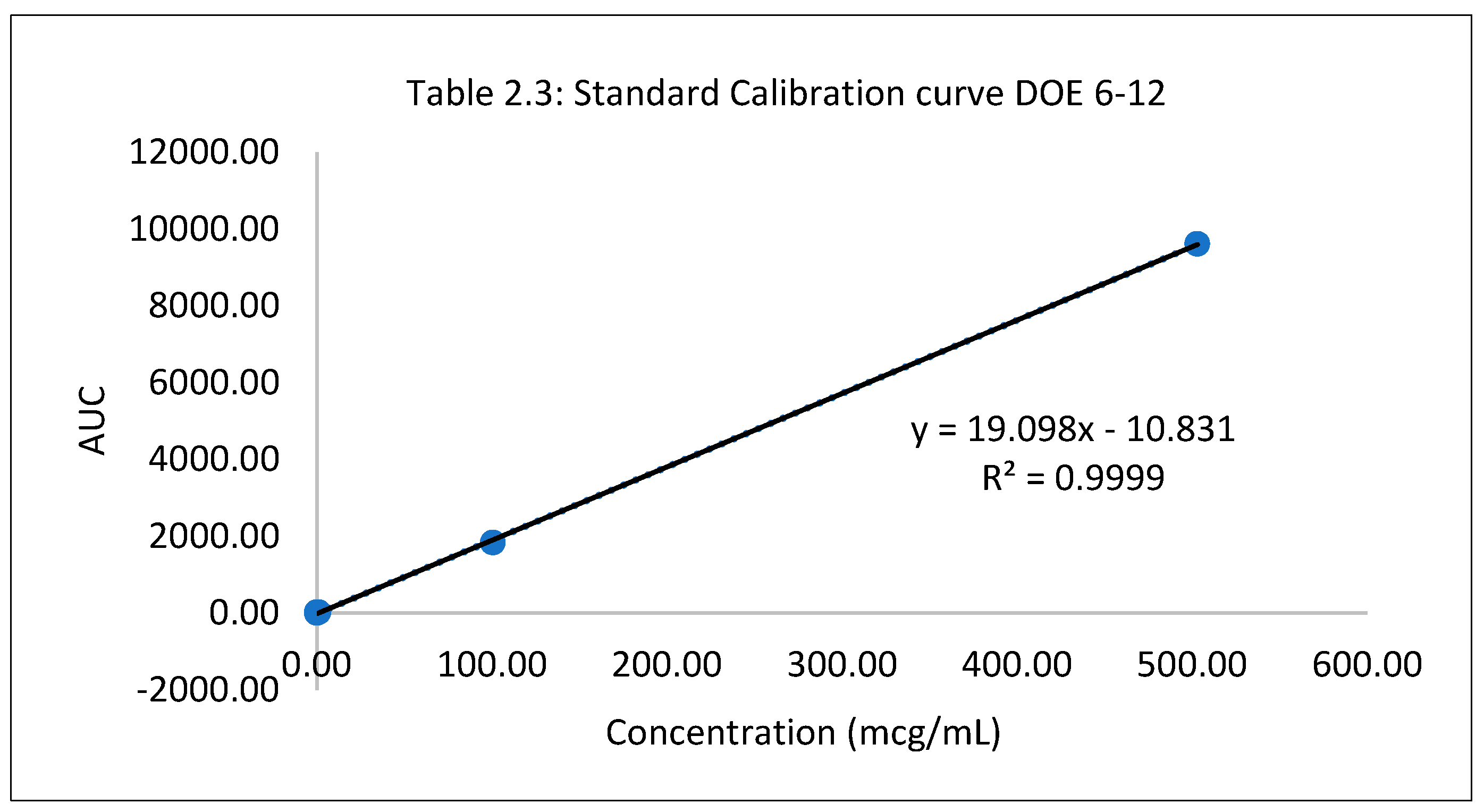
Table 2.4.
Nail extraction of DOE 6-12, N = 6.
Table 2.4.
Nail extraction of DOE 6-12, N = 6.
| Nail |
AUC |
Drug extracted (mcg/mL) |
| 7 |
0.66 |
0.6 |
| 8 |
0 |
0 |
| 9 |
0 |
0 |
| 10 |
0 |
0 |
| 11 |
0 |
0 |
| 12 |
0 |
0 |
Proof of concept study
Table 3.0.
Standard Calibration curve: Proof of Concept.
Table 3.0.
Standard Calibration curve: Proof of Concept.
| Standard conc (mcg/mL) |
AUC |
| 0 |
0 |
| 0.06 |
0.83 |
| |
0.98 |
| |
0.9 |
| 0.1046 |
1.31 |
| |
1.52 |
| |
1.41 |
| 1.046 |
5.84 |
| |
5.85 |
| |
5.84 |
| 104.6 |
556.49 |
| |
556.85 |
| |
556.67 |
| 500.9 |
2826.37 |
| |
2817.75 |
| |
2822.06 |
| Standard conc (mcg/mL) |
AUC |
| 0 |
0 |
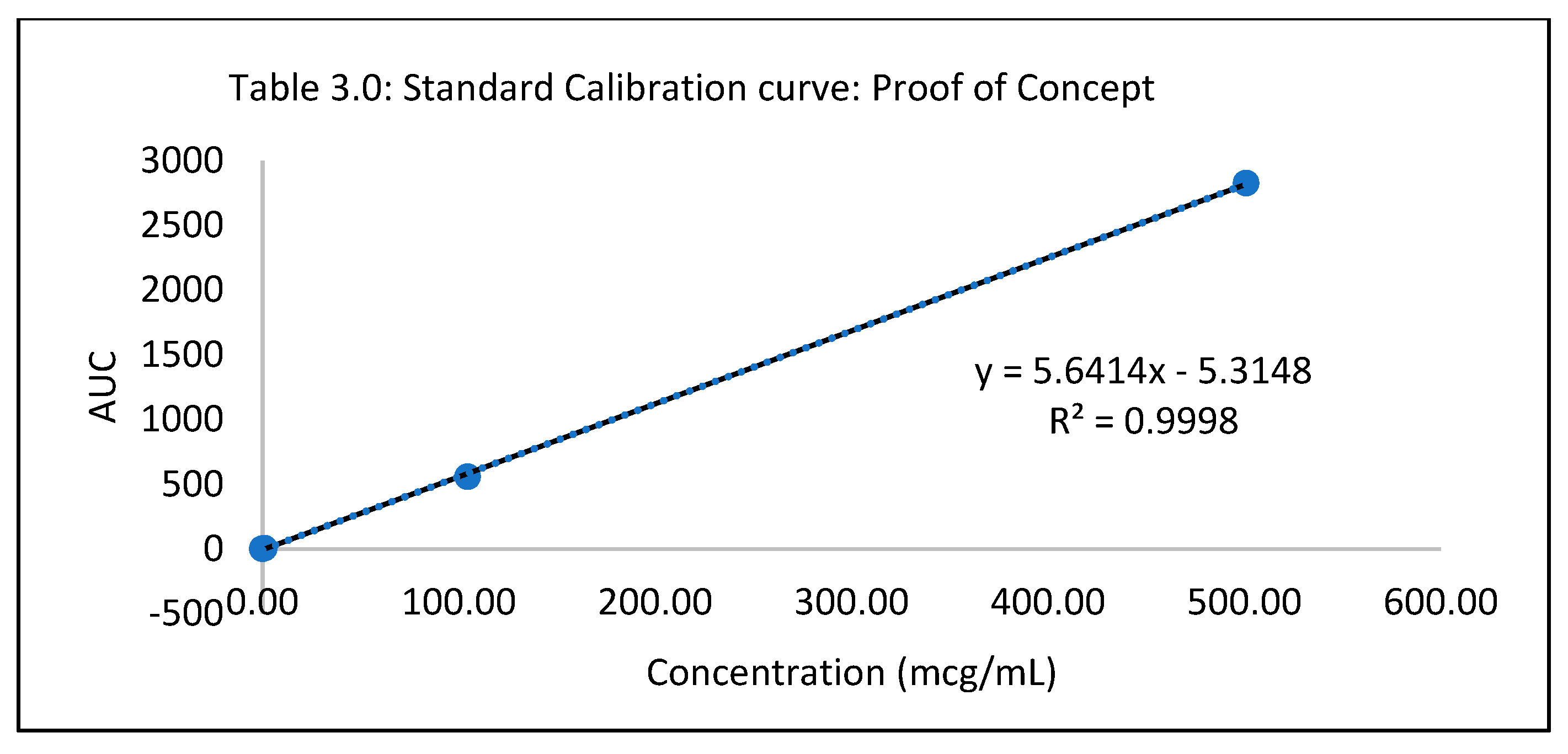
Table 3.1.
Drug extraction (Proof of concept), N = 6.
Table 3.1.
Drug extraction (Proof of concept), N = 6.
| Nail |
AUC |
Drug extracted (mcg/mL) |
Drug/mg of nail |
| 1 |
5.31 |
1.88 |
0.13 |
| |
5.39 |
1.9 |
0.13 |
| |
5.27 |
1.88 |
0.13 |
| 2 |
4.99 |
1.83 |
0.1 |
| |
5.02 |
1.83 |
0.1 |
| |
4.97 |
1.82 |
0.1 |
| 3 |
4.88 |
1.81 |
0.14 |
| |
4.86 |
1.8 |
0.14 |
| |
4.90 |
1.81 |
0.14 |
| 4 |
4.89 |
1.81 |
0.11 |
| |
4.78 |
1.79 |
0.1 |
| |
4.88 |
1.81 |
0.11 |
| 5 |
4.97 |
1.82 |
0.1 |
| |
4.95 |
1.82 |
0.1 |
| |
4.96 |
1.82 |
0.1 |
| 6 |
4.77 |
1.79 |
0.11 |
| |
4.95 |
1.82 |
0.11 |
| |
4.88 |
1.81 |
0.11 |
Quality control evaluation
Drug content for the proof of concept for the optimized nail lacquer formulations
Table 4.0.
Standard Calibration curve for drug content.
Table 4.0.
Standard Calibration curve for drug content.
| Standard conc (mg/mL) |
AUC |
| 0 |
0 |
| 0.01 |
54.69 |
| |
55.4 |
| 0.52 |
2793.87 |
| |
2781.99 |
| 1 |
5642.2 |
| |
5615.79 |
| 5.2 |
28262.6 |
| |
28268.6 |
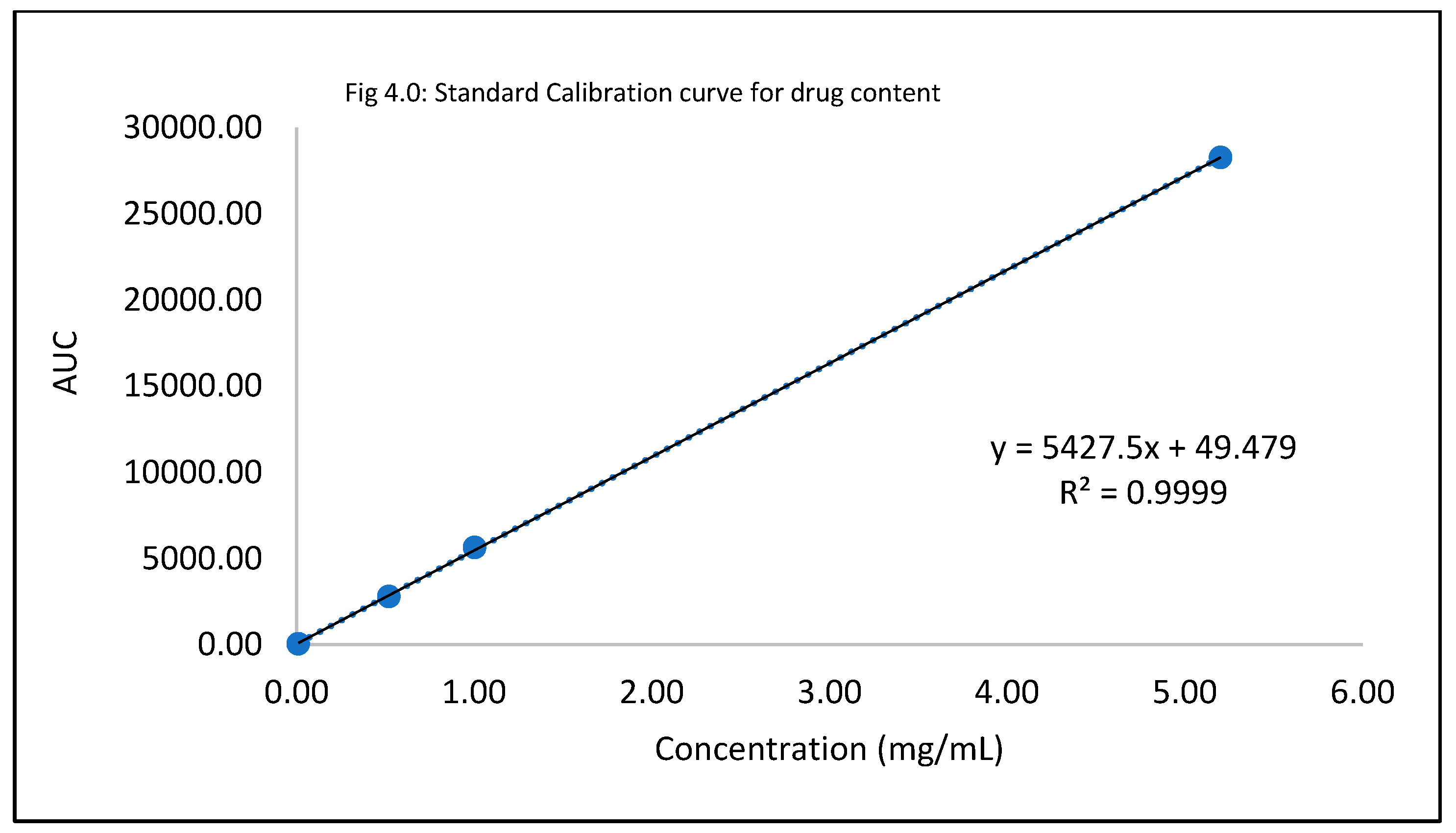
Table 4.1.
Drug content for proof of concept optimized lacquer, N = 6.
Table 4.1.
Drug content for proof of concept optimized lacquer, N = 6.
| Lacquer |
AUC |
Drug content (90-110 %) |
ITR taken(mg) |
| Lacquer 1 |
18404.04 |
98.83% |
50.13 |
| |
18609.44 |
99.98% |
50.13 |
| |
19737.48 |
106.07% |
50.13 |
| Lacquer 2 |
19426.85 |
101.27% |
51.67 |
| |
19479.56 |
101.56% |
51.67 |
| |
19464.30 |
101.48% |
51.67 |
| Lacquer 3 |
17584.06 |
94.44% |
50.07 |
| |
17774.94 |
95.61% |
50.07 |
| |
17487.45 |
93.94% |
50.07 |
| Lacquer 4 |
18920.79 |
101.73% |
50.1 |
| |
18802.34 |
101.08% |
50.1 |
| |
18691.84 |
100.47% |
50.1 |
| Lacquer 5 |
18392.47 |
98.81% |
50.14 |
| |
18299.89 |
98.32% |
50.14 |
| |
18432.00 |
99.03% |
50.14 |
| Lacquer 6 |
18209.17 |
98.00% |
50.04 |
| |
18363.04 |
98.83% |
50.04 |
| |
18374.57 |
98.90% |
50.04 |
Spreadability test
Table 5.0.
Spreadability results (7 lengths and 4 widths) .
Table 5.0.
Spreadability results (7 lengths and 4 widths) .
| Sally Hansen Ultimate Shield Base & Topcoat |
| 1 |
4cm |
| 2 |
4.1cm |
| 3 |
4cm |
| Average |
4.03cm |
Table 5.1.
Spreadability for optimized nail lacquer, N = 6.
Table 5.1.
Spreadability for optimized nail lacquer, N = 6.
| Formulation |
%match |
| Formulation 1 |
%match |
| 1 |
4.2 cm |
104.21% |
| 2 |
4.1cm |
101.73% |
| 3 |
4cm |
99.25% |
| Formulation 2 |
|
| 1 |
4.1cm |
101.66% |
| 2 |
4cm |
99.25% |
| 3 |
4cm |
99.25% |
| Formulation 3 |
|
| 1 |
4cm |
99.25% |
| 2 |
4.1cm |
101.66% |
| 3 |
3.9cm |
96.70% |
| Formulation 4 |
|
| 1 |
4cm |
99.25% |
| 2 |
4.1cm |
101.73% |
| 3 |
4.1cm |
101.73% |
| Formulation 5 |
|
| |
4cm |
99.25% |
| 2 |
4cm |
99.25% |
| 3 |
4.2cm |
104.21% |
| Formulation 6 |
|
| 1 |
4cm |
99.25% |
| 2 |
4.2cm |
104.21% |
| 3 |
4.2cm |
104.21% |
Check for precipitation: The microscope slides observed under 5X, 10X, and 20X magnification power demonstrated the absence of precipitation and crystallization of the drug.
pH: The pH of all the six proof of concept formulations were consistently within the stipulated range of 5-6.
Accelerated stability study data
Drug content
Table 6.0.
Standard Calibration curve for drug content.
Table 6.0.
Standard Calibration curve for drug content.
| Standard conc (mg/mL) |
AUC |
| 0.01 |
54.89 |
| |
55.01 |
| 0.52 |
2851.02 |
| |
2845.71 |
| 1.00 |
5697.24 |
| |
5652.42 |
| 5.22 |
28696.40 |
| |
28719.60 |
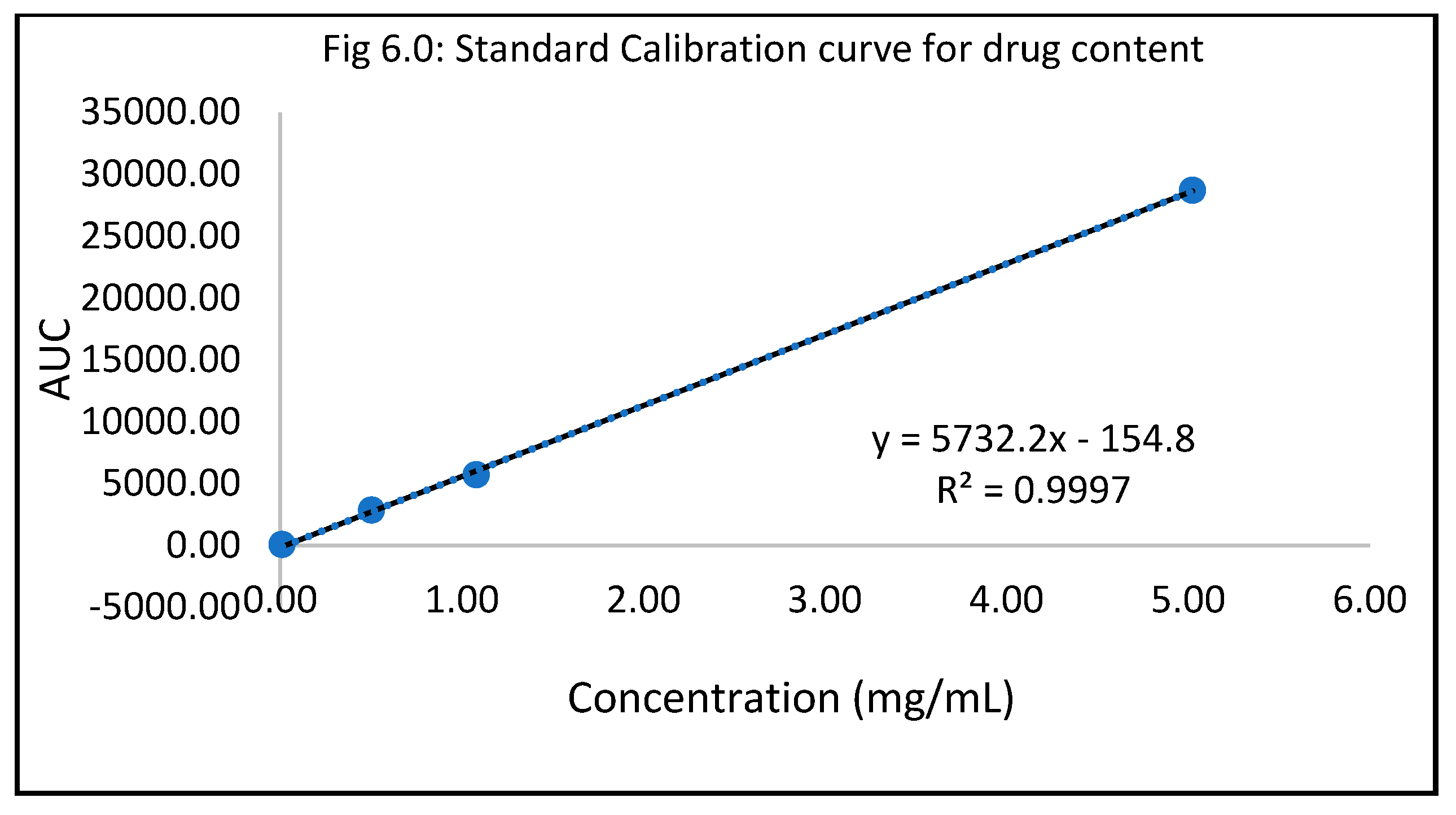
Table 6.1.
Accelerated stability drug content, N = 6.
Table 6.1.
Accelerated stability drug content, N = 6.
| Lacquer |
AUC |
Drug content (90-110 %) |
ITR taken |
| Lacquer 1 |
29397.2 |
105.96% |
50.13 |
| |
29181.4 |
105.30% |
50.13 |
| |
29290.4 |
105.69% |
50.13 |
| Lacquer 2 |
30966.6 |
108.15% |
51.67 |
| |
30914.6 |
107.97% |
51.67 |
| |
30450.8 |
106.35% |
51.67 |
| Lacquer 3 |
29903.2 |
107.89% |
50.07 |
| |
29975.2 |
108.15% |
50.07 |
| |
29733.6 |
107.28% |
50.07 |
| Lacquer 4 |
30513 |
110.08% |
50.1 |
| |
30373.4 |
109.58% |
50.1 |
| |
30019.2 |
108.31% |
50.1 |
| Lacquer 5 |
31036.2 |
111.73% |
50.14 |
| |
30755.4 |
110.73% |
50.14 |
| |
30726.8 |
110.63% |
50.14 |
| Lacquer 6 |
30829.2 |
111.21% |
50.04 |
| |
30987 |
111.78% |
50.04 |
| |
30799.6 |
111.11% |
50.04 |
Table 5.1.
Spreadability for optimized nail lacquer, N = 6.
Table 5.1.
Spreadability for optimized nail lacquer, N = 6.
| Formulation |
%match |
| Formulation 1 |
%match |
| 1 |
4.0cm |
99.25% |
| 2 |
4.0cm |
99.25% |
| 3 |
4.1cm |
101.74% |
| Formulation 2 |
|
| 1 |
4.0cm |
99.25% |
| 2 |
4.2cm |
104.21% |
| 3 |
4.1cm |
101.74% |
| Formulation 3 |
|
| 1 |
4.2 |
104.21% |
| 2 |
4.1 |
101.74% |
| 3 |
3.9 |
96.74% |
| Formulation 4 |
|
| 1 |
4.0cm |
99.25% |
| 2 |
3.9cm |
96.74% |
| 3 |
4.1cm |
101.74% |
| Formulation 5 |
|
| 1 |
4.1cm |
101.74% |
| 2 |
4cm |
99.25% |
| 3 |
4.2cm |
104.21% |
| Formulation 6 |
|
| 1 |
4cm |
99.25% |
| 2 |
4.2cm |
104.21% |
| 3 |
4.2cm |
104.21% |
4. Discussion
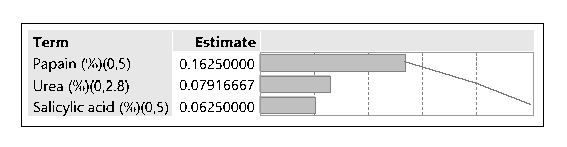
The hydrochloride salt of itraconazole was synthesized to aid the incorporation of the various combinations of penetration enhancers, namely, Papain, Salicylic acid, and Urea is an antifungal nail lacquer containing 1 %w/w Itraconazole. The synthesis process of the salt involved the use of dichloromethane, a class II residual solvent with a concentration limit of 600ppm20. Gas chromatography was conducted to verify that the synthesized Itraconazole hydrochloride salt had no traces of residual solvent present20. The chromatograms shown in the Appendix for gas chromatography indicate that the dichloromethane present in the synthesized salt was below the limit of quantitation. A high-performance liquid chromatography (HPLC) method was employed to ascertain the percent recovery of the synthesized Itraconazole hydrochloride. Based on the linearity equation generated by the standard calibration curve for the percent recovery and taking into consideration the salt factor, the yield obtained was 113%. To account for the excess yield, a thermogravimetric analysis (TGA) was conducted to determine the residual water content. The outcome of the test indicated a water content of about 17.07%. The results obtained from the TGA were by the excess percent yield of the Itraconazole hydrochloride salt. A design of experiment table was built using the JMP software to facilitate the optimization of the antifungal nail lacquer under test. The continuous input variables were the concentrations of the different penetration enhancers, Urea, Papain, and Salicylic acid. The levels of Urea used were 0%, 2.8%, for Papain were 0% and 5% and Salicylic acid were 0% and 5%. The response variable was the drug diffused from the nail clipping after five days. Two approaches were employed to quantify the amount of the drug Itraconazole hydrochloride to diffuse through the nail plate. The first approach involved conducting a transungual penetration study using a custom-made diffusion cell. The whole custom-made diffusion cell unit comprised of 8 diffusion cells within it. The procured nail clippings were altered to the dimensions of the diffusion cell and the formulated lacquers, in adherence to the DOE table were applied onto the dorsal surface of each nail clipping. The receptor chamber was filled with phosphate buffer (pH 7.4), and preservatives, namely, methylparaben and propylparaben were added to impede any microbial growth. A temperature of 34 degrees Celsius was maintained, and an aliquot of 250µL was sampled out at 0th hour, to check for the absence of drug diffused and at 72nd hour for drug diffused. The approach of using a custom-made diffusion cell was ruled out on account of the drug, Itraconazole hydrochloride having a solubility of 0.0002 mg/mL in pH 7.4. A second approach utilized the drug uptake (elaborated in the method section) on the same set of 12 nails. The results from this method indicated that the DOE runs for 3, 4, 7 had a drug uptake of 0.50 µg/mL, 0.85 µg/mL, and 0.60 µg/mL respectively.
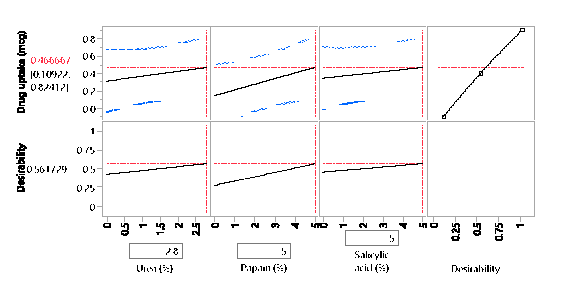
Statistical analysis was conducted on JMP to determine if the outcome, drug diffused was significant for the DOE runs. The parameter estimates indicated that there was no significant difference between the DOE formulations. Statistically, they are were the same concerning their contribution to drug diffusion across the nail plate. Though statistically insignificant, the prediction profiler, when maximized in desirability, predicted that the maximum outcome would be possible when concentrations of papain, salicylic acid, and urea would be used in a proportion of 5%w/w, 5%w/w and 2.8% respectively. The optimized formulation was considered to be that of the formulation containing 5% Salicylic acid, 5% Papain and 2.8% urea since it resulted in the highest amount of drug diffused. A proof of concept was conducted on the optimized lacquer formulation by testing it six times for drug uptake, drug content, spreadability, check for precipitation, pH and accelerated stability to establish reproducibility and quality. The drug extracted from the nails after five days was found to be within the range of 1.79 µg/mL to 1.9 µg/mL. The result indicated that the optimized lacquer formulation exceeded the MIC 90 of Itraconazole for T.rubrum (0.5 µg/mL), and the results for the drug extracted were consistent2. From the results obtained, it can be inferred that the optimized formulation containing 5% papain 5% salicylic acid and 2.8% urea in combination with 1% Itraconazole in a lacquer formulation would be useful in treating nails infected with onychomycosis. All the results for transungual penetration were obtained using healthy nails. It can be deduced that the optimized formulation would result in a greater diffusion through the nail plate in case of nails infected with onychomycosis.
Quality control evaluation tests were carried out to indicate product quality and account for its performance. A drug content study was executed to establish that the amount of itraconazole hydrochloride present in the lacquer formulation was within an acceptable range of 90% to 110%21. The linearity equation obtained from the standard calibration curve (depicted in the results section) aided in the calculation of the amount of drug present in the formulation. All the six formulations of the optimized formula conformed with the limits set for drug content. Check for precipitation gave an account for the drug’s compatibility, stability, and activity. The microscopic observations under 5X, 10X, and 20X indicated that all the six optimized formulations did not exhibit any drug crystallization or precipitation. The optimized formula conformed with the criteria for visual appearance. Another test conducted for quality was spreadability. The results for all the six formulations had a relative standard deviation of less than 1%. The texture and consistency attributes were substantiated by the test for spreadability. A pH check was conducted using a litmus strip; all the six formulated antifungal lacquers had a pH within 5-6. The specific range was chosen for pH due to the individual stability pH of the three penetration enhancers, papain, salicylic acid, urea, and the drug, Itraconazole. The results from the transungual drug uptake study corroborated the hypothesis. Drug content, spreadability, pH evaluation, and check for precipitation conformed with the standards established and thus accounted for the quality and integrity of the optimized formulation.
Accelerated stability was carried out for all the six formulations concerning drug content, spreadability, check for precipitation, and pH. The study was conducted following a period of 3 weeks from the date of formulation. The accelerated drug content evaluation tests for all the six optimized antifungal nail polishes were in adherence to the stipulated criteria of 90 to 110%21. The outcome suggested that the drug contained in the optimized lacquer did not degrade and was stable when subjected to refrigeration temperature (4 degrees Celsius) over three weeks. Tests for spreadability, check for precipitation and pH indicated that the consistency, viscosity, stability, and integrity of the lacquer was not compromised after three weeks of refrigeration. The conclusion drawn from the accelerated stability testing was that the optimized 1% antifungal nail lacquer containing papain, salicylic acid, and urea passed the test for stability. This strengthened the hypothesis by substantiating that the optimized nail lacquer adhered to the quality and stability parameters and could probably facilitate transungual diffusion even after three weeks. Comprehensively, the data generated and results obtained corroborated the approach of employing a topical antifungal nail lacquer formulation for the treatment of Onychomycosis.
5. Conclusions
The hypothesis was accepted for the current investigation as the results indicated that the optimized nail lacquer, containing 1% w/w itraconazole hydrochloride containing 5% w/w papain, 5% w/w salicylic acid, and 2.8% w/w urea had the highest transungual penetration into the nail on application to the dorsal surface of the nail plate. The antifungal nail lacquer adhered to the tests for drug content, spreadability, check for precipitation, pH, accelerated stability, and conformed as a whole to be reproducible and consistent.
Funding
This research received no external funding.
Acknowledgments
Wholehearted gratitude to Campbell University, NC and it’s research committee namely, Dr. Mali R Gupta, Dr. Antoinne Al-Achi, and Mr. Paul Johnson. Special thanks to the engineering department of Campbell University, NC for their support in building a custom nail-diffusion cell apparatus. My parents and loved ones for their moral support.
Appendix A
Appendix A.1
Appendix
JMP analysis for the design of experiment
| Urea (%w/w) |
Papain (%w/w) |
Salicylic acid (%w/w) |
Drug uptake (mcg) |
| 2.8 |
5 |
0 |
0.00 |
| 2.8 |
0 |
5 |
0.00 |
| 0 |
5 |
5 |
0.50 |
| 2.8 |
5 |
5 |
0.85 |
| 2.8 |
0 |
5 |
0.00 |
| 0 |
5 |
0 |
0.00 |
| 2.8 |
5 |
0 |
0.60 |
| 0 |
0 |
0 |
0.00 |
| 0 |
0 |
0 |
0.00 |
| 2.8 |
0 |
0 |
0.00 |
| 0 |
0 |
5 |
0.00 |
| 0 |
5 |
5 |
0.00 |
Response Drug uptake (mcg)
Actual by Predicted Plot
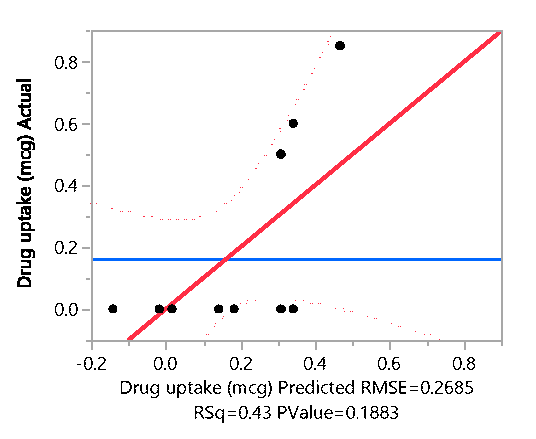
Effect Summary
| Source |
LogWorth |
|
PValue |
| Papain (%)(0,5) |
1.159 |
 |
0.06930 |
| Urea (%)(0,2.8) |
0.472 |
 |
0.33694 |
| Salicylic acid (%)(0,5) |
0.353 |
 |
0.44332 |
Lack Of Fit
| Source |
DF |
Sum of Squares |
Mean Square |
F Ratio |
| Lack Of Fit |
4 |
0.27166667 |
0.067917 |
0.8907 |
| Pure Error |
4 |
0.30500000 |
0.076250 |
Prob > F |
| Total Error |
8 |
0.57666667 |
|
0.5433 |
| |
|
|
|
Max RSq |
| |
|
|
|
0.6997 |
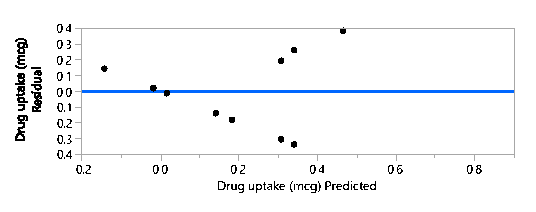
Residual by Predicted Plot
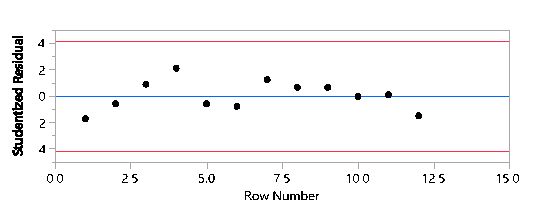
Studentized Residuals
Externally Studentized Residuals with 95% Simultaneous Limits (Bonferroni)
Summary of Fit
| RSquare |
0.432205 |
| RSquare Adj |
0.219282 |
| Root Mean Square Error |
0.268483 |
| Mean of Response |
0.1625 |
| Observations (or Sum Wgts) |
12 |
Parameter Estimates
| Term |
Estimate |
Std Error |
t Ratio |
Prob>|t| |
| Intercept |
0.1625 |
0.077504 |
2.10 |
0.0693 |
| Urea (%)(0,2.8) |
0.0791667 |
0.077504 |
1.02 |
0.3369 |
| Papain (%)(0,5) |
0.1625 |
0.077504 |
2.10 |
0.0693 |
| Salicylic acid (%)(0,5) |
0.0625 |
0.077504 |
0.81 |
0.4433 |
Prediction Profiler
Effect Screening
The parameter estimates have equal variances.
The parameter estimates are not correlated.
Parameter Estimate Population
| Term |
Estimate |
t Ratio |
Prob>|t| |
| Intercept |
0.1625000 |
2.0967 |
0.0693 |
| Urea (%)(0,2.8) |
0.0791667 |
1.0214 |
0.3369 |
| Papain (%)(0,5) |
0.1625000 |
2.0967 |
0.0693 |
| Salicylic acid (%)(0,5) |
0.0625000 |
0.8064 |
0.4433 |
Pareto Plot of Estimates
References
- Odds, F. C. (1993). Itraconazole—a new oral antifungal agent with a vast spectrum of activity in superficial and systemic mycoses. Journal of dermatological science, 5(2), 65-72.
- Jessup, C. J., Warner, J., Isham, N., Hasan, I., & Ghannoum, M. A. (2000). Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. Journal of clinical microbiology, 38(1), 341-344. [CrossRef]
- Saner, M. V., Kulkarni, A. D., & Pardeshi, C. V. (2014). Insights into drug delivery across the nail plate barrier. Journal of Drug Targeting,22(9), 769-789. [CrossRef]
- Jacobsen, A. A., & Tosti, A. (2017). Predisposing Factors for Onychomycosis. Onychomycosis,11-19. [CrossRef]
- Osborne, C. S., Leitner, I., Favre, B., & Ryder, N. S. (2004). Antifungal drug response in anin vitromodel of dermatophyte nail infection. Medical Mycology,42(2), 159-163. [CrossRef]
- Rich, P. (2011). Hydrosoluble medicated nail lacquers: In vitro drug permeation and corresponding antimycotic activity. Yearbook of Dermatology and Dermatologic Surgery,2011, 259-260. [CrossRef]
- Salicylic acid. (n.d.). Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/salicylic_acid.
- Quintanar-Guerrero, D., Ganem-Quintanar, A., Tapia-Olguin, P., Kalia, Y. N., & Buri, P. (1998). The Effect of Keratolytic Agents on the Permeability of Three Imidazole Antimycotic Drugs Through the Human Nail. Drug Development and Industrial Pharmacy,24(7), 685-690. [CrossRef]
- Gupta, A. K., & Studholme, C. (2016). Novel investigational therapies for onychomycosis: An update. Expert Opinion on Investigational Drugs,25(3), 297-305. [CrossRef]
- Gupta, A. K., Sauder, D. N., & Shear, N. H. (1994). Antifungal agents: An overview. Part II. Journal of the American Academy of Dermatology,30(6), 911-933. [CrossRef]
- Aktas, A. E., Yigit, N., Aktas, A., & Gozubuyuk, S. G. (2014). Investigation of in vitro activity of five antifungal drugs against dermatophytes species isolated from clinical samples using the E-test method. The Eurasian journal of medicine, 46(1), 26.
- https://www.drugbank.ca/drugs/DB01167.
- https://drfungus.org/knowledge-base/itraconazole/.
- Kobayashi, Y., Miyamoto, M., Sugibayashi, K., & Morimoto, Y. (1999). Drug Permeation through the Three Layers of the Human Nail Plate. Journal of Pharmacy and Pharmacology,51(3), 271-278. [CrossRef]
- Hamdan, J., & Hahn, R. (2006). Antifungal Drugs for Systemic Mycosis: An Overview of Mechanism of Action and Resistance. Anti-Infective Agents in Medicinal Chemistry,5(4), 403-412. [CrossRef]
- Itraconazole. (n.d.). Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/itraconazole.
- Kataria, P., Sharma, G., Thakur, K., Bansal, V., Dogra, S., & Katare, O. P. (2016). Emergence of nail lacquers as potential transungual delivery system in the management of onychomycosis. Expert opinion on drug delivery, 13(7), 937-952.
- Bagavatula, H., Lankalapalli, S., Tenneti, V. V. K., Beeraka, N. M. R., & Bulusu, B. T. (2014). Comparative studies on solubility and dissolution enhancement of different itraconazole salts and their complexes. Advances in Pharmacology and Pharmacy, 2(6), 85-95.
- Dr. Mali R Gupta, Sindhu Nair. Formulation of Itraconazole Emulgel as a Transungual Drug Delivery System (620 research).
- USP-467-ICH-Q3C.
- https://www.drugfuture.com/Pharmacopoeia/USP35/data/v35300/usp35nf30s0_m43724.html.
- Amrita Sawant Desai, IJPRBS, 2014; Volume 3(2): 200-214.
- USP 467 Residual Solvent Method.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).