Submitted:
08 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The mechanisms of kidney fibrosis
2.1. Main signaling of fibrosis
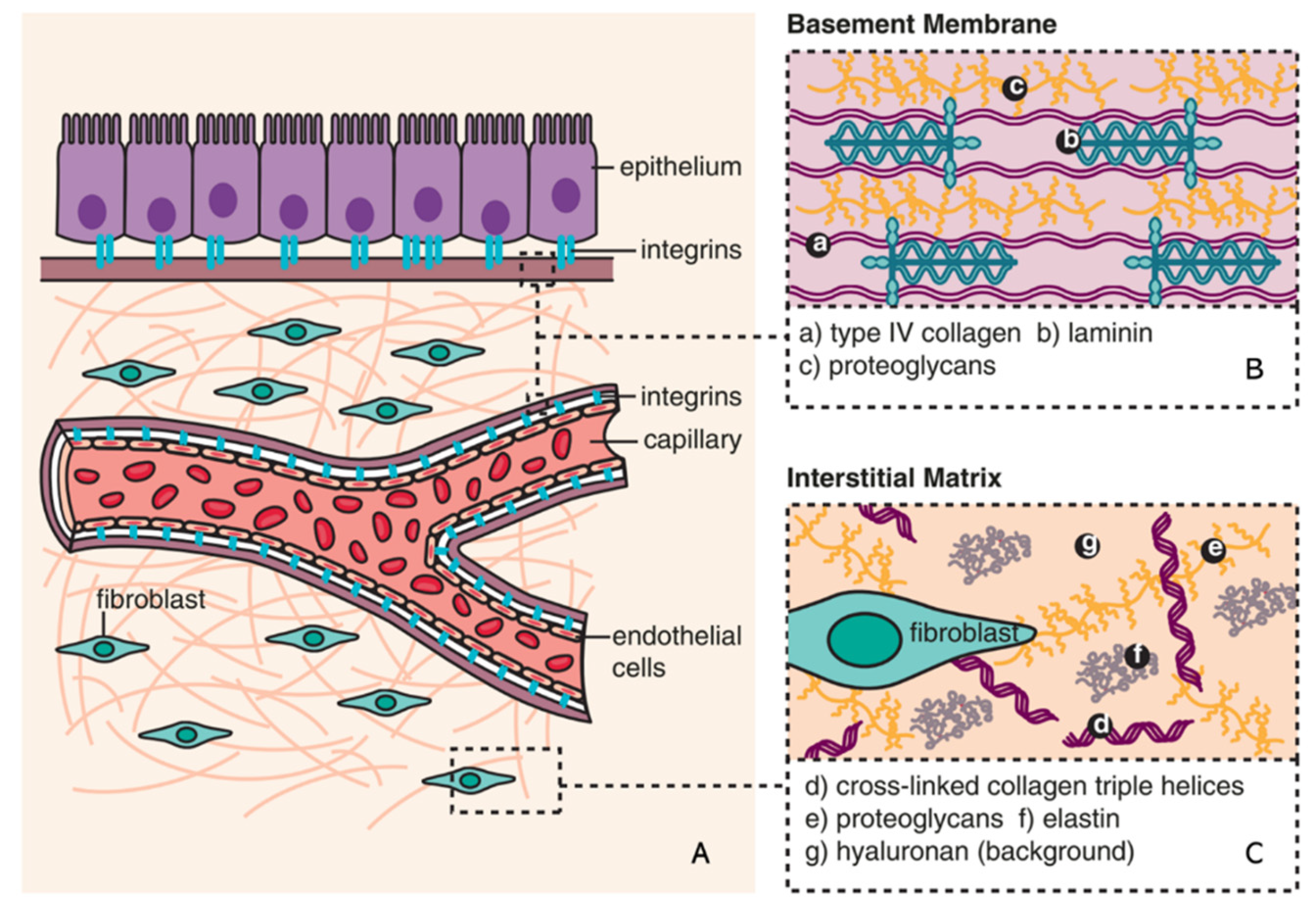
2.1. ECM in kidney fibrosis
2.2. ECM remodeling
3. Senescence
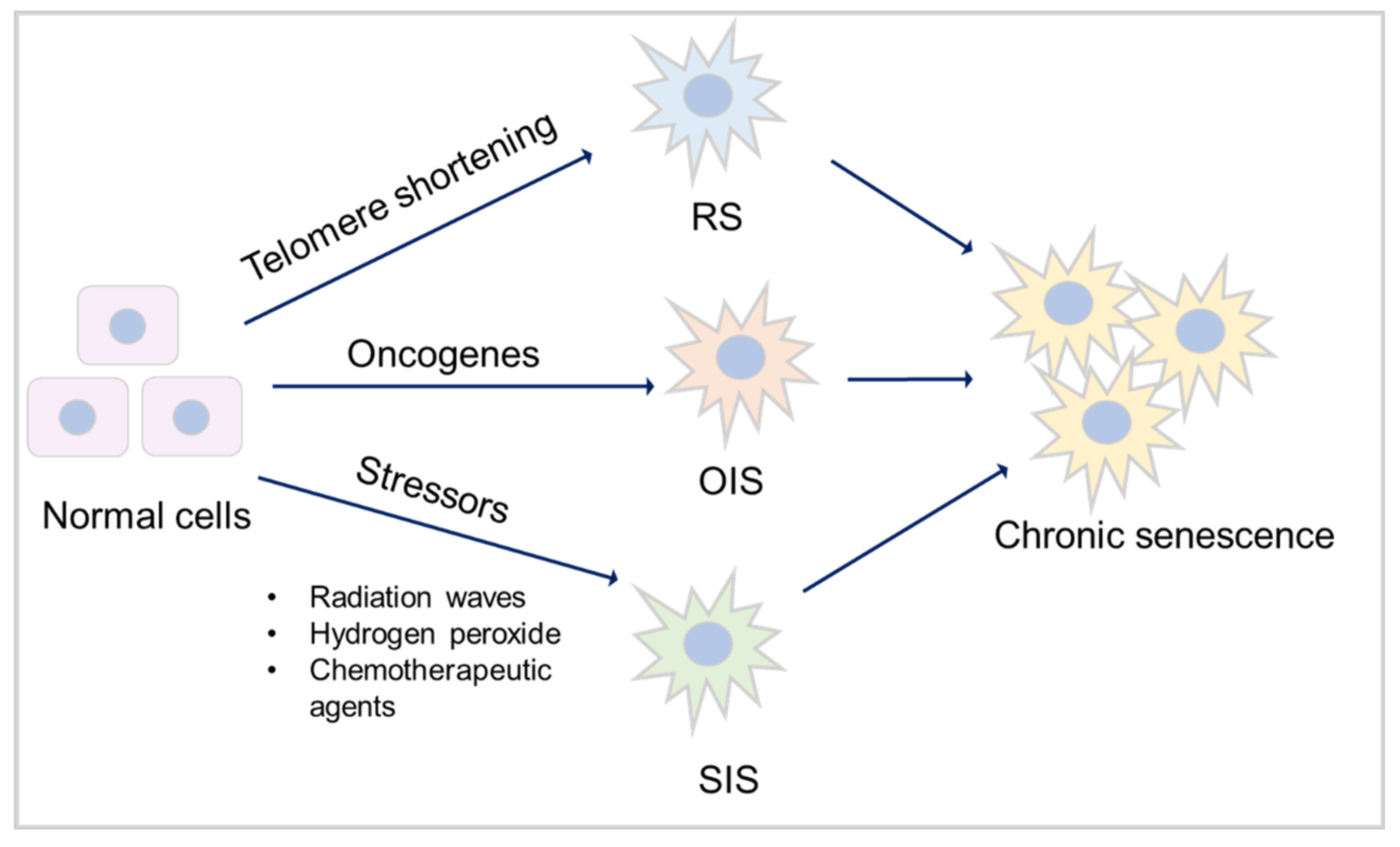
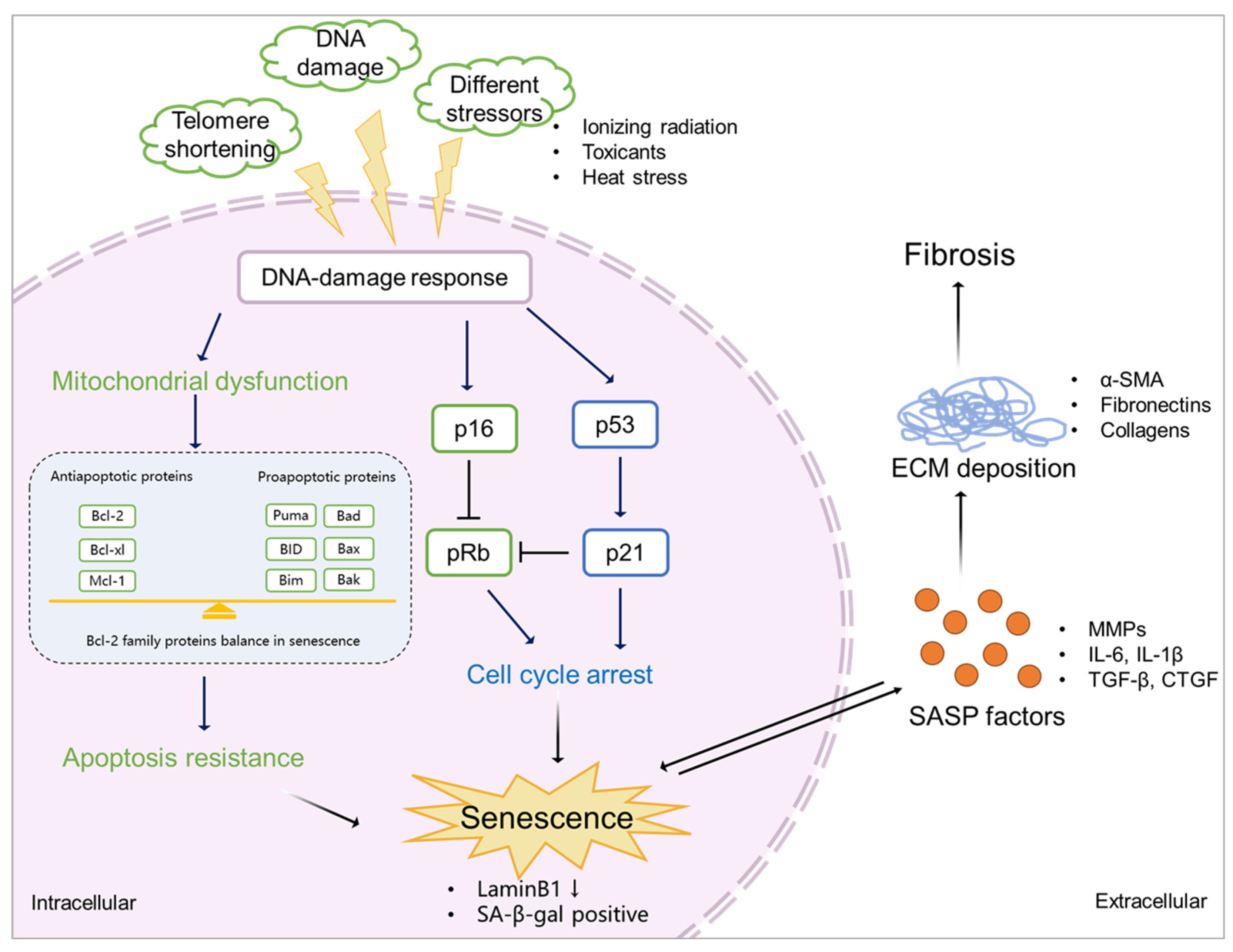
3.1. Mechanisms of senescence
3.1.1. Cell cycle arrest
3.1.2. Apoptosis resistance
3.1.3. SASP factors
3.2. Senescence and fibrosis
4. Protein-bound uremic toxins: drivers of senescence and kidney fibrosis
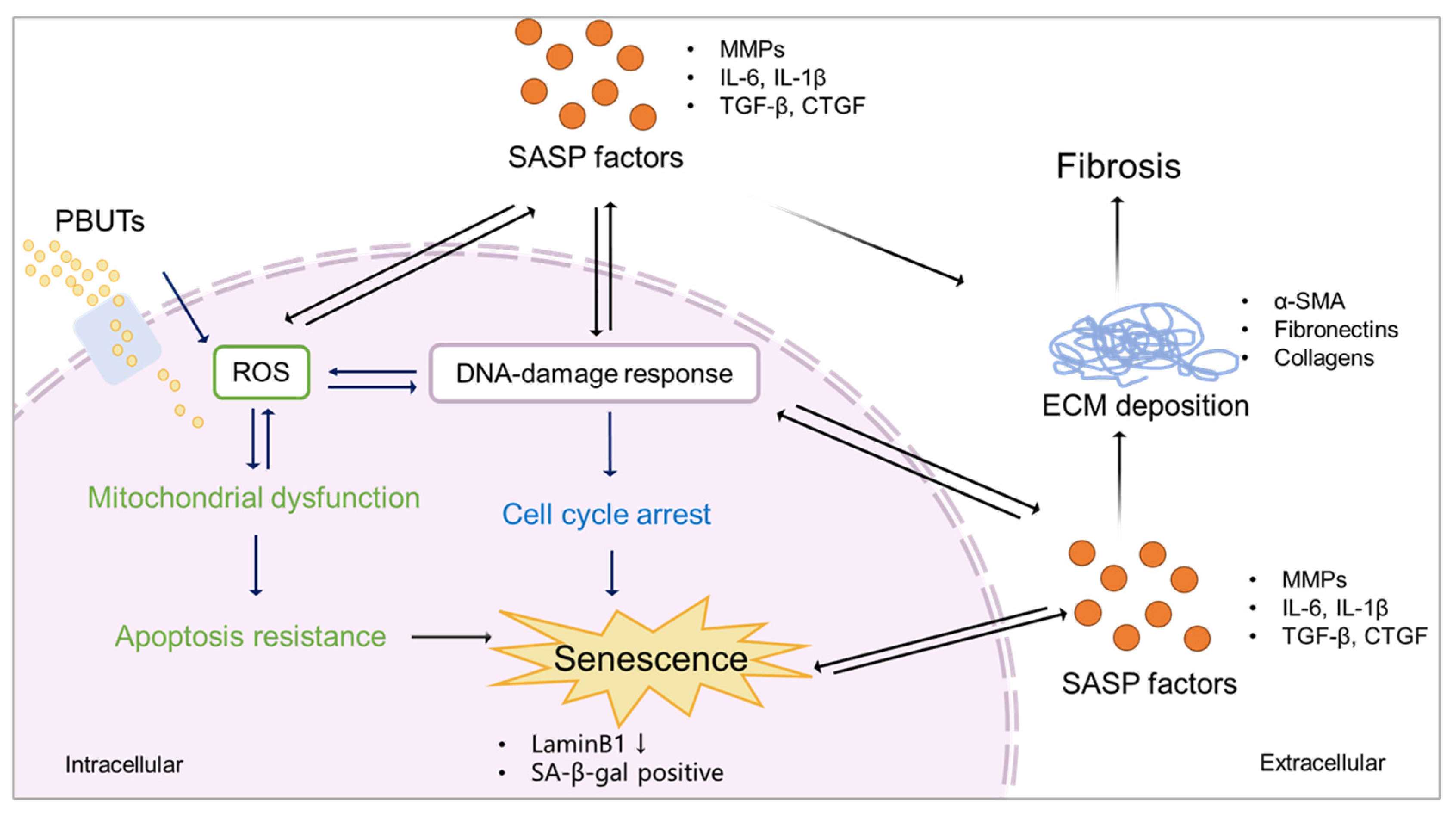
5. Protein-bound uremic toxins promote fibrosis by accelerating senescence
5.1. PBUTs accelerate senescence via mitochondrial dysfunction?
5.2. PBUTs accelerate senescence via cell cycle arrest?
5.3. PBUTs accelerate senescence via SASP factors?
5. Conclusions and future therapeutic perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rockey, D.C., P.D. Bell, and J.A. Hill, Fibrosis--a common pathway to organ injury and failure. N Engl J Med, 2015. 372(12): p. 1138-49. [CrossRef]
- Hernandez-Segura, A., J. Nehme, and M. Demaria, Hallmarks of Cellular Senescence. Trends Cell Biol, 2018. 28(6): p. 436-453. [CrossRef]
- Masereeuw, R., The Dual Roles of Protein-Bound Solutes as Toxins and Signaling Molecules in Uremia. Toxins (Basel), 2022. 14(6). [CrossRef]
- Maheshwari, V., et al., Removal of Protein-Bound Uremic Toxins Using Binding Competitors in Hemodialysis: A Narrative Review. Toxins (Basel), 2021. 13(9). [CrossRef]
- Nigam, S.K. and K.T. Bush, Uraemic syndrome of chronic kidney disease: altered remote sensing and signalling. Nat Rev Nephrol, 2019. 15(5): p. 301-316. [CrossRef]
- Sun, C.Y., S.C. Chang, and M.S. Wu, Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One, 2012. 7(3): p. e34026. [CrossRef]
- Singh, M., et al., EMT: Mechanisms and therapeutic implications. Pharmacol Ther, 2018. 182: p. 80-94. [CrossRef]
- Faheem, M.M., et al., Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: current opinions and emerging perspectives. Cell Death Discov, 2020. 6: p. 51. [CrossRef]
- Kamprom, W., et al., P-cresol and Indoxyl Sulfate Impair Osteogenic Differentiation by Triggering Mesenchymal Stem Cell Senescence. Int J Med Sci, 2021. 18(3): p. 744-755. [CrossRef]
- Kim, S.H., et al., Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest, 2012. 92(4): p. 488-98. [CrossRef]
- Piersma, B., R.A. Bank, and M. Boersema, Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med (Lausanne), 2015. 2: p. 59. [CrossRef]
- Meng, X.M., D.J. Nikolic-Paterson, and H.Y. Lan, TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol, 2016. 12(6): p. 325-38. [CrossRef]
- Rim, E.Y., H. Clevers, and R. Nusse, The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu Rev Biochem, 2022. 91: p. 571-598. [CrossRef]
- Tan, R.J., et al., Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011), 2014. 4(1): p. 84-90. [CrossRef]
- Dey, A., X. Varelas, and K.L. Guan, Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov, 2020. 19(7): p. 480-494. [CrossRef]
- Liu, F., et al., Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol, 2015. 308(4): p. L344-57. [CrossRef]
- Zhao, B., et al., TEAD mediates YAP-dependent gene induction and growth control. Genes Dev, 2008. 22(14): p. 1962-71. [CrossRef]
- Luo, C., et al., Wnt9a Promotes Renal Fibrosis by Accelerating Cellular Senescence in Tubular Epithelial Cells. J Am Soc Nephrol, 2018. 29(4): p. 1238-1256. [CrossRef]
- Tominaga, K. and H.I. Suzuki, TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int J Mol Sci, 2019. 20(20). [CrossRef]
- Genovese, F., et al., The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis & tissue repair, 2014. 7(1): p. 4 %@ 1755-1536. [CrossRef]
- Frantz, C., K.M. Stewart, and V.M. Weaver, The extracellular matrix at a glance. J Cell Sci, 2010. 123(Pt 24): p. 4195-200. [CrossRef]
- Bonnans, C., J. Chou, and Z. Werb, Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol, 2014. 15(12): p. 786-801. [CrossRef]
- Jayadev, R. and D.R. Sherwood, Basement membranes. Curr Biol, 2017. 27(6): p. R207-R211.
- Bulow, R.D. and P. Boor, Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J Histochem Cytochem, 2019. 67(9): p. 643-661. [CrossRef]
- Ariza de Schellenberger, A., et al., The Extracellular Matrix as a Target for Biophysical and Molecular Magnetic Resonance Imaging, in Quantification of Biophysical Parameters in Medical Imaging, I. Sack and T. Schaeffter, Editors. 2018, Springer International Publishing: Cham. p. 123-150.
- Herrera, J., C.A. Henke, and P.B. Bitterman, Extracellular matrix as a driver of progressive fibrosis. J Clin Invest, 2018. 128(1): p. 45-53. [CrossRef]
- Peng, W.J., et al., Matrix metalloproteinases: a review of their structure and role in systemic sclerosis. J Clin Immunol, 2012. 32(6): p. 1409-14. [CrossRef]
- Przemyslaw, L., et al., ADAM and ADAMTS family proteins and their role in the colorectal cancer etiopathogenesis. BMB Rep, 2013. 46(3): p. 139-50. [CrossRef]
- Aan, G.J., et al., Differences in protein changes between stress-induced premature senescence and replicative senescence states. Electrophoresis, 2013. 34(15): p. 2209-17. [CrossRef]
- Khokha, R., A. Murthy, and A. Weiss, Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol, 2013. 13(9): p. 649-65. [CrossRef]
- Hayflick, L. and P.S. Moorhead, The serial cultivation of human diploid cell strains. Experimental cell research, 1961. 25(3): p. 585-621. [CrossRef]
- He, S. and N.E. Sharpless, Senescence in Health and Disease. Cell, 2017. 169(6): p. 1000-1011. [CrossRef]
- Childs, B.G., et al., Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med, 2015. 21(12): p. 1424-35. [CrossRef]
- Docherty, M.H., et al., Cellular Senescence in the Kidney. J Am Soc Nephrol, 2019. 30(5): p. 726-736. [CrossRef]
- Wang, Z.N., et al., Potential Role of Cellular Senescence in Asthma. Front Cell Dev Biol, 2020. 8: p. 59. [CrossRef]
- Kobbe, C.v., Targeting senescent cells: approaches, opportunities, challenges. Aging 2019. 11: p. 18. [CrossRef]
- Munoz-Espin, D. and M. Serrano, Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol, 2014. 15(7): p. 482-96. [CrossRef]
- Marcotte R, W.E., Replicative Senescence Revisited. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2002. 57(7): p. B257-B269. [CrossRef]
- d'Adda di Fagagna, F., et al., A DNA damage checkpoint response in telomere-initiated senescence. Nature, 2003. 426(6963): p. 194-8. [CrossRef]
- Herbig, U., et al., Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell, 2004. 14(4): p. 501-13. [CrossRef]
- Rossiello, F., et al., Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol, 2022. 24(2): p. 135-147. [CrossRef]
- Liu, X.L., J. Ding, and L.H. Meng, Oncogene-induced senescence: a double edged sword in cancer. Acta Pharmacol Sin, 2018. 39(10): p. 1553-1558. [CrossRef]
- Zhu, H., et al., Oncogene-induced senescence: From biology to therapy. Mech Ageing Dev, 2020. 187: p. 111229. [CrossRef]
- Suzuki, M. and D.A. Boothman, Stress-induced premature senescence (SIPS)--influence of SIPS on radiotherapy. J Radiat Res, 2008. 49(2): p. 105-12. [CrossRef]
- Chaib, S., T. Tchkonia, and J.L. Kirkland, Cellular senescence and senolytics: the path to the clinic. Nat Med, 2022. [CrossRef]
- Krizhanovsky, R.S.a.V., Cell Senescence, DNA Damage, and Metabolism. Antioxidants & Redox Signaling, 2021. 34(4): p. 324-334. [CrossRef]
- Huang, W., et al., Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol, 2022. [CrossRef]
- Jackson, S.P. and J. Bartek, The DNA-damage response in human biology and disease. Nature, 2009. 461(7267): p. 1071-8. [CrossRef]
- Kumari, R. and P. Jat, Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol, 2021. 9: p. 645593. [CrossRef]
- Mijit, M., et al., Role of p53 in the Regulation of Cellular Senescence. Biomolecules, 2020. 10(3). [CrossRef]
- Ceccaldi, R., et al., Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell, 2012. 11(1): p. 36-49.
- Ou, H.L. and B. Schumacher, DNA damage responses and p53 in the aging process. Blood, 2018. 131(5): p. 488-495. [CrossRef]
- Rayess, H., M.B. Wang, and E.S. Srivatsan, Cellular senescence and tumor suppressor gene p16. Int J Cancer, 2012. 130(8): p. 1715-25. [CrossRef]
- Sperka, T., J. Wang, and K.L. Rudolph, DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol, 2012. 13(9): p. 579-90. [CrossRef]
- Ruan, B., et al., NVP-BEZ235 inhibits thyroid cancer growth by p53- dependent/independent p21 upregulation. Int J Biol Sci, 2020. 16(4): p. 682-693. [CrossRef]
- Zhang, Y., et al., DNMT3a plays a role in switches between doxorubicin-induced senescence and apoptosis of colorectal cancer cells. Int J Cancer, 2011. 128(3): p. 551-61. [CrossRef]
- Pietenpol, J. and Z. Stewart, Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. toxicology, 2002. 181: p. 475-481. [CrossRef]
- Childs, B.G., et al., Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep, 2014. 15(11): p. 1139-53. [CrossRef]
- D'Arcy, M.S., Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int, 2019. 43(6): p. 582-592. [CrossRef]
- Ngoi, N.Y.L., et al., Targeting Mitochondrial Apoptosis to Overcome Treatment Resistance in Cancer. Cancers (Basel), 2020. 12(3). [CrossRef]
- Van Opdenbosch, N. and M. Lamkanfi, Caspases in Cell Death, Inflammation, and Disease. Immunity, 2019. 50(6): p. 1352-1364.
- Anantram, A. and M. Degani, Targeting cancer's Achilles' heel: role of BCL-2 inhibitors in cellular senescence and apoptosis. Future Med Chem, 2019. 11(17): p. 2287-2312. [CrossRef]
- Shalini, S., et al., Old, new and emerging functions of caspases. Cell Death Differ, 2015. 22(4): p. 526-39. [CrossRef]
- Carneiro, B.A. and W.S. El-Deiry, Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol, 2020. 17(7): p. 395-417. [CrossRef]
- Korolchuk, V.I., et al., Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine, 2017. 21: p. 7-13. [CrossRef]
- Fan, Y., et al., Senescent Cell Depletion Through Targeting BCL-Family Proteins and Mitochondria. Front Physiol, 2020. 11: p. 593630. [CrossRef]
- Guo, Q., et al., Tumor Necrosis Factor-alpha (TNF-α) Enhances miR-155-Mediated Endothelial Senescence by Targeting Sirtuin1 (SIRT1). Med Sci Monit, 2019. 25: p. 8820-8835. [CrossRef]
- Li, P., et al., The inflammatory cytokine TNF-α promotes the premature senescence of rat nucleus pulposus cells via the PI3K/Akt signaling pathway. Scientific Reports, 2017. 7(1): p. 42938. [CrossRef]
- Birch, J. and J. Gil, Senescence and the SASP: many therapeutic avenues. Genes Dev, 2020. 34(23-24): p. 1565-1576. [CrossRef]
- Kuilman, T. and D.S. Peeper, Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer, 2009. 9(2): p. 81-94. [CrossRef]
- Acosta, J.C., et al., Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell, 2008. 133(6): p. 1006-18. [CrossRef]
- Kuilman, T., et al., Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell, 2008. 133(6): p. 1019-31. [CrossRef]
- You, K., et al., Moderate hyperoxia induces senescence in developing human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol, 2019. 317(5): p. L525-l536. [CrossRef]
- Li, Y., et al., Interleukin-6 Knockout Inhibits Senescence of Bone Mesenchymal Stem Cells in High-Fat Diet-Induced Bone Loss. Front Endocrinol (Lausanne), 2020. 11: p. 622950. [CrossRef]
- Effenberger, T., et al., Senescence-associated release of transmembrane proteins involves proteolytic processing by ADAM17 and microvesicle shedding. FASEB J, 2014. 28(11): p. 4847-56. [CrossRef]
- Orjalo, A.V., et al., Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A, 2009. 106(40): p. 17031-6. [CrossRef]
- Acosta, J.C., et al., A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol, 2013. 15(8): p. 978-90. [CrossRef]
- Su, L., et al., Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis, 2021. 12(6): p. 527. [CrossRef]
- Kim, Y.H., et al., Senescent tumor cells lead the collective invasion in thyroid cancer. Nat Commun, 2017. 8: p. 15208. [CrossRef]
- Jun, J.I. and L.F. Lau, CCN2 induces cellular senescence in fibroblasts. J Cell Commun Signal, 2017. 11(1): p. 15-23. [CrossRef]
- Fan, C., et al., TGF-β induces periodontal ligament stem cell senescence through increase of ROS production. Mol Med Rep, 2019. 20(4): p. 3123-3130. [CrossRef]
- Ou, S.C., et al., TGF-β Induced CTGF Expression in Human Lung Epithelial Cells through ERK, ADAM17, RSK1, and C/EBPβ Pathways. Int J Mol Sci, 2020. 21(23). [CrossRef]
- Li, X., et al., DsbA-L mediated renal tubulointerstitial fibrosis in UUO mice. Nat Commun, 2020. 11(1): p. 4467. [CrossRef]
- Zhang, Y., P.B. Alexander, and X.F. Wang, TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb Perspect Biol, 2017. 9(4). [CrossRef]
- Wang, X.-H., et al., GATA4 promotes the senescence of nucleus pulposus cells via NF-κB pathway. Archives of Gerontology and Geriatrics, 2022. 101: p. 104676. [CrossRef]
- Levi, N., et al., The ECM path of senescence in aging: components and modifiers. FEBS J, 2020. 287(13): p. 2636-2646. [CrossRef]
- Hernandez-Gonzalez, F., et al., Cellular Senescence in Lung Fibrosis. Int J Mol Sci, 2021. 22(13). [CrossRef]
- Zhang, M., et al., Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives. Mech Ageing Dev, 2021. 199: p. 111572. [CrossRef]
- Chen, M.S., R.T. Lee, and J.C. Garbern, Senescence mechanisms and targets in the heart. Cardiovasc Res, 2022. 118(5): p. 1173-1187. [CrossRef]
- Kim, K.K., D. Sheppard, and H.A. Chapman, TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb Perspect Biol, 2018. 10(4).
- Yanagihara, T., et al., Connective-Tissue Growth Factor Contributes to TGF-β1-induced Lung Fibrosis. Am J Respir Cell Mol Biol, 2022. 66(3): p. 260-270. [CrossRef]
- Nakerakanti, S.S., A.M. Bujor, and M. Trojanowska, CCN2 is required for the TGF-β induced activation of Smad1-Erk1/2 signaling network. PLoS One, 2011. 6(7): p. e21911.
- Fielding, C.A., et al., Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity, 2014. 40(1): p. 40-50. [CrossRef]
- Epstein Shochet, G., et al., TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res, 2020. 21(1): p. 56. [CrossRef]
- Zhang, S., et al., IL-1β augments TGF-β inducing epithelial-mesenchymal transition of epithelial cells and associates with poor pulmonary function improvement in neutrophilic asthmatics. Respir Res, 2021. 22(1): p. 216. [CrossRef]
- Wilson, M.S., et al., Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med, 2010. 207(3): p. 535-52. [CrossRef]
- Xu, L., D. Sharkey, and L.G. Cantley, Tubular GM-CSF Promotes Late MCP-1/CCR2-Mediated Fibrosis and Inflammation after Ischemia/Reperfusion Injury. J Am Soc Nephrol, 2019. 30(10): p. 1825-1840. [CrossRef]
- Gifford, C.C., et al., PAI-1 induction during kidney injury promotes fibrotic epithelial dysfunction via deregulation of klotho, p53, and TGF-β1-receptor signaling. Faseb j, 2021. 35(7): p. e21725. [CrossRef]
- Vanholder, R., et al., Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int, 2003. 63(5): p. 1934-43. [CrossRef]
- Rocchetti, M.T., et al., Protein-Bound Uremic Toxins and Immunity, in Cytotoxic T-Cells: Methods and Protocols, M. Gigante and E. Ranieri, Editors. 2021, Springer US: New York, NY. p. 215-227. [CrossRef]
- Fujii, H., S. Goto, and M. Fukagawa, Role of Uremic Toxins for Kidney, Cardiovascular, and Bone Dysfunction. Toxins (Basel), 2018. 10(5). [CrossRef]
- Vanholder, R., et al., Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins (Basel), 2018. 10(1). [CrossRef]
- Chen, J.H. and C.K. Chiang, Uremic Toxins and Protein-Bound Therapeutics in AKI and CKD: Up-to-Date Evidence. Toxins (Basel), 2021. 14(1). [CrossRef]
- Chmielewski, M., et al., The peptidic middle molecules: is molecular weight doing the trick? Semin Nephrol, 2014. 34(2): p. 118-34. [CrossRef]
- Smith, H.W., The kidney: structure and function in health and disease. 1951: Oxford University Press, USA.
- Chao, C.T. and C.K. Chiang, Uremic toxins, oxidative stress, and renal fibrosis: an interwined complex. J Ren Nutr, 2015. 25(2): p. 155-9. [CrossRef]
- Mihajlovic, M., et al., Protein-Bound Uremic Toxins Induce Reactive Oxygen Species-Dependent and Inflammasome-Mediated IL-1beta Production in Kidney Proximal Tubule Cells. Biomedicines, 2021. 9(10). [CrossRef]
- Sun, B., et al., Hippuric Acid Promotes Renal Fibrosis by Disrupting Redox Homeostasis via Facilitation of NRF2-KEAP1-CUL3 Interactions in Chronic Kidney Disease. Antioxidants (Basel), 2020. 9(9). [CrossRef]
- Zhang, H., et al., Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol Lett, 2018. 284: p. 29-36. [CrossRef]
- Sun, C.Y., et al., Protein-bound uremic toxins induce tissue remodeling by targeting the EGF receptor. J Am Soc Nephrol, 2015. 26(2): p. 281-90. [CrossRef]
- Schafer, M.J., et al., Targeting Senescent Cells in Fibrosis: Pathology, Paradox, and Practical Considerations. Curr Rheumatol Rep, 2018. 20(1): p. 3. [CrossRef]
- Niwa, T. and H. Shimizu, Indoxyl sulfate induces nephrovascular senescence. J Ren Nutr, 2012. 22(1): p. 102-6. [CrossRef]
- Fletcher-Sananikone, E., et al., Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment Attenuates Glioblastoma Recurrence. Cancer Res, 2021. 81(23): p. 5935-5947. [CrossRef]
- Hu, X. and H. Zhang, Doxorubicin-Induced Cancer Cell Senescence Shows a Time Delay Effect and Is Inhibited by Epithelial-Mesenchymal Transition (EMT). Med Sci Monit, 2019. 25: p. 3617-3623. [CrossRef]
- Yang, Y., et al., A Human Conditionally Immortalized Proximal Tubule Epithelial Cell Line as a Novel Model for Studying Senescence and Response to Senolytics. Front Pharmacol, 2022. 13: p. 791612. [CrossRef]
- Chang, J.F., et al., Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease. Toxins (Basel), 2020. 12(6). [CrossRef]
- Tolle, M., et al., Uremic mouse model to study vascular calcification and "inflamm-aging". J Mol Med (Berl), 2022. 100(9): p. 1321-1330. [CrossRef]
- Li, X., et al., Effects of Uremic Clearance Granules on p38 MAPK/NF-κB Signaling Pathway, Microbial and Metabolic Profiles in End-Stage Renal Disease Rats Receiving Peritoneal Dialysis. Drug Des Devel Ther, 2022. 16: p. 2529-2544. [CrossRef]
- Wang, X., et al., Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut, 2020. 69(12): p. 2131-2142. [CrossRef]
- Huang, Y., et al., Indoxyl sulfate induces intestinal barrier injury through IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics, 2020. 10(16): p. 7384-7400. [CrossRef]
- Nakano, T., et al., Indoxyl Sulfate Contributes to mTORC1-Induced Renal Fibrosis via The OAT/NADPH Oxidase/ROS Pathway. Toxins (Basel), 2021. 13(12). [CrossRef]
- Chen, C., et al., Yishen-Qingli-Huoxue formula attenuates renal fibrosis by inhibiting indoxyl sulfate via AhR/snai1 signaling. Phytomedicine, 2023. 108: p. 154546. [CrossRef]
- Hsieh, Y.H., et al., Rosmarinic acid ameliorates renal interstitial fibrosis by inhibiting the phosphorylated-AKT mediated epithelial-mesenchymal transition in vitro and in vivo. Food Funct, 2022. 13(8): p. 4641-4652. [CrossRef]
- Cai, H., et al., Lindera aggregata intervents adenine-induced chronic kidney disease by mediating metabolism and TGF-β/Smad signaling pathway. Biomed Pharmacother, 2021. 134: p. 111098. [CrossRef]
- Kim, H., et al., Lactobacillus acidophilus KBL409 Reduces Kidney Fibrosis via Immune Modulatory Effects in Mice with Chronic Kidney Disease. Molecular Nutrition & Food Research, 2022. 66(22): p. 2101105. [CrossRef]
- Barba, C., et al., A low aromatic amino-acid diet improves renal function and prevent kidney fibrosis in mice with chronic kidney disease. Sci Rep, 2021. 11(1): p. 19184. [CrossRef]
- Sun, C.Y., et al., p-Cresol Sulfate Caused Behavior Disorders and Neurodegeneration in Mice with Unilateral Nephrectomy Involving Oxidative Stress and Neuroinflammation. Int J Mol Sci, 2020. 21(18). [CrossRef]
- Miwa, S., et al., Mitochondrial dysfunction in cell senescence and aging. J Clin Invest, 2022. 132(13). [CrossRef]
- Zhao, M., et al., Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics, 2021. 11(4): p. 1845-1863. [CrossRef]
- Rossi, M., et al., Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3-4 chronic kidney disease. Arch Med Res, 2014. 45(4): p. 309-17. [CrossRef]
- Mihajlovic, M., et al., Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions. Int J Mol Sci, 2017. 18(12). [CrossRef]
- Correia-Melo, C., et al., Mitochondria are required for pro-ageing features of the senescent phenotype. Embo j, 2016. 35(7): p. 724-42. [CrossRef]
- Dibble, C.C. and L.C. Cantley, Regulation of mTORC1 by PI3K signaling. Trends Cell Biol, 2015. 25(9): p. 545-55. [CrossRef]
- Watanabe, H., et al., p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int, 2013. 83(4): p. 582-92. [CrossRef]
- Deng, M., et al., Short-Chain Fatty Acids Alleviate Hepatocyte Apoptosis Induced by Gut-Derived Protein-Bound Uremic Toxins. Front Nutr, 2021. 8: p. 756730. [CrossRef]
- Sun, C.Y., H.H. Hsu, and M.S. Wu, p-Cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol Dial Transplant, 2013. 28(1): p. 70-8. [CrossRef]
- Branzei, D. and M. Foiani, Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol, 2008. 9(4): p. 297-308. [CrossRef]
- Yang, L., et al., Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med, 2010. 16(5): p. 535-43, 1p following 143. [CrossRef]
- Passos, J.F., et al., Feedback between p21 and reactive oxygen production is necessary for cell senescence. Molecular Systems Biology, 2010. 6(1): p. 347. [CrossRef]
- Yang, Y., et al., The Uremic Toxin Indoxyl Sulfate Accelerates Senescence in Kidney Proximal Tubule Cells. Toxins, 2023. 15(4): p. 242. [CrossRef]
- Li, L., et al., Protein-bound P-cresol inhibits human umbilical vein endothelial cell proliferation by inducing cell cycle arrest at G(0)/G(1). Am J Transl Res, 2017. 9(4): p. 2013-2023.
- Shimizu, H., et al., Indoxyl sulfate enhances p53-TGF-β1-Smad3 pathway in proximal tubular cells. Am J Nephrol, 2013. 37(2): p. 97-103. [CrossRef]
- Humphreys, B.D., Mechanisms of Renal Fibrosis. Annu Rev Physiol, 2018. 80: p. 309-326. [CrossRef]
- Liu, Y., et al., The NLRP3 inflammasome in fibrosis and aging: The known unknowns. Ageing Research Reviews, 2022. 79: p. 101638. [CrossRef]
- Romero, A., et al., Pharmacological Blockade of NLRP3 Inflammasome/IL-1β-Positive Loop Mitigates Endothelial Cell Senescence and Dysfunction. Aging Dis, 2022. 13(1): p. 284-297. [CrossRef]
- Yan, M.T., C.T. Chao, and S.H. Lin, Chronic Kidney Disease: Strategies to Retard Progression. Int J Mol Sci, 2021. 22(18). [CrossRef]
- Latorre, E., et al., Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. BMC Cell Biology, 2017. 18: p. 1-15. [CrossRef]
- Herranz, N., et al., mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol, 2015. 17(9): p. 1205-17. [CrossRef]
- Knoppert, S.N., et al., Cellular Senescence and the Kidney: Potential Therapeutic Targets and Tools. Front Pharmacol, 2019. 10: p. 770. [CrossRef]
- June, C.H., et al., CAR T cell immunotherapy for human cancer. Science, 2018. 359(6382): p. 1361-1365. [CrossRef]
- Dunbar, C.E., et al., Gene therapy comes of age. Science, 2018. 359(6372): p. eaan4672. [CrossRef]
| Compartment | ECM in healthy kidney | Increased ECM in kidney fibrosis |
|---|---|---|
| Glomeruli | Mesangial Matrix: collagen IV, V, fibronectin, nidogen, laminin. | Nodular mesangial sclerosis: collagen I, III, IV, V, fibronectin, nidogen, laminin, decorin, biglycan. |
| Glomerular basement membrane: collagen I, III, VI, IV, VII, XV, XVII, agrin, perlecan, nidogen, laminin. | Focal segmental glomerulosclerosis: collagen III, IV, heparan sulfate proteoglycans. | |
| Bowman's capsule: collagen IV, laminins, nidogen, heparan sulfate proteoglycans. | Thickening of glomerular basement membrane: collagen I, III, VI, IV, VII, XV, XVII, perlecan, nidogen, laminin. | |
| Bowman's capsule: collagen IV and heparan sulfate proteoglycans. | ||
| Tubulointerstitium | Tubular basement membrane: collagen IV, agrin, perlecan, laminin. |
Thickening of tubular basement membrane: collagen IV, perlecan; |
| Interstitium: collagen I, II, III, V, VI, VII, XV, fibronectin, biglycan, decorin, versican. | Interstitial fibrosis: collagen I, II, III, V, VI, VII, XV, fibronectin, biglycan, decorin, ersican. | |
| Capillary basement membrane: N/A. | Thickening and multilayering of capillary basement membrane: N/A. |
|
| Vasculature | Intima with internal elastic lamina: elastin, perlecan, agrin, collagen XVIII, versican, biglycan, decorin. | Neointima: versican, collagen XVIII, agrin, perlecan. |
| Media with external elastic lamina: collagen I, III, XVII, elastin, agrin, perlecan, decorin, verslcan. | Intima with internal elastic lamina: elastin, perlecan, agrin, collagen XVIII, versican. | |
| Adventitia: collagen I, III, fibronectin, elastin. | Media with external elastic lamina: elastin, collagen XVII, agrin, perlecan,versican. | |
| Perivascular fibrosis (Thickening of adventitia): N/A. |
| CKD model | Species | PBUTs | Fibrosis/EMT markers | Senescence markers/ SASP factors | Invloved pathways/mechanism | Reference |
|---|---|---|---|---|---|---|
| Aristolochic acids-induced | Mouse | PCS, IS | α-SMA, collagen I, α-1 and IV | NR | TGF-β signaling | [116] |
| Adenine-induced | Mouse | NR | Collagen (masson staining) | p21, Il-6, and Il-1β | chronic inflammation | [117] |
| 5/6 nephrectomy | Rat | NR | Collagen (masson staining) | TNF-α, IL1β, and IL-6 | p38 MAPK/NF-κB signaling pathway | [118] |
| Ischemia-reperfusion injury | Mouse | NR | Collagen (masson staining), fibronectin and α-SMA | SA–β-gal, p16, p19, p53, p21, MMP-7, PAI-1 and TGF-β1 | WNT and TGF-β signaling | [18] |
| Adenine-induced | Mouse | PCS, IS, and hippuric acid | Collagen (masson staining) and α-SMA | NR | gut microbiota | [119] |
| IS-injected mouse and unilateral nephrectomy | Mouse | IS | ZO-1, occludin,claudin-1, and claudin-2 | TNF-α, IL-1β and IL-6 | mitochondrial dysfunction and mitophagy impairment | [120] |
| Adenine-induced | Mouse | IS | α-SMA, E-cadherin, and collagen I | TNF-α and IL-6 | mTOR activation | [121] |
| Adenine-induced | Rat | IS | fibronectin, collagen I, α-SMA, vimentin, and E-cadherin | NR | EMT | [122] |
| Unilateral ureteral obstruction (UUO) | Mouse | IS | Collagen (masson staining), α-SMA, collagen I, fibronectin, vimentin, and E-cadherin | TGF-β1 | EMT | [123] |
| Adenine-induced | Rat | PCS, IS, hippuric acid,p-cresyl glucuronide, and indol-3-acetic acid | NR | TGF-β1 | TGF-β signaling | [124] |
| Adenine-induced | Mouse | PCS | NR | TNF-α and IL-6 | NLRP3 inflammasome pathway | [125] |
| Adenine-induced | Mouse | PCS, IS and p-cresyl glucuronide | collagen α-1 type 1 | TGF-β1, TNF-α, MCP-1 and IL-6 | production of uremic toxins, and inflammation | [126] |
| Unilateral nephrectomy | Mouse | PCS | NR | p38 and IL-1β | Oxidative stress and inflammation | [127] |
| 5/6 nephrectomy | Rat | Hippuric acid | α-SMA, vimentin, and collagen I | MMP9 and TIMP1 | Oxidative stress and TGF-β signaling | [108] |
| NR – Not Reported. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





