Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
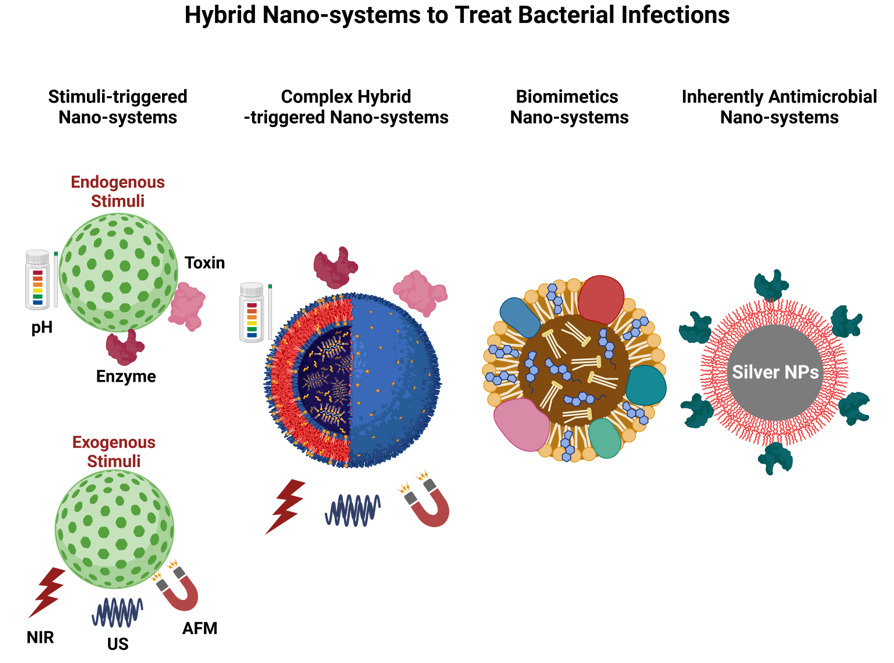
2. Hybrid Nano-systems for Combinational Therapy
2.1. Stimuli-triggered drug delivery
2.1.1. Drug Delivery Triggered by Endogenous Stimuli
| Nanomaterials | Trigger | Target Pathogen | Drug | Active Targeting | Ref. |
|---|---|---|---|---|---|
| Endogenous Triggered DDS | |||||
| PLGA-PLH-PEG nanoparticles | pH |
S. aureus E. coli |
Vancomycin | Electrostatic | 21 |
| Squalenoylated Penicillin Bioconjugates | pH | S. aureus | β-lactam antibiotics | NA | 32 |
| Chitosan modified gold nanoparticles, Liposome | pH | H. Pylori | Doxycycline | NA | 17 |
| Mesoporous silica nanoparticle, pH sensitive Nanovalves | pH | F. tularemia | Moxifloxacin | NA | 33 |
| Ureido-conjugated chitosan/TPP multifunctional nanoparticle | pH | H. Pylori | Amoxicillin | Ureido targeting groups | 18 |
| Triblock polymers PEG-b-PCL-b-PAE | pH | S. aureus | Vancomycin | Electrostatic | 22 |
| Mg-based micromotor | pH | H. Pylori | pH sensitive polymer coating | NA | 15 |
| Mg-based micromotor | pH | H. Pylori | Ciprofloxacin | NA | 34 |
| Solid lipid Np | pH | MSSA, MRSA | Vancomycin | NA | 35 |
| AMX-PLGA/UCCs-2 nanoparticles | pH | H. Pylori | Amoxicillin | UCCs-2 as targeting moiety | 20 |
| Cysteine conjugated chitosan/PMLA | pH | H. Pylori | Amoxicillin | NA | 19 |
| AMP (LL37) and lipid (OA) selfassembly | pH | E. coli | LL 37 | Electrostatic | 24 |
| Antimicrobial Peptide-Reduced Gold Nanoclusters | pH | E. coli, P. aeruginosa, S. aureus, S. epidermidis | Antimicrobial peptide | Electrostatic | 25 |
| Hyaluronic acid (HA)-based nanocapsules | Enzyme (hyaluronidase) |
S. aureus,E. coli | polyhexanide | NA | 27 |
| Chitosan-modified gold nanoparticles (AuChi-liposome) | Enzyme (phospholipase A2) | H. Pylori | Doxycycline | NA | 28 |
| PGA and Bla-responsive polymeric vesicles | Enzyme (penicillin Gamidase (PGA) and b-lactamase (Bla) | MRSA, B. longum, L. acidophilus,and E. faecalis | Vancomycin, Gentamycine, quinupristin/dalfopristin (Synercid) | NA | 36 |
| MSNP/LIPID Bilayer | Enzyme (Lipase) | S. aureus | Gentamycin | bacteria-targeting peptide ubiquicidin (UBI29−41) | 29 |
| Monoolein liquid crystal nanoparticles (MO-LCNPs) | Enzyme (Lipase) | P. aeruginosa S. aureus | Rifampicin Ciprofloxacin |
NA | 30 |
| Gold nanoparticle-stabilized phospholipid liposomes. | Alpha-toxin | MRSA | Vancomycin | NA | 37 |
| Liposome-based nanoreactors | Alpha-toxin | MRSA | Rifampicin | NA | 38 |
| Exogenous Triggered DDS | |||||
| Hollow microspheres (HMs) shell PLGA Core- Van, polypyrrole nanoparticles |
PPT (808 nm, 0.5 W/cm2, 15 min |
S. aureus (subcutaneous bacterial abscesses) | Vancomycin | NA | 39 |
| Reduced graphene oxide (rGO)-embedded polymeric nanofiber mats | PPT (980 nm, 1 W/cm2), 10 min) |
E. coli K12 S. aureus S. epidermidis |
ampicillin cefepime | NA | 40 |
| (PDA-PEG-Van | PPT (808 nm, 0.78 W/cm210 min | MRSA | Vancomycin | 41 | |
| Vancomycin (Van)-modified gold nanostars | PPT | MRSA | Vancomycin | Vancomycin | 42 |
| Bubble liposomes | US (0.15 or 0.44 W/ cm2) | C. trachomatis | Doxycycline Ceftizoxime | NA | 43 |
| microbubble-mediated low-intensity ultrasound | US (100mW/cm2; 46.5 KHz; 33% duty cycle; 12 h. | E. coli | Gentamycin | NA | 44 |
| dextran sulfate-shelled perfluoropentane (PFP)-cored NBs | US (f = 2.5 MHz; P = 5 W; t = 10 min) | MRSA | Vancomycin | NA | 45 |
| microbubble suspension | US (1.1 MHz, 2.5 Mpa, 5500 cycles at 20 ms pulse duration) for 20 s) | UTI (E. faecalis) | Gentamycin | NA | 46 |
| (Pd@Pt-T790) | US | MRSA | T790 as sonosensitizer | NA | 47 |
| iron oxide nanoparticles (NPs) encapsulated into polymeric microspheres | Magnetic | S. aureus | ciprofloxacin | NA | 48 |
| MnFe2O4 superparamagnetic nanoparticles, pegalyated chitosan as shell | Magnetic |
S. aureus S. Epidermitis, B. subtilis , E. coli, P.aeruginosa, and MRSA |
Vancomycin | NA | 49 |
| iron oxide nanoparticles | Magnetic | S. aureus, B. subtilis , E. coli, andP.aeruginosa | Gentamicin. | NA | 50 |
| Fe3O4 nanoparticles, chitosan microbeads cross-linked with varying lengths of polyethylene glycol dimethacrylate. | Magnetic | S. aureus | vancomycin | NA | 51 |
| MNPs@Ag@HA | Magnetic |
S. aureus, E. coli S. aureus biofilm |
gentamicin | NA | 52 |
| Hybrid Nano-systems | |||||
| MNP Eudragit®S100 |
pH Magnetic |
H. Pylori | Amoxicillin | NA | 53 |
| SiO2-Cy-Van | Bacteria-activated polyelectrolyte dissociation | MRSA | Vancomycin conjugate poly(acrylic acid) | Vancomycin | 54 |
| Amphiphilic block copolymer consisting of biotinylated poly(ethylene glycol)-b-poly(β-amino ester)-b-poly(ethylene glycol) grafted with PEGylated lipid (Biotin-PEG-b-PAE(-g-PEGb-DSPE)-b-PEG-Biotin) | pH, Enzyme |
P. aeruginosa, (Sepsis) |
Ciprofloxacin, and an anti-inflammatory agent (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide, TPCA-1) | Intercellular adhesion molecule-1 antibodies | 55 |
| amphiphilic poly (ethylene glycol)−poly(ε-caprolactone) (PECL) copolymers, | pH, Enzyme (Lipase) | P. aeruginosa | Ciprofloxacin | Vancomycin as targeting ligand | 56 |
| Amp-MSN@FA@CaP@FA | pH | E. coli, S. aureus | AMP | Folic acid | 57 |
| Multimetallic microparticles (MMPs), PLGA, AgNP, ZnO NP | NA | M. tuberculosis | Rifampicin | NA | 58 |
| MSN, PGEDA, CB[7],TPE-(COOH)4 | NA | S. aureus, E. coli | Amoxicillin | Electrostatic | 59 |
| Mesoporous silica nanoparticles decorated with polycationic dendrimers | NA | E. coli | Levofloxacin | Electrostatic | 60 |
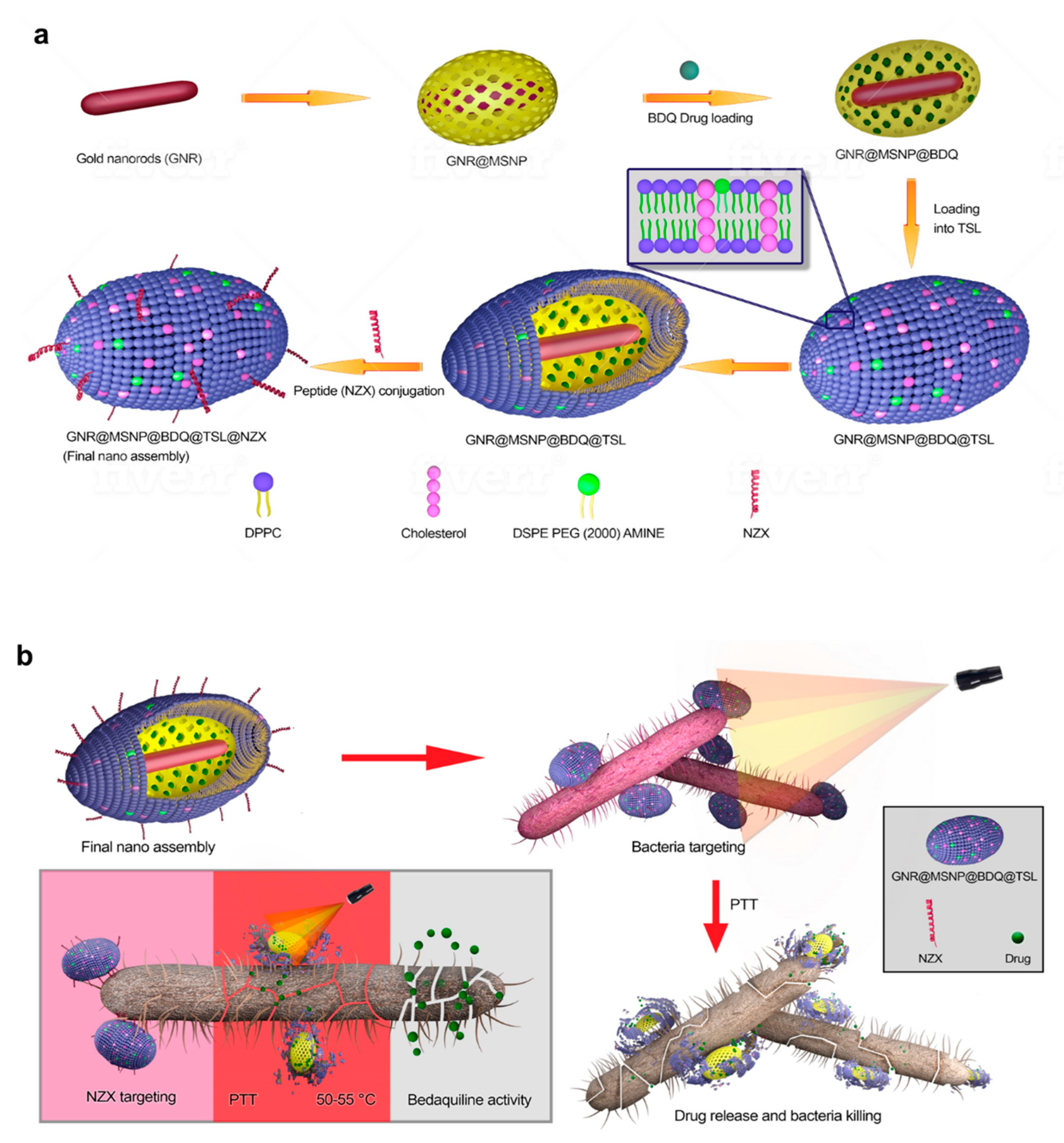
| DAP-GCS-PDA@GNRs) | pH, PPT808 nm Laser (0.5 W cm−2) for 0–8 min | MRSA | Daptomycin (DAP | Electrostatic | 61 |
| Porous silicon np, Peptide carg peptide identified by phage library |
NA |
S. aureus, P. aeruginosa |
VAN | CARG Peptide | 62 |
| Fusogenic pSi nanoparticle system (F-pSi) | NA | S. aureus | siRNA | Macrophage-targeting peptide (F-siIRF5-CRV) | 63 |
| Au@AgNP@SiO2@Nc-Van | PTT, (780 nm, 30 mW/cm2 for 30 min) | Van-sensitive B. subtilis, Van-resistant E. faecium, E. faecalis, E. coli |
Van | NA | 64 |
| TRIDENT, Natural fatty acid, Lecitin, DSPEPEG2000,IR780 | PTT (808 nm, 0.5W/cm2) | MDR S. aureus E. coli (Sepsis) |
Imipenem | NA | 65 |
| Black phosphorus quantum dots (BPQDs) and thermal-sensitive liposome. | PTT (1 W, 808nm, 15 min) | MRSA | Vancomycin | NA | 66 |
| AA@Ru@HA-MoS | Enzyme, PTT (808 nm, 0.5 W/cm2 for 7 min) | S. aureus and MDR P. aeruginosa | Pro drug Ascorbic acid | Ciprofloxacin as a catalyst with targeting effect | 67 |
| Lipid−dendrimer hybrid nanoparticles (LDH-NPs) | pH | MRSA | Vancomycin | Electrostatic | 68 |
| Maltohexaose-decorated cholesterol and bacteria-responsive lipid compositions, a smart nanoliposomes platform (MLP18) | Enzyme, US | MRSA | Purpurin 18 as sonosensitizer | Bacteria-targeting maltohexaose | 69 |
| Metal organic frameworks(MOFs)/antibiotics | pH | S. aureus | Tetracycline | Hyaluronic acid (HA) targeting | 70 |
| P(HEMA-co-DMA) as templet, Van-OA@PPy | PTT (808 nm, 1.0 W/cm2 for 5 min) |
MRSA | Vancomycin | Vancomycin conjugated oleic acid | 71 |
| AIE flurophore TTD, Micelle | White light irradiation (250 mW/ cm2 | M. tuberculosis | Rifampicin | TTD targeting | 72 |
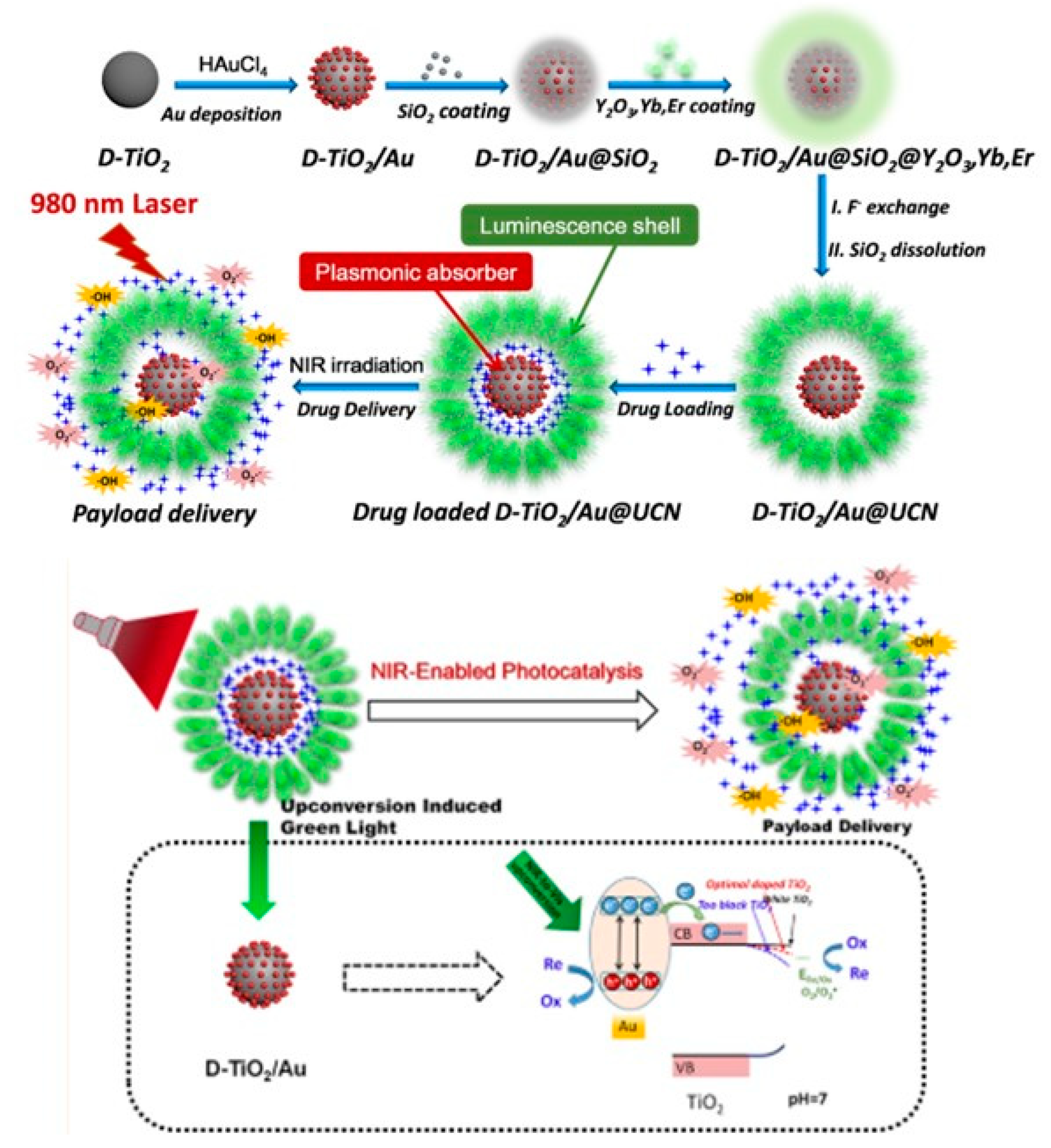
| D-TiO2/Au@UCN nanocomposites. | PTT (980 nm laser 0.68 W/cm2) |
E. coli and MRSA |
Ampicillin | NA | 73 |
| Ison@Man-Se NPs | M. tuberculosis | Isoniazid | Mannose targeting | 74 | |
2.1.2. Drug Delivery Triggered by Exogenous Stimuli
2.2. Complex Hybrid Triggered Nano-systems
2.3. Biomimetic Nano-systems
| Drug delivery NP | Biomimetic membrane | Pathogen | Drug | Ref. |
|---|---|---|---|---|
| Polymeric cores | plasma membranes of gastric epithelial cells | H. Pylori | clarithromycin | 139 |
| Polymeric nanoparticles, poly(lactic-co-glycolic acid)(PLGA) nanoparticle | membrane of extracellular vesicle secreted by S. aureus | S. aureus | Vancomycin (Van) and rifampicin (Rif) | 140 |
| Polymeric nanoparticles | red blood cells | Methicillin-resistant Staphylococcus aureus (MRSA) |
N/A | 141 |
| Polymeric nanoparticles | red blood cells | bacterial pore-forming toxin | N/A | 142 |
| neutrophil membrane-coated nanoparticles | MRSA | Sparfloxacin (SPX) | 143 | |
| Gold nanoparticle | bacteria outer membrane | E. coli | N/A | 144 |
| BSA nanoparticles | hollow outer membrane vesicles of bacteria | carbapenem-resistant K. pneumoniae | N/A | 145 |
3. Applications of Inherently Antimicrobial Nano-systems
3.1. Wound Healing
| Material | Infection | Ref. |
|---|---|---|
| Silver-based Nanomaterials | ||
| AgNP in hyaluronic acid hydrogel | E. coli, S. aureus, P. aeruginosa | 146 |
| GA-AgNP hydrogel + NIR laser | E. coli, S. aureus | 147 |
| SWCNTs@mSiO2-TSD@Ag | MDR E. coli, MDR S. aureus | 148 |
| BPN-AgNP | E. coli | 149 |
| Ag2S QD/mSiO2 NP hydrogel + NIR laser | E. coli, MRSA | 150 |
| CG/PDA@Ag + NIR laser | E. coli, S. aureus | 151 |
| Au/AgNPs | E. coli, MRSA | 152 |
| IM-POP-AgNPs | E. coli, S. aureus | 155 |
| S-nitroso-MSA/AgNP in alginate hydrogel | E. coli, S. aureus, S. mutans | 156 |
| Biogenic AgNPs/PLA/PEG nanofilm | S. aureus, P. aeruginosa | 157 |
| OCOS-AgNPs-pADMs | E. coli, S. aureus | 158 |
| Electrospun CA/SSD nanofibers | E. coli, B. subtilis | 159 |
| Copper-based Nanomaterials | ||
| PATA-C4@CuS nanoclusters | Levoflaxin-resistant S. aureus, E. coli, P. aeruginosa, B. amyloloquefaciens | 125 |
| CuS NDs + NIR laser | MRSA, ESBL-producing E. coli | 153 |
| BSA-CuS + NIR laser | S. aureus, A. baumannii, S. haemolyticus | 160 |
| Polyphenol-crosslinked CMCS-CuNPs | E. coli, S. aureus | 161 |
| Molybdenum-based Nanomaterials | ||
| CF-MoS2 + NIR laser | E. coli, S. aureus | 162 |
| PEG-MoS2 NFs + NIR laser | B. subtilis, AmpR E. coli | 163 |
| MoS2-BNN6 + NIR laser | AmpR E. coli, E. faecalis, & S. aureus | 164 |
| Gold-based Nanomaterials | ||
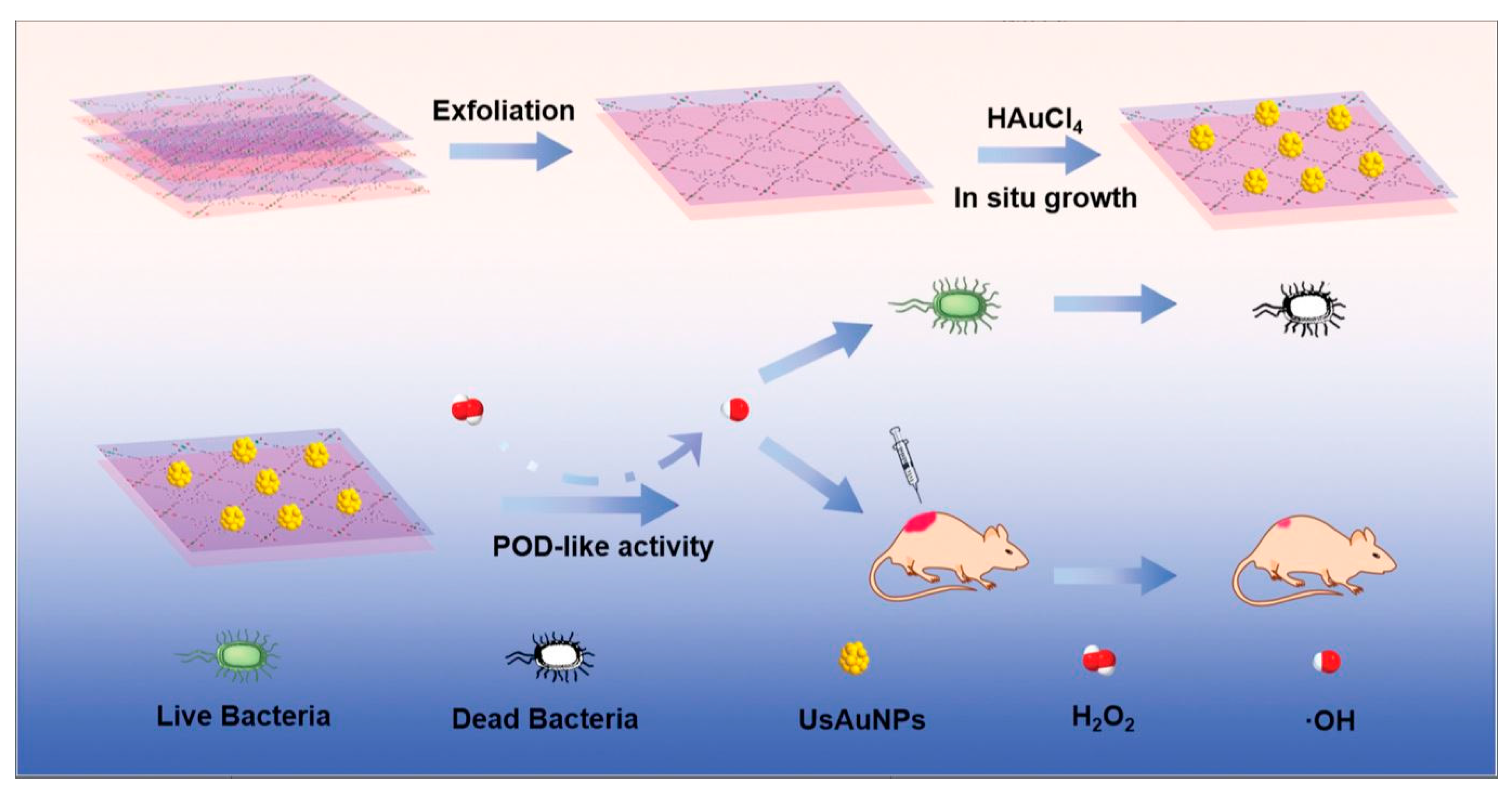
| UsAuNPs/MOFs | E. coli, S. aureus | 165 |
| CSAu@ MMT/gelatin | E. coli, S. aureus, MRSA | 166 |
| PDA@Au-HAp NPs + NIR laser | E. coli, S. aureus | 167 |
| Polymer-based Nanomaterials | ||
| Guanidine nanogel | E. coli, S. aureus | 168 |
| PDMAPS-co-PMA-Ade/Chitosan hydrogel | E. coli, S. aureus | 169 |
| PHCI hydrogel | E. coli, S. aureus | 170 |
| rGB/QCS/PDA-PAM | MRSA | 171 |
| Other Nanomaterials | ||
| Y2O3 in lauric acid-peptide conjugate gel | E. coli, S. aureus | 172 |
3.2. Surface Modification of Implants
| Material | Infection | Ref. |
|---|---|---|
| Nanomaterial-Modified Implants | ||
| Ag-coated Ti joint implants | N/A | 175 |
| nZnO-coated implants | S. enteric | 174 |
| TNTs-AgNPs-(CHI/ADA)10 | E. coli, S. aureus | 177 |
| TNT/AgNP composite coated Ti6Al4V surface | E. coli, S. aureus | 178 |
| PDA-AgNP coated titanium surface | S. aureus | 179 |
| Nanomaterial-Modified Stents | ||
| TiO2 NT@AgNP stents | S. aureus | 180 |
| AgNP biliary stents | E. coli, S. aureus Quail chicken enterococcus D, E. cloacae, K. pneumoniae, E. faecalis | 181 |
| PU/PU-PTX-PCL/PU-AgNP tri-layer membrane stents | E. coli & S. aureus | 182 |
| JQ alloy stents | N/A | 183 |
| hCOLIII-based ECM-mimetic coated stents | N/A | 184 |
| EVA/BS@SN ureteral J-shaped stents | E. coli | 185 |
| SF/CS/Cu coating for cardiovascular stents | N/A | 186 |
| PVP-AgNPs coated on silicone hydrogel | E. coli | 187 |
| Nanomaterial-Modified Catheters | ||
| Ag/Cu-coated catheters | MRSA | 188 |
| ACPs@AgNP-coated catheter | Drug resistant S. aureus | 189 |
| AgPEI NP-coated catheter | Candida species | 190 |
| PDA-CMC-AgNP-coated urinary catheter | E. coli, S. aureus | 191 |
| ZnO coated central venous catheter | P. aeruginosa, E. coli, S. aureus | 192 |
| ZnO NP-grafted silicone catheter | P. aeruginosa | 193 |
| AgNP-coated mini catheters | P. aeruginosa | 194 |
| GO/CU coating | C. parapsilosis | 195 |
| Ag/TiOx-PDMS nanoflim | P. aeruginosa, E. coli, S. aureus | 196 |
| Nanomaterial Modified Tissue Scaffolds | ||
| AgNP-silk fibroin scaffold | E. coli, S. aureus | 176 |
| PCL/AgNP-coated tissue scaffold | E. coli | 197 |
| Chitosan-CMC-FZO@Hap scaffold | E. coli, S. paratyphi, S. aureus, & L. monocytogenes | 198 |
| Hap/AgNP loaded cellulose scaffold | E. coli, S. aureus | 199 |
| CuFe2O4-MXene/PLLA tracheal scaffold | S. aureus, P. aeruginosa | 200 |
| Ag/MBG scaffold | E. coli, S. aureus | 201 |
| LgNP/PCL nanofiber scaffold | S. aureus | 202 |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- World Health Organization Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed June 8). 8 June.
- Abushaheen, M. A.; Muzaheed; Fatani, A. J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D. D.; Jhugroo, C.; Vellappally, S.; Khan, A. A.; Shaik, J.; Jhugroo, P. Antimicrobial resistance, mechanisms and its clinical significance. Disease-a-Month 2020, 66, 100971.
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, R.; Xia, T. Adaption/resistance to antimicrobial nanoparticles: Will it be a problem? Nano Today 2020, 34, 100909. [Google Scholar] [CrossRef]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- Patel, U. S. Nanomaterials in Theranostics: Therapeutics and Diagnosis against Infectious Diseases. Ph.D., The University of Alabama in Huntsville, Ann Arbor, 2022.
- Mu, Y.; Gong, L.; Peng, T.; Yao, J.; Lin, Z. Advances in pH-responsive drug delivery systems. OpenNano 2021, 5, 100031. [Google Scholar] [CrossRef]
- Morey, M.; Pandit, A. Responsive triggering systems for delivery in chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Angsantikul, P.; Liu, W.; Esteban-Fernández de Ávila, B.; Thamphiwatana, S.; Xu, M.; Sandraz, E.; Wang, X.; Delezuk, J.; Gao, W.; Zhang, L.; Wang, J. Micromotors Spontaneously Neutralize Gastric Acid for pH-Responsive Payload Release. Angewandte Chemie International Edition 2017, 56, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Ding, X.; Liu, Y.; Liu, W.; Li, J.; Wang, B.; Gu, Z. Gastric acid powered micromotors for combined-drug delivery to eradiate helicobacter pylori. Appl. Mater. Today 2023, 31. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Fu, V.; Zhu, J.; Lu, D.; Gao, W.; Zhang, L. Nanoparticle-Stabilized Liposomes for pH-Responsive Gastric Drug Delivery. Langmuir 2013, 29, 12228–12233. [Google Scholar] [CrossRef]
- Jing, Z.-W.; Jia, Y.-Y.; Wan, N.; Luo, M.; Huan, M.-L.; Kang, T.-B.; Zhou, S.-Y.; Zhang, B.-L. Design and evaluation of novel pH-sensitive ureido-conjugated chitosan/TPP nanoparticles targeted to Helicobacter pylori. Biomaterials 2016, 84, 276–285. [Google Scholar] [CrossRef]
- Arif, M.; Dong, Q. J.; Raja, M. A.; Zeenat, S.; Chi, Z.; Liu, C. G. Development of novel pH-sensitive thiolated chitosan/PMLA nanoparticles for amoxicillin delivery to treat Helicobacter pylori. Mater Sci Eng C Mater Biol Appl 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Luo, M.; Jia, Y.-Y.; Jing, Z.-W.; Li, C.; Zhou, S.-Y.; Mei, Q.-B.; Zhang, B.-L. Construction and optimization of pH-sensitive nanoparticle delivery system containing PLGA and UCCs-2 for targeted treatment of Helicobacter pylori. Colloids and Surfaces B: Biointerfaces 2018, 164, 11–19. [Google Scholar] [CrossRef]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef]
- Chu, L.; Gao, H.; Cheng, T.; Zhang, Y.; Liu, J.; Huang, F.; Yang, C.; Shi, L.; Liu, J. A charge-adaptive nanosystem for prolonged and enhanced in vivo antibiotic delivery. Chem. Commun. 2016, 52, 6265–6268. [Google Scholar] [CrossRef]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. From Structure to Function: pH-Switchable Antimicrobial Nano-Self-Assemblies. ACS Applied Materials & Interfaces 2019, 11, 2821–2829. [Google Scholar]
- Pranantyo, D.; Liu, P.; Zhong, W.; Kang, E.-T.; Chan-Park, M.B. Antimicrobial Peptide-Reduced Gold Nanoclusters with Charge-Reversal Moieties for Bacterial Targeting and Imaging. Biomacromolecules 2019, 20, 2922–2933. [Google Scholar] [CrossRef] [PubMed]
- Rahme, L.G.; Stevens, E.J.; Wolfort, S.F.; Shao, J.; Tompkins, R.G.; Ausubel, F.M. Common Virulence Factors for Bacterial Pathogenicity in Plants and Animals. Science 1995, 268, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Baier, G.; Cavallaro, A.; Vasilev, K.; Mailänder, V.; Musyanovych, A.; Landfester, K. Enzyme Responsive Hyaluronic Acid Nanocapsules Containing Polyhexanide and Their Exposure to Bacteria To Prevent Infection. Biomacromolecules 2013, 14, 1103–1112. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Gao, W.; Pornpattananangkul, D.; Zhang, Q.; Fu, V.; Li, J.; Li, J.; Obonyo, M.; Zhang, L. Phospholipase A2-responsive antibiotic delivery via nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Mater. Chem. B 2014, 2, 8201–8207. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-Targeting Nanoparticles with Microenvironment-Responsive Antibiotic Release To Eliminate Intracellular Staphylococcus aureus and Associated Infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311. [Google Scholar] [CrossRef]
- Thorn, C.R.; Clulow, A.J.; Boyd, B.J.; Prestidge, C.A.; Thomas, N. Bacterial lipase triggers the release of antibiotics from digestible liquid crystal nanoparticles. J. Control. Release 2019, 319, 168–182. [Google Scholar] [CrossRef]
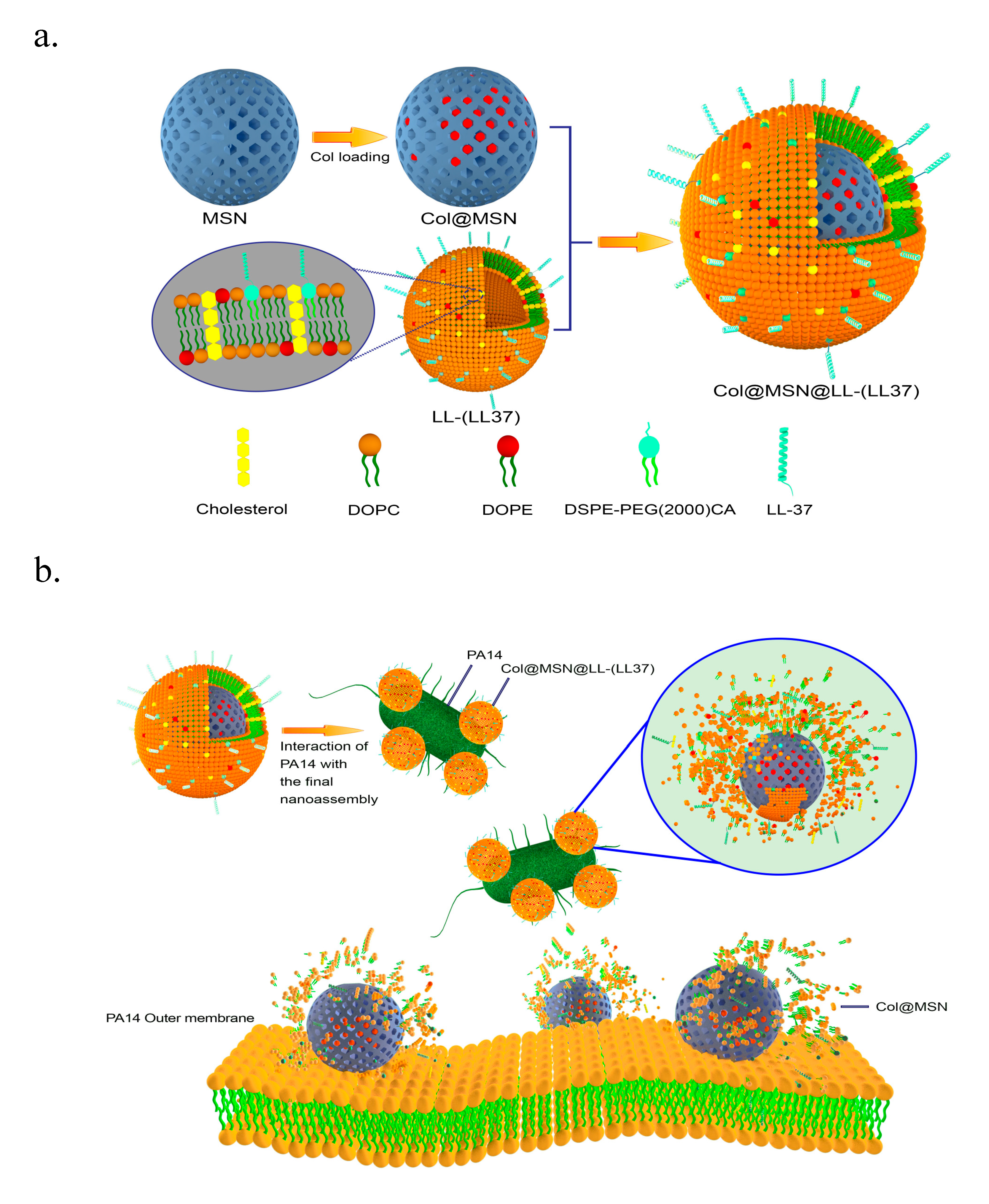
- Rathnayake, K.; Patel, U.; Pham, C.; McAlpin, A.; Budisalich, T.; Jayawardena, S.N. Targeted Delivery of Antibiotic Therapy to Inhibit Pseudomonas aeruginosa Using Lipid-Coated Mesoporous Silica Core–Shell Nanoassembly. ACS Appl. Bio Mater. 2020, 3, 6708–6721. [Google Scholar] [CrossRef]
- Sémiramoth, N.; Di Meo, C.; Zouhiri, F.; Saïd-Hassane, F.; Valetti, S.; Gorges, R.; Nicolas, V.; Poupaert, J.H.; Chollet-Martin, S.; Desmaële, D.; et al. Self-Assembled Squalenoylated Penicillin Bioconjugates: An Original Approach for the Treatment of Intracellular Infections. ACS Nano 2012, 6, 3820–3831. [Google Scholar] [CrossRef]
- Li, Z.; Clemens, D. L.; Lee, B.-Y.; Dillon, B. J.; Horwitz, M. A.; Zink, J. I. Mesoporous Silica Nanoparticles with pH-Sensitive Nanovalves for Delivery of Moxifloxacin Provide Improved Treatment of Lethal Pneumonic Tularemia. ACS Nano 2015, 9, 10778–10789. [Google Scholar] [CrossRef]
- de Ávila, B. E.-F.; Angsantikul, P.; Li, J.; Angel Lopez-Ramirez, M.; Ramírez-Herrera, D. E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V.; Obonyo, M.; Zhang, L.; Wang, J. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nature Communications 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomedicine: Nanotechnology, Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-Responsive Polymeric Vesicles for Bacterial-Strain-Selective Delivery of Antimicrobial Agents. Angew. Chem. Int. Ed. 2015, 55, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.-M.; Zhang, L. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Z.; Wang, H.; Han, H. Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Chiang, W.-L.; Lin, T.-T.; Sureshbabu, R.; Chia, W.-T.; Hsiao, H.-C.; Liu, H.-Y.; Yang, C.-M.; Sung, H.-W. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. J. Control. Release 2015, 199, 53–62. [Google Scholar] [CrossRef]
- Altinbasak, I.; Jijie, R.; Barras, A.; Golba, B.; Sanyal, R.; Bouckaert, J.; Drider, D.; Bilyy, R.O.; Dumych, T.; Paryzhak, S.; et al. Reduced Graphene-Oxide-Embedded Polymeric Nanofiber Mats: An “On-Demand” Photothermally Triggered Antibiotic Release Platform. ACS Appl. Mater. Interfaces 2018, 10, 41098–41106. [Google Scholar] [CrossRef]
- Hu, D.; Zou, L.; Li, B.; Hu, M.; Ye, W.; Ji, J. Photothermal Killing of Methicillin-Resistant Staphylococcus aureus by Bacteria-Targeted Polydopamine Nanoparticles with Nano-Localized Hyperpyrexia. ACS Biomater. Sci. Eng. 2019, 5, 5169–5179. [Google Scholar] [CrossRef]
- Wang, H.; Song, Z.; Li, S.; Wu, Y.; Han, H. One Stone with Two Birds: Functional Gold Nanostar for Targeted Combination Therapy of Drug-Resistant Staphylococcus aureus Infection. ACS Appl. Mater. Interfaces 2019, 11, 32659–32669. [Google Scholar] [CrossRef]
- Ikeda-Dantsuji, Y.; Feril, L.B.; Tachibana, K.; Ogawa, K.; Endo, H.; Harada, Y.; Suzuki, R.; Maruyama, K. Synergistic effect of ultrasound and antibiotics against Chlamydia trachomatis-infected human epithelial cells in vitro. Ultrason. Sonochemistry 2011, 18, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-X.; Cai, X.-Z.; Shi, Z.-L.; Hu, B.; Yan, S.-G. Microbubble-Mediated Ultrasound Enhances the Lethal Effect of Gentamicin on Planktonic Escherichia coli. BioMed Research International 2014, 2014, 142168. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G.; et al. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int. J. Pharm. 2017, 523, 176–188. [Google Scholar] [CrossRef]
- Horsley, H.; Owen, J.; Browning, R.; Carugo, D.; Malone-Lee, J.; Stride, E.; Rohn, J. Ultrasound-activated microbubbles as a novel intracellular drug delivery system for urinary tract infection. J. Control. Release 2019, 301, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Pang, X.; Cheng, Y.; Ming, J.; Xiang, S.; Zhang, C.; Lv, P.; Chu, C.; Chen, X.; Liu, G.; et al. Ultrasound-Switchable Nanozyme Augments Sonodynamic Therapy against Multidrug-Resistant Bacterial Infection. ACS Nano 2020, 14, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Sirivisoot, S.; Harrison, B.S. Magnetically stimulated ciprofloxacin release from polymeric microspheres entrapping iron oxide nanoparticles. Int. J. Nanomed. 2015, ume 10, 4447–4458. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ghobadianpour, S. Vancomycin loaded superparamagnetic MnFe 2 O 4 nanoparticles coated with PEGylated chitosan to enhance antibacterial activity. Int. J. Pharm. 2016, 501, 326–330. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Neogi, S. Gentamicin coated iron oxide nanoparticles as novel antibacterial agents. Mater. Res. Express 2017, 4, 095005. [Google Scholar] [CrossRef]
- Harris, M.; Ahmed, H.; Barr, B.; LeVine, D.; Pace, L.; Mohapatra, A.; Morshed, B.; Bumgardner, J.D.; Jennings, J.A. Magnetic stimuli-responsive chitosan-based drug delivery biocomposite for multiple triggered release. Int. J. Biol. Macromol. 2017, 104, 1407–1414. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Li, P.; Wang, L.; Zhou, J.; Zhang, G.; Li, X.; Hu, B.; Xing, X. Microenvironment-Responsive Magnetic Nanocomposites Based on Silver Nanoparticles/Gentamicin for Enhanced Biofilm Disruption by Magnetic Field. ACS Appl. Mater. Interfaces 2018, 10, 34905–34915. [Google Scholar] [CrossRef]
- Silva-Freitas, E.L.; Pontes, T.R.F.; Araújo-Neto, R.P.; Damasceno, .H.M.; Silva, K.L.; Carvalho, J.F.; Medeiros, A.C.; Silva, R.B.; Silva, A.K.A.; Morales, M.A.; et al. Design of Magnetic Polymeric Particles as a Stimulus-Responsive System for Gastric Antimicrobial Therapy. AAPS PharmSciTech 2016, 18, 2026–2036. [CrossRef]
- Zhao, Z.; Yan, R.; Yi, X.; Li, J.; Rao, J.; Guo, Z.; Yang, Y.; Li, W.; Li, Y.-Q.; Chen, C. Bacteria-Activated Theranostic Nanoprobes against Methicillin-Resistant Staphylococcus aureus Infection. ACS Nano 2017, 11, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management. Adv. Mater. 2018, 30, e1803618. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, S.; Wei, J.; Song, X.; Ding, Z.; Li, X. Antibacterial Micelles with Vancomycin-Mediated Targeting and pH/Lipase-Triggered Release of Antibiotics. ACS Applied Materials & Interfaces 2018, 10, 36814–36823. [Google Scholar]
- Chen, X.; Liu, Y.; Lin, A.; Huang, N.; Long, L.; Gang, Y.; Liu, J. Folic acid-modified mesoporous silica nanoparticles with pH-responsiveness loaded with Amp for an enhanced effect against anti-drug-resistant bacteria by overcoming efflux pump systems. Biomaterials Science 2018, 6, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Chiappi, M.; García-Trenco, A.; Al-Ejji, M.; Sarkar, S.; Georgiou, T.K.; Shaffer, M.S.P.; Tetley, T.D.; Schwander, S.; Ryan, M.P.; et al. Multimetallic Microparticles Increase the Potency of Rifampicin against Intracellular Mycobacterium tuberculosis. ACS Nano 2018, 12, 5228–5240. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Lu, H.; Wu, X.; Chen, S.; Song, N.; Yang, Y.-W.; Gao, H. Construction of Supramolecular Nanoassembly for Responsive Bacterial Elimination and Effective Bacterial Detection. ACS Appl. Mater. Interfaces 2017, 9, 10180–10189. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- He, D.; Yang, T.; Qian, W.; Qi, C.; Mao, L.; Yu, X.; Zhu, H.; Luo, G.; Deng, J. Combined photothermal and antibiotic therapy for bacterial infection via acidity-sensitive nanocarriers with enhanced antimicrobial performance. Appl. Mater. Today 2018, 12, 415–429. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Kim, B.; Pang, H.-B.; Kang, J.; Park, J.-H.; Ruoslahti, E.; Sailor, M.J. Immunogene therapy with fusogenic nanoparticles modulates macrophage response to Staphylococcus aureus. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Peng, S.; Sui, M.; Chen, S.; Huang, L.; Xu, H.; Jiang, T. Multifunctional nanocomplex for surface-enhanced Raman scattering imaging and near-infrared photodynamic antimicrobial therapy of vancomycin-resistant bacteria. Colloids Surfaces B: Biointerfaces 2018, 161, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.; Zhao, X.; Gong, N.; Chen, J.; Li, X.; Gan, Y.; Wang, Y.; Zhang, Z.; Zhang, Y.; Guo, W.; et al. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wang, J.; Wang, Y.; Chen, A.; Wang, C.; Mo, W.; Li, Y.; Yuan, Q.; Zhang, Y. Photon-Responsive Antibacterial Nanoplatform for Synergistic Photothermal-/Pharmaco-Therapy of Skin Infection. ACS Applied Materials & Interfaces 2019, 11, 300–310. [Google Scholar]
- Liu, Y.; Lin, A.; Liu, J.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Enzyme-Responsive Mesoporous Ruthenium for Combined Chemo-Photothermal Therapy of Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26590–26606. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C. A.; Agrawal, N.; Maduray, K.; Hassan, D.; Mokhtar, C.; Mackhraj, I.; Govender, T. , pH-Responsive Lipid–Dendrimer Hybrid Nanoparticles: An Approach To Target and Eliminate Intracellular Pathogens. Molecular Pharmaceutics 2019, 16, 4594–4609. [Google Scholar] [CrossRef]
- Pang, X.; Xiao, Q.; Cheng, Y.; Ren, E.; Lian, L.; Zhang, Y.; Gao, H.; Wang, X.; Leung, W.; Chen, X.; et al. Bacteria-Responsive Nanoliposomes as Smart Sonotheranostics for Multidrug Resistant Bacterial Infections. ACS Nano 2019, 13, 2427–2438. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Huang, L.; Zhang, W.; Wang, R.; Yue, T.; Sun, J.; Li, G.; Wang, J. The highly efficient elimination of intracellular bacteria via a metal organic framework (MOF)-based three-in-one delivery system. Nanoscale 2019, 11, 9468–9477. [Google Scholar] [CrossRef]
- Guo, X.; Cao, B.; Wang, C.; Lu, S.; Hu, X. In vivo photothermal inhibition of methicillin-resistant Staphylococcus aureus infection by in situ templated formulation of pathogen-targeting phototheranostics. Nanoscale 2020, 12, 7651–7659. [Google Scholar] [CrossRef]
- Liao, Y.; Li, B.; Zhao, Z.; Fu, Y.; Tan, Q.; Li, X.; Wang, W.; Yin, J.; Shan, H.; Tang, B.Z.; et al. Targeted Theranostics for Tuberculosis: A Rifampicin-Loaded Aggregation-Induced Emission Carrier for Granulomas Tracking and Anti-Infection. ACS Nano 2020, 14, 8046–8058. [Google Scholar] [CrossRef]
- Xu, J.; Liu, N.; Wu, D.; Gao, Z.; Song, Y.-Y.; Schmuki, P. Upconversion Nanoparticle-Assisted Payload Delivery from TiO2 under Near-Infrared Light Irradiation for Bacterial Inactivation. ACS Nano 2019, 14, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-Targeted Isoniazid–Selenium Nanoparticles Promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angew. Chem. Int. Ed. 2019, 59, 3226–3234. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–51. [Google Scholar] [CrossRef] [PubMed]
- Füssle, R.; Bhakdi, S.; Sziegoleit, A.; Tranum-Jensen, J.; Kranz, T.; Wellensiek, H.J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J. Cell Biol. 1981, 91, 83–94. [Google Scholar] [CrossRef]
- Bantel, H.; Sinha, B.; Domschke, W.; Peters, G.; Schulze-Osthoff, K.; Jänicke, R.U. α-Toxin is a mediator of Staphylococcus aureus–induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 2001, 155, 637–648. [Google Scholar] [CrossRef]
- Xu, J.-W.; Yao, K.; Xu, Z.-K. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef]
- Tang, H.; Shen, S.; Guo, J.; Chang, B.; Jiang, X.; Yang, W. Gold nanorods@mSiO2 with a smart polymer shell responsive to heat/near-infrared light for chemo-photothermal therapy. Journal of Materials Chemistry 2012, 22, 16095–16103. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, B.; Chen, B.; Wang, J.; Xu, L.; Zhang, S.; Song, H. Multifunctional Au@mSiO2/rhodamine B isothiocyanate nanocomposites: cell imaging, photocontrolled drug release, and photothermal therapy for cancer cells. Small 2013, 9, 604–12. [Google Scholar] [CrossRef]
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte Membrane Is an Alternative Coating to Polyethylene Glycol for Prolonging the Circulation Lifetime of Gold Nanocages for Photothermal Therapy. ACS Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, S.; Feng, J.; Jiang, X.; Yang, W. Mussel-Inspired Gold Hollow Superparticles for Photothermal Therapy. Adv. Heal. Mater. 2015, 4, 1009–1014. [Google Scholar] [CrossRef]
- Luo, G.-F.; Chen, W.-H.; Lei, Q.; Qiu, W.-X.; Liu, Y.-X.; Cheng, Y.-J.; Zhang, X.-Z. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Li, A.; Xu, S.; Manghnani, P.N.; Liu, C.; Guo, L.; Zheng, H.; Liu, B. Molecular Engineering of Conjugated Polymers for Biocompatible Organic Nanoparticles with Highly Efficient Photoacoustic and Photothermal Performance in Cancer Theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J. L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Patel, U.; Rathnayake, K.; Singh, N.; Hunt, E.C. Dual Targeted Delivery of Liposomal Hybrid Gold Nano-Assembly for Enhanced Photothermal Therapy against Lung Carcinomas. ACS Appl. Bio Mater. 2023, 6, 1915–1933. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Yang, C.; Ren, C.; Zhou, J.; Liu, J.; Zhang, Y.; Huang, F.; Ding, D.; Xu, B.; Liu, J. Dual Fluorescent- and Isotopic-Labelled Self-Assembling Vancomycin for in vivo Imaging of Bacterial Infections. Angew. Chem. Int. Ed. 2017, 56, 2356–2360. [Google Scholar] [CrossRef]
- Patel, U.; Rathnayake, K.; Jani, H.; Jayawardana, K.W.; Dhakal, R.; Duan, L.; Jayawardena, S.N. Near-infrared responsive targeted drug delivery system that offer chemo-photothermal therapy against bacterial infection. Nano Sel. 2021, 2, 1750–1769. [Google Scholar] [CrossRef]
- Yumita, N.; Iwase, Y.; Nishi, K.; Komatsu, H.; Takeda, K.; Onodera, K.; Fukai, T.; Ikeda, T.; Umemura, S.-I.; Okudaira, K.; et al. Involvement of Reactive Oxygen Species in Sonodynamically Induced Apoptosis Using a Novel Porphyrin Derivative. Theranostics 2012, 2, 880–888. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy??a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochemistry 2004. [Google Scholar] [CrossRef] [PubMed]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, J.F. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. In Therapeutic Ultrasound; Springer Science and Business Media LLC: Cham, Switzerland, 2016; Volume 880, pp. 429–450. [Google Scholar]
- Chen, H.; Zhou, X.; Gao, Y.; Zheng, B.; Tang, F.; Huang, J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov. Today 2014, 19, 502–509. [Google Scholar] [CrossRef]
- Rengeng, L.; Qianyu, Z.; Yuehong, L.; Zhongzhong, P.; Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hall, D. O.; Selfridge, A. R. Multi-frequency ultrasound therapy systems and methods. Google Patents: 1995.
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH -Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. WIREs Nanomed. Nanobiotechnology 2016, 8, 696–716. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.C.; McCREERY, T.P.; Sweitzer, R.H. Ultrasound Enhances Gene Expression of Liposomal Transfection. Investig. Radiol. 1997, 32, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D. Ultrasound contrast agents: An overview. Eur. J. Radiol. 2006, 60, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Peregrino, M.; Arvanitis, C.D.; Rifai, B.; Seymour, L.W.; Coussios, -C.C. Ultrasound-induced cavitation enhances the delivery and therapeutic efficacy of an oncolytic virus in an in vitro model. J. Control. Release 2012, 157, 235–242. [CrossRef]
- Xiang, Y.; Lu, J.; Mao, C.; Zhu, Y.; Wang, C.; Wu, J.; Liu, X.; Wu, S.; Kwan, K.Y.H.; Cheung, K.M.C.; et al. Ultrasound-triggered interfacial engineering-based microneedle for bacterial infection acne treatment. Sci. Adv. 2023, 9, eadf0854. [Google Scholar] [CrossRef]
- Freeman, M. W.; Arrott, A.; Watson, J. H. L. Magnetism in Medicine. Journal of Applied Physics 1960, 31, S404–S405. [Google Scholar] [CrossRef]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef]
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.-H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Heal. Mater. 2018, 7, e1701392. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, H. S.; Rathnayake, K. M.; Patel, U.; Sentell, A.; Johnson, J.; Mustain, M.; Devarasetty, V. V. N. M. Rapid diagnostics of mycobacteria with lectin conjugated particles. U.S. Patent No. 20210311049A1, 7 October 2021.
- Mabrouk, M.; El-Wahab, R.M.A.; Abo-Elfadl, M.T.; Beherei, H.H.; Selim, M.M.; Ibrahim, A.M.; Das, D.B. Magnetic nanosystems substituted with zinc for enhanced antibacterial, drug delivery and cell viability behaviours. Colloids Surfaces A: Physicochem. Eng. Asp. 2022, 650. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of Iron Oxide-Based Magnetic Nanoparticles in the Diagnosis and Treatment of Bacterial Infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Estévez, M.; Gallo-Cordova, A.; González, B.; Castillo, R.R.; Morales, M.d.P.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy. Pharmaceutics 2022, 14, 163. [Google Scholar] [CrossRef]
- Patel, U.; Rathnayake, K.; Hunt, E.C.; Singh, N. Role of Nanomaterials in COVID-19 Prevention, Diagnostics, Therapeutics, and Vaccine Development. J. Nanotheranostics 2022, 3, 151–176. [Google Scholar] [CrossRef]
- Thwaites, G. E.; Gant, V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nature Reviews Microbiology 2011, 9, 215–222. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F. L.; Scicluna, B. P.; Netea, M. G. The immunopathology of sepsis and potential therapeutic targets. Nature Reviews Immunology 2017, 17, 407. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management. Adv. Mater. 2018, 30, e1803618. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Shi, Z.; Zhu, H.; Li, M.; Yu, Q. Pathogen infection-responsive nanoplatform targeting macrophage endoplasmic reticulum for treating life-threatening systemic infection. Nano Res. 2022, 15, 6243–6255. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; Von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Foster, T. J. Immune evasion by staphylococci. Nature reviews microbiology 2005, 3, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B. T.; Chambers, R. C.; Liu, K. D. Acute respiratory distress syndrome. New England Journal of Medicine 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J. R. Acute infective endocarditis. Infectious disease clinics of North America 2009, 23, 643–664. [Google Scholar] [CrossRef]
- Wright, J. A.; Nair, S. P. Interaction of staphylococci with bone. International journal of medical microbiology 2010, 300, 193–204. [Google Scholar] [CrossRef]
- Serpe, L.; Giuntini, F. Sonodynamic antimicrobial chemotherapy: First steps towards a sound approach for microbe inactivation. J. Photochem. Photobiol. B: Biol. 2015, 150, 44–49. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, Y.; Yu, Y.; Chen, X.; Wei, X.; Zhang, X.; Li, C. Single Continuous Near-Infrared Laser-Triggered Photodynamic and Photothermal Ablation of Antibiotic-Resistant Bacteria Using Effective Targeted Copper Sulfide Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 30470–30479. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation To Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xu, C.; Jiang, Y.; Xiao, Q.; Leung, A.W. Natural products in the discovery of novel sonosensitizers. Pharmacol. Ther. 2016, 162, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Zhao, Y.; Hu, M.; Ma, D.; Zhang, P.; Xue, Y.; Li, X. Photomagnetic nanoparticles in dual-modality imaging and photo-sonodynamic activity against bacteria. Chem. Eng. J. 2018, 356, 811–818. [Google Scholar] [CrossRef]
- Cai, S.; Jia, X.; Han, Q.; Yan, X.; Yang, R.; Wang, C. Porous Pt/Ag nanoparticles with excellent multifunctional enzyme mimic activities and antibacterial effects. Nano Res. 2017, 10, 2056–2069. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 2017, 113, 145–157. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Bifunctionalized Mesoporous Silica-Supported Gold Nanoparticles: Intrinsic Oxidase and Peroxidase Catalytic Activities for Antibacterial Applications. Adv. Mater. 2014, 27, 1097–1104. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 1–46. [Google Scholar] [CrossRef]
- Yang, G.; Chen, S.; Zhang, J. Bioinspired and Biomimetic Nanotherapies for the Treatment of Infectious Diseases. Front. Pharmacol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Ai, X.; Hu, M.; Wang, Z.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Recent Advances of Membrane-Cloaked Nanoplatforms for Biomedical Applications. Bioconjugate Chem. 2018, 29, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y.; Cottam, E.; Pierini, R.; Roberts, R.; Wileman, T.; Kim, S.-W.; Kim, H.; et al. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating Nanoparticles with Gastric Epithelial Cell Membrane for Targeted Antibiotic Delivery against Helicobacter pylori Infection. Adv. Ther. 2018, 1. [Google Scholar] [CrossRef]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier. ACS Infect. Dis. 2018, 5, 218–227. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, M.; Zhuang, J.; Fang, R.H.; Gao, W.; Zhang, L. Biomimetic Nanosponges Suppress In Vivo Lethality Induced by the Whole Secreted Proteins of Pathogenic Bacteria. Small 2019, 15, e1804994. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Meyer, R.A.; Yu, H.; Smith, J.T.; Pardoll, D.M.; Green, J.J. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 2020, 6, eaay9035. [Google Scholar] [CrossRef]
- Wang, K.; Lei, Y.; Xia, D.; Xu, P.; Zhu, T.; Jiang, Z.; Ma, Y. Neutrophil membranes coated, antibiotic agent loaded nanoparticles targeting to the lung inflammation. Colloids Surfaces B: Biointerfaces 2020, 188, 110755. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.-M.J.; Zhang, L. Modulating Antibacterial Immunity via Bacterial Membrane-Coated Nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Wu, G.; Ji, H.; Guo, X.; Li, Y.; Ren, T.; Dong, H.; Liu, J.; Liu, Y.; Shi, X.; He, B. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomedicine: Nanotechnology, Biol. Med. 2020, 24, 102148. [Google Scholar] [CrossRef]
- Massironi, A.; Franco, A. R.; Babo, P. S.; Puppi, D.; Chiellini, F.; Reis, R. L.; Gomes, M. E. Development and Characterization of Highly Stable Silver NanoParticles as Novel Potential Antimicrobial Agents for Wound Healing Hydrogels International Journal of Molecular Sciences [Online], 2022.
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2019, 382, 122990. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, M.; Yu, S.; Wu, Q.; Ma, K.; Chen, Y.; Liu, X.; Li, C.; Wang, F. Silver-Laden Black Phosphorus Nanosheets for an Efficient In Vivo Antimicrobial Application. Small 2020, 16, e1905938. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Xiao, Z.; Cao, J.; Wei, L.; Li, C.; Jiao, J.; Song, Z.; Liu, J.; Du, X.; Wang, S. NIR-activated multi-hit therapeutic Ag2S quantum dot-based hydrogel for healing of bacteria-infected wounds. Acta Biomater. 2022, 145, 88–105. [Google Scholar] [CrossRef]
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, e2106015. [Google Scholar] [CrossRef]
- Kim, T.; Zhang, Q.; Li, J.; Zhang, L.; Jokerst, J.V. A Gold/Silver Hybrid Nanoparticle for Treatment and Photoacoustic Imaging of Bacterial Infection. ACS Nano 2018, 12, 5615–5625. [Google Scholar] [CrossRef]
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y.; Xie, T.; Li, W.; Zhong, D.; Zhang, Y.; et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Luo, H.; Huang, T.; Li, X.; Wang, J.; Lv, T.; Tan, W.; Gao, F.; Zhang, J.; Zhou, B. Synergistic antibacterial and wound-healing applications of an imidazole-based porous organic polymer encapsulated silver nanoparticles composite. Microporous Mesoporous Mater. 2022, 337. [Google Scholar] [CrossRef]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef]
- Bardania, H.; Mahmoudi, R.; Bagheri, H.; Salehpour, Z.; Fouani, M.H.; Darabian, B.; Khoramrooz, S.S.; Mousavizadeh, A.; Kowsari, M.; Moosavifard, S.E.; et al. Facile preparation of a novel biogenic silver-loaded Nanofilm with intrinsic anti-bacterial and oxidant scavenging activities for wound healing. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Dan, N.; Dan, W.; Liu, X.; Cong, L. A novel antibacterial acellular porcine dermal matrix cross-linked with oxidized chitosan oligosaccharide and modified by in situ synthesis of silver nanoparticles for wound healing applications. Mater. Sci. Eng. C 2018, 94, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Khan, M. Q.; Kharaghani, D.; Sanaullah; Shahzad, A.; Saito, Y.; Yamamoto, T.; Ogasawara, H.; Kim, I. S. Fabrication of antibacterial electrospun cellulose acetate/ silver-sulfadiazine nanofibers composites for wound dressings applications. Polym. Test. 2019, 74, 39-44.
- Zhao, Y.; Cai, Q.; Qi, W.; Jia, Y.; Xiong, T.; Fan, Z.; Liu, S.; Yang, J.; Li, N.; Chang, B. BSA-CuS Nanoparticles for Photothermal Therapy of Diabetic Wound Infection In Vivo. ChemistrySelect 2018, 3, 9510–9516. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, Y.; Wei, Y.; Wang, Y.; Jin, Z.; Ma, G.; Jiang, Y.; Zhang, W.; Hu, Z. Facile preparation of polyphenol-crosslinked chitosan-based hydrogels for cutaneous wound repair. Int. J. Biol. Macromol. 2023, 228, 99–110. [Google Scholar] [CrossRef]
- Tang, Y.; Qin, Z.; Zhong, Y.; Yan, X.; Kong, L.; Yang, X.; Yin, S.; Li, M.; Liu, Z.; Sun, H. Bioinspired MoS2 Nanosheet-Modified Carbon Fibers for Synergetic Bacterial Elimination and Wound Disinfection. Adv. Heal. Mater. 2022, 12, e2202270. [Google Scholar] [CrossRef]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, X.; Yin, W.; Ma, D.; Xie, C.; Zheng, L.; Dong, X.; Mei, L.; Yu, J.; Wang, C.; et al. Functionalized MoS2Nanovehicle with Near-Infrared Laser-Mediated Nitric Oxide Release and Photothermal Activities for Advanced Bacteria-Infected Wound Therapy. Small 2018, 14, 1802290. [Google Scholar] [CrossRef]
- Hu, W.-C.; Younis, M. R.; Zhou, Y.; Wang, C.; Xia, X.-H. In Situ Fabrication of Ultrasmall Gold Nanoparticles/2D MOFs Hybrid as Nanozyme for Antibacterial Therapy. Small 2020, n/a (n/a), 2000553.
- Lu, B.; Ye, H.; Shang, S.; Xiong, Q.; Yu, K.; Li, Q.; Xiao, Y.; Dai, F.; Lan, G. Novel wound dressing with chitosan gold nanoparticles capped with a small molecule for effective treatment of multiantibiotic-resistant bacterial infections. Nanotechnology 2018, 29, 425603. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K. W. K.; Chu, P. K.; Wu, S. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomaterialia 2018, 77, 352–364. [Google Scholar] [CrossRef]
- Han, H.; Zhu, J.; Wu, D.-Q.; Li, F.-X.; Wang, X.-L.; Yu, J.-Y.; Qin, X.-H. Inherent Guanidine Nanogels with Durable Antibacterial and Bacterially Antiadhesive Properties. Adv. Funct. Mater. 2019, 29, n/a. [Google Scholar] [CrossRef]
- Yan, J.; Ji, Y.; Huang, M.; Li, T.; Liu, Y.; Lü, S.; Liu, M. Nucleobase-Inspired Self-Adhesive and Inherently Antibacterial Hydrogel for Wound Dressing. ACS Mater. Lett. 2020, 2, 1375–1380. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Chen, X.; Xu, C.; Guo, J.; Deng, M.; Qu, X.; Huang, P.; Feng, Z.; Zhang, J. Versatile Hydrogel Dressing with Skin Adaptiveness and Mild Photothermal Antibacterial Activity for Methicillin-Resistant Staphylococcus Aureus-Infected Dynamic Wound Healing. Adv. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Chawla, V.; Sharma, S.; Singh, Y. Yttrium Oxide Nanoparticle-Loaded, Self-Assembled Peptide Gel with Antibacterial, Anti-Inflammatory, and Proangiogenic Properties for Wound Healing. ACS Biomater. Sci. Eng. 2023, 9, 2647–2662. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold–silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials 2020, 234, 119763. [Google Scholar] [CrossRef]
- Karbowniczek, J.; Cordero-Arias, L.; Virtanen, S.; Misra, S.K.; Valsami-Jones, E.; Tuchscherr, L.; Rutkowski, B.; Górecki, K.; Bała, P.; Czyrska-Filemonowicz, A.; et al. Electrophoretic deposition of organic/inorganic composite coatings containing ZnO nanoparticles exhibiting antibacterial properties. Mater. Sci. Eng. C 2017, 77, 780–789. [Google Scholar] [CrossRef]
- Geng, H.; Poologasundarampillai, G.; Todd, N.; Devlin-Mullin, A.; Moore, K.L.; Golrokhi, Z.; Gilchrist, J.B.; Jones, E.; Potter, R.J.; Sutcliffe, C.; et al. Biotransformation of Silver Released from Nanoparticle Coated Titanium Implants Revealed in Regenerating Bone. ACS Appl. Mater. Interfaces 2017, 9, 21169–21180. [Google Scholar] [CrossRef]
- Patil, S.; Singh, N. Antibacterial silk fibroin scaffolds with green synthesized silver nanoparticles for osteoblast proliferation and human mesenchymal stem cell differentiation. Colloids Surfaces B: Biointerfaces 2018, 176, 150–155. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, P.; Hao, Y.; Ding, Y.; Cai, K. Construction of Ag-incorporated coating on Ti substrates for inhibited bacterial growth and enhanced osteoblast response. Colloids Surfaces B: Biointerfaces 2018, 171, 597–605. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhao, X.; He, H.; Sun, G.; Li, W.; Wang, X. Spray-deposited Ag nanoparticles on micro/nano structured Ti6Al4V surface for enhanced bactericidal property and cytocompatibility. Surf. Coatings Technol. 2021, 431, 128010. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Wang, Y.; Hong, H.; Zhang, L.; Chen, Z.; Zhang, P.; Chen, Z.; Zhang, W.; Zheng, S.; et al. A homogeneous dopamine–silver nanocomposite coating: striking a balance between the antibacterial ability and cytocompatibility of dental implants. Regen. Biomater. 2022, 10, rbac082. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Jiang, L.; Liu, L.; Chen, J.; Liao, Y.; He, S.; Cui, J.; Liu, X.; Zhao, A.-S.; Yang, P.; et al. Photofunctionalized and Drug-Loaded TiO2 Nanotubes with Improved Vascular Biocompatibility as a Potential Material for Polymer-Free Drug-Eluting Stents. ACS Biomater. Sci. Eng. 2020, 6, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ren, Z.; Chai, Q.; Cui, G.; Jiang, L.; Chen, H.; Feng, Z.; Chen, X.; Ji, J.; Zhou, L.; et al. A novel biliary stent coated with silver nanoparticles prolongs the unobstructed period and survival via anti-bacterial activity. Sci. Rep. 2016, 6, 21714. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Park, J.; Moon, J.Y.; Lee, S.; Park, C.H.; Kim, C.S. A Novel Design of Tri-Layer Membrane with Controlled Delivery of Paclitaxel and Anti-Biofilm Effect for Biliary Stent Applications. Nanomaterials 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Tie, D.; Hort, N.; Chen, M.; Guan, R.; Ulasevich, S.; Skorb, E.V.; Zhao, D.; Liu, Y.; Holt-Torres, P.; Liu, H. In vivo urinary compatibility of Mg-Sr-Ag alloy in swine model. Bioact. Mater. 2022, 7, 254–262. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM) - Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ebrahimi, N.G.; Rouhi, H. Investigation of radiopacity and antibacterial properties of ethylene vinyl acetate hollow fiber utilizable in ureteral J-shaped stents. Mater. Today Commun. 2023, 34. [Google Scholar] [CrossRef]
- Hua, J.; Yang, H.; Wang, B.; Dai, Y.; Li, X.; Yan, K.; You, R.; Ma, L. Silk fibroin/chitosan coating with tunable catalytic nitric oxide generation for surface functionalization of cardiovascular stents. Int. J. Biol. Macromol. 2023, 228, 261–272. [Google Scholar] [CrossRef]
- Wuppaladhodi, V.; Yang, S.; Pouri, H.; Zhang, J. Laser-assisted process for the deposition of nanostructured anti-microbial coatings on hydrogels. Opt. Laser Technol. 2023, 164. [Google Scholar] [CrossRef]
- Ballo, M.K.S.; Rtimi, S.; Pulgarin, C.; Hopf, N.; Berthet, A.; Kiwi, J.; Moreillon, P.; Entenza, J.M.; Bizzini, A. In Vitro and In Vivo Effectiveness of an Innovative Silver-Copper Nanoparticle Coating of Catheters To Prevent Methicillin-Resistant Staphylococcus aureus Infection. Antimicrob. Agents Chemother. 2016, 60, 5349–5356. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, S.; Ma, L.; Sun, M.; Wei, Z.; Wang, J.; Chen, Z.; Guo, Y.; Shi, J.; Wu, Q. An Amphiphilic Carbonaceous/Nanosilver Composite-Incorporated Urinary Catheter for Long-Term Combating Bacteria and Biofilms. ACS Appl. Mater. Interfaces 2021, 13, 38029–38039. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.P.; López-Marín, L.M.; Millán-Chiu, B.; Manzano-Gayosso, P.; Acosta-Torres, L.S.; García-Contreras, R.; Manisekaran, R. Polymer mediated synthesis of cationic silver nanoparticles as an effective anti-fungal and anti-biofilm agent against Candida species. Colloid Interface Sci. Commun. 2021, 43, 100449. [Google Scholar] [CrossRef]
- Cai, Y.; Gu, R.; Dong, Y.; Zhao, Q.; Zhang, K.; Cheng, C.; Yang, H.; Li, J.; Yuan, X. Fabrication of antibacterial polydopamine-carboxymethyl cellulose-Ag nanoparticle hydrogel coating for urinary catheters. J. Biomater. Appl. 2023, 38, 73–84. [Google Scholar] [CrossRef]
- Malhotra, A.; Chauhan, S.R.; Rahaman, M.; Tripathi, R.; Khanuja, M.; Chauhan, A. Phyto-assisted synthesis of zinc oxide nanoparticles for developing antibiofilm surface coatings on central venous catheters. Front. Chem. 2023, 11. [Google Scholar] [CrossRef]
- Elzahaby, D. A.; Farrag, H. A.; Haikal, R. R.; Alkordi, M. H.; Abdeltawab, N. F.; Ramadan, M. A. Inhibition of Adherence and Biofilm Formation of Pseudomonas aeruginosa by Immobilized ZnO Nanoparticles on Silicone Urinary Catheter Grafted by Gamma Irradiation Microorganisms [Online], 2023.
- Gallingani, T.; Resca, E.; Dominici, M.; Gavioli, G.; Laurita, R.; Liguori, A.; Mari, G.; Ortolani, L.; Pericolini, E.; Sala, A.; et al. A new strategy to prevent biofilm and clot formation in medical devices: The use of atmospheric non-thermal plasma assisted deposition of silver-based nanostructured coatings. PLOS ONE 2023, 18, e0282059. [Google Scholar] [CrossRef]
- Cacaci, M.; Squitieri, D.; Palmieri, V.; Torelli, R.; Perini, G.; Campolo, M.; Di Vito, M.; Papi, M.; Posteraro, B.; Sanguinetti, M.; Bugli, F. Curcumin-Functionalized Graphene Oxide Strongly Prevents Candida parapsilosis Adhesion and Biofilm Formation Pharmaceuticals [Online], 2023.
- Bletsa, E.; Merkl, P.; Thersleff, T.; Normark, S.; Henriques-Normark, B.; Sotiriou, G.A. Highly durable photocatalytic titanium suboxide–polymer nanocomposite films with visible light-triggered antibiofilm activity. Chem. Eng. J. 2023, 454. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; Teyssier, C.; Balme, S.; Miele, P.; Cornu, D.; Kalkura, N.; Cavaillès, V.; Bechelany, M. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Materials Science and Engineering: C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Saxena, V.; Hasan, A.; Pandey, L.M. Antibacterial nano-biocomposite scaffolds of Chitosan, Carboxymethyl Cellulose and Zn & Fe integrated Hydroxyapatite (Chitosan-CMC-FZO@HAp) for bone tissue engineering. Cellulose 2021, 28, 9207–9226. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2020, 118, 111547. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, L.; Shuai, Y.; Wu, X.; Zeng, Z.; Peng, S.; Shuai, C. 3D-printed CuFe2O4-MXene/PLLA antibacterial tracheal scaffold against implantation-associated infection. Appl. Surf. Sci. 2023, 614. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; García, A.; González-Jiménez, A.; Vallet-Regí, M. Antibacterial effect of 3D printed mesoporous bioactive glass scaffolds doped with metallic silver nanoparticles. Acta Biomater. 2023, 155, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Kharaghani, D.; Sun, L.; Ullah, S.; Sarwar, M.N.; Ullah, A.; Khatri, M.; Yoshiko, Y.; Gopiraman, M.; Kim, I.S. Synthesized bioactive lignin nanoparticles/polycaprolactone nanofibers: A novel nanobiocomposite for bone tissue engineering. Mater. Sci. Eng. C 2023, 144, 213203. [Google Scholar] [CrossRef]
- Chen, J.; Yang, P.; Liao, Y.; Wang, J.; Chen, H.; Sun, H.; Huang, N. Effect of the Duration of UV Irradiation on the Anticoagulant Properties of Titanium Dioxide Films. ACS Appl. Mater. Interfaces 2015, 7, 4423–4432. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, A.; Chen, H.; Liao, Y.; Yang, P.; Sun, H.; Huang, N. The effect of full/partial UV-irradiation of TiO 2 films on altering the behavior of fibrinogen and platelets. Colloids Surfaces B: Biointerfaces 2014, 122, 709–718. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





