Running title: Prognostic value of ePWV after PCI
Introduction
Coronary Artery Disease (CAD) remains a leading cause of mortality worldwide, despite significant advances in medical science [
1]. Characterized by the blockage of the coronary arteries, CAD can lead to debilitating health complications such as myocardial infarction and sudden cardiac death [
2]. Modern treatments have enhanced short-term survival rates, but there is room for improvement in the long-term prognosis, particularly given the chronic and progressive characteristics of the disease [
1]. This situation underscores the essential need for an effective prognostic tool for CAD. Such a tool would provide a more precise risk assessment, enabling timely interventions, personalized treatment strategies, and, ultimately, better patient outcomes.
Arterial stiffening refers to a reduction in the elasticity of arteries, typically caused by aging and unmanaged risk factors such as hypertension, hyperglycemia, dyslipidemia, smoking, inflammation, and oxidative stress [
3,
4]. Increased arterial stiffness augments cardiovascular load and adversely affects cardiac function, making it a key factor in cardiovascular disease progression, including CAD [
5,
6,
7]. A growing body of evidence suggests that arterial stiffness, measured through methods like pulse wave velocity (PWV), can serve as a significant predictive marker for CAD prognosis [
6,
8,
9,
10]. Understanding and monitoring arterial stiffness could thus become an integral part of CAD prognosis prediction.
Pulse Wave Velocity (PWV) is a clinical indicator of arterial stiffness, reflecting the rate at which pressure waves traverse the arteries [
4]. Traditionally, PWV is measured directly, but an estimation can be made using age and blood pressure (BP), main factors known to influence arterial elasticity [
11,
12,
13,
14]. In this study, we utilized this “estimated PWV (ePWV)” as a prognostic tool for patients who had undergone PCI. The aim was to investigate whether ePWV, derived from simple, readily available clinical parameters, could provide a reliable prediction of long-term outcomes post-PCI. This could potentially enhance personalized care and improve health outcomes for CAD patients.
Materials and Methods
Study design and population
This study is a retrospective analysis conducted at the cardiovascular center of Boramae Medical Center, a general hospital situated in Seoul, South Korea. Between August 2008 and June 2020, we examined a total of 4,270 consecutive patients who visited the center and underwent successful drug-eluting stent (DES) implantation for conditions such as angina pectoris or myocardial infarction. Following the exclusion of in-hospital mortality cases (n = 151), a total of 4,119 patients were included in the final analysis. The study protocol was approved by the Institutional Review Board (IRB) of Boramae Medical Center. Due to the retrospective nature of the study, the IRB waived the requirement for obtaining informed consent.
Clinical data collection
Body mass index (BMI) was calculated by dividing an individual’s weight in kilograms by the square of their height in meters. Hypertension was identified if any of the following criteria were met: a prior diagnosis of hypertension by a physician, ongoing anti-hypertensive medication use, or consistent systolic/diastolic BP readings above 140/90 mmHg. Diabetes mellitus was similarly identified based on any of the following: a prior diagnosis by a physician, current use of anti-diabetic medication, repeated fasting blood glucose levels of ≥ 126 mg/dL, or at least one instance of glycated hemoglobin level of ≥ 6.5%. Current smoking status was determined by regular and active smoking within the preceding year. Acute myocardial infarction was diagnosed based on chest pain, electrocardiographic alterations, troponin elevation, and angiographic findings. Previous CAD was determined by a history of myocardial infarction or coronary revascularization. After overnight fasting, venous blood samples were drawn from the antecubital vein to measure parameters such as hemoglobin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, creatinine, glycated hemoglobin, uric acid, and C-reactive protein. Glomerular filtration rate (GFR) was estimated using the equation from the Modification of Diet in Renal Disease study. Left ventricular ejection fraction was acquired through transthoracic echocardiography using the biplane method. Additionally, data regarding the use of cardiovascular medications at discharge, including beta-blockers, renin-angiotensin system blockers, and statins, were collected.
Invasive coronary angiography and PCI procedure
Invasive coronary angiography (ICA) was conducted in line with standard guideline recommendations [
15]. A luminal stenosis exceeding 50% in the epicardial coronary artery was considered an obstructive lesion. The terms one-vessel disease, two-vessel disease, and three-vessel disease were used to describe the condition based on the number of epicardial coronary arteries exhibiting stenotic lesions beyond 50%. A 50% or more stenosis in the left main coronary artery was classified as a two-vessel disease. The decision to PCI was based on the patient’s symptoms, stress test outcomes, and ICA results, at the discretion of the supervising cardiologist. The PCI procedure was executed following the current international guidelines [
15].
ePWV calculation
BP was measured using an automatic sphygmomanometer on the right upper arm while the patient was in a stable sitting position during their hospital stay. The average systolic BP (SBP) and diastolic BP (DBP) were calculated by taking the mean of three measurements obtained just prior to the patient’s stable discharge. Mean BP (MBP) was determined using the formula: MBP = DBP + [0.4 × (SBP-DBP)]. ePWV was computed using the patient’s age and MBP according to the following equation: ePWV (m/s) = 9.587 - (0.402 × age) + (4.560 × 0.001 × age
2) - (2.621 × 0.00001 × age
2 × MBP) + 3.176 × 0.001 × age × MBP) - (1.832 × 0.01 × MBP) [
11].
Clinical outcome
Details regarding major adverse cardiovascular events (MACE) were evaluated during the clinical follow-up after PCI. MACE encompassed cardiac death, non-fatal myocardial infarction, coronary revascularization, and ischemic stroke. Cardiac death was characterized as death resulting from acute coronary syndrome, fatal ventricular arrhythmia, or heart failure. Unexplained sudden death was likewise classified under cardiac death. A myocardial infarction was recognized based on symptoms such as chest pain, elevated troponin levels, electrocardiographic changes, and the presence of obstructive coronary lesions as detected by coronary angiography. Coronary revascularization included procedures like PCI and coronary bypass surgery. Ischemic stroke was confirmed based on the sudden onset of neurological symptoms, verified by detecting areas of infarction in brain imaging studies. If multiple events were experienced simultaneously, the first event was categorized as the MACE. Events occurring within 30 days from the date of the PCI procedure were attributed to the underlying condition necessitating the intervention, and thus were not classified as MACE.
Statistical analysis
Continuous variables are shown as mean ± standard deviation, while categorical variables are expressed as numbers (percentages). The Student t-test and chi-square test were used to compare continuous and categorical variables between patients with and without MACE, respectively. For grouping, median value and tertiles of ePWV were utilized. MACE incidence in relation to ePWV tertiles was assessed using the chi-square test for linear association. The receiver operating characteristic (ROC) curve analysis determined the optimal ePWV cut-off for MACE prediction. Multivariable Cox regression analyses established the independent relationship between ePWV and MACE, adjusting for various clinical covariates such as age, sex, body mass index, hypertension, diabetes mellitus, prior history of CAD, acute myocardial infarction diagnosis, CAD severity, smoking, glomerular filtration rate, left ventricular ejection fraction, and usage of beta-blockers and renin-angiotensin system blockers. The Kaplan-Meier survival curve analysis was applied to demonstrate the prognostic significance of ePWV, and the log-rank test was used for statistical significance. The incremental predictive value of ePWV over clinical factors (age, sex, body mass index, hypertension, diabetes mellitus, previous CAD, diagnosis of acute myocardial infarction, cigarette smoking, glomerular filtration rate, and the use of beta-blockers, and renin-angiotensin system blockers) for MACE prediction was evaluated by assessing changes in global chi-squares. All analyses were two-tailed, with a P-value less than 0.05, denoting statistical significance. All statistical computations were performed using SPSS version 23.0 (IBM Co., Armonk, NY, USA).
Results
Baseline clinical characteristics in patients with and without MACE
Throughout a median follow-up period of 3.51 years (interquartile range, 1.35 ~ 6.37 years), a total of 746 cases of MACE (18.1%) were reported. The MACE included 100 cases of cardiac death (2.4%), 102 non-fatal myocardial infarctions (2.4%), 530 instances of coronary revascularization (12.8%), and 114 occurrences of ischemic stroke (2.7%).
Table 1 presents the comparative analysis of baseline clinical characteristics between patients who experienced MACE and those who did not. Patients who experienced MACE were generally older (69.1 ± 11.4
vs. 66.5 ± 11.5 years;
P < 0.001). Both groups had similar gender proportions (female: 32.9%
vs. 33.2%) and comparable body mass indices (
P > 0.05 for each). Compared to patients who did not experience MACE, those who did had a more significant number of cardiovascular risk factors, including hypertension, diabetes mellitus, and prior CAD. Laboratory findings revealed lower levels of hemoglobin and high-density lipoprotein cholesterol, and higher levels of low-density lipoprotein cholesterol, glomerular filtration rate, glycated hemoglobin, uric acid, and C-reactive protein in the MACE group. The MACE group also had a lower left ventricular ejection fraction (57.9% ± 14.8%
vs. 60.3%
± 12.4%;
P < 0.001), and more severe CAD, evidenced by a higher prevalence of three-vessel disease (55.1%
vs. 37.3%,
P < 0.001). No significant differences were observed in discharge medications between the two groups.
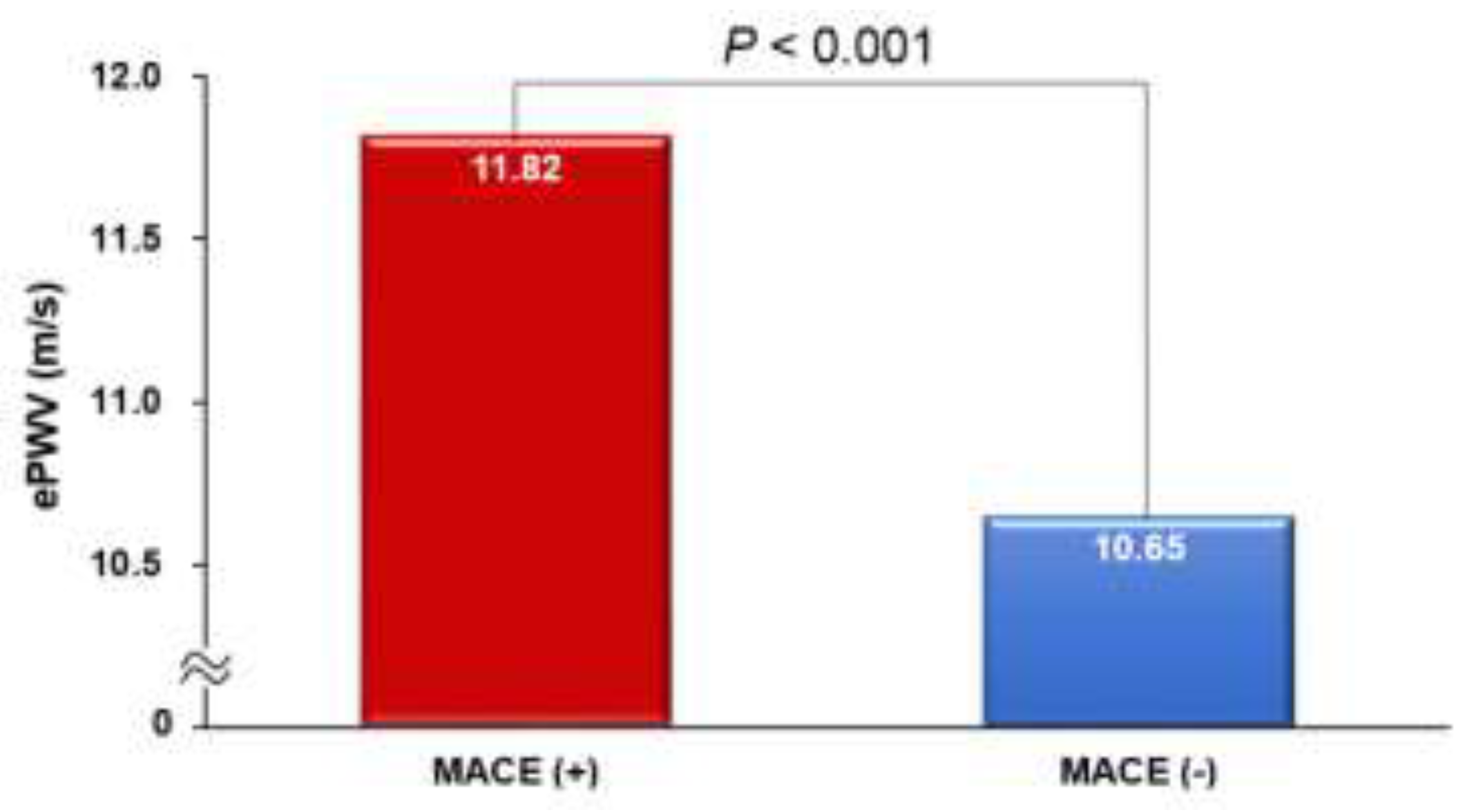
The ePWV was notably higher in patients who experienced MACE compared to those who did not (11.82 ± 2.13 vs. 10.65 ± 2.13 m/s;
P < 0.001) (
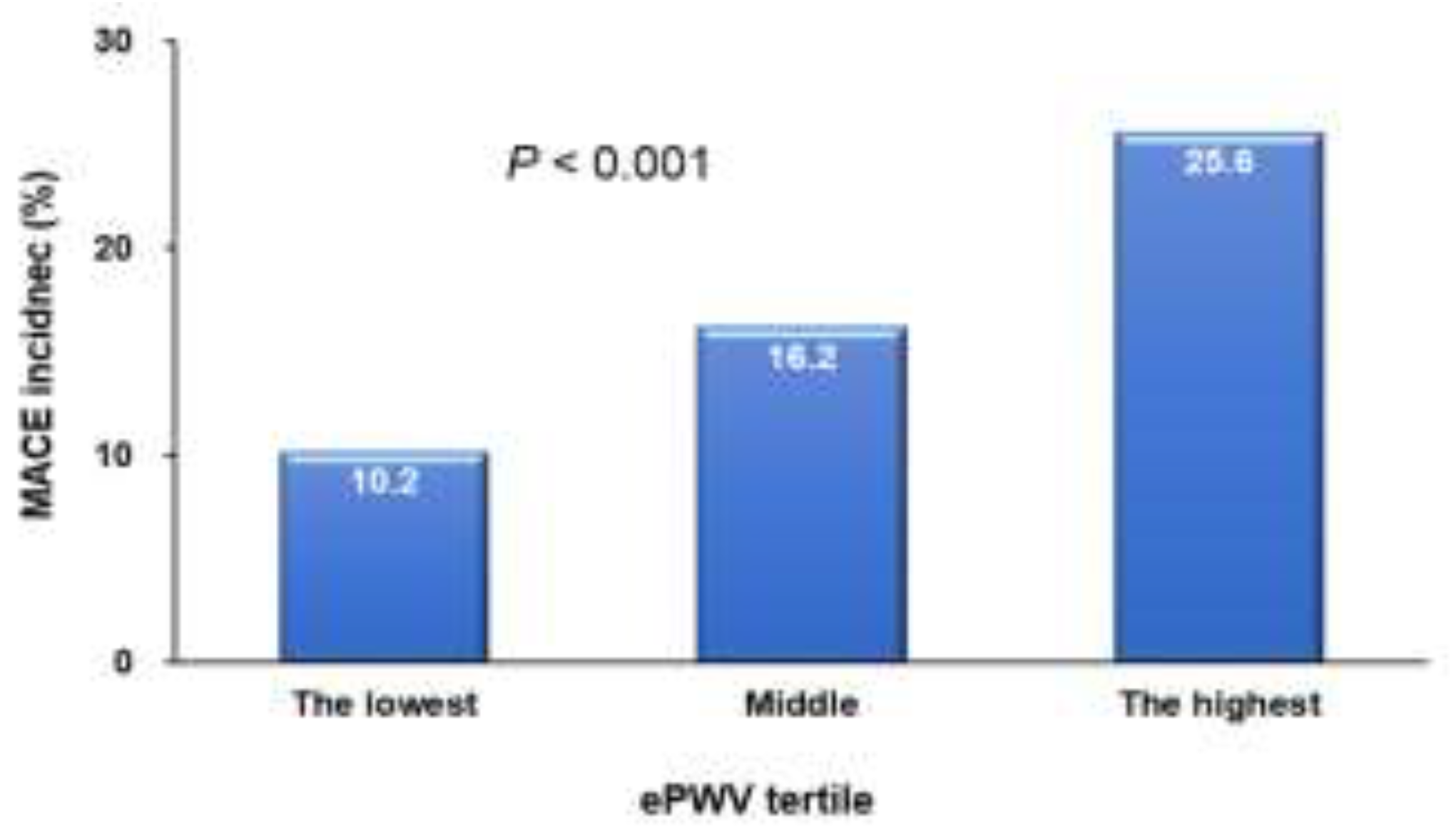
Figure 1). There was a proportional increase in the incidence of MACE from the lowest to the highest tertile of ePWV (
P < 0.001) (
Figure 2).
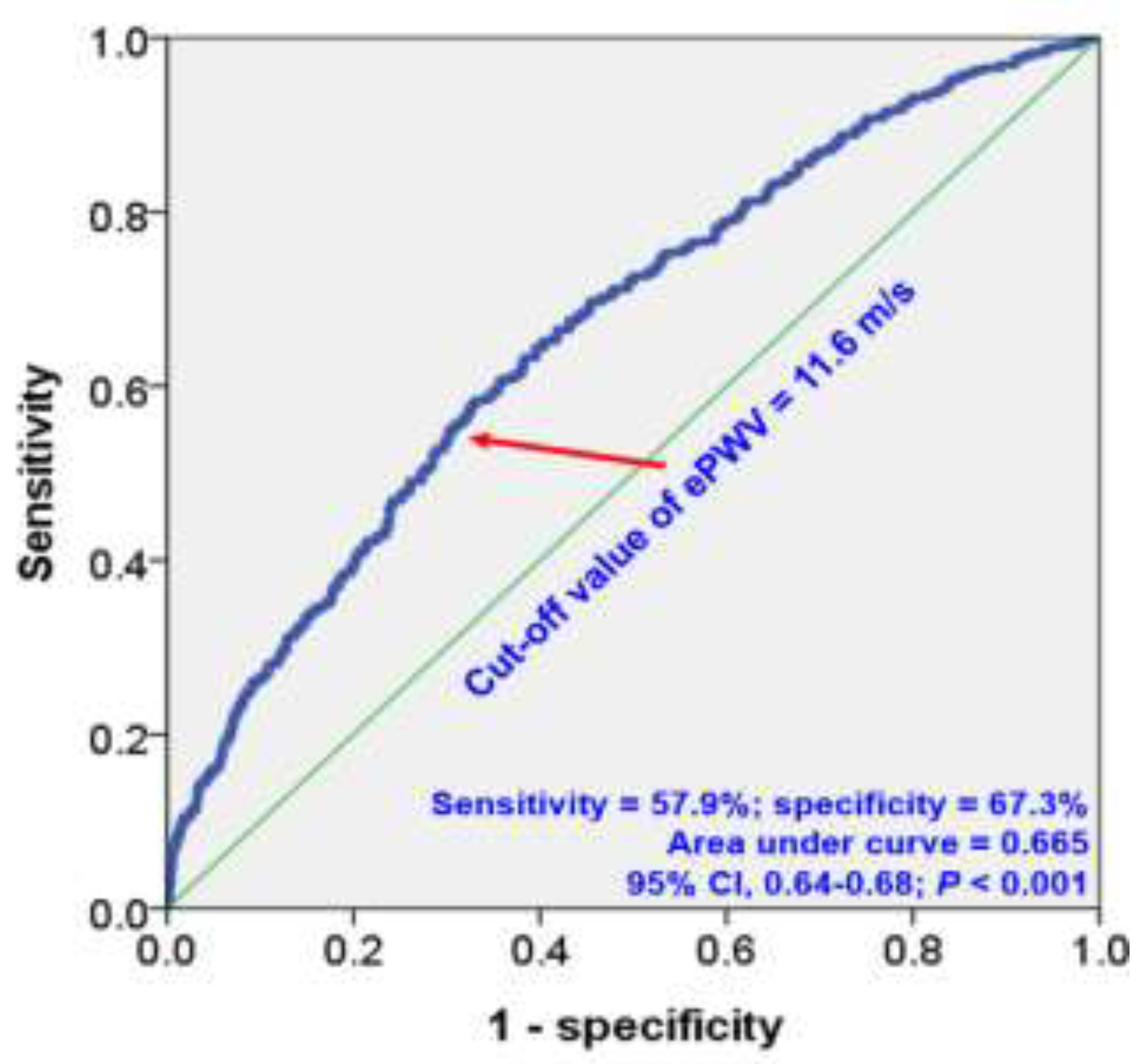
The ROC curve analysis identified an ePWV cut-off value of 11.6 m/s for predicting MACE, yielding a sensitivity of 57.6% and a specificity of 67.3%. (area under curve, 0.665; 95% confidence interval, 0.64-0.68;
P < 0.001) (
Figure 3).
Multivariable analyses established that a higher ePWV served as a significant predictor for MACE (
Table 2). When ePWV was divided into different thresholds – the median value (ePWV ≥ 10.9 m/s: hazard ratio [HR], 3.78; 95% confidence interval [CI]; 2.83-4.91;
P < 0.001), the cut-off value from ROC curve analysis (ePWV ≥ 11.6 m/s: HR, 3.56; 95% CI, 2.77-4.58;
P < 0.001), and tertile criteria (middle tertile vs. the lowest tertile: HR, 2.49; 95% CI, 1.81-3.42;
P < 0.001; the highest tertile
vs. the lowest tertile: HR, 6.18; 95% CI, 4.33-8.80;
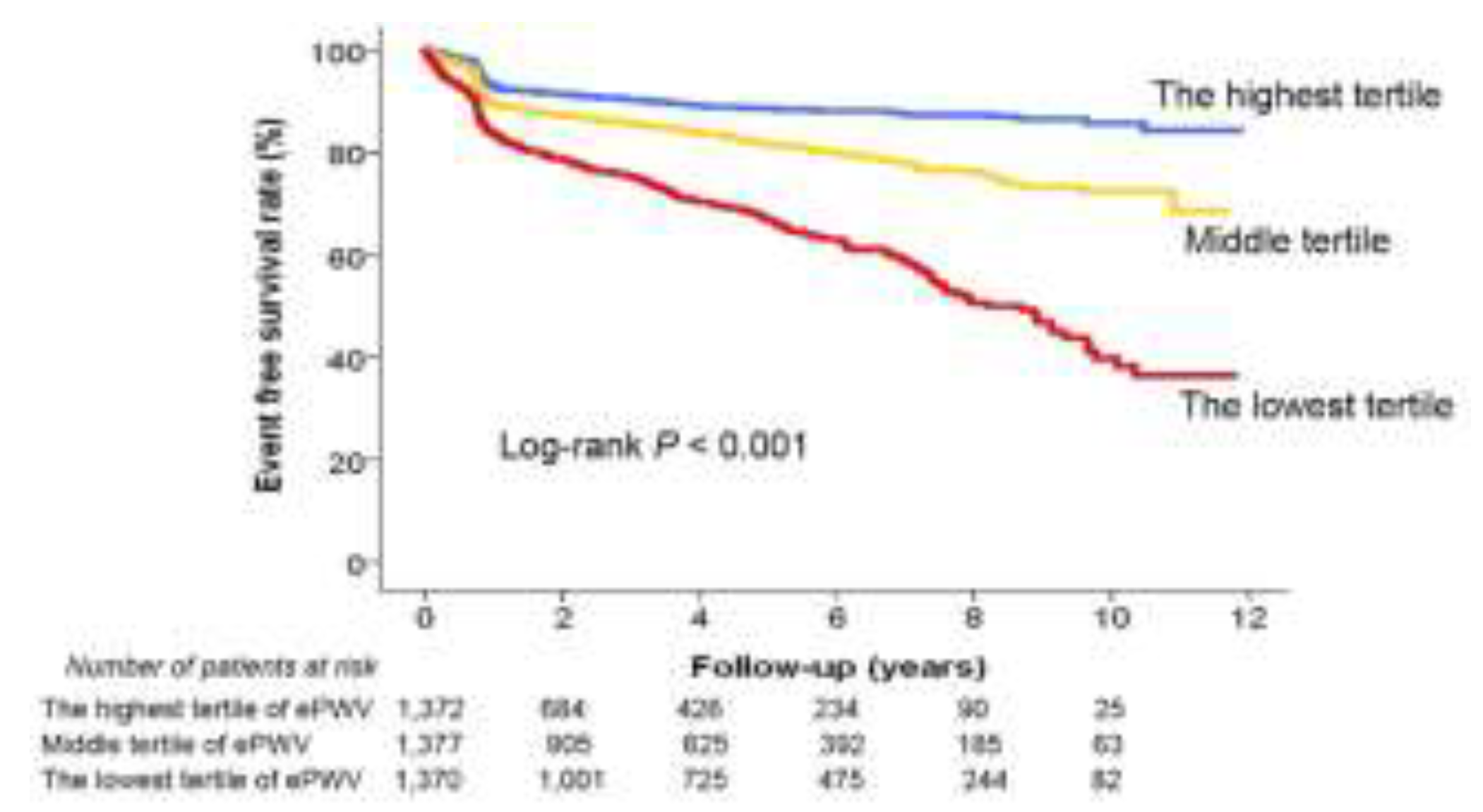
P < 0.001) – a high ePWV under all conditions independently predicted MACE. Kaplan-Meier survival curve analysis further highlighted significant differences in patients’ MACE risk across ePWV tertiles (log-rank
P < 0.001) (
Figure 4).
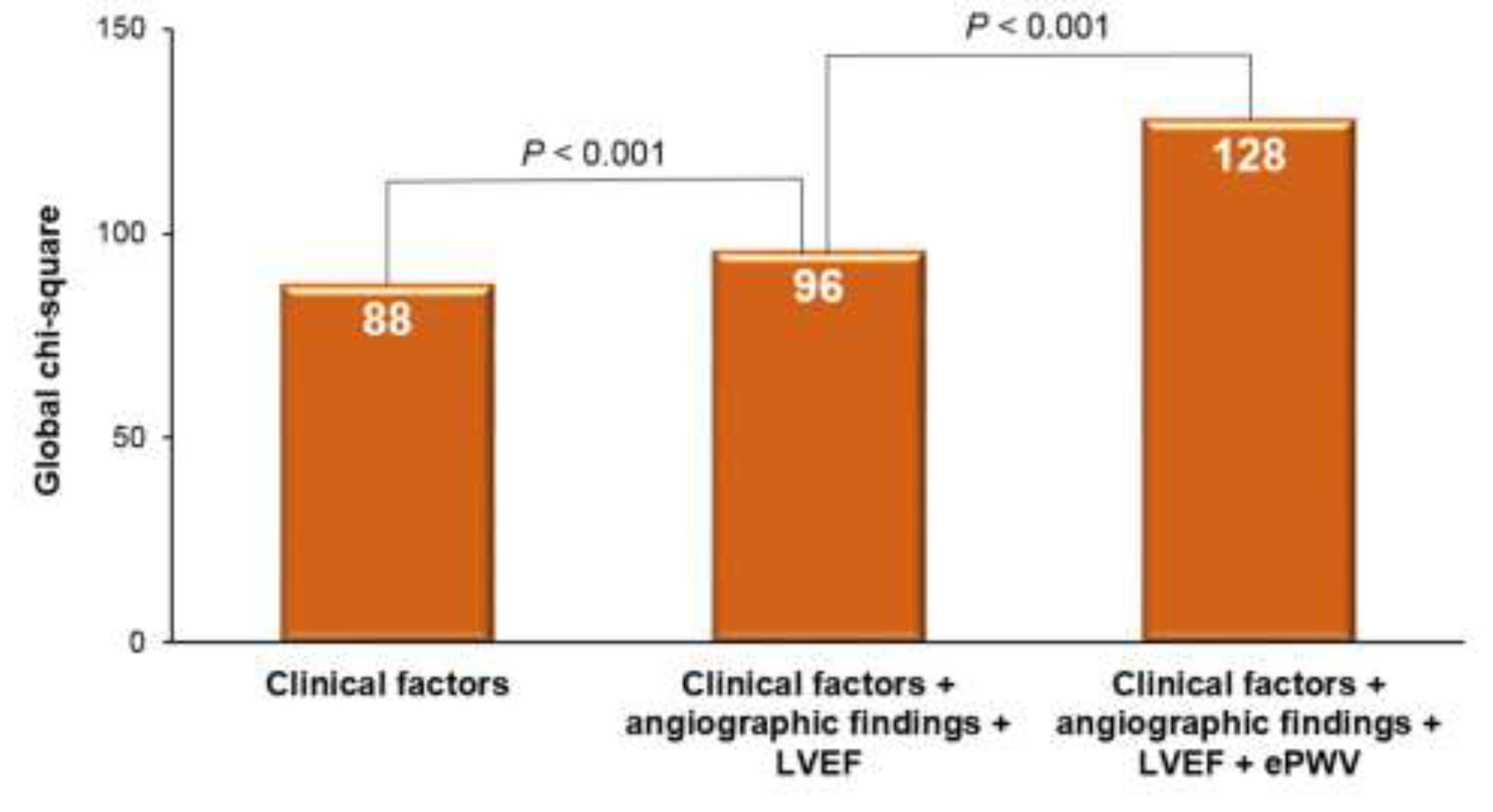
The predictive power for MACE increased significantly when angiographic findings and left ventricular ejection fraction were added to other clinical factors, as evidenced by a rise in the global chi-square from 88 to 96 (P < 0.001). Further inclusion of ePWV information to the clinical factors, angiographic findings, and left ventricular ejection fraction resulted in an even greater enhancement of MACE prediction, elevating the global chi-square from 96 to 128 (P < 0.001). These results are illustrated in Figure 5.
Discussion
The principal discovery of this study is the association between ePWV and MACE in patients who received DES implantation. As far as we know, this study is the first to confirm the prognostic significance of ePWV in patients undergoing PCI.
Prior studies have published findings regarding the prognostic value of ePWV. In the general population, an elevated baseline ePWV has been linked to an increased likelihood of subsequent cardiovascular events or mortality [
16,
17,
18]. Additionally, the prognostic value of ePWV in patients at moderate or high risk has been revealed by some research. For example, one study involving 1,040 individuals undergoing ICA found a significant association between ePWV and clinical outcomes, even after adjusting for potential confounders [
8]. In the Systolic Blood Pressure Intervention Trial, which included 9,361 participants with high risk profiles, an elevated ePWV was associated with cardiovascular risk independently of Framingham Risk scores [
12]. Further studies have underscored the prognostic value of ePWV in stroke patients [
13] and those with diabetes mellitus [
19]. Yet, the significance of ePWV for patients undergoing PCI has not been firmly established. Given the high mortality rate associated with CAD, our study, which demonstrates the prognostic value of ePWV in CAD patients, is both novel and of great importance.
The mechanistic link between elevated arterial stiffness and a patient's cardiovascular risk is well established [
3,
4]. Greater arterial stiffness, indicated by an elevated PWV, is associated with an increased likelihood of cardiovascular incidents and mortality across diverse patient groups. This is because arterial stiffness can cause an upsurge in systolic BP, augment cardiac afterload, leading to left ventricular hypertrophy, and inhibit coronary perfusion, all of which can precipitate cardiovascular events. Moreover, escalated arterial stiffness shares cardiovascular risk factors such as hyperglycemia, dyslipidemia, inflammation, and oxidative stress.[
4] As such, assessing arterial stiffness offers crucial insights into a patient’s vascular health and risk for future adverse occurrences [
5].
The findings from this study have potential implications for patient care. Incorporating ePWV assessments into standard post-PCI evaluations could assist in early risk identification, expedite therapeutic intervention, and enhance the management of patients at high risk for MACE. The evidence pointing towards substantial prognostic improvement by adding ePWV data to other clinical parameters, as revealed by this study, underscores the promising potential of this approach. Additionally, managing arterial stiffness could emerge as a vital therapeutic target. Lifestyle modifications, management of BP, and specific medication may decrease arterial stiffness and lower cardiovascular risk. Moreover, regular monitoring of arterial stiffness can provide valuable insights into these interventions’ effectiveness, thereby helping optimize patient care. ePWV can be computed only if BP values are available, which could further enhance its economic utility. The practicality of routine ePWV measurements in various clinical settings will require further exploration in future research.
Despite ePWV being derived from Western data [
11], as demonstrated in our study and several others, it appears to be highly relevant for Asian and Korean populations as well [
17,
18]. Through our research, we anticipate that ePWV could be applied to other patient groups or different ethnic populations.
Study limitations
Our study has several limitations. Firstly, being a retrospective study, the incidence of MACE may have been underestimated due to potential unrecognized occurrences. For similar reasons, we may not have accounted for all major confounding variables. Secondly, we used BP measurements taken when the patient was stable at the time of discharge. Given that BP is a primary determinant of ePWV, accurate measurement methods are vital. Our study, however, being retrospective in nature, did not offer stringent guidelines for BP measurement. We attempted to mitigate this limitation by taking an average of three readings. Lastly, as our study population consisted of Koreans who underwent DES implantation, directly extrapolating the results to other populations might be challenging.
Study conclusions
ePWV exhibited a significant association with MACE in patients who underwent DES implantation. Given its ease of calculation, ePWV could potentially serve as a valuable tool for stratifying cardiovascular risk within this high-risk patient population.
Author Contributions
Conceptualization, H.L.K.; Data acquisition and curation, H.S.J., W.H.L., J.B.S., S.H.K., J.H.Z. and M.A.K.; Formal analysis, H.L.K.; Resources, M.A.K.; Supervision, M.A.K.; Writing-original draft, H.L.K.; and Writing-review and editing, H.S.J., W.H.L., J.B.S., S.H.K., J.H.Z. and M.A.K.. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, B.H. Aging and arterial stiffness. Circ J 2010, 74, 2257–2262. [Google Scholar] [CrossRef]
- Kim, H.L.; Kim, S.H. Pulse Wave Velocity in Atherosclerosis. Front Cardiovasc Med 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Weber, T. Pulsatile Hemodynamics and Coronary Artery Disease. Korean Circ J 2021, 51, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Weber, T. The Role of Arterial Stiffness and Central Hemodynamics in Heart Failure. Int J Heart Fail 2020, 2, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Hametner, B.; Wassertheurer, S.; Mayer, C.C.; Danninger, K.; Binder, R.K.; Weber, T. Aortic Pulse Wave Velocity Predicts Cardiovascular Events and Mortality in Patients Undergoing Coronary Angiography: A Comparison of Invasive Measurements and Noninvasive Estimates. Hypertension 2021, 77, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Jin, K.N.; Kim, H.L.; Kim, Y.N.; Im, M.S.; Lim, W.H.; Seo, J.B.; Kim, S.H.; Zo, J.H.; Kim, M.A. Additional prognostic value of brachial-ankle pulse wave velocity to coronary computed tomography angiography in patients with suspected coronary artery disease. Atherosclerosis 2018, 268, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Koji, Y.; Yambe, M.; Shiina, K.; Motobe, K.; Yamada, J.; Shido, N.; Tanaka, N.; Chikamori, T.; Yamashina, A. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J 2005, 69, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Greve, S.V.; Blicher, M.K.; Kruger, R.; Sehestedt, T.; Gram-Kampmann, E.; Rasmussen, S.; Vishram, J.K.; Boutouyrie, P.; Laurent, S.; Olsen, M.H. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J Hypertens 2016, 34, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Terentes-Printzios, D.; Laurent, S.; Nilsson, P.M.; Protogerou, A.D.; Aznaouridis, K.; Xaplanteris, P.; Koutagiar, I.; Tomiyama, H.; Yamashina, A.; et al. Association of Estimated Pulse Wave Velocity With Survival: A Secondary Analysis of SPRINT. JAMA Netw Open 2019, 2, e1912831. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bu, X.; Pan, H.; Yang, S.; Cheng, W.; Shubhra, Q.T.H.; Ma, N. Estimated pulse wave velocity is associated with all-cause and cardio-cerebrovascular disease mortality in stroke population: Results from NHANES (2003-2014). Front Cardiovasc Med 2023, 10, 1140160. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.D.; Chu, P.; Kong, C.H.; Shi, Y.; Zhu, M.H.; Xia, Y.Y.; Li, Z.; Zhang, J.X.; Chen, S.L. Estimated pulse wave velocity is associated with all-cause mortality and cardiovascular mortality among adults with diabetes. Front Cardiovasc Med 2023, 10, 1157163. [Google Scholar] [CrossRef] [PubMed]
- ACC/AHA guidelines for cardiac catheterization and cardiac catheterization laboratories. American College of Cardiology/American Heart Association Ad Hoc Task Force on Cardiac Catheterization. J Am Coll Cardiol 1991, 18, 1149–1182. [Google Scholar]
- Vishram-Nielsen, J.K.K.; Laurent, S.; Nilsson, P.M.; Linneberg, A.; Sehested, T.S.G.; Greve, S.V.; Pareek, M.; Palmieri, L.; Giampaoli, S.; Donfrancesco, C.; et al. Does Estimated Pulse Wave Velocity Add Prognostic Information?: MORGAM Prospective Cohort Project. Hypertension 2020, 75, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, Y.; Park, J.K.; Lim, Y.H.; Shin, J.H. Association of the Estimated Pulse Wave Velocity with Cardio-Vascular Disease Outcomes among Men and Women Aged 40-69 Years in the Korean Population: An 18-Year Follow-Up Report on the Ansung-Ansan Cohort in the Korean Genome Environment Study. J Pers Med 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Gao, J.; Huang, Z.; Chen, S.; Wang, G.; Wu, S.; Jonas, J.B. Estimated pulse wave velocity and cardiovascular events in Chinese. Int J Cardiol Hypertens 2020, 7, 100063. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, H.; Kong, F.; Yang, S.; Shubhra, Q.T.H.; Li, D.; Chen, S. Association of arterial stiffness with all-cause and cause-specific mortality in the diabetic population: A national cohort study. Front Endocrinol (Lausanne) 2023, 14, 1145914. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).