Submitted:
03 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathophysiology
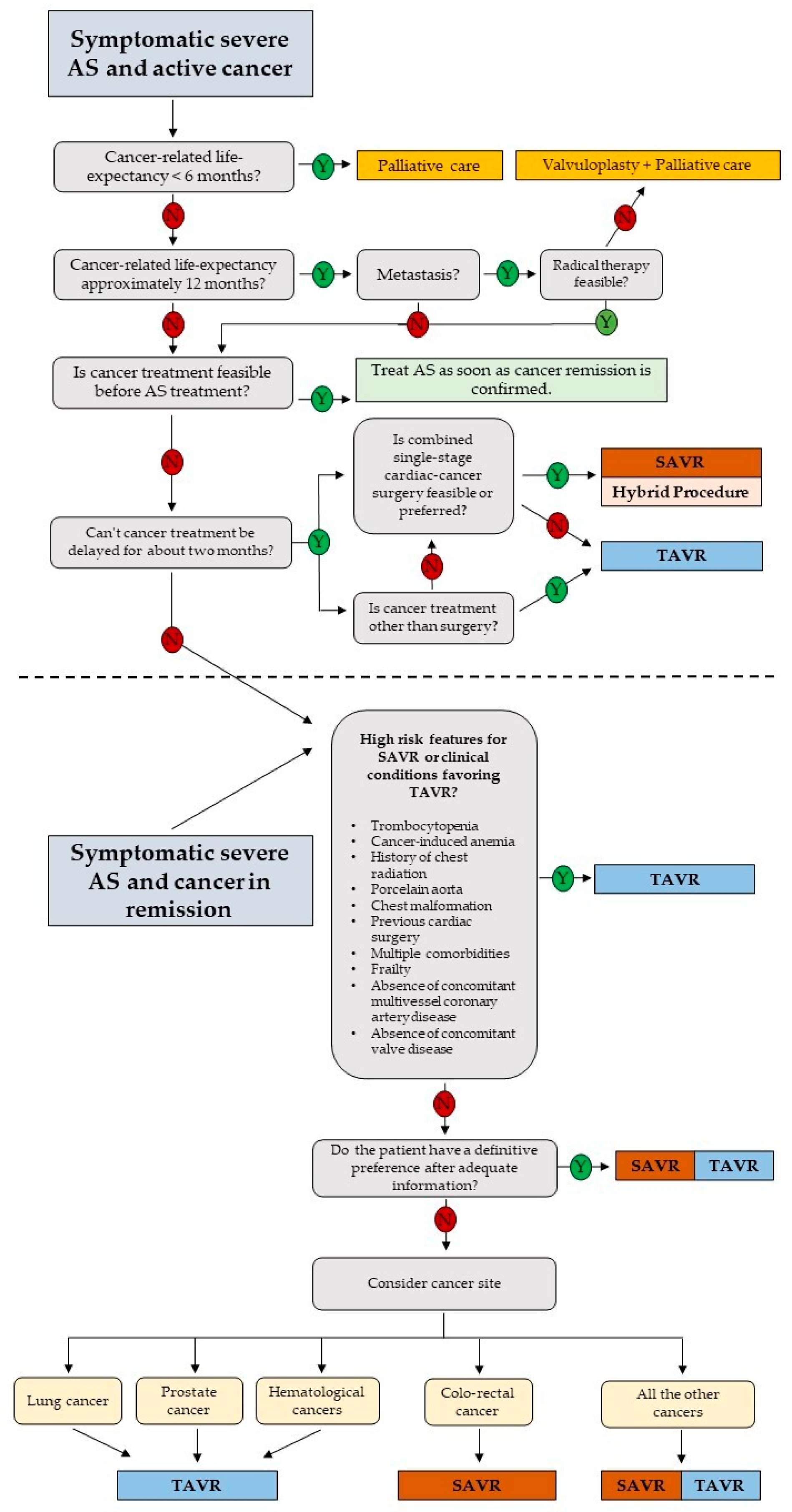
3. Treatment
3.1. Radiation therapy and Aortic stenosis treatment
3.2. Aortic stenosis, coronary artery disease (CAD) and mitral regurgitation
3.3. Aortic stenosis and cardiac Amyloidosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Disclosure of Conflict of Interests
Abbreviations:
| AKI: | acute kidney injury |
| AS: | aortic stenosis |
| CA: | cardiac amyloidosis |
| CAD: | coronary artery disease |
| CCTA: | coronary computed tomography angiography |
| CTA: | computed tomography angiography |
| C-XRT: | chest radiation therapy |
| DAPT: | dual antiplatelet therapy |
| HF: | heart failure |
| LV: | left ventricular |
| MR: | mitral regurgitation |
| PCI: | percutaneous coronary interventions |
| SAVR: | surgical valve replacement |
| TAVR: | transcatheter valve replacement |
References
- Giza, D.E.; Iliescu, G.; Hassan, S.; Marmagkiolis, K.; Iliescu, C. Cancer as a Risk Factor for Cardiovascular Disease. Curr. Oncol. Rep. 2017, 19, 39. [Google Scholar] [CrossRef]
- Faggiano, P.; Frattini, S.; Zilioli, V.; Rossi, A.; Nistri, S.; Dini, F.L.; Lorusso, R.; Tomasi, C.; Cas, L.D. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision-making process. Int. J. Cardiol. 2012, 159, 94–99. [Google Scholar] [CrossRef]
- Mangner, N.; Woitek, F.J.; Haussig, S.; Holzhey, D.; Stachel, G.; Schlotter, F.; Höllriegel, R.; Mohr, F.W.; Schuler, G.; Linke, A. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 188–196. [Google Scholar] [CrossRef]
- Guha, A.; Dey, A.K.; Arora, S.; Cavender, M.A.; Vavalle, J.P.; Sabik, J.F., III; Jimenez, E.; Jneid, H.; Addison, D. Contemporary Trends and Outcomes of Percutaneous and Surgical Aortic Valve Replacement in Patients With Cancer. J. Am. Hear. Assoc. 2020, 9, e014248. [Google Scholar] [CrossRef]
- Okura, Y.; Ishigaki, S.; Sakakibara, S.; Yumoto, C.; Hashitate, M.; Sekine, C.; Fujita, T.; Takayama, T.; Ozaki, K.; Sato, N.; et al. Prognosis of Cancer Patients with Aortic Stenosis Under Optimal Cancer Therapies and Conservative Cardiac Treatments. Int. Hear. J. 2018, 59, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Minamino-Muta, E.; Kato, T.; Morimoto, T.; Taniguchi, T.; Nakatsuma, K.; Kimura, Y.; Inoko, M.; Shirai, S.; Kanamori, N.; Murata, K.; et al. Malignant disease as a comorbidity in patients with severe aortic stenosis: clinical presentation, outcomes, and management. Eur. Hear. J. - Qual. Care Clin. Outcomes 2018, 4, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Marmagkiolis, K.; Monlezun, D.J.; Cilingiroglu, M.; Grines, C.; Herrmann, J.; Toutouzas, K.P.; Ates, I.; Iliescu, C. TAVR in Cancer Patients: Comprehensive Review, Meta-Analysis, and Meta-Regression. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Sarfaraz, A.; Durand, J.-B.; Swafford, J.; Daher, I.N. Management and outcomes of severe aortic stenosis in cancer patients. Am. Hear. J. 2011, 161, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Khrais, A.; Gilani, N.; Sapin, J.; Abboud, Y.; Kahlam, A.; Le, A.; Shah, M.; Palani, A.; Javed, J. Differential Rates of Lower Gastrointestinal Bleeding and Other Outcomes in Colorectal Cancer Patients With Aortic Stenosis. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef]
- Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017, 19, 9–42.
- Ezaz G, Long JB, Gross CP, Chen J. Risk Prediction Model for Heart Failure and Cardiomyopathy After Adjuvant Trastuzumab Therapy for Breast Cancer. J. Am. Hear. Assoc. 2014, 3, e000472. [CrossRef] [PubMed]
- Lenihan, D.J.; Oliva, S.; Chow, E.J.; Cardinale, D. Cardiac toxicity in cancer survivors. Cancer 2013, 119 (Suppl. 11), 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-Induced Cardiomyopathy: Clinical Relevance and Response to Pharmacologic Therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Murbraech K, Wethal T, Smeland KB, Holte H, Loge JH, Holte E, et al. Valvular Dysfunction in Lymphoma Survivors Treated With Autologous Stem Cell Transplantation: A National Cross-Sectional Study. JACC Cardiovasc Imaging 2016, 9, 230–239. [Google Scholar] [CrossRef]
- Bravo-Jaimes, K.; Palaskas, N.L.; Banchs, J.; Abelhad, N.I.; Altaf, A.; Gouni, S.; Song, J.; Hassan, S.A.; Iliescu, C.; Deswal, A.; et al. Rate of Progression of Aortic Stenosis in Patients With Cancer. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Balanescu SM, Balanescu DV, Donisan T, Yang EH, Palaskas N, Lopez-Mattei J, et al. The Onco-cardiologist Dilemma: to Implant, to Defer, or to Avoid Transcatheter Aortic Valve Replacement in Cancer Patients with Aortic Stenosis? Curr Cardiol Rep. 2019, 21, 83. [Google Scholar] [CrossRef]
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [CrossRef]
- Paris, S.; Tarantini, L.; Navazio, A.; Faggiano, P. Cardio-oncology: the new frontier of clinical and preventive cardiology. Monaldi Arch. Chest Dis. 2020, 90. [Google Scholar] [CrossRef]
- Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [CrossRef]
- Kamp, D.W.; Shacter, E.; A Weitzman, S. Chronic inflammation and cancer: the role of the mitochondria. Oncology 2011, 25, 400–410. [Google Scholar] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Revista espanola de cardiologia 2022, 75, 524. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, S.M.; Lee, K.S.; Park, S.W.; Chung, M.J.; Cho, H.; Jung, J.I.; Jang, H.W.; Jung, S.-H.; Goo, J. Quantification of Aortic Valve Calcifications Detected During Lung Cancer-Screening CT Helps Stratify Subjects Necessitating Echocardiography for Aortic Stenosis Diagnosis. Medicine 2016, 95, e3710–e3710. [Google Scholar] [CrossRef] [PubMed]
- Schechter, M.; Balanescu, D.V.; Donisan, T.; Dayah, T.J.; Kar, B.; Gregoric, I.; Giza, D.E.; Song, J.; Lopez-Mattei, J.; Kim, P.; et al. An update on the management and outcomes of cancer patients with severe aortic stenosis. Catheter. Cardiovasc. Interv. 2019, 94, 438–445. [Google Scholar] [CrossRef]
- Kojima, Y.; Higuchi, R.; Hagiya, K.; Saji, M.; Takamisawa, I.; Iguchi, N.; Takanashi, S.; Doi, S.; Okazaki, S.; Sato, K.; et al. Prognosis of patients with active cancer undergoing transcatheter aortic valve implantation: An insight from Japanese multicenter registry. IJC Hear. Vasc. 2022, 40, 101045. [Google Scholar] [CrossRef]
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Sakai, T.; Yahagi, K.; Miura, S.; Hoshino, T.; Yokota, T.; Tanabe, K.; Ikeda, S. Transcatheter aortic valve implantation for patients with lung cancer and aortic valve stenosis. J. Thorac. Dis. 2018, 10, E387–E390. [Google Scholar] [CrossRef]
- Iliescu, C.A.; Grines, C.L.; Herrmann, J.; Yang, E.H.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.P.; Leesar, M.A.; Marmagkiolis, K. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa intervencionista). Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2016, 87, E202–E223. [Google Scholar]
- Bendary, A.; Ramzy, A.; Bendary, M.; Salem, M. Transcatheter aortic valve replacement in patients with severe aortic stenosis and active cancer: a systematic review and meta-analysis. Open Heart 2020, 7, e001131. [Google Scholar] [CrossRef]
- Diaz-Arocutipa, C.; Torres-Valencia, J.; Zavaleta-Camacho, G.; Vicent, L. Association Between Previous or Active Cancer and Clinical Outcomes in TAVR Patients: A Systematic Review and Meta-Analysis of 255,840 Patients. Front. Cardiovasc. Med. 2021, 8, 763557. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Thalambedu, N.; Zahid, S.; Muhammadzai, H.Z.U.; Sandhyavenu, H.; Kumar, A.; Alraies, M.C.; Vishnevsky, A.; Ruggiero, N.J.; Mamas, M.A.; et al. Trends and Outcomes of TAVI and SAVR in Cancer and Noncancer Patients. JACC Adv. 2023, 2, 100167. [Google Scholar] [CrossRef]
- Faggiano, P.; Lorusso, R.; Carugo, S.; Faggiano, A. Heart Valve Team Conundrum. JACC: Adv. 2023, 2. [Google Scholar] [CrossRef]
- Desai, M.Y.; Wu, W.; Masri, A.; Popovic, Z.B.; Agarwal, S.; Smedira, N.G.; Lytle, B.W.; Griffin, B.P. Increased Aorto-Mitral Curtain Thickness Independently Predicts Mortality in Patients With Radiation-Associated Cardiac Disease Undergoing Cardiac Surgery. Ann. Thorac. Surg. 2014, 97, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Belzile-Dugas, E.; Michel, C.; Morin, J.-F.; Eisenberg, M.J. The Heart-Team Approach for the Treatment of Radiation-Induced Aortic Stenosis and Coronary Artery Disease: A Case Report. CJC Open 2021, 3, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Mitchell JD, Cehic DA, Morgia M, Bergom C, Toohey J, Guerrero PA, et al. Cardiovascular Manifestations From Therapeutic Radiation: A Multidisciplinary Expert Consensus Statement From the International Cardio-Oncology Society. JACC CardioOncol. 2021, 3, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Bijl JM, Roos MM, van Leeuwen-Segarceanu EM, Vos JM, Bos WW, Biesma DH, et al. Assessment of Valvular Disorders in Survivors of Hodgkin's Lymphoma Treated by Mediastinal Radiotherapy +/- Chemotherapy. Am J Cardiol. 2016, 117, 691–696. [Google Scholar] [CrossRef]
- Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013, 127, 1476–1485. [Google Scholar] [CrossRef]
- Donnellan E, Griffin BP, Johnston DR, Popovic ZB, Alashi A, Kapadia SR, et al. Rate of Progression of Aortic Stenosis and its Impact on Outcomes in Patients With Radiation-Associated Cardiac Disease: A Matched Cohort Study. JACC Cardiovasc Imaging 2018, 11, 1072–1080. [Google Scholar] [CrossRef]
- Shahian DM, Jacobs JP, Badhwar V, Kurlansky PA, Furnary AP, Cleveland JC, Jr. , et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1-Background, Design Considerations, and Model Development. Ann Thorac Surg. 2018, 105, 1411–1418. [Google Scholar] [CrossRef]
- Donnellan, E.; Masri, A.; Johnston, D.R.; Pettersson, G.B.; Rodriguez, L.L.; Popovic, Z.B.; Roselli, E.E.; Smedira, N.G.; Svensson, L.G.; Griffin, B.P.; et al. Long-Term Outcomes of Patients With Mediastinal Radiation–Associated Severe Aortic Stenosis and Subsequent Surgical Aortic Valve Replacement: A Matched Cohort Study. J. Am. Hear. Assoc. 2017, 6, e005396. [Google Scholar] [CrossRef]
- Ghoneim, A.; Bouhout, I.; Perrault, L.P.; Bouchard, D.; Pellerin, M.; Lamarche, Y.; Demers, P.; Carrier, M.; Cartier, R.; El-Hamamsy, I. Reexamining the Role of Surgical Aortic Valve Replacement After Mediastinal Radiation Therapy. Ann. Thorac. Surg. 2017, 104, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Renker, M.; Schoepf, U.J.; Kim, W.K. Combined CT Coronary Artery Assessment and TAVI Planning. Diagnostics 2023, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.R.; Mustafa, S.F.; Miller, T.W.; Alkhawlani, T.; Sharma, U.C. Outcomes after transcatheter aortic valve replacement in cancer survivors with prior chest radiation therapy: a systematic review and meta-analysis. Cardio-Oncology 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Bartoli, A.; Deharo, P.; Vaillier, A.; Ferrara, J.; Barral, P.-A.; Jaussaud, N.; Morera, P.; Porto, A.; Collart, F.; et al. Feasibility of Non-Invasive Coronary Artery Disease Screening with Coronary CT Angiography before Transcatheter Aortic Valve Implantation. J. Clin. Med. 2023, 12, 2285. [Google Scholar] [CrossRef]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021.
- Mack, M.J.; Brennan, J.M.; Brindis, R.; Carroll, J.; Edwards, F.; Grover, F.; Shahian, D.; Tuzcu, E.M.; Peterson, E.D.; Rumsfeld, J.S.; et al. Outcomes Following Transcatheter Aortic Valve Replacement in the United States. JAMA 2013, 310, 2069–2077. [Google Scholar] [CrossRef]
- Tarantini, G.; Tang, G.; Fovino, L.N.; Blackman, D.; Van Mieghem, N.M.; Kim, W.-K.; Karam, N.; Carrilho-Ferreira, P.; Fournier, S.; Pręgowski, J.; et al. Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery. EuroIntervention 2023, 19, 37–52. [Google Scholar] [CrossRef]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Waisbren, E.C.; Stevens, L.-M.; Avery, E.G.; Picard, M.H.; Vlahakes, G.J.; Agnihotri, A.K. Changes in Mitral Regurgitation After Replacement of the Stenotic Aortic Valve. Ann. Thorac. Surg. 2008, 86, 56–62. [Google Scholar] [CrossRef]
- Ternacle J, Krapf L, Mohty D, Magne J, Nguyen A, Galat A, et al. Aortic Stenosis and Cardiac Amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2019, 74, 2638–2651. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Galat A, Guellich A, Bodez D, Slama M, Dijos M, Zeitoun DM, et al. Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Eur Heart J. 2016, 37, 3525–3531. [Google Scholar] [CrossRef]
- Java AP, Greason KL, Dispenzieri A, Grogan M, King KS, Maleszewski JJ, et al. Aortic valve replacement in patients with amyloidosis. J Thorac Cardiovasc Surg. 2018, 156, 98–103. [Google Scholar] [CrossRef]
- Cavalcante JL RS, Abdelkarim I, et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson 2017, 19, 98. [Google Scholar] [CrossRef] [PubMed]
| Author | Reference | All population, n | Cancer, n (%) |
|---|---|---|---|
| Faggiano et al. | Int J Cardiol.2012;159(2):94-9 | 240 | 64(26.6%) |
| Mangner et al. | J Interv Cardiol. 2018;31(2):188-96 | 1821 | 99(5.4%) |
| Minamino-Muta et al. | Eur Heart J Qual Care Clin Outcomes. 2018;4(3):180-8. | 3815 | 513 (13.4%) |
| Okura et al. | Int Heart J. 2018;59(4):750-8. | 26325 | 111(0.42%) |
| Guha et al. | J Am Heart Assoc. 2020;9(2):e014248 | 47295 | 27960 (37.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





