1. Introduction
Catalysis plays a vital role in modern chemical synthesis, efficiently converting simple starting materials into valuable complex compounds. Bimetallic catalysis, involving two different metal species working together synergistically, has emerged as a powerful tool for various chemical reactions [
1,
2,
3,
4]. Sequential bimetallic catalysis represents a cutting-edge approach, enabling the synthesis of valuable compounds with higher efficiency, selectivity, and atom economy. It opens new synthetic possibilities for more complex compounds by activating and transforming substrates that may be unreactive or challenging for a single-metal catalyst, aligning with green chemistry principles, and reducing the formation of unwanted by-products [
5].
Pioneering studies on transition-metal-catalyzed cross-coupling reactions, such as Suzuki, Heck, and Negishi reactions, laid the foundation for bimetallic catalysis by demonstrating selective coupling of different organic fragments [
6]. Early investigations into tandem catalysis, performing consecutive reactions without intermediate isolation, inspired the concept of sequential bimetallic catalysis for increased efficiency and shorter reaction times. The cooperative action of two metal catalysts to activate a substrate showcased the potential of combining multiple metals for improved reactivity and selectivity. Cascade reactions, where bond-forming events occur in a one-pot manner, influenced the design of sequential bimetallic catalysis, promoting multiple cyclical reactions for complex molecule synthesis [
3,
7].
One particularly promising area of bimetallic catalysis is the synthesis of
N-heterocycles, essential building blocks in numerous biologically active compounds and pharmaceuticals [
8]. Traditional synthetic routes for
N-heterocycles have some drawbacks, involving multiple steps, harsh conditions, and generating significant waste. Therefore, bimetallic catalysis offers several key advantages in
N-heterocycle synthesis. In particular, the use of two different metal catalysts in tandem allows for intricate control over the reaction pathways, enhancing selectivity and improving yields of the desired
N-heterocyclic products. This is particularly important in complex molecules where traditional methods may lead to competing side reactions.
Moreover, bimetallic catalysis facilitates the activation of less reactive substrates, enabling the synthesis of
N-heterocycles that were previously challenging or unattainable through mono-metallic catalysis. This expands the synthetic toolbox, providing access to a broader range of
N-heterocyclic structures. In addition, the efficient use of bimetallic catalysis reduces waste and increases atom efficiency, making the process more environmentally friendly and economically viable [
3,
7].
As the field continues to evolve, this review presents an overview of the recent developments concerning bimetallic sequential catalysis for the synthesis of N-heterocycles, which represents a promising avenue in catalysis.
2. Bimetallic Approaches to N,O-Aminals and Related Spiro N,O-Heterocycles
N,O-aminals are an interesting class of substituted molecules bearing a geminally
N,O-substituted (stereogenic) carbon center, and have been recently recognized as an important class of building blocks in organic synthesis [
9]. In the conventional approach,
N,O-aminals are derived either from the corresponding α-amido sulfones via nucleophilic substitution, replacing the sulfonyl group with an alkoxy moiety [
10,
11,
12,
13,
14], or from the corresponding imines by means of nucleophilic addition, introducing an alkoxy group [
15,
16,
17,
18,
19,
20,
21,
22,
23].
While several methods exist for synthesizing these compounds, enantioselective examples are still limited, particularly for the construction of chiral tetrasubstituted carbon centers [
21]. Moreover, some of these methods do come with certain disadvantages such as the requirement of harsh reaction conditions, such as the use of strong acids or high temperatures, which may lead to side reactions or limited substrate compatibility. Additionally, the formation of unwanted by-products and the potential for racemization can pose challenges, particularly in the synthesis of chiral
N,O-aminals. Despite these drawbacks, researchers continue to explore new strategies to overcome these limitations and develop more sustainable and selective routes for
N,O-aminal synthesis, where bimetallic catalysis has shown important perspectives [
24].
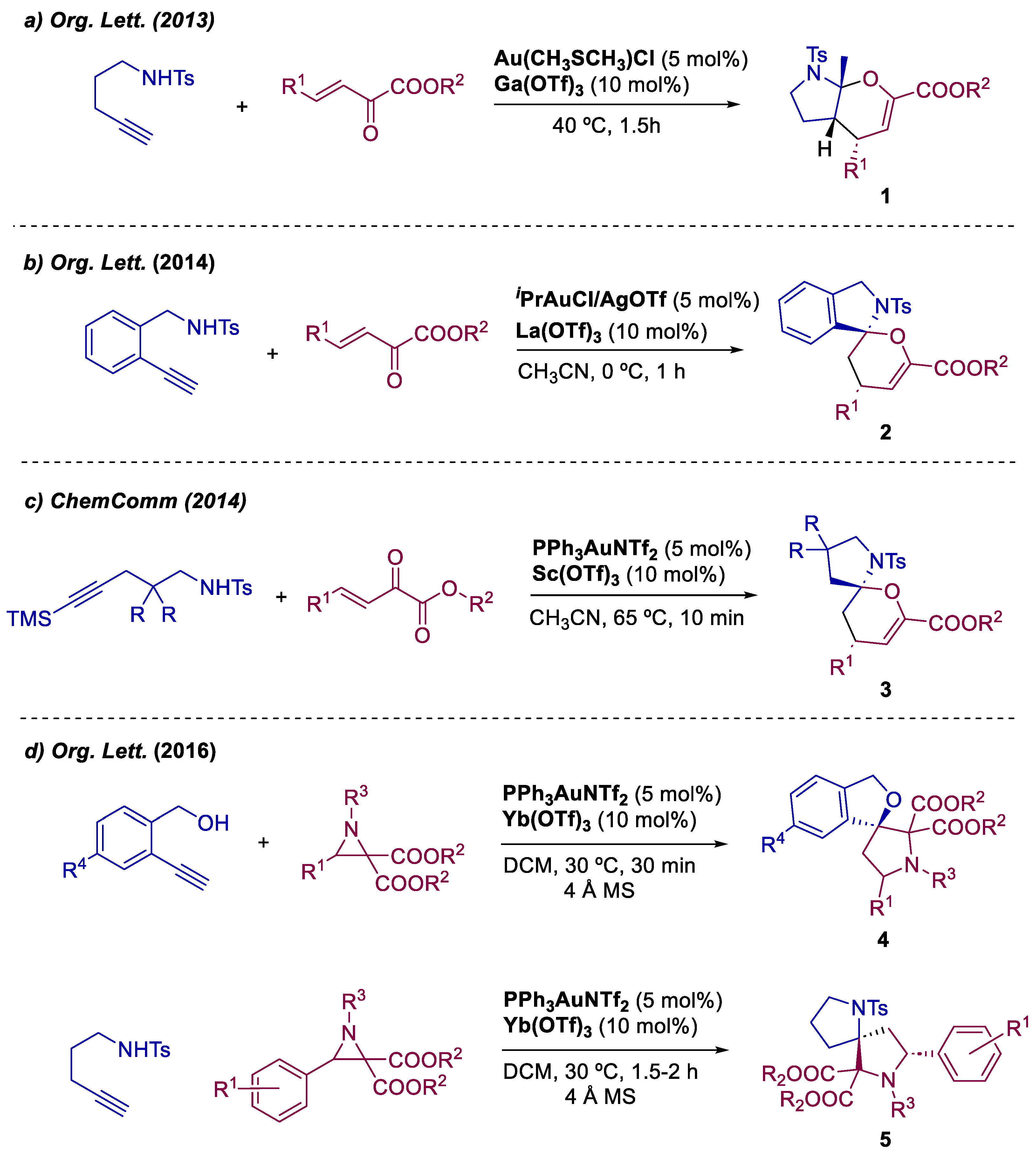
Xu and co-workers has been actively developing synthetic processes for fused bicyclic
N,O-aminals and spiro-
N,O-aminals. The authors focused on bimetallic catalysis under mild conditions, utilizing Au(I) as a catalyst and a metallic Lewis acid as a co-catalyst. In 2013, the group successfully achieved the synthesis of aromatic and allyl-substituted fused bicyclic aminals
1 through a Au(I)/Ga(III)-catalyzed [4+2] cycloaddition cascade reaction, with 13 examples scope and yields up to 90% (
Scheme 1a) [
25]. Subsequently, in 2014, the same group disclosed the synthesis of spiro-
N,O-aminals
2 by employing a Au(I)/La(III)-catalyzed [4+2] cycloaddition bimetallic approach (
Scheme 1b) [
24]. To prevent inward isomerization of the generated enamide, a fused aromatic ring was introduced in the same alkyne amine, enabling the reaction with activated electrophiles and yielding spirocyclic products in 12 examples scope with yields up to 90%. In the same year, Xu's group successfully synthesized aromatic and allyl-substituted spiro-aminals
3 using a bimetallic Au(I)/Sc(III)-catalyzed [4+2] cycloaddition process (
Scheme 1c) [
26]. In this work, trimethylsilyl (TMS) was employed as a traceless controlling group to stabilize the derived enamide and inhibit its isomerization, resulting in a scope of 14 examples and yields up to 89%. Additionally, in 2016, Xu and co-workers reported the preparation of spiro-heterocycles through a Au(I)/Yb(III)-catalyzed diastereoselective [3+2] cycloaddition between aziridines and alkynyl substrates (
Scheme 1d) [
27]. Various
N-protecting groups and aliphatic and aromatic alkynyl substrates were tested, resulting in a scope of substituted spiro-
N,O-heterocycles
4 with 16 examples and yields up to 99%. Furthermore, by switching from alkynyl alcohols to alkynyl amides, a similar reaction with aziridines afforded aromatic-substituted spiro-
N,N-heterocycles
5 with a scope of 9 examples and yields up to 80%.
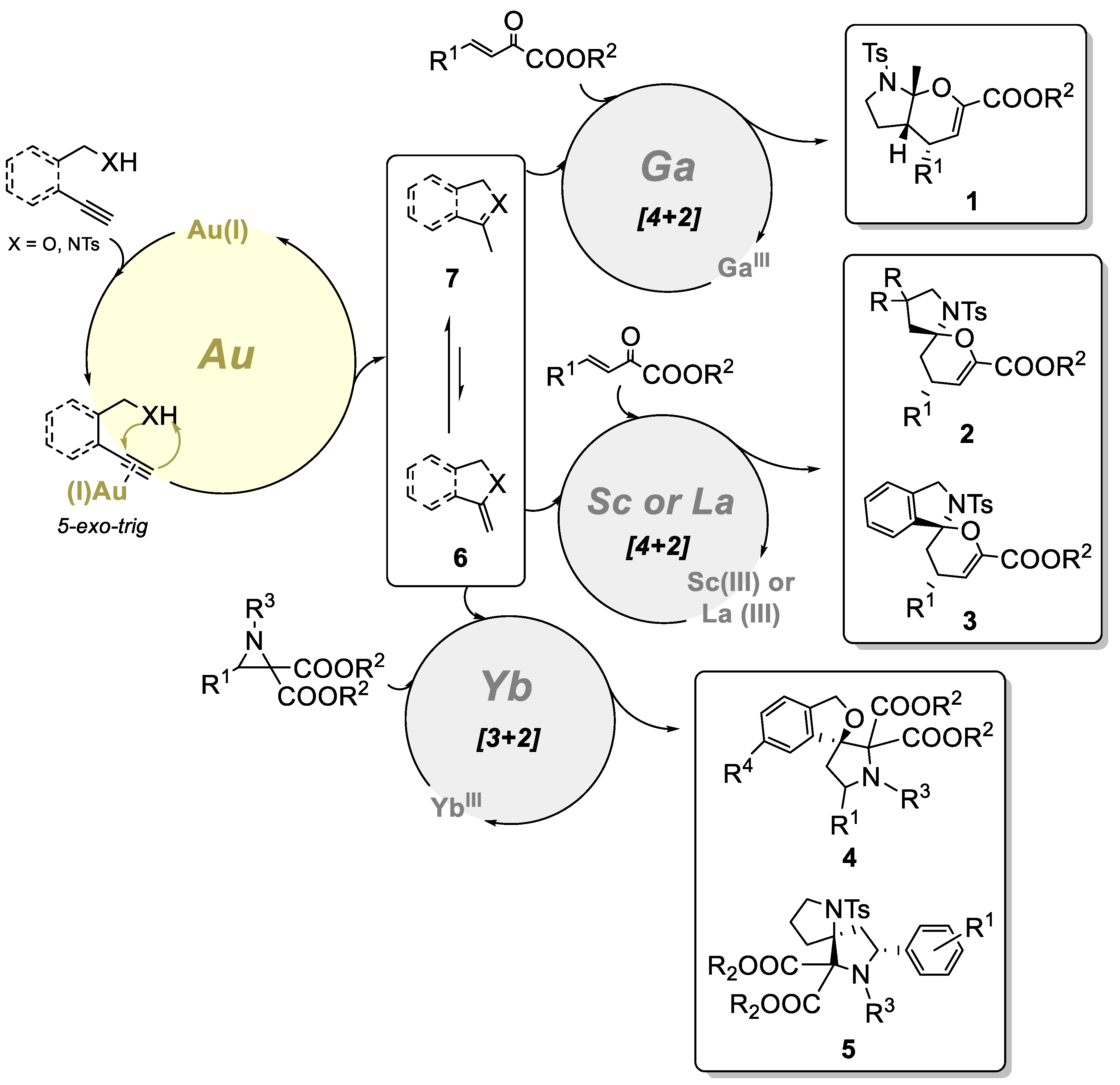
To gain insights into the reactions’ mechanisms (
Scheme 2), Xu and co-workers conducted deuteration experiments that showed that the alkynyl substrate undergoes a Au(I)-catalyzed 5-
exo-dig cyclization affording the enamide
6, followed by isomerization into another enamide
7. Although
7 is more stable, depending on the reaction conditions and the type or loading of the Lewis acid, either spiro or fused aminals can be afforded by a [4+2] cycloaddition. Thus, the Lewis acid activates the electrophile and generates the final product. When in contact with aziridines, enamide
6 can also undergo a Lewis acid-catalyzed [3+2] cycloaddition.
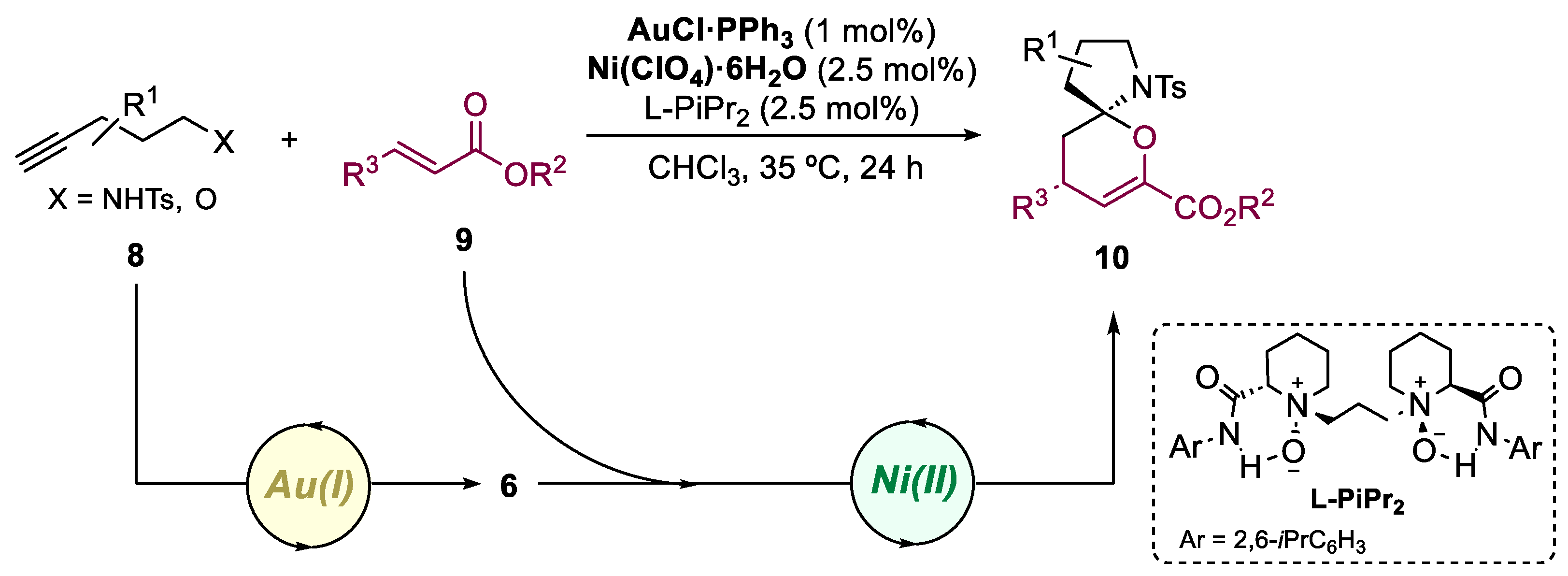
In 2016, Li and co-workers described an innovative asymmetric cascade reaction between alkynyl amides
8 and keto esters
9, employing a bimetallic catalytic system of achiral π-acid Au(I) and chiral Lewis acid
N,N'-dioxide Ni(II) complex (
Scheme 3) [
28]. This approach enabled the synthesis of spiroaminals
10 with high yields (up to 99%), excellent enantioselectivity (over 99% ee), and moderate to high diastereoselectivity (19:1 d.r.) under mild reaction conditions. The bimetallic catalytic system facilitated the sequential activation of the carbonyl and alkyne moieties, with the
N,N'-dioxide ligand playing a crucial role.
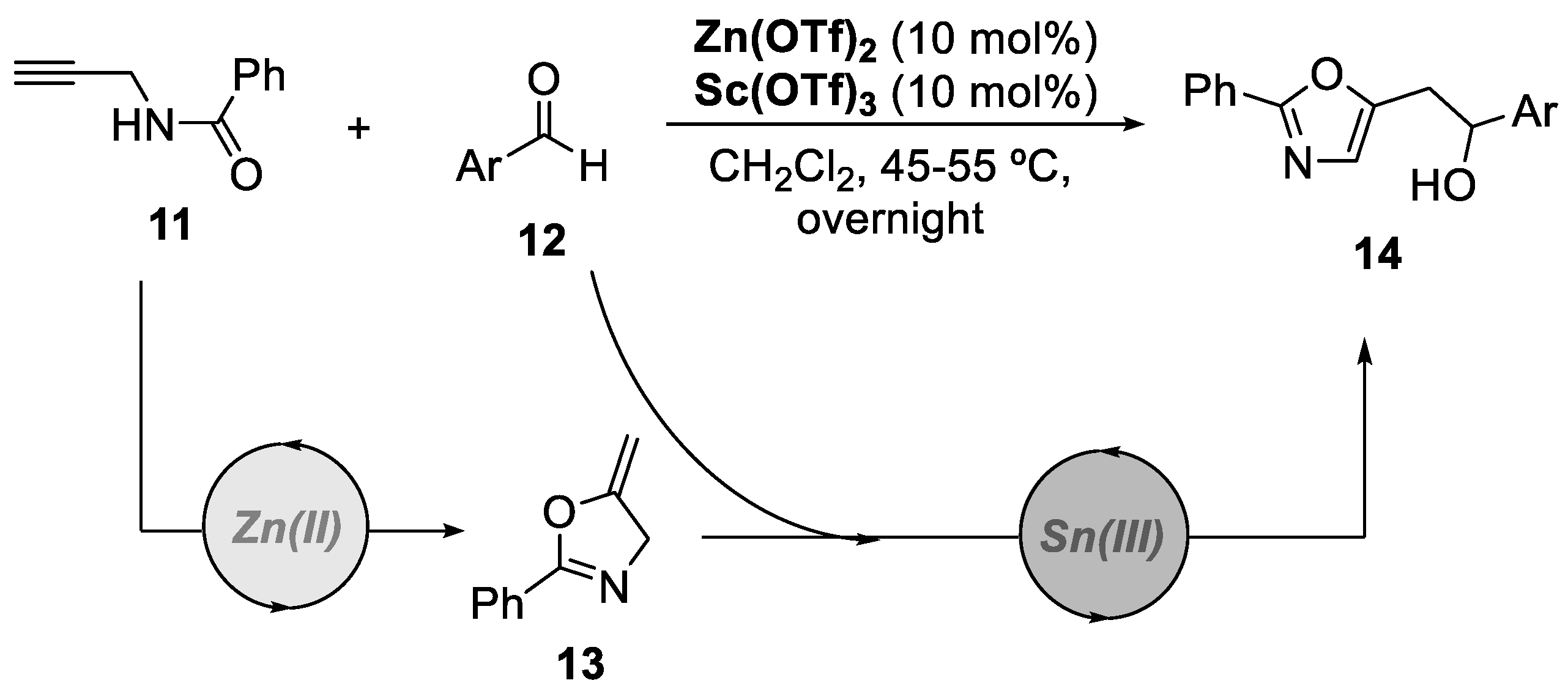
In 2016, another interesting bimetallic relay catalytic system was developed by the group of Xu, enabling the synthesis of oxazole derivatives from readily available
N-(propargyl)-aryl amides and aldehydes under mild reaction conditions (
Scheme 4) [
29]. The system consists of Zn(OTf)
2 and Sc(OTf)
3, which act as a π acid and a σ acid, respectively. The reaction proceeds through a cascade of reactions, beginning with an intramolecular 5-
exo-dig cyclization of alkynyl amide
11 catalyzed by Zn(OTf)
2. Simultaneously, Sc(OTf)
3 coordinates with the carbonyl group of the aldehyde
12, promoting the subsequent carbonyl-ene reaction with the oxazoline intermediate
13, yielding the desired oxazole product
14. This method showed a great atom-economy and straightforwardness, possessing the potential for applications in organic synthesis and medicinal chemistry.
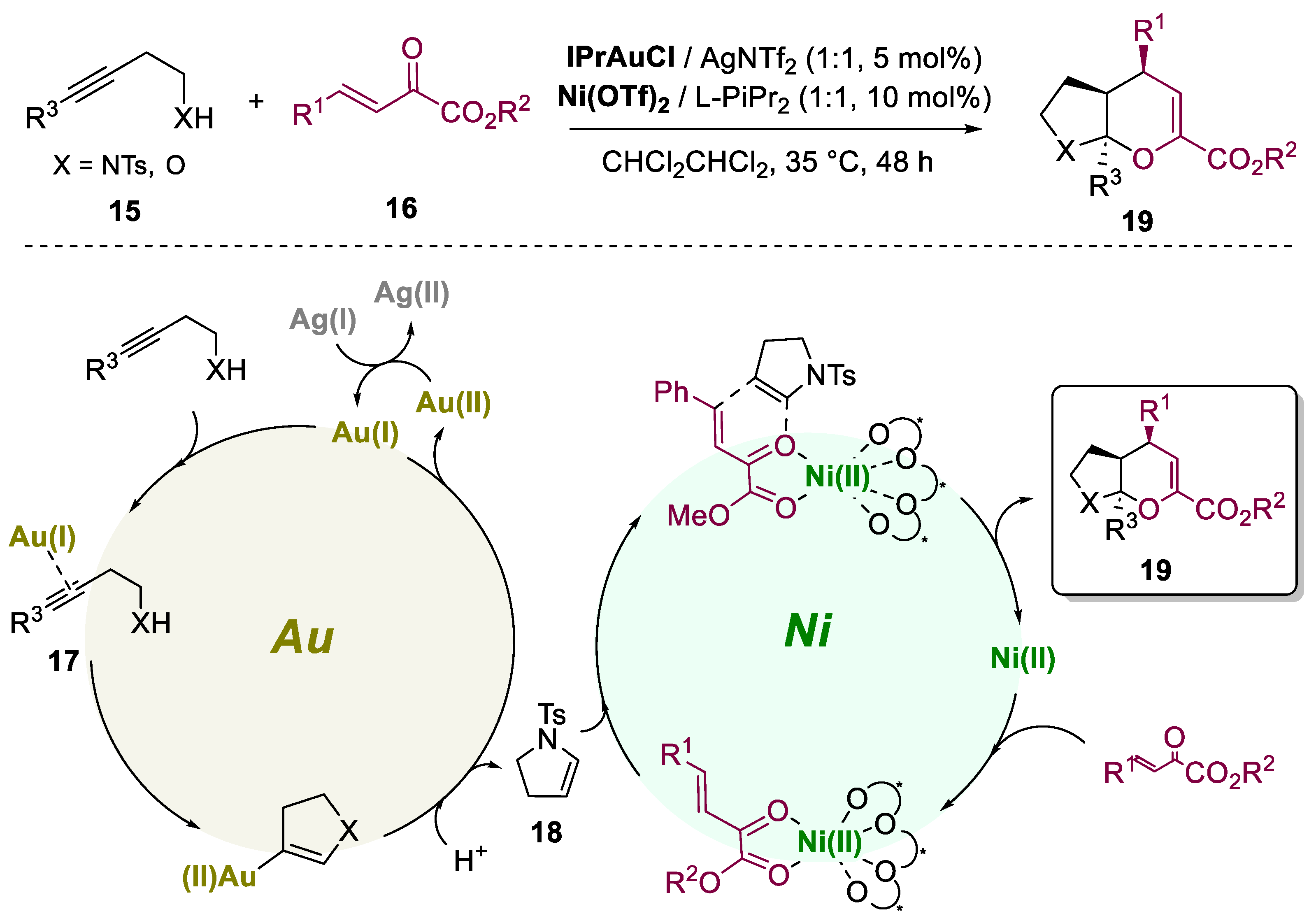
A similar work was reported by Feng's group in 2018 [
30]. In this work, a similar efficient catalytic asymmetric cyclization reaction (
Scheme 5) was conducted by a bimetallic system that involved an achiral Au(I) catalyst and the same chiral
N,N'-dioxide ligand/Ni(II) catalyst. This reaction also involved the cyclization of alkyl amides or alcohols
15 with, β,γ-unsaturated α-ketoesters
16 leading to the formation of fused bicyclic
N,O-acetals or
O,O-acetals. The researchers employed a 5-
endo-dig cyclization process based on Baldwin's rules, resulting in a cycloalkene intermediate capable of reacting with β,γ-unsaturated α-ketoesters to form the desired fused bicyclic products through an inverse-electron-demand hetero-Diels-Alder (IEDHDA) reaction. The optimal conditions for the reaction were determined, yielding products with good yields (77-99%) and excellent enantioselectivities (96-99% ee). The reaction tolerated various substrates, including those with 2-naphthyl and heteroaromatic groups. The substrate scope was expanded to alkynyl alcohols and alkynyl amines, yielding the desired fused bicyclic
N,O-acetals with excellent yield and enantioselectivity. Based on control experiments, a reaction mechanism is proposed: initially, the gold catalyst coordinates with the alkynyl substrate
15 to form a p-gold-alkyne complex
17 and, in situ, generated the key cyclic intermediate
18. On the other hand, chiral Lewis acid Ni(II)/L-PiPr
2 activated b,g-unsaturated a-ketoester
16 reacts with
18, giving the fused bicyclic acetal
19.
3. Bimetallic Approaches Involving Indole Scaffolds
Indoles and indolines are of great importance in medicinal chemistry, pharmaceuticals, and natural product synthesis due to their involvement in various biological processes and structural significance. Several common methods for synthesizing indoles, including the Fischer indole synthesis, Bischler-Möhlau indole synthesis, Madelung indole synthesis, Reissert indole synthesis, and Buchwald-Hartwig amination, have been widely employed [
31]. However, some traditional routes suffer from drawbacks such as harsh reaction conditions, multistep processes, limited substrate scope, and regioselectivity issues, which can lead to the formation of unwanted by-products or the need for expensive catalysts. Thus, bimetallic catalytic routes that involve the formation of an indole intermediate or product has emerged as a desirable approach.
The direct formation of pyrrolo[2,3-b]indoles via catalyzed bicycloaddition reactions is a very attractive yet challenging process. Isocyanides happen to be a simple group and, taking advantage of their electrophilicity, can be inserted either by Lewis acid [
32], or transition metal catalysis, undoubtedly with palladium as the most popular choice [
33,
34,
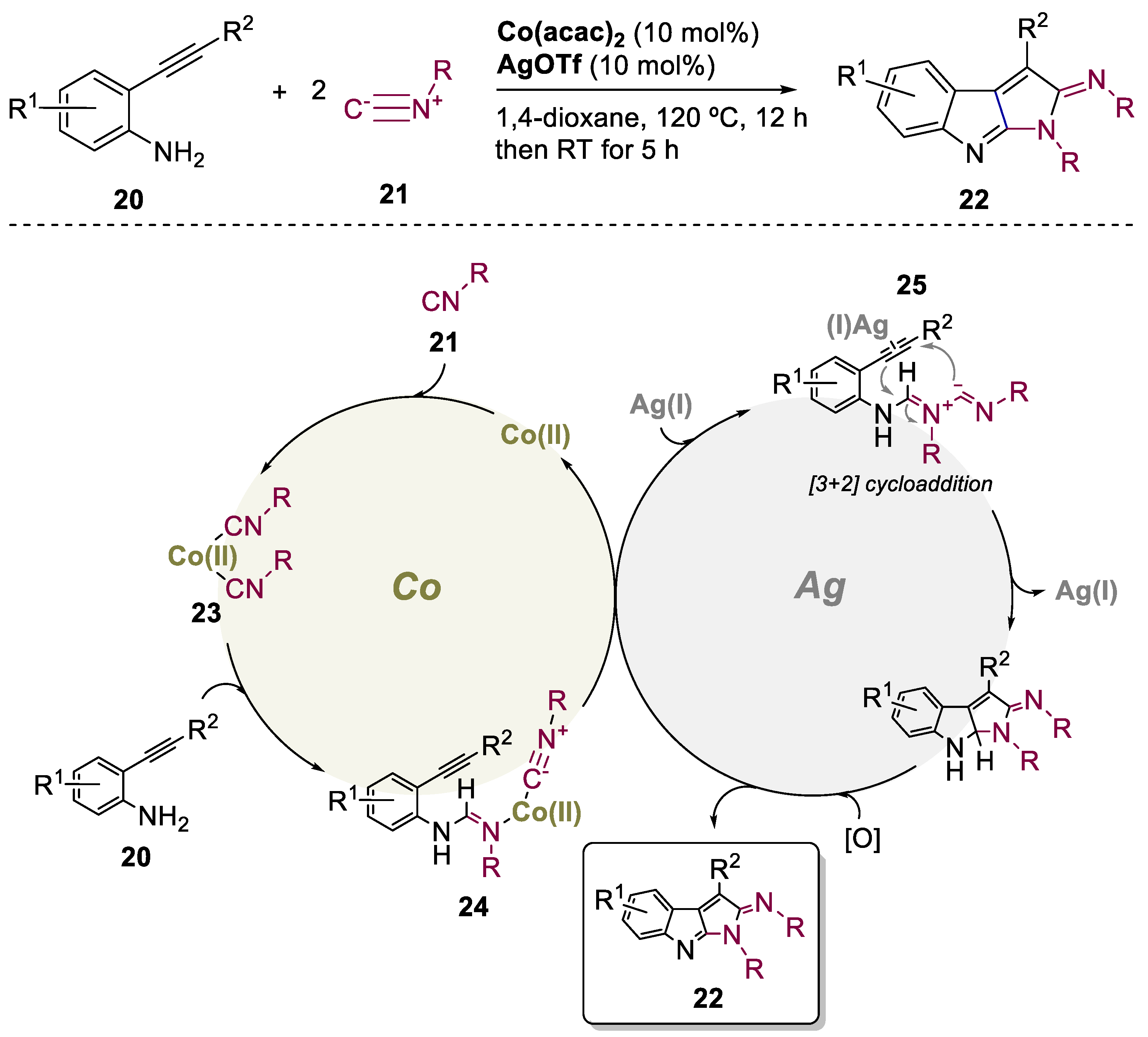
35]. In contrast, Gao and co-workers, in 2015, explored the preparation of pyrrolo[2,3-b]indoles by an inexpensive Co(II)-enabled process
(Scheme 6) [
36]. The authors demonstrated that the combination of Co(acac)
2 and AgOTf promoted a bimetallic relay catalysis reaction between 2-ethynylanilines
20 and isocyanides
21, allowing access to new densely functionalized pyrrolo[2,3-b]indoles
22. Overall 26 examples were reported, in yields up to 86%. The authors suggested that the reaction pathway involves a Co(II)-catalyzed double isocyanide insertion followed by a Ag(I)-catalyzed 1,3-dipolar cycloaddition. A suitable mechanism for the formation of pyrrolo[2,3-b]indoles can be described by a ligand exchange, affording the intermediate complex
23, which activates the electrophile isocyanides, a key step in this process. It then undergoes an N-H insertion to obtain the enyne-imine species
24, detected using GC-MS. Then, a second migratory insertion affords the 1,3-dipole
25 and regenerates the Co(II) catalyst. The presence of Ag(I) allows an intramolecular 1,3-dipolar cycloaddition followed by dehydrogenation under air conditions, that leads to the desired thermodynamically stable pyrrolo[2,3-b]indole
22.
Scheme 6.
General conditions and a plausible mechanism for the preparation of pyrrolo[2,3-β]indoles by a Co(II)/Ag(I)-catalyzed bimetallic approach.
Scheme 6.
General conditions and a plausible mechanism for the preparation of pyrrolo[2,3-β]indoles by a Co(II)/Ag(I)-catalyzed bimetallic approach.
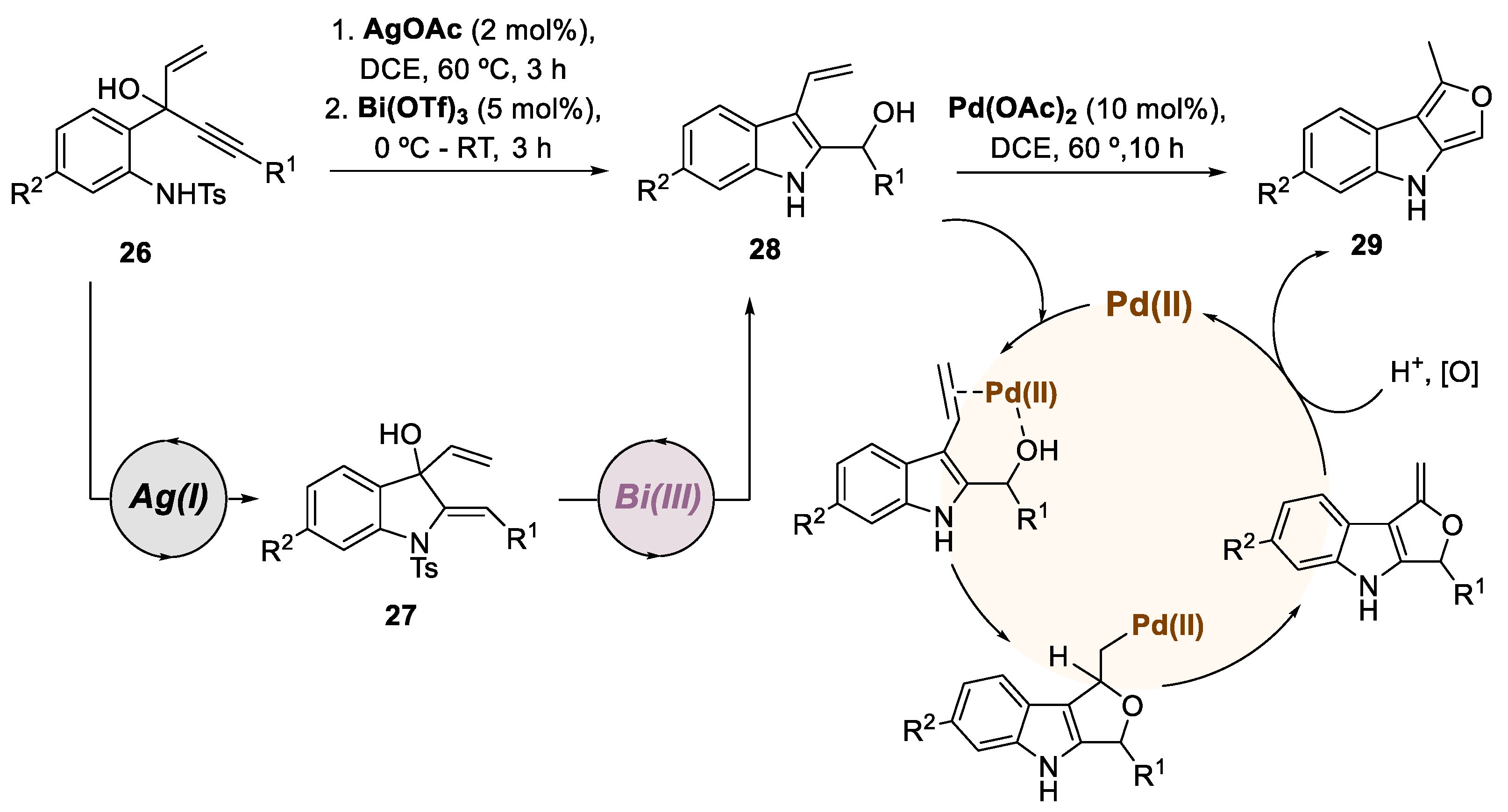
In 2016, Ramasastry and co-workers published two research works involving indole synthesis and subsequent transformation through a trimetallic catalytic system. In the first study, the authors reported a successful three orthogonal metal relay catalytic system for preparing furo[3,4-b] indoles (
Scheme 7) [
37]. This approach utilized a sequential Ag(I)/Bi(III)/Pd(II) trimetallic catalysis, demonstrating its versatility with 10 examples and yields up to 57%. The preparation of cyclopenta[b]indoles was also investigated using a one-pot Ag(I)/Brønsted acid catalysis from 3-(2-aminophenyl)-4-pentenyn-3-ols, although not involving a multimetallic catalytic approach. The reaction mechanism involved Ag(I)-catalyzed 5-
exo-dig cyclization of substrate
26 to generate intermediate
27, followed by Bi(III)-catalyzed 1,3-allylic alcohol isomerization leading to
38. Finally, a Pd(II)-catalyzed intramolecular etherification through a 5-
exo-trig cyclization under oxidative conditions resulted in the desired furo[3,4-b]indole
29.
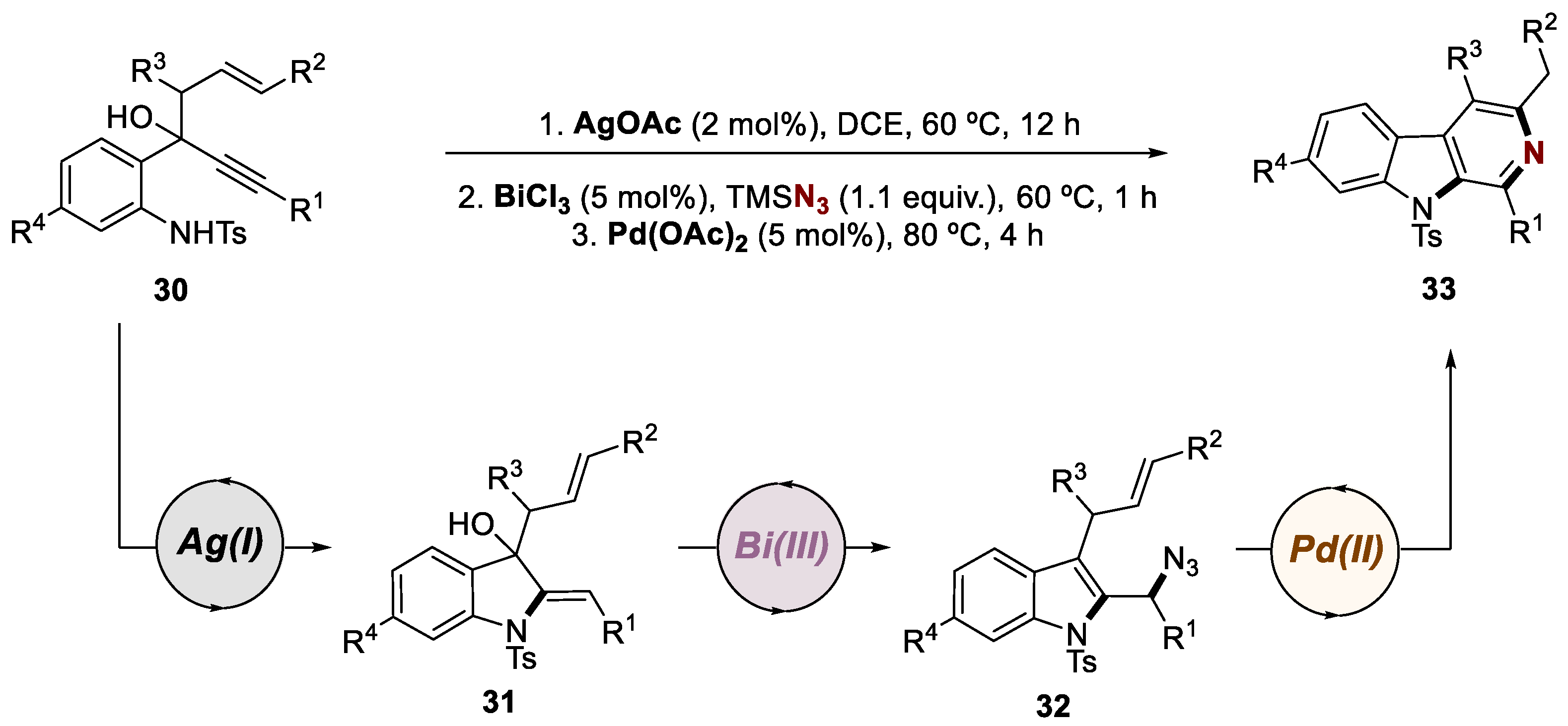
In the second work of 2016, Ramasastry and co-workers presented a similar one-pot triple-orthogonal-metal relay catalysis strategy for synthesizing 1,3-di- and 1,3,4-trisubstituted β-carbolines, also employing silver, bismuth, and palladium catalysts in a sequential manner (
Scheme 8) [
38]. The synthetic pathway included intramolecular hydroamination, Friedel–Crafts-type dehydrative azidation, and a unique annulation step leading to the formation of the pyridine ring. Starting from
30, they achieved indoline intermediate
31 through a Ag(I)-catalyzed 5-
exo-dig cyclization and protodemetalation. A subsequent Bi(III)-promoted cascade reaction involving 1,3-allylic alcohol isomerization and nucleophilic azidation led to the formation of azide
32. This intermediate underwent a Pd(II)-mediated aziridine formation, followed by deprotonation, ring opening of the aziridine, and aromatization, resulting in the desired substituted
N-heterocycle
33. This innovative approach provided access to a diverse range of distinct β-carbolines, offering new possibilities for the synthesis of these valuable compounds.
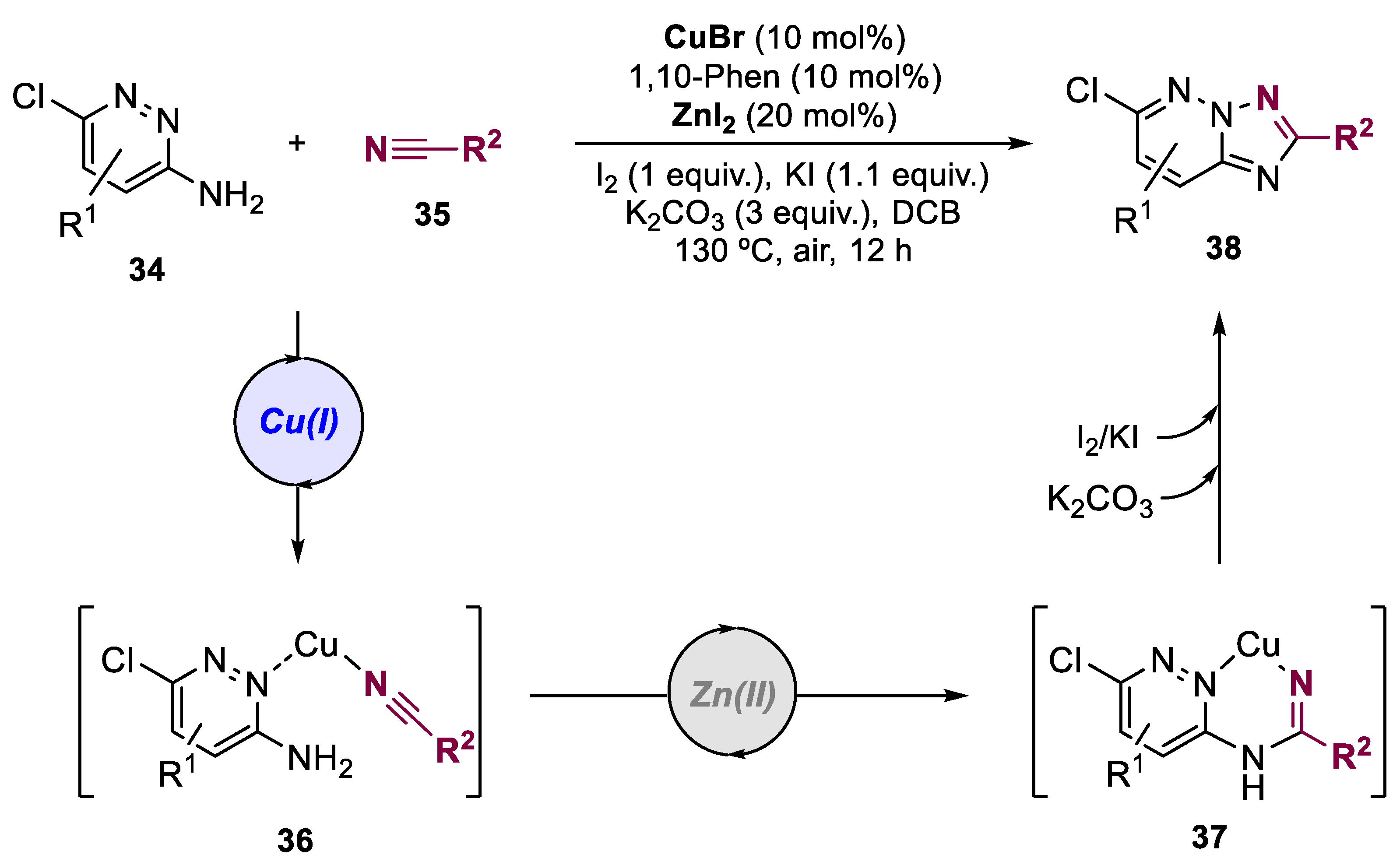
In 2017, Mu and co-workers reported a new and efficient protocol for the preparation of chlorine-containing 1,2,4-triazolo[1,5-
b]pyridazine scaffolds (
Scheme 9) [
39]. The authors developed a one-pot oxidative cycloaddition reaction of 3-aminopyridazine derivatives
34 and nitriles
35, involving cooperative Cu(I) and Zn(II)-catalyzed tandem C-N addition to achieve intermediate
36 followed by the amidine
37. Then, a I
2/KI-mediated intramolecular oxidative N-N bond formation affords the final 1,2,4-triazolo[1,5-
b] pyridazine derivatives
38.
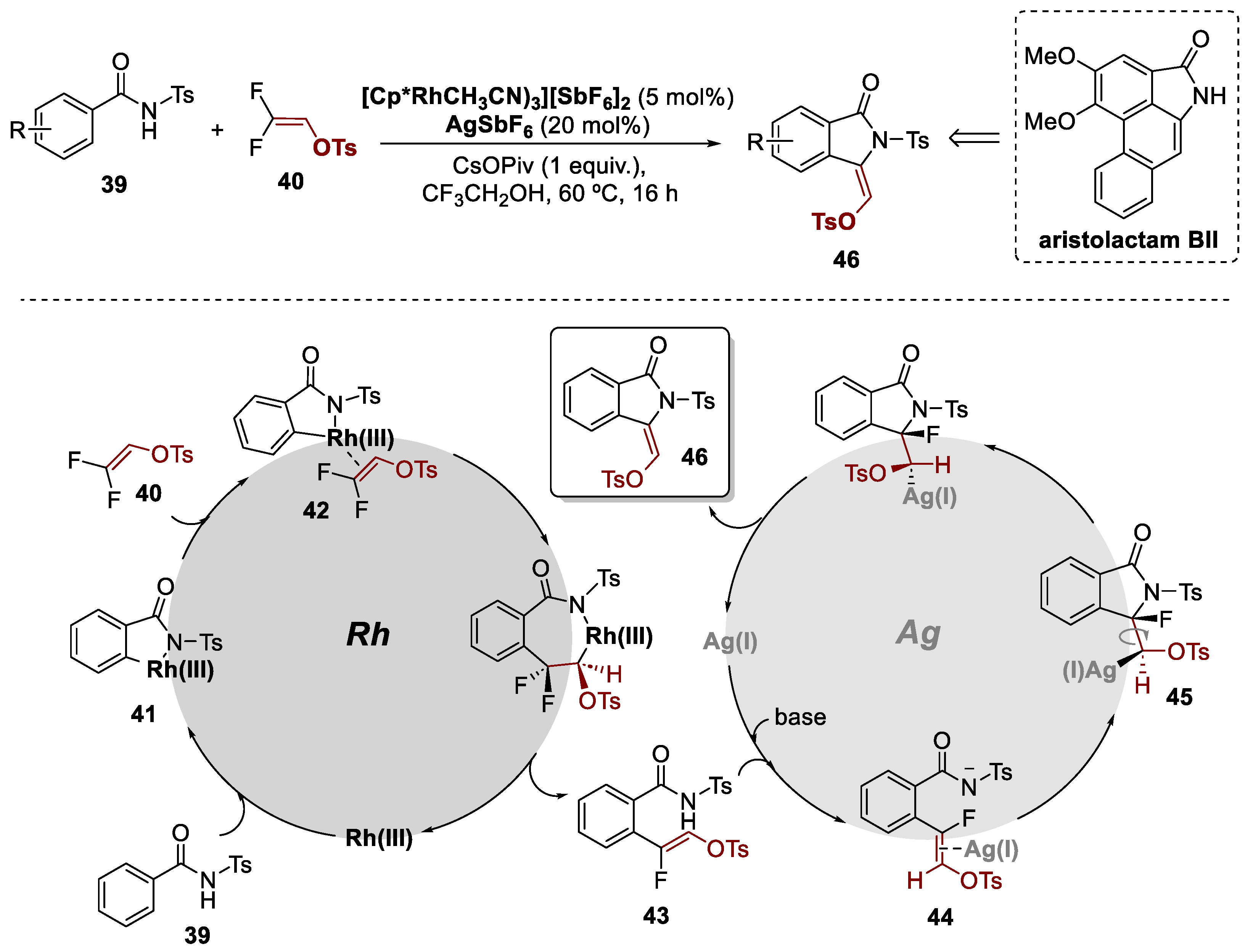
In 2017, Wang and co-workers reported the construction of 3-alkylidene isoindolinones. This was efficiently achieved through a redox-neutral bimetallic Rh(III)/Ag(I) relay catalysis between
N-tosyl benzamides
39 and 2,2-difluorovinyl tosylate
40 (
Scheme 10) [
40]. The Rh(III) catalyst facilitates the C-H monofluoroalkenylation reaction, while the Ag(I) salt acts as an activator for the subsequent cyclization step. According to the authors, the mechanism starts with the Rh(III)-catalyzed C-H activation in
N-tosylbenzamide
39, assisted by the NTs group, generating intermediate
41. The subsequent coordination of
40, that leads to intermediate
42, underwent a regioselective olefin insertion, followed by an anti-coplanar β-F elimination, resulting in the
Z-type monofluoroalkenylation product
43 with notable stereoselectivity. The Ag(I) salt was suggested to act as a π acid, promoting the activation of the olefin (
44) and facilitating the intramolecular cyclization reaction. Consequently, the anti-addition to the double bond induced 5-
exo cyclization, resulting in the formation of intermediate
45. The selective attack at the α-position of the fluorine atom was attributed to the low-lying LUMO with a significant coefficient at this position. Finally, a stereospecific formation of the
E-type 3-alkylidene isoindolinone product
46 was achieved through an anti-coplanar β-F elimination process. The methodology described in this study was used to rapidly synthesize aristolactam BII, a natural product with potential pharmaceutical applications. The results demonstrate the potential of difluorovinyl tosylate and Rh(III)/Ag(I) relay catalysis for the efficient synthesis of a variety of biologically active compounds.
X. Feng and co-workers reported a highly efficient asymmetric cascade reaction of alkenyloxindoles with pyridines and diazoacetates via a bimetallic iron(III)/chiral N,N′-dioxide-scandium(III) complex catalyst [
41]. Tetrahydroindolizines were obtained with good to excellent diastereo- and enantioselectivities.
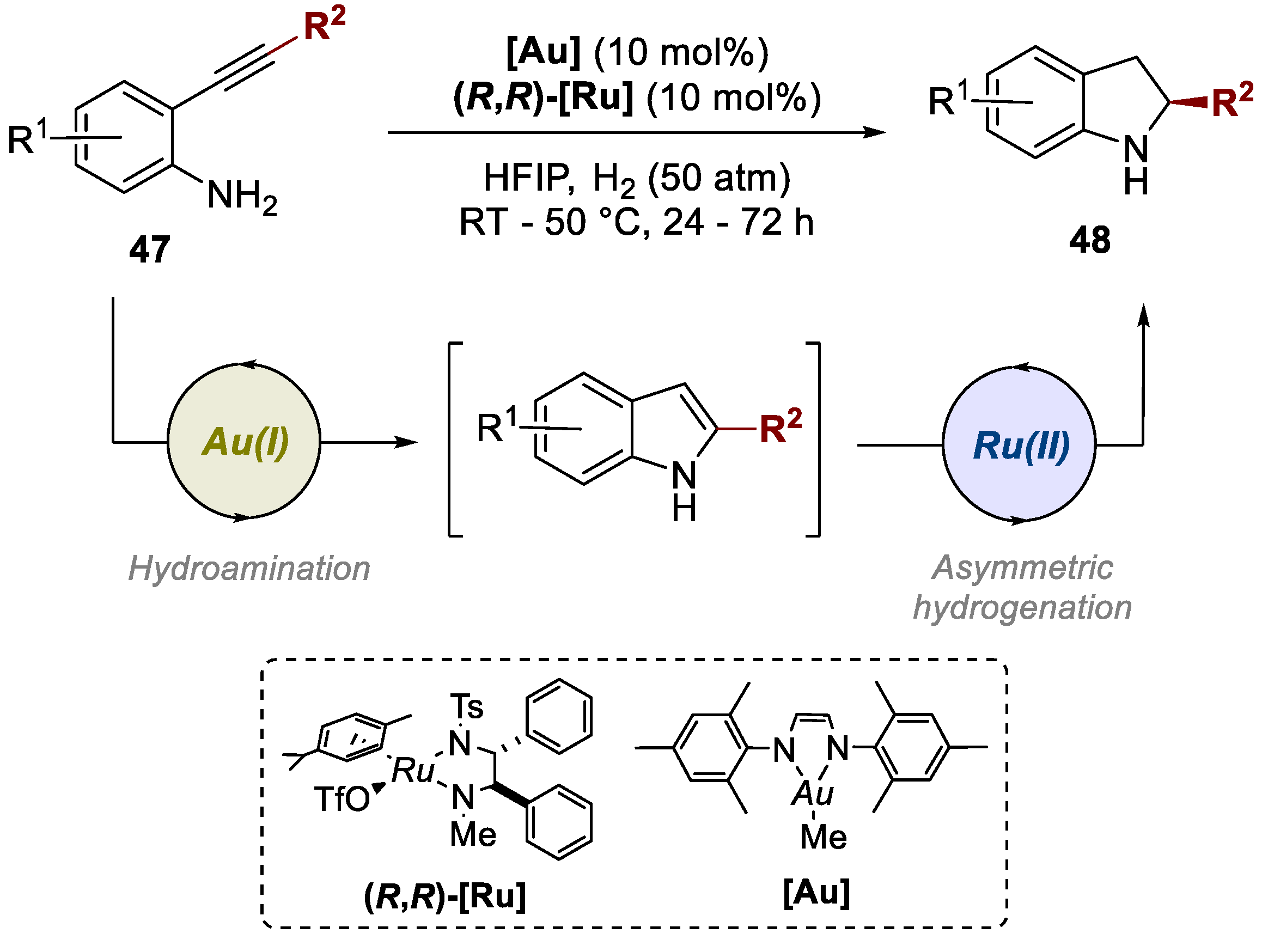
In 2019, Fan and co-workers published a one-pot cascade reaction strategy to afford functionalized indolines. This process involved a sequential Au(I)-catalyzed intramolecular hydroamination followed by a Ru(II)-catalyzed asymmetric hydrogenation of various anilino-alkynes
47 (
Scheme 11) [
42]. This enabled the access to chiral indolines
48. Optimal reaction conditions were determined, and the reactions proceeded smoothly, achieving full conversions, with high yields (up to 98%) and moderate to excellent enantioselectivities (up to 97% ee). This work was also extended to chiral 1,2,3,4-tetrahydroquinolines, although only requiring one ruthenium catalyst, and not a bimetallic approach.
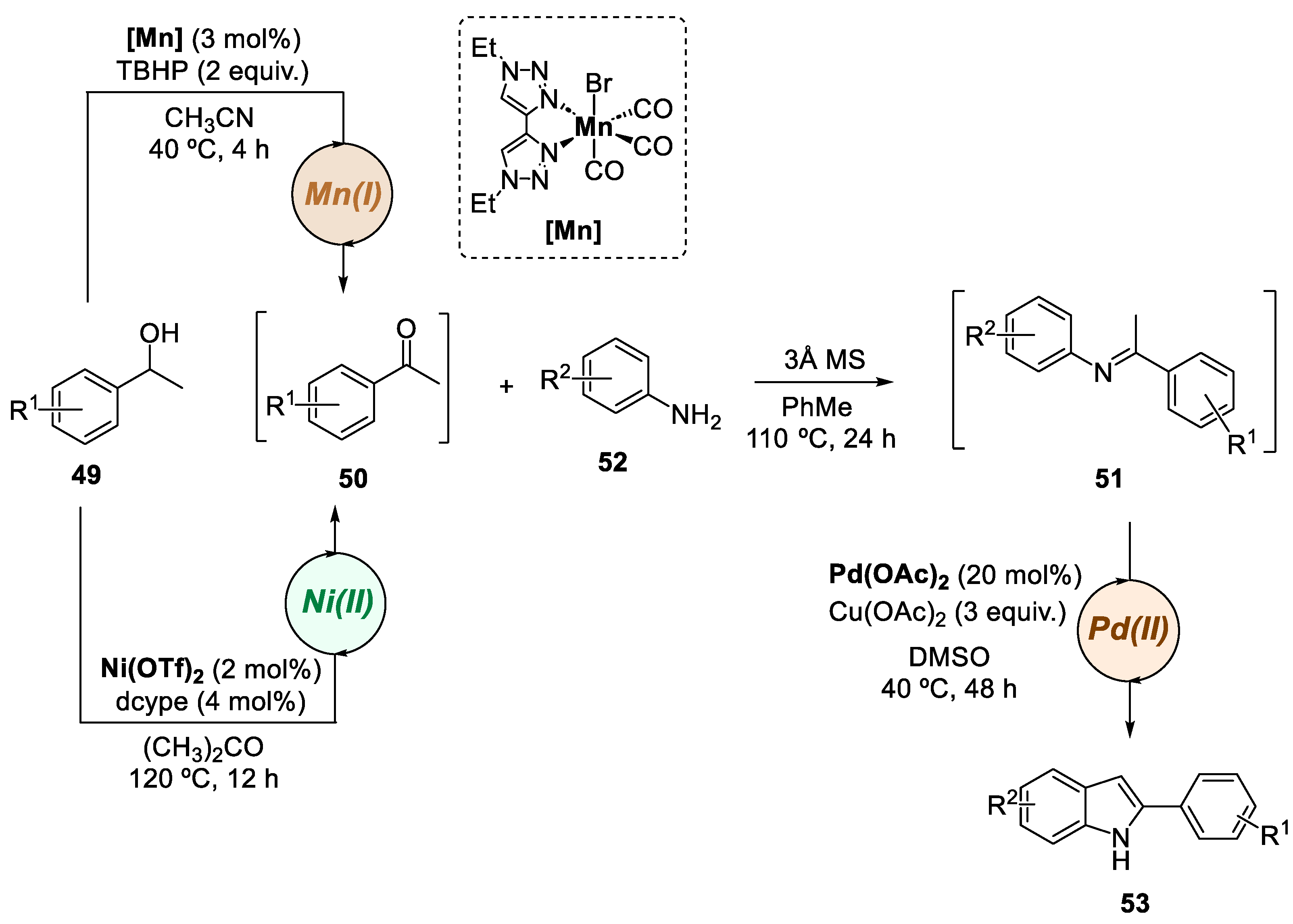
Marques and co-workers have recently disclosed a novel one-pot bimetallic catalytic approach for the synthesis of indole derivatives, using secondary alcohols and anilines as starting materials (
Scheme 12) [
8]. A commercially available nickel catalyst combined with a simple phosphine was investigated for the dehydrogenation of alcohol
49, while a phosphine-free manganese complex was also synthesized to achieve this oxidation step. Both systems were studied to obtain the desired ketone
50, which was subsequently converted to an imine
51 through condensation with an aniline
52, followed by an
in-situ palladium-catalyzed oxidative cyclization. This system achieved several 2-arylindoles
53, with a 3-step synthetic pathway and overall yields of up to 45%. This process had the advantage of avoiding the isolation of sensitive intermediates and presented a sustainable pathway for the preparation of functionalized indoles.
4. Bimetallic Approaches Involving Lactam Scaffolds
The β-lactam is the common core structure of clinically used drugs such as penicillin, cephalosporin, and monocyclic antibiotics such as aztrenam [
43,
44,
45,
46]. Development of novel methods to access new β-lactams is important in further studies of this heterocyclic system, including structure-activity relationships and the discovery of new antibiotics, and is thus important in global health problems. Synthetic methods that construct the β-lactam ring have been developing exorbitantly over the last century from almost every imaginable set of synthons. Yet, there is still a necessity for innovation and improvement in the field, even within each category of well-established reactions [
47]. Despite all of the synthetic methods to date for obtaining achiral or racemic lactams, asymmetric methodologies remain largely limited to chiral auxiliary-based systems [
48]. This review presents below two examples of asymmetric bimetallic catalytic approaches to afford substituted β-lactams.
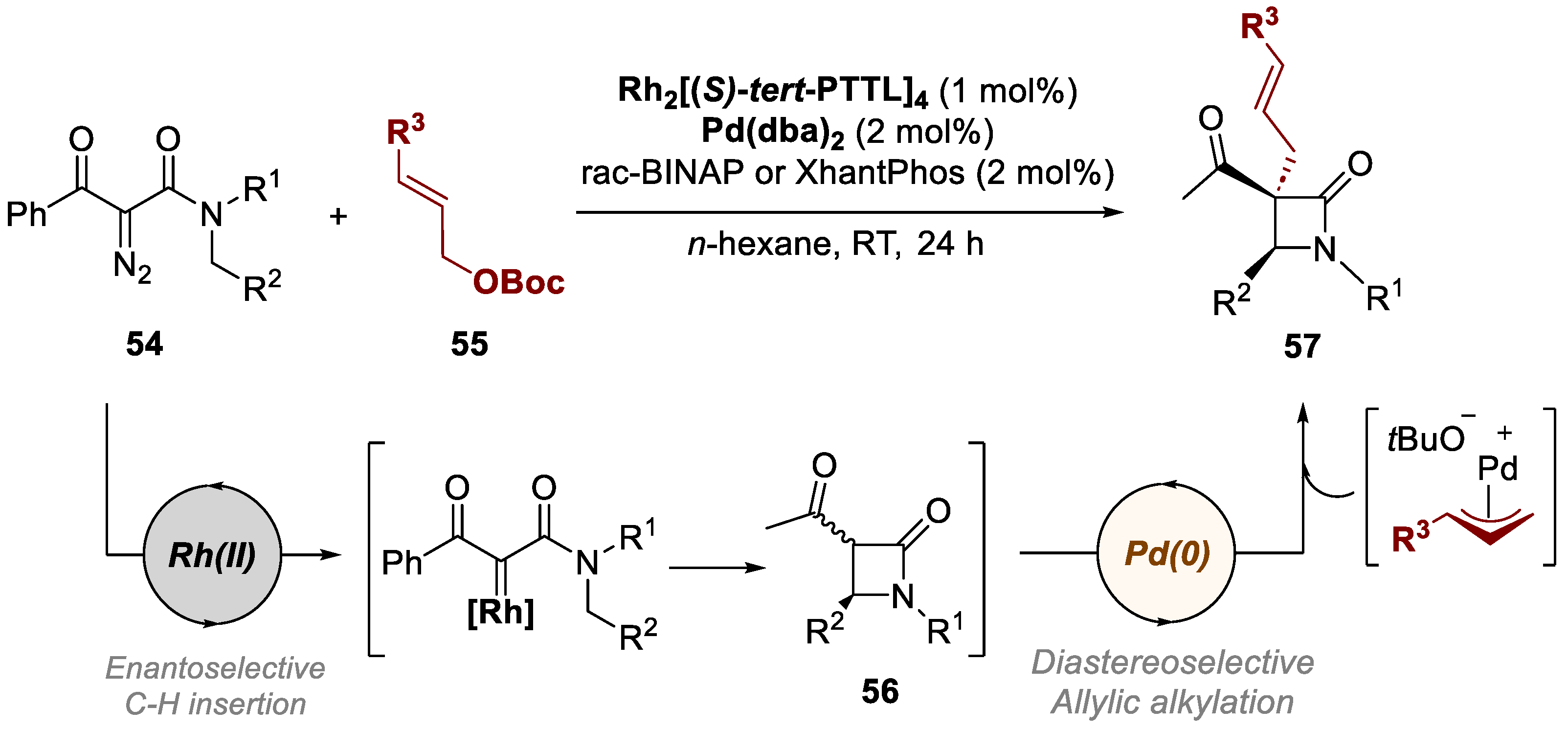
In 2018, Lee and co-workers developed a novel asymmetric dual Rh(II)/Pd(0) relay catalysis for synthesizing α-quaternary allylated chiral β-lactams, by reacting
N-benzyl α-diazoamides
54 and allyl
tert-butyl carbonates
55. (
Scheme 13) [
49]. The experiments conducted in this work supported a relay reaction with the formation of β-lactam intermediate
56 that resulted on the desired α-quaternary allylated chiral β-lactams
57. Optimization showed that nonhalogenated and nonpolar solvents yielded superior results compared to halogenated solvents. Different electron-donating and withdrawing groups on the phenyl ring were well-tolerated. The position of substituents on the aromatic ring affected the reaction, with ortho substituents exhibiting reduced reactivity due to steric hindrance. Heteroaromatic rings and naphthyl rings demonstrated favorable performance. This method provided a wide scope, achieving yields up to 99% and with good diastereomeric ratios (up to >91:1 dr) and enantioselectivities (up to 98% ee). Furthermore, by varying the allylic substrates, given the widespread availability of the diazo compounds and allyl carbonates, this asymmetric dual relay catalysis strategy may be a cornerstone for many new reactions exploiting multimetallic transformations of Rh(II) carbenoids and π-allyl Pd(II) complexes.
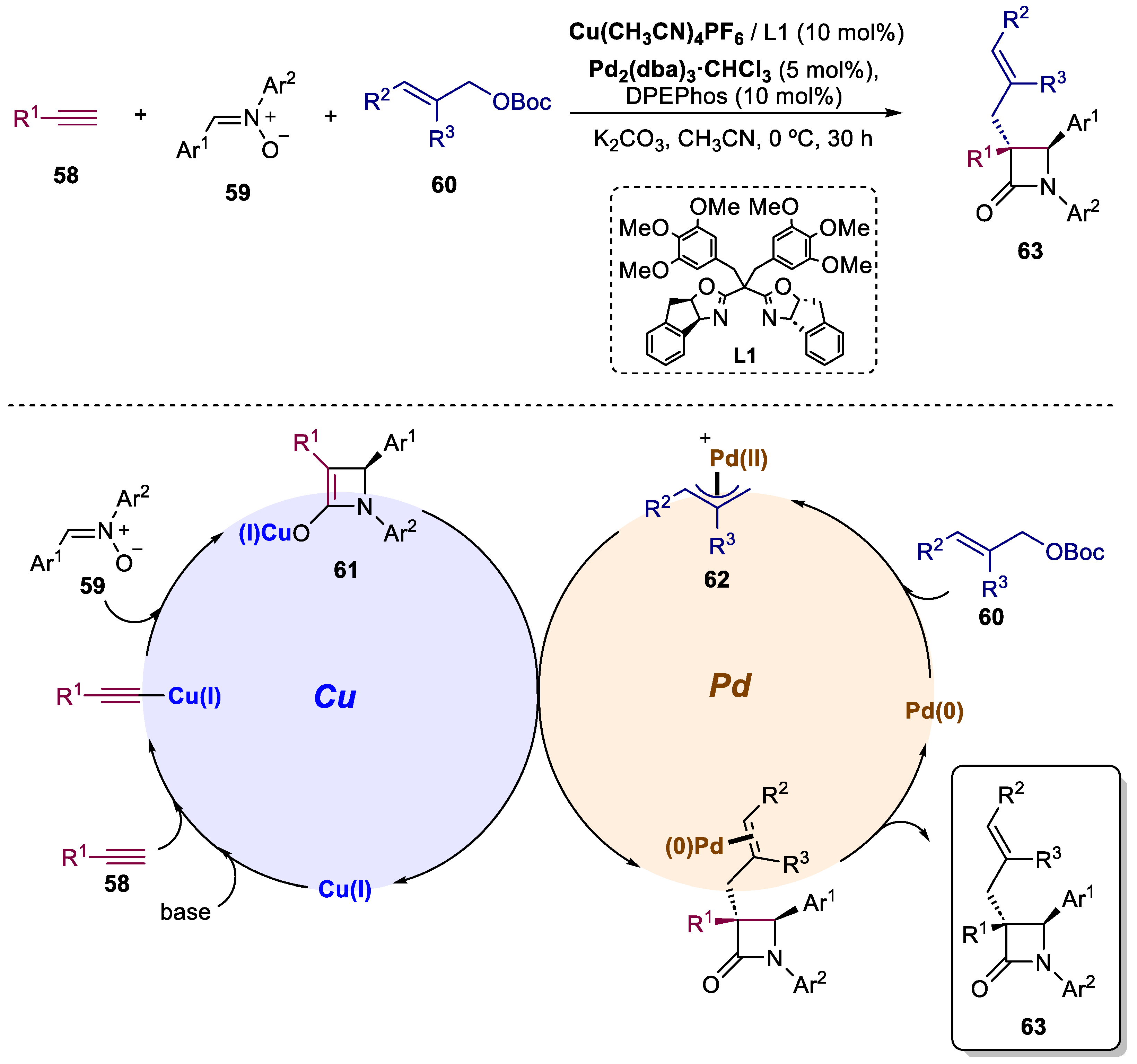
Xu and co-workers have successfully disclosed a groundbreaking asymmetric multicomponent reaction: the interrupted Kinugasa allylic alkylation (
Scheme 14) [
50]. This methodology synergistically merges a copper-catalyzed Kinugasa system with a palladium-catalyzed allylic alkylation. This remarkable reaction enables the synthesis of α-quaternary chiral β-lactams from simple and readily available alkynes
58, nitrones
59, and allylic carbonates
60, with high yield (up to 87%) and excellent stereoselectivity (up to 96:4 er). The most plausible mechanism can be initiated with the cycloaddition of Cu(I) acetylide and nitrones, a pivotal chiral four-membered enolate Cu(I) intermediate (
61) is formed. Simultaneously, the palladium catalyst reacts with the allylic electrophile, resulting in the creation of an allylic palladium intermediate (
62). Subsequent stereo-controlled allylic substitution between
61 and
62 leads to the desired α-quaternary chiral β-lactams
63, while concurrently regenerating both the Cu(I) and Pd(0) catalysts. This one-pot approach is distinguished by a well-programmed reaction sequence, highly efficient formation of multiple bonds in asymmetric multicomponent reactions, and the construction of medicinally important α-quaternary chiral β-lactams, thus anticipating its utility in the synthesis of other biologically attractive molecules. Furthermore, in 2023, the same research group reported a novel bimetallic system, substituting the palladium with an iridium catalyst, capable of accomplishing the synthesis of the same type of compounds [
51].
5. Bimetallic Approaches Involving Triazole and Tetrazole Scaffolds
Triazoles and their derivatives are important
N-heterocyclic scaffolds in medicinal chemistry, presenting significant biological properties such as antimicrobial, antiviral, antitubercular, anticancer, antioxidant and anti-inflammatory activities, among others [
52]. The wide range of bioactivity displayed by these N-heterocycles has stimulated the development of many synthetic strategies.
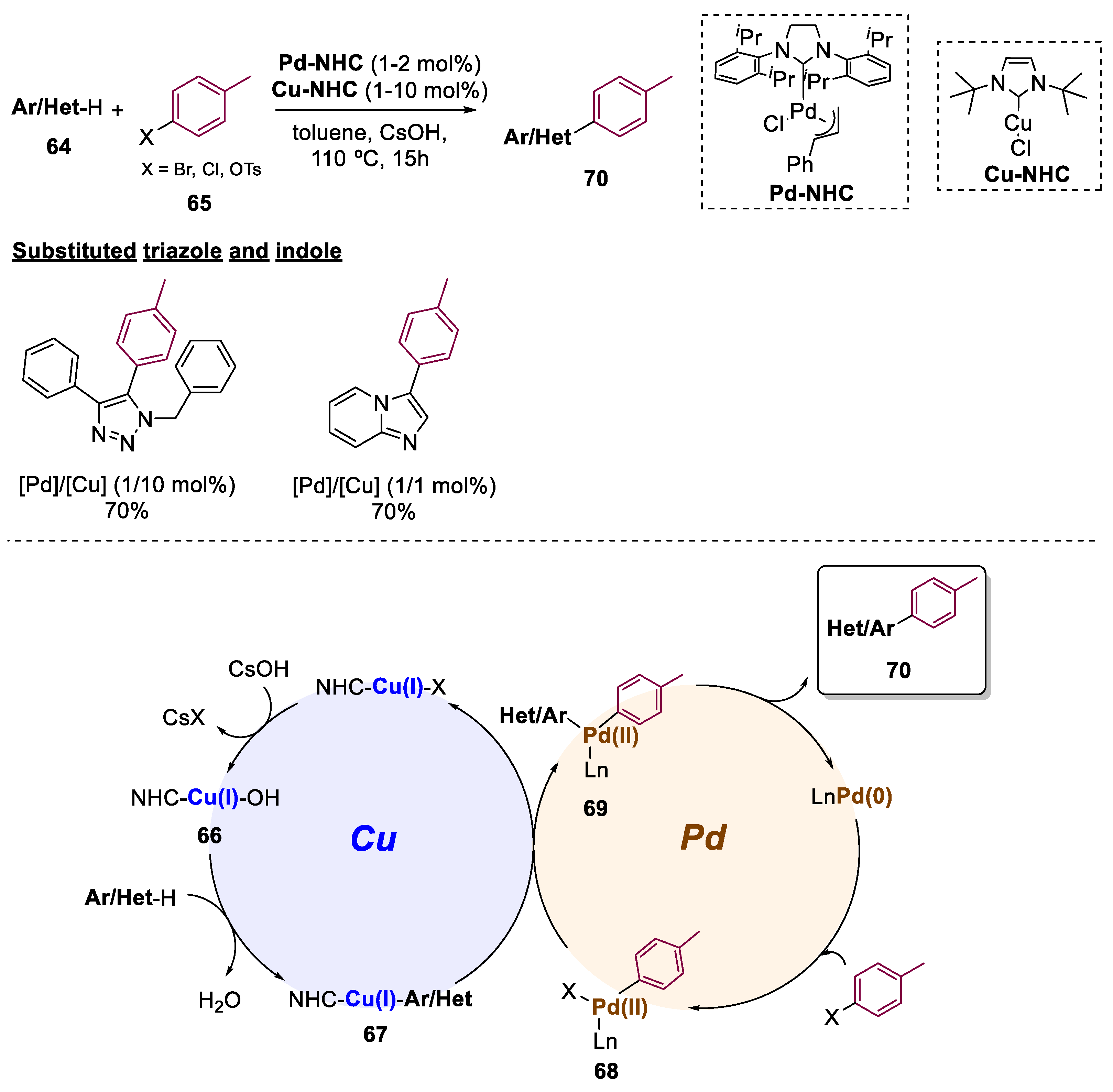
In comparison to the more established cross-coupling reactions, C−H activation is becoming a hot topic in all areas of chemistry, dismissing the pre-functionalization of both coupling partners [
53]. Considering the inferior reactivity of arenes, when compared to aryl halides, their selective and direct arylation remains a challenge. These issues can be addressed by the presence of directing groups on the arene substrate, however, may provide a limited scope since the directing group strictly facilitates the activation in the
ortho-positioned C–H bond. In this context, in 2014, Cazin and co-workers developed a process that promotes the construction of C–C bonds through an intermolecular direct arylation, that eliminated the need for directing groups (
Scheme 15) [
54]. The scope of this reaction included the functionalization of
N-heterocycles, respectively, triazole and indole rings. The authors reported a novel Cu/Pd bimetallic catalytic system to promote C-H activation from arenes or heteroarenes
64 using aryl halides
65. Both Pd and Cu complexes were composed of imidazole-based ligands (Pd/Cu-NHC), with a scope constituted by 20 examples and yields of up to 98%. The authors performed mechanistic studies that allowed them to propose a catalytic cycle, firstly including the formation of the hydroxide [Cu(OH)(NHC)] (
66) by transmetallation involving CsOH. Then, an acid-base reaction promoted the C–H activation of the heteroaryl, producing
67 and H
2O. The transmetallation between
67 and the Pd(II) catalyst
68 leads to the regeneration of the Cu(I) catalyst and the Pd(II) intermediate
69. Finally, product
70 is released after reductive elimination and the Pd(0) catalyst is regenerated, thus completing the catalytic cycle.
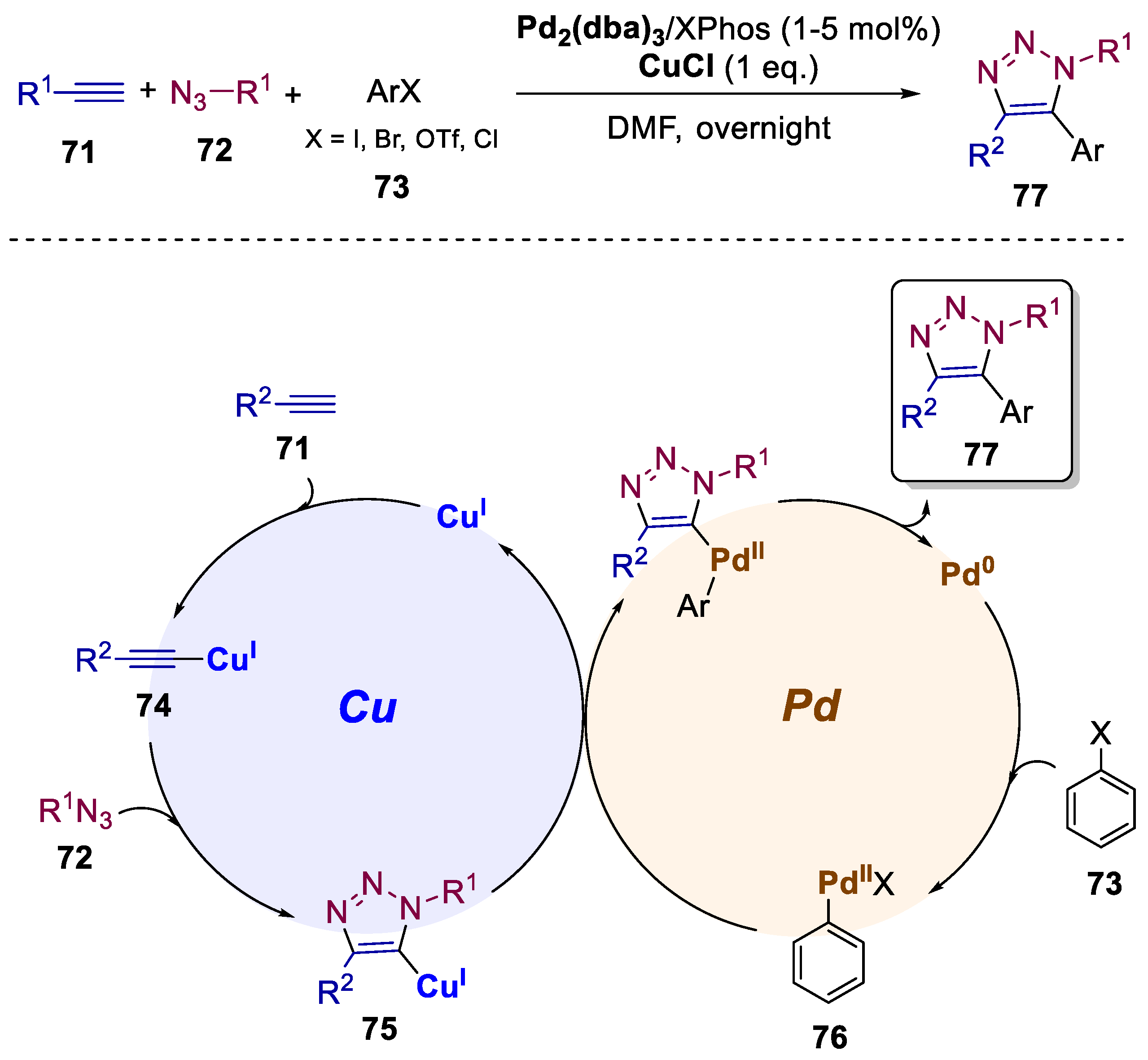
A crucial limitation of the famous click reaction remains the difficulty to obtain fully substituted 1,2,3-triazoles from internal alkynes as substrates, owing to the increased energy barrier and difficulty in regiocontrol, particularly for intermolecular reactions [
55]. Nevertheless, in 2015, Xu and coworkers successfully synthesized 1,4,5-trisubstituted 1,2,3-triazoles by relying on a Cu/Pd transmetallation relay catalysis, a modular synthesis that afforded trisubstituted triazoles from the reaction between alkynyl substrates
71 with azides
72 and aryl halides
73 (
Scheme 16) [
56]. This reaction makes it possible to freely install three different substituents onto the triazole ring in one step, with a total of 33 examples with yields up to 98%. The most plausible mechanism starts with the cycloaddition of Cu(I) acetylide
74 with azide and generates cuprate−triazole intermediate
75. Simultaneously, oxidative addition of aryl halide to Pd(0) catalyst forms the palladium intermediate
76. The transmetalation reaction between
75 and
76, followed by reductive elimination produced the target trisubstituted triazole
77 and regenerate the Pd(0) catalyst.
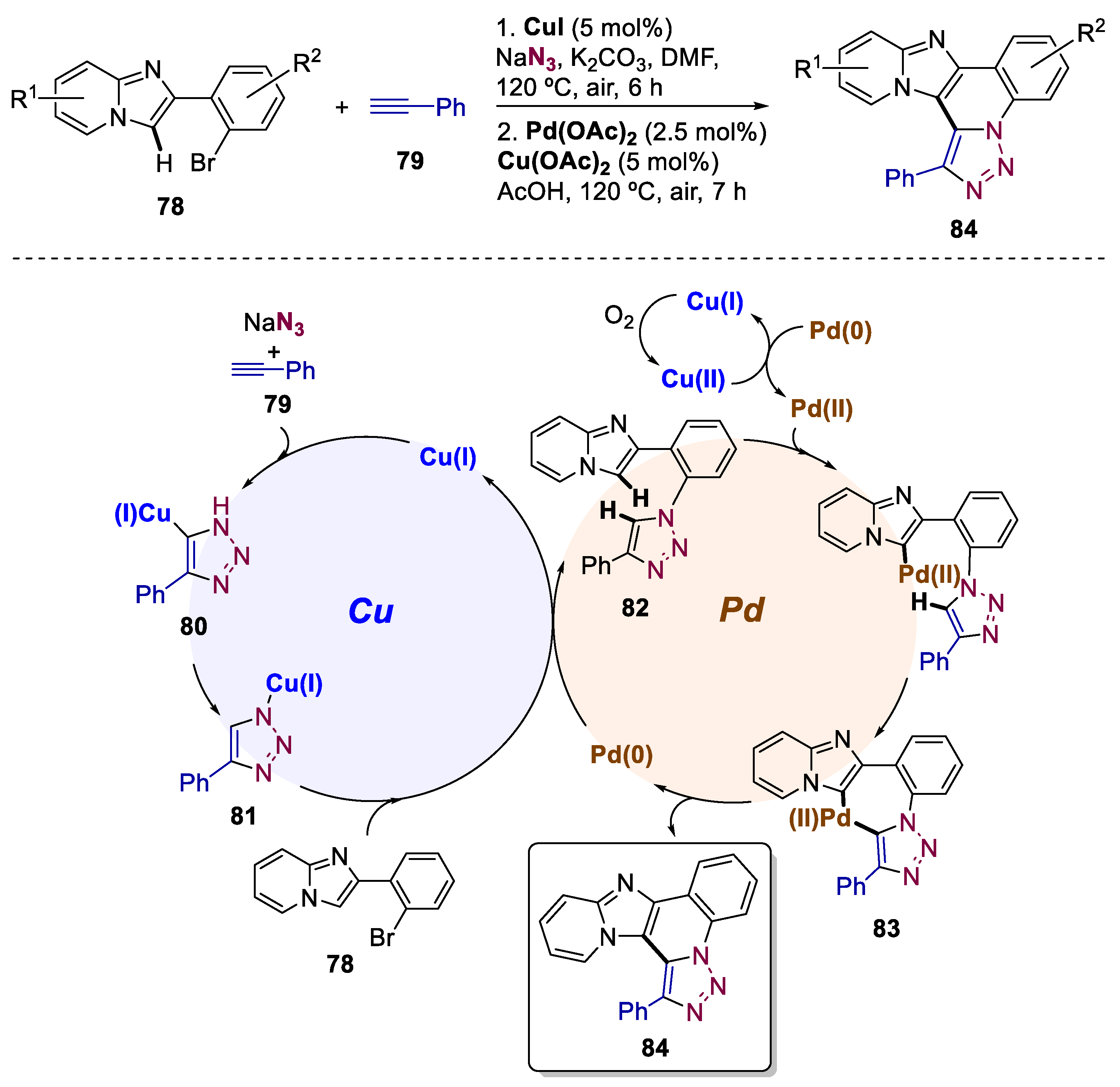
Imidazo[1,2-a]pyridine constitutes a valuable skeleton for a variety of pharmaceuticals [
57,
58]. Hence, different strategies for the preparation of these scaffolds have been implemented [
59,
60,
61]. Although there is already a report bearing a Cu-catalyzed process to obtain 1,2,3-triazole-fused imidazo[1,2-a]pyridines, it relies on brominated imidazo[1,2-a]pyridines as substrates [
62]. To optimize the atom economy and environmental aspects of this process, Fan and co-workers reported, in 2016, an efficient one-pot synthesis of 1,2,3-triazole/quinoline-fused imidazo[1,2-a]pyridines starting from 2-(2-bromophenyl)imidazo[1,2-a]pyridines
78, alkynes
79, and sodium azide (
Scheme 17) [
63]. This involved a cascade one-pot bimetallic Cu/Pd relay-catalyzed process combining azide-alkyne cycloaddition, C-N coupling, and cross-dehydrogenative C-C coupling. They built a scope of different alkynes and 2-(2-bromophenyl)imidazo[1,2-a]pyridines with a total of 24 examples and yields up to 74%. Fan suggested that the mechanism started with a Cu(I)-catalyzed azide−alkyne cycloaddition to afford intermediate
80.
81 is then formed by a copper-hydrogen exchange. C−N coupling between
78 and
81 results in the formation of the key intermediate
82. In the second phase of this cascade process, aromatic palladation of
82 by a sequence of C-H bond cleavage yields the seven-membered palladacycle
83. Finally, reductive elimination affords the final 1,2,3-triazole/quinoline-fused imidazo[1,2-a]pyridine
84 and Pd(0) is re-oxidized into Pd(II) by Cu(II)/atmospheric O
2.
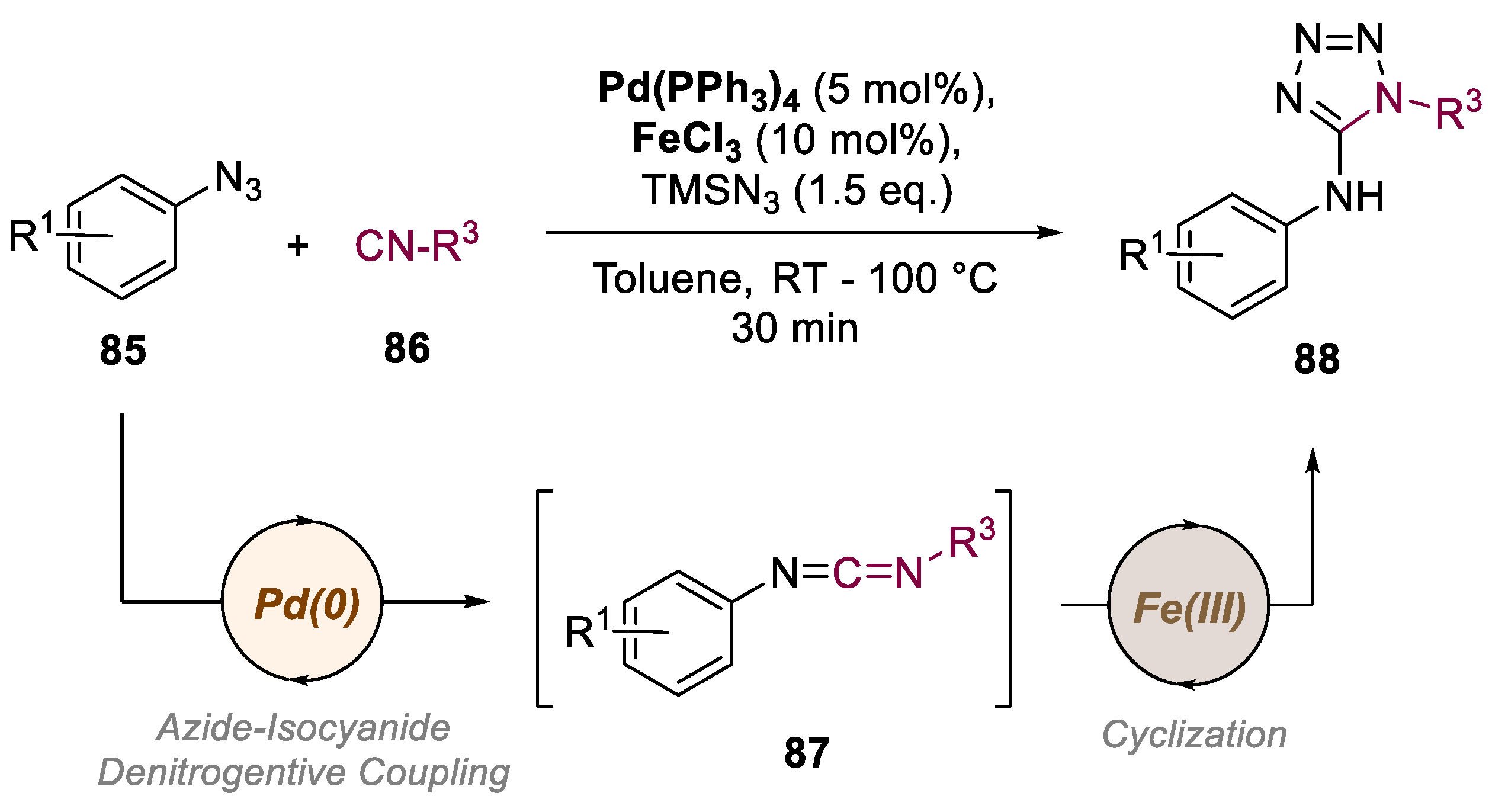
In 2018, Sawant and colleagues developed a fast and efficient method to produce aminotetrazoles (
Scheme 18) [
64]. The authors used aryl azides
85, isocyanides
86, and TMSN
3 in a sequential Pd(0)/Fe(III) catalyzed reaction. The process involved a Pd-catalyzed reaction to generate carbodiimide
87 in situ, which then reacts with TMSN
3 in the presence of FeCl
3, all in one pot, and finally yields the respective aminotetrazole
88. This approach has advantages over traditional methods that use toxic Hg and Pb salts in large amounts. With the optimized conditions in hand, they investigated the reaction's versatility showing various aryl azides with different substituents, including electron-donating and electron-withdrawing groups, which reacted well with different isocyanides and TMS-N
3 to produce the corresponding 5-amino-1
H-tetrazoles. Substituents at all three positions of the aryl azides were well-tolerated, even ortho-substituents that usually pose steric hindrance. Alkyl-, cycloalkyl-, and aryl-substituted isocyanides reacted successfully under the optimized conditions, obtaining a scope with 19 examples and yields of up to 90%, although aryl isocyanides with electron-donating groups and aliphatic azides did not react under the standard conditions.
6. Bimetallic Approaches Involving Pyridine, Pyrimidine, and Related Scaffolds
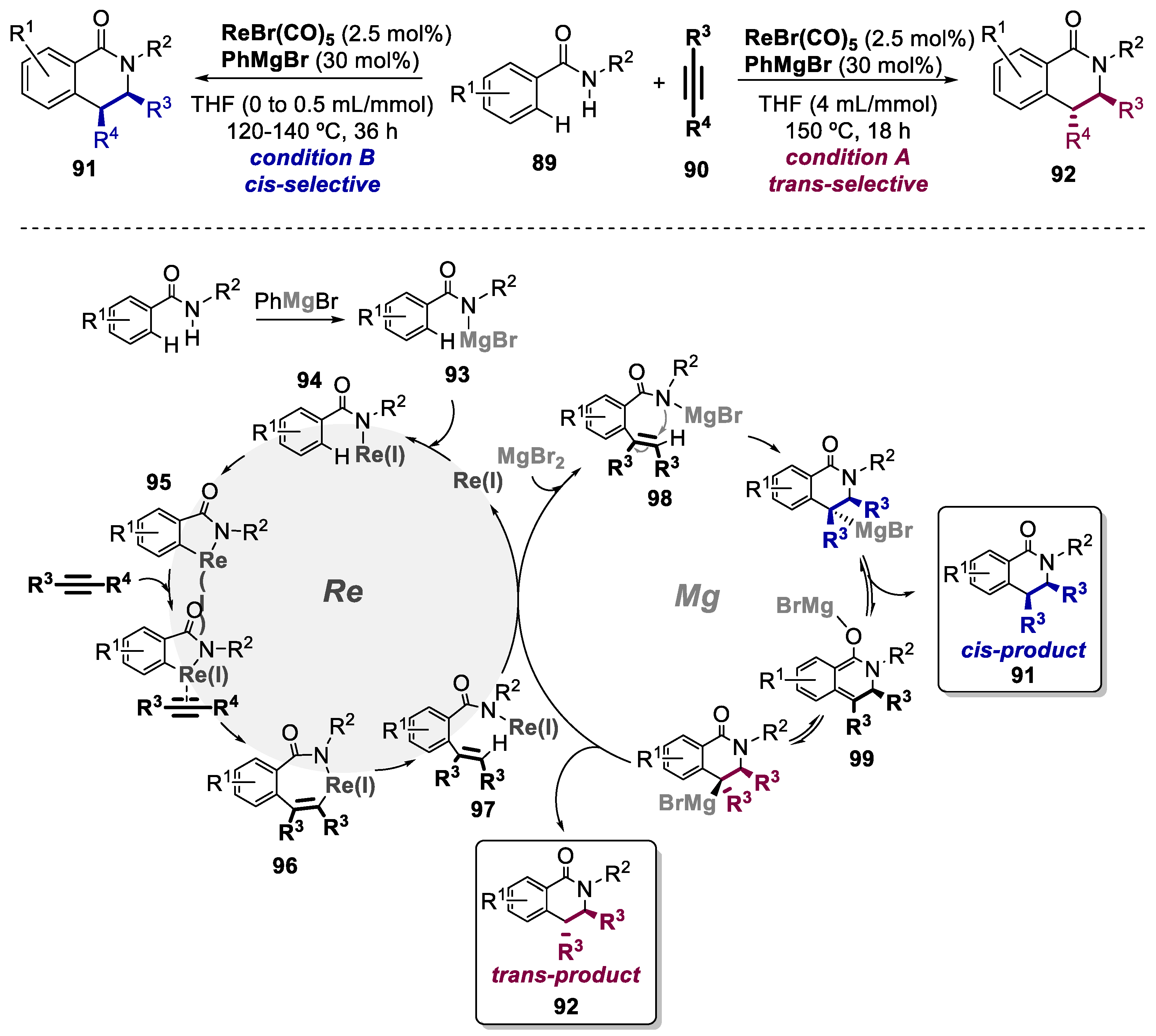
Isoquinolinones can be prepared via metal-catalyzed C-H activation [
65,
66,
67]. However, this process bears the requirement of internal/external oxidants and, in the case of 3,4-disubstituted isoquinolinones, poor diastereoselectivity is encountered. To overcome this limitations, in 2013, Wang and co-workers disclosed the first redox-neutral Re
I/Mg
II cocatalyzed [4+2] annulation of benzamides
89 and alkynes
90 via C-H/N-H functionalization to afford both
cis- and
trans-3,4-dihydroisoquinolinones (
91 and
92, respectively) in a highly diastereoselective fashion (
Scheme 19) [
68]. This was accessed in a simply by subtle tuning of reaction conditions, which adds further values to this bimetallic catalyst system. The scope for the formation of both
cis- and
trans-disubstituted scaffolds involved a total of 44 examples, affording yields up 90% and isomer ratios up to 36:1 and 16:1, respectively. Mechanistic experiments were conducted and allowed the authors to formulate a plausible reaction mechanism. With the aid of PhMgBr, via amido-magnesium
93, amido-rhenium
94 is initially formed, which then undergoes a deprotonative cyclorhenation affording rhenacycle
95. The ensuing coordination and insertion of an alkyne gives rise to seven-membered rhenacycle
96, which further leads to intermediate
97 upon protonation. Transmetalation results into a new amido-magnesium intermediate
98 which undergoes an intramolecular nucleophilic addition/cyclization generating the cis product, or further leading to the trans product via intermediate
99. Protonation of these species affords the final products and regenerates the amido-magnesium
93, thus closing the entire Re/Mg bimetallic tandem catalytic cycles.
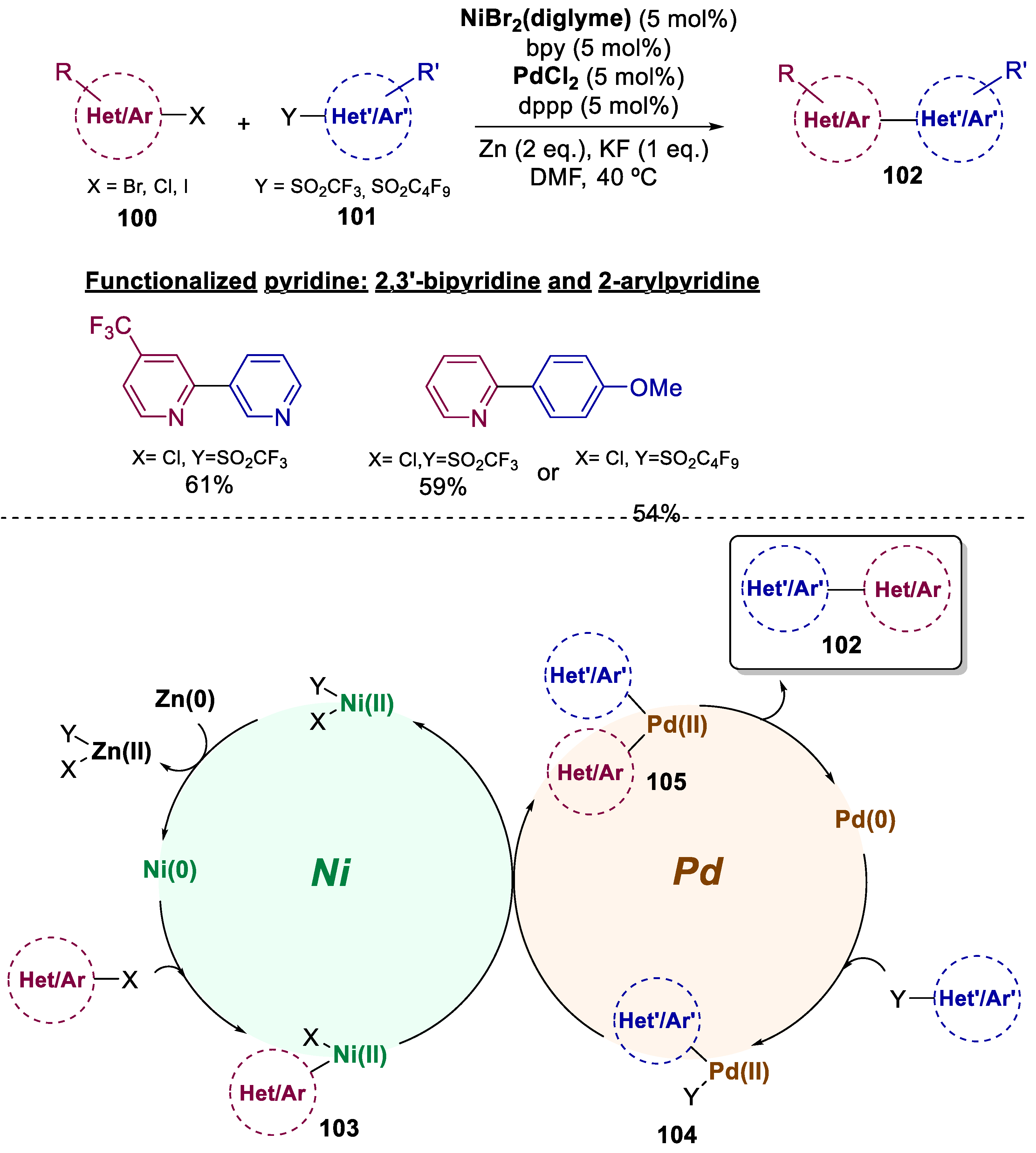
Cross-Ullmann couplings are amongst the most common procedures to obtain biaryls. Nevertheless, a crucial challenge of cross-Ullmann reactions remains the achievement of selectivity for the hetero-coupling product over the homo-coupling [
69]. For this matter, in 2015, Ackerman and co-workers developed a method to couple aryl halides
100 with aryl triflates
101 and affording hetero-coupled bi(hetero)aryls
102 by directly by a Ni/Pd bimetallic-catalyzed cross-Ullmann (
Scheme 20) [
70]. The selectivity was envisioned by the orthogonal reactivity of the two catalysts and the relative stability of the two arylmetal intermediates. Initially, each catalyst formed less than a 5% yield of the cross-coupled product. Yet, a total of 20 examples were described achieving yields of up to 94%. This new method can obtain biaryls, heteroaryls, dienes, and, specifically,
N-heterocycles by functionalization of the pyridine ring, affording 2,3’-bipyridine and 2-arylpyridine. The authors suggest that the mechanism of this cross-Ullmann reaction undergoes a bimetallic approach, where the Ni catalyst reacts preferentially with aryl bromides to form a transient, reactive intermediate
103, while the Pd catalyst reacts preferentially with aryl triflates to afford a persistent intermediate
104. When each of the two catalysts activates only one of the two substrates, transmetallation occurs from nickel to palladium (
105), which affords the final product by reductive elimination, thus regenerating the Pd
0 catalyst. Ni
0 catalyst is reobtained with the help of a Zn
0 reductant.
Copper-catalyzed electrophilic cyclization is highly attractive due to its low cost, easy availability, and high tolerance towards diverse functional groups. Nevertheless, there are relatively few synthetic studies on the Cu-catalyzed three component tandem reaction for the synthesis of polyheterocycles. Moreover, there has been no report which shows the dual behaviour of a Cu(I) catalyst, as well as
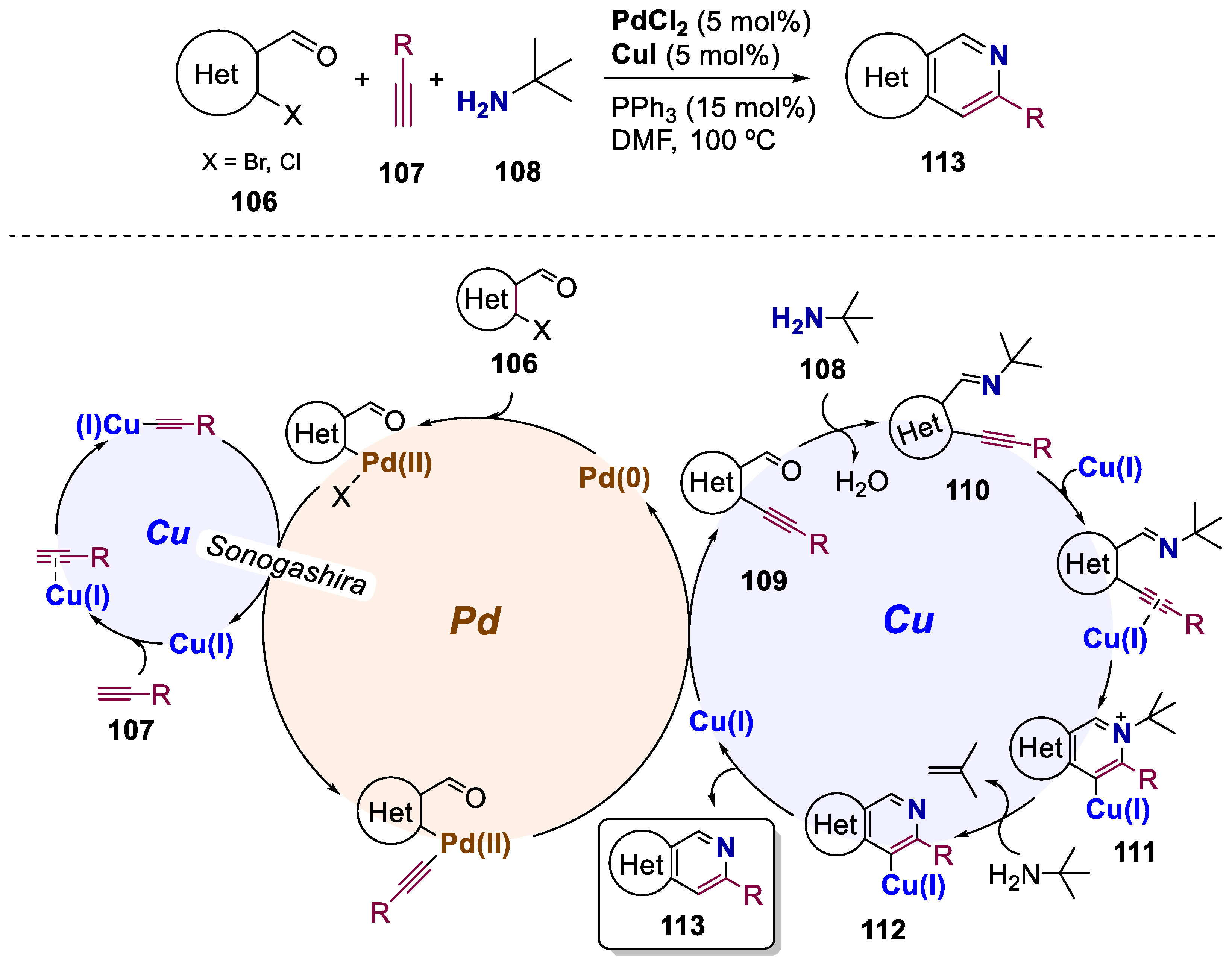
tert-butylamine, for the synthesis of polyheterocycles. For this matter, in 2016, Verma’s group reported the synthesis of heterocyclic scaffolds starting from substrates
106 and alkynyls 107 via Pd(II)/Cu(I)-catalyzed Sonogashira coupling followed by a Cu(I)-catalyzed
6-endo-dig cyclization, with
tert-butylamine
108 as a nitrogen source (
Scheme 21) [
71]. The scope of this process included the preparation of naphthyridines, isoquinolines, benzothieno- and benzofuropyridines with a total of 33 examples and yields up to 83%. To give insights into the mechanism, an array of preliminary control experiments was performed and revealed the dual role of Cu: to enhance the rate of Sonogashira coupling and to assist electrophilic cyclization. The catalytic system involves the formation of C–C and C–N bonds via Sonogashira coupling and electrophilic cyclization, respectively. The authors suggest that, initially, the
ortho-halo aldehyde reacts with terminal alkynes under Sonogashira coupling conditions generating the
ortho-alkynyl aldehyde intermediate
109. The latter reacts with
tert-butylamine and leads to the formation of imine
110. π-Complexation between
110 and Cu(I) facilitates the electrophilic cyclization and affords
111. The presence of a
tert-butyl group enhances the formation of intermediate
112 by the elimination of an isobutylene fragment. Finally, protodemetalation yields the desired cyclized pyridine-containing heterocycle
113.
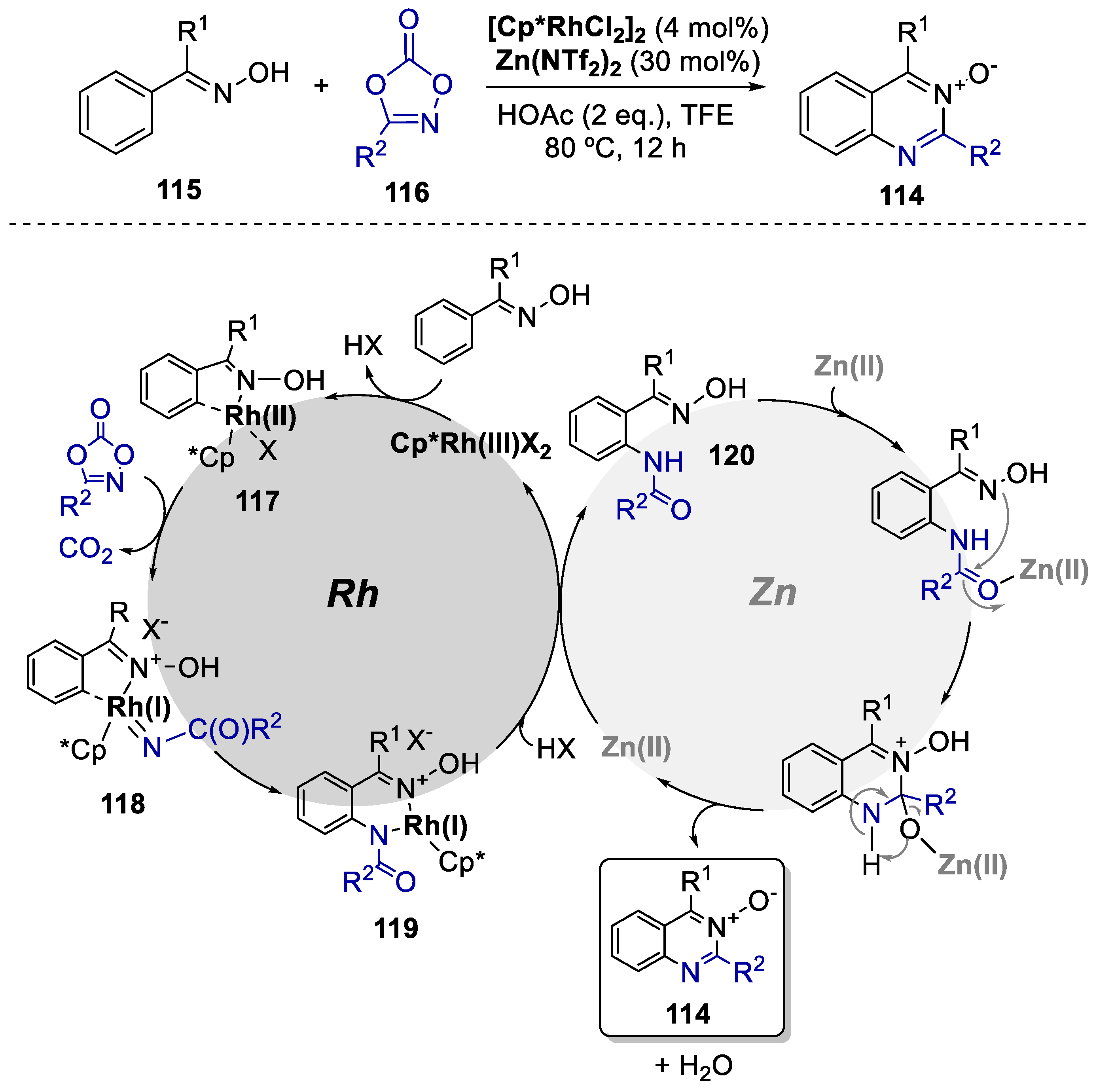
Until nowadays, the preparation of
N-oxides of azacycles via direct C−H activation of arenes remains a challenge and highly underexplored, with only a few reports on the synthesis of
N-oxides of isoquinoline [
72,
73]. For this reason, in 2016, Li and co-workers prepared quinazoline-
N-oxides
114 via a single-step C−H activation approach by a Rh(III)/Zn(II) bimetallic-catalyzed process. They selected simple substrates, such as ketoximes
115 and 1,4,2-dioxazol-5-ones
116 (
Scheme 22) [
74]. This annulation system proceeded with high efficiency under mild conditions with H
2O and CO
2 as the coproducts, obviating any need for oxidants. A total of 32 examples of both omixes and dioxazolones was obtained in yields of up to 95%. Preliminary mechanistic studies were constructed to gain insight into the mechanism of this annulation reaction) and concluded that Rh(III) participated in the C−H activation-amidation of the ketoximes and Zn(II) in the cyclization. The authors suggest that, first, an active rhodium catalyst Cp*RhX
2 (where X = NTf
2 or OAc) is generated from the anion exchange between [RhCp*Cl
2]
2 and ZnNTf
2 or HOAc. Next, the oxime reagent undergoes a ciclometalation to afford the rhodacyclic intermediate
117 and an acid via a concerted metalation−deprotonation mechanism. Coordination of dioxazolone is followed by a decarboxylation with CO
2 elimination, yielding a nitrenoid species
118, and subsequent migratory insertion of the Rh-aryl bond produces the amidate
119. Protonolysis of the latter releases the amidated intermediate
120 and regenerates the Rh(III) complex. Zn(II) will then catalyze the cyclization and condensation of
120, furnishing the final quinazoline
N-oxide
114.
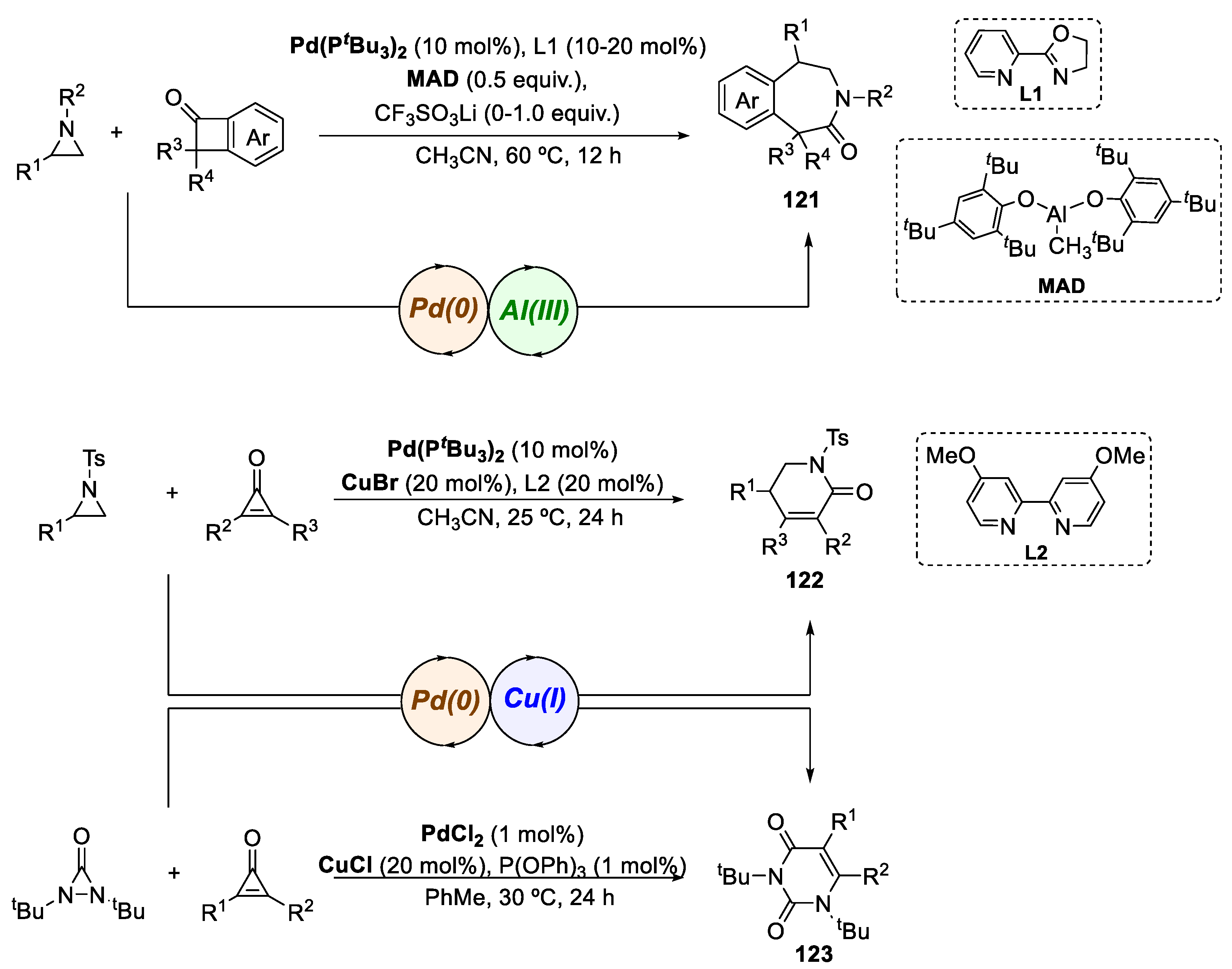
In 2021, Zhao and co-workers developed a general ring expansion strategy that enables the efficient and scalable synthesis of diverse
N-heterocycles (
Scheme 23), such as 3-benzazepinones
121 (up to 90% yield), dihydropyridinones
122 (up to 91% yield) and uracils
123 (up to 98% yield) [
75]. The designed concept is based on the use of synergistic bimetallic catalysis to promote a formal cross-dimerization reaction between three-membered aza-heterocycles and three- and four-membered-ring ketones. The authors present a novel methodology that combines strain-release-induced oxidative C-C bond cleavage and C-N bond cleavage, effectively expanding the scope for stereospecific
N-heterocycle synthesis. In this route, the palladium complex serves as the main catalyst for the reactions, although aluminum or copper, which function as Lewis acids, are also highlighted as critical components in this pathway. This approach provides a versatile and reliable method for the synthesis of 3-benzazepinones, dihydropyridinones and uracils, showcasing its flexibility and significant potential in the synthesis of complex molecules through transition-metal-catalyzed formal cross-dimerization of cyclic compounds.
7. Bimetallic Approaches Involving Other N-heterocyclic Scaffolds
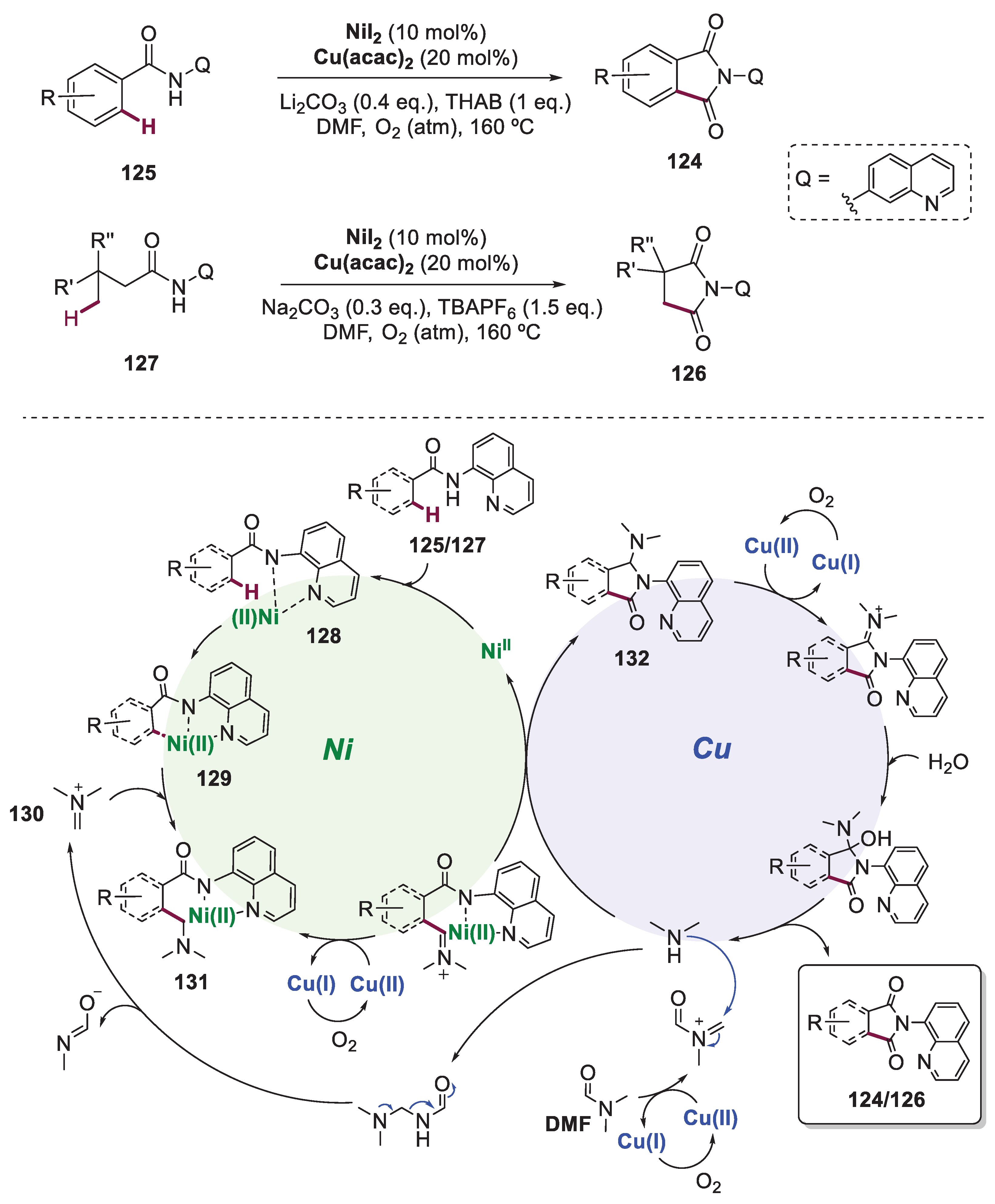
Whitin the metal-catalyzed C−H functionalization reaction’s class, direct carbonylation has attracted considerable attention in recent years due to the prevalent presence of the carbonyl group in organic molecules. The direct carbonylation of C(sp
3)−H bonds has been demonstrated by transition metal-catalyzed processes, however, mainly rely on the use of toxic CO gas at high pressure [
76,
77,
78]. To overcome this limitation, Ge and co-workers described, in 2015, the direct carbonylation of aromatic sp
2 and unactivated sp
3 C−H bonds of amides via a Ni/Co bimetallic catalysis with
N,N-dimethylformamide (DMF) as the carbonyl source (
Scheme 24) [
79]. The reactions are performed under atmospheric O
2 and the substrates are constituted by a bidentate directing group (Q). The authors provided a scope of aryl-substituted phthalimides
124, from aromatic amides
125 achieving yields up to 90% with the optimized conditions, as well as 3,3’-disubstituted succinimides
126, from aliphatic amides
127, with yields up to 81%. Preliminary control experiments elucidated that both nickel and copper catalysts are required for this process, suggesting that this reaction is performed via synergistic catalysis. The authors suggested that the process starts with the coordination of amide
125/127 to Ni(II) via a ligand exchange under basic conditions, forming complex
128. Then, cyclometalation of
128 occurs via either sp
2 or sp
3 C−H bond activation to generate the intermediate
129, keeping in mind that sp
2 C−H bond cleavage is a reversible step while sp
3 is irreversible. Electrophile
130 is then inserted into the catalytic cycle, generated in situ from DMF. This suffers decarboxylation or an elimination process via Cu(II)/O
2. These intermediates react through a nucleophilic addition and sequential decarbonylation. The nucleophilic addition of the intermediate
C to the iminium ion intermediate
130 provides
131. Oxidation of the latter followed by intramolecular nucleophilic addition gives rise to the intermediate
132 which then produces the product
124 or
126 via oxidation by Cu(II) and hydrolysis.
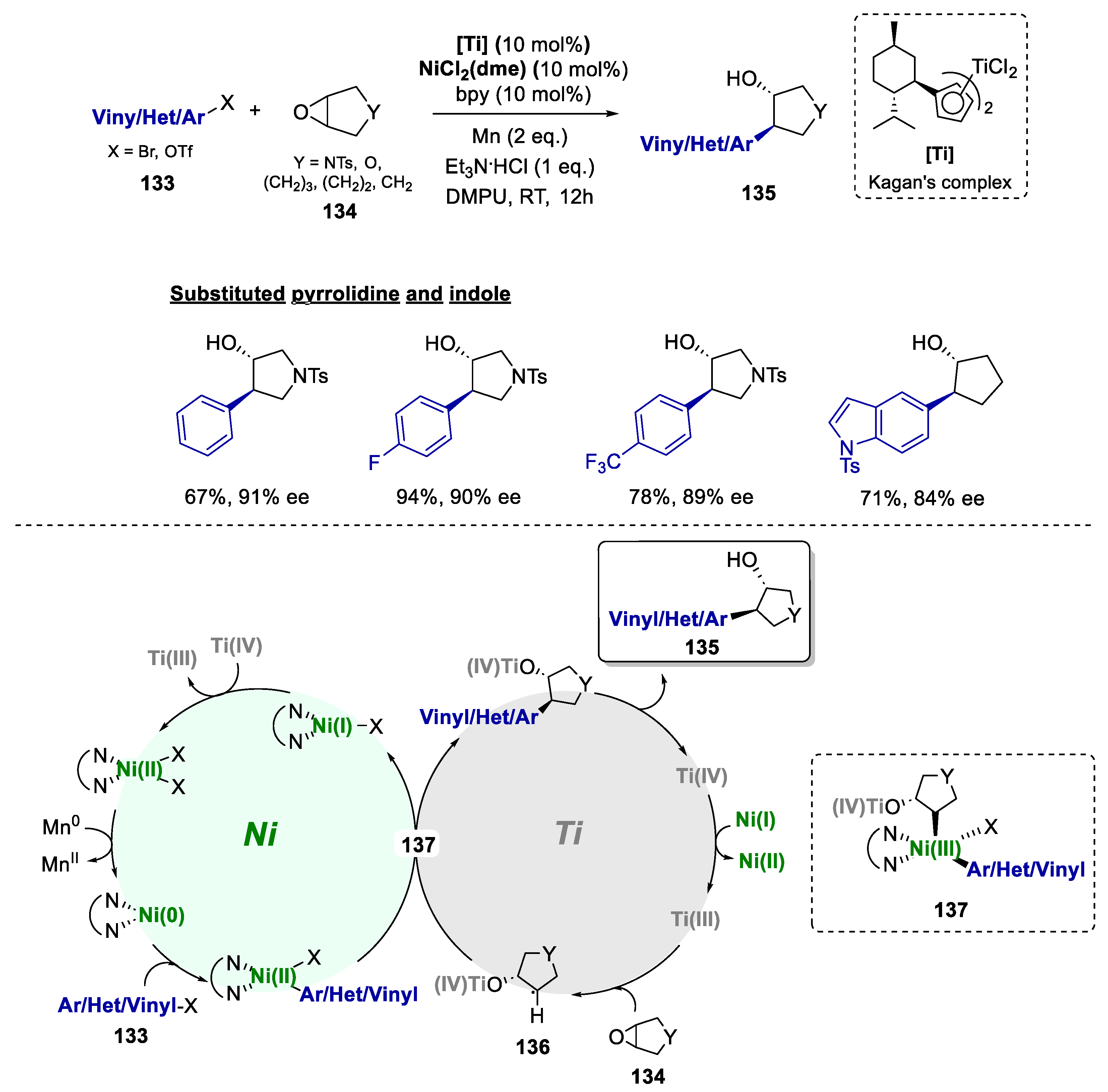
The enantioselective coupling of aryl and vinyl nucleophiles with
meso-epoxides is considered to be highly challenging, with the best results to date resorting to aryl lithium reagents and chiral ligands [
80,
81]. In this context, in 2015, Zhao and Weix reported an enantioselective cross-electrophile coupling of aryl halides
133 with meso-epoxides
134 to form
trans-β-arylcycloalkanols
135, from a novel bimetallic Ni(II)/Ti(III) catalytic system (
Scheme 25) [
82]. The reaction was catalyzed by a combination of (bpy)NiCl
2 and a chiral titanocene under reducing conditions. Different titanocenes were tested, and the one first reported by Cesarotti and co-workers [
83] showed the highest yield and enantioselectivity . This combination enantioselectively coupled aryl, heteroaryl, or vinyl halides with meso-epoxides with a scope constituted by 28 examples. It also included examples on the functionalization of pyrrolidine scaffolds and one example for indole, with yields up to 94% and enantiomeric excesses up to 91% ee. These authors suggested that the coupling mechanism (
Scheme 25) would be initiated by the enantioselective formation of a β-titanoxy carbon radical from the meso-epoxide (
136). Then, the oxidative addition of a β-titanoxy carbon radical to an arylnickel(II) intermediate would form a diorganonickel(III) species (
137), and the reductive elimination of the product (
135). Finally, the reduction of both catalysts closes the catalytic cycle.
Scheme 1.
Xu’s contributions towards the bimetallic synthesis of fused bicyclic- and spiroaminals.
Scheme 1.
Xu’s contributions towards the bimetallic synthesis of fused bicyclic- and spiroaminals.
Scheme 2.
A plausible mechanism of the Au/Lewis acid bimetallic synthesis of fused bicyclic- and spiroaminals by Xu’s group.
Scheme 2.
A plausible mechanism of the Au/Lewis acid bimetallic synthesis of fused bicyclic- and spiroaminals by Xu’s group.
Scheme 3.
General conditions for the preparation of spiroaminals by a Au(I)/Ni(II)-catalyzed bimetallic approach.
Scheme 3.
General conditions for the preparation of spiroaminals by a Au(I)/Ni(II)-catalyzed bimetallic approach.
Scheme 4.
General conditions for the preparation of oxazoles by a Zn(II)/Sc(III)-catalyzed bimetallic approach.
Scheme 4.
General conditions for the preparation of oxazoles by a Zn(II)/Sc(III)-catalyzed bimetallic approach.
Scheme 5.
General conditions and a plausible mechanism for the preparation of fused bicyclic acetals or aminals by a Au(I)/Ni(II)-catalyzed bimetallic approach.
Scheme 5.
General conditions and a plausible mechanism for the preparation of fused bicyclic acetals or aminals by a Au(I)/Ni(II)-catalyzed bimetallic approach.
Scheme 7.
General conditions and a plausible mechanism for the preparation of furo[3,4-]indoles by a Ag(I)/Bi(III)/Pd(II)-catalyzed trimetallic approach.
Scheme 7.
General conditions and a plausible mechanism for the preparation of furo[3,4-]indoles by a Ag(I)/Bi(III)/Pd(II)-catalyzed trimetallic approach.
Scheme 8.
General conditions and a plausible mechanism for the preparation of 1,3-di- and 1,3,4-trisubstituted β-carbolines by a Ag(I)/Bi(III)/Pd(II)-catalyzed trimetallic approach.
Scheme 8.
General conditions and a plausible mechanism for the preparation of 1,3-di- and 1,3,4-trisubstituted β-carbolines by a Ag(I)/Bi(III)/Pd(II)-catalyzed trimetallic approach.
Scheme 9.
General conditions and a plausible mechanism for the preparation of 1,2,4-triazolo[1,5-b] pyridazines by a Cu(I)/Zn(II)-catalyzed bimetallic approach.
Scheme 9.
General conditions and a plausible mechanism for the preparation of 1,2,4-triazolo[1,5-b] pyridazines by a Cu(I)/Zn(II)-catalyzed bimetallic approach.
Scheme 10.
General conditions and a plausible mechanism for the preparation of 3-alkylidene isoindolinones by a Rh(III)/Ag(I)-catalyzed bimetallic approach.
Scheme 10.
General conditions and a plausible mechanism for the preparation of 3-alkylidene isoindolinones by a Rh(III)/Ag(I)-catalyzed bimetallic approach.
Scheme 11.
General conditions for the preparation of asymmetrically-substituted indolines by a Au(I)/Ru(II)-catalyzed bimetallic approach.
Scheme 11.
General conditions for the preparation of asymmetrically-substituted indolines by a Au(I)/Ru(II)-catalyzed bimetallic approach.
Scheme 12.
General conditions for the preparation of 2-arylindoles by a Mn(I)/Pd(II)- or Ni(II)/Pd(II)- catalyzed bimetallic approaches.
Scheme 12.
General conditions for the preparation of 2-arylindoles by a Mn(I)/Pd(II)- or Ni(II)/Pd(II)- catalyzed bimetallic approaches.
Scheme 13.
General conditions and plausible mechanism for the preparation of allylated chiral β-lactams by a Rh(II)/Pd(0)-catalyzed bimetallic approach.
Scheme 13.
General conditions and plausible mechanism for the preparation of allylated chiral β-lactams by a Rh(II)/Pd(0)-catalyzed bimetallic approach.
Scheme 14.
General conditions and plausible mechanism for the preparation of α-quaternary chiral β-lactams by a Cu(I)/Pd(0)-catalyzed bimetallic approach.
Scheme 14.
General conditions and plausible mechanism for the preparation of α-quaternary chiral β-lactams by a Cu(I)/Pd(0)-catalyzed bimetallic approach.
Scheme 15.
General conditions and plausible mechanism for the preparation of functionalized N-heterocycles, including triazole and indole scaffolds by a Cu(I)/Pd(II)-catalyzed bimetallic approach.
Scheme 15.
General conditions and plausible mechanism for the preparation of functionalized N-heterocycles, including triazole and indole scaffolds by a Cu(I)/Pd(II)-catalyzed bimetallic approach.
Scheme 16.
General conditions and plausible mechanism for the preparation of 1,4,5-trisubstituted 1,2,3-triazoles by a Cu(I)/Pd(0)-catalyzed bimetallic approach.
Scheme 16.
General conditions and plausible mechanism for the preparation of 1,4,5-trisubstituted 1,2,3-triazoles by a Cu(I)/Pd(0)-catalyzed bimetallic approach.
Scheme 17.
General conditions and plausible mechanism for the preparation of 1,2,3-triazole/quinoline-fused imidazo[1,2-a]pyridines by a Cu(I)/Pd(II)-catalyzed bimetallic approach.
Scheme 17.
General conditions and plausible mechanism for the preparation of 1,2,3-triazole/quinoline-fused imidazo[1,2-a]pyridines by a Cu(I)/Pd(II)-catalyzed bimetallic approach.
Scheme 18.
General conditions and plausible mechanism for the preparation of aminotetrazoles by a Pd(0)/Fe(III)-catalyzed bimetallic approach.
Scheme 18.
General conditions and plausible mechanism for the preparation of aminotetrazoles by a Pd(0)/Fe(III)-catalyzed bimetallic approach.
Scheme 19.
General conditions and plausible mechanism for the preparation of cis- and trans-3,4-dihydroisoquinolinones by a Re(I)/Mg-catalyzed bimetallic approach.
Scheme 19.
General conditions and plausible mechanism for the preparation of cis- and trans-3,4-dihydroisoquinolinones by a Re(I)/Mg-catalyzed bimetallic approach.
Scheme 20.
General conditions and plausible mechanism for the preparation of hetero-coupled bi(hetero)aryls by a Ni(II)/Pd(II)-catalyzed bimetallic approach.
Scheme 20.
General conditions and plausible mechanism for the preparation of hetero-coupled bi(hetero)aryls by a Ni(II)/Pd(II)-catalyzed bimetallic approach.
Scheme 21.
General conditions and plausible mechanism for the preparation of polyheterocycles by a Cu(I)/Pd(II)-catalyzed bimetallic approach.
Scheme 21.
General conditions and plausible mechanism for the preparation of polyheterocycles by a Cu(I)/Pd(II)-catalyzed bimetallic approach.
Scheme 22.
General conditions and plausible mechanism of the preparation of quinazoline N-oxides by a Rh(III)/Zn(II)-catalyzed bimetallic approach.
Scheme 22.
General conditions and plausible mechanism of the preparation of quinazoline N-oxides by a Rh(III)/Zn(II)-catalyzed bimetallic approach.
Scheme 23.
General conditions for the preparation of 3-benzazepinones by a Pd(0)/Al(III)-catalyzed bimetallic approach and dihydropyridinones and uracils by a Pd(0)/Cu(I)-catalyzed bimetallic approach.
Scheme 23.
General conditions for the preparation of 3-benzazepinones by a Pd(0)/Al(III)-catalyzed bimetallic approach and dihydropyridinones and uracils by a Pd(0)/Cu(I)-catalyzed bimetallic approach.
Scheme 24.
General conditions and plausible mechanism of the preparation of phthalimides and 3,3’-disubstituted succinimides by a Ni(II)/Cu(I)-catalyzed bimetallic approach.
Scheme 24.
General conditions and plausible mechanism of the preparation of phthalimides and 3,3’-disubstituted succinimides by a Ni(II)/Cu(I)-catalyzed bimetallic approach.
Scheme 25.
General conditions and plausible mechanism of the preparation of trans-β-arylcycloalkanols by a Ni(II)/Ti(IV)-catalyzed bimetallic approach.
Scheme 25.
General conditions and plausible mechanism of the preparation of trans-β-arylcycloalkanols by a Ni(II)/Ti(IV)-catalyzed bimetallic approach.