1. Introduction
Pelvic organ prolapse (POP) is a common disease adversely affecting the quality of life of many women1. It affects 50% of parous women2 and 37% of women over the age of 803. The lifetime risk of undergoing surgery for prolapse is 11%4. POP is defined as the descent of one or more of the anterior vaginal wall, posterior vaginal wall, the uterus (cervix), or the apex of the vagina (vaginal vault or cuff scar after hysterectomy)5. It has been attributed both to damage to the levator ani muscle6, (where the weakness of the levator ani may cause widening of the levator hiatus and descent of the central portion of the pelvic diaphragm) and to endopelvic fascial defect7. However, DeLancey8, 9 described the interaction between pelvic floor muscles and endopelvic fascia and maintained that it is not possible to determine which of these is responsible for prolapse because they are intimately interdependent.Clinical investigation of the female pelvic floor has advanced significantly in recent years and nowadays prolapse can be staged from I to IV through the Pelvic Organ Prolapse Quantification (POP-Q) introduced in 199610,11,12. Even though a careful physical examination is a predominant method for evaluating pelvic floor defects, even the most experienced clinicians can sometimes be misled by multiple findings13 and by many variables (time of the day in which the clinician makes the examination, the position of the examination, etc.) and this could compromise the success of the treatment. To gain more certainty the clinician can choose among a wide range of imaging modalities, which can confirm clinical suspicions and reveal unsuspected defects. Despite Magnetic Resonance Imaging (MRI) being an expensive radiological technique its advantages are well known and include the lack of radiation, excellent soft tissue contrast, and multiplanar imaging without superimposition of structures. These advantages can be used to visualize the morphology of the pelvic floor in great detail14. Functional MRI (fMRI) of the pelvic floor with a depiction of organ movement was first introduced by Yang et al.6 and Kruyt et al.15 in 1991. Radiologists can accurately identify and report the underlying structural defects, allowing clinicians to individually tailor surgical techniques for each patient. This is important because even those patients presenting with the same clinical symptoms may have different underlying structural derangements or abnormalities that may warrant a different treatment plan or approach. The primary endpoint of our study was to find a correspondence between the clinical and radiological classification of pelvic organ prolapse before and after vaginal surgery. A secondary objective was to demonstrate any correlation between vaginal surgery and continence, through analysis of some pelvic measurements at MRI.
2. Materials and Methods
This study was performed at the Urogynecological Service of Gynaecological Clinic and Radiodiagnostic and Radiotherapy Unit, University of Catania, Italy. It was a prospective study including 25 women recruited who underwent surgery for urogenital prolapse between October 2018 and February 2019. The institutional ethical committee of the department approved the study. All the subjects provided written informed consent before entering the study, which was conducted under the Declaration of Helsinki. The study was not advertised and no remuneration was offered.
Inclusion criteria were:
Presence of prolapse (cystocele and/or hysterocele and/or rectocele) ≥ stage 2 POP-Q
Indication for vaginal surgery (vaginal hysterectomy in case of hysterocele and anterior and posterior fascial repair, respectively for cystocele and rectocele)
Exclusion criteria were previous vaginal surgery.
Twenty-five women were recruited, 5 of these dropped out, 2 because of anxiety during MRI examination, 1 didn’t give any reason and 2 rejected postoperative MR. Twenty women completed the study. Women underwent pre-operative clinical examination by the same doctor (MGM) to collect history, and evaluate urinary symptoms and stage prolapse through the POP-Q system; women, also, underwent urodynamic examination. Then they underwent an MRI performed by a radiologist who didn't know the clinical staging of prolapse. Urogynecological examination and MRI were repeated 3 months after vaginal surgery for pelvic organ prolapse.
MRI imaging protocol
MR examinations were performed with a closed-configuration superconducting unit with 1.5-T field strength (GESigna HDx 1.5 T, GE Medical Systems, Milwaukee, WI, USA) with 57.2 mT/m gradient strength and 120 T/m/s slew rate, by using an 8-channel high-resolution torso coil with array spatial sensitivity technique (ASSET) parallel acquisition.
Preparation
Patient preparation and cooperation are essential for the success of the study. Before the examination, patients are given an enema and instructed regarding the maneuvers to be performed inside the magnet. Patients are invited to wear a large pad, a stratagem that has the dual purpose of preventing soiling of the MR bed and reducing psychological discomfort. The bladder should be half full. Inside the gantry, the rectum is distended with approximately 120 ml of ultrasound gel (hyperintense on T2 and FIESTA sequences) introduced through a Nelaton catheter (20 Ch, 6.67 mm×360 mm) (Bicakcilar, Istanbul, Turkey) and a 50-ml catheter-tip syringe. The degree of straining is monitored with a respiratory gating device placed around the patient's waist. Inside the gantry, the patient lies supine (feet first), with knees slightly flexed, as this position facilitates evacuation of rectal contrast agent during defecation.
Sequences
Our protocol includes the acquisition of:
- -
High-spatial-resolution static sequences to study the morphology of the levator ani;
- -
Dynamic sequences to study abnormalities of the pelvic organs during contraction, rest, straining, and defecation.
Static sequences included T2-weighted fast spin-echo (FSE) sequences in the sagittal, axial, and coronal planes. The technical parameters for this sequence were time to repetition (TR)/time to echo (TE), 4,675/100; flip angle, 90°; section thickness, 4 mm; interslice gap, 1 mm; bandwidth, 41.67 kHz; field of view (FOV), 32 cm; matrix, 320×224; several averages, 4; number of images, 26; acquisition time, 3 min 49 s.
Dynamic sequences were performed in the midsagittal plane identified on the T2-weighted FSE static images, with the pubic symphysis, urethra, vagina, rectum, and coccyx included in the FOV. In the dynamic phase, two types of sequences were used: T2-weighted single-shot fast spin-echo (SSFSE) and fast imaging employing steady-state acquisition (FIESTA) sequences acquired with the following parameters:
- -
SSFSE (TR/TE, 708/90; flip angle, 90°; section thickness, 8 mm; bandwidth, 83.3 kHz; FOV, 34 cm; matrix, 384×224; several averages, 0.5; acquisition time for each image, 0.3 s) in the midsagittal plane, with sequential acquisition during contraction, rest and straining;
- -
FIESTA (TR/TE, 3.3/1.4; flip angle, 45°; section thickness, 8 mm; bandwidth, 125 kHz; FOV, 35 cm; matrix, 224×224; a number of averages, 1; number of images, 20; acquisition time, 20 s) in the midsagittal plane, with continuous multiphase acquisition during contraction, rest, straining and defecation.
Overall examination time, including patient preparation, was approximately 40 min.
Parameters observed at MR were:
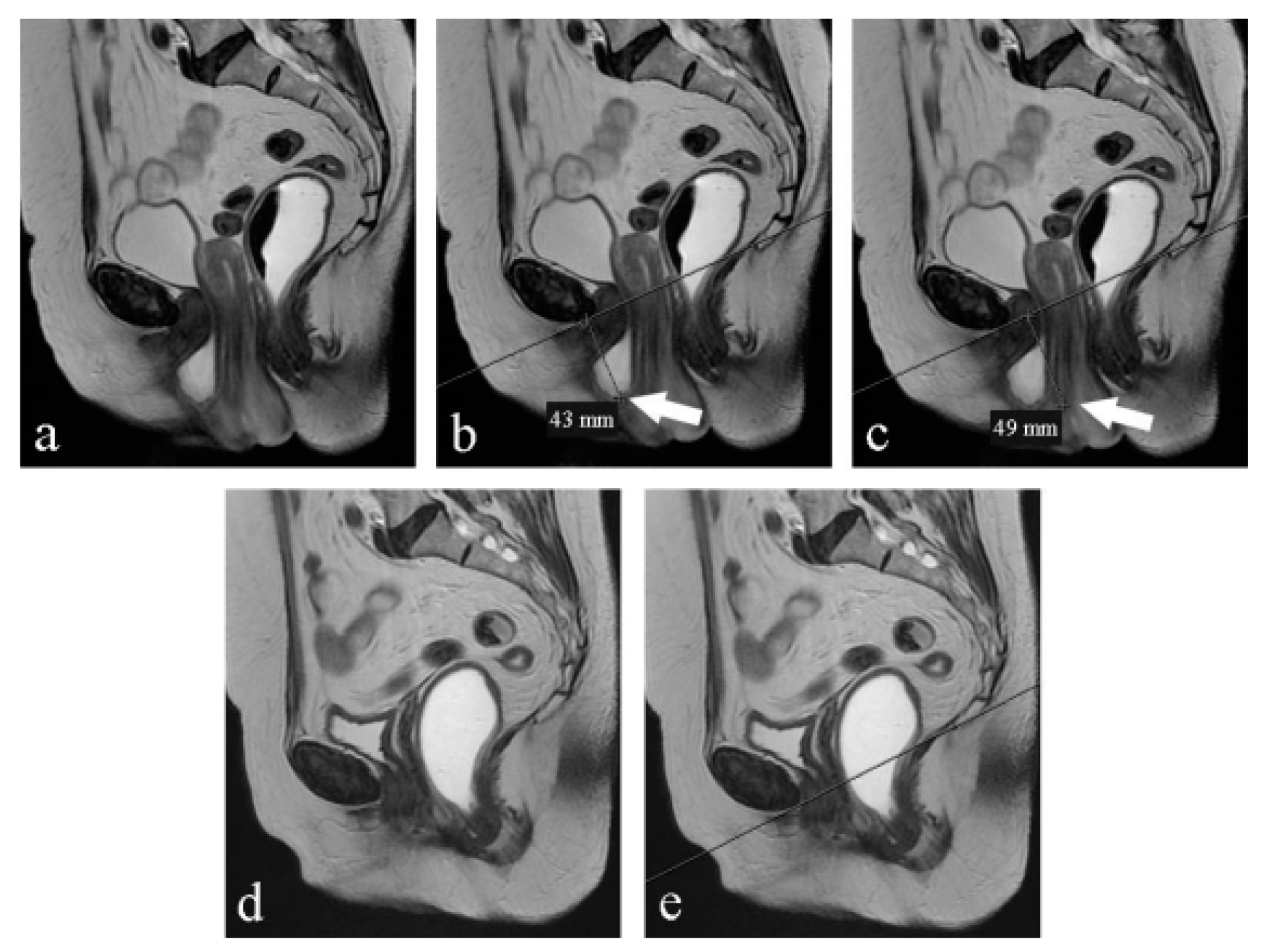
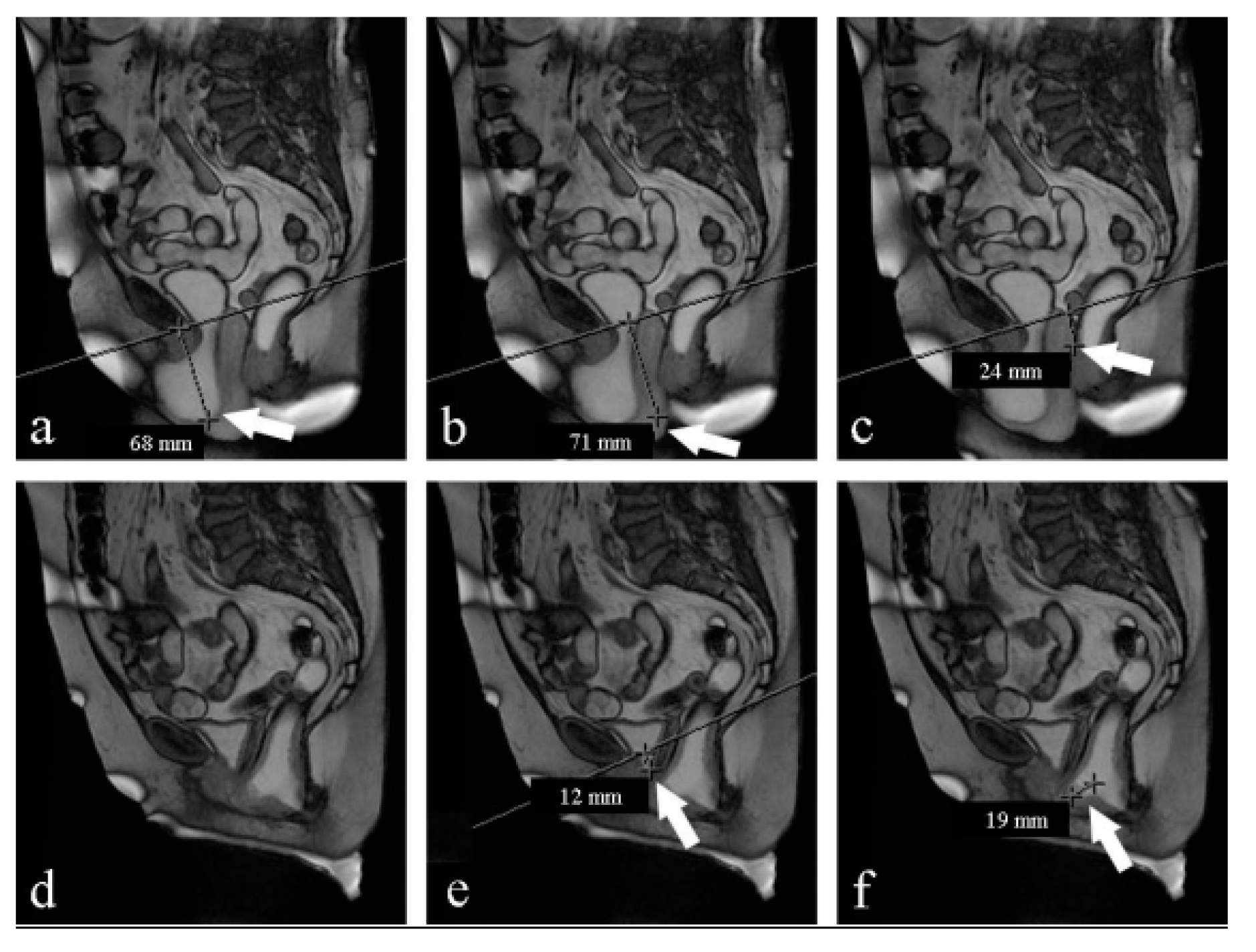
PCL (pubococcygeal line): a line drawn from the inferior margin of the pubic symphysis to the last coccygeal joint16;
Radiological evaluation of prolapse: Prolapse severity can be easily graded according to the ‘‘rule of three’’: prolapse of an organ below the PCL ≤3 cm is mild, 3–6 cm is moderate, and >6 cm is severe; 17,18 (Farouk El Sayed, 2013)
The levator plate angle (LPA): enclosed between the levator plate and the PCL where the ileo-coccygeus touches the coccyx. It is usually 11,7° ± 4,8 in healthy women under the Valsalva maneuver. (Rosenkrantz, Lewis, Yalamanchili, Lim, Wong, & Bennett, 2013)(Farouk El Sayed, 2013);
Posterior vesicoureteral angle (VUA): the angle between the axis of the urethra and the bladder base. In normal conditions, it is about 90°. (Tunn, Goldammer, Gauruder-Burmester, Wildt, & Beyersdorff, 2005);
The H-line: which extends from the inferior aspect of the pubic symphysis to the anorectal junction, represents the genital hiatus: on the effort it is 5,8 cm 0,5 (Ansquer, Fernandez, Aimot, Bennis, Salomon, & Carbonne, 2007)(Farouk El Sayed, 2013);
The M-line: dropped as a perpendicular line from the PCL to the posterior aspect of the H-line: normally on effort, it is 1,3 cm 0,5 (Ansquer, Fernandez, Aimot, Bennis, Salomon, & Carbonne, 2007)(Farouk El Sayed, 2013);
The total vaginal length (TVL): distance from the posterior part of the introitus to the proximal vagina, if the cervix is present it corresponds to the posterior fornix, if the woman had a hysterectomy it corresponds to the vaginal vault. (Humphries, Simpson, Creighton, & Hall-Craggs, 2008);
Funneling: the opening of the proximal urethra on effort (Tunn, Goldammer, Gauruder-Burmester, Wildt, & Beyersdorff, 2005);
The thickness of the mid urethra.
Surgery
Surgery for pelvic organ prolapse consisted of vaginal hysterectomy in cases of hysterocele and anterior and posterior fascial repair, respectively for the anterior compartment and posterior compartment. Women were given spinal anesthesia and antibiotic prophylaxis and then placed in the lithotomy position. After vaginal hysterectomy, suspension of the vaginal apex to the uterosacral ligaments and McCall culdoplasty (with uterosacral ligaments incorporated into the closure of the peritoneum and upper vagina) were performed for the prevention of vaginal vault
prolapse. Surgical treatment of cystocele is based on the reconstruction of the anterior vaginal wall, and anterior colporrhaphy, which is performed by placing an interrupted suture with vicryl 2-0 that plicate the weakened fascial tissues. Surgical treatment of rectocele can be approached either transvaginally or transperineally and can be repaired with native tissue, with the plication of rectovaginal fascia in cases of central and lateral defects. We used native tissue for pelvic floor repair according to the FDA Safety Communication of 201119
Statistical analysis was descriptive qualitative and quantitative made by Kolmogorov-Smirnov test. The comparison between preoperative and postoperative data was made by concordance correlation coefficient for qualitative parameters and by T-student paired test for quantitative parameters. Wilcoxon signed-rank test was used to compare related samples. The statistical significance was for p<0,05.
3. Results
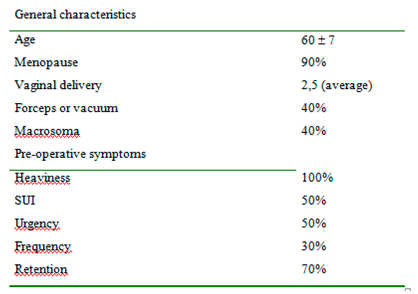
Of the 20 women (medium age 60) 12 underwent vaginal hysterectomy, anterior and posterior pelvic floor repair, and 8 women underwent vaginal hysterectomy and anterior pelvic floor repair. Fifty percent complaint of urgency, 50% of stress urinary incontinence (SUI), and 100% heaviness. (
Table 1).
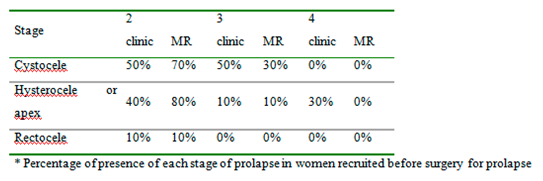
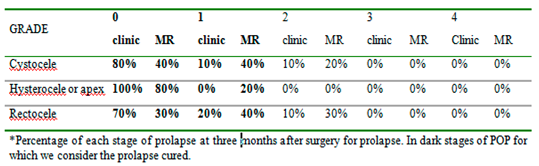
Table 2 reports the staging of prolapse for each compartment in women recruited according to clinical examination and magnetic resonance evaluation before surgery. We included women with clinical stage ≥2 POP-Q because otherwise there was no indication for surgery. (
Table 2). At 3 months follow up women reported an improvement in urinary symptoms, no complaints of heaviness, and a reduction in urgency (40% vs 50%) and SUI (20% vs 50%). The postoperative evaluation through MR gave few differences as shown in
Table 4 that compare the two staging techniques (
Table 3) (
Figure 1) From the analysis of
Table 2 and
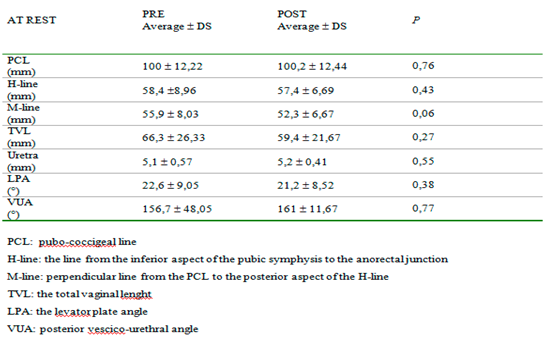
Table 3, we can observe that preoperatively clinical examination tends to overestimate the degree of prolapse in comparison to MR; otherwise, postoperatively clinical examination tends to underestimate the degree of prolapse. Moreover, MR examination showed hypotrophy of puborectalis muscle in 12 women and hypotrophy of ileo-coccygeus muscle in 14 women. One woman had damage and loss of convexity of the levator ani.Qualitative analysis between pre and post-surgery showed no statistically significant difference in all the parameters considered at rest (
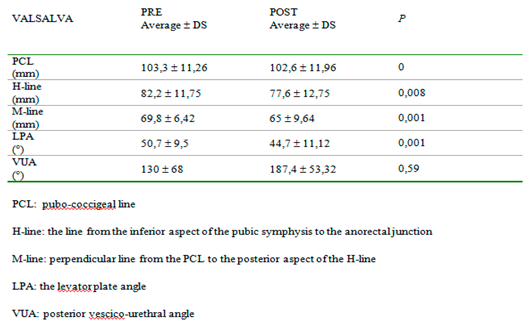
Table 4).On the contrary, under the Valsalva maneuver, we found some significant differences: PCL was reduced (p<0,05), H-line was 7 mm shorter (p<0,05), M-line was 5 mm shorter (p<0,05), LPA was 6° smaller (p<0,05). VUA was 57° wider but the difference was not statistically significant (
Table 5) (
Figure 2).
4. Discussion
Even though magnetic resonance imaging is not required for diagnosis there is evidence that MRI can play a role in more accurately guiding the choice of surgical technique in 41.6% – 75% of patients with different spectra of pelvic floor dysfunction20,21,22. From our data, the comparison between the clinical and radiological evaluation of prolapse showed some discrepancies both pre and postoperatively, especially for stages first and second. MR makes staging through objective measurements so it is more accurate. We think that clinicians can go through this bias of staging if they admit to vaginal surgery women based on symptoms and desire for improvement, not only on prolapse quantification. Postoperatively we had improvement in the questionnaire on quality of life which is the real objective of surgery for prolapse. For this reason, we agree with the literature, that considers MR not required to make a diagnosis of prolapse even if this method is more accurate and it could be indicated in complex pelvic floor disorders. Many studies were made using this imaging technique to better visualize pelvic structures. In literature, MR findings were compared to other imaging techniques in the study of the pelvic floor: Foti et al23 found that in outlet obstruction syndrome MR imaging can provide a morphological and functional study of pelvic floor structures and may offer an imaging tool complementary to conventional defecography in the multicompartment evaluation of the pelvis. Other functional studies24 using MR has been published on continence mechanisms: they show that during a cough, normal pelvic floor muscle function produces timely compression of the pelvic floor and additional external support to the urethra, reducing displacement, velocity, and acceleration. In women with SUI, who have weaker urethral attachments, this shortening contraction does not occur; consequently, the urethras of women with SUI move further and faster for a longer duration. Our radiologic data, besides, showed a post-operative reduction of PCL, H-line, M-line, and LPA at rest, which was not statistically significant. On the contrary, this reduction was significant under the Valsalva maneuver, which demonstrated the anatomical changes caused by surgical treatment, which correlate with improvement in symptomatology. The VUA, the angle between the axis of the urethra and the bladder base is usually a rectal angle at rest; during micturition, it becomes opened and disappears to permit the passage of urine. The disappearance of this angle, as a consequence of surgery, causes a permanent funneling of the bladder neck and the proximal urethra which could cause de novo incontinence. In 1952 Roberts25 and Jeffcoate26,27, reported a series of observations on the posterior urethrovesical angle and its relationship to urinary continence in the female. These authors used as their main method of observation the technique of lateral cystourethrography and they concluded that urinary continence depends upon a posterior urethrovesical angle of about 100 degrees which is maintained by the intrinsic musculature of the bladder neck. Stress incontinence is characterized by loss of the posterior urethrovesical angle that can rarely be restored by anterior colporrhaphy. Also, Dutton found a marked correlation between the absence of the posterior urethrovesical angle and stress incontinence (91.5%) but only a moderate correlation between the presence of the angle and urinary continence (66.7%). It was concluded that the posterior urethrovesical angle is not the fundamental factor concerned with urinary control but that it is probably very closely linked to such a factor28. We found that the VUA, in a supine position before surgery for prolapse, was about 156° at rest. After the correction of the cystocele, it was wider and this difference was not statistically significant either at rest or on effort. Moreover, our data reported a significant improvement in SUI (50% pre versus 20% post). We found that the widening of VUA after correction of cystocele is not statistically significant and it didn't alter the static nor the dynamic balance of pelvic organs. The limits of our study are the small number of women recruited and the lack of comparison with a different treatment of prolapse. These are preliminary data useful to understand if clinicians and radiologists can speak a common language about prolapse and to understand the potentiality of MRI as a diagnostic technique. Some larger studies should be performed to better understand if MRI could predict recurrence.
5. Conclusions
Based on our data static magnetic resonance imaging does not add any further information on the management and efficacy of surgical treatment for prolapse. Dynamic magnetic resonance imaging otherwise can be useful to further investigate on balance and functionality of pelvic organs, which can be compromised by prolapse. Moreover, radiologic measurements demonstrated that vaginal correction of prolapse is efficient and it does not alter urethral mobility and continence mechanisms.
Author Contributions
Conceptualization, G.S. and M.G.M; methodology, G.S and PVF; writing—original draft preparation, G.S and CC; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kelleher CJ, Cardozo LD, Khullar V, et al. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997, 104, 1374–1379. [CrossRef] [PubMed]
- Tinelli A, Malvasi A, Rahimi S, et al. Age-related pelvic floor modifications and prolapse risk factors in postmenopausal women. Menopause. 2010, 17, 204–212. [CrossRef] [PubMed]
- Morley GW. Treatment of uterine and vaginal prolapse. Clin Obstet Gynecol. Clin Obstet Gynecol. 1996, 39, 959–69. [CrossRef] [PubMed]
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997, 89, 501–506. [CrossRef] [PubMed]
- et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Yang A, Mostwin JL, Rosenshein NB, et al. Pelvic floor descent in women: dynamic evaluation with fast MR imaging and cinematic display. Radiology. 1991, 179, 25–33. [CrossRef] [PubMed]
- Mengert WF. Mechanics of uterine support and position. Am J Obstet Gynecol. 1936, 31, 775–782. [CrossRef]
- DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993, 36, 897–909. [CrossRef] [PubMed]
- DeLancey, J.O. Functional anatomy of the pelvic floor. In Imaging pelvic floor disorders; Bartram, C.I., DeLancey, J.O., Halligan, S., et al., Eds.; Springer: New York, NY, USA, 2003; pp. 27–38. [Google Scholar]
- Abrams P, Cardozo L, Fall M, et al The standardization of terminology of lower urinary tract function. Report from the standardization subcommittee of the International Continence Society. Neurourol Urodyn. 2002, 21, 167–178. [CrossRef] [PubMed]
- Bump RC, Cundiff GW. Pelvic organ prolapse. In Clinical urogynaecology; Stanton, S.L., Monga, A.K., Eds.; Churchill Livingstone. London, UK, 2000; pp. 357–372. In Clinical urogynaecology; Stanton, S.L., Monga, A.K., Eds.; Churchill Livingstone: London, UK, 2000; pp. 357–372. [Google Scholar]
- Bump RC, Mattiasson A, Bo K, et al The standardization of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996, 175, 10–11. [CrossRef] [PubMed]
- Kelvin FM, Maglinte DD Dynamic cystoproctography of female pelvic floor defects and their interrelationships. AJR Am J Roentgenol. 1997, 169, 769–774. [CrossRef] [PubMed]
- Fielding, JR. MR imaging of pelvic floor relaxation. Radiol Clin North Am. 2003, 41, 747–756. [CrossRef] [PubMed]
- Kruyt RH, Delemarre JBVM, Doornbos J, et al. Normal anorectum: dynamic MR imaging anatomy. Radiology. 1991; 179(1):159-63. [CrossRef]
- Rosenkrantz AB, Lewis MT, Yalamanchili S, et al. Prevalence of pelvic organ prolapse detected at dynamic MRI in women without history of pelvic floor dysfunction: comparison of two reference lines. Clin Radiol. 2014; 69(2):e71-7. [CrossRef]
- Lienemann A, Anthuber C, Baron A, et al Dynamic MR colpocystorectography assessing pelvic floor descent. Eur Radiol. 1997, 7, 1309–1317. [CrossRef]
- Goh V, Halligan S, Kaplan G, et al. Dynamic MRI of the pelvic floor in asymptomatic subjects. AJR Am J Roentgenol. 2000;174:661–666. [CrossRef]
- FDA Safety communication: Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Trans-vaginal Placement for Pelvic Organ Prolapse. July 2011.
- El Sayed RF, Fielding JR, El Mashed S, et al. Preoperative and postoperative magnetic resonance imaging of female pelvic floor dysfunction: correlation with clinical findings. J Women’s Imaging. 2005; 7:163–180. [CrossRef]
- Kaufman HS, Buller JL, Thompson JR, et al Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis Colon Rectum. 2001, 44, 1575–1584. [CrossRef]
- Altringer WE, Saclarides TJ, Dominguez JM, et al. Fourcontrast defecography: pelvic‘‘floor-oscopy’’. Dis Colon Rectum. 1995; 38:695–699. [CrossRef]
- Foti PV, Farina R, Riva G, et al. Pelvic floor imaging: comparison between magnetic resonance imaging and conventional defecography in studying outlet obstruction syndrome. Radiol Med. 2013;118(1):23-39. [CrossRef]
- Lovegrove Jones RC, Peng Q, Stokes M, et al Mechanisms of pelvic floor muscle function and the effect on the urethra during a cough. Eur Urol. 2010, 57, 1101–1110. [CrossRef]
- Roberts, H. Cystourethrography in women. Brit. J. Radiol. 1952, 25, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate TN, Roberts H Observations on stress incontinence of urine Observations on stress incontinence of urine. Am J Obst Gynec. 1952, 64, 721–738. [CrossRef]
- Aldridge A, Jeffcoate TN, Roberts H Stress incontinence of urine Stress incontinence of urine. J Obstet Gynaecol Br Emp. 1952, 59, 681–720. [CrossRef] [PubMed]
- Dutton, W.A. The urethrovesical angle and stress incontinence. Can Med Assoc J. 1960, 10, 1242–5. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).