Submitted:
24 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Procedure
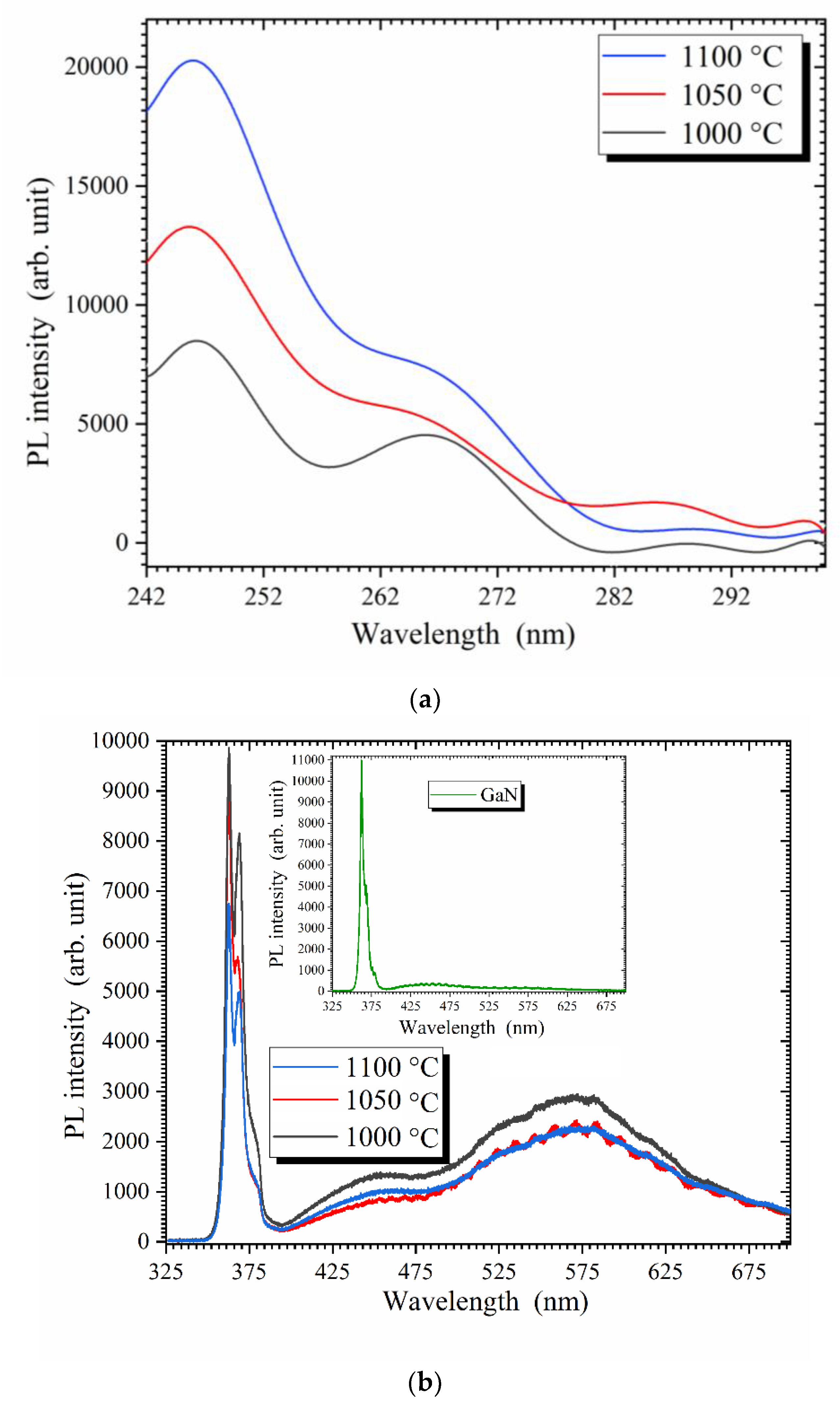
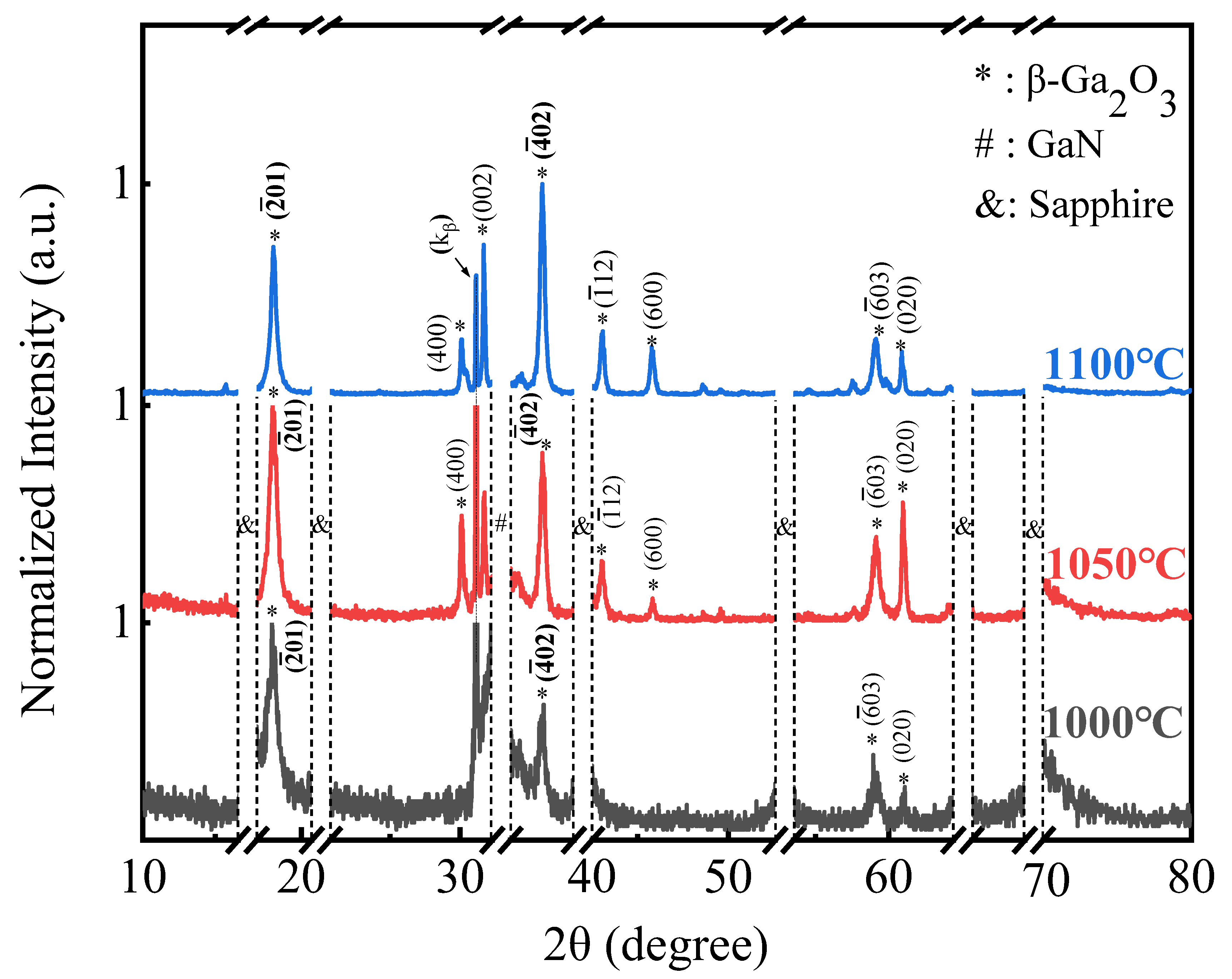
3. Results and discussion
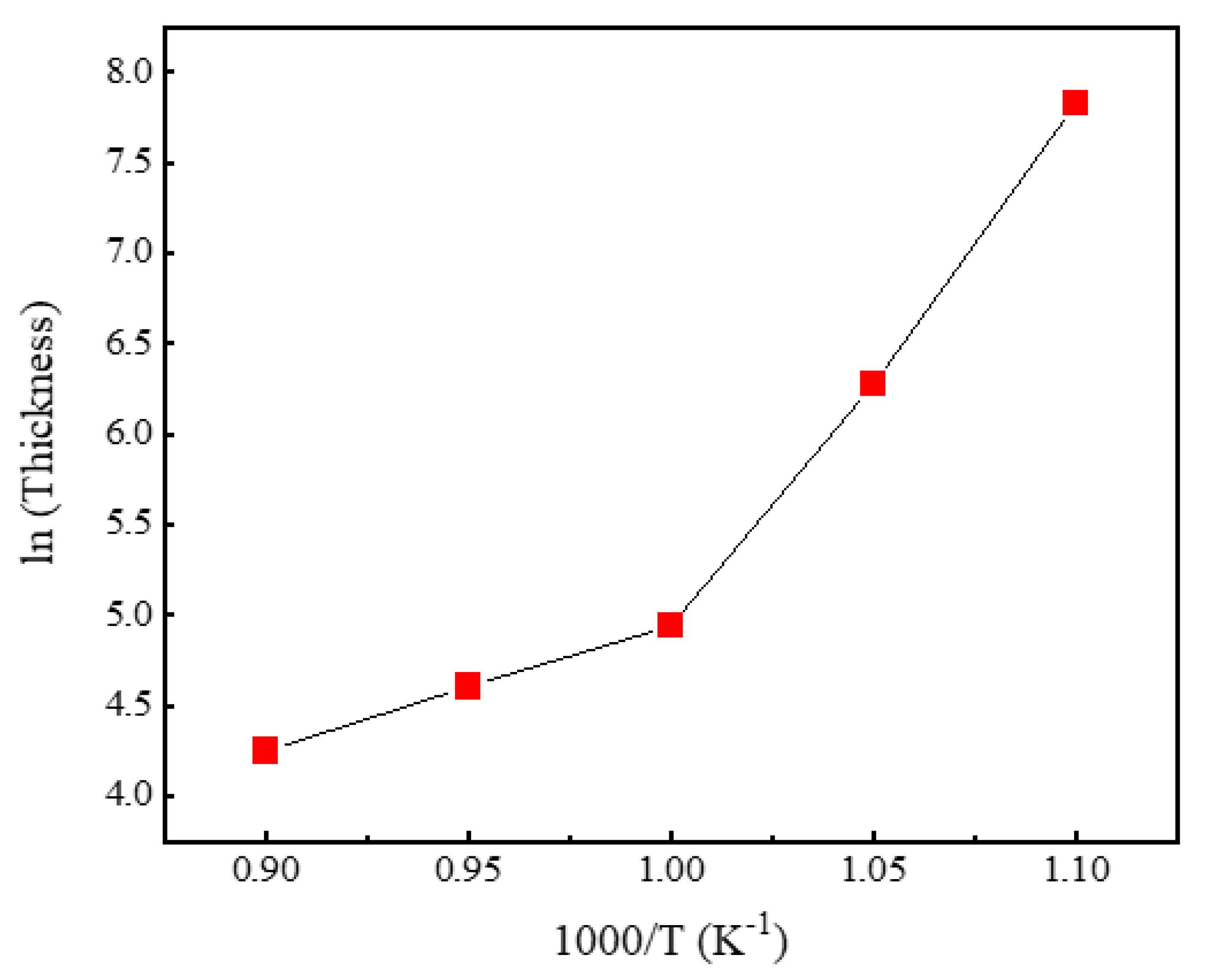
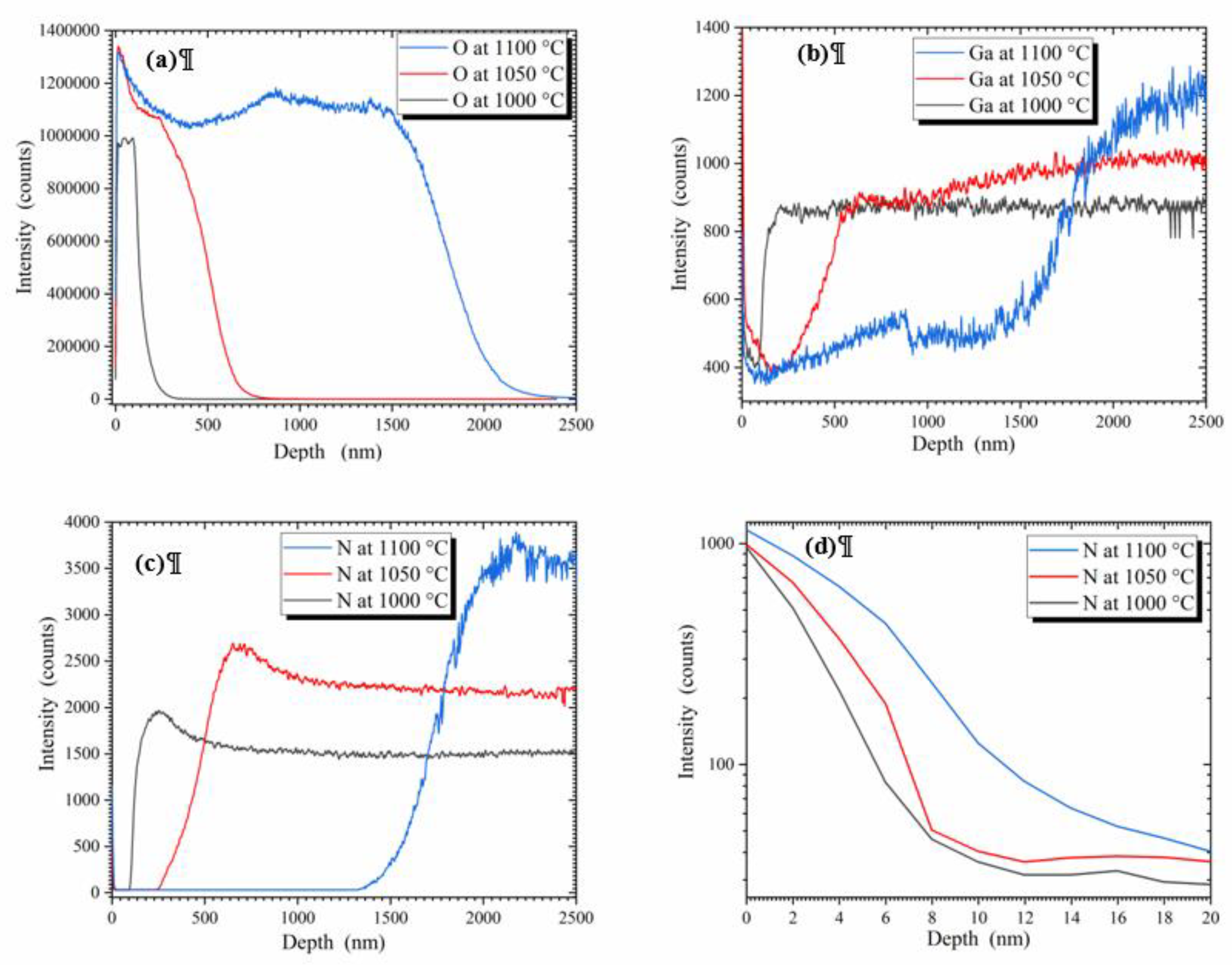
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearton, S.J.; Yang, J.; Cary, P.H., IV; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Murakami, H.; Kumagai, Y.; Koukitu, A.; Kuramata, A.; Masui, T.; Yamakoshi, S. Recent progress in Ga2O3power devices. Semicond. Sci. Technol. 2016, 31, 034001. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Mastro, M.A.; Kuramata, A.; Calkins, J.; Kim, J.; Ren, F.; Pearton, S.J. Perspective—Opportunities and Future Directions for Ga2O3. ECS J. Solid State Sci. Technol. 2017, 6, P356–P359. [Google Scholar] [CrossRef]
- Konishi, K.; Goto, K.; Murakami, H.; Kumagai, Y.; Kuramata, A.; Yamakoshi, S.; Higashiwaki, M. 1-kV vertical Ga2O3 field-plated Schottky barrier diodes. Appl. Phys. Lett. 2017, 110. [Google Scholar] [CrossRef]
- Sasaki, K.; Higashiwaki, M.; Kuramata, A.; Masui, T.; Yamakoshi, S. $\hbox{Ga}_{2} \hbox{O}_{3}$ Schottky Barrier Diodes Fabricated by Using Single-Crystal $\beta$– $\hbox{Ga}_{2} \hbox{O}_{3}$ (010) Substrates. IEEE Electron Device Lett. 2013, 34, 493–495. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Konishi, K.; Sasaki, K.; Goto, K.; Nomura, K.; Thieu, Q.T.; Togashi, R.; Murakami, H.; Kumagai, Y.; Monemar, B.; et al. Temperature-dependent capacitance–voltage and current–voltage characteristics of Pt/Ga2O3 (001) Schottky barrier diodes fabricated on n––Ga2O3 drift layers grown by halide vapor phase epitaxy. Appl. Phys. Lett. 2016, 108, 133503. [Google Scholar] [CrossRef]
- Hu, Z.; Nomoto, K.; Li, W.; Tanen, N.; Sasaki, K.; Kuramata, A.; Nakamura, T.; Jena, D.; Xing, H.G. Enhancement-Mode Ga2O3 Vertical Transistors With Breakdown Voltage >1 kV. IEEE Electron Device Lett. 2018, 39, 869–872. [Google Scholar] [CrossRef]
- Chabak, K.D.; Moser, N.; Green, A.J.; Walker, D.E.; Tetlak, S.E.; Heller, E.; Crespo, A.; Fitch, R.; McCandless, J.P.; Leedy, K.; et al. Enhancement-mode Ga2O3 wrap-gate fin field-effect transistors on native (100) β-Ga2O3 substrate with high breakdown voltage. Appl. Phys. Lett. 2016, 109, 213501. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Kamimura, T.; Wong, M.H.; Krishnamurthy, D.; Kuramata, A.; Masui, T.; Yamakoshi, S. Depletion-mode Ga2O3 metal-oxide-semiconductor field-effect transistors on β-Ga2O3 (010) substrates and temperature dependence of their device characteristics. Appl. Phys. Lett. 2013, 103, 123511. [Google Scholar] [CrossRef]
- Dang, G.T.; Kawaharamura, T.; Furuta, M.; Allen, M.W. Mist-CVD Grown Sn-Doped $\alpha $ -Ga2O3 MESFETs. IEEE Trans. Electron Devices 2015, 62, 3640–3644. [Google Scholar] [CrossRef]
- Kaur, D.; Kumar, M. A Strategic Review on Gallium Oxide Based Deep-Ultraviolet Photodetectors: Recent Progress and Future Prospects. Adv. Opt. Mater. 2021, 9, 2002160. [Google Scholar] [CrossRef]
- Oh, S.; Jung, Y.; Mastro, M.A.; Hite, J.K.; Eddy, C.R.; Kim, J. Development of solar-blind photodetectors based on Si-implanted β-Ga_2O_3. Opt. Express 2015, 23, 28300–5. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, W.; Huang, F. Gallium oxide solar-blind ultraviolet photodetectors: a review. J. Mater. Chem. C 2019, 7, 8753–8770. [Google Scholar] [CrossRef]
- Chiang, J.-L.; Shang, Y.-G.; Yadlapalli, B.K.; Yu, F.-P.; Wuu, D.-S. Ga2O3 nanorod-based extended-gate field-effect transistors for pH sensing. Mater. Sci. Eng. B 2021, 276, 115542. [Google Scholar] [CrossRef]
- Das, M.; Chakraborty, T.; Lin, C.Y.; Lin, R.-M.; Kao, C.H. Screen-printed Ga2O3 thin film derived from liquid metal employed in highly sensitive pH and non-enzymatic glucose recognition. Mater. Chem. Phys. 2022, 278. [Google Scholar] [CrossRef]
- Minami, T.; Nishi, Y.; Miyata, T. High-Efficiency Cu2O-Based Heterojunction Solar Cells Fabricated Using a Ga2O3Thin Film as N-Type Layer. Appl. Phys. Express 2013, 6, 044101. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, X.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic Overall Water Splitting Promoted by an α-β phase Junction on Ga2O3. Angew. Chem. 2012, 124, 13266–13269. [Google Scholar] [CrossRef]
- Galazka, Z.; Uecker, R.; Klimm, D.; Irmscher, K.; Naumann, M.; Pietsch, M.; Kwasniewski, A.; Bertram, R.; Ganschow, S.; Bickermann, M. Scaling-Up of Bulk β-Ga2O3Single Crystals by the Czochralski Method. ECS J. Solid State Sci. Technol. 2016, 6, Q3007–Q3011. [Google Scholar] [CrossRef]
- Kuramata, A.; Koshi, K.; Watanabe, S.; Yamaoka, Y.; Masui, T.; Yamakoshi, S. High-quality β-Ga2O3 single crystals grown by edge-defined film-fed growth. Jpn. J. Appl. Phys. 2016, 55, 1202A2. [Google Scholar] [CrossRef]
- Chou, T.-S.; Bin Anooz, S.; Grüneberg, R.; Irmscher, K.; Dropka, N.; Rehm, J.; Tran, T.T.V.; Miller, W.; Seyidov, P.; Albrecht, M.; et al. Toward Precise n-Type Doping Control in MOVPE-Grown β-Ga2O3 Thin Films by Deep-Learning Approach. Crystals 2021, 12, 8. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuramata, A.; Masui, T.; Víllora, E.G.; Shimamura, K.; Yamakoshi, S. Device-Quality $\beta$-Ga$_{2}$O$_{3}$ Epitaxial Films Fabricated by Ozone Molecular Beam Epitaxy. Appl. Phys. Express 2012, 5. [Google Scholar] [CrossRef]
- Neal, A.T.; Mou, S.; Rafique, S.; Zhao, H.; Ahmadi, E.; Speck, J.S.; Stevens, K.T.; Blevins, J.D.; Thomson, D.B.; Moser, N.; et al. Donors and deep acceptors in β-Ga2O3. Appl. Phys. Lett. 2018, 113, 062101. [Google Scholar] [CrossRef]
- Ahmadi, E.; Koksaldi, O.S.; Kaun, S.W.; Oshima, Y.; Short, D.B.; Mishra, U.K.; Speck, J.S. Ge doping of β-Ga2O3 films grown by plasma-assisted molecular beam epitaxy. Appl. Phys. Express 2017, 10. [Google Scholar] [CrossRef]
- Sasaki, K.; Higashiwaki, M.; Kuramata, A.; Masui, T.; Yamakoshi, S. Si-Ion Implantation Doping in β-Ga2O3and Its Application to Fabrication of Low-Resistance Ohmic Contacts. Appl. Phys. Express 2013, 6, 086502. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Qi, D.-C.; Chen, L.; Zhang, K.H.L. Recent progress on the electronic structure, defect, and doping properties of Ga2O3. APL Mater. 2020, 8, 020906. [Google Scholar] [CrossRef]
- Wong, M.H.; Lin, C.-H.; Kuramata, A.; Yamakoshi, S.; Murakami, H.; Kumagai, Y.; Higashiwaki, M. Acceptor doping of β-Ga2O3 by Mg and N ion implantations. Appl. Phys. Lett. 2018, 113, 102103. [Google Scholar] [CrossRef]
- Lyons, J.L. A survey of acceptor dopants forβ-Ga2O3. Semicond. Sci. Technol. 2018, 33, 05LT02. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, Z.; Ma, C.; Ruan, W.; Chen, Y.; Zhang, H.; Zhang, G.; Fang, Z.; Kang, J.; Zhang, T.-Y. Energy-driven multi-step structural phase transition mechanism to achieve high-quality p-type nitrogen-doped β-Ga2O3 films. Mater. Today Phys. 2021, 17, 100356. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, Z.; Ma, C.; Deng, J.; Zhang, H.; Xu, Y.; Ye, J.; Fang, Z.; Zhang, G.; Kang, J.; et al. P-type β-Ga2O3 metal-semiconductor-metal solar-blind photodetectors with extremely high responsivity and gain-bandwidth product. Mater. Today Phys. 2020, 14, 100226. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, Z.; Song, P.; Tian, P.; Hu, L.; Liu, R.; Fang, Z.; Kang, J.; Zhang, T. Nanowire-Seeded Growth of Single-Crystalline (010) β-Ga 2 O 3 Nanosheets with High Field-Effect Electron Mobility and On/Off Current Ratio. Small 2019, 15, e1900580. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, Z.; Shen, X.; Kang, J.; Fang, Z.; Zhang, T.-Y. Self-consistent growth of single-crystalline (2̄01)β-Ga2O3nanowires using a flexible GaN seed nanocrystal. CrystEngComm 2016, 19, 625–631. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Shan, C.; Zhao, M.; Lien, S.-Y.; Lee, M.-K. Compositions and properties of high-conductivity nitrogen-doped p-type β-Ga2O3 films prepared by the thermal oxidation of GaN in N2O ambient. J. Mater. Res. Technol. 2022, 21, 3113–3128. [Google Scholar] [CrossRef]
- Wolter, S.D.; Mohney, S.E.; Venugopalan, H.; Wickenden, A.E.; Koleske, D.D. Kinetic Study of the Oxidation of Gallium Nitride in Dry Air. J. Electrochem. Soc. 1998, 145, 629–632. [Google Scholar] [CrossRef]
- Readinger, E.D.; Wolter, S.D.; Waltemyer, D.L.; Delucca, J.M.; Mohney, S.E.; Prenitzer, B.I.; Giannuzzi, L.A.; Molnar, R.J. Wet thermal oxidation of GaN. J. Electron. Mater. 1999, 28, 257–260. [Google Scholar] [CrossRef]
- Lee, I.-H.; Lee, C.-R.; Shin, D.C.; Nam, O.; Park, Y. Correlations between photoluminescence and Hall mobility in GaN/sapphire grown by metalorganic chemical vapor deposition. J. Cryst. Growth 2004, 260, 304–308. [Google Scholar] [CrossRef]
- Yang J, Zhao D, Jiang D, Chen P, Liu Z, Zhu J, et al. Different variation behaviors of resistivity for high-temperature-grown and low-temperature-grown p-GaN films. Chin. Phys. B. 2016;25,027102-1-4. [CrossRef]
- Lu, W.; Terazawa, M.; Han, D.-P.; Sone, N.; Goto, N.; Iida, K.; Murakami, H.; Iwaya, M.; Tekeuchi, T.; Kamiyama, S.; et al. Structural and optical impacts of AlGaN undershells on coaxial GaInN/GaN multiple-quantum-shells nanowires. Nanophotonics 2019, 9, 101–111. [Google Scholar] [CrossRef]
- Cocchi, C.; Zschiesche, H.; Nabok, D.; Mogilatenko, A.; Albrecht, M.; Galazka, Z.; Kirmse, H.; Draxl, C.; Koch, C.T. Atomic signatures of local environment from core-level spectroscopy inβ−Ga2O3. Phys. Rev. B 2016, 94, 075147. [Google Scholar] [CrossRef]
- He, H.; Orlando, R.; Blanco, M.A.; Pandey, R.; Amzallag, E.; Baraille, I.; Rérat, M. First-principles study of the structural, electronic, and optical properties ofGa2O3in its monoclinic and hexagonal phases. Phys. Rev. B 2006, 74, 195123. [Google Scholar] [CrossRef]
- Navarro-Quezada, A.; Galazka, Z.; Alamé, S.; Skuridina, D.; Vogt, P.; Esser, N. Surface properties of annealed semiconducting β-Ga2O3 (1 0 0) single crystals for epitaxy. Appl. Surf. Sci. 2015, 349, 368–373. [Google Scholar] [CrossRef]
- Navarro-Quezada, A.; Alamé, S.; Esser, N.; Furthmüller, J.; Bechstedt, F.; Galazka, Z.; Skuridina, D.; Vogt, P. Near valence-band electronic properties of semiconductingβ−Ga2O3(100) single crystals. Phys. Rev. B 2015, 92. [Google Scholar] [CrossRef]
- Korhonen, E.; Tuomisto, F.; Gogova, D.; Wagner, G.; Baldini, M.; Galazka, Z.; Schewski, R.; Albrecht, M. Electrical compensation by Ga vacancies in Ga2O3 thin films. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Chikoidze, E.; Sartel, C.; Mohamed, H.; Madaci, I.; Tchelidze, T.; Modreanu, M.; Vales-Castro, P.; Rubio, C.; Arnold, C.; Sallet, V.; et al. Enhancing the intrinsic p-type conductivity of the ultra-wide bandgap Ga2O3 semiconductor. J. Mater. Chem. C 2019, 7, 10231–10239. [Google Scholar] [CrossRef]
- Chikoidze, E.; Fellous, A.; Perez-Tomas, A.; Sauthier, G.; Tchelidze, T.; Ton-That, C.; Huynh, T.T.; Phillips, M.; Russell, S.; Jennings, M.; et al. P-type β-gallium oxide: A new perspective for power and optoelectronic devices. Mater. Today Phys. 2017, 3, 118–126. [Google Scholar] [CrossRef]
- Ho, C.-H.; Tseng, C.-Y.; Tien, L.-C. Thermoreflectance characterization of β-Ga_2O_3 thin-film nanostrips. Opt. Express 2010, 18, 16360–16369. [Google Scholar] [CrossRef]
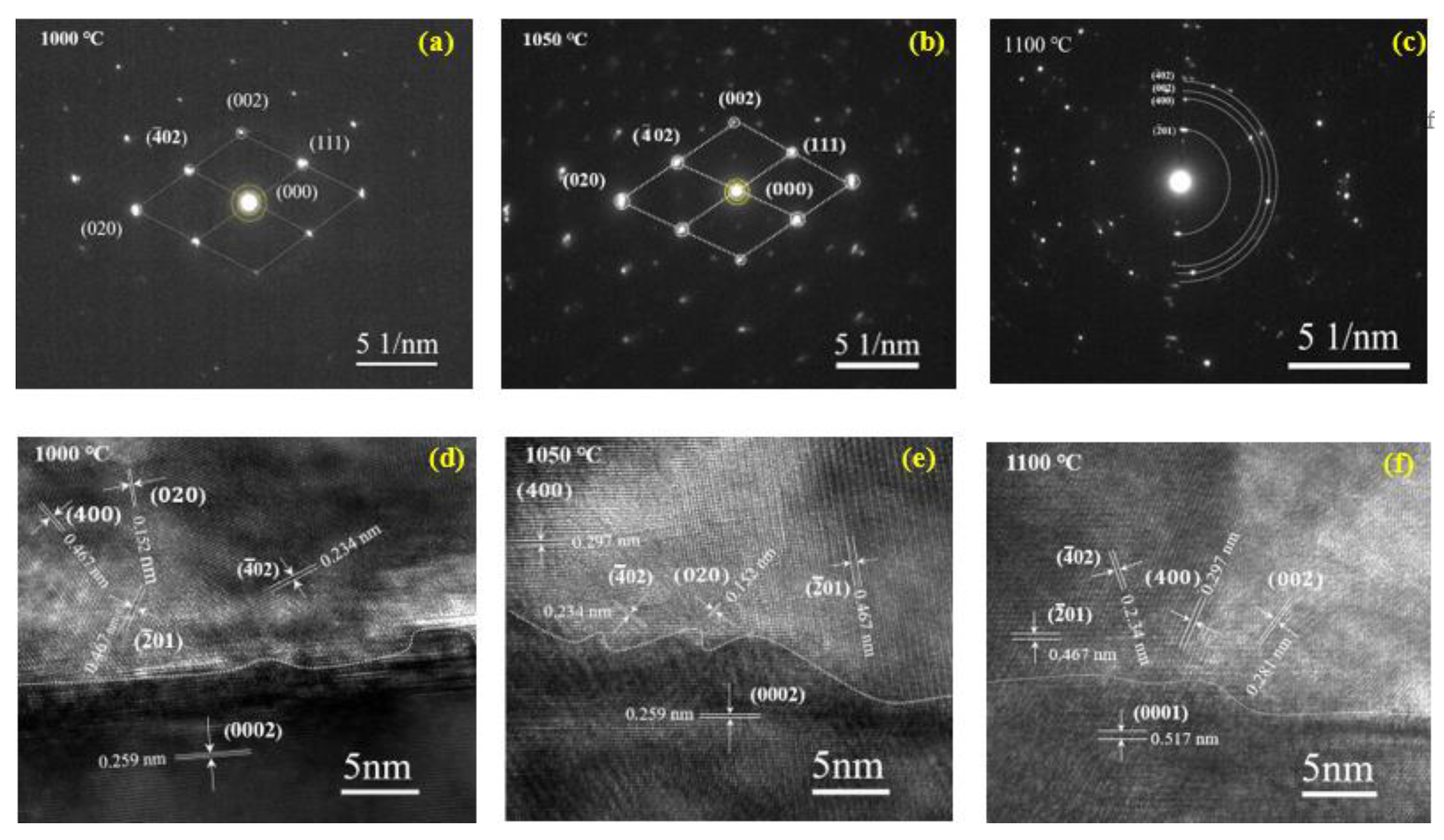
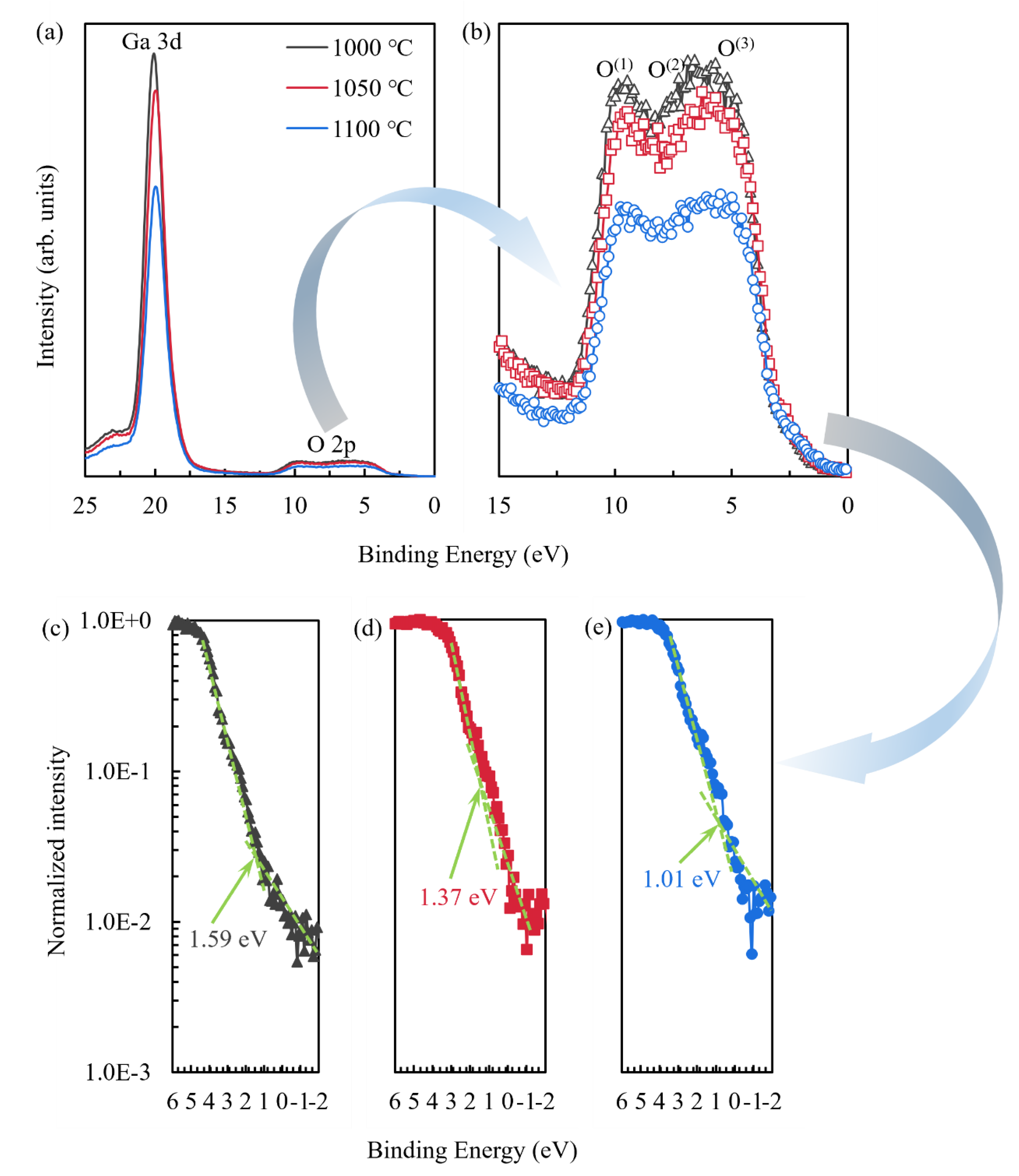
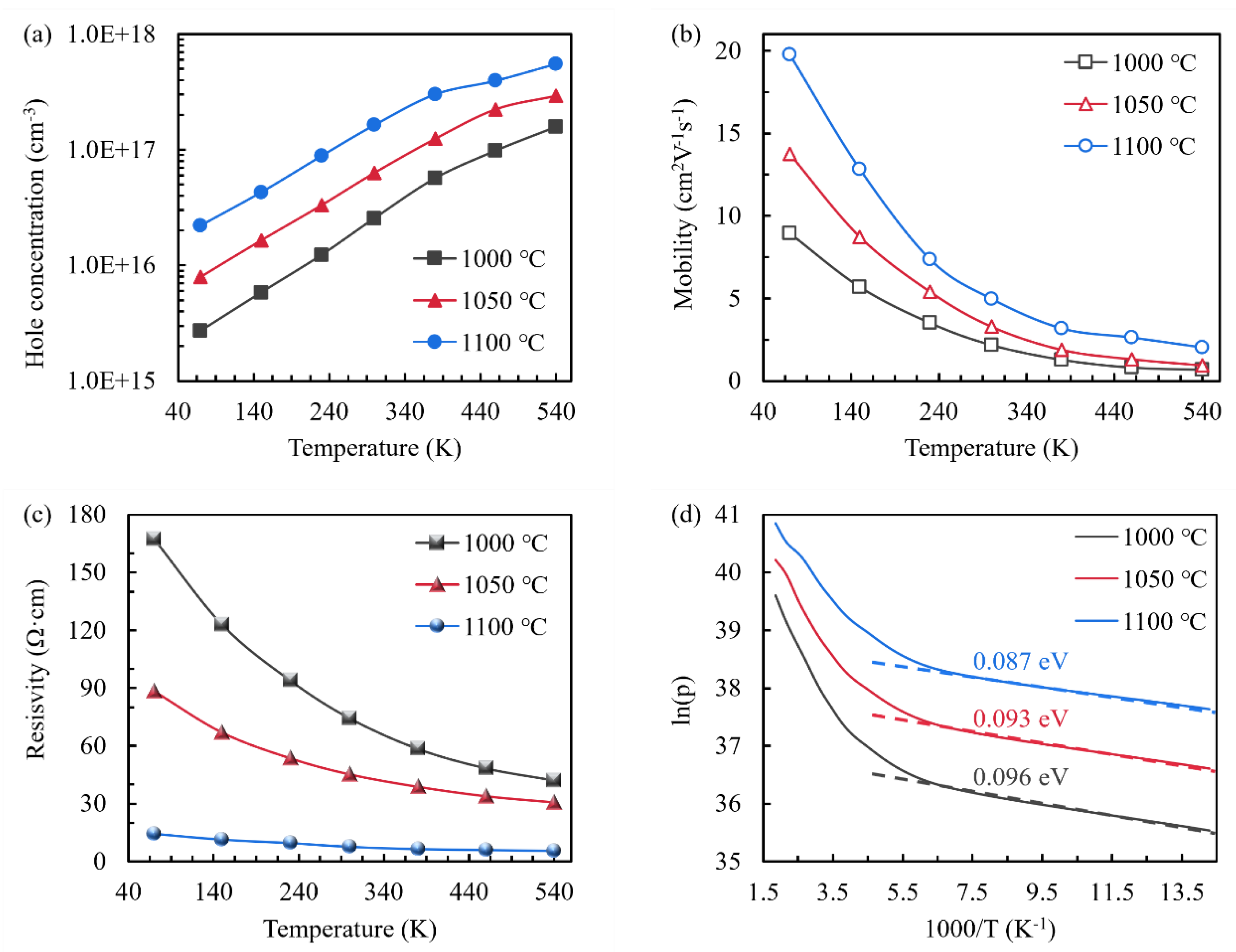
| Oxidation temperature (°C) | 1000 | 1050 | 1100 |
|---|---|---|---|
| Ga/O ratio | 0.831 | 0.821 | 0.818 |
| N elemental ratio (%) | 0.0323 | 0.0369 | 0.0878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




