Submitted:
24 July 2023
Posted:
25 July 2023
Read the latest preprint version here
Abstract
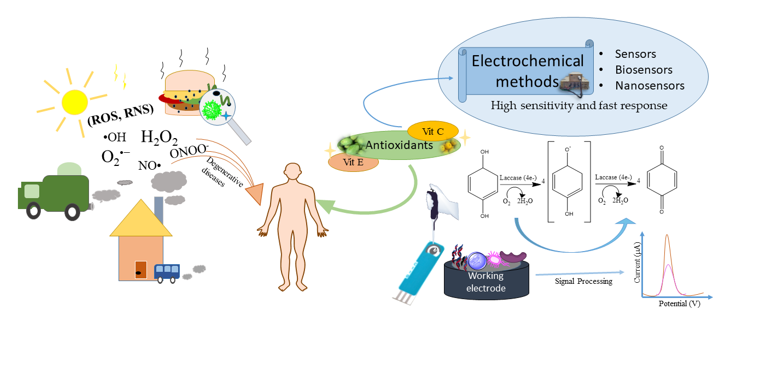
Keywords:
1. Introduction
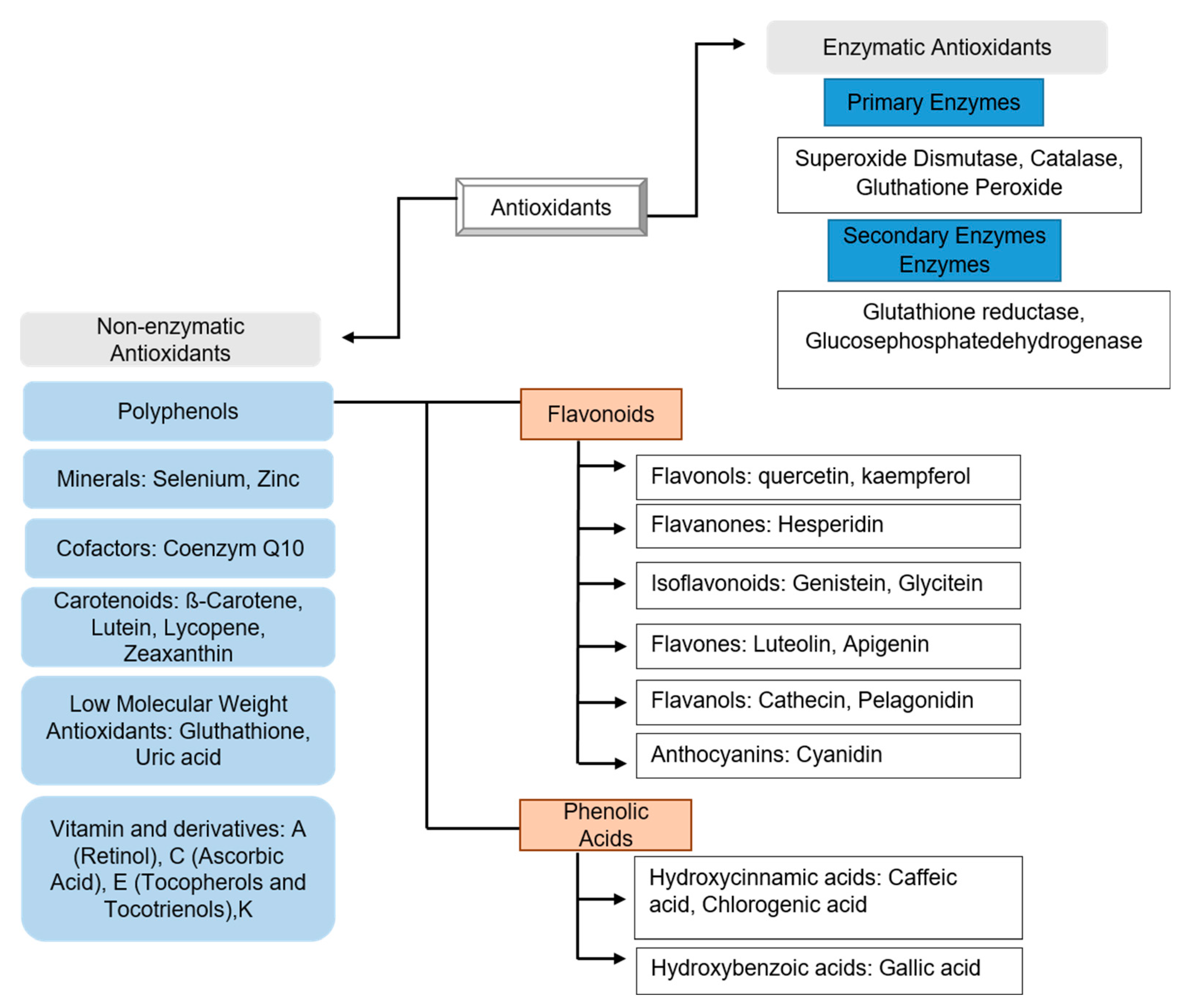
2. Antioxidants
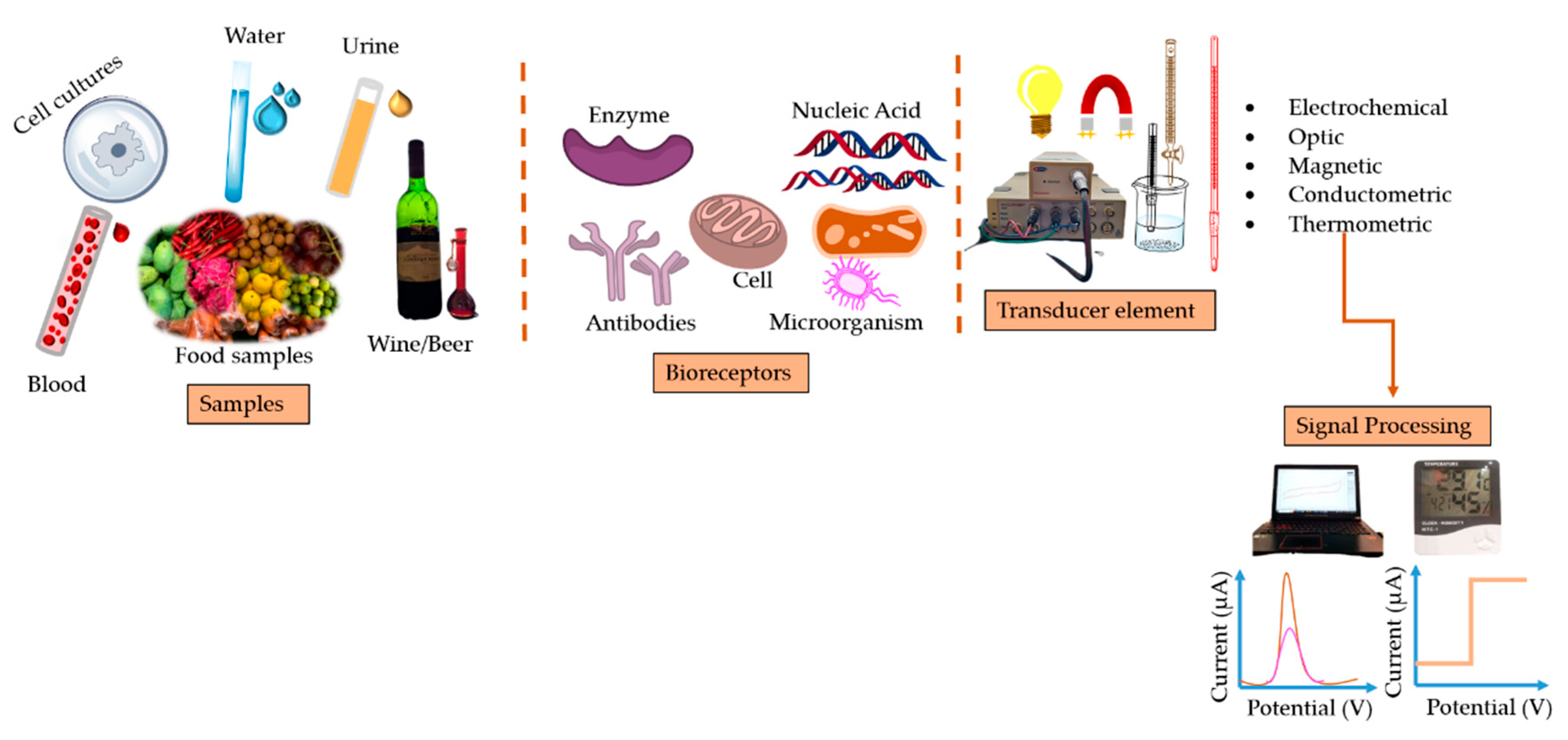
3. Electrochemical Sensors
| Electrochemical detection method | Electrode | Sample | Antioxidant | Detection limit |
Range linear | Ref |
|---|---|---|---|---|---|---|
| Cyclic Voltammetry | Glassy carbon | Spices | Curcumin | 4.1 × 10–6 M | 9.9 × 10-6 – 1.07 × 10–4 M | [53] |
| Amperometry | AgNP/Delph/GCE | Apple juice, lemon juice, peach juice, orange juice, green tea | Gallic acid | 0.28 µmol/L | 6×10-7– 8.68×10-6 M | [59] |
| Differential Pulse Voltammetry | GCE | Human serum blood | Total antioxidant capacity | - | - | [60] |
| Differential Pulse Voltammetry | GCE/ZnAl-NO3 layered double hydroxide film | - | Gallic Acid | 1.6 µM | 4–600 µM 7.0–180 µM |
[44] |
| Caffeic Acid | 2.6 µM | |||||
| Differential Pulse Voltammetry, Cyclic voltammetry | GCE | - | Tertiary butyl hydroquinone | 67 nM | 1.0 µM–1.1 mM | [54] |
| Differential pulse polarography | Dropping mercury | - | Gallic acid | 0.3 µM | 1.0–50 µM | [61] |
| Differential pulse polarography | Ti3Al0.5Cu0.5C2/GCE | Kiwi | Rutin | 0.015 μmol L− 1 . | 0.02–50.00 μmol/L | [62] |
| Square wave voltammetry | SPCE | - | • Ascorbic acid | • 0.09 mmol/I | [56] | |
| Square wave voltammetry | SPCE | - | • N-acetylcysteine | • 0.04 mmol/I | - | [56] |
| Square wave voltammetry | SPCE | - | • Melatonin | • 0.07 mmol/I | - | [56] |
| Square wave voltammetry | 4-[(4-decyloxyphenyl)- ethynyl]-1- methylpyridinium iodide modified glassy carbon | Mate herb extracts | Caffeic acid standard | 9.0×10-7 M 8.7×10-6 M | 9.9×10-7 M−3.8×10-5 M 4.7×10-5 M−9.9×10-5 M | [57] |
| Differential Pulse Voltammetry | (ZrO2/Co3O4/rG) | Tea, juice and urine | Gallic acid and uric acid | 2.5 × 10-8M | 2.2×10-7–5.5×105M | [52] |
| Differential Pulse Voltammetry | Am-ZrO2-CPE | Wine | Gallic acid | 1.24 × 10-7M | 1×10-6–1×10-3 M | (46] |
| Differential Pulse Voltammetry | Nano-GO-SiO2- nanoparticles-GCE | Red wine | Gallic acid | 6.25 × 10-6M | 1×10-6–1×10-3M | [63] |
4. Antioxidants-based Biosensors
4.1. Enzyme-based biosensor
4.1.1. Tyrosinase
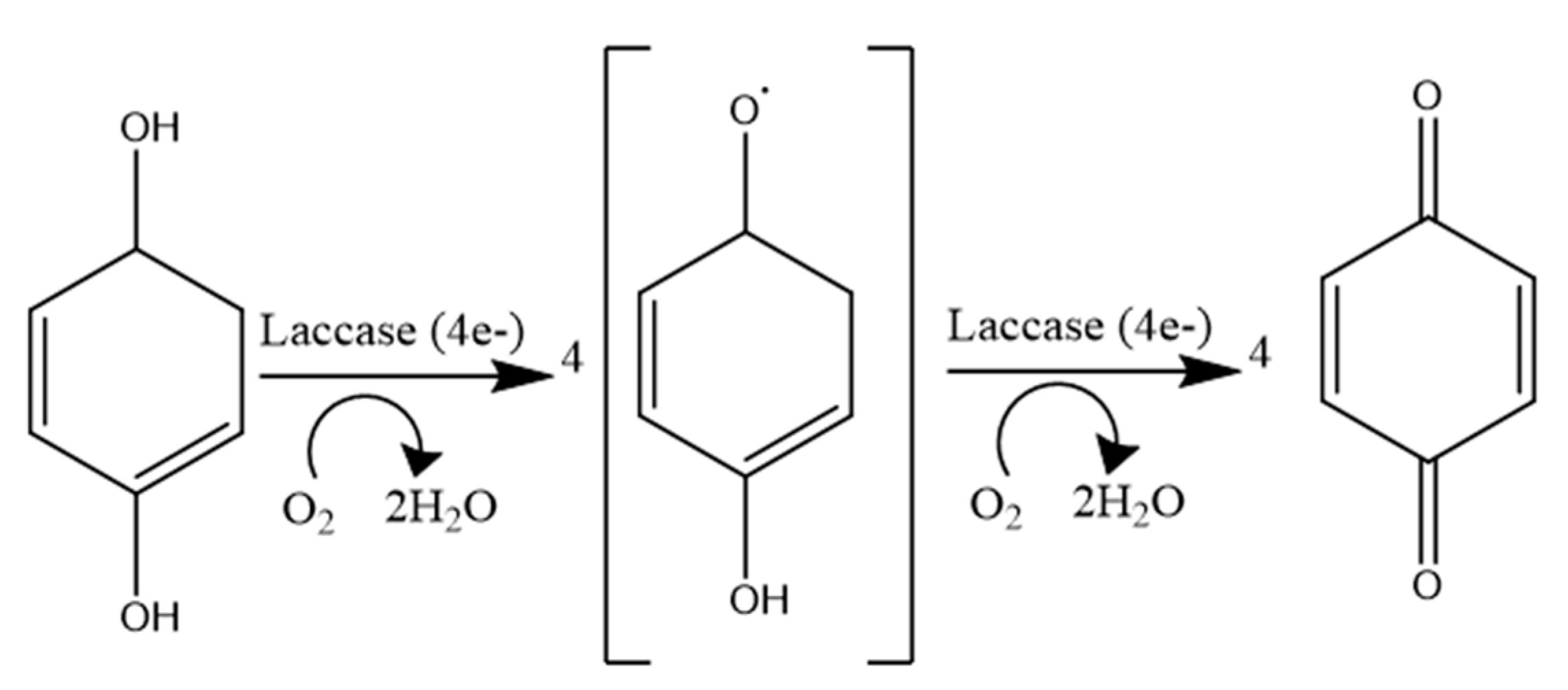
4.1.2. Laccase
4.1.3. Peroxidase-based biosensors
4.2. Cell/microorganism-based biosensors
4.3. DNA-based-biosensors
5. Nanosensors
| Method | Electrode | Medium | Antioxidants | Matrix | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|---|
| DPV | MIM-PACO/GCE | 0.25 M ABS (pH 6.5) | Curcumin | Turmeric extract | 5.0 nM | 10 nM–2.0 µM | [127] |
| DPV | SNO NRs/GCE | 0.1 M PBS (pH 5.0) | Quercetin | Apple and grape juice | 1.98 nM | 0.01–68.53 µM | [128] |
| DPV | MMIP | 0.1 M PBS (pH 1.0) | Rosmarinic acid | Salvia officinalis, Zataria multiflor, Mentha longifolia, and Rosmarinus officinalis | 0.085 µM | 0.1–100 µM | [129] |
| 100–500 µM | |||||||
| DPV | EGDMA-MIP/IL-GR/GCE | 0.04 M BRBS (pH 2.0) | Rutin | Tablet | 0.12 µM | 0.3–1 µM | [130] |
| DPV | GCE/rGO/ZIF-8/MIP | Rutin | Tablet and orange juice | 0.0001 µM | 0.05–100 µM | [131] | |
| 0.0005 -0.05µM | |||||||
| DPV | MIP/AuNPs/EGP | 0.1 M PBS (pH 5.0) | TBHQ | Edible oil | 0.07 µM | 0.08–100 µM | [122] |
| DPV | MIP/CHIT + AuNPs/SPCE | PBS (pH 7.0) | BHA | Chewing gum, mayonnaise and potato chips | 0.001 µg/mL | 0.01–20 µg/mL | [124] |
| DPV | CuO.NFs/NH2-CNTs/SPCE | 0.05 M PBS (pH 7.0) | TBHQ | Coconut oil, sesame oil, soybean oil | 3 nM | 0.013.9 µM | [125] |
| 3.9–147.6 µM | |||||||
| DPV | VMSF/ErGO/GCE | 0.1 M PBS (pH 4.0) | TBHQ | Edible oil, Toning lotion | 0.23 nM | 0.001–0.5 µM | [126] |
| 0.5–120 µM | |||||||
| AMP | poly O-cresolphthalein/MWCNT electrode | 0.1 M PBS (pH 7.0) | BHA | Potato chips | 0.11 µM | 0.33–110 µM | [121] |
| AMP | poly(carminic acid)/MWNT/GCE | BRBS (pH 2.0) | BHA | Linseed oil | 0.23 µM | 0.25–75 µM | [123] |
| TBHQ | 0.36 µM | 0.50–75 µM | |||||
| AMP | 4-aminobenzoic acid/Toray carbon fiber electrode | 0.1 M PBS (pH 7.0) | Bilirubin | Serum | 15 µM | 150–890 µM | [132] |
| AMP | AuNPs/RGO/SPCE | 100 mM PBS (pH 3.5) | Vitamin C | Commercial, pasteurized, and skimmed cow’s milk | 0.088 µg/mL | 50–500 µM | [133] |
| AMP | Mesoporous CuCo2O4/GCE | 0.15 M NaOH solution | Vitamin C | Vitamin C tablets Effervescent tablets | 0.21 µM | 1–100 µM | [134] |
| CV | H-BDDP-printed electrode | 1/15 M PBS (pH 7.0) | L-Cysteine | Bovine plasma | 0.620 µM | 1–194 µM | [135] |
| CV | GOCuNP/CPE | 1.0 M KCl (pH 7.0 | N-acetylcysteine | - | 2.97 × 10-5 M | 3.0×10-4–6.0× 10-3 M | [136] |
6. Biosensors Trends and Perspective
- Stability and immobilization
- Several kinds of enzymes
- Interference with the matrix
- Sensitivity and usage
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020, 382, 1199–207. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014, 5, 164–75. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in cardiovascular diseases: still a therapeutic target. Nutrients. 2019, 11, 2090. [Google Scholar] [CrossRef]
- Prasad, M.; Sedlarova, A.; Balukova, M.; Rac, P. Pospisil. Reactive Oxygen Species as a Response to Wounding: In Vivo Imaging in Arabidopsis thaliana. Front Plant Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020, 92, 479–90. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, H. A review on free radicals and antioxidants. Infect Disord Drug Targets. 2020, 20, 16–26. [Google Scholar] [CrossRef]
- Marrocco I, Altieri F, Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid Med Cell Longev. 2017, 2017, 1–32. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative: A review. European J Med Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Sandrasari, D.A.; Andarwulan, N.; Faridah, D.N.; Dewi, F.N.A. Indonesian indigenous plants as a source of antioxidants to treat gastrointestinal disorders. Food Res. 2021, 5, 195–204. [Google Scholar] [CrossRef]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.J.F.R. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y.J. Perspectives of the potential implications of polyphenols in influencing the interrelationship between oxi-inflammatory stress, cellular senescence and immunosenescence during aging. Trends Food Sci & Technol. 2020, 98, 41–52. [Google Scholar]
- Iswantini, D.; Yulian, M.; Mulijani, S.; Trivadila. Inhibition kinetics of Sida rhombifolia L. extract toward xanthine oxidase electrochemical method. Indo. J. Chem.. 2014, 14, 71–77. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.M. Oxidative stress mitigation by antioxidants an overview on their chemistry and influences on health status. Eur J Med Chem. 2021, 112891. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Budić-Leto, I.; Corral-Jara, K.F.; Ajdžanović, V.; Arola-Arnal, A.; Bravo, F.I.; Deligiannidou, G.E.; Havlik, J.; Janeva, M.; Kistanova, E.; Kontogiorgis, C.; Krga, I.; Massaro, M.; Miler, M.; Milosevic, V.; Morand, C.; Scoditi, E.; Suarez, M.; Vauzour, D.; Milenkovic, D. Systematic bioinformatic analyses of nutrigenomic modifications by polyphenols associated with cardiometabolic health in humans—Evidence from targeted nutrigenomic studies. Nutrients. 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, J.; Sun, Z.; Shen, P.; Sun, X. Recent advances in electrochemical biosensors for antioxidant analysis in foodstuff. TrAC - Trends Anal Chem, 1157. [Google Scholar] [CrossRef]
- Srinivasan, K. Antioxidant Potential of Spices and Their Active Constituents. Crit Rev Food Sci Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants. 2017, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Ojha, K.; Dubey, S.; Chandrakar, J.; Minj, R.A.; Dehariya, R.; Dixit, A.K. A review on different methods of determination of antioxidant activity assay of herbal plant. J of Sci Bio Pharm and chem sci. 2018, 4, 707–732. [Google Scholar]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int J Cosmet Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int J Mol Sci. 2016, 17, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Cisowska, J.; Flaczyk, E.; Rudzińska, M.; Kmiecik, D. Antioxidant properties of extracts from Ginkgo biloba leaves in meatballs. Meat Sci. 2014, 97, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl Surf Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Vinci, G.; D’ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The Influence of Green and Black Tea Infusion Parameters on Total Polyphenol Content and Antioxidant Activity by ABTS and DPPH Assays. Beverages. 2022, 8. [Google Scholar] [CrossRef]
- Thongsuk, P.; Sameenoi, Y. Colorimetric determination of radical scavenging activity of antioxidants using Fe3O4 magnetic nanoparticles. Arab J Chem. 2022, 15, 103475. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Huang, K.; Cheng, N. Advanced Agrochem Biosensor for agriculture and food safety : Recent advances and future perspectives. Adv Agrochem. 2022, 1, 3–6. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Chlorogenic Acid Electroanalysis. Int J Mol Sci. 2021, 22, 13138. [Google Scholar] [CrossRef]
- Barroso, M.F.; Ramalhosa, M.J.; Alves, R.C.; Dias, A.; Soares, C.M.D.; Oliva-Teles, M.T.; Delerue-Matos, C. Total antioxidant capacity of plant infusions: Assessment using electrochemical DNA-based biosensor and spectrophotometric methods. Food Control. 2016, 68, 153–161. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; Rajendran, S.; Ayati, A.; Fu,L. ; Sanati, A.L.; Tanhaei, B.; Sen, F.; Shabani-nooshabadi, M.; Asrami, P.N.; Al-Othman, A. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, X.; Zhang, Y.; Han, X.; Sun, X. 2022. A novel cell-based electrochemical biosensor based on MnO2 catalysis for antioxidant activity evaluation of anthocyanins. Biosens Bioelectron. 2021, 202, 113990. [Google Scholar] [CrossRef] [PubMed]
- Romero, K.J.; Galliher, M.S.; Raycroft, M.A.R.; Chauvin, J.P.R.; Bosque, I.; Pratt, D.A.; Stephenson, C.R.J. Electrochemical Dimerization of Phenylpropenoids and the Surprising Antioxidant Activity of the Resultant Quinone Methide Dimers. Angew Chemie - Int Ed. 2018, 57, 17125–17129. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Parpinello, G.P.; Teslić, N.; Kilmartin, P.A.; Versari, A. Suitability of the cyclic voltammetry measurements and DPPH• spectrophotometric assay to determine the antioxidant capacity of food-grade oenological tannins. Molecules. 2019, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petković, B.B.; Stanković, D.; Milčić, M.; Sovilj, S.P.; Manojlović, D. Dinuclear copper(II) octaazamacrocyclic complex in a PVC coated GCE and graphite as a voltammetric sensor for determination of gallic acid and antioxidant capacity of wine samples. Talanta. 2015, 132, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D. ; Harish.; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology (Basel), 10. [CrossRef]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur J Med Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Chu, P.M.; Tuan, V.P.; Te, J.S.L.; Lee, I.T. The promising role of antioxidant phytochemicals in the prevention and treatment of periodontal disease via the inhibition of oxidative stress pathways: Updated insights. Antioxidants. 2020, 9, 1–28. [Google Scholar] [CrossRef]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int J Pharm Sci Res. 2015, 6, 546–566. https://doi.org/10.13040/IJPSR.0975-8232.6(2).546-66. Martínez, A.; Galano, A.; Vargas, R. Free radical scavenger properties of α-mangostin: Thermodynamics and kinetics of HAT and RAF mechanisms. J Phys Chem, B. 2011, 115, 12591–12598. doi:10.1021/jp205496u.
- Martinez-Perez, C.; Ward, C.; Cook, G.; Mullen, P.; McPhail, D.; Harrison, D.J.; Langdon, S.P. Novel flavonoids as anti-cancer agents: Mechanisms of action and promise for their potential application in breast cancer. Biochem Soc Trans. 2014, 42, 1017–1023. [Google Scholar] [CrossRef]
- Martinez-Perez, C.; Ward, C.; Cook, G.; Mullen, P.; McPhail, D.; Harrison, D.J.; Langdon, S.P. Novel flavonoids as anti-cancer agents: Mechanisms of action and promise for their potential application in breast cancer. Biochem Soc Trans. 2014, 42, 1017–1023. [Google Scholar] [CrossRef]
- Awouafack, Maurice, D., et al. ‘Isolation and Structure Characterization of Flavonoids’. Flavonoids - From Biosynthesis to Human Health, InTech, 23 Aug. 2017. [CrossRef]
- Tiwari, S.C.; Husain, N. Biological Activities and Role of Flavonoids in Human Health-A Review. Indian J.Sci.Res. 2017, 12, 193–196. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Kahl, M.; Golden, T.D. Electrochemical determination of phenolic acids at a Zn/Al layered double hydroxide film modified glassy carbon electrode. Electroanalysis. 2014, 26, 1664–1670. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chikere, C.O.; Faisal, N.H.; Kong-Thoo-Lin, P.; Fernandez, C. Interaction between amorphous zirconia nanoparticles and graphite: Electrochemical applications for gallic acid sensing using carbon paste electrodes in wine. Nanomaterials. 2020, 10, 1–25. [Google Scholar] [CrossRef]
- Zheng, Y.; Karimi-maleh, H.; Fu, L. Evaluation of Antioxidants Using Electrochemical Sensors: A Bibliometric Analysis. Sensors. 2022, 22, 1–25. [Google Scholar] [CrossRef]
- Nejad, F.G.; Tajik, S.; Beitollahi, H.; Sheikhshoaie, I. Magnetic nanomaterials based electrochemical (bio)sensors for food analysis. Talanta. 2021, 228, 122075. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors. 2021, 21, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Gajdár, J.; Goněc, T.; Jampílek, J.; Brázdová, M.; Bábková, Z.; Fojta, M.; Barek, J.; Fischer, J. Voltammetry of a Novel Antimycobacterial Agent 1-Hydroxy-N-(4-nitrophenyl)naphthalene-2-carboxamide in a Single Drop of a Solution. Electroanalysis. 2018, 30, 38–47. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis. 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Shahamirifard, S.A.; Ghaedi, M.; Razmi, Z.; Hajati, S. A simple ultrasensitive electrochemical sensor for simultaneous determination of gallic acid and uric acid in human urine and fruit juices based on zirconia-choline chloride-gold nanoparticles-modified carbon paste electrode. Biosens Bioelectron. 2018, 114, 30–36. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Nizamova, A.M.; Budnikov, H.C. Voltammetric determination of curcumin in spices. J Anal Chem. 2012, 67, 591–594. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, C.; Yu, Z. Electrochemical biosensor for assessment of the total antioxidant capacity of orange juice beverage based on the immobilizing DNA on a poly l-glutamic acid doped silver hybridized membrane. Sensors Actuators, B Chem. 2014, 192, 628–633. [Google Scholar] [CrossRef]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.G.; Marcolino-Júnior, L.H.; Bergamini, M.F. Voltammetric determination of the antioxidant capacity in wine samples using a carbon nanotube modified electrode. J. Agric. Food. Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef]
- Kračmarová, A.; Pohanka, M. Elektrochemické stanovení nízkomolekulárních antioxidantů v séru. Chem List. 2014, 108, 64–69. [Google Scholar]
- Silva, T.R.; Westphal, E.; Gallardo, H.; Vieira, I.C. Ionic organic film sensor for determination of phenolic compounds. Electroanalysis. 2014, 26, 1801–1809. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Electrochemical determination of chlorogenic acid in nutraceuticals using voltammetric sensors based on screen-printed carbon electrode modified with graphene and gold nanoparticles. Int. J. Mol. Sci. 2021, 22, 8897. [Google Scholar] [CrossRef]
- Ghaani, M.; Nasirizadeh, N.; Ardakani, S.A.Y.; Mehrjardi, F.Z.; Scampicchio, M.; Farris, S. Development of an electrochemical nanosensor for the determination of gallic acid in food. Anal Methods. 2016, 8, 1103–1110. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Freinbichler, W.; Linert, W.; Dorozhko, E.V.; Bukkel, M.V.; Plotnikov, E.V.; Voronova, O.A. Study of total antioxidant activity of human serum blood in the pathology of alcoholism. Molecules. 2013, 18, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ü.T.; Kekillioglu, A.; Mert, R. Determination of Gallic acid by differential pulse polarography: Application to fruit juices. J Anal Chem. 2013, 68, 1064–1069. [Google Scholar] [CrossRef]
- Senocak, A.; Sanko, V.; Tumay, S.O.; Orooji, Y.; Demirbas, E.; Yoon, Y.; Khataee, A. Ultrasensitive electrochemical sensor for detection of rutin antioxidant by layered Ti3Al0.5Cu0.5C2 MAX phase. Food Chem. Toxicol. 2022, 164, 1–9. [Google Scholar] [CrossRef]
- Chikere, C.; Faisal, N.H.; Lin, P.K.T.; Fernandez, C. Zinc oxide nanoparticles modified-carbon paste electrode used for the electrochemical determination of Gallic acid. J Phys Conf Ser. 2019, 1310. [Google Scholar] [CrossRef]
- Wardak, C.; Paczosa-Bator, B.; Malinowski, S. Application of cold plasma corona discharge in preparation of laccase-based biosensors for dopamine determination. Materials Science and Engineering: C. 2020, 116, 1–10. [Google Scholar] [CrossRef]
- Azad, M.S. Experiences of Domestic Abuse within the South Asian Community. J glob faultlines. 2021, 8, 50–68. [Google Scholar] [CrossRef]
- Gil, D.M.A.; Rebelo, M.J.F. Evaluating the antioxidant capacity of wines: A laccase-based biosensor approach. Eur Food Res Technol. 2010, 231, 303–308. [Google Scholar] [CrossRef]
- Gahlaut, A.; Gothwal, A.; Chhillar, K.; Hooda, V. Electrochemical Biosensors for Determination of Organophosphorus Compounds : Review. Sci Res J. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- de Oliveira Neto, J.R.; Rezende, S.G.; Lobón, G.S.; Garcia, T.A.; Macedo, I.Y.L.; Garcia, L.F.; Alves, V.F.; Torres, I.M.S.; Santiago, M.F.; Schmidt, F.; de Souza Gil, E. Electroanalysis and laccase-based biosensor on the determination of phenolic content and antioxidant power of honey samples. Food Chem. 2017, 237, 1118–1123. [Google Scholar] [CrossRef]
- Tomac, I. , Šeruga, M., Labuda, J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Sun, X.; Zhang, Y.; Han, X.; Sun, X. A novel cell-based electrochemical biosensor based on MnO2 catalysis for antioxidant activity evaluation of anthocyanins. Biosens Bioelectron. 2022, 202, 113990. [Google Scholar] [CrossRef] [PubMed]
- Narita, F.; Wang, Z.; Kurita, H.; Li, Z.; Shi, Y.; Jia, Y.; Soutis, C. A Review of Piezoelectric and Magnetostrictive Biosensor Materials for Detection of COVID-19 and Other Viruses. Adv. Mater. 2021, 33, 2005448. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. ; Exercise, Sport, and Bioanalytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Dzyadevych, S.; Jaffrezic-Renault, N. Conductometric Biosensors. In Biological Identification; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–193. [Google Scholar]
- Fu, Y.; You, Z.; Xiao, A.; Liu, L.; Zhou, W. Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene. Open Chem. 2020, 18, 1054–1063. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; Pividori, M.I.; Gutiérrez, J.M.; Del Valle, M. Resolution of phenolic antioxidant mixtures employing a voltammetric bio-electronic tongue. Analyst. 2012, 137, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Vlamidis, Y.; Gualandi, I.; Tonelli, D. Amperometric biosensors based on reduced GO and MWCNTs composite for polyphenols detection in fruit juices. J Electroanal Chem. 2017, 799, 285–292. [Google Scholar] [CrossRef]
- Nadifiyine, S.; Calas-Blanchard, C.; Amine, A.; Marty, J.L. Tyrosinase Biosensor Used for the Determination of Catechin Derivatives in Tea: Correlation with HPLC/DAD Method. Food Nutr Sci. 2013, 4, 108–118. [Google Scholar] [CrossRef]
- Sýs, M.; Pekec, B.; Kalcher, K.; Vytřas, K. Amperometric enzyme carbon paste-based biosensor for quantification of hydroquinone and polyphenolic antioxidant capacity. Int J Electrochem Sci. 2013, 8, 9030–9040. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Tyrosinase-Based Biosensor—A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations. Materials (Basel) 2022a, 15, 3221. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Laccase and tyrosinase biosensors used in the determination of hydroxycinnamic acids. Int. J. Mol. Sci. 2021, 22, 4881. [Google Scholar] [CrossRef]
- Kadam, A.A.; Saratale, G.D.; Ghodake, G.S.; Saratale, R.G.; Shahzad, A.; Magotra, V.K.; Kumar, M.; Palem, R.R.; Sung, J.S. Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques. Chemosensors. 2022, 10, 1–27. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC - Trends Anal Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Assessment of the Antioxidant Activity of Catechin in Nutraceuticals: Comparison between a Newly Developed Electrochemical Method and Spectrophotometric Methods. Int J Mol Sci. 2022a, 23, 8110. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, H.; Zhang, Q.; Bai, X.; Liu, C.; Zhang, Y.H. Direct electron transfer and sensing performance for catechin of nano-gold particles-polymer nano-composite with immobilized Laccase. Chem Phys Lett. 2016, 658, 259–269. [Google Scholar] [CrossRef]
- Steevensz, A.; Villegas, L.G.C.; Feng, W.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Soybean peroxidase for industrial wastewater treatment: a mini review. J Environ Eng Sci. 2014, 9, 181–186. [Google Scholar] [CrossRef]
- Neumann, B.; Wollenberger, U. Electrochemical Biosensors Employing Natural and Artificial Heme Peroxidases on Semiconductors. Sensors 2020, 20, 3692. [Google Scholar] [CrossRef]
- Smutok, O.; Kavetskyy, T.; Prokopiv, T.; Serkiz, R.; Šauša, O.; Novák, I.; Švajdlenková, H.; Maťko, I.; Gonchar, M.; Katz, E. Biosensor Based on Peroxidase-Mimetic Nanozyme and Lactate Oxidase for Accurate L-Lactate Analysis in Beverages. Biosensors. 2022, 12, 1042. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, M.J.; Choi, J.H.; Song, J.; Kim, T.H.; Oh, B.K. Electrochemical H2O2 biosensor based on horseradish peroxidase encapsulated protein nanoparticles with reduced graphene oxide-modified gold electrode. Nano converg. 2020, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yin, W.; Tang, K.; Li, D.; Shao, K.; Zuo, Y.; Ma, J.; Liu, J.; Han, H. Enzymatic biosensor of horseradish peroxidase immobilized on Au-Pt nanotube/Au-graphene for the simultaneous determination of antioxidants. Anal Chim Acta. 2016, 933, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. DNA-stabilized, fluorescent, metal nanoclusters for biosensor development. TrAC - Trends Anal Chem. 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef]
- Inda, M.E.; Mimee, M.; Lu, T.K. Cell-based biosensors for immunology, inflammation, and allergy. J Allergy Clin Immunol. 2019, 144, 645–647. [Google Scholar] [CrossRef]
- Ge, Q.; Ge, P.; Jiang, D.; Du, N.; Chen, J.; Yuan, L.; Yu, H.; Xu, X.; Wu, M.; Zhang, W.; Zhou, G. A novel and simple cell-based electrochemical biosensor for evaluating the antioxidant capacity of Lactobacillus plantarum strains isolated from Chinese dry-cured ham. Biosens Bioelectron. 2018, 99, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K. , et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors guideline from the college of American pathologists, the international association for the study of lung cancer, and the a. Arch Pathol Lab Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Iswantini, D.; Weniarti. ; Nurhidayat, N.; Abidin, Z.; Trivadila. Antioxidant biosensor based on superoxide dismutase from Indonesian microbes immobilized in Indonesian natural zeolite. J Appl Pharm Sci. 2019, 9, 104–109. [Google Scholar] [CrossRef]
- Iswantini, D.; Nurhidayat, N.; Trivadila; Widiyatmoko, O. Activity and stability of uricase from Lactobacillus plantarum immobilizated on natural zeolite for uric acid biosensor. Pak J Biol Sci. 2014, 17, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Febryanti, A.; Mulijani, S.; Iswantini, D. Antioxidant biosensor based on Deinococcus radiodurans biofilm immobilized on screen printed carbon electrode (SPCE) surface. IJRASB, 2018; 5, 1–7. [Google Scholar]
- Reder-Christ, K.; Bendas, G. Biosensor applications in the field of antibiotic research-a review of recent developments. Sensors. 2011, 11, 9450–9466. [Google Scholar] [CrossRef]
- Liang, G.; Man, Y.; Li, A.; Jin, X.; Liu, X.; Pan, L. DNAzyme-based biosensor for detection of lead ion: A review. Microchem J. 2017, 131, 145–153. [Google Scholar] [CrossRef]
- Saidur, M.R.; Aziz, A.R.A.; Basirun, W.J. Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A review. Biosens Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef]
- Dehghani, S.; Nosrati, R.; Yousefi, M.; Nezami, A.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Alibolandi, M.; Ramezani, M. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): A review. Biosens Bioelectron. 2018, 110, 23–37. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J, Zhong,Q. Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. J Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Liu, W.; Wang, R. Nucleic acid-based electrochemical biosensor: Recent advances in probe immobilization and signal amplification strategies. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2022, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-based biosensors for antibiotic detection: A review. Biosensors. 2018, 8, 54. [Google Scholar] [CrossRef]
- Li, N.; Du, M.; Liu, Y.; Ji, X.; He, Z. Multipedal DNA Walker Biosensors Based on Catalyzed Hairpin Assembly and Isothermal Strand-Displacement Polymerase Reaction for the Chemiluminescent Detection of Proteins. ACS Sensors. 2018, 3, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Kataoka, Y.; Kuwahara, M.; Horii, K.; Shiratori, I.; Waga, I. A high affinity modified DNA aptamer containing base-appended bases for human β-defensin. Anal Biochem. 2020, 594, 8–11. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Liu, J. G-quadruplex DNA for construction of biosensors. TrAC - Trends Anal Chem. 2020, 132, 116060. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, S.; Yu, X.; Hu, S.; Lu, Y.; Wu, Z.S. Periodically Ordered, Nuclease-Resistant DNA Nanowires Decorated with Cell-Specific Aptamers as Selective Theranostic Agents. Angew Chemie - Int Ed. 2020, 59, 17540–17547. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. DNA-stabilized, fluorescent, metal nanoclusters for biosensor development. TrAC - Trends Anal Chem. 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Applications of a DNA-electrochemical biosensor. TrAC - Trends Anal Chem. 2016, 79, 23–36. [Google Scholar] [CrossRef]
- Barroso, M.F.; Delerue-Matos, C.; Oliveira, M.B.P.P. Electrochemical evaluation of total antioxidant capacity of beverages using a purine-biosensor. Food Chem. 2012, 132, 1055–1062. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Li, M.; Zhang, L.; Liu, M.; Fu, S. N-P-Zn-containing 2D supermolecular networks grown on MoS2 nanosheets for mechanical and flame-retardant reinforcements of polyacrylonitrile fiber. Chem Eng J. 2019, 372, 873–885. [Google Scholar] [CrossRef]
- Tang, P.; Tang, X.; Mei, S.; Xie, Y.; Liu, L.; Ren, L. Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE). Open Chem. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Tomac, I.; Šeruga, M.; Labuda, J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemnadi, N.; Amini, M.; Rezaei, B. Impedimetric DNA-biosensor for the study of dopamine induces DNA damage and investigation of inhibitory and repair effects of some antioxidants. Bioelectrochemistry. 2015, 104, 71–78. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr Rev Food Sci Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Manoranjitham, J.J.; Narayanan, S.S. Electrochemical sensor for determination of butylated hydroxyanisole (BHA) in food products using poly O-cresolphthalein complexone coated multiwalled carbon nanotubes electrode. Food Chem. 2021, 342, 128246. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Kan, X. Sensitive detection of butylated hydroxyanisole based on free-standing paper decorated with gold and NiO nanoparticles. Microchem J. 2020, 159, 105511. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Budnikov, H. Amperometric sensor based on MWNT and electropolymerized carminic acid for the simultaneous quantification of TBHQ and BHA. J Electroanal Chem. 2020, 859, 113885. [Google Scholar] [CrossRef]
- Motia, S.; Bouchikhi, B.; El Bari, N. An electrochemical molecularly imprinted sensor based on chitosan capped with gold nanoparticles and its application for highly sensitive butylated hydroxyanisole analysis in foodstuff products. Talanta. 2020, 223, 121689. [Google Scholar] [CrossRef] [PubMed]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Rasana, N. Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J Hazard Mater. 2021, 406, 124792. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, M.; Jin, Q.; Zhou, H.; Xie, L.; Tang, H.; Liu, J. Vertically-ordered mesoporous silica films on graphene for anti-fouling electrochemical detection of tert-butylhydroquinone in cosmetics and edible oils. J Electroanal Chem. 2021, 881, 114969. [Google Scholar] [CrossRef]
- Gao, Q.; Zang, Y.; Zhang, Y.; Xie, J.; Li, J.; Gao, J.; Xue, H. Composite polymerized molecular imprinting membrane-based electrochemical sensor for sensitive determination of curcumin by using 4-pentenoyl-aminoacyl-chitosan oligosaccharide as functional monomer oligomer. J Electroanal Chem. 2020, 879, 114793. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sangili, A.; Chen, S.M.; Veerakumar, P.; Lin, K.C. Sr-Doped NiO3 nanorods synthesized by a simple sonochemical method as excellent materials for voltammetric determination of quercetin. New J Chem. 2020, 44, 2821–2832. [Google Scholar] [CrossRef]
- Alipour, S.; Azar, P.A.; Husain, S.W.; Rajabi, H.R. Determination of Rosmarinic acid in plant extracts using a modified sensor based on magnetic imprinted polymeric nanostructures. Sensors Actuators, B Chem. 2020, 323, 128668. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, J.; Zeng, Y.; Zhu, Y.; Wang, H.; Lei, X.; Huang, S.; Guo, L.; Li, L. Electrochemical determination of rutin based on molecularly imprinted poly (ionic liquid) with ionic liquid-graphene as a sensitive element. Sensors Actuators, B Chem. 2020, 311, 127911. [Google Scholar] [CrossRef]
- El Jaouhari, A. .; Yan, L.; Zhu, J.; Zhao, D.; Khan, M.Z.H.; Liu, X. Enhanced molecular imprinted electrochemical sensor based on zeolitic imidazolate framework/reduced graphene oxide for highly recognition of rutin. Anal Chim Acta. 2020, 1106, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, B.; Lingagouder, J.; Berchmans, S.; Ganesh, V. Electrochemical grafting of 4-aminobenzoic acid onto toray carbon – Interfacial investigation and fabrication of non-enzymatic bilirubin sensor. Sensors Actuators, B Chem. 2021, 344, 130292. [Google Scholar] [CrossRef]
- Bettazzi, F.; Ingrosso, C.; Sfragano, P.S.; Pifferi, V.; Falciola, L.; Curri, M.L.; Palchetti, I. Gold nanoparticles modified graphene platforms for highly sensitive electrochemical detection of vitamin C in infant food and formulae. Food Chem. 2021, 344, 128692. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Z.; Nan, F.; Zhao, Y.; Wang, P.; He, F.; Wang, Y. Mesoporous CuCo2O4 rods modified glassy carbon electrode as a novel non-enzymatic amperometric electrochemical sensors with high-sensitive ascorbic acid recognition. J Alloys Compd. 2021, 852, 157045. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kondo, T.; Shitanda, I.; Hoshi, Y.; Itagaki, M.; Tojo, T.; Yuasa, M. Sensitive electrochemical detection of L-Cysteine at a screen-printed diamond electrode. Carbon N Y. 2021, 173, 395–402. [Google Scholar] [CrossRef]
- Maraldi, V.A.; Colmenares, Y.N.; Barbosa, P.P.F. , Mastelaro, V.; do Carmo, D.R. Graphene Oxide as a Platform for Copper Pentacyanonitrosylferrate Nanoparticles and their Behavior in the Electro-oxidation of N-Acetylcysteine. Electroanalysis. 2020, 32, 1408–1416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





