Submitted:
08 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
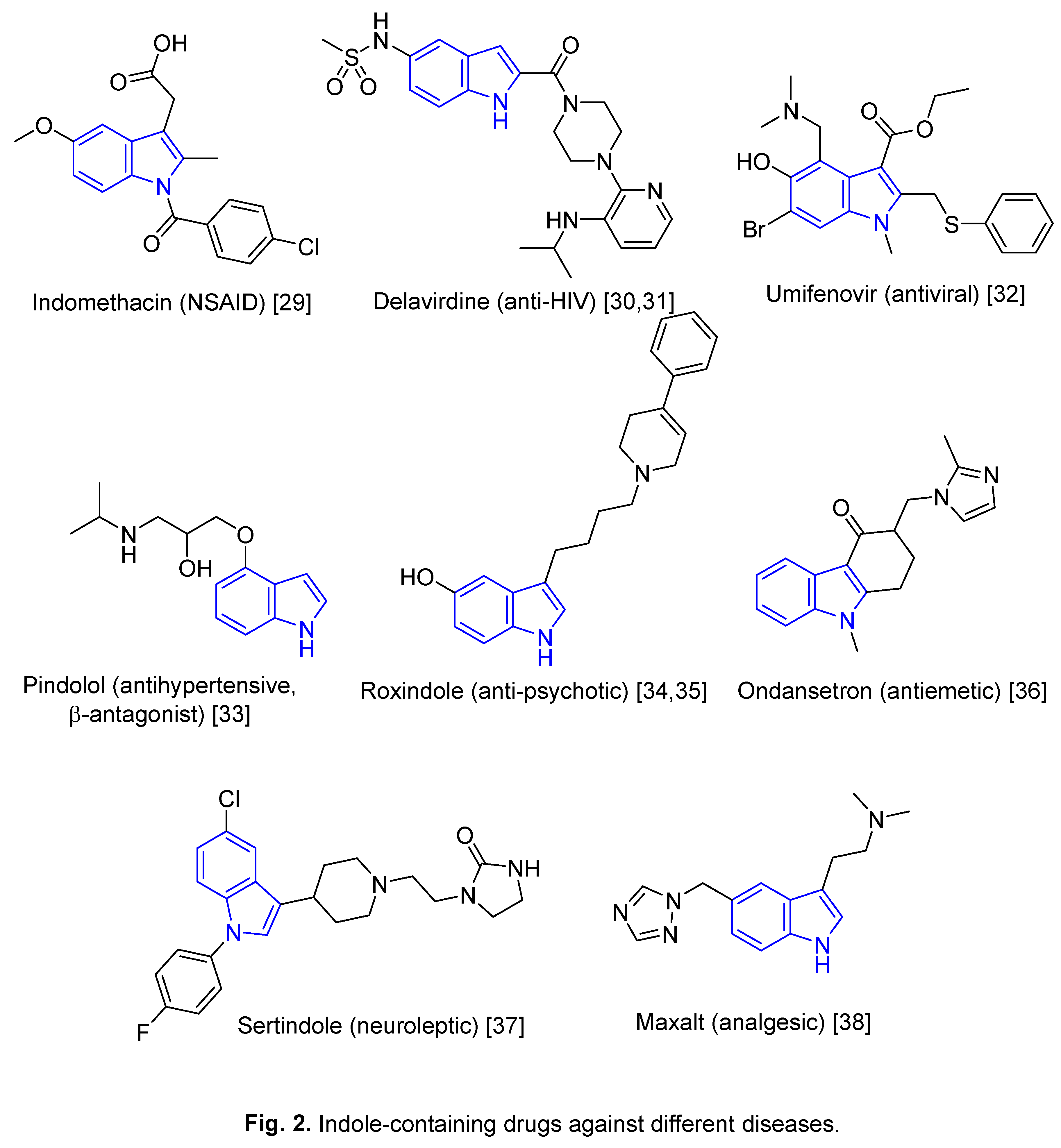
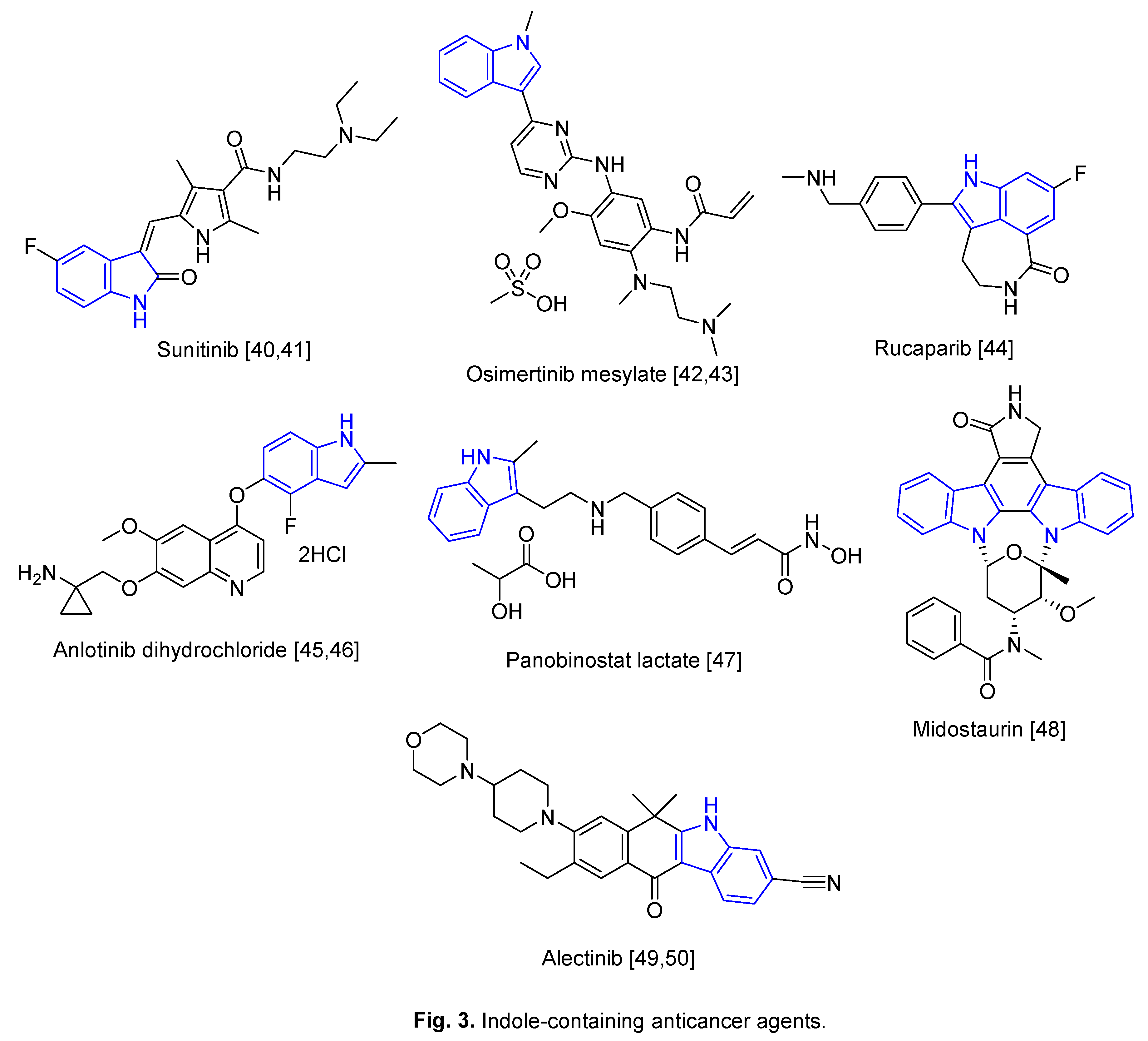
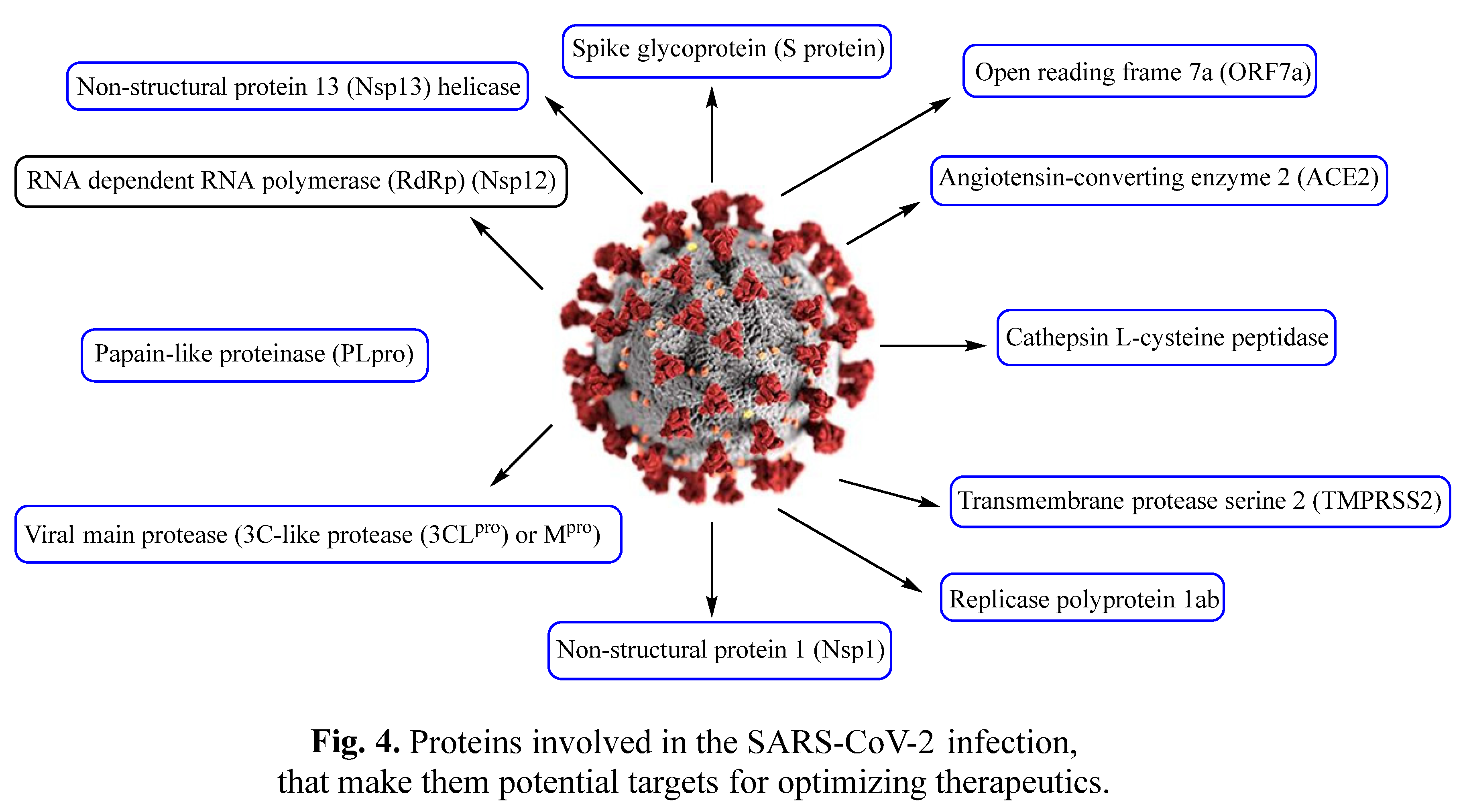
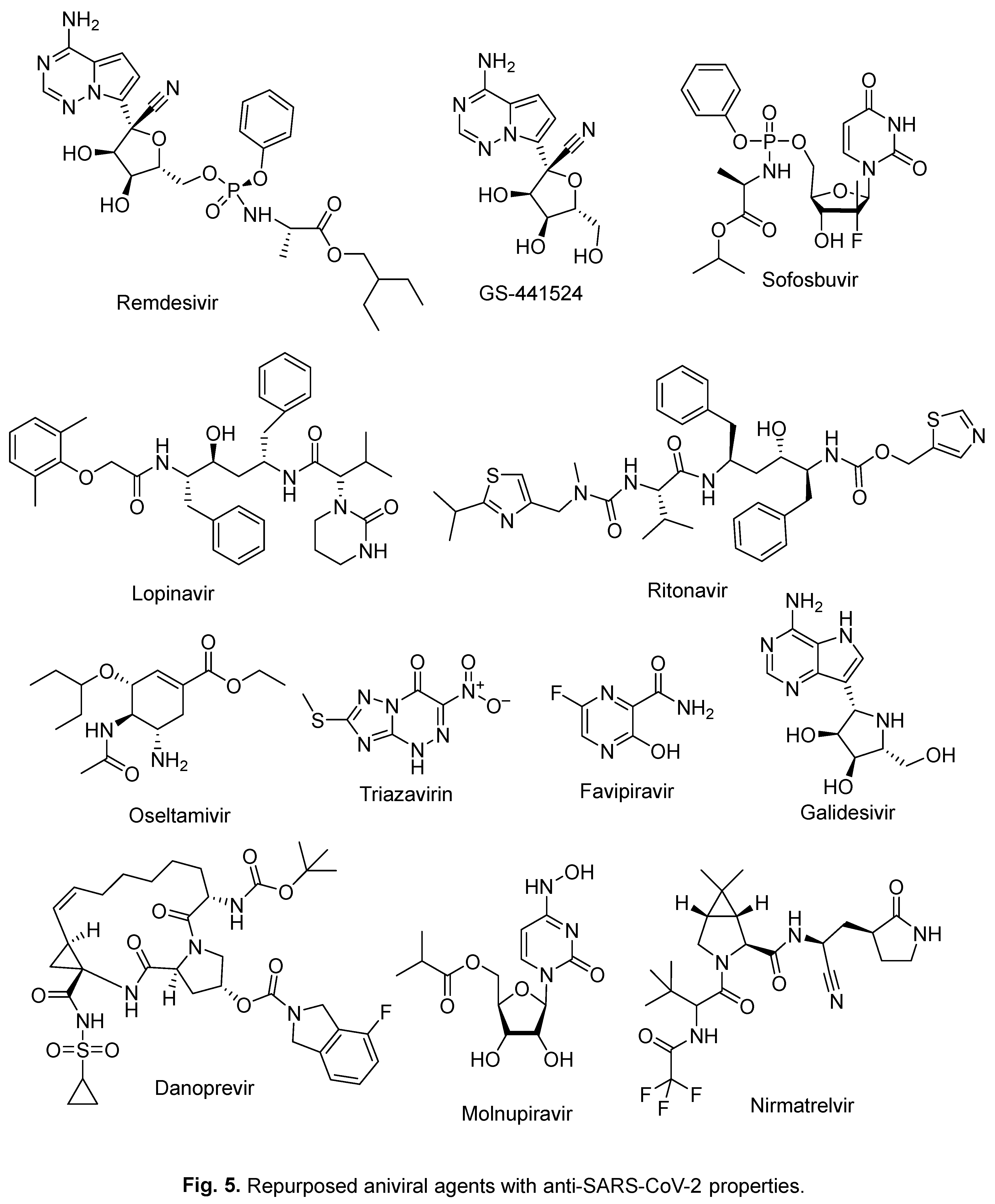
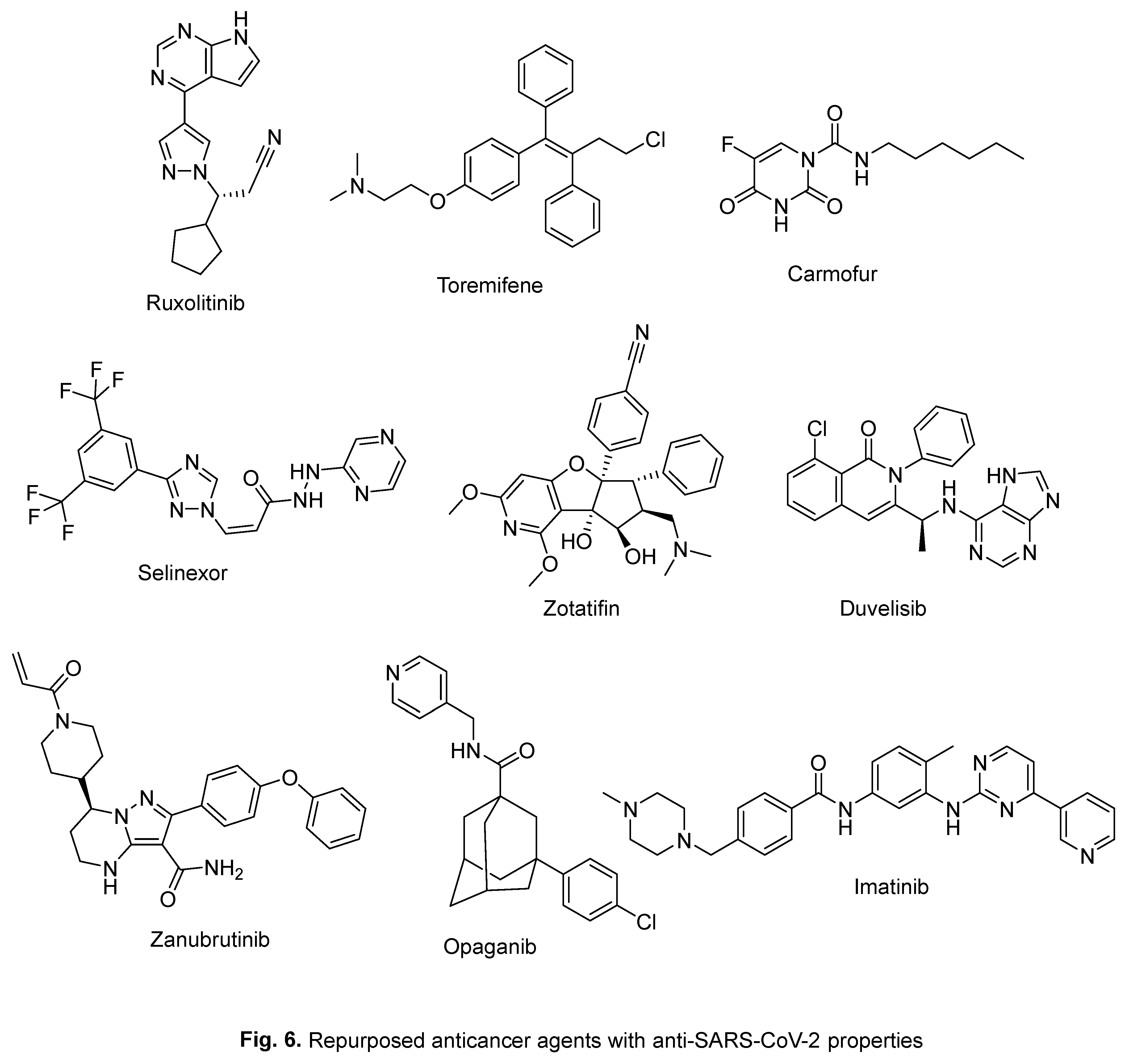
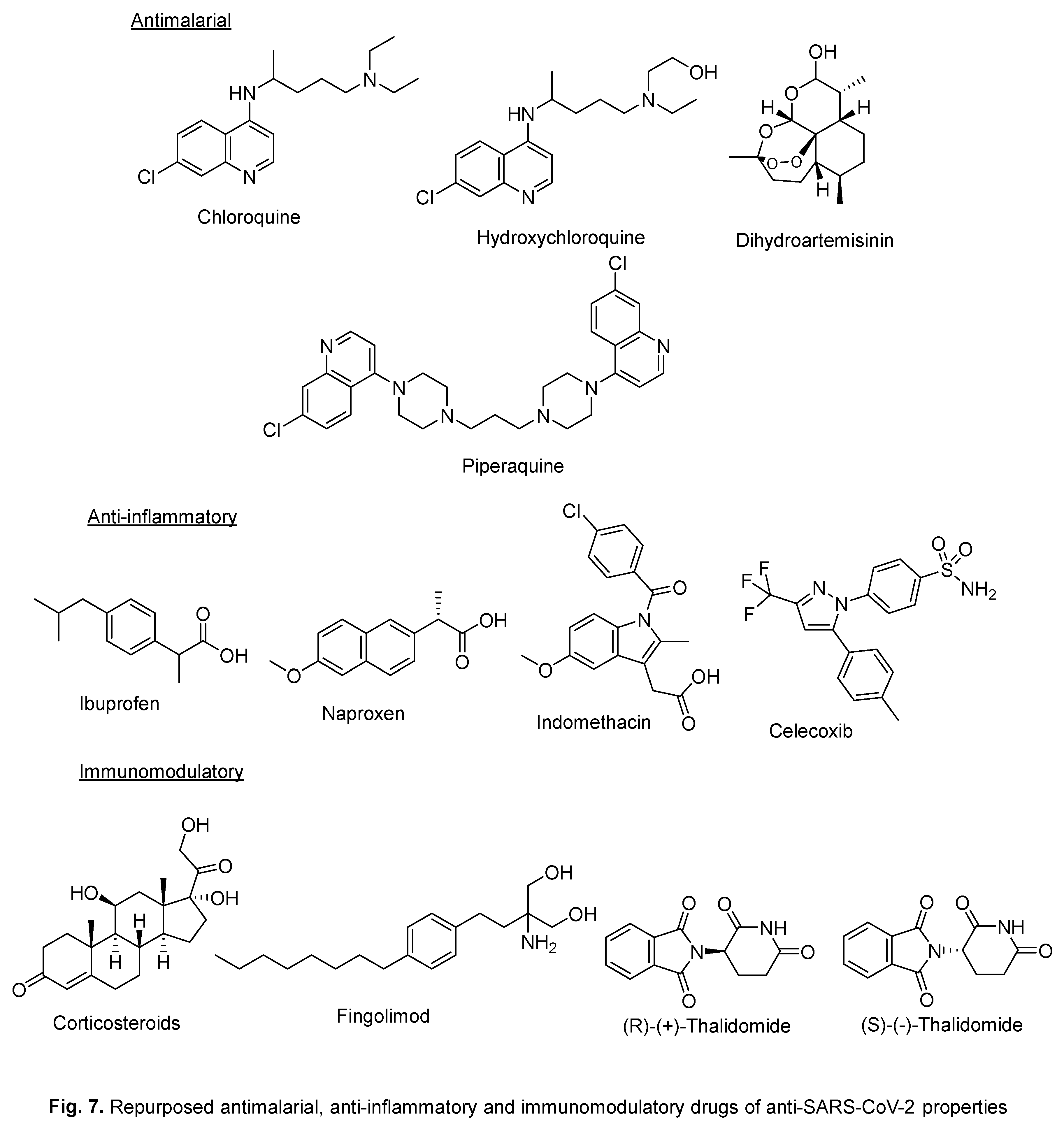
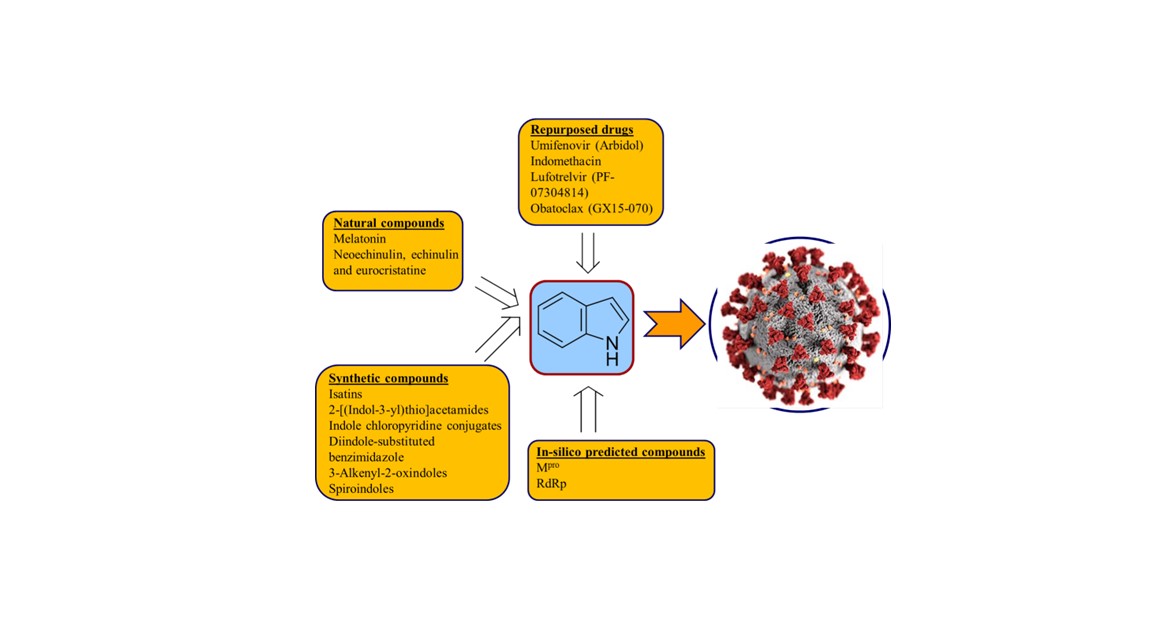
2. Repurposed indole-containing drugs
2.1. Umifenovir (Arbidol)

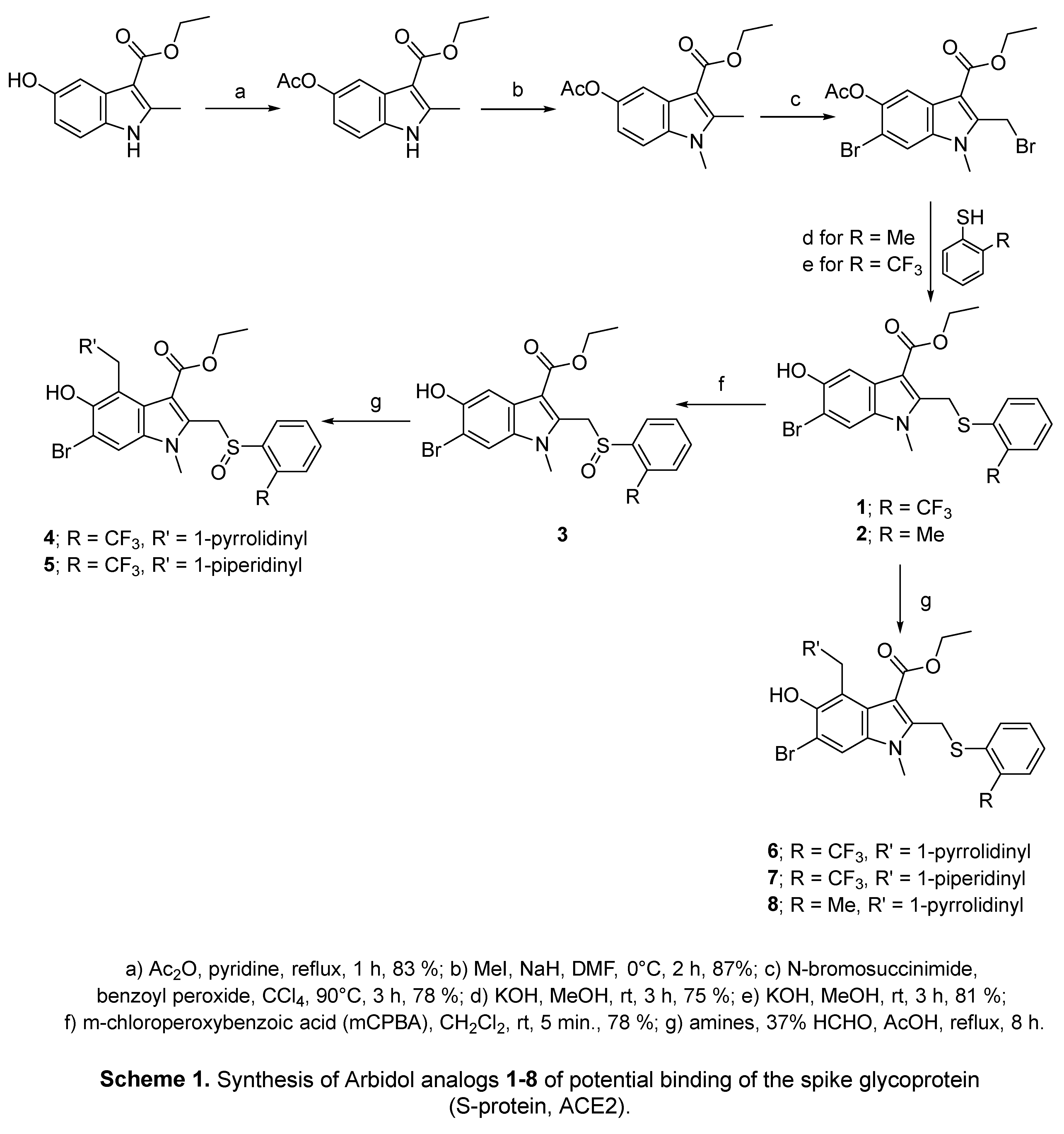
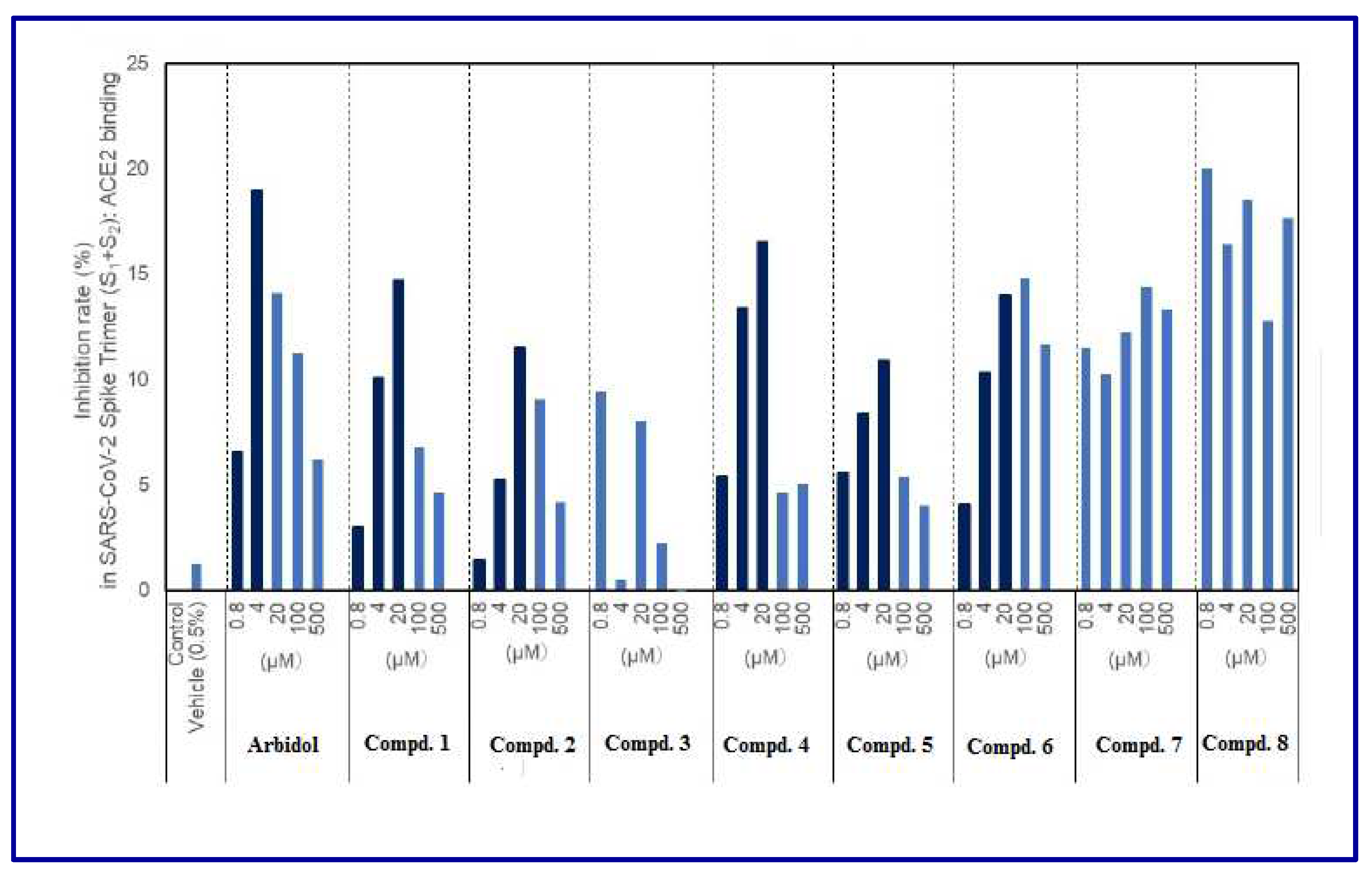
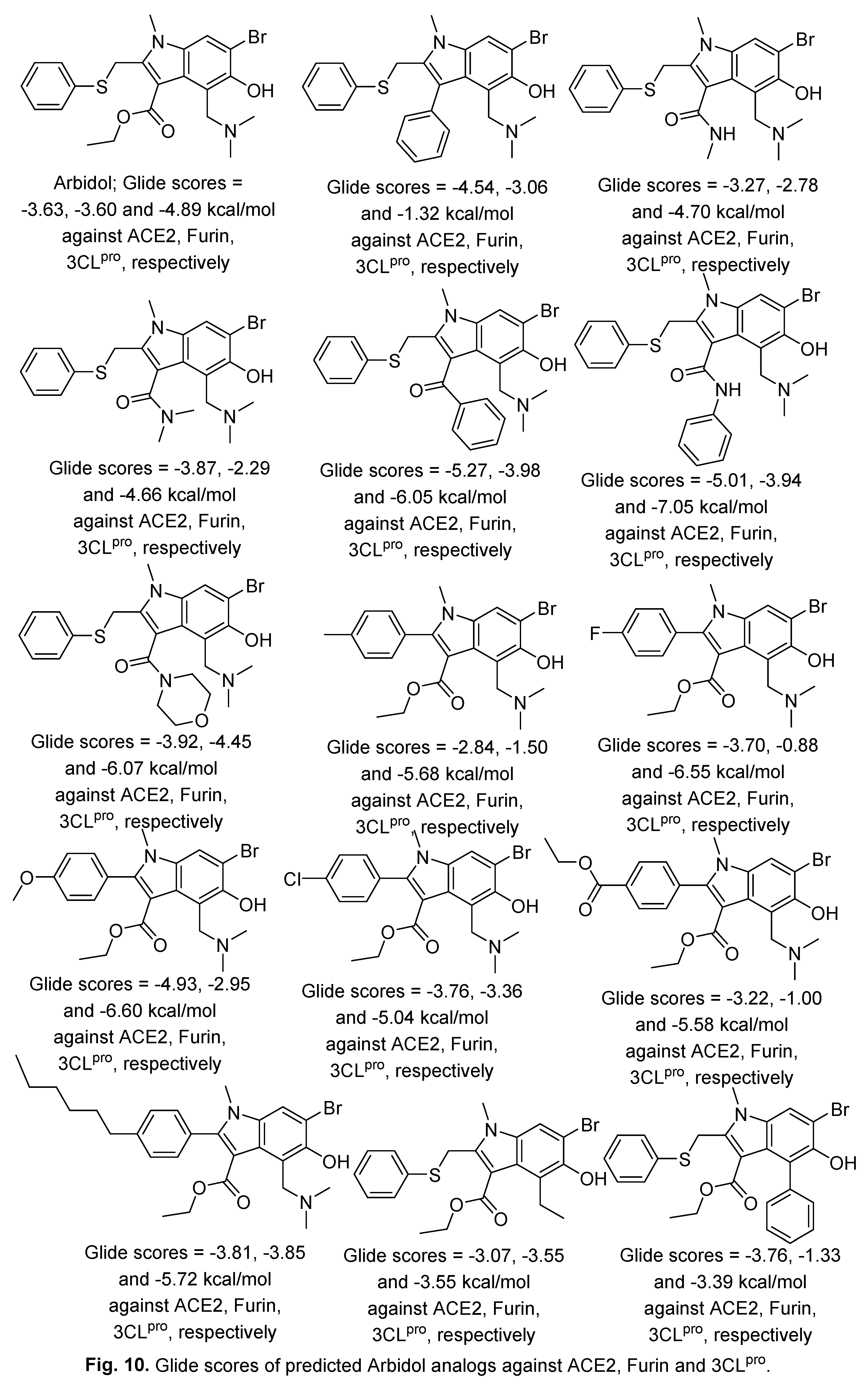
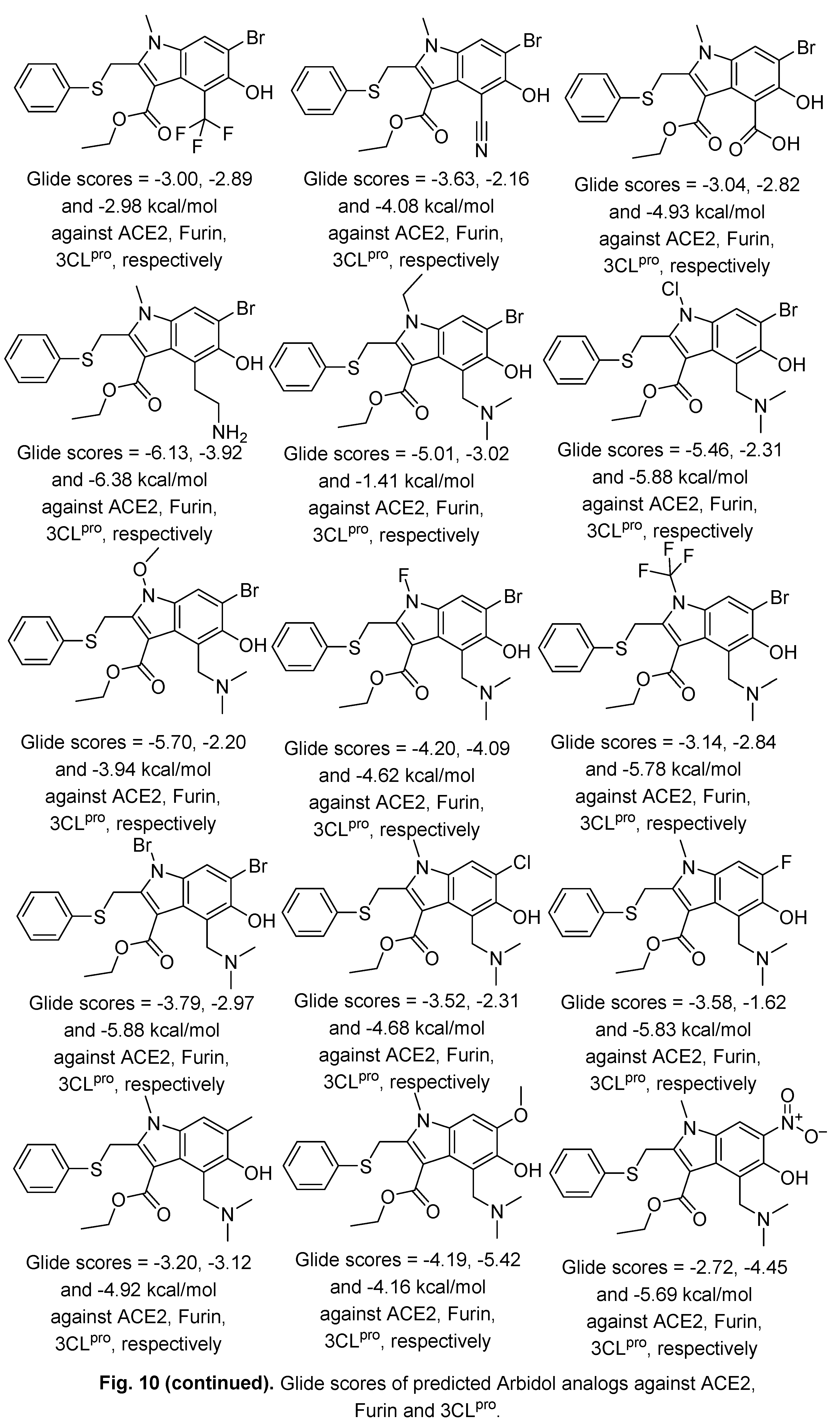
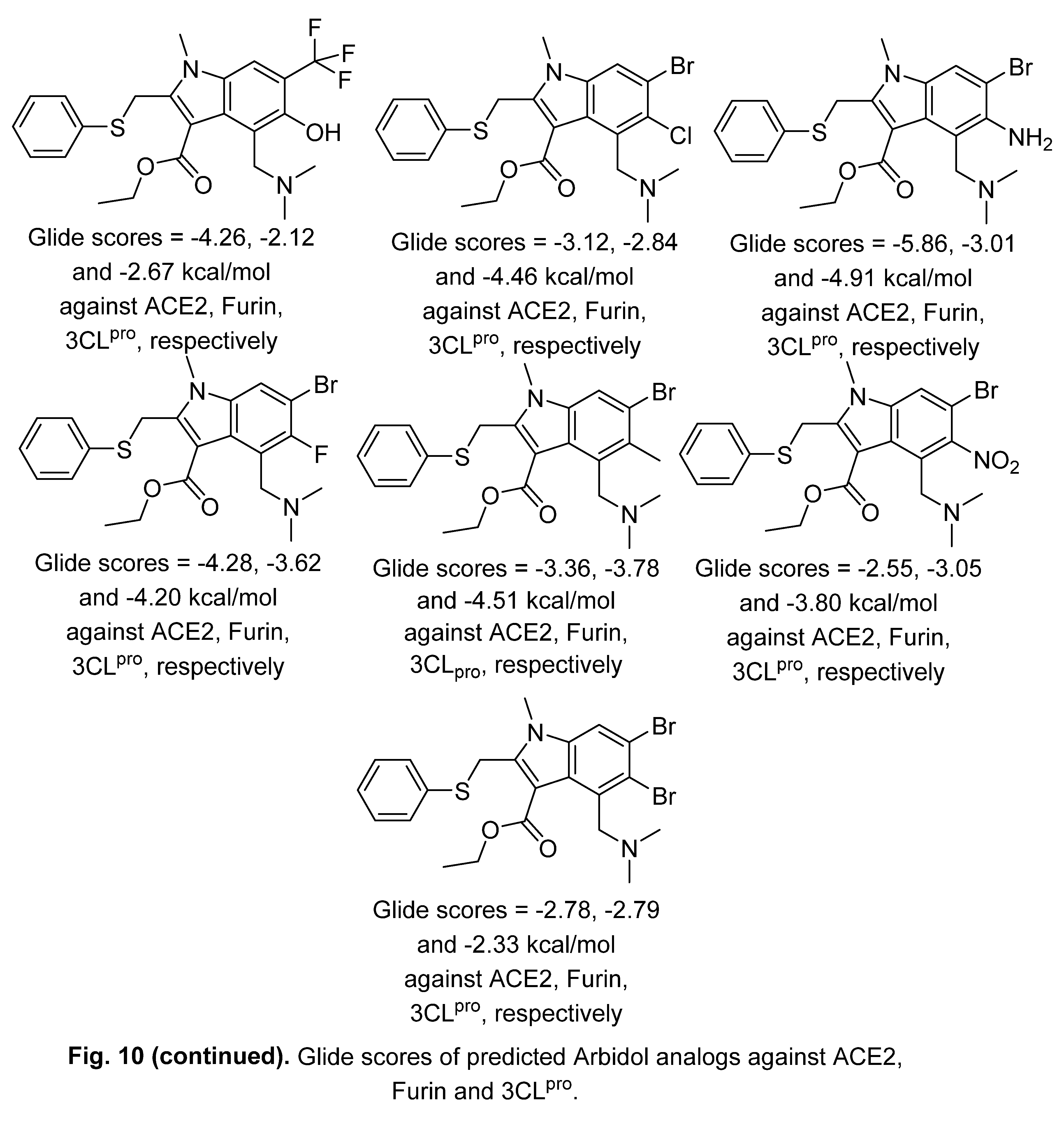
2.2. Indomethacin
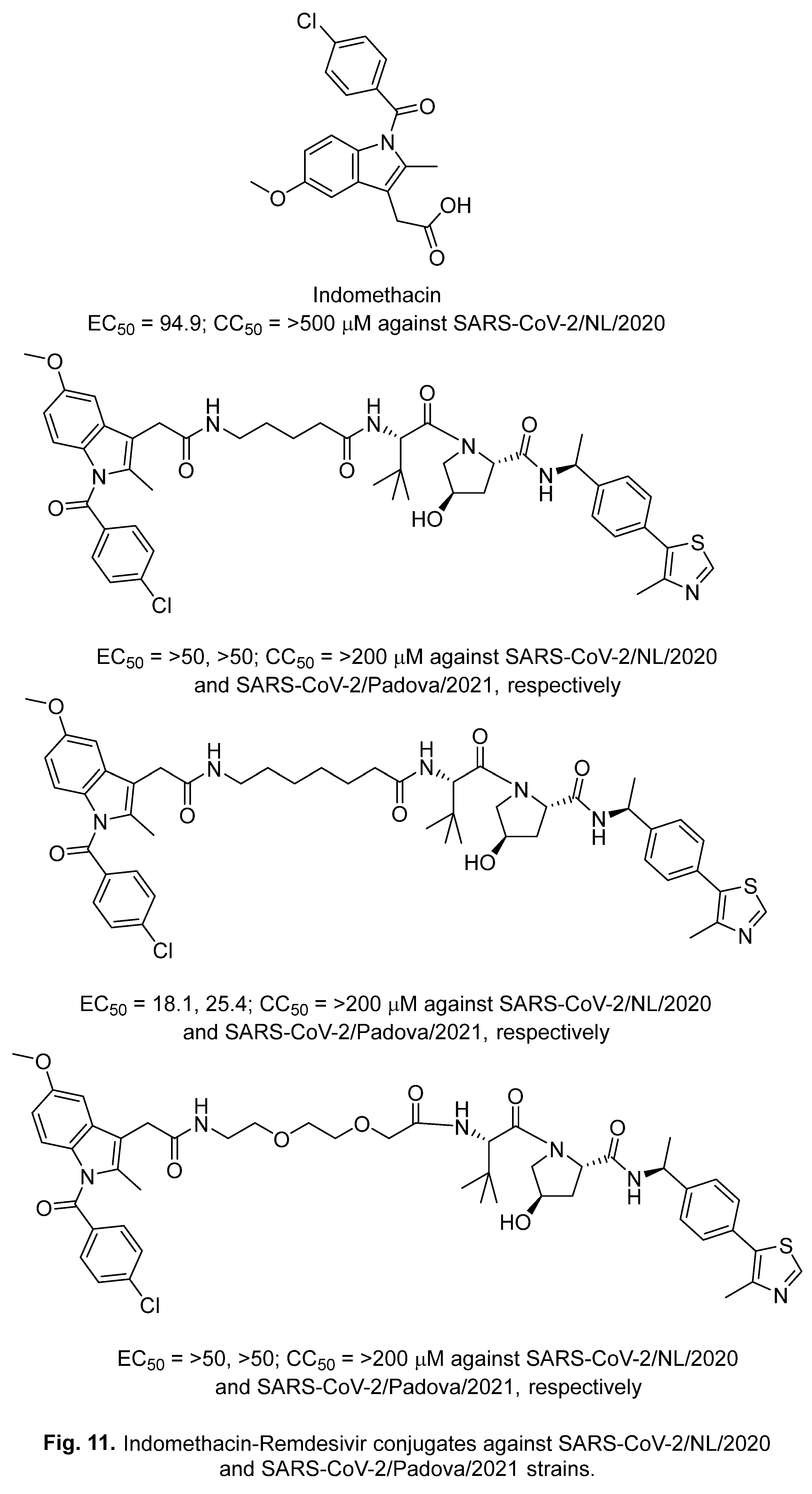
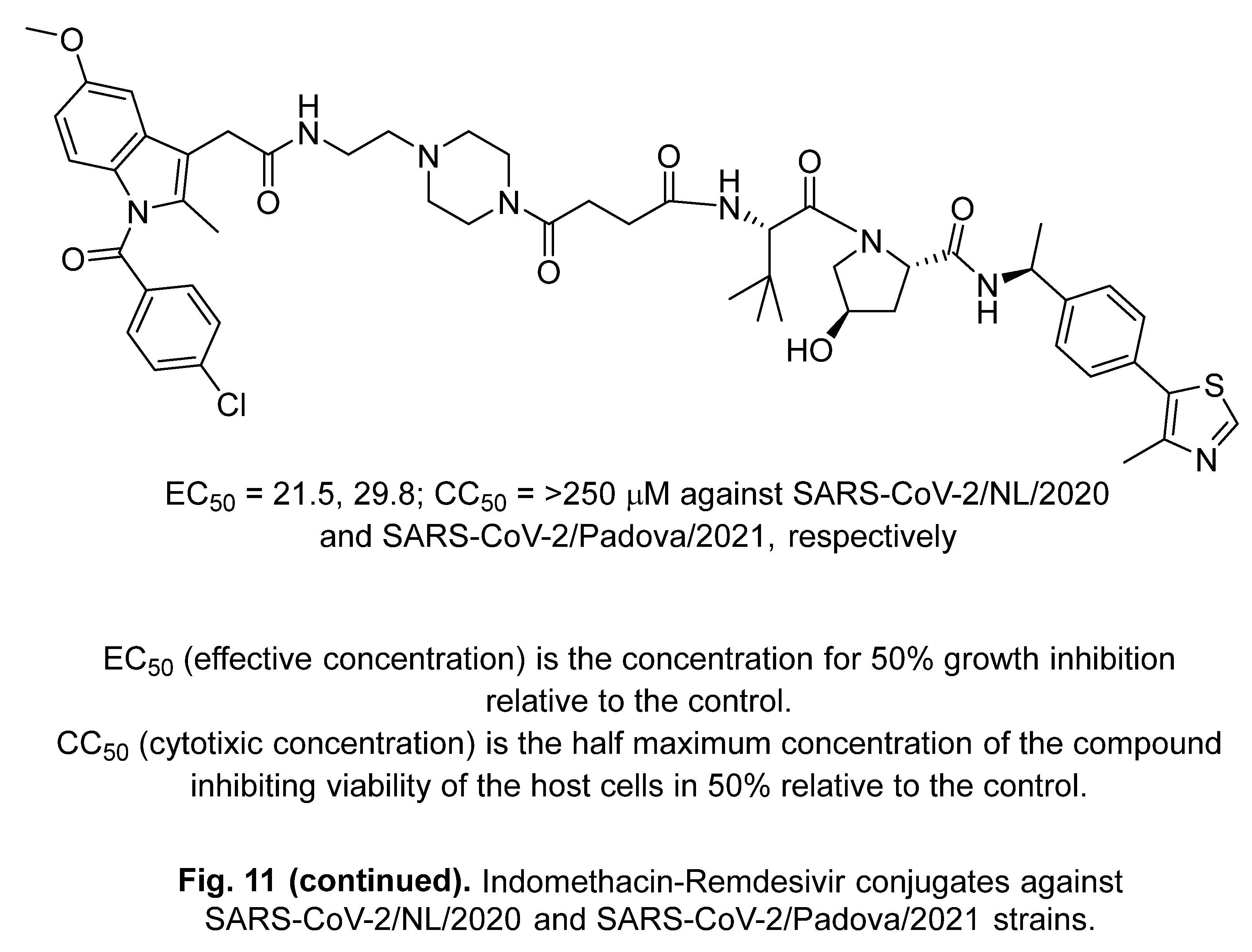
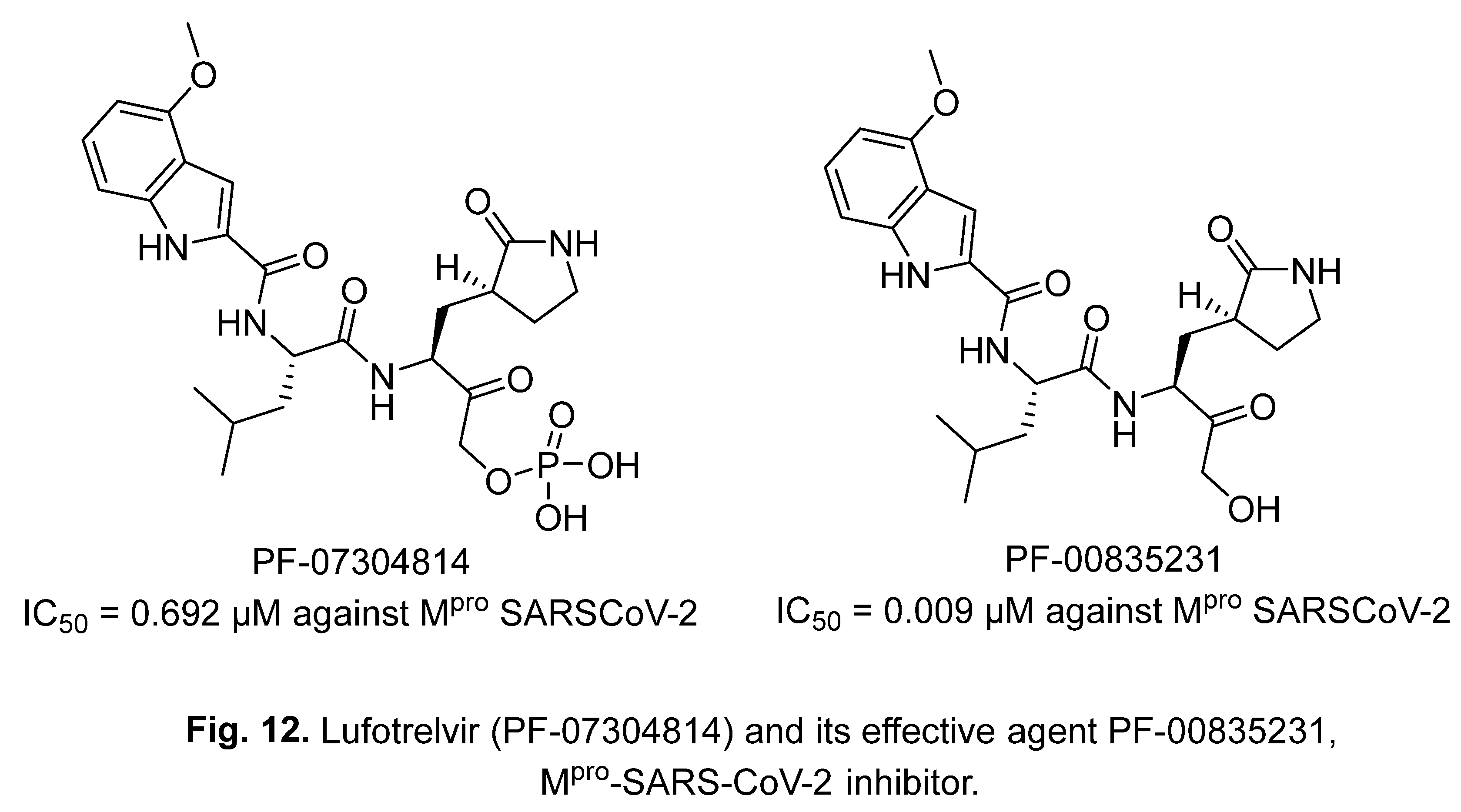
2.3. Lufotrelvir (PF-07304814)
2.4. Obatoclax (GX15-070)
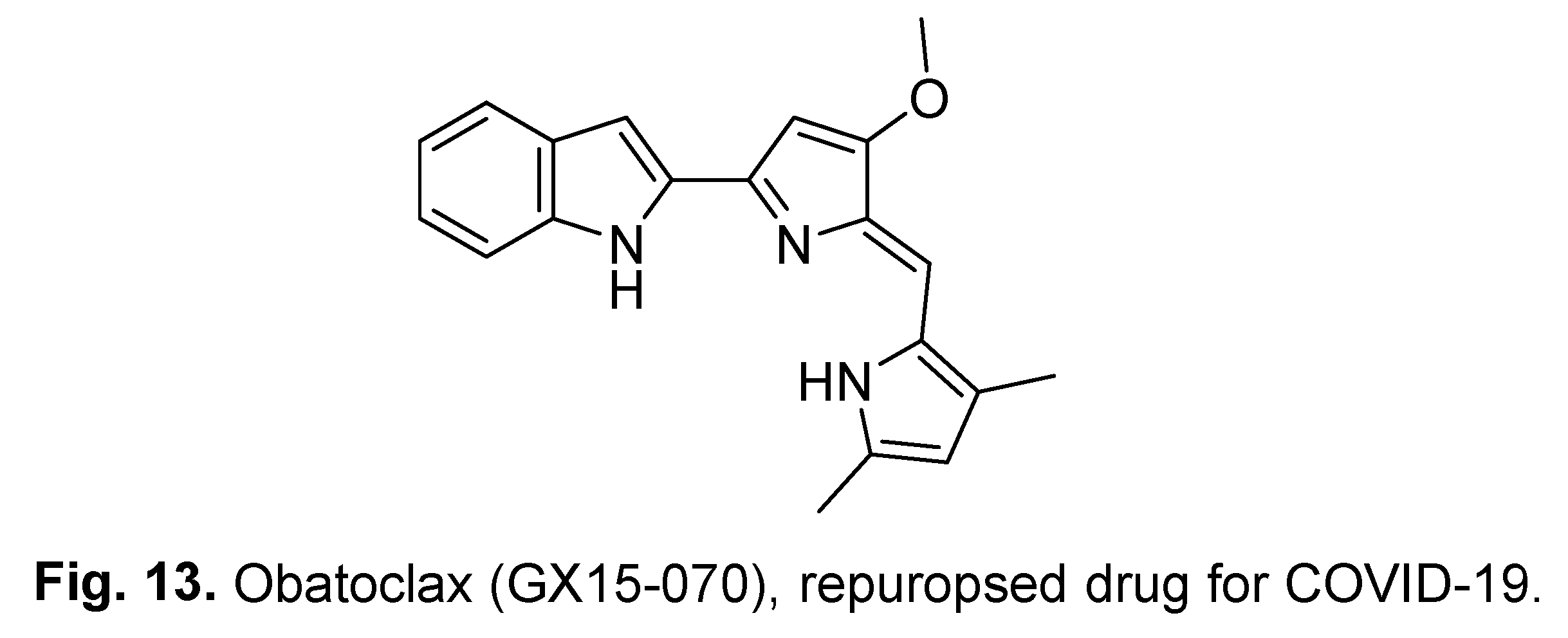
3. Natural indole-containing compounds
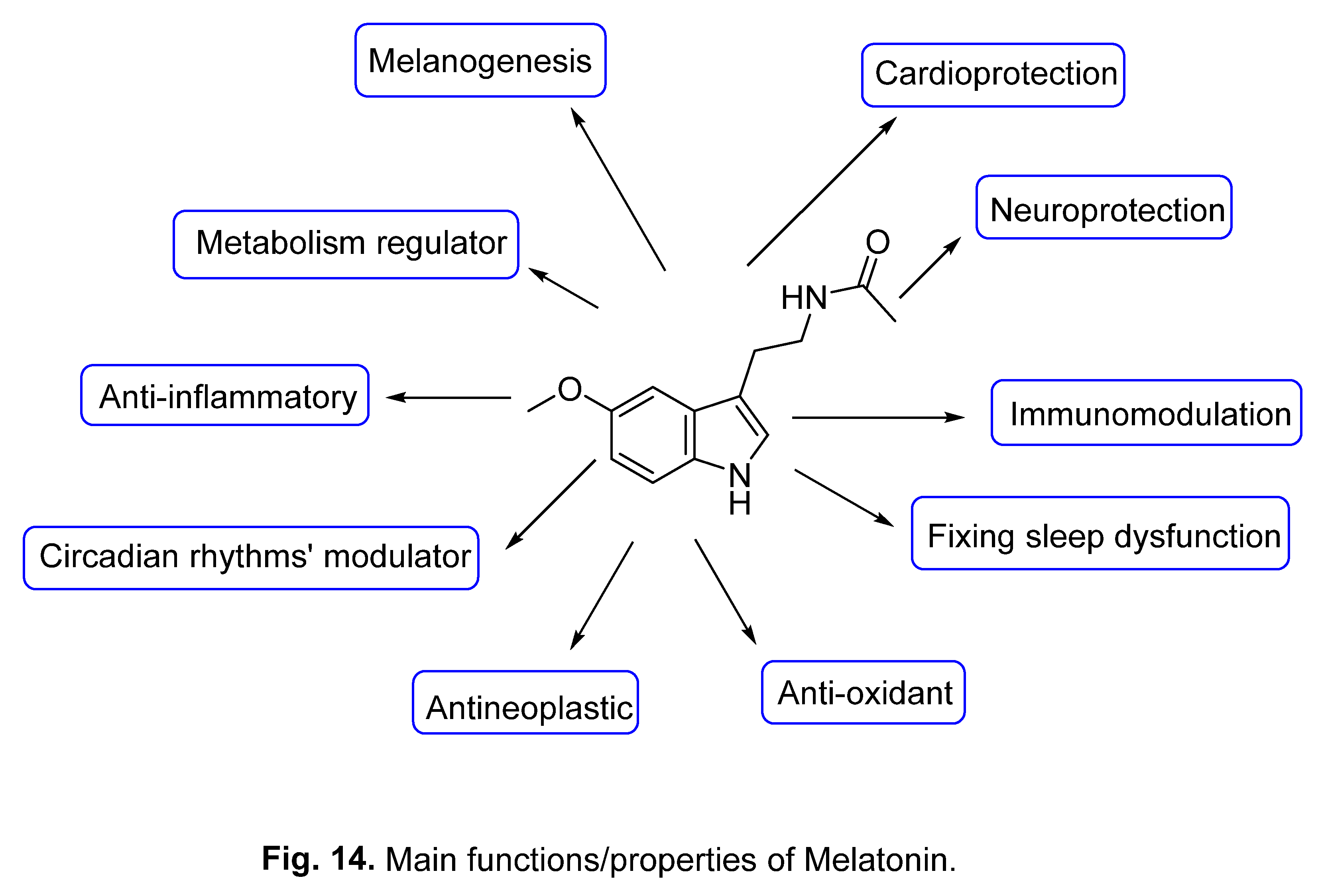
3.1. Melatonin
3.2. Neoechinulin A, echinulin and eurocristatine
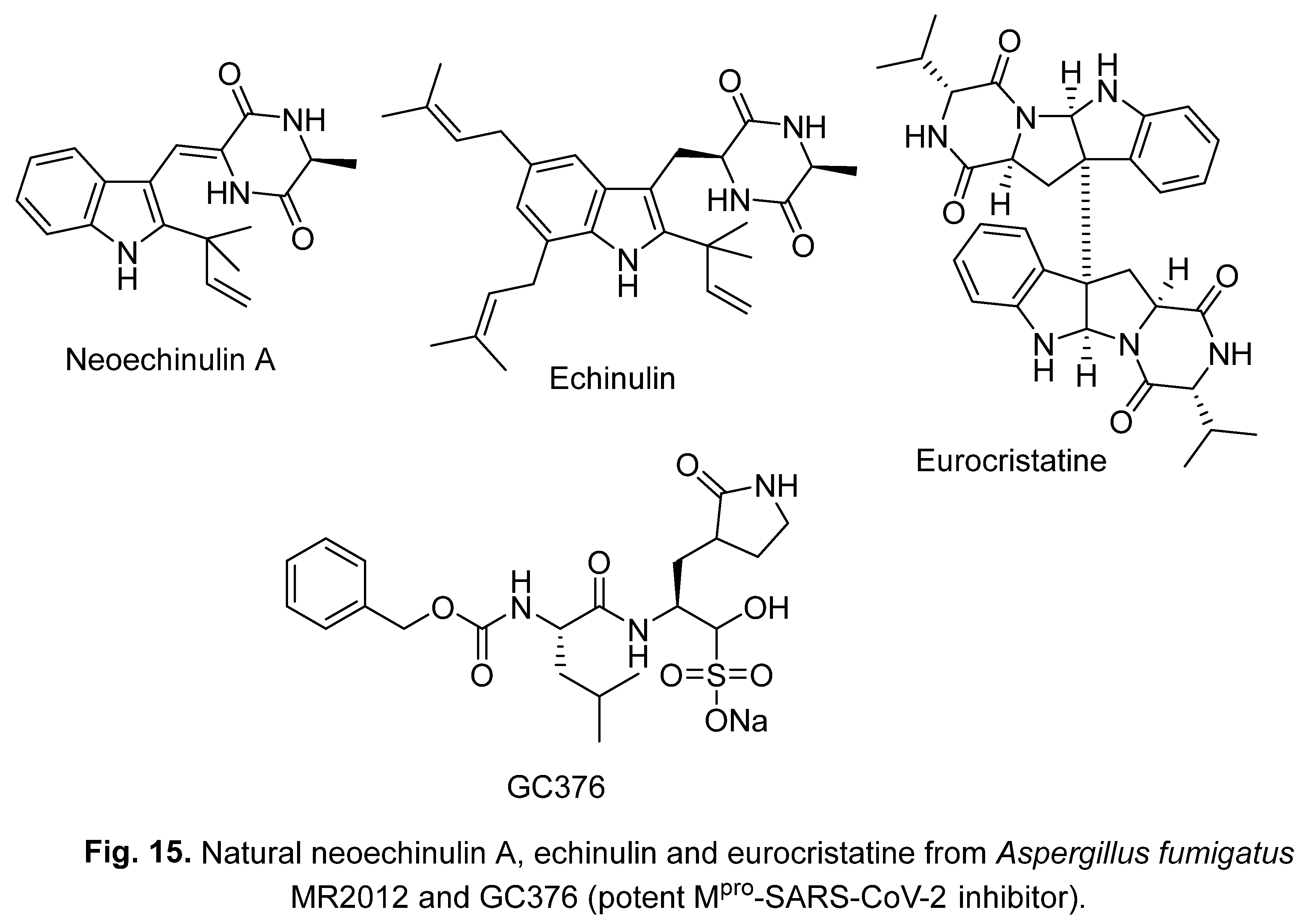
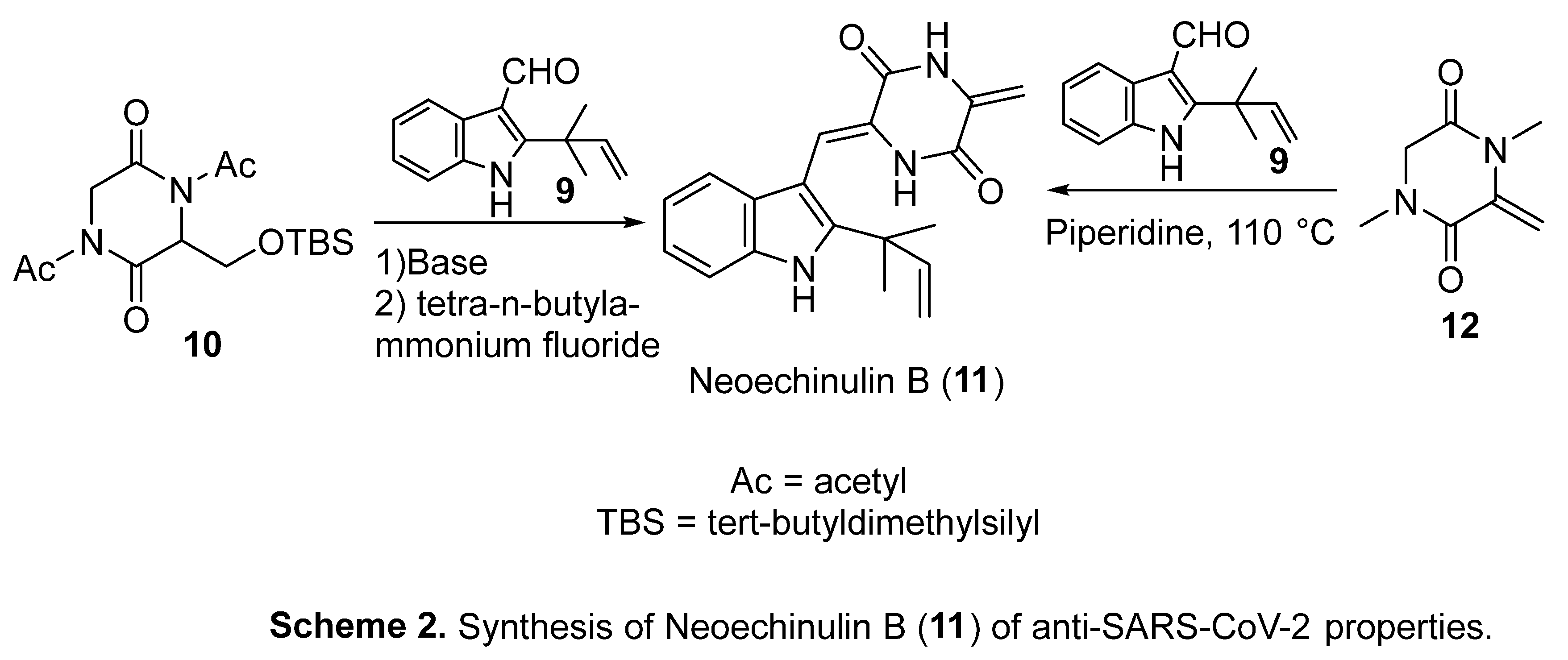
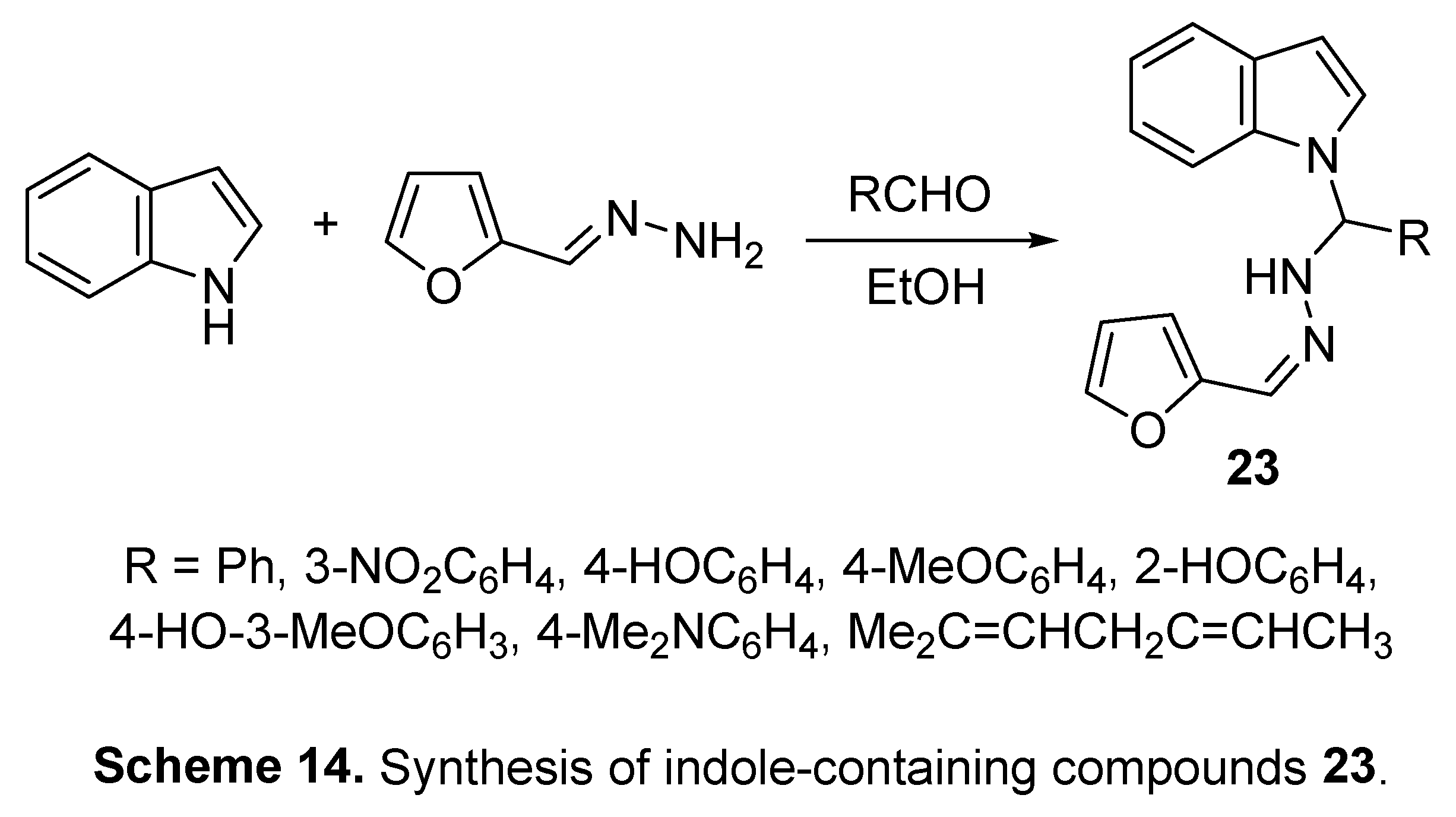
4. Synthetic indole-containing compounds
4.1. Isatins
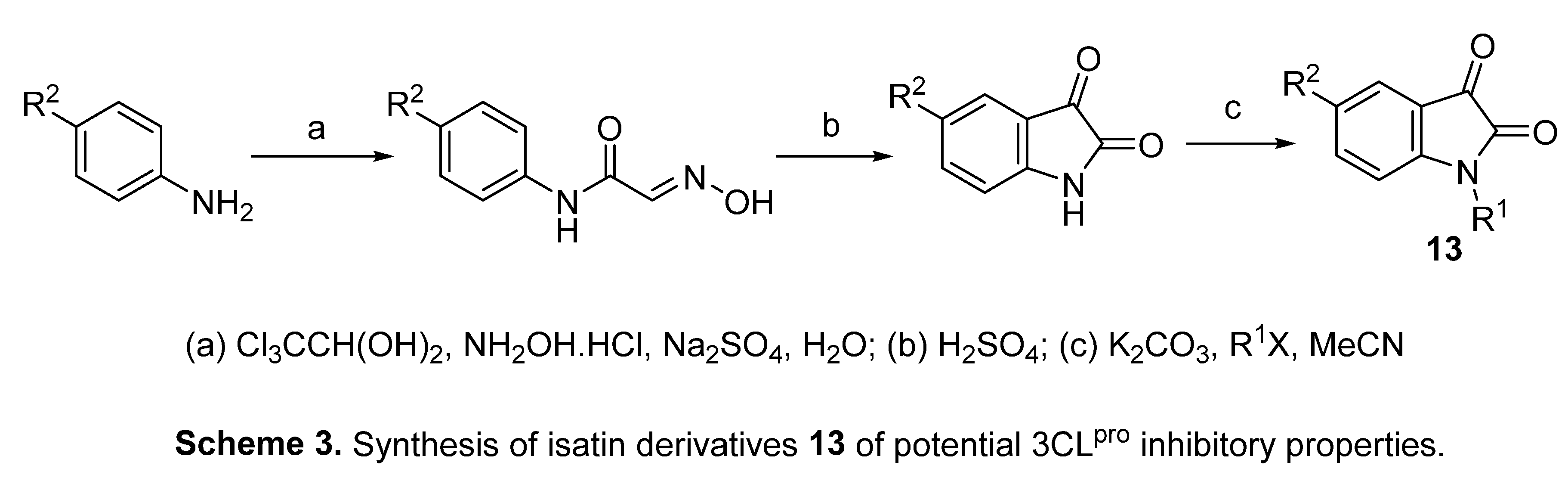
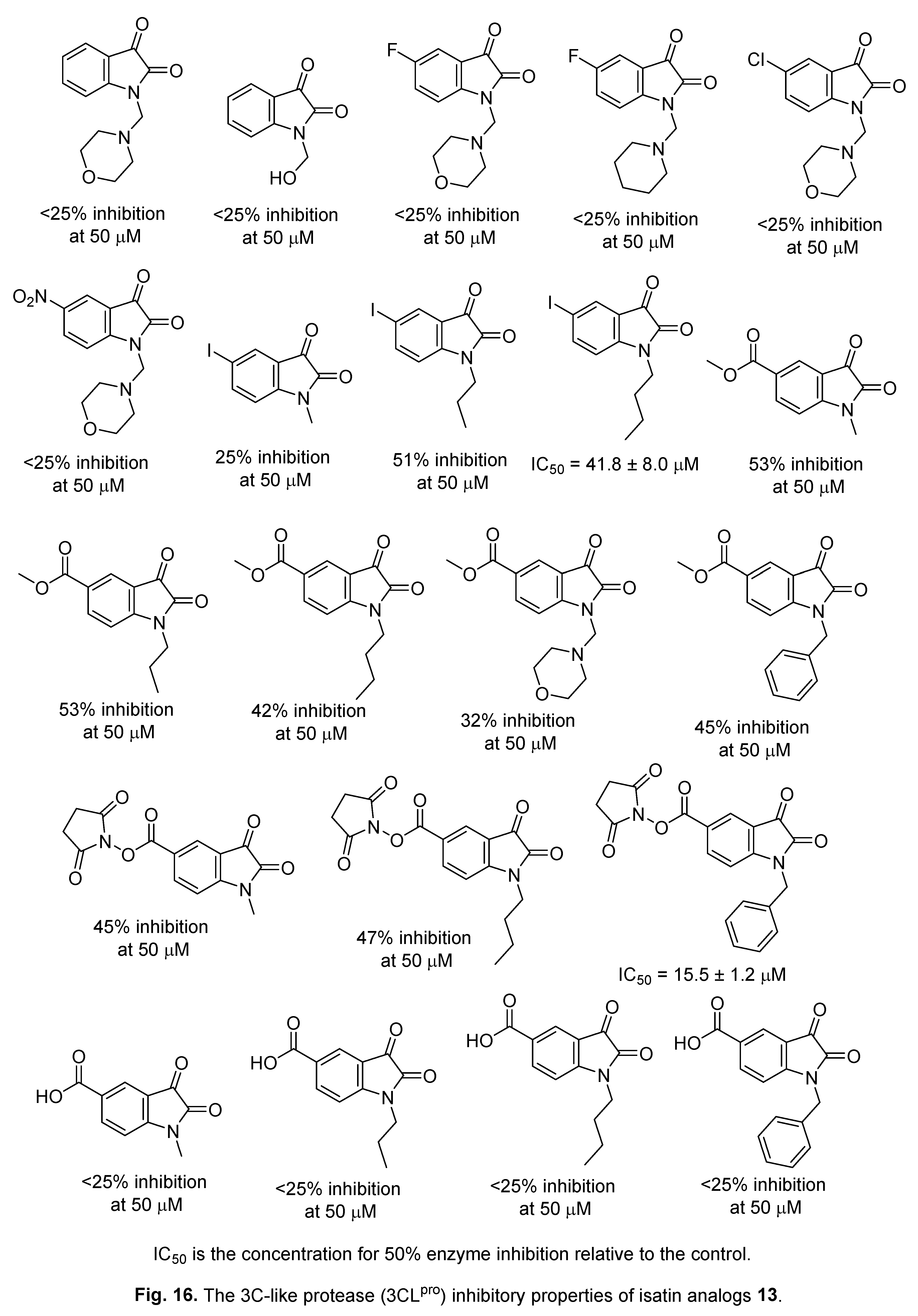
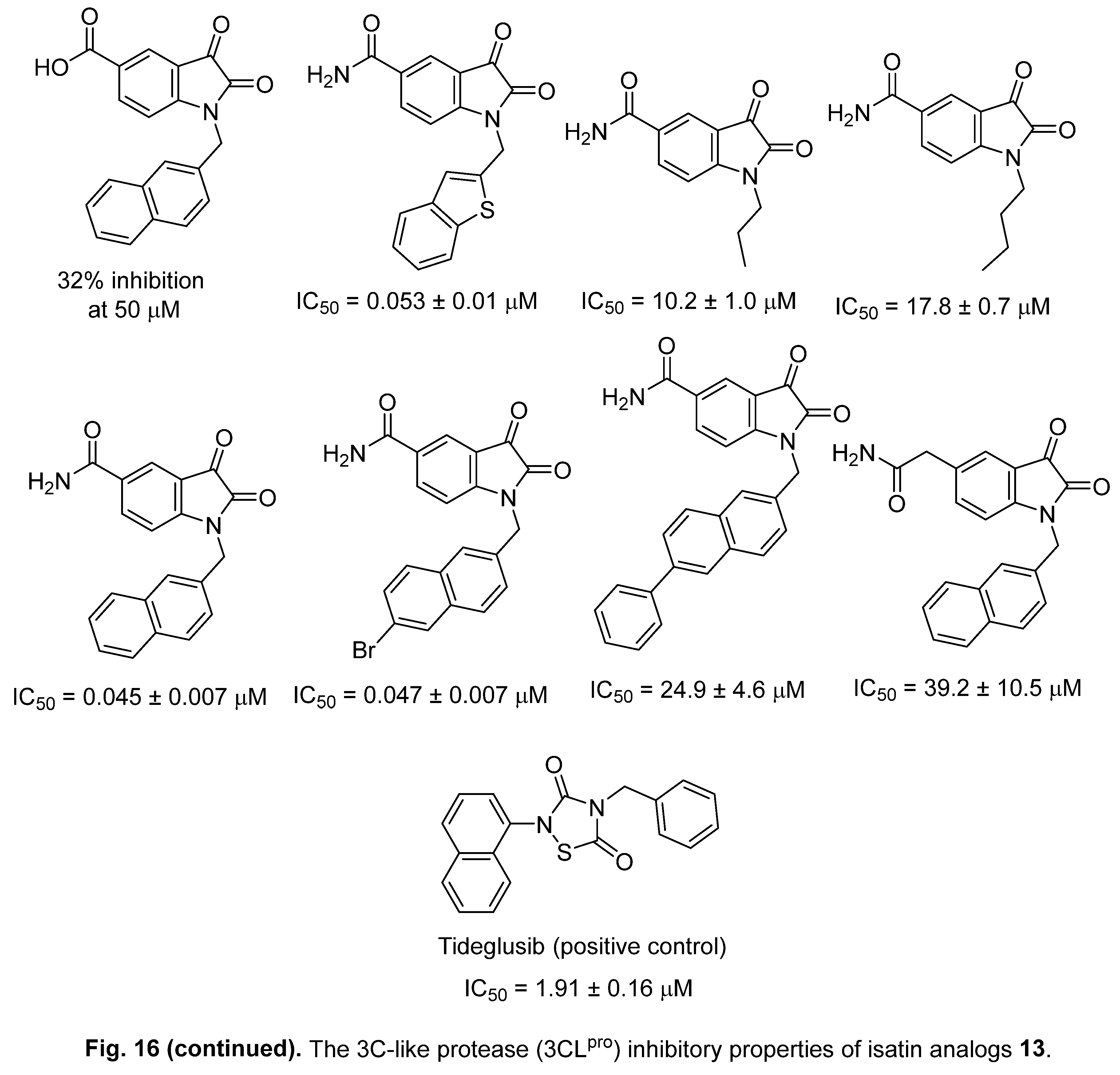
4.2. 2-[(Indol-3-yl)thio]acetamides
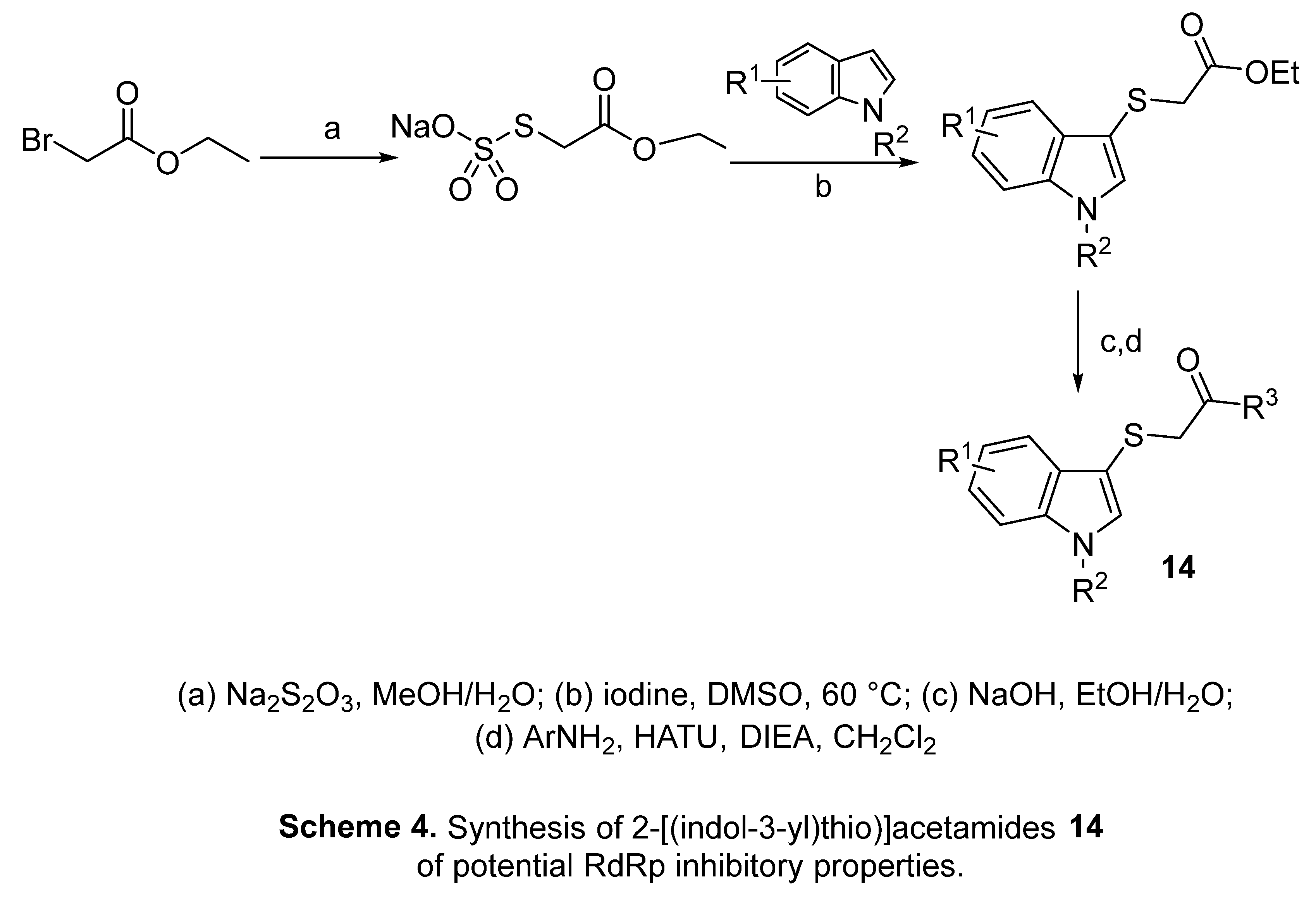
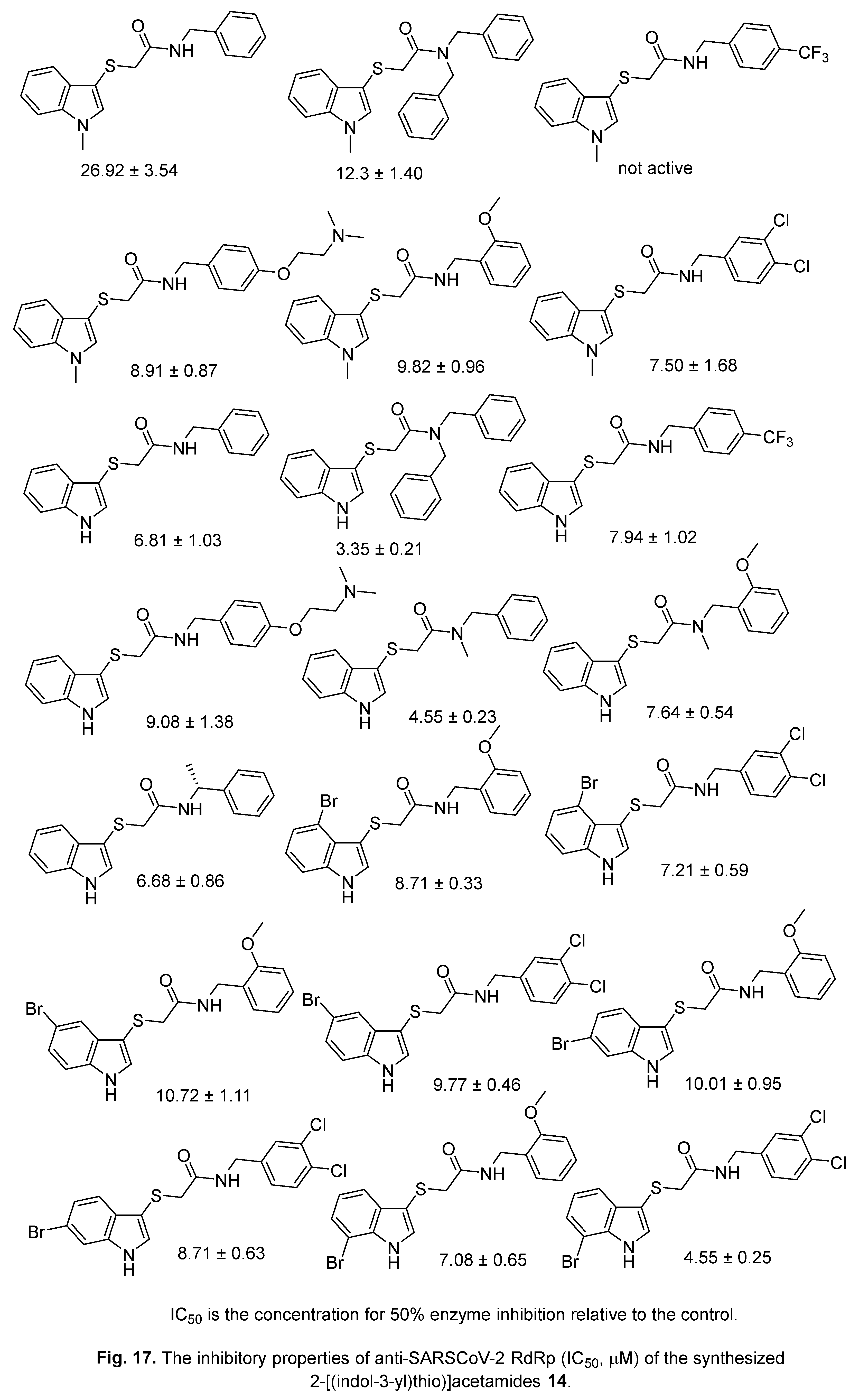
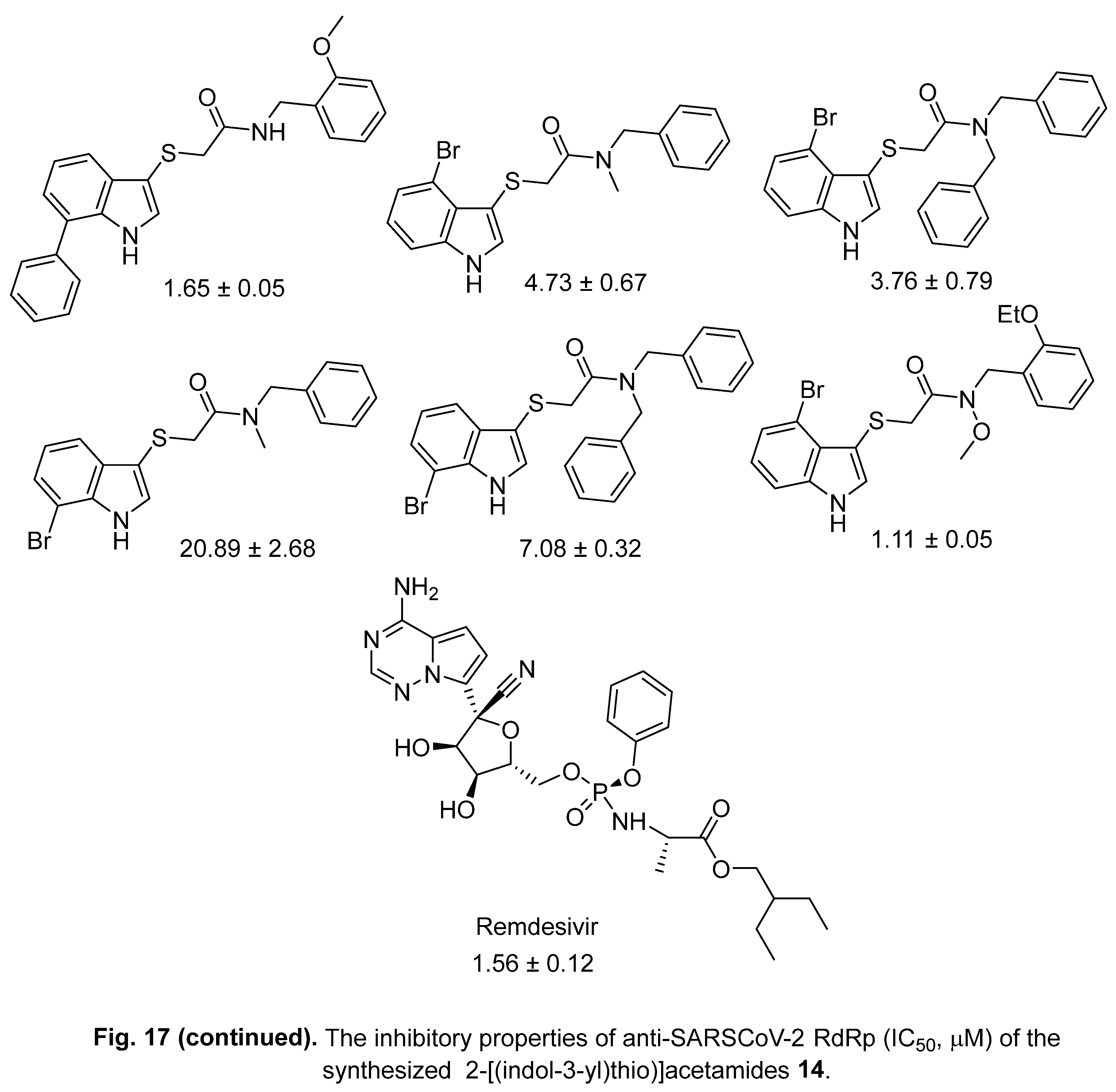
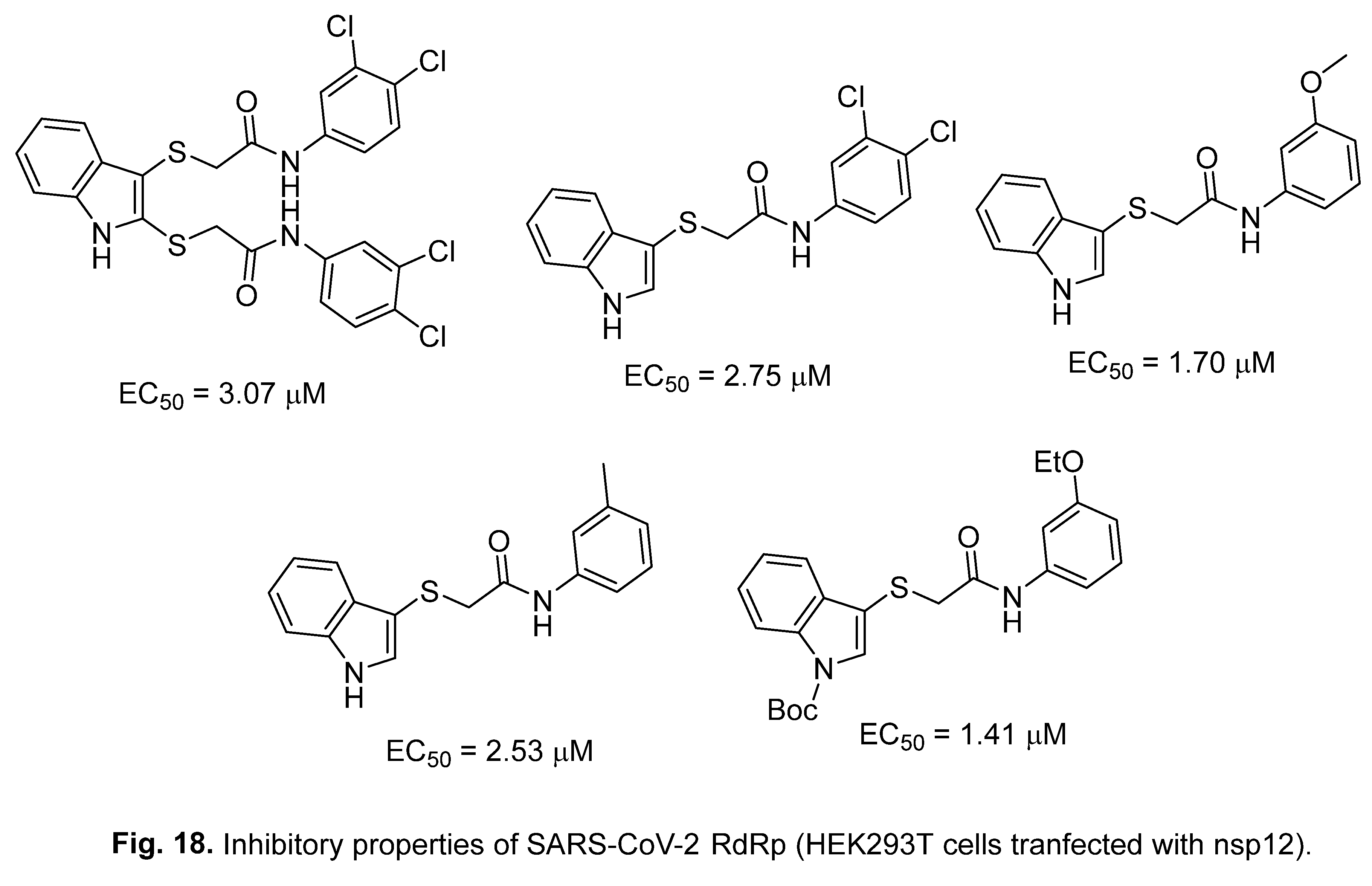
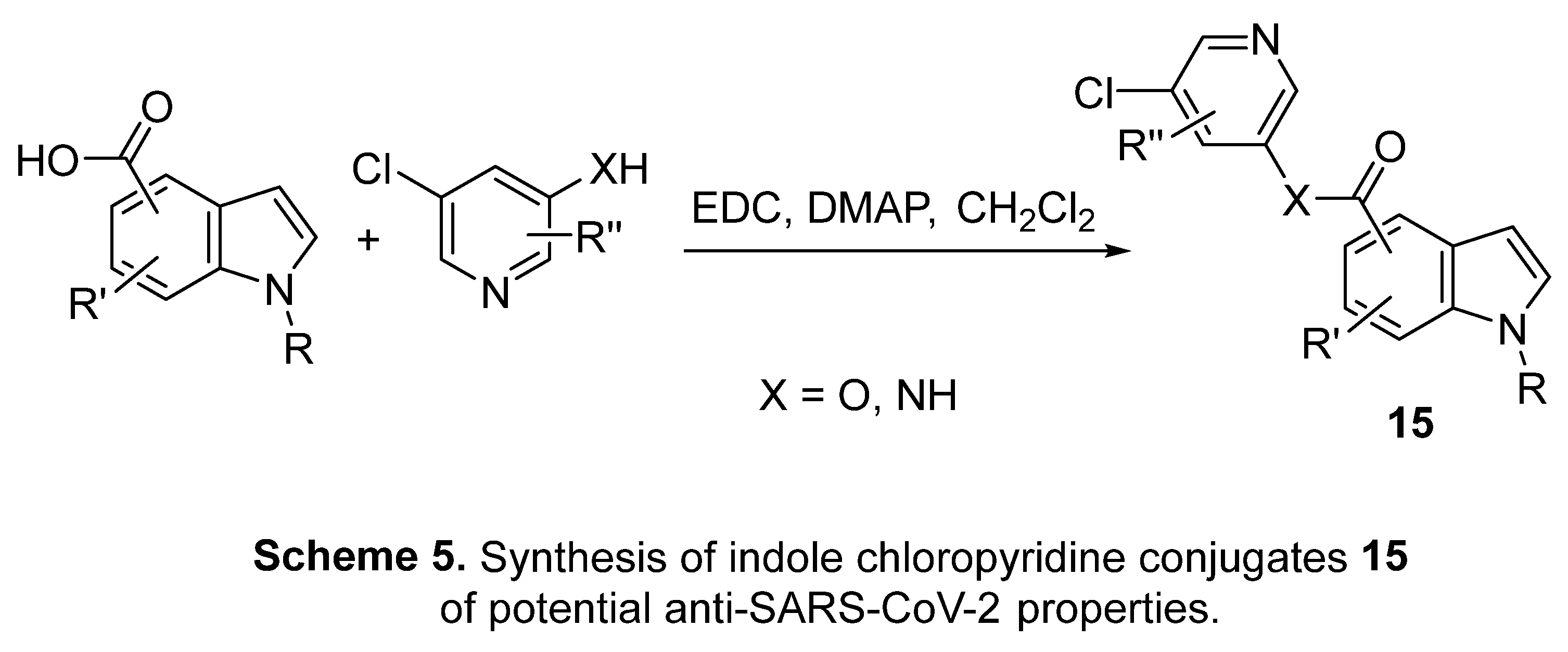
4.3. Indole chloropyridine conjugates
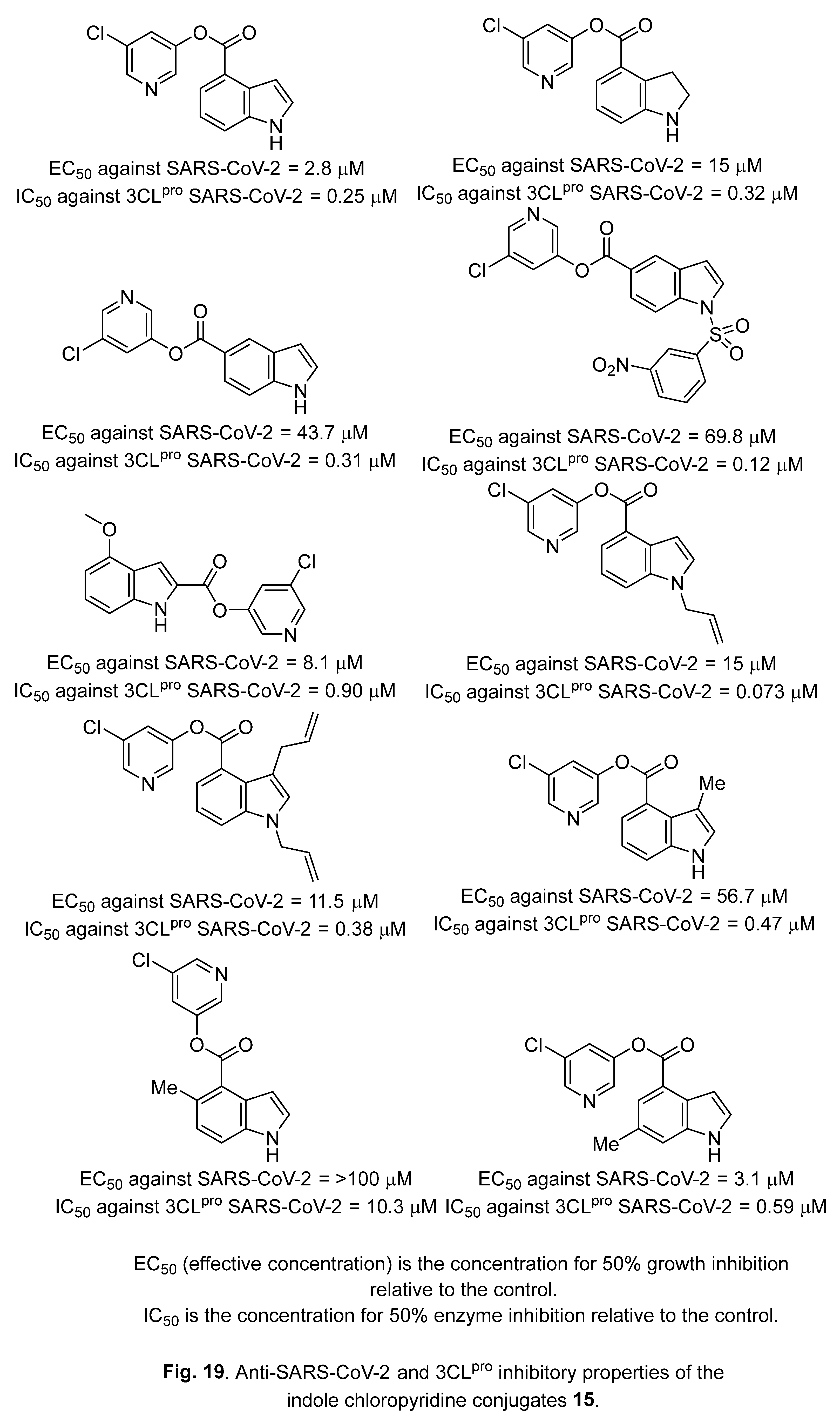
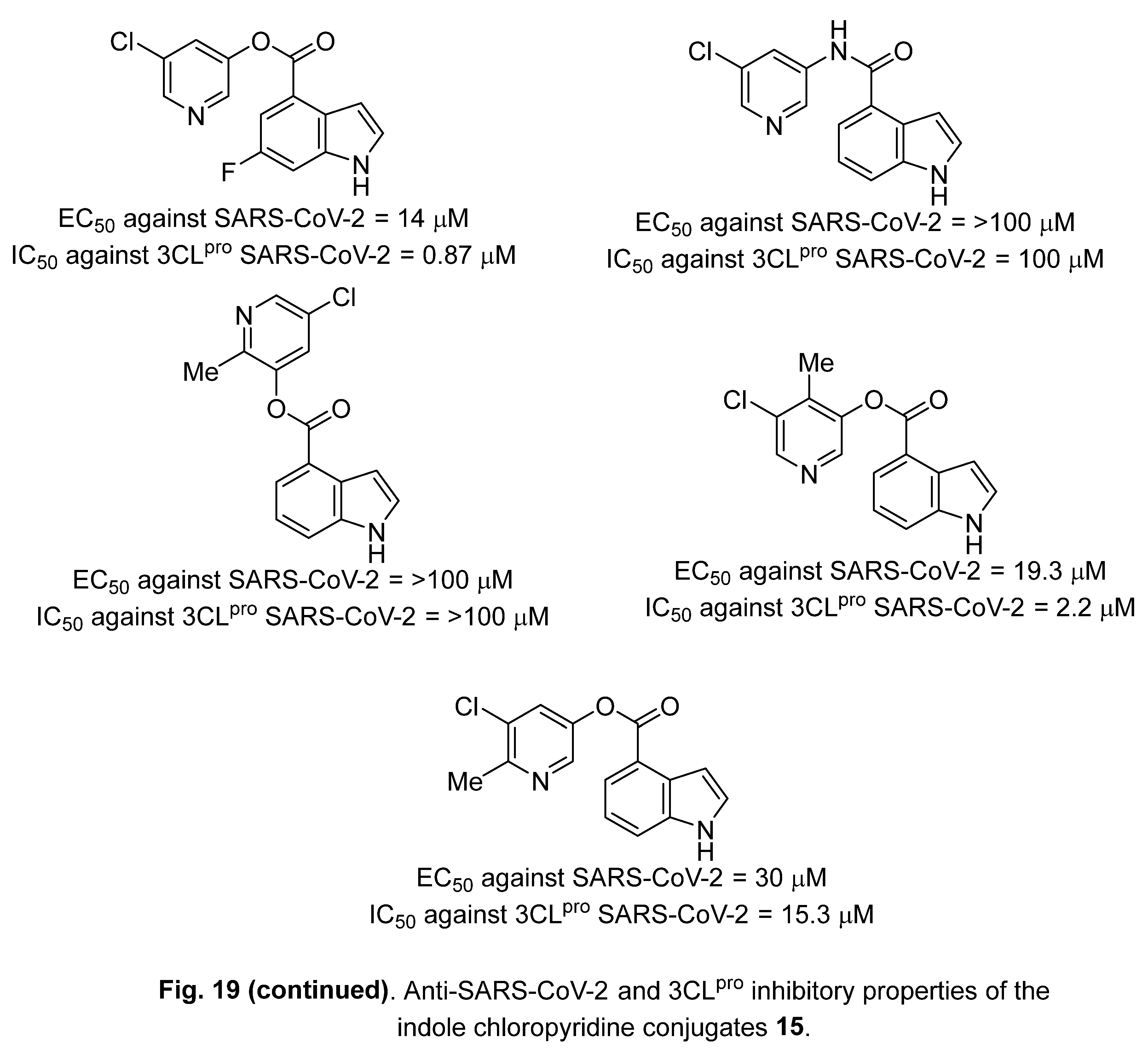
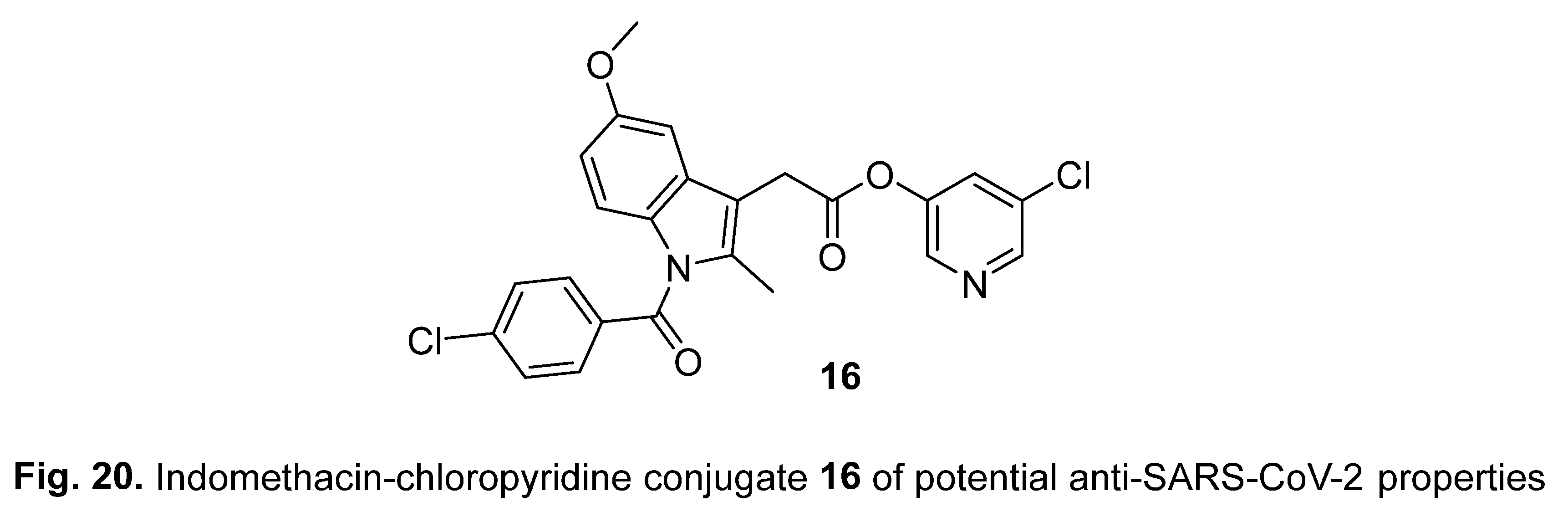
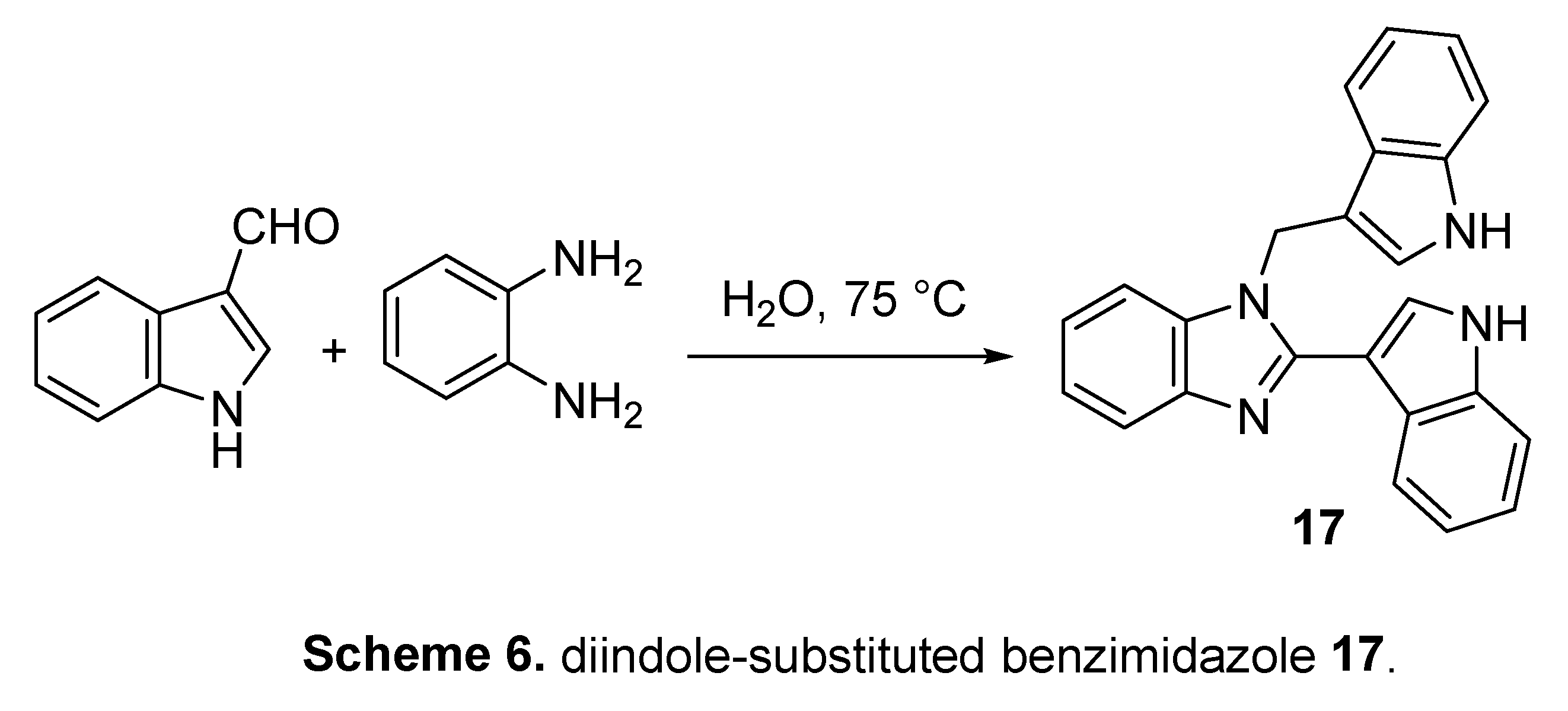
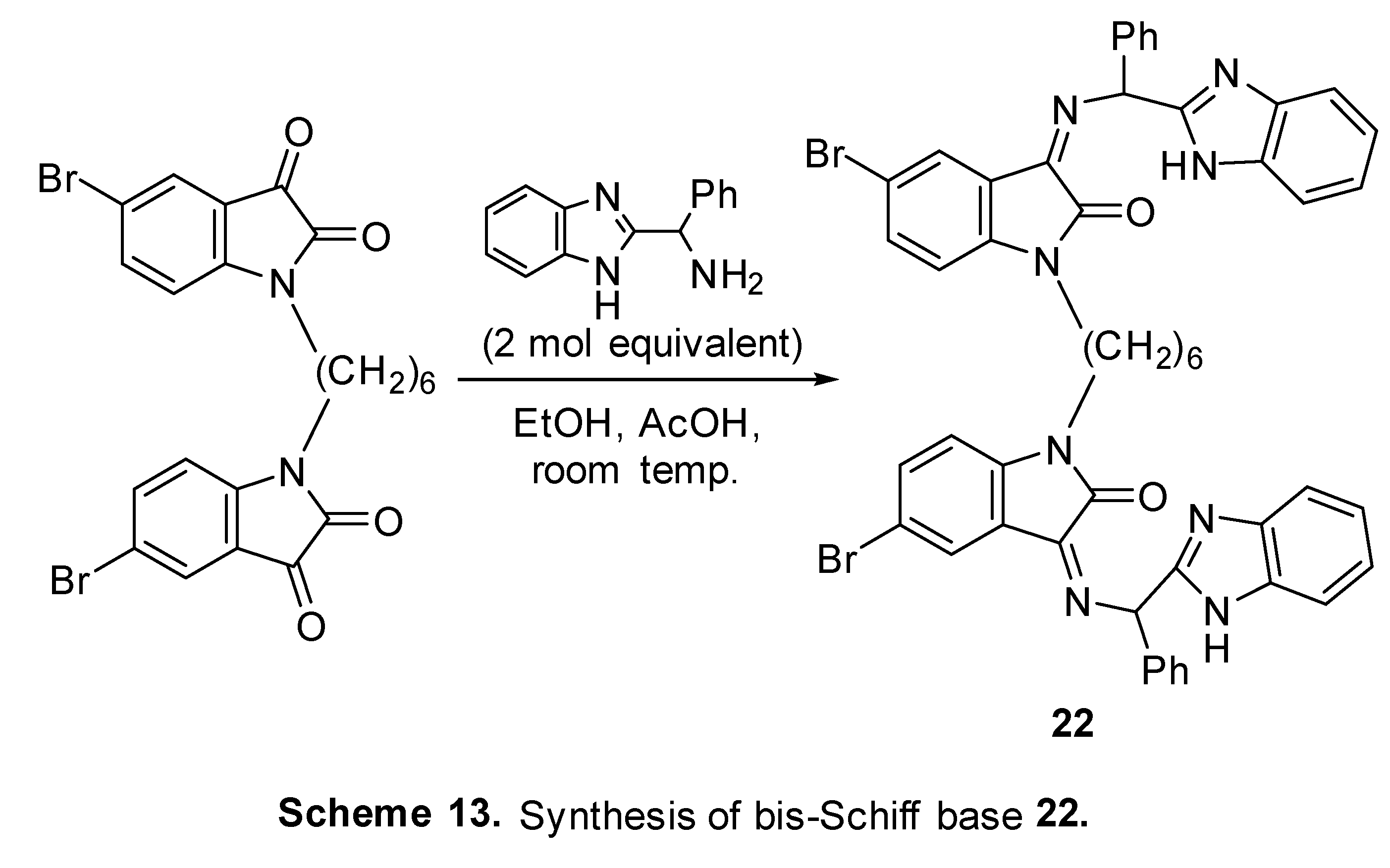
4.4. Diindole-substituted benzimidazole
4.5. 3-Alkenyl-2-oxindoles
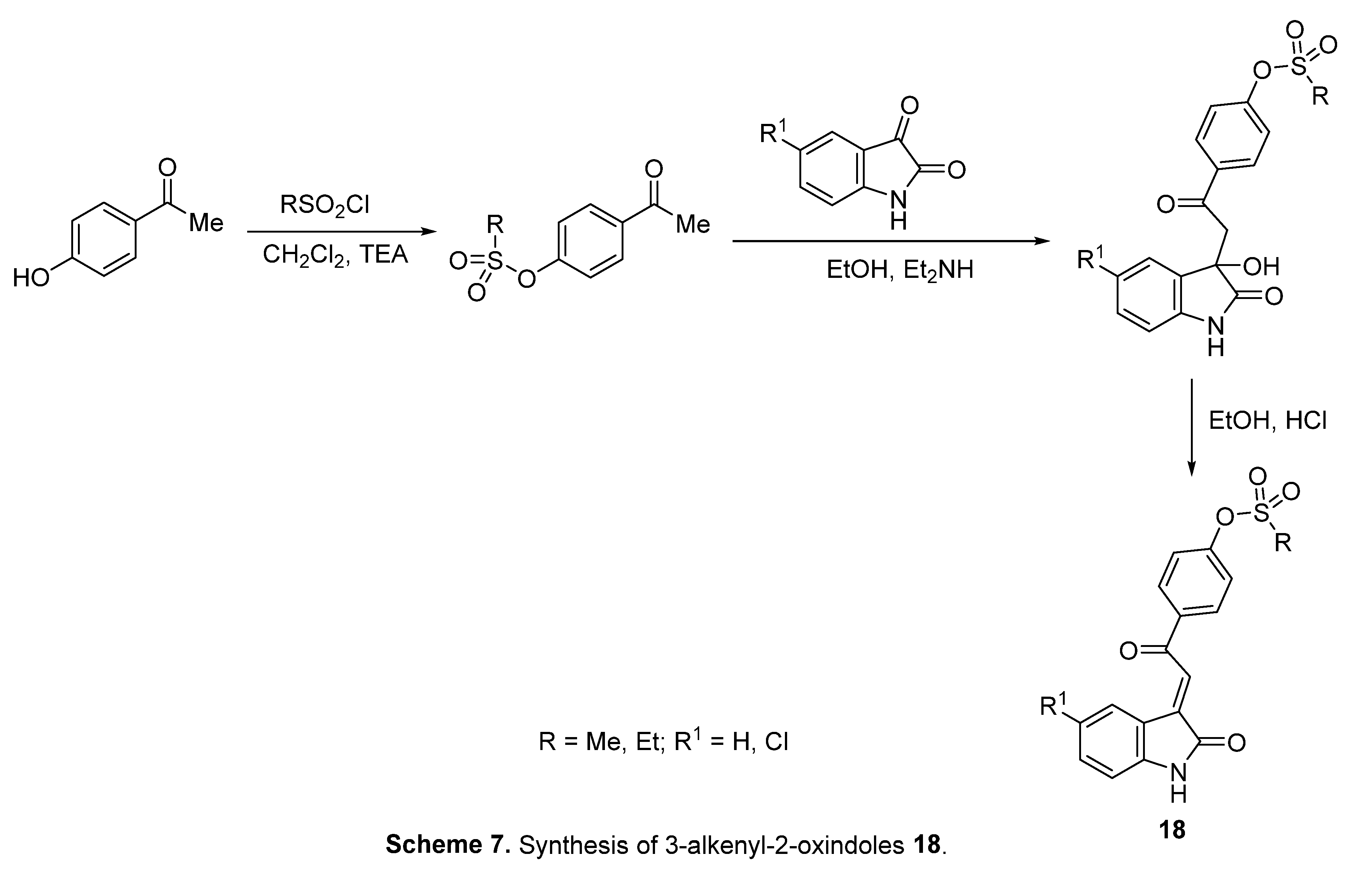
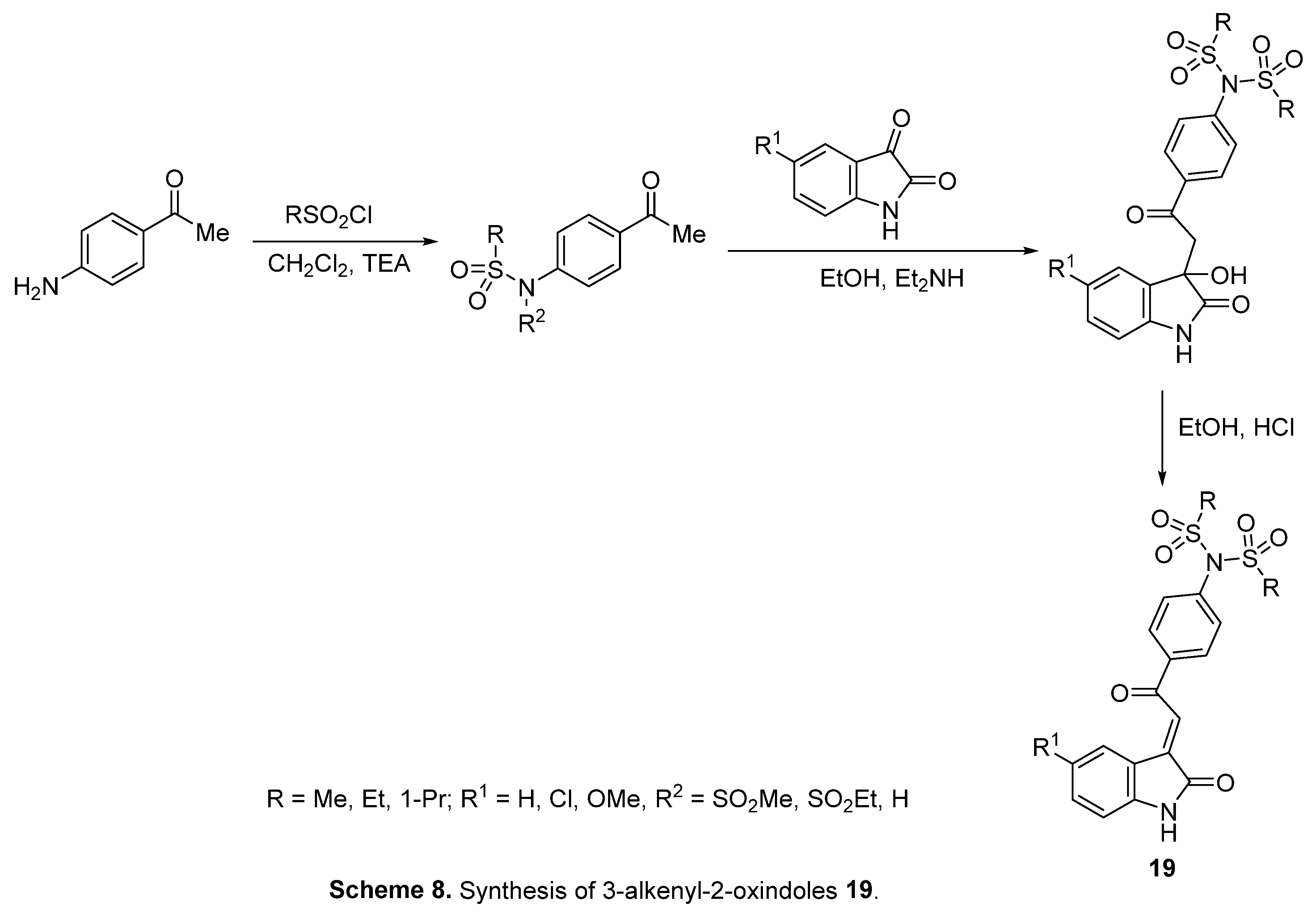
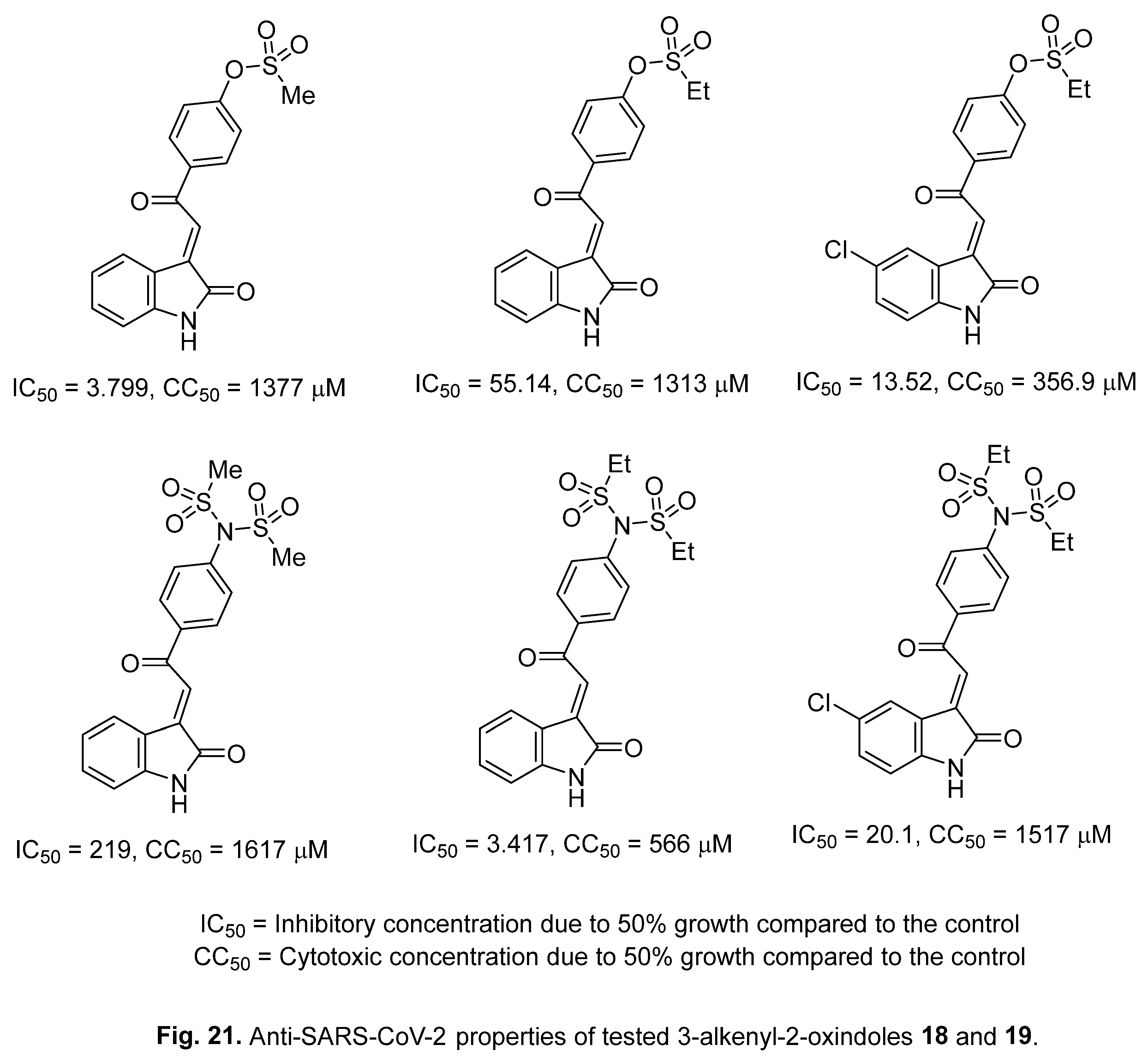
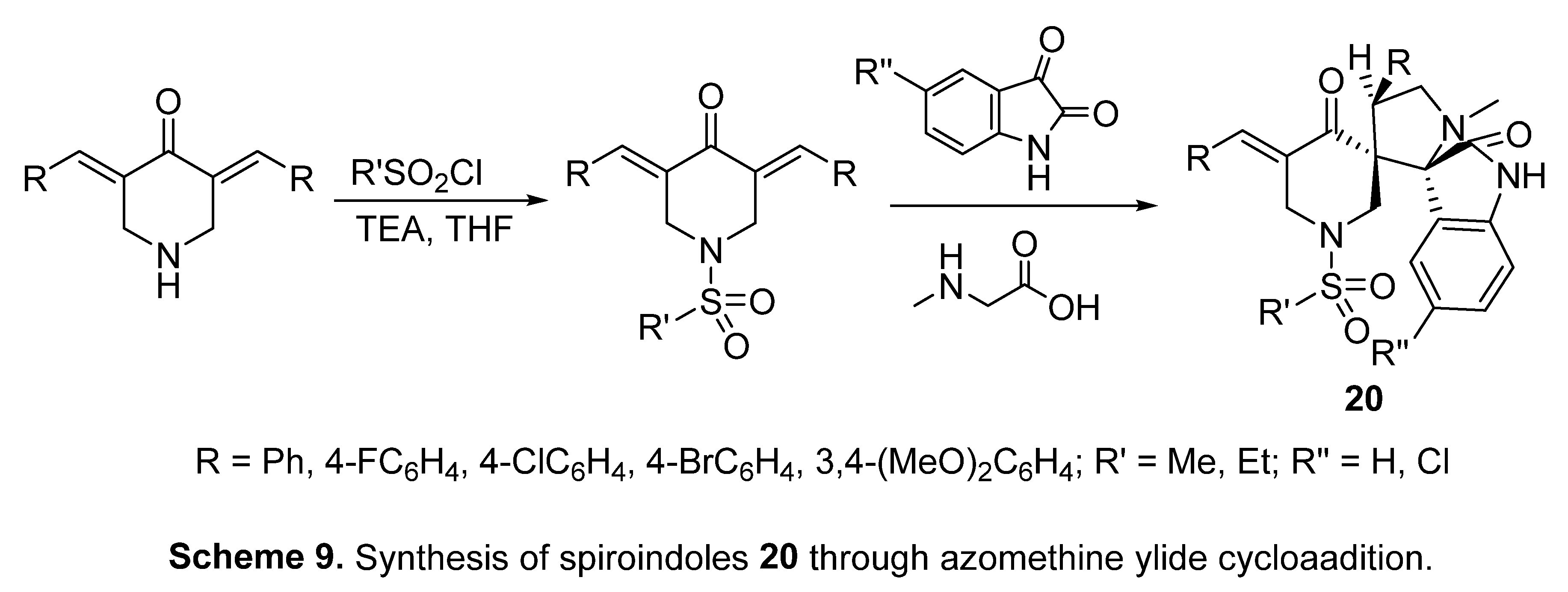
4.6. Spiroindoles
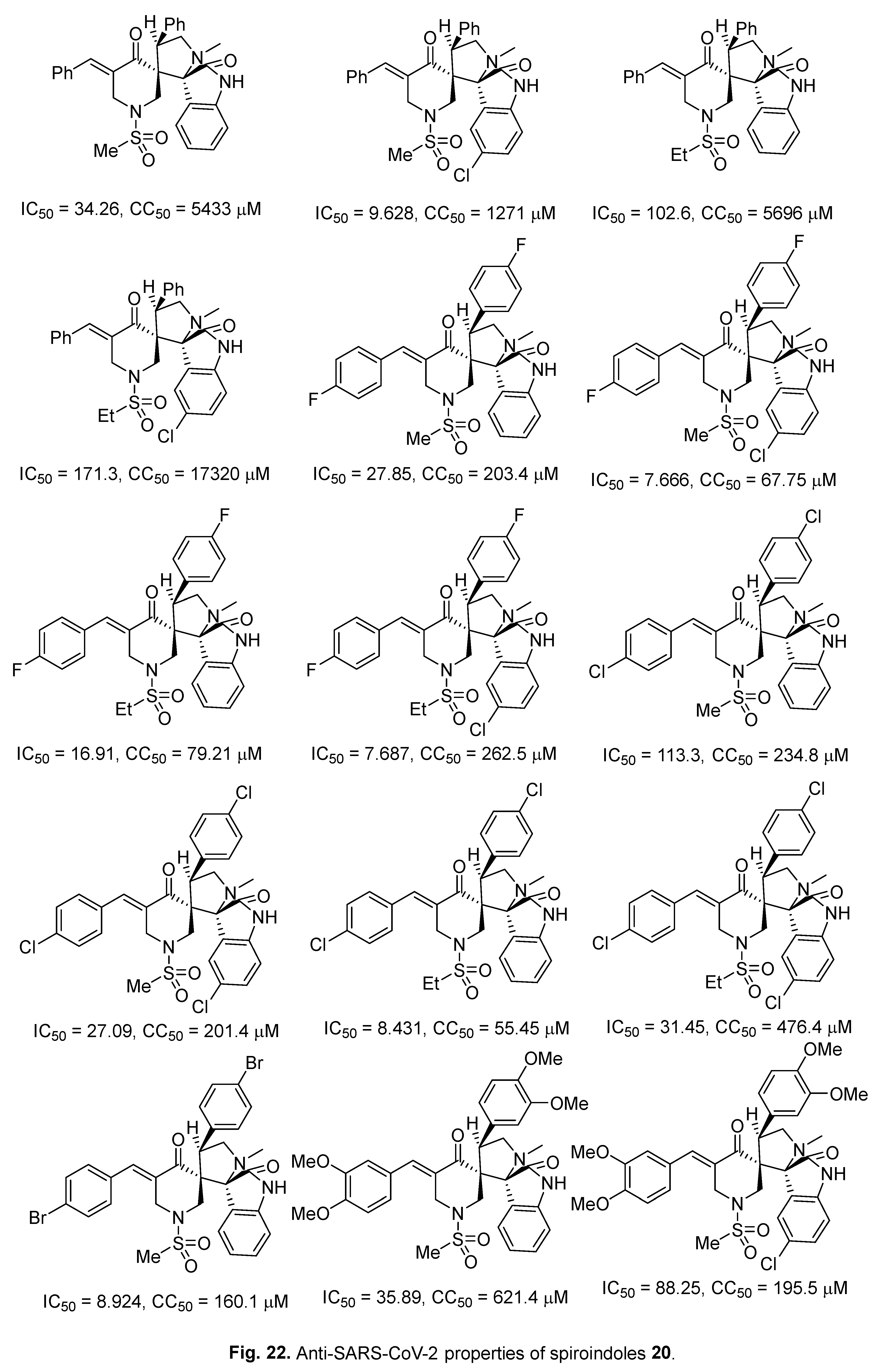
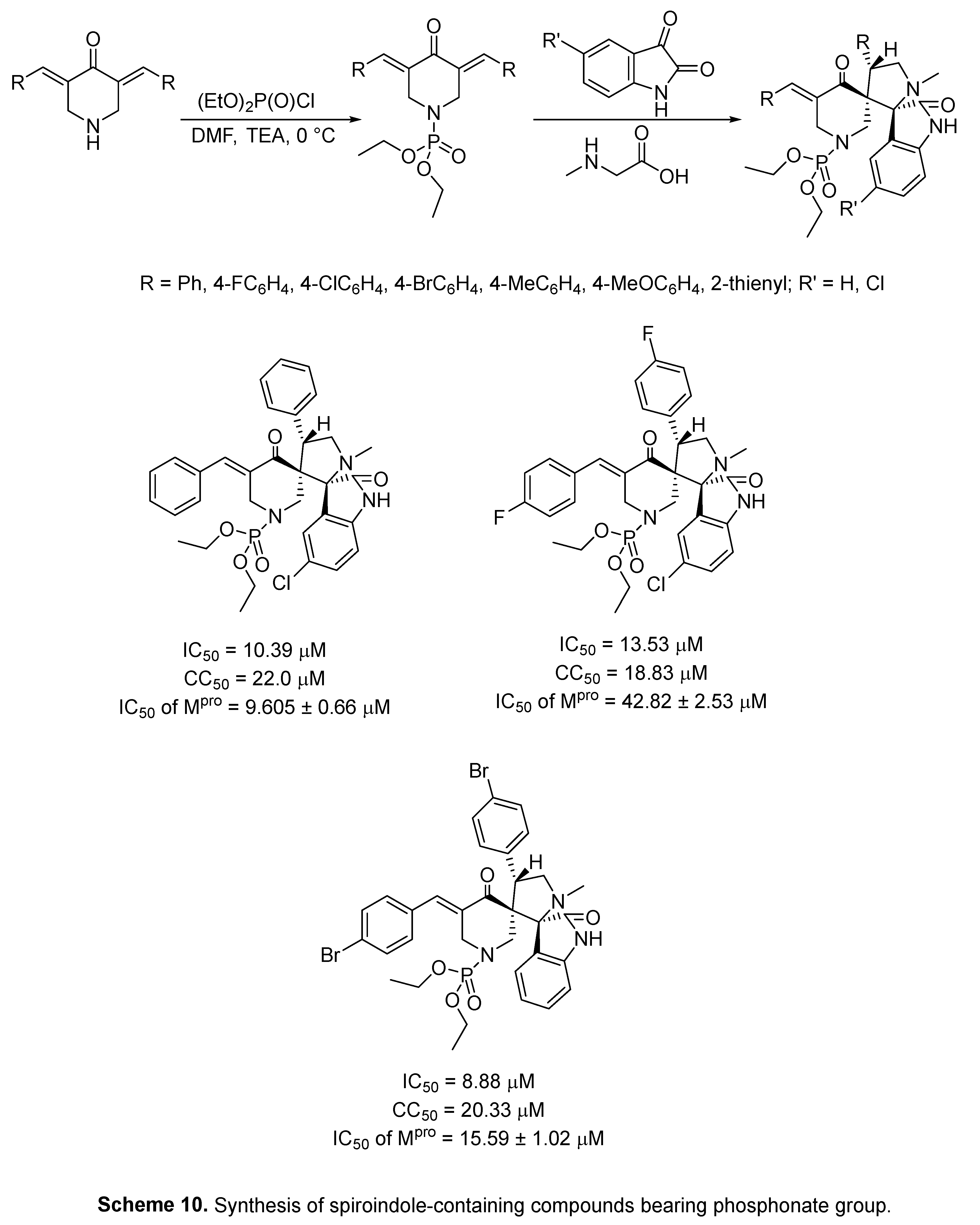
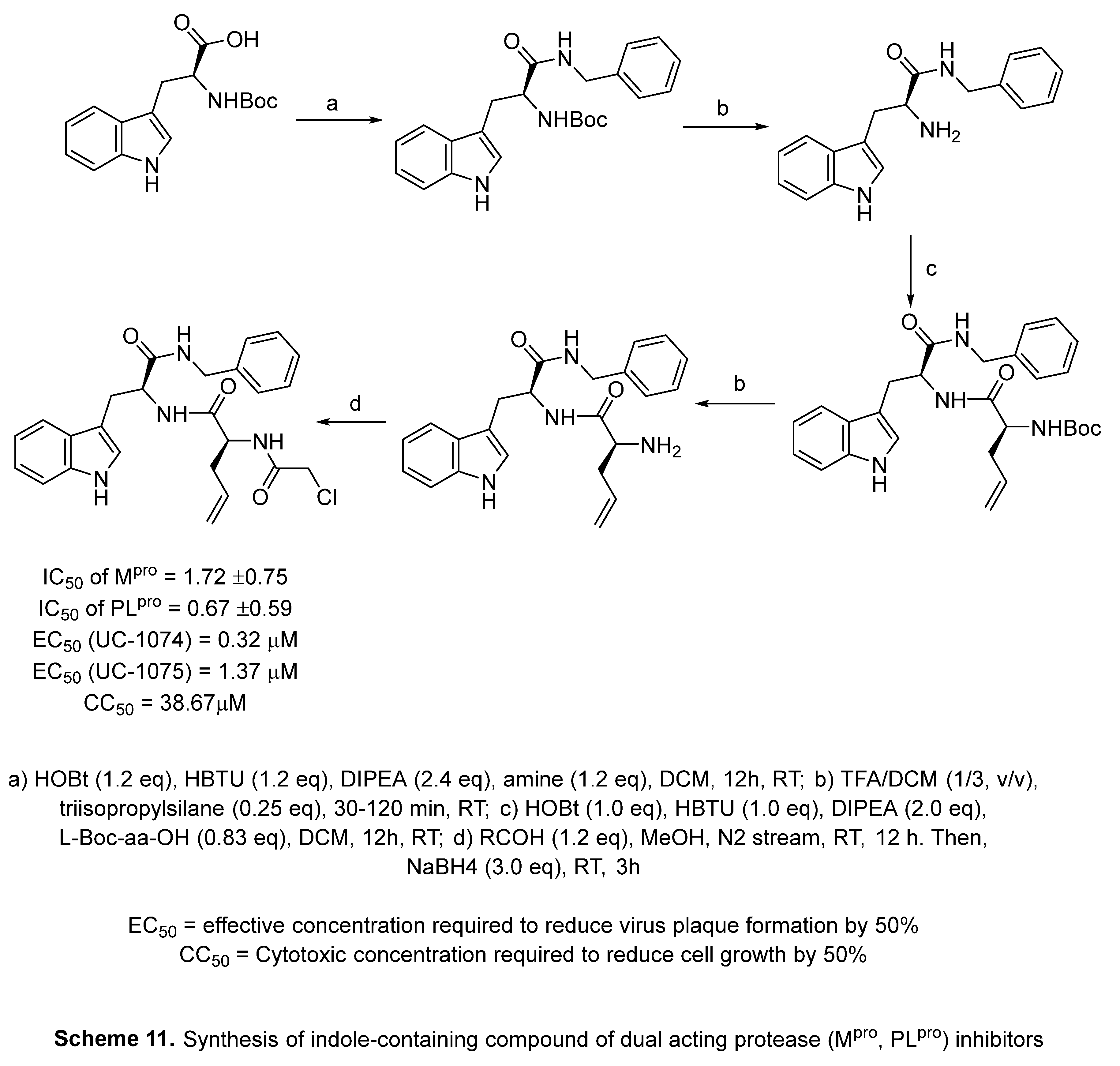
4.7. Indole with dual acting proteases inhibitor
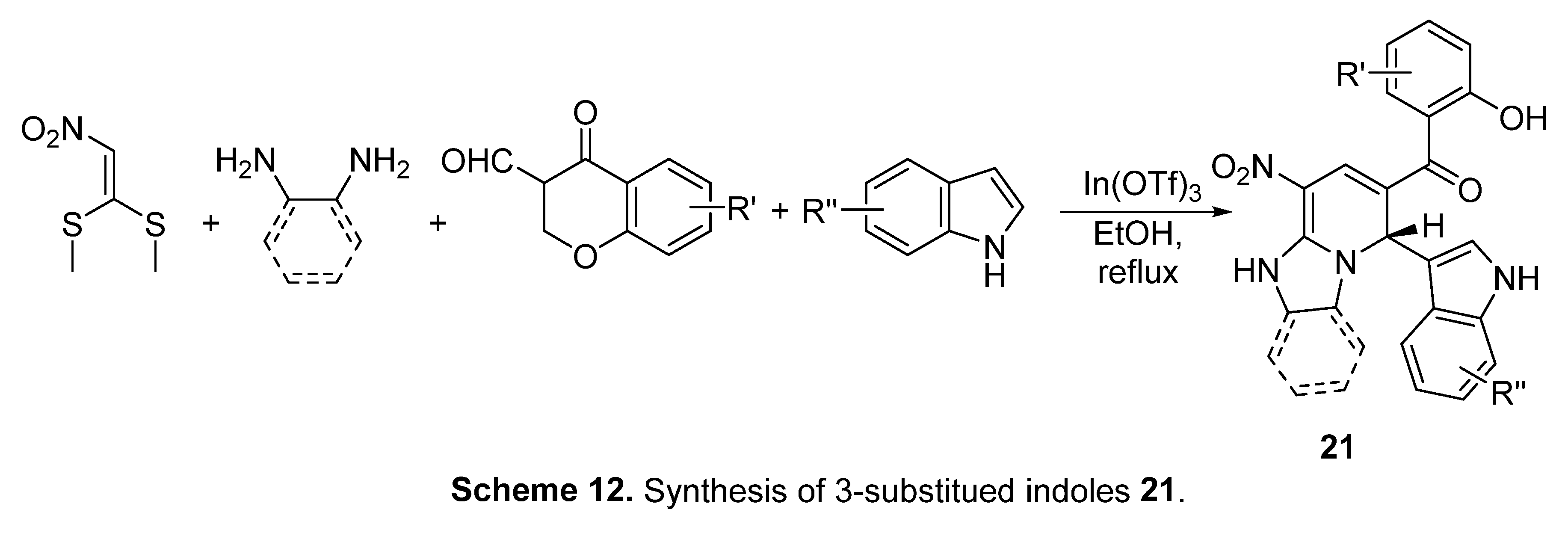
5. In-silico predicted anti-SARS-CoV-2 indoles
5.1. SARS-CoV-2 (main protease, Mpro) inhibitor
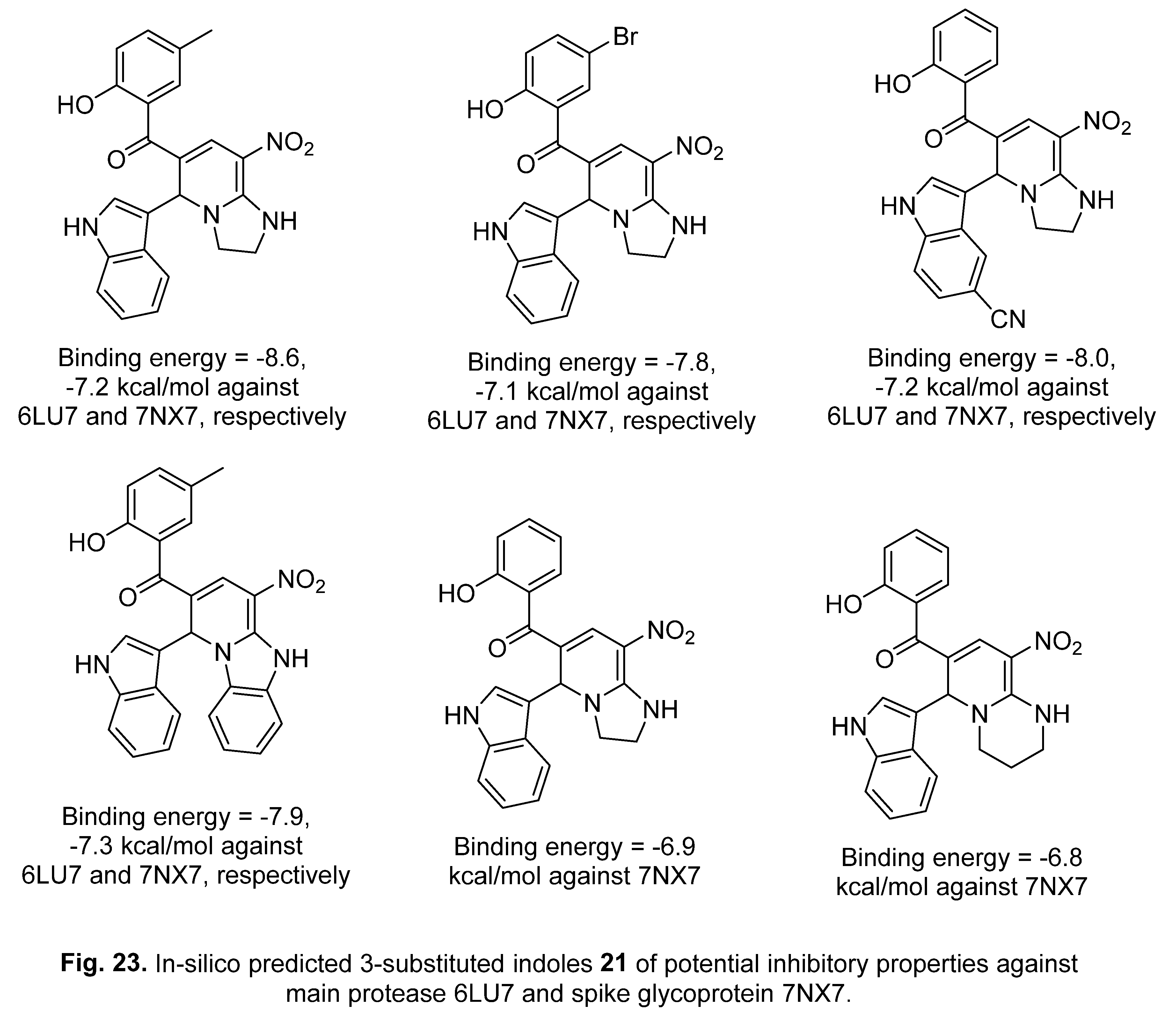
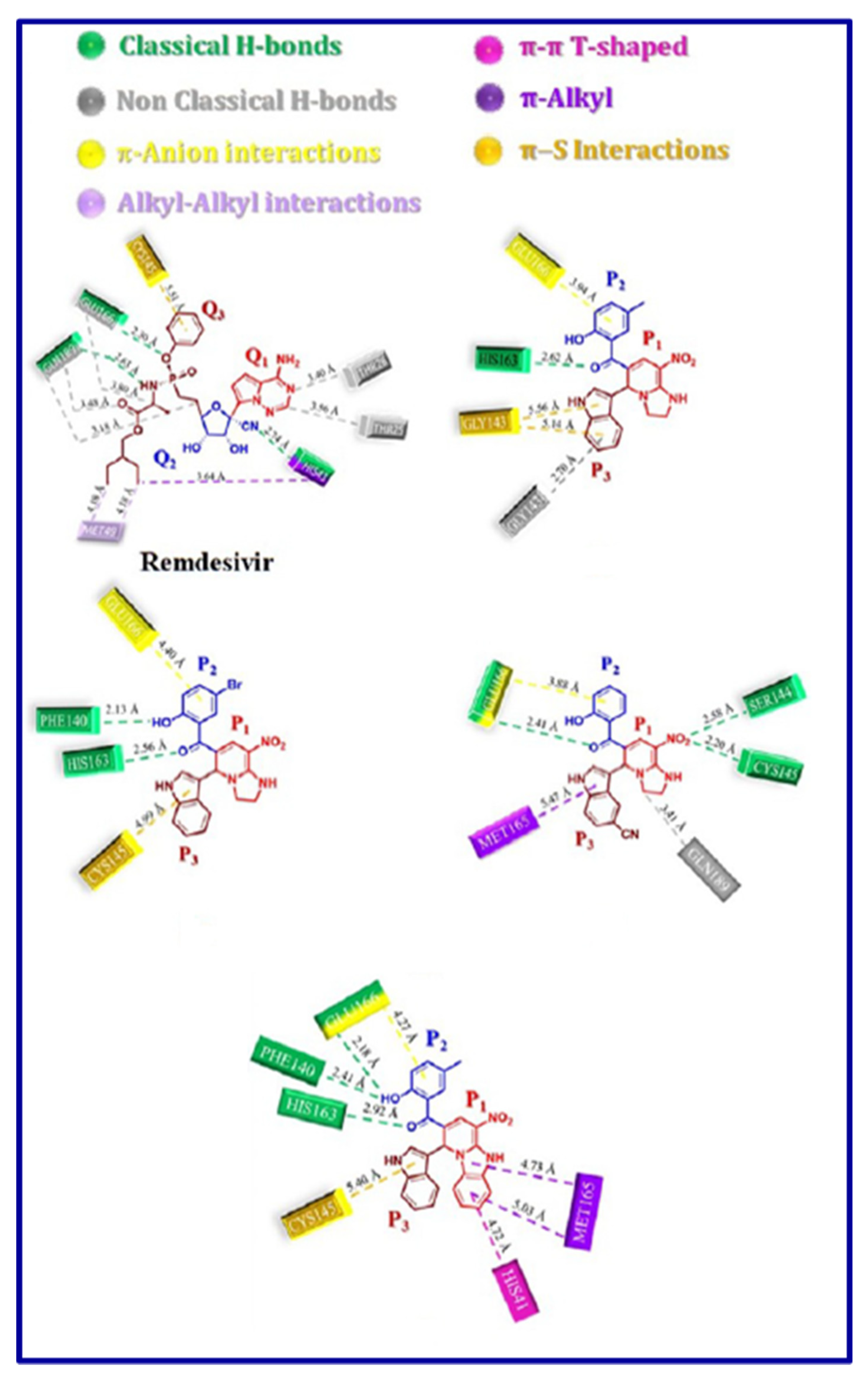
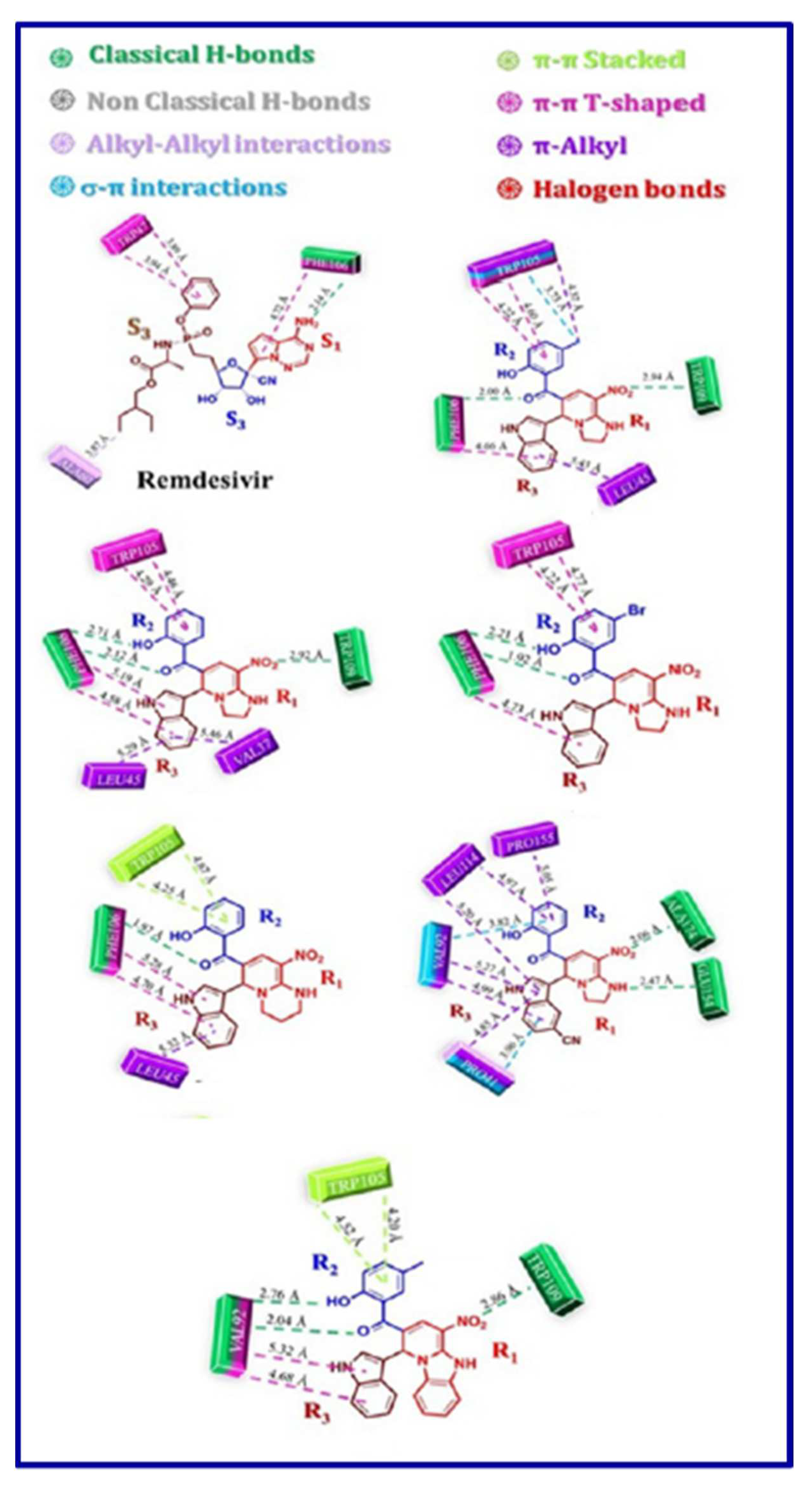
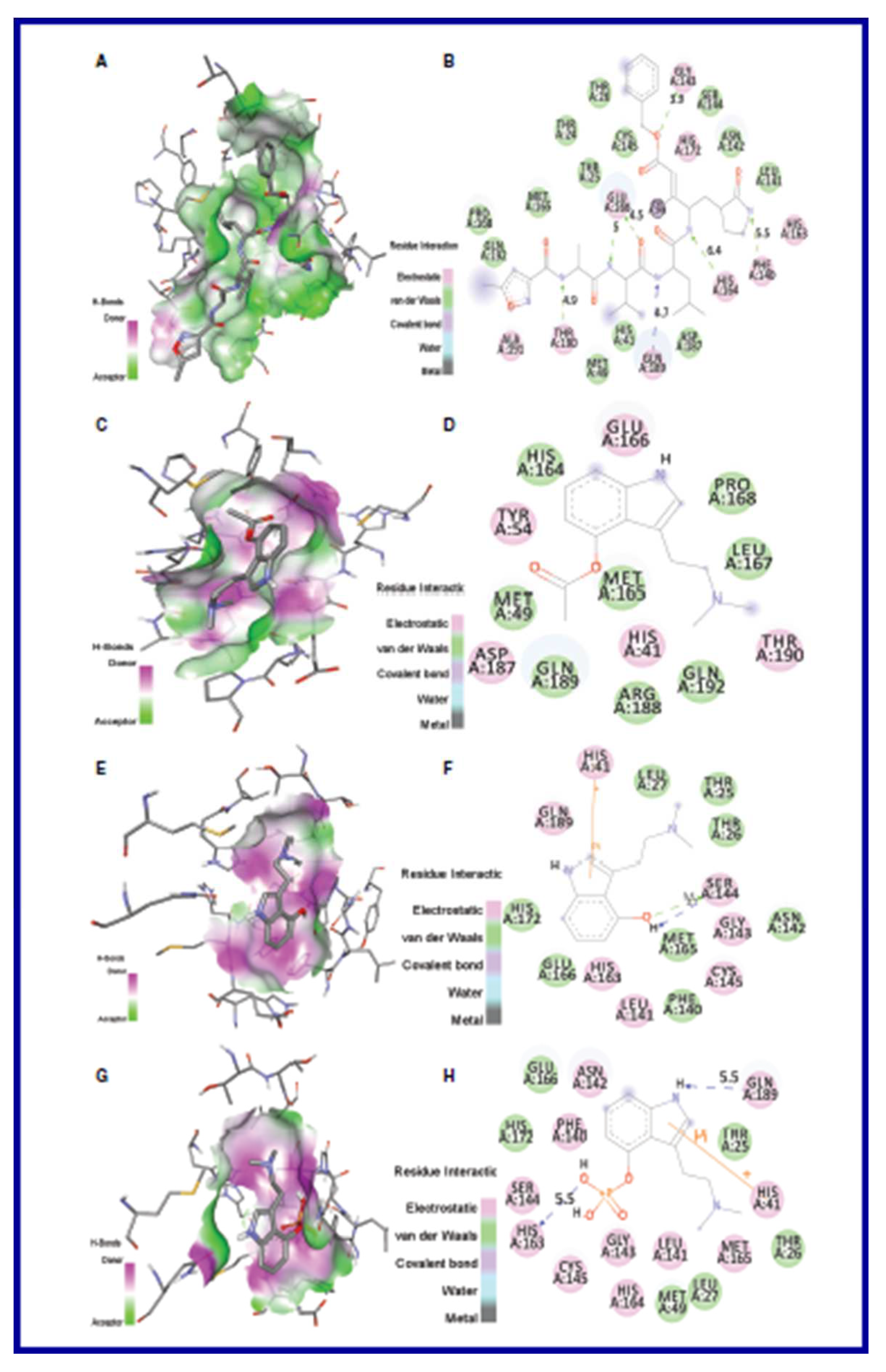
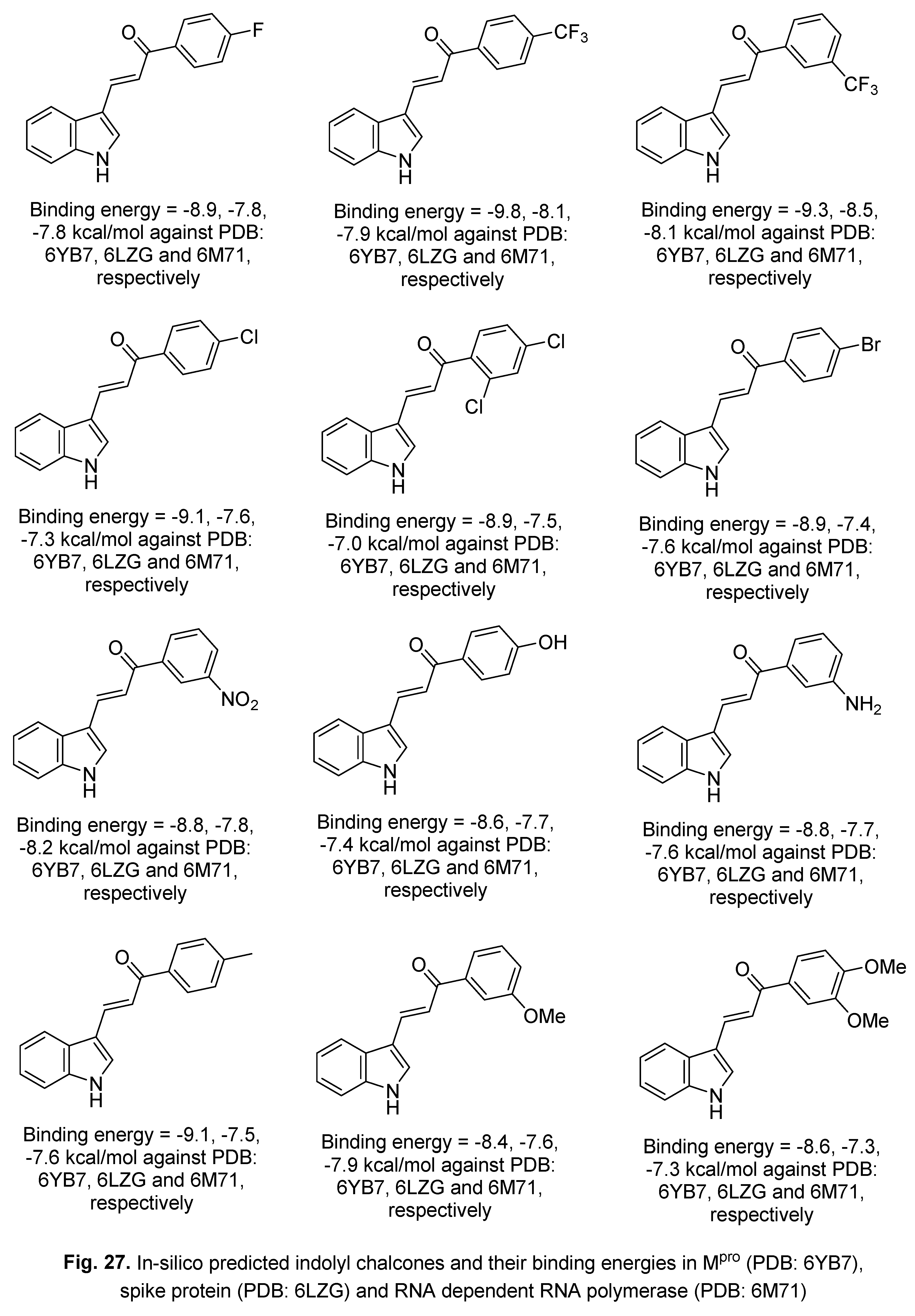
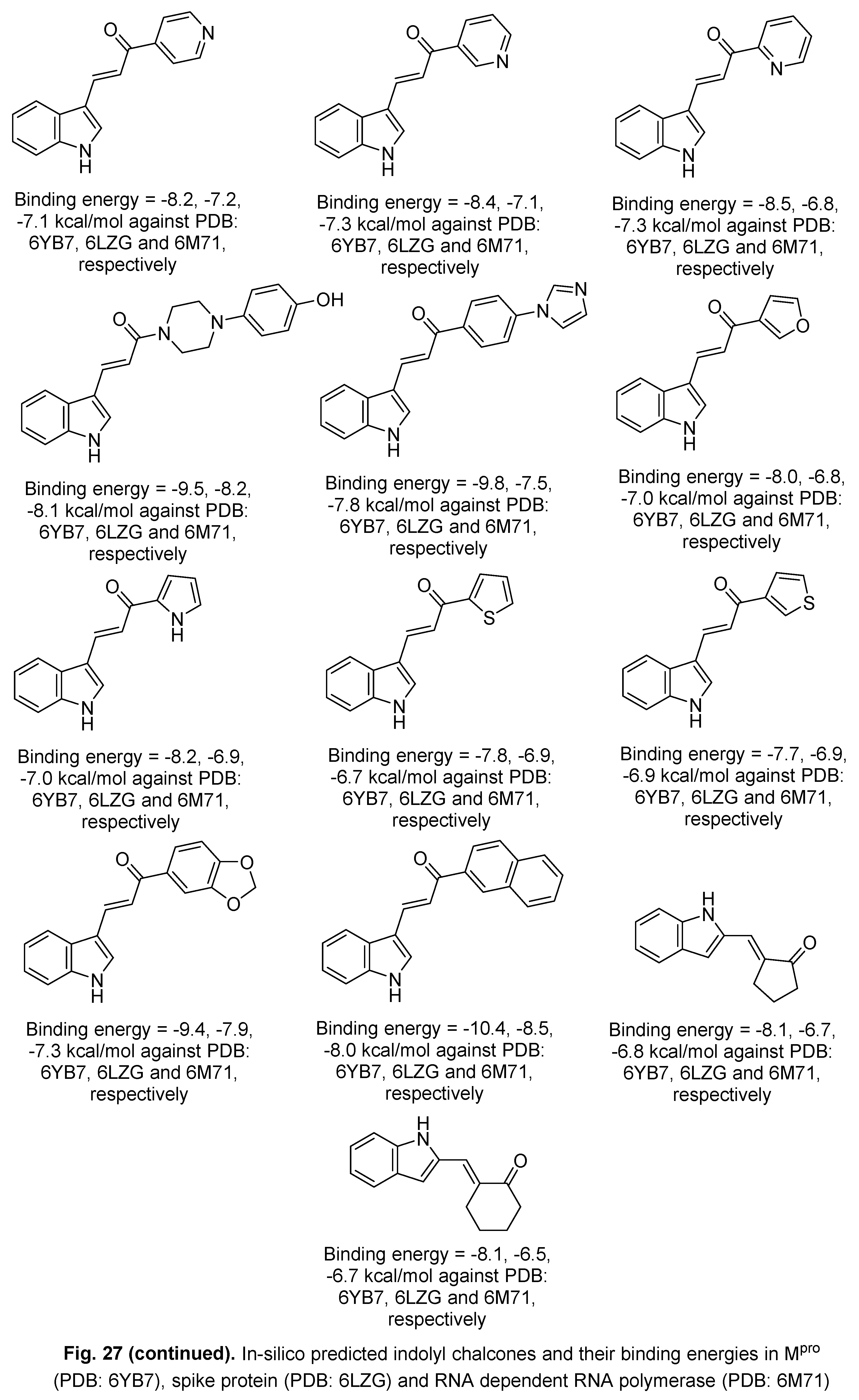
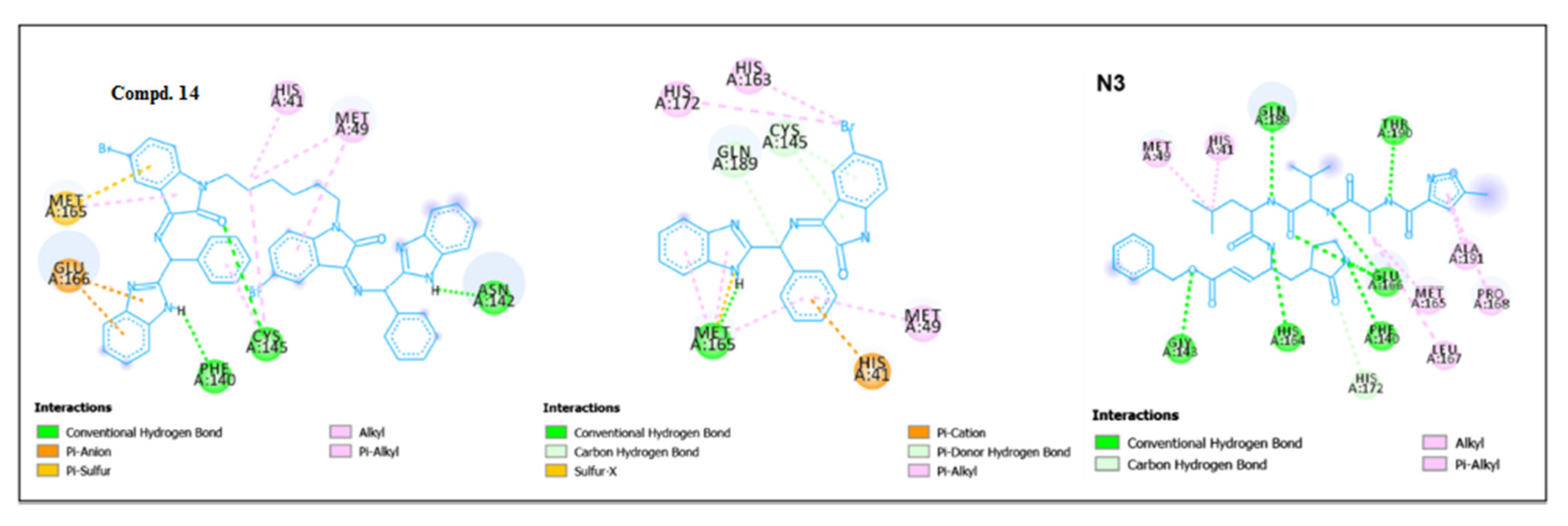
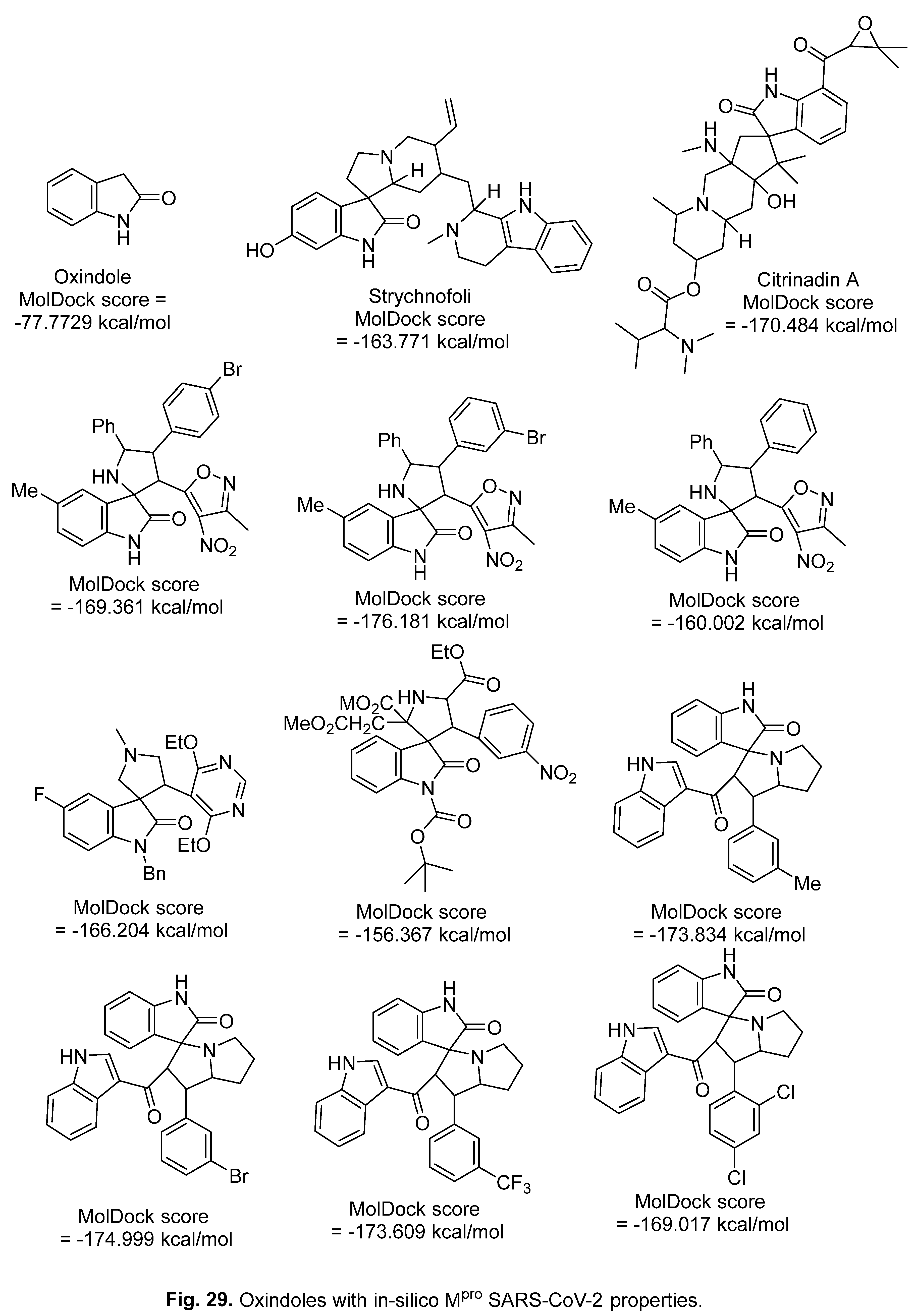
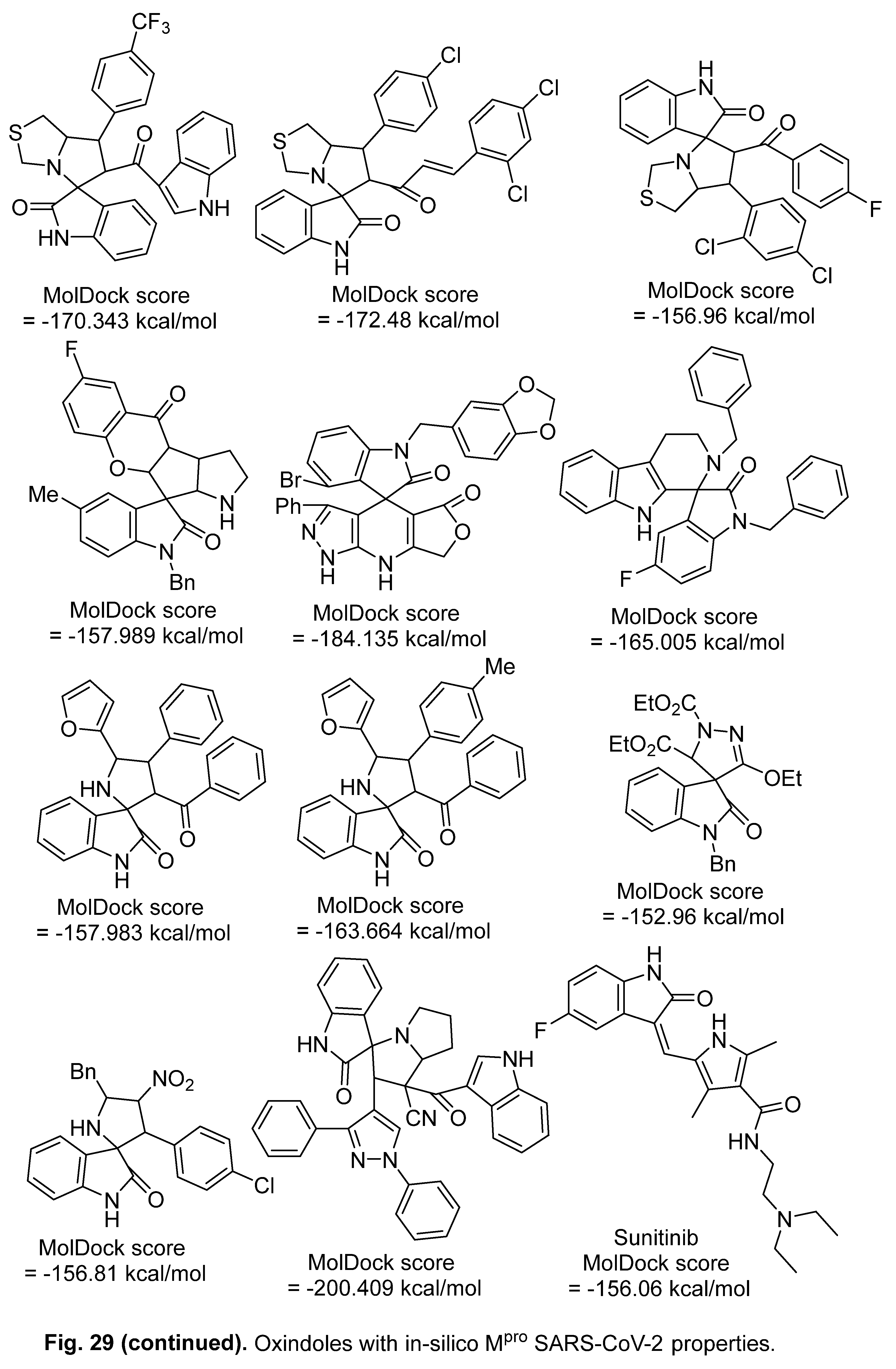
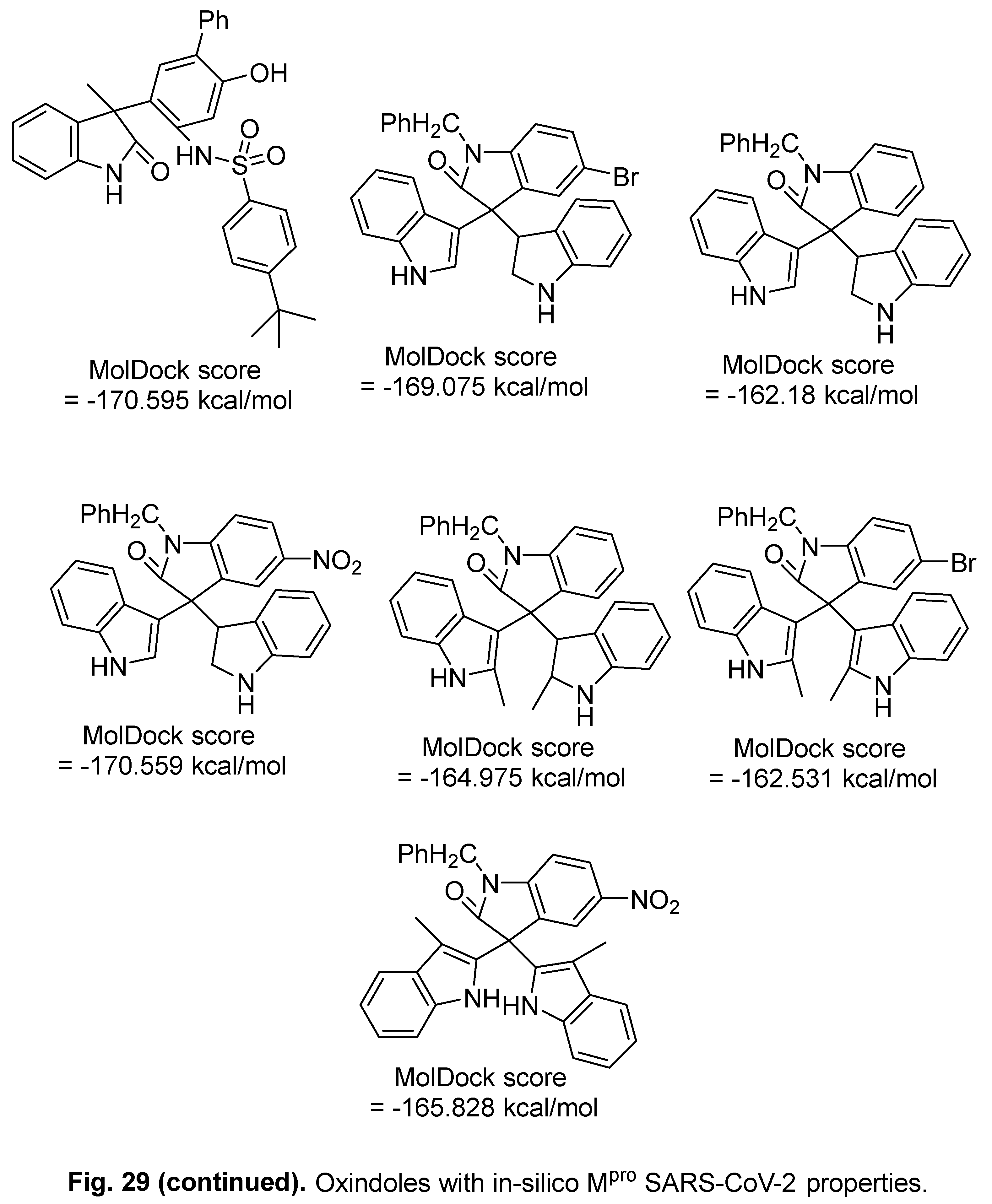
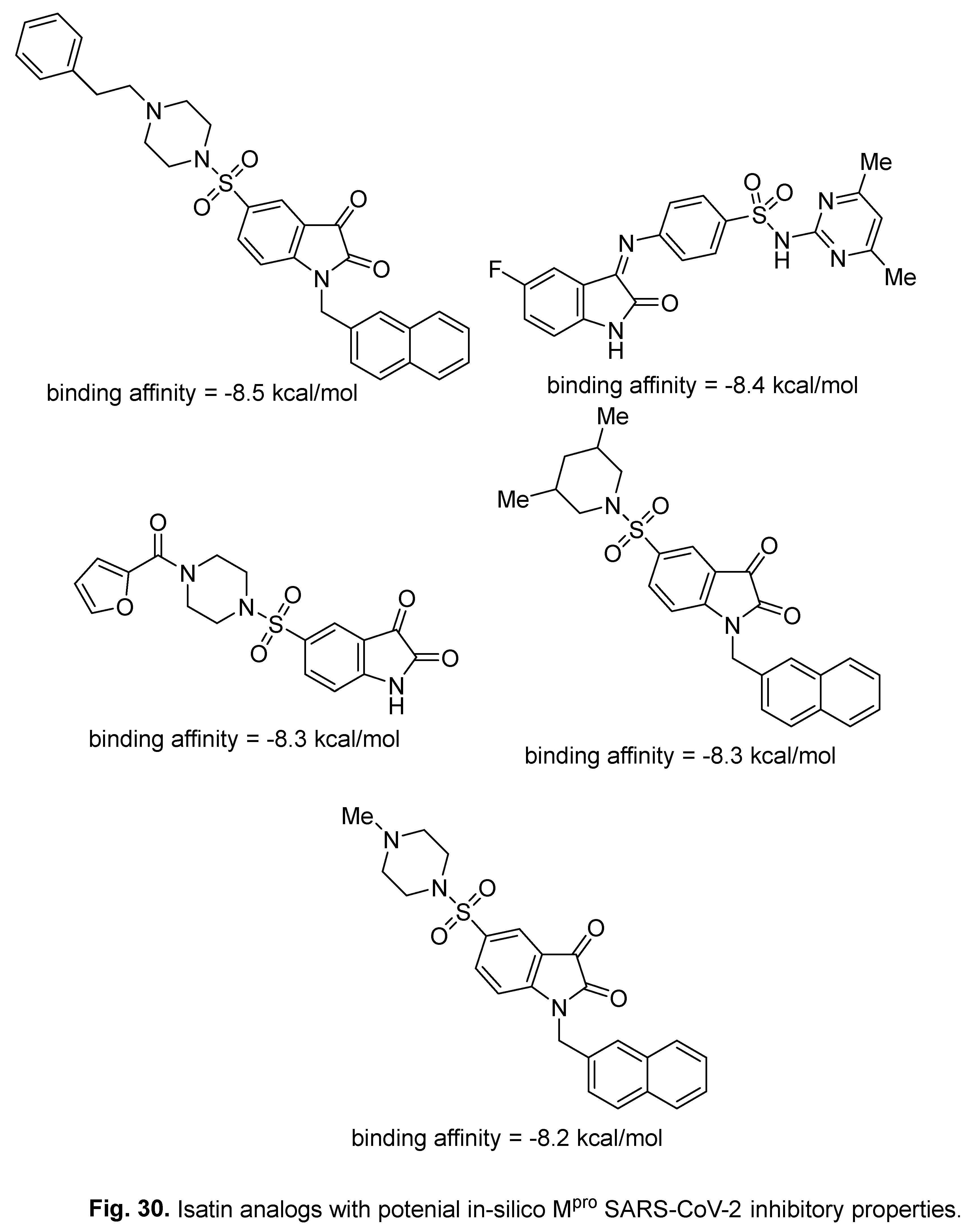
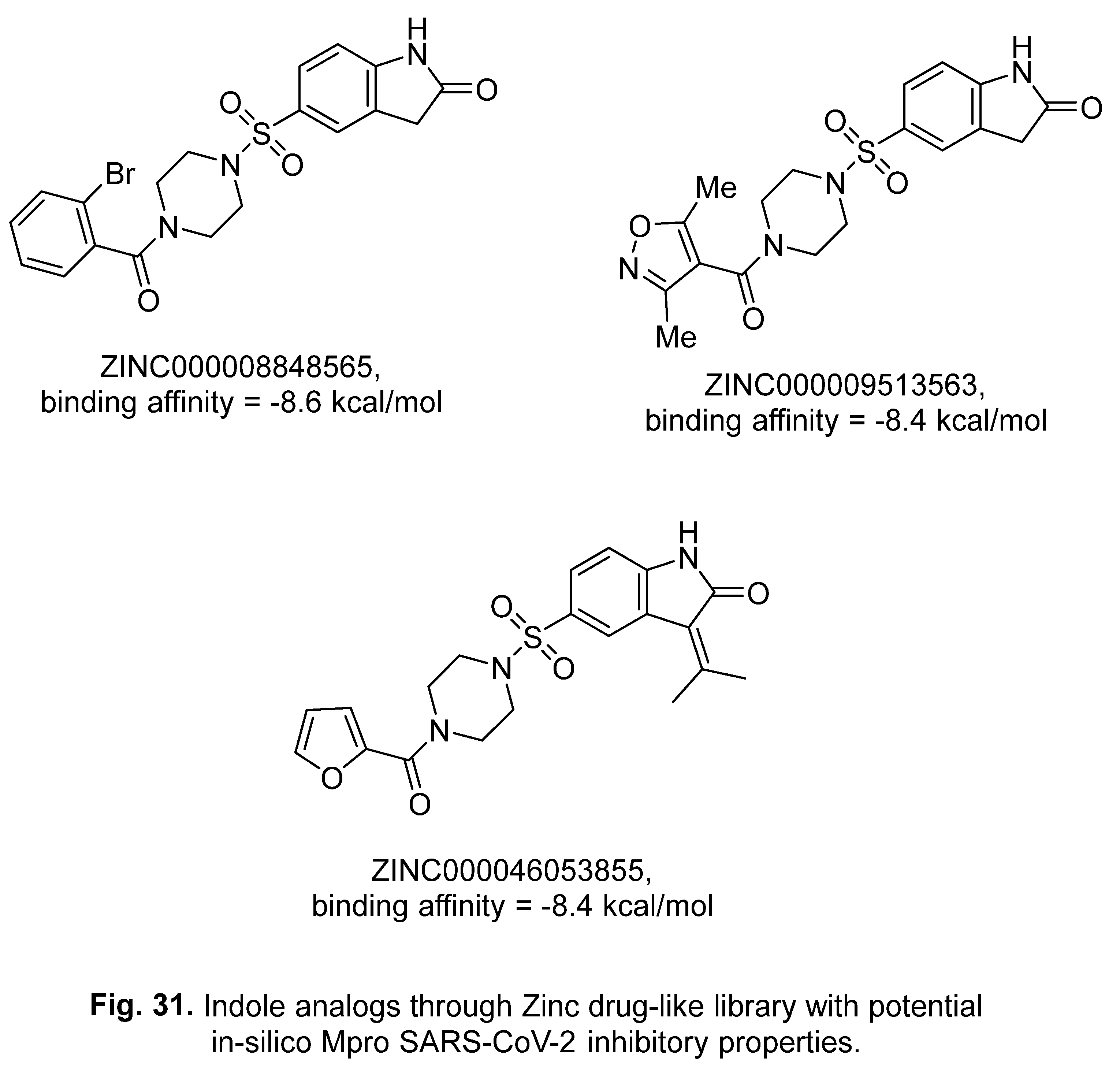

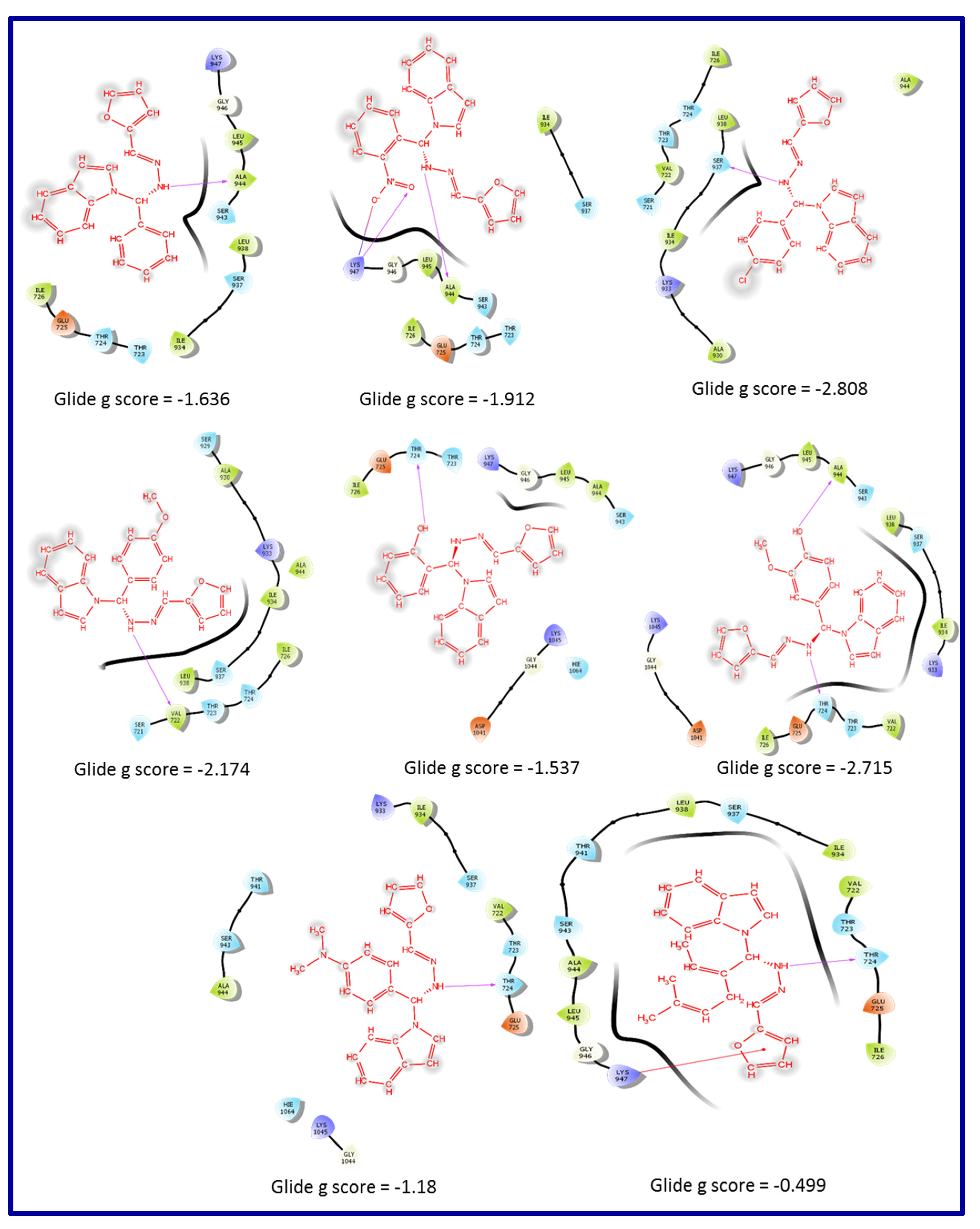
5.2. RdRp (RNA-dependent RNA polymerase) inhibitor
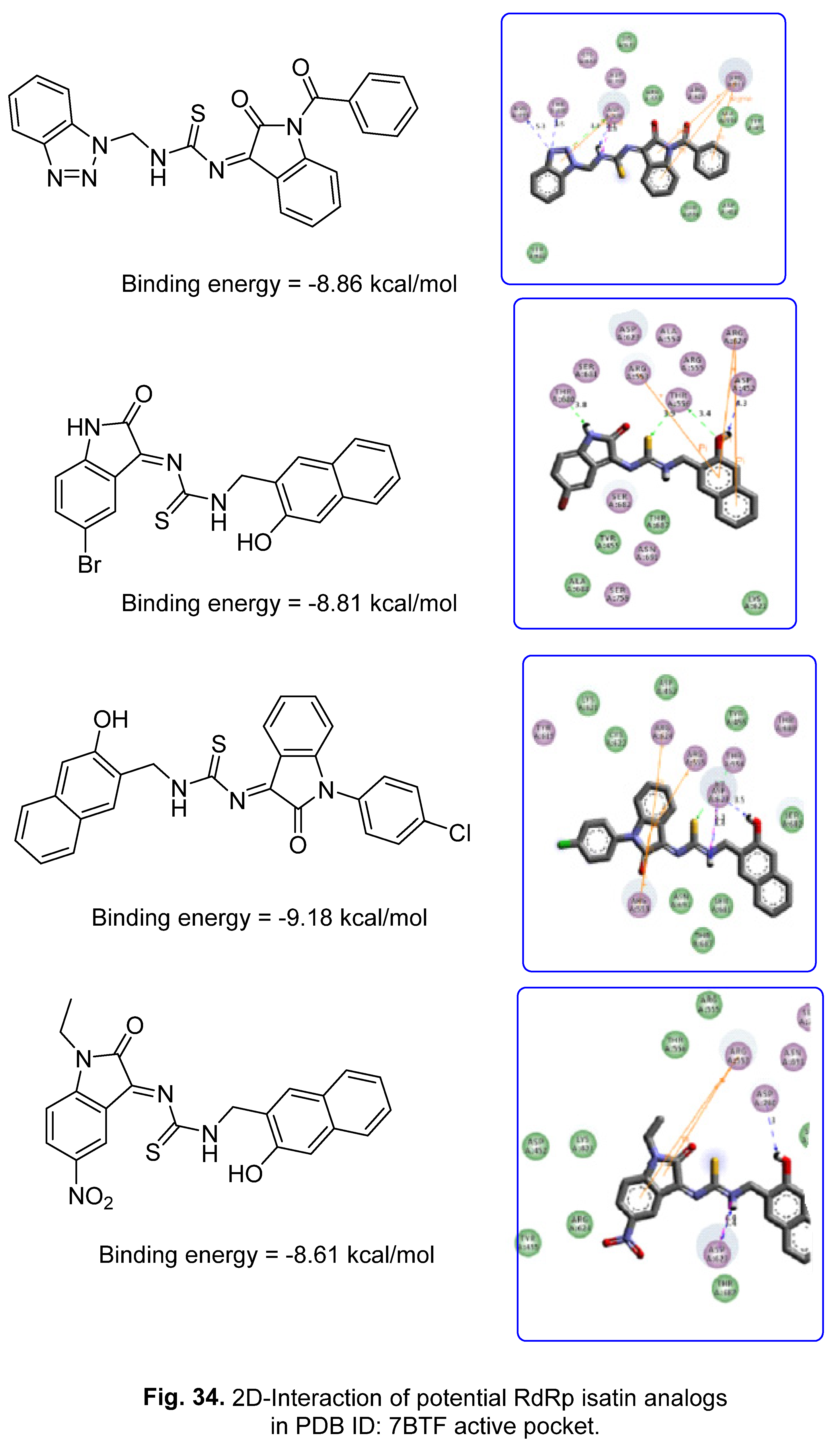
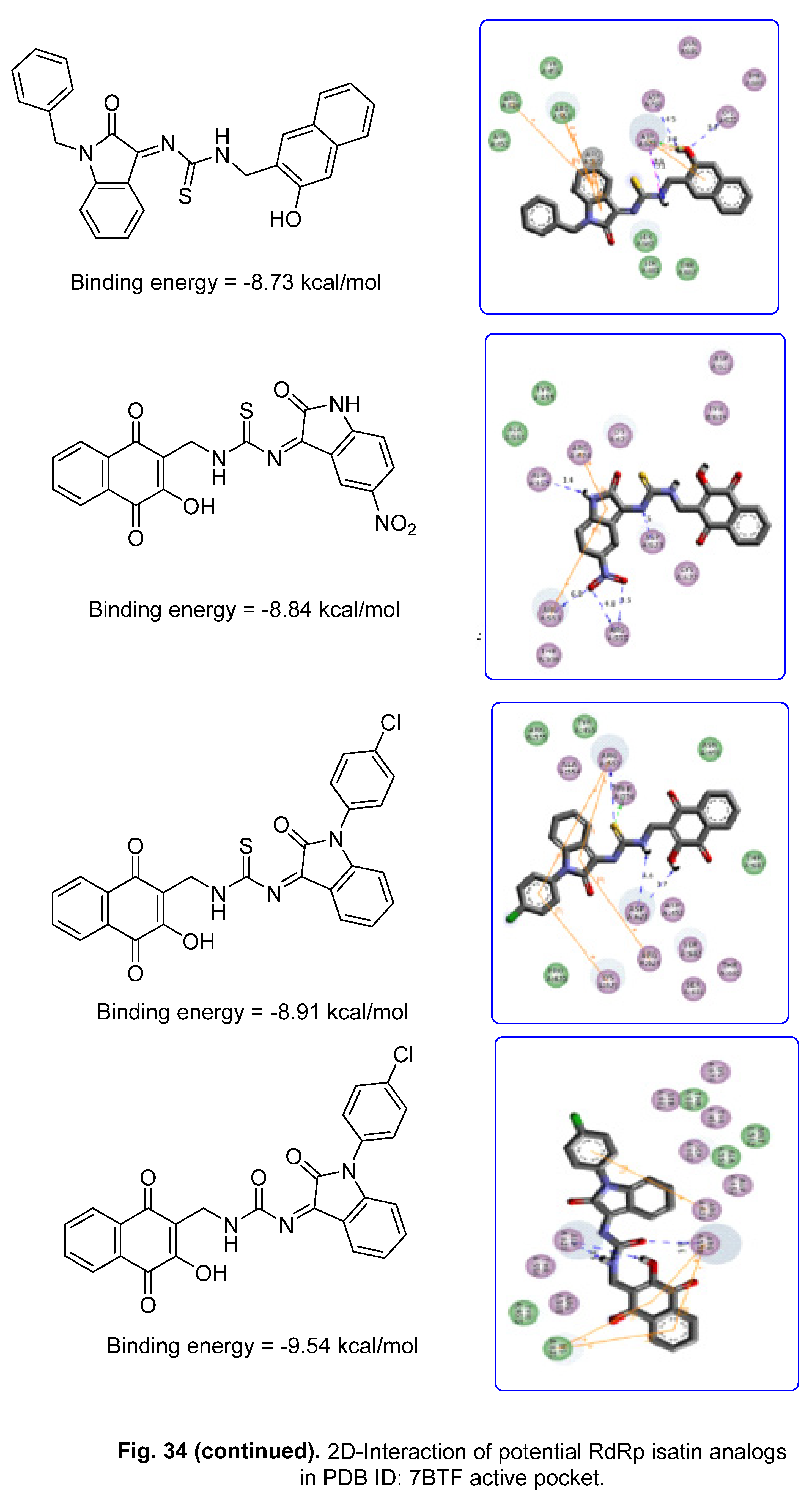
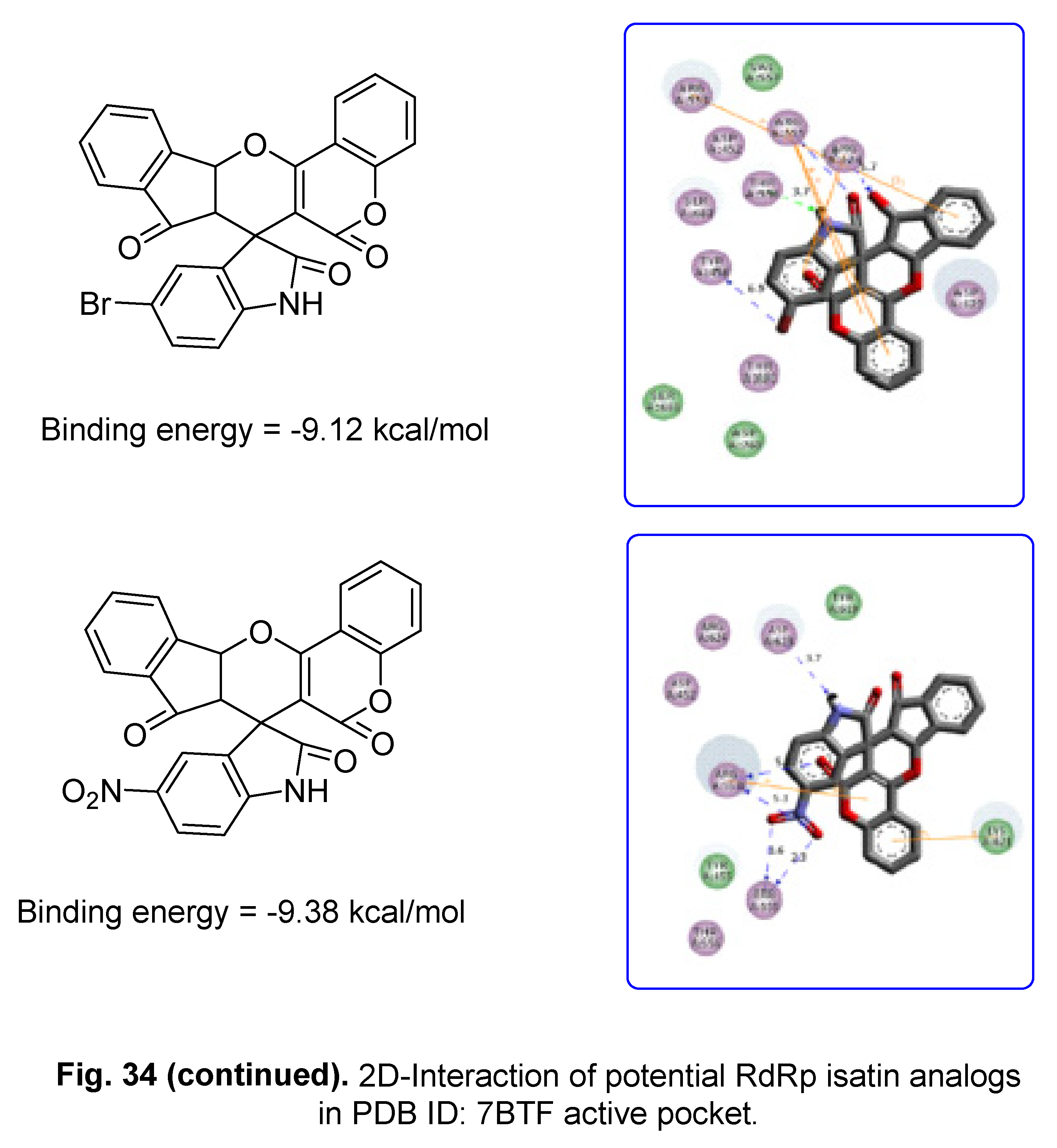
6. Conclusion
Acknowledgments
Declaration of competing interest
References
- N. George, M. J. Akhtar, K. A. Al Balushi, S. A. Khan, Rational drug design strategies for the development of promising multi-target directed indole hybrids as anti-Alzheimer agents, Bioorg. Chem. 127 (2022) 105941. [CrossRef]
- H.-L. Qin, J. Liu, W.-Y. Fang, L. Ravindar, K. P. Rakesh, Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA), Eur. J. Med. Chem. 194 (2020) 112245. [CrossRef]
- A. Ramkissoon, M. Seepersaud, A. Maxwell, J. Jayaraman, A. Ramsubhag, Isolation and antibacterial activity of indole alkaloids from Pseudomonas aeruginosa UWI-1, Molecules 25 (2020) 3744. [CrossRef]
- Y. Liu, Y. Cui, L. Lu, Y. Gong, W. Han, G. Piao, Natural indole-containing alkaloids and their antibacterial activities, Arch Pharm. 353 (2020) e2000120. [CrossRef]
- T. Meng, Y. Hou, C. Shang, J. Zhang, B. Zhang, Recent advances in indole dimers and hybrids with antibacterial activity against methicillin-resistant Staphylococcus aureus, Arch Pharm. 354 (2021) e2000266. [CrossRef]
- J. Ma, Y. Jiang, X. Zhuang, H. Chen, Y. Shen, Z. Mao, G. Rao, R. Wang, Discovery of novel indole and indoline derivatives against Candida albicans as potent antifungal agents, Bioorg. Med. Chem. Lett. 71 (2022) 128826. [CrossRef]
- M. Bolous, N. Arumugam, A. I. Almansour, R. S. Kumar, K. Maruoka, V. C. Antharam, S. Thangamani, Broad-spectrum antifungal activity of spirooxindolo-pyrrolidine tethered indole/imidazole hybrid heterocycles against fungal pathogens, Bioorg. Med. Chem. Lett. 29 (2019) 2059–2063. [CrossRef]
- M.-L. Yang J. Chen, M. Sun, D.-B. Zhang, K. Gao, Antifungal indole alkaloids from Winchia calophylla, Planta Med. 82 (2016) 712‒716. /: 82 (2016) 712‒716, https. [CrossRef]
- M. S. Bekheit, S. S. Panda, A. S. Girgis, Potential RNA-dependent RNA polymerase (RdRp) inhibitors as prospective drug candidates for SARS-CoV-2, Eur. J. Med. Chem. 252 (2023) 115292. [CrossRef]
- S. Nie, J. Zhao, X. Wu, Y. Yao, F. Wu, Y.-L. Lin, X. Li, A. R. Kneubehl, M. B. Vogt, R. Rico-Hesse, Y. Song, Synthesis, structure-activity relationship and antiviral activity of indole-containing inhibitors of Flavivirus NS2B-NS3 protease, Eur. J. Med. Chem. 225 (2021) 113767. /: 225 (2021) 113767, https. [CrossRef]
- C. Wei, L. Zhao, Z. Sun, D. Hu, B. Song, Discovery of novel indole derivatives containing dithioacetal as potential antiviral agents for plants, Pestic. Biochem. Physiol. 166 (2020) 104568. [CrossRef]
- M.-Z. Zhang, Q. Chen, G.-F. Yang, A review on recent developments of indole-containing antiviral agents, Eur. J. Med. Chem. 89 (2015) 421‒441. 2015. [CrossRef]
- J. Blaising, S. J. Polyak, E.-I. Pecheur, Arbidol as a broad-spectrum antiviral: An update, Antiviral Res. 107 (2014) 84–94. [CrossRef]
- M. Giampieri, A. Balbi, M. Mazzei, P. La Collab, C. Ibba, R. Loddo, Antiviral activity of indole derivatives, Antiviral Res. 3 (2009) 179–185. /. [CrossRef]
- V. Mashayekhi, K. H. M. E. Tehrani, P. Azerang, S. Sardari, F. Kobarfard, Synthesis, antimycobacterial and anticancer activity of novel indole-based thiosemicarbazones, Arch. Pharm. Res. 44 (2021) 764–776. /. [CrossRef]
- B. Yu, D.-Q. Yu. H.-M. Liu, Spirooxindoles: Promising scaffolds for anticancer agents, Eur. J. Med. Chem. 97 (2015) 673‒698. [CrossRef]
- M. Chauhan, A. Saxena, B. Saha, An insight in the anti-malarial potential of indole scaffold: A review, Eur. J. Med. Chem. 218 (2021) 113400. [CrossRef]
- A. S. Surur, S. A. Huluka, M. L. Mitku, K. Asres, Indole: The after next scaffold of antiplasmodial agents?, Drug Des. Devel. Ther. 14 (2020) 4855–4867. /. [CrossRef]
- J.-Y. Li, X.-F. Sun, J.-J. Li, F. Yu, Y. Zhang, X.-J. Huang, F.-X. Jiang, The antimalarial activity of indole alkaloids and hybrids, Arch Pharm. 353 (2020) e2000131. [CrossRef]
- S. N. S. Vasconcelos, K. A. Meissner, W. R. Ferraz, G. H. G. Trossini, C. Wrenger, H. A. Stefani, Indole-3-glyoxyl tyrosine: synthesis and antimalarial activity against Plasmodium falciparum, Future Med. Chem. 11 (2019) 525–538. /, 2019. [CrossRef]
- Y. Tamura, I. Morita, Y. Hinata, E. Kojima, H. Ozasa, H. Ikemoto, M. Asano, T. Wada, Y. Hayasaki-Kajiwara, T. Iwasaki, K. Matsumura, Identification of novel indole derivatives as highly potent AMPK activators with anti-diabetic profiles, Bioorg. Med. Chem. Lett. 68 (2022) 128769. [CrossRef]
- M. Nazir, M. A. Abbasi, Aziz-ur-Rehman, S. Z. Siddiqui, K. M. Khan, Kanwal, U. Salar, M. Shahid, M. Ashraf, M. A. Lodhi, F. A. Khan, New indole based hybrid oxadiazole scaffolds with N-substituted acetamides: As potent anti-diabetic agents, Bioorg. Chem. 81 (2018) 253–263. [CrossRef]
- A. Kumari, R. K. Singh, Synthesis, molecular docking and biological evaluation of N-substituted indole derivatives as potential anti-inflammatory and antioxidant agents, Chem. Biodivers. 19 (2022) e202200290. [CrossRef]
- Ì. T. T. Jacob, F. O. S. Gomes, M. D. S. de Miranda, S. M. V. de Almeida, I. J. da Cruz-Filho, C. A. Peixoto, T. G. da Silva, D. R. M. Moreira, C. M. L. de Melo, J. F. de Oliveira, M. C. A. de Lima, Anti-inflammatory activity of novel thiosemicarbazone compounds indole-based as COX inhibitors, Pharmacol. Rep. 73 (2021) 907‒925. [CrossRef]
- L.-L. Song, Y.-L. Mu, H.-C. Zhang, G.-Y. Wu, J.-Y. Sun, A new indole alkaloid with anti-inflammatory from the branches of Nauclea officinalis, Nat. Prod. Res. 34 (2020) 2283‒2288. [CrossRef]
- S. Sharma, D. Kumar, G. Singh, V. Monga, B. Kumar, Recent advancements in the development of heterocyclic anti-inflammatory agents, Eur. J. Med. Chem. 200 (2020) 112438.
- L. Jiang, H. Pu, X. Qin, J. Liu, Z. Wen, Y. Huang, J. Xiang, Y. Xiang, J. Ju, Y. Duan, Y. Huang, Syn-2, 3-diols and anti-inflammatory indole derivatives from Streptomyces sp. CB09001. Nat. Prod. Res. 35 (2021) 144‒151. [CrossRef]
- Y. Hong, Y.-Y. Zhu, Q. He, S.-X. Gu, Indole derivatives as tubulin polymerization inhibitors for the development of promising anticancer agents, Bioorg. Med. Chem. 55 (2022) 116597. 116597. [CrossRef]
- https://www.drugs.com/history/tivorbex.html (accessed on , 2023). 6 July.
- G. Li, Y. Wang, E. De Clercq, Approved HIV reverse transcriptase inhibitors in the past decade, Acta Pharm. Sin. B 12 (2022) 1567‒1590. [CrossRef]
- https://pubmed.ncbi.nlm.nih.gov/11364363/ (accessed on , 2023). 6 July.
- https://go.drugbank.com/drugs/DB13609 (accessed on , 2023). 6 July.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/018285s034lbl.pdf (accessed on , 2023). 6 July.
- W. J. Cronenwett, Schizophrenia pharmacology: Past, present, and future targets for intervention, Focus 14 (2016) 308–314. [CrossRef]
- R. B. Mailman, V. Murthy, Third generation antipsychotic drugs: partial agonism or receptor functional selectivity?, Curr Pharm Des. 16 (2010) 488–501. /: 16 (2010) 488–501, https. [CrossRef]
- https://www.drugs.com/history/zuplenz.html (accessed on , 2023). 6 July.
- https://www.drugs.com/history/serdolect.html (accessed on , 2023). 6 July.
- https://www.thepharmaletter.com/article/merck-s-maxalt-approved-in-usa (accessed on , 2023). 6 July.
- Y. Han, W. Dong, Q. Guo, X. Li, L. Huang, The importance of indole and azaindole scaffold in the development of antitumor agents, Eur. J. Med. Chem. 203 (2020) 112506. [CrossRef]
- https://www.drugs.com/history/sutent.html (accessed on , 2023). 6 July.
- https://www.cancer.gov/about-cancer/treatment/drugs/sunitinibmalate (accessed on , 2023). 6 July.
- https://www.drugs.com/history/tagrisso.html (accessed on , 2023). 6 July.
- https://www.cancer.gov/about-cancer/treatment/drugs/osimertinib (accessed on , 2023). 6 July.
- https://www.cancer.gov/about-cancer/treatment/drugs/rucaparibcamsylate (accessed on , 2023). 6 July.
- S. Li, Anlotinib: A novel targeted drug for bone and soft tissue sarcoma, Front. Oncol. 11 (2021) 664853. [CrossRef]
- Y. Y. Syed, Anlotinib: First global approval, Drugs 78 (2018) 1057–1062. [CrossRef]
- https://www.drugs.com/history/farydak.html (accessed on , 2023). 6 July.
- https://www.drugs.com/history/rydapt.html (accessed on , 2023). 6 July.
- https://www.drugs.com/history/alecensa.html (accessed on , 2023). 6 July.
- https://www.cancer.gov/about-cancer/treatment/drugs/alectinib (accessed on , 2023). 6 July.
- S. Dadashpour, S. Emami, Indole in the target-based design of anticancer agents: A versatile scaffold with diverse mechanisms, Eur. J. Med. Chem. 150 (2018) 9‒29. [CrossRef]
- R. Patil, S. A. Patil, K. D. Beaman, S. A. Patil, Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, an update (2013–2015), Future Med. Chem. 8 (2016) 1291–1316. /: 8 (2016) 1291–1316, https. [CrossRef]
- S. A. Patil, R. Pati D. D. Miller, Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, Future Med. Chem. 4 (2012) 2085–2115. [CrossRef]
- A. Ahmad, W. A. Sakr, K. M. W. Rahman, Anticancer properties of indole compounds: Mechanism of apoptosis induction and role in chemotherapy, Curr. Drug Targets 11 (2010) 652‒666. /. [CrossRef]
- S. S. Panda, A. S. Girgis, M. N. Aziz, M. S. Bekheit, Spirooxindole: A versatile biologically active heterocyclic scaffold, Molecules 28 (2023) 618. [CrossRef]
- I. A. Seliem, S. S. Panda, A. S. Girgis, Q. L. Tran, M. F. Said, M. S. Bekheit, A. Abdelnaser, S. Nasr, W. Fayad, A. A. F. Soliman, R. Sakhuja, T. S. Ibrahim, Z. K. M. Abdel-Samii, A. M. M. Al-Mahmoudy, Development of isatin-based Schiff bases targeting VEGFR-2 inhibition: Synthesis, characterization, antiproliferative properties, and QSAR studies, ChemMedChem 17 (2022) e202200164. [CrossRef]
- A. S. Girgis, S. S. Panda, M. N. Aziz, P. J. Steel, C. D. Hall, A. R. Katritzky, Rational design, synthesis, and 2D-QSAR study of anti-oncological alkaloids against hepatoma and cervical carcinoma, RSC Adv. 5 (2015) 28554–28569. [CrossRef]
- Y. Y. Lee, H. H. Park, W. Park, H. Kim, J. G. Jang, K. S. Hong, J.-Y. Lee, H. S. Seo, D. H. Na, T.-H. Kim, Y. B. Choy, J. H. Ahn, W. Lee, C. G. Park, Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm, Biomaterials 267 (2021) 120389. [CrossRef]
- A. K. Ghosh, J. Raghavaiah, D. Shahabi, M. Yadav, B. J. Anson, E. K. Lendy, S.-i. Hattori, N. Higashi-Kuwata, H. Mitsuya, A. D. Mesecar, Indole chloropyridinyl ester-derived SARS-CoV-2 3CLpro Inhibitors: Enzyme inhibition, antiviral efficacy, structure‒activity relationship, and X-ray structural studies, J. Med. Chem. 64 (2021) 14702–14714. [CrossRef]
- S. K. Mishra, T. Tripathi, One year update on the COVID-19 pandemic: Where are we now?, Acta Tropica 214 (2021) 105778. 2021. [CrossRef]
- https://covid19.who.int/ (accessed on , 2023). 5 July.
- K. Jayabal, D. Elumalai, S. Leelakrishnan, S. Bhattacharya, V. Rengarajan, T. Kannan, S.-C. Chuang, Green and regioselective approach for the synthesis of3-substituted indole based 1,2-dihydropyridine and azaxanthone derivatives as a potential lead for SARS-CoV-2 and delta plus mutant virus: DFT and docking studies, ACS Omega 7 (2022) 43856−43876. [CrossRef]
- Shagufta, I. Ahmad, The race to treat COVID-19: Potential therapeutic agents for the prevention and treatment of SARS-CoV-2, Eur. J. Med. Chem. 213 (2021) 113157. [CrossRef]
- J. M. Sanders, M. L. Monogue, T. Z. Jodlowski, J. B. Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19), A Review, JAMA 323 (2020) 1824‒1836. [CrossRef]
- L. Zheng, L. Zhang, J. Huang, K.S. Nandakumar, S. Liu, K. Cheng, Potential treatment methods targeting 2019-nCoV infection, Eur. J. Med. Chem. 205 (2020) 112687. [CrossRef]
- I. A. Seliem, S. S. Panda, A. S. Girgis, Y. Moatasim, A. Kandeil, A. Mostafa, M. A. Ali, E. S. Nossier, F. Rasslan, A. M. Srour, R. Sakhuja, T. S. Ibrahim, Z. K. M. Abdel-samii, A. M. M. Al-Mahmoudy, New quinoline-triazole conjugates: Synthesis, and antiviral properties against SARS-CoV-2, Bioorg. Chem. 114 (2021) 105117. [CrossRef]
- P. Zhou, X.-L. Yang, X.-G. Wang, B. Hu, L. Zhang, W. Zhang, H.-R. Si, Y. Zhu, B. Li, C.-L. Huang, H.-D. Chen, J. Chen, Y. Luo, H. Guo, R.-D. Jiang, M.-Q. Liu, Y. Chen, X.-R. Shen, X. Wang, X.-S. Zheng, K. Zhao, Q.-J. Chen, F. Deng, L.-L. Liu, B. Yan, F.-X. Zhan, Y.-Y. Wang, G.-F. Xiao, Z.-L. Shi, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature 579 (2020) 270–273. [CrossRef]
- R. Banerjee, L. Perera, L. M. V. Tillekeratne, Potential SARS-CoV-2 main protease inhibitors, Drug Discov. Today, 26 (2021) 804‒816. [CrossRef]
- Y. Araf, F. Akter, Y.-d. Tang, R. Fatemi, S. A. Parvez, C. Zheng, G. Hossain, Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines, J. Med. Virol. 94 (2022) 1825–1832. [CrossRef]
- M. He, Y. Huang, Y. Wang, J. Liu, M. Han, Y. Xiao, N. Zhang, H. Gui, H. Qiu, L. Cao, W. Jia, S. Huang, Metabolomics-based investigation of SARS-CoV-2 vaccination (Sinovac) reveals an immune-dependent metabolite biomarker, Front. Immunol. 13 (2022) 954801. [CrossRef]
- R. Yapasert, P. Khaw-on, R. Banjerdpongchai, Coronavirus infection-associated cell death signaling and potential therapeutic targets, Molecules 26 (2021) 7459. [CrossRef]
- V. Varadharajan, G. S. Arumugam, S. Shanmugam, Isatin-based virtual high throughput screening, molecular docking, DFT, QM/MM, MD and MM-PBSA study of novel inhibitors of SARS-CoV-2 main protease, J. Biomol. Struct. Dyn. 40 (2022) 7852–7867. [CrossRef]
- Y. Hua, X. Dai, Y. Xu, G. Xing, H. Liu, T. Lu, Y. Chen, Y. Zhang, Drug repositioning: Progress and challenges in drug discovery for various diseases, Eur. J. Med. Chem. 234 (2022) 114239. [CrossRef]
- T. Pillaiyar, S. Meenakshisundaram, M. Manickam. M. Sankaranarayanan, A medicinal chemistry perspective of drug repositioning: Recent advances and challenges in drug discovery, Eur. J. Med. Chem. 195 (2020) 112275. [CrossRef]
- S. Kumar, S. Kovalenko, S. Bhardwaj, A. Sethi, N. Y. Gorobets, S. M. Desenko, Poonam, B. Rathi, Drug repurposing against SARS-CoV-2 using computational approaches, Drug Discov. Today 27 (2022) 2015‒2027. [CrossRef]
- K. Mohamed, N. Yazdanpanah, A. Saghazadeh, N. Rezaei, Computational drug discovery and repurposing for the treatment of COVID-19: A systematic review, Bioorg. Chem. 106 (2021) 104490. [CrossRef]
- W.-C. Chiou, M.-S. Hsu, Y.-T. Chen, J.-M. Yang, Y.-G. Tsay, H.-C. Huang, C. Huang, Repurposing existing drugs: identification of SARS-CoV-2 3C-like protease inhibitors, J. Enzyme Inhib. Med. Chem. 36 (2021) 147–153. [CrossRef]
- T. Asselah, D. Durantel, E. Pasmant, G. Lau, R. F. Schinazi, COVID-19: Discovery, diagnostics and drug development, J. Hepatology 74 (2021) 168–184. [CrossRef]
- A. Sarkar, K. Mandal, Repurposing an antiviral drug against SARS-CoV-2 main protease, Angew. Chem. Int. Ed. 60 (2021) 23492–23494. [CrossRef]
- P. N. Batalha, L. S. M. Forezi, C. G. S. Lima, F. P. Pauli, F. C. S. Boechat, M. C. B. V. de Souza, A. C. Cunha, V. F. Ferreira, F. d. C. da Silva, Drug repurposing for the treatment of COVID-19: Pharmacological aspects and synthetic approaches, Bioorg. Chem. 106 (2021) 104488. [CrossRef]
- J. Dowarah, B. N. Marak, U. C. S. Yadav, V. P. Singh, Potential drug development and therapeutic approaches for clinical intervention in COVID-19, Bioorg. Chem. 114 (2021) 105016. [CrossRef]
- N. Trivedi, A. Verna, D. Kumar, Possible treatment and strategies for COVID-19: review and assessment, Eur. Rev. Med. Pharmacol. Sci. 24 (2020) 12593‒12608. [CrossRef]
- N. A. Ashour, A. A. Elmaaty, A. A. Sarhan, E. B. Elkaeed, A. M. Moussa, I. A. Erfan, A. A. Al-Karmalawy, A systematic review of the global intervention for SARS-CoV-2 combating: From drugs repurposing to Molnupiravir approval, Drug Des. Devel. Ther. 16 (2022) 685–715. [CrossRef]
- J. Santos, S. Brierley, M. J. Gandhi, M. A. Cohen, P. C. Moschella, A. B. L. Declan, Repurposing therapeutics for potential treatment of SARS-CoV-2: A review, Viruses 12 (2020) 705. [CrossRef]
- X. Zhang, Y. Yang, M. Grimstein, G. Liu, E. Kitabi, J. Fan, Y.-H. Wang, J. Earp, J. L. Weaver, H. Zhu, J. Liu, K. S. Reynolds, S.-M. Huang, Y. Wang, Anti–SARS-CoV-2 repurposing drug database: Clinical pharmacology considerations, CPT Pharmacometrics Syst. Pharmacol. 10 (2021) 973–982. [CrossRef]
- A. Simonis, S. J. Theobald, G. Fätkenheuer, J. Rybniker, J. J. Malin, A comparative analysis of remdesivir and other repurposed antivirals against SARS-CoV-2, EMBO Mol. Med. 13 (2021) e13105. [CrossRef]
- https://go.drugbank.com/drugs/DB15661 (accessed on , 2023). 6 July.
- https://www.drugs.com/history/molnupiravir.html (accessed on , 2023). 6 July.
- https://www.drugs.com/history/paxlovid.html (accessed on , 2023). 6 July.
- A. M. Srour, S. S. Panda, A. Mostafa, W. Fayad, M. A. El-Manawaty, A. A. F. Soliman, Y. Moatasim, A. El Taweel, M. F. Abdelhameed, M. S. Bekheit, M. A. Ali, A. S. Girgis, Synthesis of aspirin-curcumin mimic conjugates of potential antitumor and anti-SARS-CoV-2 properties, Bioorg. Chem. 117 (2021) 105466. [CrossRef]
- I. A. Seliem, A. S. Girgis, Y. Moatasim, A. Kandeil, A. Mostafa, M. A. Ali, M. S. Bekheit, S. S. Panda, New pyrazine conjugates: Synthesis, computational studies, and antiviral properties against SARS-CoV-2, ChemMedChem 16 (2021) 3418–3427. [CrossRef]
- M. A. Youssef, S. S. Panda, D. R. Aboshouk, M. F. Said, A. El Taweel, M. GabAllah, W. Fayad, A. A. F. Soliman, A. Mostafa, N. G. Fawzy, A. S. Girgis, Novel curcumin mimics: Design, synthesis, biological properties and computational studies of piperidone-piperazine conjugates, ChemistrySelect 7 (2022) e202201406. [CrossRef]
- K. A. Wyman, A. S. Girgis, P. S. Surapaneni, J. M. Moore, N. M. Abo Shama, S. H. Mahmoud, A. Mostafa, R. F. Barghash, Z. Juan, R. D. Dobaria, A. J. Almalki, T. S. Ibrahim, S. S. Panda, Synthesis of potential antiviral agents for SARS-CoV-2 using molecular hybridization approach, Molecules 27 (2022) 5923. [CrossRef]
- C. Li, L. Wang, L. Ren, Antiviral mechanisms of candidate chemical medicines and traditional Chinese medicines for SARS-CoV-2 infection, Virus Res. 286 (2020) 198073. [CrossRef]
- I. Leneva, N. Kartashova, A. Poromov, A. Gracheva, E. Korchevaya, E. Glubokova, O. Borisova, A. Shtro, S. Loginova, V. Shchukina, R. Khamitov, E. Faizuloev, Antiviral activity of Umifenovir in vitro against a broad spectrum of coronaviruses, including the novel SARS-CoV-2 Virus, Viruses 13 (2021) 1665. [CrossRef]
- H. Tanaka, S. Miyagi, Y. Yoshida, J. S. Lamb, C. N. Chick, L. P. Luhata, M. Shibata, E. Tanaka, Y. Suzuki, T. Usuki, Synthesis and biological evaluation of Umifenovir analogues as anti-SARS-CoV-2 agents, ChemistrySelect 7 (2022) e202202097. [CrossRef]
- A. Shuster, D. Pechalrieu, C. B. Jackson, D. Abegg, H. Choe, A. Adibekian, Clinical antiviral drug Arbidol inhibits infection by SARS-CoV-2 and variants through direct binding to the spike protein, ACS Chem. Biol. 16 (2021) 2845–2851. [CrossRef]
- A. K. Yadav, S. Wen, X. Xu, L. Yu, Antiviral treatment in COVID-19: which is the most promising? - a narrative review, Ann. Palliat. Med. 10 (2021) 707‒720. [CrossRef]
- X. Pan, L. Dong, L. Yang, D. Chen, C. Peng, Potential drugs for the treatment of the novel coronavirus pneumonia (COVID-19) in China, Virus Res. 286 (2020) 198057. [CrossRef]
- D. Wang, Z. Li, Y. Liu, An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics, J. Infect. Public Health 13 (2020) 1405–1414. [CrossRef]
- A. K. Padhi, A. Seal, J. M. Khan, M. Ahamed, T. Tripathi, Unraveling the mechanism of arbidol binding and inhibition of SARS-CoV-2: Insights from atomistic simulations, Eur. J. Pharmacol. 894 (2021) 173836. [CrossRef]
- S. S. Borisevich, E. M. Khamitov, M. A. Gureev, O. I. Yarovaya, N. B. Rudometova, A. V. Zybkina, E. D. Mordvinova, D. N. Shcherbakov, R. A. Maksyutov, N. F. Salakhutdinov, Simulation of molecular dynamics of SARS-CoV-2 S-protein in the presence of multiple Arbidol molecules: Interactions and binding mode insights, Viruses 14 (2022) 119. [CrossRef]
- N. Vankadari, Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein, Int. J. Antimicrob. Agents 56 (2020) 105998. [CrossRef]
- Z. Zhu, Z. Lu, T. Xu, C. Chen, G. Yang, T. Zha, J. Lu, Y. Xue, Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19, J. Infection 81 (2020) e21–e23. [CrossRef]
- M. Nojomi, Z. Yassin, H. Keyvani, M. J. Makiani, M. Roham, A. Laali, N. Dehghan, M. Navaei, M. Ranjbar, Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial, BMC Infectious Diseases 20 (2020) 954. [CrossRef]
- Y. Li, Z. Xie, W. Lin, W. Cai, C. Wen, Y. Guan, X. Mo, J. Wang, Y. Wang, P. Peng, X. Chen, W. Hong, G. Xiao, J. Liu, L. Zhang, F. Hu, F. Li, F. Zhang, Efficacy and safety of Lopinavir/Ritonavir or Arbidol in adult patients with mild/moderate COVID-19: An exploratory randomized controlled trial, Med. 1 (2020) 105‒113. [CrossRef]
- Z. Wang, B, Yang, Q. Li, L. Wen, R. Zhang, Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China, Clin. Infect. Dis. 71 (2020) 769‒777. . [CrossRef]
- Z. Zhu, Z. Lu, T. Xu, C. Chen, G. Yang, T. Zha, J. Lu, Y. Xue, Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19, J. Infect. 81 (2020) e21‒23. [CrossRef]
- Z. Wang, X. Chen, Y. Lu, F. Chen, W. Zhang, Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment, Biosci. Trends 14 (2020) 64‒68. [CrossRef]
- R. Ramachandran, V. Bhosale, H. Reddy, V. Atam, M. M. A. Faridi, J. Fatima, V. Shukla, Z. A. Khan, H. Khan, V. Singh, M. P. S. Negi, M. Srivastava, A. K. Srivastava, C. B. Tripathi, N. Ghosh, N. Majumdar, R. K. Tripathi, S. K. Rath, P. R. Mishra, S. Sharma, T. K. Kundu, Phase III, randomized, double-blind, Placebo controlled trial of efficacy, safety and tolerability of antiviral drug Umifenovir vs standard care of therapy in non-severe COVID-19 patients, Int. J. Infect. Dis. 115 (2022) 62–69. [CrossRef]
- M. Yu, D.-C. Wang, S. Li, Y.-H. Lei, J. Wei, L.-Y. Huang, Meta-analysis of arbidol versus lopinavir/ritonavir in the treatment of coronavirus disease 2019, J. Med. Virol. 94 (2022) 1513–1522. [CrossRef]
- C. Yang, C. Ke, D. Yue, W. Li, Z. Hu, W. Liu, S. Hu, S. Wang, J. Liu, Effectiveness of Arbidol for COVID-19 prevention in health professionals, Front. Public Health 8 (2020) 249. [CrossRef]
- M. Li, T. Yu, J. Zhu, Y. Wang, Y. Yang, K. Zhao, Y. Yi, J. He, C. Li, J. He, Comparison of the antiviral effect of Arbidol and Chloroquine in treating COVID-19, Ann. Palliat. Med. 10 (2021) 3307‒3312. [CrossRef]
- X.-L He, Y.-Y. Zhou, W. Fu, Y. Xue, M.-Y. Liang, B.-H. Yang, W.-I. Ma, Q. Zhou, L. Chen, J.-C. Zhang, X.-R. Wang, Prolonged SARS-CoV-2 viral shedding in patients with COVID-19 was associated with delayed initiation of Arbidol treatment and consulting doctor later: A retrospective cohort study, Curr. Med. Sci. 41 (2021) 1096‒1104. [CrossRef]
- N. V. Ul’yanovskii, D. S. Kosyakov, S. A. Sypalov, I. S. Varsegov, I. S. Shavrina, A. T. Lebedev, Antiviral drug Umifenovir (Arbidol) in municipal wastewater during the COVID-19 pandemic: Estimated levels and transformation, Sci. Total Environ. 805 (2022) 150380. [CrossRef]
- S. Choudhary, O. Silakari, Scaffold morphing of arbidol (umifenovir) in search of multi-targeting therapy halting the interaction of SARS-CoV-2 with ACE2 and other proteases involved in COVID-19, Virus Res. 289 (2020) 198146. [CrossRef]
- F. D. Hart, P. L. Boardman, Indomethacin: A new non-steroid anti-inflammatory agent, British Medical J. (1963) 965–970. /. [CrossRef]
- A. M. Ghanim, A. S. Girgis, B. M. Kariuki, N. Samir, M. F. Said, A. Abdelnaser, S. Nasr, M. S. Bekheit, M. F. Abdelhameed, A. J. Almalki, T. S. Ibrahim, S. S. Panda, Design and synthesis of ibuprofen-quinoline conjugates as potential anti-inflammatory and analgesic drug candidates, Bioorg. Chem. 119 (2022) 105557. [CrossRef]
- R. M. Bokhtia, S. S. Panda, A. S. Girgis, N. Samir, M. F. Said, A. Abdelnaser, S. Nasr, M. S. Bekheit, A. S. Dawood, H. Sharma, M. Wade, S. K. Sharma, A. M. Ghanim, New NSAID conjugates as potent and selective COX-2 inhibitors: Synthesis, molecular modeling and biological investigation, Molecules 28 (2023) 1945. [CrossRef]
- R. Ravichandran, S. K. Mohan, S. K. Sukumaran, D. Kamaraj, S. S. Daivasuga, S. O. A. S. Ravi, S. Vijayaraghavalu, R. K. Kumar, An open label randomized clinical trial of Indomethacin for mild and moderate hospitalized Covid-19 patients, Sci. Rep. 12 (2022) 6413. [CrossRef]
- J. Desantis, B. Mercorelli, M. Celegato, F. Croci, A. Bazzacco, M. Baroni, L. Siragusa, G. Cruciani, A. Loregian, L. Goracci, Indomethacin-based PROTACs as pan-coronavirus antiviral agents, Eur. J. Med. Chem. 226 (2021) 113814. [CrossRef]
- R. Gomeni, T. Xu, X. Gao, F. Bressolle-Gomeni, Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS CoV-2, J. Pharmacokinet. Pharmacodyn. 47 (2020) 189–198. [CrossRef]
- A. V. Krymchantowski, R. P. Silva-Néto, C. Jevoux, A. G. Krymchantowski, Indomethacin for refractory COVID or post-COVID headache: a retrospective study, Acta Neurol. Belg. 122 (2022) 465–469. [CrossRef]
- N. Shekhar, H. Kaur, P. Sarma , A. Prakash, B. Medhi, Indomethacin: an exploratory study of antiviral mechanism and host-pathogen interaction in COVID-19, Expert Rev. Anti-infect. Ther. 20 (2022) 383‒390. [CrossRef]
- J. Li, C. Lin, X. Zhou, F. Zhong, P. Zeng, P. J. McCormick, H. Jiang, J. Zhang, Structural basis of main proteases of coronavirus bound to drug candidate PF-07304814, J. Mol. Biol. 434 (2022) 167706. [CrossRef]
- B. Mao, V. T. K. Le-Trilling, K. Wang, D. Mennerich, J. Hu, Z. Zhao, J. Zheng, Y. Deng, B. Katschinski, S. Xu, G. Zhang, X. Cai, Y. Hu, J. Wang, M. Lu, A. Huang, N. Tang, M. Trilling, Y. Lin, Obatoclax inhibits SARS-CoV-2 entry by altered endosomal acidification and impaired cathepsin and furin activity in vitro, Emerg. Microbes Infect. 11 (2022) 483‒497. [CrossRef]
- R. Begum, A. N. M. Mamun-Or-Rashid, T. T. Lucy, K. Pramanik, B. K. Sil, N. Mukerjee, P. Tagde, M. Yagi, Y. Yonei, Potential therapeutic approach of melatonin against omicron and some other variants of SARS-CoV-2, Molecules 27 (2022) 6934. [CrossRef]
- M. Vlachou, A. Siamidi, A. Dedeloudi, S. K. Konstantinidou, I. P. Papanastasiou, Pineal hormone melatonin as an adjuvant treatment for COVID-19 (Review), Int. J. Mol. Med. 47 (2021) 47. /. [CrossRef]
- H. Parlakpinar, S. Polat, H. A. Acet, Pharmacological agents under investigation in the treatment of coronavirus disease 2019 and the importance of melatonin, Fundam. Clin. Pharmacol. 35 (2021) 62–75. [CrossRef]
- E. L. Feitosa, F. T. D. S. S. Júnior, J. A. D. Neto, L. F. L. Matos, M. H. D. Moura, T. O. Rosales, G. B. L. De Freitas, COVID-19: Rational discovery of the therapeutic potential of Melatonin as a SARS-CoV-2 main protease inhibitor, Int. J. Med. Sci. 17 (2020) 2133-2146. [CrossRef]
- R. Zhang, X. Wang, L. Ni, X. Di, B. Ma, S. Niu, C. Liu, R. J. Reiter, COVID-19: Melatonin as a potential adjuvant treatment, Life Sci. 250 (2020) 117583. [CrossRef]
- G. Öztűrk, K. G. Akbulut, Ş. Gűney, Melatonin, aging, and COVID-19: Could melatonin be beneficial for COVID-19 treatment in the elderly?, Turk. J. Med. Sci. 50 (2020) 1504‒1512. /: 50 (2020) 1504‒1512, https. [CrossRef]
- A. Wichniak, A. Kania, M. Siemiński, W. J. Cubała, Melatonin as a potential adjuvant treatment for COVID-19 beyond sleep disorders, Int. J. Mol. Sci. 22 (2021) 8623. [CrossRef]
- R. J. Reiter, R. Sharma, F. Simko, A. Dominguez-Rodriguez, J. Tesarik, R. L. Neel, A. T. Slominski, K. Kleszczynski, V. M. Martin-Gimenez, W. Manucha, D. P. Cardinali, Melatonin: highlighting its use as a potential treatment for SARS-CoV-2 infection, Cell. Mol. Life Sci. 79 (2022) 143. [CrossRef]
- I. G. García, M. Rodriguez-Rubio, A. R. Mariblanca, L. M. de Soto, L. D. García, J. M. Villatoro, J. Q. Parada, E. S. Meseguer, M. J. Rosales, J. González, J. R. Arribas, A. J. Carcas, P. de la Oliva, A. M. Borobia, A randomized multicenter clinical trial to evaluate the efficacy of melatonin in the prophylaxis of SARS-CoV-2 infection in high-risk contacts (MeCOVID Trial): A structured summary of a study protocol for a randomised controlled trial, Trials 21(2020) 466. [CrossRef]
- Z. T. Hasan, M. Q. Y. M. A. Al Atrakji, A. K. Mehuaiden, The effect of Melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients, Int. J. Infect. Dis. 114 (2022) 79–84. [CrossRef]
- S.-H. Lan, H.-Z. Lee, C.-M. Chao, S.-P. Chang, L.-C. Lu, C.-C. Lai, Efficacy of melatonin in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials, J. Med. Virol. 94 (2022) 2102–2107. [CrossRef]
- G. Farnoosh, M. Akbariqomi, T. Badri, M. Bagheri, M. Izadi, A. Saeedi-Boroujeni, E. Rezaie, H. E. G. Ghaleh, H. Aghamollaei, M. Fasihi-ramandi, K. Hassanpour, G. Alishiri, Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical trial, Arch. Med. Res. 53 (2022) 79–85. [CrossRef]
- A. Ziaei, P. Davoodian, H. Dadvand, O. Safa, S. Hassanipour, M. Omidi, M. Masjedi, F. Mahmoudikia, B. Rafiee, M. Fathalipour, Evaluation of the efficacy and safety of Melatonin in moderately ill patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial, Trials 21 (2020) 882. [CrossRef]
- H. A. Alhadrami, G. Burgio, B. Thissera, R. Orfali, S. E. Jiffri, M. Yaseen, A. M. Sayed, M. E. Rateb, Neoechinulin A as a promising SARS-CoV-2Mpro inhibitor: In vitro and in silico study showing the ability of simulations in discerning active from inactive enzyme inhibitors, Mar. Drugs 20 (2022) 163. [CrossRef]
- W. Vuong, M. B. Khan, C. Fischer, E. Arutyunova, T. Lamer, J. Shields, H. A. Saffran, R. T.McKay, M. J. van Belkum, M. A. Joyce, H. S. Young, D. L. Tyrrell, J. C. Vederas, M. J. Lemieux, Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication, Nat. Commun. 11 (2020) 4282. [CrossRef]
- K. Nishiuchi, H. Ohashi, K. Nishioka, M. Yamasaki, M. Furuta, T. Mashiko, S. Tomoshige, K. Ohgane, S. Kamisuki, K. Watashi, K. Kuramochi, Synthesis and antiviral activities of Neoechinulin B and its derivatives, J. Nat. Prod. 85 (2022) 284−291. [CrossRef]
- P. Liu, H. Liu, Q. Sun, H. Liang, C. Li, X. Deng, Y. Liu, L. Lai, Potent inhibitors of SARS-CoV-2 3C-like protease derived from N-substituted isatin compounds, Eur. J. Med. Chem. 206 (2020) 112702. [CrossRef]
- G.-N. Zhang, J. Zhao, Q. Li, M. Wang, M. Zhu, J. Wang, S. Cen, Y. Wang, Discovery and optimization of 2-((1H-indol-3-yl)thio)-N-benzyl-acetamides as novel SARS-CoV-2 RdRp inhibitors, Eur. J. Med. Chem. 223 (2021) 113622. [CrossRef]
- J. Zhao, G. Zhang, Y. Zhang, D. Yi, Q. Li, L. Ma, S. Guo, X. Li, F. Guo, R. Lin, G. Luu, Z. Liu, Y. Wang, S. Cen, 2-((1H-indol-3-yl)thio)-N-phenyl-acetamides: SARS-CoV-2 RNA-dependent RNA polymerase inhibitors, Antiviral Res. 196 (2021) 105209. [CrossRef]
- A. K. Ghosh , D. Shahabi, M. Yadav, S. Kovela, B. J. Anson, E. K. Lendy, C. Bonham, D. Sirohi, C. A. Brito-Sierra, S-i. Hattori, R. Kuhn, H. Mitsuya, A. D. Mesecar, Chloropyridinyl esters of nonsteroidal anti-inflammatory agents and related derivatives as potent SARS-CoV-2 3CL protease inhibitors, Molecules 26 (2021) 5782. [CrossRef]
- P. K. Mudi, A. K. Mahanty, M. Kotakonda, S. Prasad, S. Bhattacharyya, B. Biswas, A benzimidazole scaffold as a promising inhibitor against SARS-CoV-2, J. Biomol. Struct. Dyn. 41 (2023) 1798–1810. [CrossRef]
- A. S. Girgis, S. S. Panda, A. M. Srour, A. Abdelnaser, S. Nasr, Y. Moatasim, O. Kutkat, A. El Taweel, A. Kandeil, A. Mostafa, M. A. Ali, N. G. Fawzy, M. S. Bekheit, E. M. Shalaby, L. Gigli, W. Fayad, A. A. F. Soliman, 3-Alkenyl-2-oxindoles: Synthesis, antiproliferative and antiviral properties against SARS-CoV-2, Bioorg. Chem. 114 (2021) 105131. [CrossRef]
- N. G. Fawazy, S. S. Panda, A. Mostafa, B. M. Kariuki, M. S. Bekheit, Y. Moatasim, O. Kutkat, W. Fayad, M. A. El-Manawaty, A. A. F. Soliman, R. A. El-Shiekh, A. M. Srour, R. F. Barghash, A. S. Girgis, Development of spiro-3-indolin-2-one containing compounds of antiproliferative and ant-SARS-CoV-2 properties, Sci. Rep. 12 (2022) 13880. [CrossRef]
- M. S. Bekheit, S. S. Panda, B. M. Kariuki, S. H. Mahmoud, A. Mostafa, A. S. Girgis, Spiroindole-containing compounds bearing phosphonate group of potential Mpro-SARS-CoV-2 inhibitory properties, Eur. J. Med. Chem. 258 (2023) 115563. [CrossRef]
- V. Di Sarno, G. Lauro, S. Musella, T. Ciaglia, V. Vestuto, M. Sala, M. C. Scala, G. Smaldone, F. Di Matteo, S. Novi, M. F. Tecce, O. Moltedo, G. Bifulco, P. Campiglia, I. M. Gomez-Monterrey, R. Snoeck, G. Andrei, C. Ostacolo, A. Bertamino, Identification of a dual acting SARS-CoV-2 proteases inhibitor through in silico design and step-by-step biological characterization, Eur. J. Med. Chem. 226 (2021) 113863. [CrossRef]
- A. Serra, M. Fratello, A. Federico, R. Ojha, R. Provenzani, E. Tasnadi, L. Cattelani, G. del Giudice, P. A. S. Kinaret, L. A. Saarimäki, A. Pavel, S. Kuivanen, V. Cerullo, O. Vapalahti, P. Horvath, A. Di Lieto, J. Yli-Kauhaluoma, G. Balistreri, D. Greco, Computationally prioritized drugs inhibit SARS-CoV-2 infection and syncytia formation, Brief. Bioinfor. 23 (2022) 1–20. [CrossRef]
- S. S. Panda, A. S. Girgis, S. J. Thomas, J. E. Capito, R. F. George, A. Salman, M. A. El-Manawaty, A. Samir, Synthesis, pharmacological profile and 2D-QSAR studies of curcumin-amino acid conjugates as potential drug candidates, Eur. J. Med. Chem. 196 (2020) 112293. [CrossRef]
- M. Mohseni, H. Bahrami, B. Farajmand, F. S. Hosseini, M. Amanlou, H. Salehabadi, Indole alkaloids as potential candidates against COVID-19: an in silico study, J. Mol. Model. 28 (2022) 144. [CrossRef]
- V. Raj, J.-H. Lee, J.-J. Shim, J. Lee, Antiviral activities of 4H-chromen-4-one scaffold-containing flavonoids against SARS–CoV–2 using computational and in vitro approaches, J. Mol. Liq. 353 (2022) 118775. [CrossRef]
- https://www.drugs.com/history/paxlovid.html (accessed on , 2023). 6 July.
- https://go.drugbank.com/unearth/q?utf8=%E2%9C%93&searcher=drugs&query=Paxlovid (accessed on , 2023). 6 July.
- J. Reina, C. Iglesias, Nirmatrelvir más ritonavir (Paxlovid) una potente combinación inhibidora de la proteasa 3CLpro del SARS-CoV-2, Rev. Esp. Quimioter 35 (2022) 236‒240. [CrossRef]
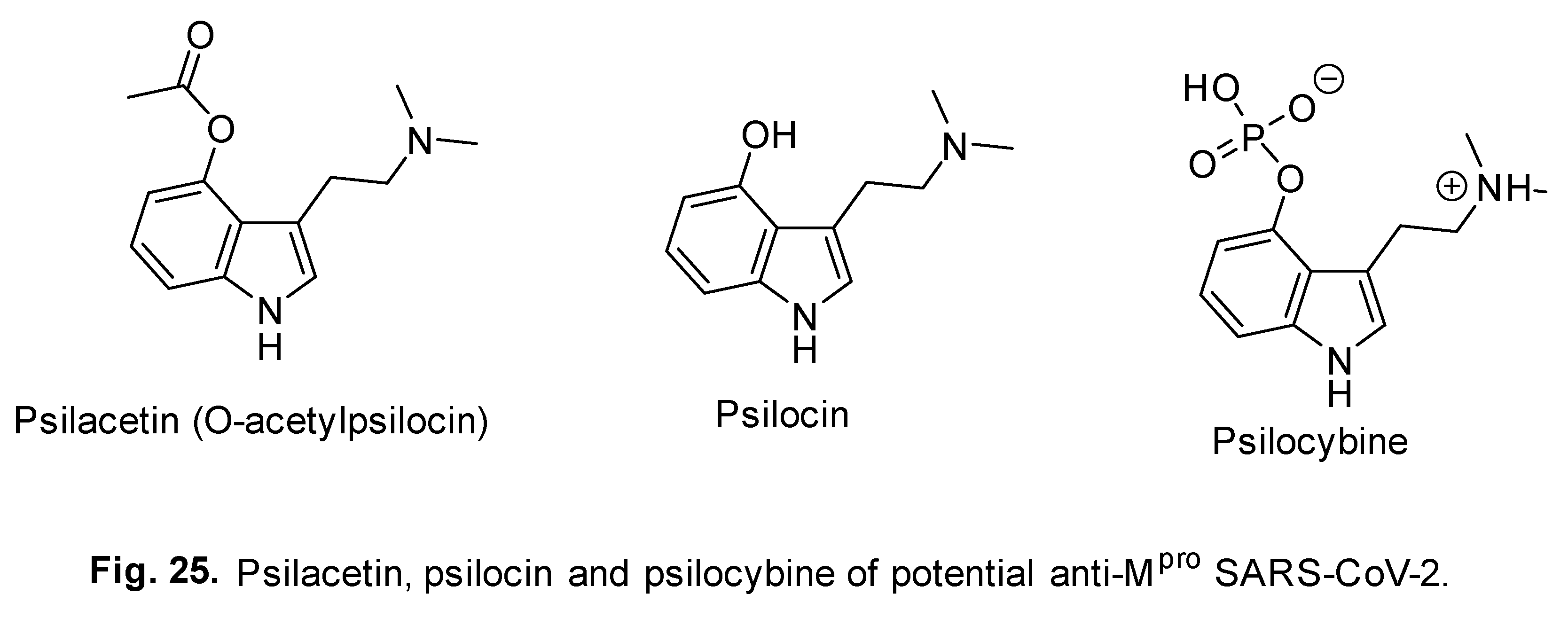
- F. I. Khan, F. Hassan, D. Lai, In silico studies on psilocybin drug derivatives against SARS-CoV-2 and cytokine storm of human interleukin-6 receptor, Front. Immunol. 12 (2022) 794780. [CrossRef]
- B. G. Vijayakumar, D. Ramesh, A. Joji, J. J. prakasan, T. Kannan, In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2, Eur. J. Pharm. 886 (2020) 173448. [CrossRef]
- S. Singhal, P. Khanna, L. Khanna, Synthesis, comparative in vitro antibacterial, antioxidant and UV fluorescence studies of bis indole Schiff bases and molecular docking with ct-DNA and SARS-CoV-2 Mpro, Luminescence 36 (2021) 1531–1543. [CrossRef]
- V. R. Shah, J. D. Bhaliya, G. M. Patel, In silico approach: docking study of oxindole derivatives against the main protease of COVID-19 and its comparison with existing therapeutic agents, J. Basic Clin. Physiol. Pharmacol. 32 (2021) 197–214. [CrossRef]
- S.-i. Hattori, N. Higashi-Kuwata, H. Hayashi, S. R. Allu, J. Raghavaiah, H. Bulut, D. Das, B. J. Anson, E. K. Lendy, Y. Takamatsu, N. Takamune, N. Kishimoto, K. Murayama, K. Hasegawa, M. Li, D. A. Davis, E. N. Kodama, R. Yarchoan, A. Wlodawer, S. Misumi, A. D. Mesecar, A. K. Ghosh, H. Mitsuya, A small molecule compound with an indole moiety inhibits the main protease of SARS-CoV-2 and blocks virus replication, Nat. Commun. 12 (2021) 668. [CrossRef]
- P. Gobinath, P. Packialakshmi, K. Vijayakumar, M. H. Abdellattif, M. Shahbaaz, A. Idhayadhulla, R. Surendrakumar, Synthesis and cytotoxic activity of novel indole derivatives and their in silico screening on spike glycoprotein of SARS-CoV-2, Front. Mol. Biosci. 8 (2021) 637989. [CrossRef]
- X. Xu, Y. Chen, X. Lu, W. Zhang, W. Fang, L. Yuan, X. Wang, An update on inhibitors targeting RNA-dependent RNA polymerase for COVID-19 treatment: promises and challenges, Biochem. Pharmacol. 205 (2022) 115279. [CrossRef]
- R. Kumar M., D. A. Gideon, R. Mariadasse, V. Nirusimhan, S. Rosita A., J. C. Edward, J. Jeyaraman, V. Dhayabaran, In silico evaluation of isatin-based derivatives with RNA-dependent RNA polymerase of the novel coronavirus SARS-CoV-2, J. Biomol. Struct. Dyn. 40 (2022) 6710–6724. 2022. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




