1. Introduction
Autism Spectrum Disorder (ASD), a group of pediatric neurodevelopmental disorders, affects about 1% of the general population worldwide [
1]. It is characterized by communication, social interaction, and cognitive impairments with repetitive behaviors [
2]. It is often associated with other comorbidities like hyperactivity and attention disorders, sleep disorders, anxiety, depression, epilepsy, and abnormal functioning of the digestive system (chronic constipation and/or diarrhea) [
3]. The possible neuropathology of ASD includes cerebral hypoperfusion, atypical neural connectivity, cerebellum alterations like decreased density of Purkinje cells (PCs), cerebral cortex defects, altered density of dendritic spines and atypical myelination [
4,
5] along with immune dysfunction, increased oxidative stress, inflammation and apoptosis, hypoxia, atypical excitatory-inhibitory signaling, neuronal migration defects, and synaptic dysfunction [
5,
6].
Conventional treatments available for ASD such as psychological interventions, behavioral therapy, occupational therapy, speech therapy, and pharmacotherapy have limited therapeutic efficacy in addressing the core neuropathology [
2]. Hence, there is a requirement of newer treatments which address the unmet medical needs. Cell therapy has shown promising therapeutic efficacy in the treatment of ASD based on its regenerative and restorative properties [
7]. Autologous Bone Marrow Mononuclear Cells (BMMNCs) were chosen as they do not possess tumorigenicity and eliminate the risk of immune rejection [
8]. These cells are easy to procure and do not involve complex processing. Once administered, they migrate to the dysfunctional areas and stimulate neurogenesis, angiogenesis, anti-inflammatory response, and amelioration of immune system dysregulation via paracrine mechanisms [
9]. These cells also promote endogenous neural stem cell proliferation which enhances the brain repair process [
10].
Aim of this study was to investigate the safety and efficacy of cell therapy with standard neurorehabilitation in a larger population with confirmed ASD diagnosis. Additionally, this study describes various factors influencing the outcome of the intervention. Outcome measures used to study the therapeutic effects of cell therapy were Indian Scale of Autism Assessment (ISAA) and Childhood Autism Rating Scale (CARS). Comparative 18-Fluorodeoxyglucose Brain Positron Emission Computed Tomography (18-FDG Brain PET CT) scan was also used to evaluate effects of cell therapy on brain metabolism.
2. Materials and Methods
2.1. Study Design
This study is an observational clinical study conducted on patients diagnosed with ASD based on The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria who underwent autologous BMMNCs transplantation. A total of 1011 autism patients were included in the study. The primary aim of this study was to evaluate the safety and efficacy of cell transplantation in combination with neurorehabilitation as a treatment measure for ASD. The intervention consisted of intrathecal administration of autologous bone marrow mononuclear cells along with standard neurorehabilitation. Neurorehabilitation included behavioral therapy, psychological intervention, occupational therapy, activities of daily living [ADL] training, physiotherapy, aquatic therapy, speech therapy, special education and dietary recommendations.
2.2. Patient selection
Patient selection was based on the World Medical Association’s Helsinki Declaration for Ethical Principles for medical research involving human subjects [
11]. The protocol was reviewed by the Central Drugs Standard Control Organization (CDSCO) registered Institutional Ethics Committee (IEC).
2.3. Inclusion criteria
The inclusion criteria were patients of both genders who were diagnosed with autism spectrum disorder based on DSM-V criteria with age above 2 years.
2.4. Exclusion criteria
The exclusion criteria were presence of acute infections, severe anemia (Hemoglobin <8), pyrexia, HIV/HBV/HCV, malignancies, bleeding tendencies, renal failure, severe liver dysfunction and other acute medical conditions such as respiratory infections. Patients having other comorbid neurological conditions such cerebral palsy, intellectual disability, learning disability, etc were also excluded from the study.
2.5. Informed consent
Detailed protocol of the treatment along with possible adverse events were explained to the parents/caregivers of the patients and written informed consent was taken before the intervention. Consent was also video recorded.
2.6. Intervention
2.6.1. Pre-intervention assessment:
All the patients underwent detailed serological, biochemical and hematological tests before the intervention. They also underwent brain Magnetic resonance imaging (MRI), brain PET CT scan and electroencephalography (EEG). Patients were further evaluated via a team of neurologist, neurosurgeon, pediatrician, physician, psychologist, occupational therapist, physiotherapist, speech therapist and special educator to check the severity of the condition in patients.
2.6.2. Aspiration of BMMNCs
Patients were administered Granulocyte Colony Stimulating Factor (GCSF) subcutaneously, 48 hours and 24 hours prior to BMMNCs aspiration. On the day of transplantation, bone marrow was aspirated under general anesthesia with sedation in the operation theater under aseptic conditions. 80-100 ml of bone marrow (depending on the age and body weight of the patient) was aspirated from the anterior superior iliac bone using bone marrow aspiration needle and collected in heparinized tubes.
2.6.3. Isolation of BMMNCs
MNCs are separated from the bone marrow by density gradient separation method. The bone marrow was diluted in the ratio of 1:1 with normal saline and subjected to centrifugation at 440×g rpm for 35 minutes in a swinging bucket rotor without brake at 20°C. MNCs were then obtained as a buffy coat. The MNCs were washed thrice with normal saline by centrifuging at 300×g for 15 minutes in a swinging bucket rotor without brake at 20°C and finally resuspended in 1 ml of normal saline. Viability of the isolated MNCs was checked using trypan blue vital dye which was mixed in 1:1 proportion and loaded onto the haemocytometer for the total cell count and viability. CD34+ analysis of the samples was also done using Invitrogen Attune NXT flow cytometer.
2.6.4. Administration of BMMNCs
The isolated BMMNCs were injected immediately using a 25G spinal needle between fourth and fifth lumbar vertebrae under general anesthesia with sedation. Simultaneously 20 mg/kg body weight of Methylprednisolone in 100 ml Isolyte P or normal saline was given intravenously to reduce local inflammation and enhance survival of transplanted BMMNCs [
12]. Patients were monitored for adverse events. In the present study, 543 patients underwent more than one dose of cell therapy and each time the cell therapy protocol was the same as above.
2.7. Neurorehabilitation
Every patient underwent extensive neurorehabilitation for 4 days after cell transplantation. The rehabilitation protocols were personalized for individual patients. This consisted of applied behavioral analysis (ABA), psychological intervention, occupational therapy, sensory integration therapy, activities of daily living (ADL) training, physiotherapy, aquatic therapy, speech therapy, special education and dietary recommendations. Patients were also given a home program to continue rehabilitation at home.
2.8. Follow up
The mean follow up duration in this study was 19.3 (±12.8) months. A detailed neuro-evaluation and outcome measures i.e ISAA and CARS scales were performed for patients on each follow up.
2.9. Outcome Measures
The outcome measures used to analyze the effect of intervention at follow up were Indian Scale for Assessment of Autism (ISAA) [
13] and Childhood Autism Rating Scale (CARS) [
14]. Symptomatic improvements were also recorded. Brain PET CT scan was repeated after 6 months and compared to the pre-intervention scan to find the effect of intervention on brain function. Parents/caregivers of 401 patients gave their consent to repeat the PET CT scan.
2.10. Brain PET CT scan imaging, processing, and quantification
Brain PET CT imaging was performed for all the patients as a pre-intervention protocol. 18-FDG Brain PET CT scan was done to assess the brain glucose uptake on a combined PET CT scanner (GE Discovery IQ PET CT scanner, USA). Advantage Workstation (ADW) software was used for the reconstruction of images. The percent hypometabolism of different brain regions were analyzed using Oasis Cerquant quantification software (Segami corporations, Columbia). The percent hypometabolism of 9 brain regions that are usually hypometabolic in autism were determined and analyzed in this study [
15]. These 9 brain regions include amygdala, hippocampus, parahippocampal gyrus, caudate nucleus, cerebellum, mesial temporal lobe, thalamus, superior and middle temporal poles. Based on percent hypometabolism shown by these distinct brain regions, they were categorized in grades from 1 to 10 as shown in
Table 1. 401 out of 1011 patients underwent a comparative repeat PET CT scan after cell therapy to determine the effect of intervention on brain metabolism.
2.11. Adverse Event (AE) Monitoring
All major and minor adverse events were monitored throughout the procedure during their hospital stay and at follow ups. Long term adverse reactions were also monitored. Adverse events were grouped into two categories: procedure related adverse events and cell therapy related adverse events. Procedure related AEs involve events related to bone marrow aspiration and cells administration via lumbar puncture. These include fever, spinal headache, nausea, vomiting, pain at the site of aspiration/injection, back pain, skin rashes etc. Cell therapy related AEs included seizures, increased hyperactivity and aggressiveness etc.
2.12. Methodology of analysis
A detailed analysis was performed to study the outcome of intervention. This included percentage analysis of changes in symptoms and mean follow up was calculated. Subgroup analysis was also performed to study the effect of age, severity of illness and number of cell therapies taken by patients. Degree of improvement was estimated on ISAA score with respect to the number of cell therapies administered to the patients.
2.13. Statistical analysis
Wilcoxon’s Signed-Rank Test at level of significance < 0.05 was used to statistically analyze the outcome of intervention on ISAA and CARS. The test was also done to determine statistical significance on each domain of ISAA. Statistical tests were performed for subgroup analysis based on age, severity of illness and the number of cell therapies taken by the patients. Change in the percent hypometabolism grade in the PET CT scan performed before and after intervention was also statistically evaluated using Wilcoxon’s Signed-Rank Test (P<0.05).
3. Results
A total of 1011 patients were included in the analysis, out of which 841 were males and 170 were females (ratio 5:1) with an age range of 3 to 31 years (
Table 2).
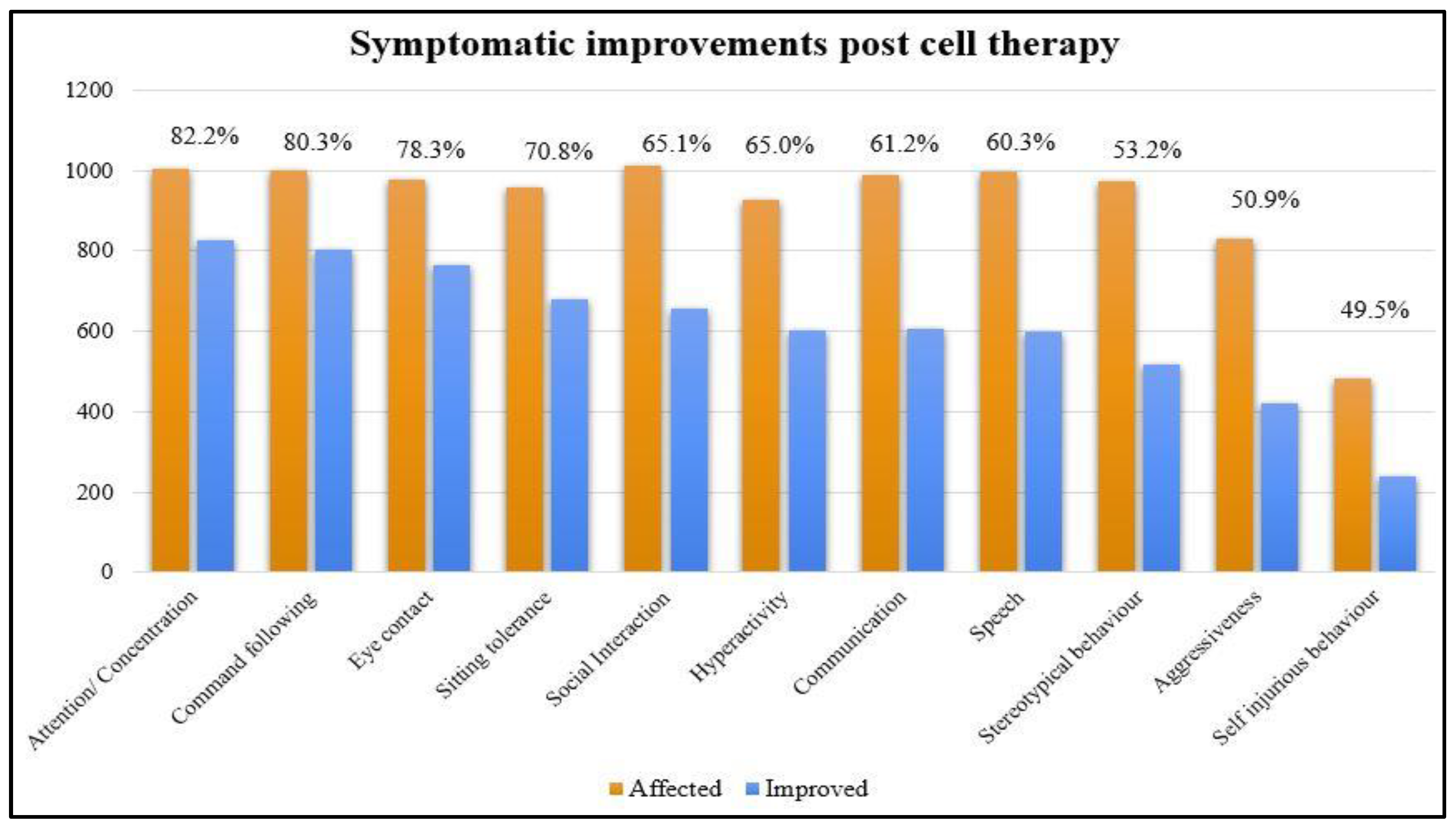
The results of percentage analysis of the symptomatic changes at a mean follow up of 19.3 months were shown in
Table 3 and
Figure 1.
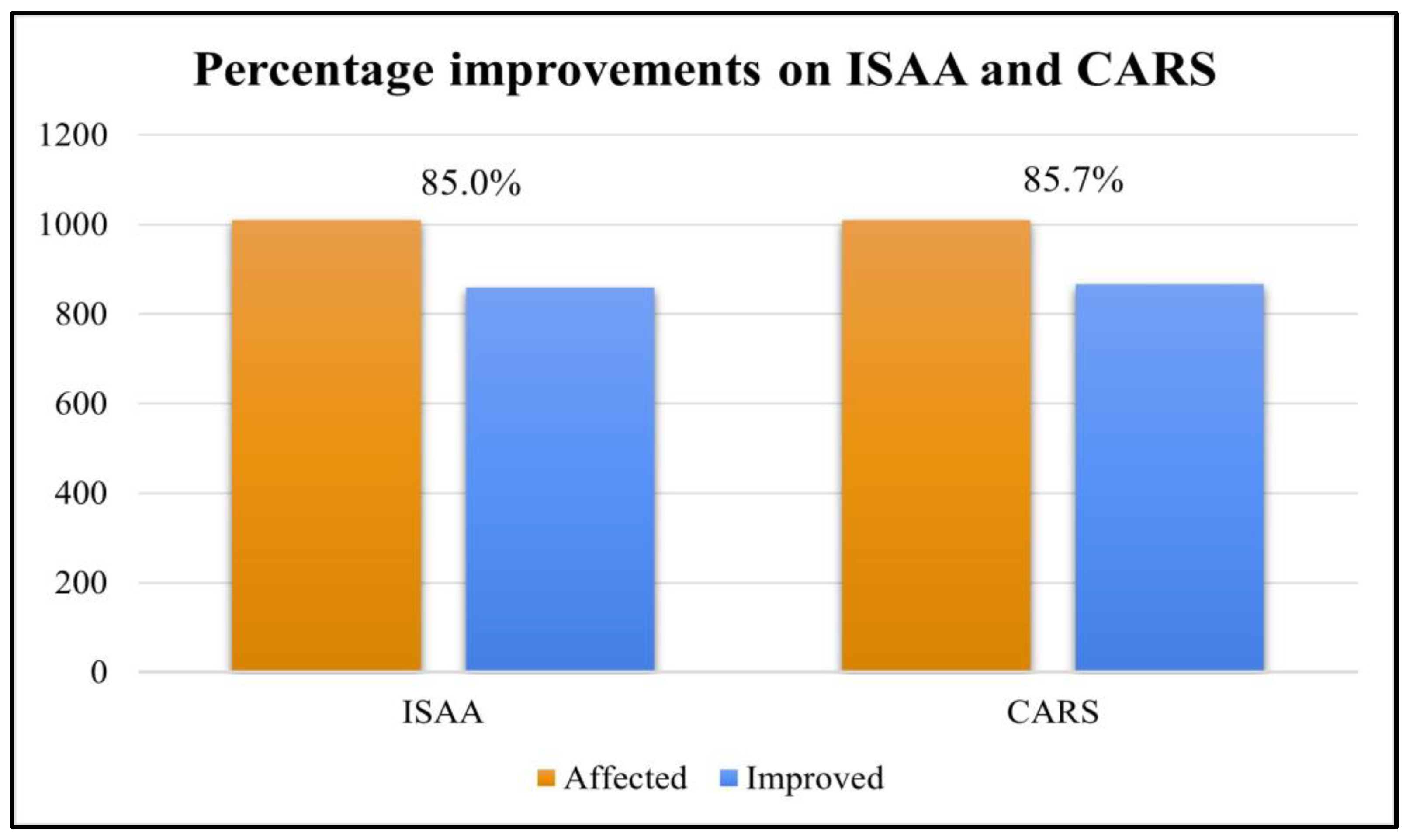
Overall, 90.6% (916 out of 1011) patients improved on at least one outcome measure after cell therapy. The scores on ISAA and CARS showed positive change after cell transplantation, it was found that 85% patients showed improvement in ISAA scores while 85.8% patients showed improvement in CARS scores (
Table 4 and
Figure 2).
On statistically analyzing the data using Wilcoxon’s Signed-Rank Test with level of significance as P<0.05, it was found that the improvements on ISAA and CARS were statistically significant (
Table 5).
3.1. Analysis of individual domains of ISAA
The change in scores of individual domains of ISAA before and after the intervention were also analyzed. The ISAA has 6 domains including social relationship and reciprocity, emotional responsiveness, speech-language and communication, behavior patterns, sensory aspects and cognitive components. It was found that scores of each domain of ISAA showed statistically significant improvement after cell therapy (
Table 6).
3.2. Factors Affecting outcome of intervention
Sub-group analysis was performed based on age, severity of illness on ISAA, number of cell administrations.
3.2.1. Age
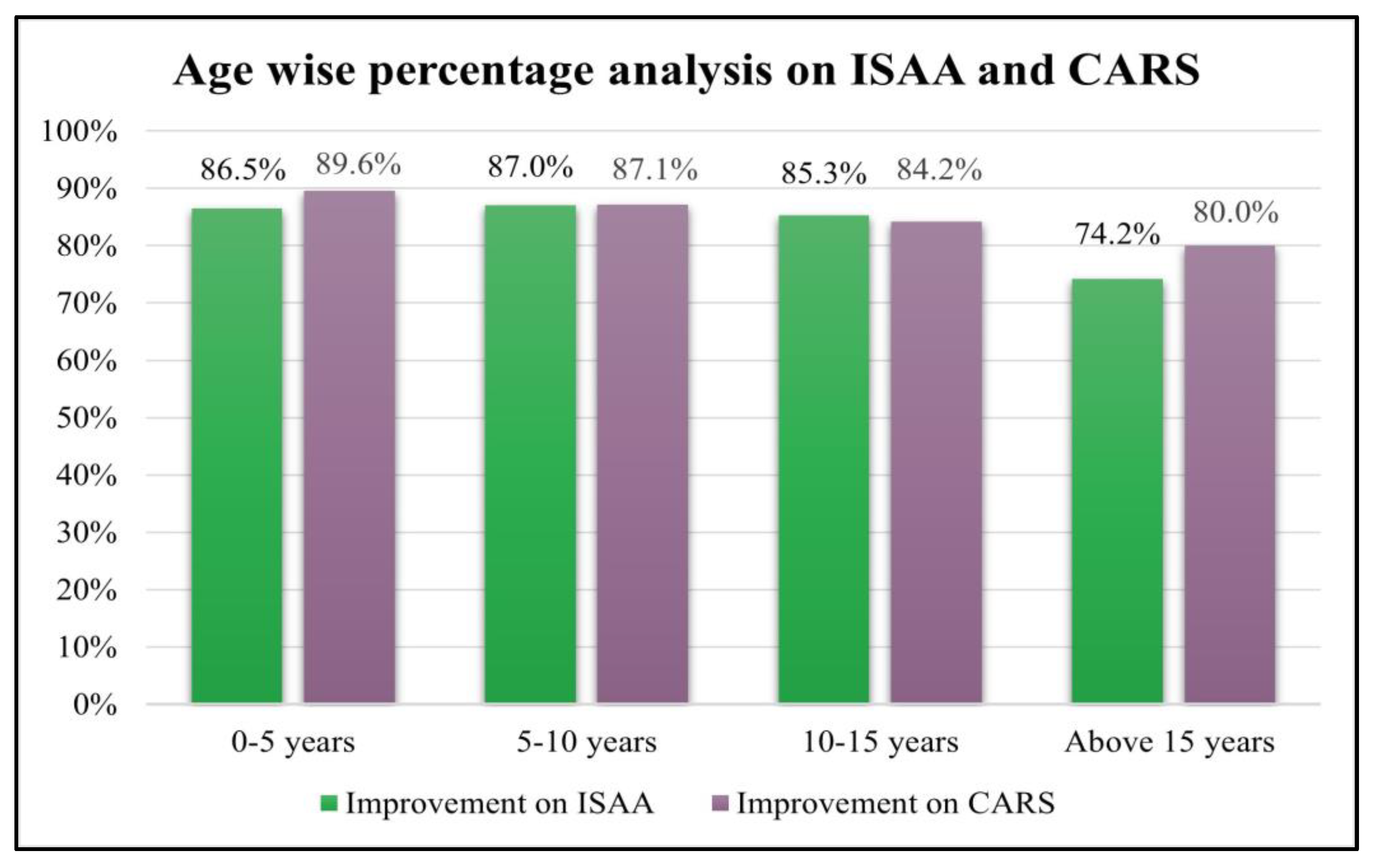
Age wise analysis was performed on ISAA and CARS. It was observed that all the groups showed statistically significant improvement irrespective of age (
Table 7 and
Table 8;
Figure 3).
3.2.2. Severity of illness on ISAA
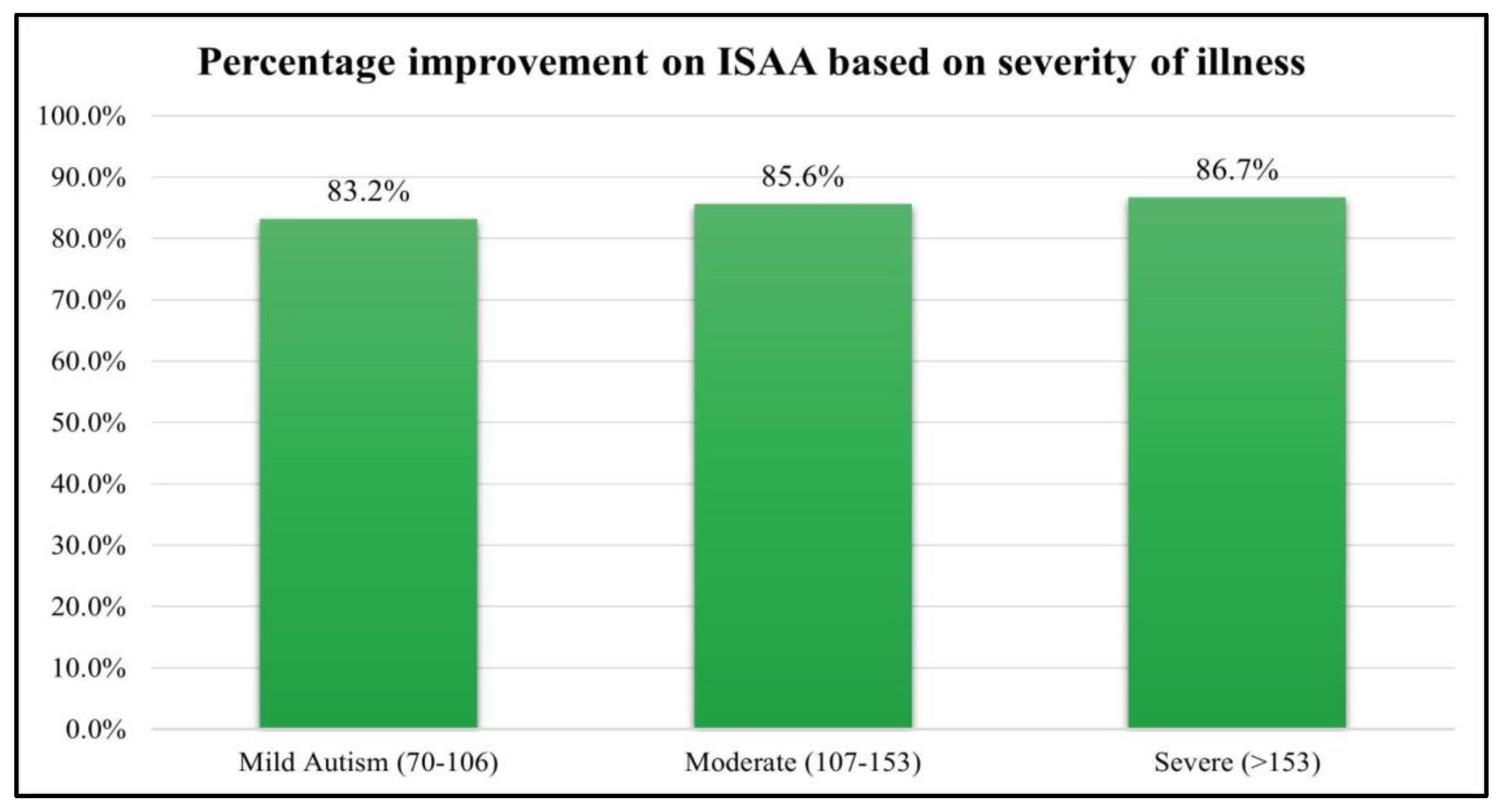
The patients were also analyzed based on their severity of illness based on their ISAA score. The patients who scored between 70-106 on ISAA were mild, 107-153 were moderate and ISAA scores >153 were severe autism. All the groups showed statistically significant improvements. However, there were only 15 cases of severe autism. The results of which are depicted in
Table 9 and
Figure 4.
3.2.3. Number of cell therapies taken
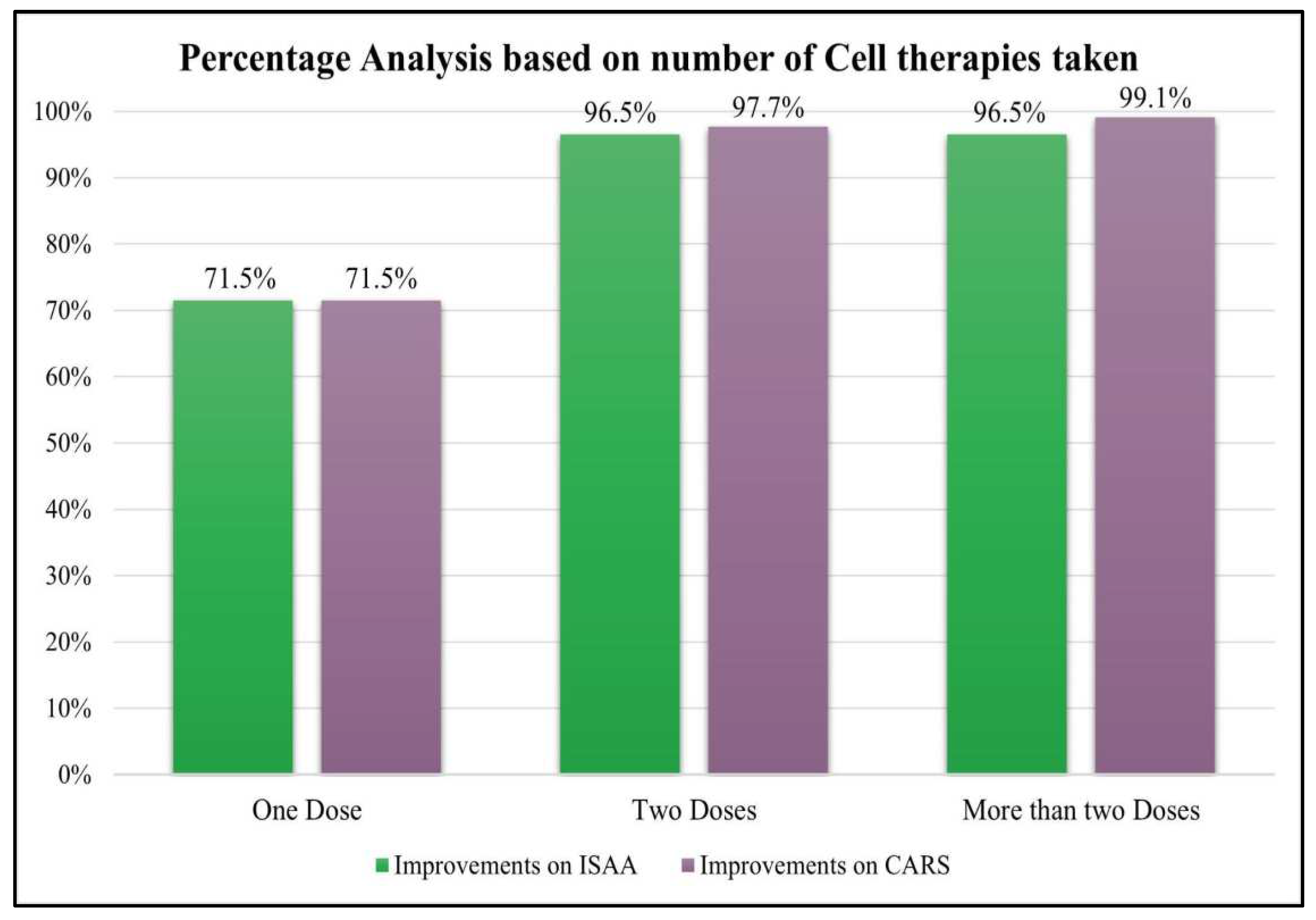
- 1)
Percentage analysis based on ISAA and CARS score.
Patients were categorized into three groups based on the number of cell therapies administered, those who have taken one dose, two doses and more than two doses of BMMNCs. It was observed that patients who underwent cell therapy >/=2 times showed better outcomes i.e. more than 96% patients improved as compared to those who underwent cell transplantation only once (
Table 10 and
Table 11;
Figure 5).
- 2)
Correlation of degree of improvement on ISAA and number of cell therapies
The number of cell therapies and degree of improvements on ISAA score was statistically analyzed by Wilcoxon’s Signed rank test. All the groups showed statistically significant improvements (P<0.05) in the ISAA score after cell therapy (
Table 12). It can also be observed that the maximum change in the mean ISAA score before and after the intervention was shown by the patients who underwent two or more than two cell therapies.
3.2.4. Comparative analysis of Brain FDG PET scan
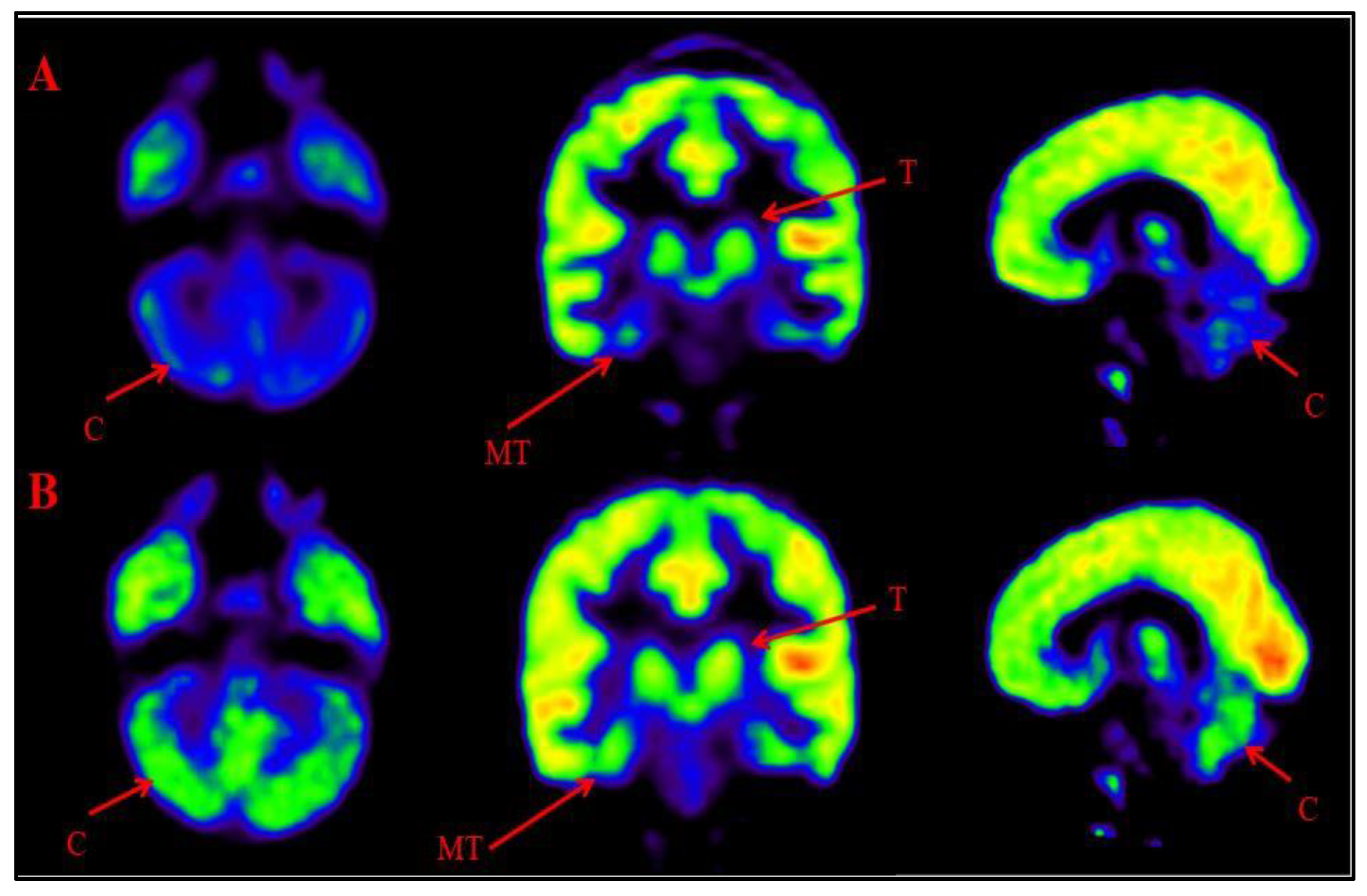
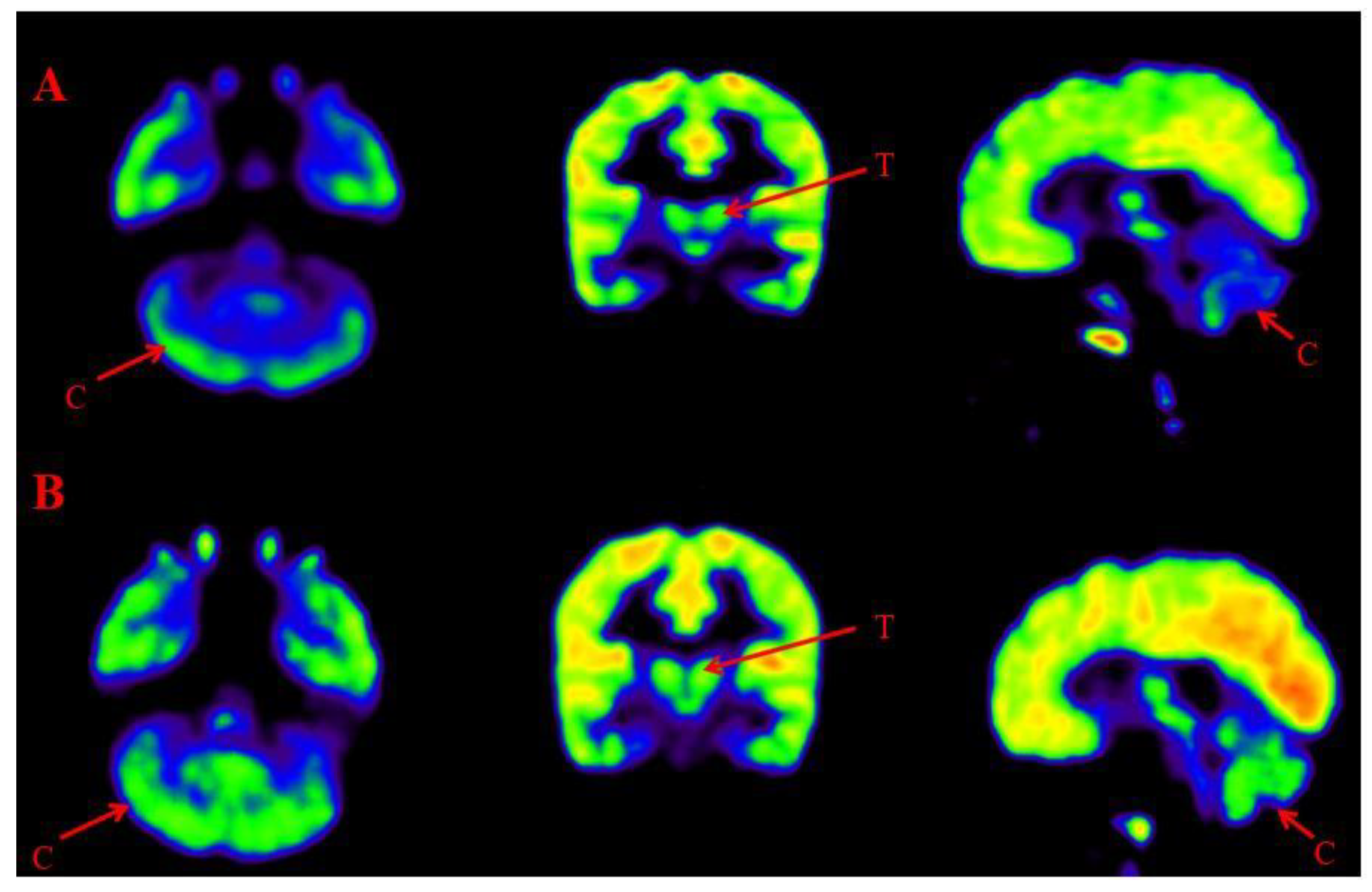
Comparison of Brain FDG PET scans performed before and after intervention was done to observe the metabolic changes in the brain after BMMNCs administration. 18-FDG PET scan shows glucose uptake in different brain areas and also depicts efficacy of treatment in ASD patients via change in metabolic rate [
16]. A comparative PET CT scan was performed in 401 patients. Improved metabolism, reduction in percent hypometabolism, was observed in brain regions including amygdala, hippocampus, parahippocampal gyrus, caudate nucleus, cerebellum, mesial temporal lobe, thalamus, superior and middle temporal poles (
Figure 6,
Figure 7). The grading of these distinct nine regions showed statistically significant improvement on Wilcoxon’s signed rank test (p<0.05) after cell therapy.
4. Discussion
Cell therapy has gained considerable importance and has been found beneficial in the treatment of various neurological conditions including ASD, cerebral palsy, intellectual disability etc [
17,
18,
19,
20]. ASD has a complex multifaceted underlying neuropathophysiology which can be ameliorated by cell therapy.
4.1. Pathophysiology of autism
There are multiple mechanisms which are associated with the pathophysiology of autism. Mitochondrial dysfunction is observed in autism. The damage in the mitochondrial complex leads to increased reactive oxygen species (ROS) production and oxidative stress [
21]. Higher oxidative stress contributes to neuronal damage in ASD patients [
22]. ASD is also marked by immune system dysregulation. Patients with autism have increased microglial and astroglial activation. Both microglia and astroglia modulate the immune system by releasing pro-inflammatory cytokines such as IL-17. Increased activation of both neuroglial cells alters cytokine profile of the brain that increases neuro-inflammatory process and causes neuronal and synaptic dysfunctions [
7,
9]. Altered activation of microglia is also associated with ASD behavioral phenotype such as impaired social interaction, cognitive functions, anxiety etc [
23]. Higher levels of IL-17 induce production of IL-1β, TNF-α, and MMP9 causing neurotoxicity and increased apoptosis in neurons [
24]. Structural and functional MRI studies have shown that ASD individuals have atypical brain connectivity, gray matter volume and activation [
25]. They also have imbalance in excitatory and inhibitory (E/I) neural connections. The hypofunctioning of inhibitory GABAergic neurotransmission leads to higher E/I ratio in patients with ASD [
26]. Atypical brain networks and reduced inhibitory control in ASD may also contribute to behavioral and social dysfunctions in autism [
25,
26]. They also have constricted blood vessels leading to reduced blood flow to the brain resulting in brain damage. Diminished blood circulation also limits oxygenation in the brain causing hypoxia [
27]. Reduced blood flow has been observed in brain SPECT studies of autism patients [
28]. Previous Brain PET-CT studies have shown that children with ASD have relatively lower metabolism in amygdala, hippocampus, parahippocampal gyrus, caudate nucleus, cerebellum, mesial temporal lobe, thalamus, superior and middle temporal pole [
15].
4.2. Mechanism of action of BMMNCs
BMMNCs are mixture of Mesenchymal stem cells (MSCs), hematopoietic cells, monocytes, macrophages, stromal cells, very-small embryonic like stem cells, progenitor cells, hemangioblasts, endothelial progenitor cells. Hence, they can offer a cumulative benefit as compared to individual sub-fractions [
29,
30]. Upon administration, these cells recognize and home towards the site of injury/damage and carry out the repair and regeneration process [
31].
In ASD, these cells act through various mechanisms including paracrine effects, neuroprotection, immunomodulation, anti-inflammatory effects, angiogenesis and synaptogenesis. The cells also stimulate endogenous neural stem cell proliferation which enhances the neurogenesis leading to functional recovery [
10]. These cells secrete various growth factors such as vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and nerve growth factors (NGF) which help in neuroprotection and neuroplasticity [
7,
32,
33]. These neurotrophins have the potential to modulate synaptic and structural plasticity which enhances learning and memory [
34]. A study including rat models of cerebral infarction demonstrated that BMMNCs activate PI3K/AKT/NRF2 pathways that directly activate cellular defense mechanisms, increases biliverdin expression and superoxide dismutase and glutathione peroxidase levels that are known to reduce oxidative stress. It inhibits apoptosis, and thus improves various neurological functions [
32]. The reduction in oxidative stress ameliorates ASD symptoms like communication issues, repetitive behavior, irritability, and sensorimotor functions [
35]. BMMNCs induce Hypoxia Inducing Factor (Hif-1α) which increases the uptake of VEGF by cells, thus activating angiogenesis [
36]. These cells also act as immunomodulators by suppressing production of pro-inflammatory cytokines such as TNF-α and increasing the production of anti-inflammatory cytokines such as IL-10, consequently reducing the inflammation and alleviating symptoms associated with ASD [
7,
37]. MSCs, a subpopulation of BMMNCs have the property to release synaptic transmitters which reinforce excitatory and inhibitory neural connectivity [
23]. Improved connectivity may improve underlying social cognition, language, and executive functions [
38]. Thus, the varied mechanism of action of BMMNCs can help in amelioration of symptoms of autism.
4.3. Rationale for intrathecal administration of BMMNCs
The intrathecal route of administration was chosen as it is easy, safe, and relatively less invasive. Administered cells are mobilized directly to the site of injury via cerebrospinal fluid (CSF) [
31]. The cells from CSF in the subarachnoid space travel into the perivascular space around the blood vessels and then enter brain parenchyma via diffusion. Furthermore, Aquaporin-4 (AQP4), are channels that facilitate entry of cells into the brain parenchyma [
39,
40]. Patients with autism have altered blood-brain barrier permeability enabling the transplanted cells to reach the dysfunctional site of the brain [
41]. It improves the migration of cells to the desired region and limits unwanted entrapment of transplanted cells into other organs such as lungs, spleen, liver etc [
31].
4.4. Rationale for neurorehabilitation along with cell therapy
Rehabilitation improves neural plasticity and facilitates neuroprotection [
42]. Exercise and physical activity are linked to the activation and proliferation of varied cell types along with improved oxygenation and angiogenesis [
43]. Rehabilitation helps in mobilization and homing of the transplanted cells to the site of damage and inflammation [
44]. The cell proliferation expands the pool of cells for mobilization [
44]. Lv et al demonstrated that in autism patients, intrathecal and intravenous transplantation of human cord blood cells along with rehabilitation showed better outcomes than patients receiving only rehabilitation [
45]. Thus, it can be postulated that cell therapy in combination with neurorehabilitation shows better outcomes in patients having ASD.
4.5. Supporting evidence
Our two previously published clinical studies have shown the beneficial effects of BMMNCs along with standard neurorehabilitation in 32 and 254 patients diagnosed with ASD respectively [
17,
18]. The first study, published in 2013 showed statistically significant improvements on ISAA and CGI-I (Clinical Global Impression) at a mean follow up of 12.7 months. On CGI-II 96% of patients showed global improvement with few adverse events including seizures in three patients and were controlled with medications [
17]. The other study published in 2020 included 254 patients diagnosed with ASD [
18]. Symptomatic improvements along with statistically significant improvements on ISAA and CARS were observed. Brain PET CT scan also showed improved brain metabolism after intervention. Villarreal-Martinez et al and Nguyen Thanh et al showed that intrathecal (IT) administration of autologous BMMNCs in ASD patients improves their condition with no serious adverse events [
46,
47]. Kobinia GS demonstrated that administration of autologous, bone marrow derived cells is a safe therapeutic option having no adverse events for patients with autism [
48]. Previous research also demonstrated that intrathecal injection of autologous bone marrow MSC seems to be safe and feasible in treatment of children with ASD [
49]. A large meta-analysis and systematic review published by Villarreal-Martinez L et al in 2022 examined the safety and efficacy of different types of cell therapies in patients with ASD diagnosis and result suggested that cell therapy is safe and clinically improves patients with ASD, regardless of the cell source, dosage, and delivery routes [
50].
4.6. Clinical findings
In the present study, BMMNCs were administered to 1011 patients having ASD along with standard neurorehabilitation. This is the first clinical study to demonstrate safety and efficacy of cell therapy in such a large cohort. The outcome measures used to study the efficacy of the intervention were ISAA and CARS. Although for severity of illness and degree of improvement analysis, ISAA was preferred because components dealing with delayed response, cognitive and savant abilities are not included in CARS [
13]. It was found that statistically significant improvements were observed on both the outcome measures post intervention. Symptomatic improvements were observed in attention and concentration, command following, eye contact, sitting tolerance, social interaction, hyperactivity, communication, speech, stereotypical behavior, aggressiveness, and self-injurious behavior. The improvements on all the domains of ISAA showed statistically significant improvement after cell therapy. The symptomatic improvement is correlated with the improved metabolism observed on Brain PET CT scan of the distinct nine brain regions which is depicted in
Table 13. Thus, cell therapy's ability to reduce cerebral hypometabolism in patients with ASD is crucial for the clinical improvements.
The results of the study showed that improvements were observed in patients regardless of age, although younger age is always preferred as they show better treatment effects [
51]. Significant improvement was also observed in patients irrespective of disease severity. The present study also showed that a greater number of cell therapy doses lead to better therapeutic effects.
One patient in the study group, who improved after cell therapy, after 10 months suffered from severe jaundice which led to mortality. This incident was unrelated to cell therapy.
4.7. Adverse event (AE) monitoring
Adverse events were grouped into two categories: procedure related adverse events and cell therapy related adverse events.
4.8. Procedure related AEs
Procedure related AEs involve events related to bone marrow aspiration and cells administration via lumbar puncture. These include spinal headache, nausea, vomiting, pain at the site of aspiration/injection, back pain, fever, skin rashes etc. In this study 3.27% patients experienced procedure related AEs which were managed with medications during their hospital stay.
4.9. Cell therapy related AEs
In the present study, cell therapy related AEs were reported in 8.3% patients which included increased hyperactivity (4.3%), increased aggressiveness (3.1%). Seizures were also one of the adverse events observed but it was considered as AE only if there was any new episode of seizures or was an increase in frequency or duration of the seizure. In this study, 116 patients had a history of seizures before intervention while 62 patients had an abnormal electroencephalogram (EEG). Patients who already had abnormal EEG or had a history of seizures were advised prophylactic antiepileptic protocol which reduces the chances of occurrence of seizures post cell therapy [
52]. In this study, only 9 out of 1011 (0.9%) patients had seizures as an AE post cell therapy. Out of these patients 4 already had a history of seizures. 5 patients (0.5%) had new onset seizures which were managed by antiepileptic medications. However, occurrence of seizures did not affect the outcome of intervention as these patients still demonstrated improvements.
Limitations: The study lacks a control group to demonstrate the effects of neurorehabilitation alone. However, these patients were already receiving rehabilitation prior to cell therapy, therefore they may serve as self-control. The number of females included in the study are less than males. The Comparative Brain PET CT scan could not be performed for all the patients as only 401 patients gave the consent for repeat scan.
5. Conclusion
This study demonstrates safety and efficacy of intrathecal transplantation of bone marrow mononuclear cell therapy along with neurorehabilitation in a large cohort of patients affected with ASD. Symptomatic and objective improvements on ISAA, CARS scales and Brain PET CT scan provide evidence for the efficacy of the treatment. These improvements result in reduced degree of impairment and improved quality of life of patients with ASD. This study also suggests that multiple cellular therapies increase the effectiveness of cell therapy. Thus, we can conclude that intrathecal BMMNCs are safe and feasible clinical options to treat autism.
Author Contributions
Conceptualization, Dr. Alok Sharma. and Dr. Hemangi Sane.; Methodology, Dr. Alok Sharma. Dr. Nandini Gokulchandran. and Dr. Prerna Badhe; Investigation, Ms. Krishnaveni Kannan. Dr. Hema Biju. Ms. Amruta Paranjape. and Ms. Myola D’sa.; Data Curation, Ms. Krishnaveni Kannan. Ms. Zubiya Shaikh. Dr. Hema Biju. Ms. Amruta Paranjape. and Ms. Myola D’sa.; Project Administration, Dr. Hemangi Sane. and Ms. Pooja Kulkarni.; Supervision, Ms. Pooja Kulkarni.; Data Analysis, Ms. Zubiya Shaikh.; Writing-Original Draft Preparation, Ms. Zubiya Shaikh.; Writing – Review & Editing, Ms. Pooja Kulkarni.; Final review and approval, Dr. Alok Sharma. Dr. Nandini Gokulchandran. Dr. Hemangi Sane. and Dr. Prerna Badhe.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of NeuroGen Brain & Spine Institute (NGBSI/IEC/AT-CS-01/2018/ISSUE-01/REVISION-01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient’s confidentiality policy.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, J.; Kaminiów, K.; Kozak, S.; Sztuba, K.; Emich-Widera, E. Stem Cell Therapies for Cerebral Palsy and Autism Spectrum Disorder—A Systematic Review. Brain Sciences 2021, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.; Jones, R.M.; Pickles, A.; State, M.W.; Taylor, J.L. Autism spectrum disorder. Nat Rev Dis Primers 2020, 6, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; Hof, P.R. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol 2017, 134, 537–566. [Google Scholar] [CrossRef] [PubMed]
- Ecker, C.; Schmeisser, M.J.; Loth, E.; Murphy, D.G. Neuroanatomy and neuropathology of autism spectrum disorder in humans. Translational Anatomy and Cell Biology of Autism Spectrum Disorder 2017, 27–48. [Google Scholar]
- Fetit, R.; Hillary, R.F.; Price, D.J.; Lawrie, S.M. The neuropathology of autism: A systematic review of post-mortem studies of autism and related disorders. Neuroscience & Biobehavioral Reviews 2021, 129, 35–62. [Google Scholar]
- Siniscalco, D.; Kannan, S.; Semprún-Hernández, N.; Eshraghi, A.A.; Brigida, A.L.; Antonucci, N. Stem cell therapy in autism: Recent insights. Stem Cells Cloning 2018, 11, 55–67. [Google Scholar] [CrossRef]
- Goel, R.K.; Suri, V.; Suri, A.; Sarkar, C.; Mohanty, S.; Sharma, M.C.; Yadav, P.K.; Srivastava, A. Effect of bone marrow-derived mononuclear cells on nerve regeneration in the transection model of the rat sciatic nerve. Journal of clinical neuroscience 2009, 16, 1211–1217. [Google Scholar] [CrossRef]
- Forest, E.D.; Gallicchio, V.S. The Effect of Stem Cell Therapy in Autism Spectrum Disorder. J Regen Biol Med 2020, 2, 1–8. [Google Scholar]
- Nakano-Doi, A.; Nakagomi, T.; Fujikawa, M.; Nakagomi, N.; Kubo, S.; Lu, S.; Yoshikawa, H.; Soma, T.; Taguchi, A.; Matsuyama, T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells 2010, 28, 1292–1302. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- hong Jing, Y.; ping Hou, Y.; feng Song, Y.; Yin, J. Methylprednisolone improves the survival of new neurons following transient cerebral ischemia in rats. Acta Neurobiol Exp (Wars) 2012, 72, 240–252. [Google Scholar]
- Chakraborty, S.; Thomas, P.; Bhatia, T.; Nimgaonkar, V.L.; Deshpande, S.N. Assessment of severity of autism using the Indian scale for assessment of autism. Indian J Psychol Med 2015, 37, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, C.; Green, J.A.; Barton, M.L.; Fein, D. Using the childhood autism rating scale to diagnose autism spectrum disorders. J Autism Dev Disord 2010, 40, 787–799. [Google Scholar] [CrossRef]
- Sharma, A.; Gokulchandran, N.; Sane, H.; Nivins, S.; Paranjape, A.; Badhe, P. The baseline pattern and age-related developmental metabolic changes in the brain of children with autism as measured on positron emission tomography/computed tomography scan. World journal of nuclear medicine 2018, 17, 94. [Google Scholar] [CrossRef]
- Tan, Z.; Wei, H.; Song, X.; Mai, W.; Yan, J.; Ye, W.; Ling, X.; Hou, L.; Zhang, S.; Yan, S.; Xu, H. Positron Emission Tomography in the Neuroimaging of Autism Spectrum Disorder: A Review. Front Neurosci 2022, 16, 806876. [Google Scholar] [CrossRef]
- Sharma, A.; Gokulchandran, N.; Sane, H.; Nagrajan, A.; Paranjape, A.; Kulkarni, P.; Shetty, A.; Mishra, P.; Kali, M.; Biju, H.; Badhe, P. Autologous bone marrow mononuclear cell therapy for autism: An open label proof of concept study. Stem cells international 2013, 623875. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gokulchandran, N.; Kulkarni, P.P.; Sane, H.M.; Sharma, R.; Jose, A.; Badhe, P.B. Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: A clinical study. American Journal of Stem Cells 2020, 9, 89. [Google Scholar]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Kulkarni, P.; Gandhi, S.; Sundaram, J.; Paranjape, A.; Shetty, A.; Bhagwanani, K.; Biju, H.; Badhe, P. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: A new frontier. Stem Cells International 2015, 905874, 1–11. [Google Scholar] [CrossRef]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Pai, S.; Kulkarni, P.; Ganwir, V.; Maheshwari, M.; Sharma, R.; Raichur, M.; Nivins, S.; Badhe, P. An open-label proof-of-concept study of intrathecal autologous bone marrow mononuclear cell transplantation in intellectual disability. Stem Cell Res Ther 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Balachandar, V.; Rajagopalan, K.; Jayaramayya, K.; Jeevanandam, M.; Iyer, M. Mitochondrial dysfunction: A hidden trigger of autism? Genes & Diseases 2021, 8, 629–639. [Google Scholar]
- Chen, L.; Shi, X.J.; Liu, H.; Mao, X.; Gui, L.N.; Wang, H.; Cheng, Y. Oxidative stress marker aberrations in children with autism spectrum disorder: A systematic review and meta-analysis of 87 studies (N= 9109). Translational psychiatry 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, M.X.; Sun, L.; Wallis, C.U.; Zhou, J.S.; Ao, L.J.; Li, Q.; Sham, P.C. Rational use of mesenchymal stem cells in the treatment of autism spectrum disorders. World J Stem Cells 2019, 11, 55–72. [Google Scholar] [CrossRef]
- Inga Jácome, M.C.; Morales Chacòn, L.M.; Vera Cuesta, H.; Maragoto Rizo, C.; Whilby Santiesteban, M.; Ramos Hernandez, L.; Noris García, E.; González Fraguela, M.E.; Fernandez Verdecia, C.I.; Vegas Hurtado, Y.; Siniscalco, D. Peripheral Inflammatory Markers Contributing to Comorbidities in Autism. Behavioral Sciences 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Uono, S. The atypical social brain network in autism: Advances in structural and functional MRI studies. Current Opinion in Neurology 2019, 32, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.Y.; Liu, F.C. Molecular pathology and pharmacological treatment of autism spectrum disorder-like phenotypes using rodent models. Frontiers in cellular neuroscience 2018, 12, 422. [Google Scholar] [CrossRef]
- Bjørklund, G.; Kern, J.; Urbina, M.; Saad, K.; El-Houfey, A.; Geier, D.; Chirumbolo, S.; Geier, M.; Mehta, J.; Aaseth, J. Cerebral hypoperfusion in autism spectrum disorder. Acta Neurobiol Exp (Wars) 2018, 78, 21–29. [Google Scholar] [CrossRef]
- Yang, W.H.; Jing, J.; Xiu, L.J.; Cheng, M.H.; Wang, X.; Bao, P.; Wang, Q.X. Regional cerebral blood flow in children with autism spectrum disorders: A quantitative ⁹⁹mTc-ECD brain SPECT study with statistical parametric mapping evaluation. Chin Med J (Engl) 2011, 124, 1362–1366. [Google Scholar]
- Cuende, N.; Rico, L.; Herrera, C. Concise review: Bone marrow mononuclear cells for the treatment of ischemic syndromes: Medicinal product or cell transplantation? Stem Cells Transl Med 2012, 1, 403–408. [Google Scholar] [CrossRef]
- Brenes, R.A.; Bear, M.; Jadlowiec, C.; Goodwin, M.; Hashim, P.; Protack, C.D.; Ziegler, K.R.; Li, X.; Model, L.S.; Lv, W.; Collins, M.J. Cell-based interventions for therapeutic angiogenesis: Review of potential cell sources. Vascular 2012, 20, 360–368. [Google Scholar] [CrossRef]
- Maric, D.M.; Velikic, G.; Maric, D.L.; Supic, G.; Vojvodic, D.; Petric, V.; Abazovic, D. Stem Cell Homing in Intrathecal Applications and Inspirations for Improvement Paths. Int J Mol Sci 2022, 23, 4290. [Google Scholar] [CrossRef]
- Chen, N.N.; Wang, J.P.; Liu, H.F.; Zhang, M.; Zhao, Y.Z.; Fu, X.J.; Yu, L. The bone marrow mononuclear cells reduce the oxidative stress of cerebral infarction through PI3K/AKT/NRF2 signaling pathway. Eur Rev Med Pharmacol Sci 2017, 21, 5729–5735. [Google Scholar] [PubMed]
- Zanirati, G.; Azevedo, P.N.; Marinowic, D.R.; Rodrigues, F.; de Oliveira Dias, A.C.; Venturin, G.T.; Greggio, S.; Simão, F.; DaCosta, J.C. Transplantation of bone marrow mononuclear cells modulates hippocampal expression of growth factors in chronically epileptic animals. CNS neuroscience & therapeutics. 2015, 21, 463–471. [Google Scholar]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Frontiers in cellular neuroscience 2019, 363. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative stress and immune system dysfunction in autism spectrum disorders. International journal of molecular sciences 2020, 21, 3293. [Google Scholar] [CrossRef]
- Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Yasui, T.; Kimura, T.; Gul, S.; Claussen, C.; Boltze, J. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction–Mediated Cell-Cell Interaction. Stroke 2020, 51, 1279–1289. [Google Scholar] [CrossRef]
- Wong, R.S. Neuroinflammation in autism spectrum disorders: Potential target for mesenchymal stem cell-based therapy. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2022, 58, 1–3. [Google Scholar] [CrossRef]
- Maximo, J.O.; Cadena, E.J.; Kana, R.K. The implications of brain connectivity in the neuropsychology of autism. Neuropsychology review 2014, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Derk, J.; Jones, H.E.; Como, C.; Pawlikowski, B.; Siegenthaler, J.A. Living on the edge of the CNS: Meninges cell diversity in health and disease. Frontiers in Cellular Neuroscience 2021, 15, 703944. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids and Barriers of the CNS 2014, 11, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Aragón-González, A.; Shaw, P.J.; Ferraiuolo, L. Blood–Brain Barrier Disruption and Its Involvement in Neurodevelopmental and Neurodegenerative Disorders. International Journal of Molecular Sciences 2022, 23, 15271. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Feng, R.; Li, W.B.; Ren, S.Q.; Zhang, J.; Zhang, F. Physical exercise training and neurovascular unit in ischemic stroke. Neuroscience 2014, 271, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Brixius, K.; Bloch, W. Exercise-induced stem cell activation and its implication for cardiovascular and skeletal muscle regeneration. Minimally Invasive Therapy & Allied Technologies 2008, 17, 91–99. [Google Scholar]
- Emmons, R.; Niemiro, G.M.; De Lisio, M. Exercise as an Adjuvant Therapy for Hematopoietic Stem Cell Mobilization. Stem Cells Int 2016, 2016, 7131359. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.T.; Zhang, Y.; Liu, M.; Qiuwaxi, J.N.T.; Ashwood, P.; Cho, S.C.; Huan, Y.; Ge, R.C.; Chen, X.W.; Wang, Z.J.; Kim, B.J. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. Journal of translational medicine 2013, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Martinez, L.; MartÍnez-Garza, L.E.; Rodriguez-Sanchez, I.P.; Alvarez-Villalobos, N.; Guzman-Gallardo, F.; Pope-Salazar, S.; Salinas-Silva, C.; Cepeda-Cepeda, M.G.; Garza-Bedolla, A.; Dominguez-Varela, I.A.; Villarreal-Martinez, D.Z. Correlation Between CD133+ Stem Cells and Clinical Improvement in Patients with Autism Spectrum Disorders Treated with Intrathecal Bone Marrow-derived Mononuclear Cells. InnovClin Neurosci 2022, 19, 78–86. [Google Scholar]
- Nguyen Thanh, L.; Nguyen, H.P.; Ngo, M.D.; Bui, V.A.; Dam, P.T.; Bui, H.T.P.; Ngo, D.V.; Tran, K.T.; Dang, T.T.T.; Duong, B.D.; Nguyen, P.A.T. Outcomes of bone marrow mononuclear cell transplantation combined with interventional education for autism spectrum disorder. Stem cells translational medicine 2021, 10, 14–26. [Google Scholar] [CrossRef]
- Kobinia, G.S.; Zaknun, J.J.; Pabinger, C.; Laky, B. Case Report: Autologous Bone Marrow Derived Intrathecal Stem Cell Transplant for Autistic Children - A Report of Four Cases and Literature Review. Front Pediatr 2021, 9, 620188. [Google Scholar] [CrossRef]
- Sharifzadeh, N.; Ghasemi, A.; Tavakol Afshari, J.; Moharari, F.; Soltanifar, A.; Talaei, A.; Pouryousof, H.R.; Nahidi, M.; Fayyazi Bordbar, M.R.; Ziaee, M. Intrathecal autologous bone marrow stem cell therapy in children with autism: A randomized controlled trial. Asia Pac Psychiatry 2021, 13, e12445. [Google Scholar] [CrossRef]
- Villarreal-Martínez, L.; González-Martínez, G.; Sáenz-Flores, M.; Bautista-Gómez, A.J.; González-Martínez, A.; Ortiz-Castillo, M.; Robles-Sáenz, D.A.; Garza-López, E. Stem Cell Therapy in the Treatment of Patients With Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Stem Cell Rev Rep 2022, 18, 155–164. [Google Scholar] [CrossRef]
- Dawson, G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and psychopathology 2008, 20, 775–803. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sane, H.; Paranjape, A.; Gokulchandran, N.; Takle, M.; Badhe, P. Seizures as an adverse event of cellular therapy in pediatric neurological disorders and its prevention. J Neurol Disord 2014, 2, 164. [Google Scholar] [CrossRef]
Figure 1.
Graph representing symptomatic improvements after cell therapy.
Figure 1.
Graph representing symptomatic improvements after cell therapy.
Figure 2.
Graph representing improvements on ISAA and CARS post cell therapy.
Figure 2.
Graph representing improvements on ISAA and CARS post cell therapy.
Figure 3.
Graph representing age wise analysis of patients on ISAA and CARS post cell therapy.
Figure 3.
Graph representing age wise analysis of patients on ISAA and CARS post cell therapy.
Figure 4.
Graph representing analysis based on severity of illness on ISAA post cell therapy.
Figure 4.
Graph representing analysis based on severity of illness on ISAA post cell therapy.
Figure 5.
Graph representing analysis of patients on ISAA and CARS based on number of cell therapy taken.
Figure 5.
Graph representing analysis of patients on ISAA and CARS based on number of cell therapy taken.
Figure 6.
Representative images of Comparative Brain FDG PET scan of ASD patients performed before and after Cell therapy.
Figure 6A: Top Row (a): Arrow marked blue areas represent hypometabolism. Bottom Row (B): Arrow marked green areas showing improved metabolism post Cell therapy. Cerebellum (C), Medial temporal cortex (MT) and Thalamus (T).
Figure 6.
Representative images of Comparative Brain FDG PET scan of ASD patients performed before and after Cell therapy.
Figure 6A: Top Row (a): Arrow marked blue areas represent hypometabolism. Bottom Row (B): Arrow marked green areas showing improved metabolism post Cell therapy. Cerebellum (C), Medial temporal cortex (MT) and Thalamus (T).
Figure 7.
Representative images of Comparative Brain FDG PET scan of ASD patients performed before and after Cell therapy. Top Row (A): Arrow marked blue areas represent hypometabolism. Bottom Row (B): Arrow marked green areas showing improved metabolism post Cell therapy. Cerebellum (C) and Thalamus (T).
Figure 7.
Representative images of Comparative Brain FDG PET scan of ASD patients performed before and after Cell therapy. Top Row (A): Arrow marked blue areas represent hypometabolism. Bottom Row (B): Arrow marked green areas showing improved metabolism post Cell therapy. Cerebellum (C) and Thalamus (T).
Table 1.
Percent hypometabolism grading for brain regions.
Table 1.
Percent hypometabolism grading for brain regions.
| Grades |
Percent Hypometabolism of brain regions |
| 1 |
0 to 10% hypometabolic brain region |
| 2 |
10.01 to 20% hypometabolic brain region |
| 3 |
20.01 to 30% hypometabolic brain region |
| 4 |
30.01 to 40% hypometabolic brain region |
| 5 |
40.01 to 50% hypometabolic brain region |
| 6 |
50.01 to 60% hypometabolic brain region |
| 7 |
60.01 to 70% hypometabolic brain region |
| 8 |
70.01 to 80% hypometabolic brain region |
| 9 |
80.01 to 90% hypometabolic brain region |
| 10 |
90.01 to 100 hypometabolic brain region |
Table 2.
Demographic Data.
Table 2.
Demographic Data.
| Total |
|
1011 |
| Gender |
Male |
841 |
| |
Female |
170 |
| Age |
0-5 years |
96 |
| |
5-10 years |
529 |
| |
10-15 years |
266 |
| |
Above 15 years |
120 |
| Severity based on ISAA |
Mild autism (70-106) |
268 |
| |
Moderate autism (107-153) |
728 |
| |
Severe autism (>153) |
15 |
Table 3.
Symptomatic improvements observed post cell therapy.
Table 3.
Symptomatic improvements observed post cell therapy.
| Symptoms |
Affected |
Improved |
Percent (%) improved |
| Attention/Concentration |
1006 |
827 |
82.2 |
| Command following |
1000 |
803 |
80.3 |
| Eye contact |
978 |
766 |
78.3 |
| Sitting tolerance |
959 |
679 |
70.8 |
| Social Interaction |
1011 |
658 |
65.1 |
| Hyperactivity |
927 |
603 |
65.0 |
| Communication |
988 |
605 |
61.2 |
| Speech |
995 |
600 |
60.3 |
| Stereotypical behavior |
973 |
518 |
53.3 |
| Aggressiveness |
829 |
422 |
50.9 |
| Self injurious behavior |
483 |
239 |
49.5 |
Table 4.
Improvements on ISAA and CARS post cell therapy.
Table 4.
Improvements on ISAA and CARS post cell therapy.
| Outcome measures |
Affected |
Improved |
Percent (%) improved |
| ISAA |
1011 |
859 |
85.0 |
| CARS |
1011 |
867 |
85.8 |
Table 5.
Wilcoxon’s Signed-Rank Test (Level of significance P<0.05).
Table 5.
Wilcoxon’s Signed-Rank Test (Level of significance P<0.05).
| Scales |
Pre Mean score |
Post Mean score |
Significance (p<0.05) |
| ISAA |
116.93 |
107.17 |
Significant |
| CARS |
38.24 |
35.73 |
Significant |
Table 6.
Wilcoxon’s Signed-Rank Test for domains of ISAA (Level of significance p<0.05).
Table 6.
Wilcoxon’s Signed-Rank Test for domains of ISAA (Level of significance p<0.05).
| Category |
Pre Mean score |
Post Mean score |
Significance (p<0.05) |
| Social relationship and Reciprocity |
35.28 |
32.74 |
Significant |
| Emotional Responsiveness |
14.03 |
12.43 |
Significant |
| Speech-Language and communication |
23.57 |
22.19 |
Significant |
| Behavior Patterns |
18.55 |
16.60 |
Significant |
| Sensory Aspects |
15.09 |
13.72 |
Significant |
| Cognitive Components |
10.41 |
9.46 |
Significant |
Table 7.
Wilcoxon’s Signed-Rank Test for age-wise analysis on ISAA.
Table 7.
Wilcoxon’s Signed-Rank Test for age-wise analysis on ISAA.
| Age Groups |
Affected |
Improved |
Percent (%) improved |
Significance (p<0.05) |
| 0-5 years |
96 |
83 |
86.5 |
Significant |
| 5-10 years |
529 |
460 |
87.0 |
Significant |
| 10-15 years |
266 |
227 |
85.3 |
Significant |
| Above 15 years |
120 |
89 |
74.2 |
Significant |
Table 8.
Wilcoxon’s Signed-Rank Test for age-wise analysis on CARS.
Table 8.
Wilcoxon’s Signed-Rank Test for age-wise analysis on CARS.
| Age Groups |
Affected |
Improved |
Percent (%) improved |
Significance (p<0.05) |
| 0-5 years |
96 |
86 |
89.6 |
Significant |
| 5-10 years |
529 |
461 |
87.1 |
Significant |
| 10-15 years |
266 |
224 |
84.2 |
Significant |
| Above 15 years |
120 |
96 |
80.0 |
Significant |
Table 9.
Wilcoxon’s Signed-Rank Test based on severity of illness on ISAA.
Table 9.
Wilcoxon’s Signed-Rank Test based on severity of illness on ISAA.
| Severity on ISAA |
Affected |
Improved |
Percent (%) improved |
Significance (p<0.05) |
| Mild |
268 |
223 |
83.2 |
Significant |
| Moderate |
728 |
623 |
85.6 |
Significant |
| Severe |
15 |
13 |
86.7 |
Significant |
Table 10.
Wilcoxon’s Signed-Rank Test based on number of cell therapies taken on ISAA.
Table 10.
Wilcoxon’s Signed-Rank Test based on number of cell therapies taken on ISAA.
| Cell therapies taken |
Affected |
Improved |
Percent (%) improved |
Significance (p<0.05) |
| One dose |
467 |
334 |
71.5 |
Significant |
| Two doses |
429 |
414 |
96.5 |
Significant |
| More than two doses |
115 |
111 |
96.5 |
Significant |
Table 11.
Wilcoxon’s Signed-Rank Test based on number of cell therapies taken on CARS.
Table 11.
Wilcoxon’s Signed-Rank Test based on number of cell therapies taken on CARS.
| Cell therapies taken |
Affected |
Improved |
Percent (%) improved |
Significance (p<0.05) |
| One dose |
467 |
334 |
71.5 |
Significant |
| Two doses |
429 |
419 |
97.7 |
Significant |
| More than two doses |
115 |
114 |
99.1 |
Significant |
Table 12.
Analysis degree of improvement on ISAA scores based on number of cell therapies taken.
Table 12.
Analysis degree of improvement on ISAA scores based on number of cell therapies taken.
| |
N |
Pre Mean ISAA score |
Post Mean ISAA score |
Mean Difference |
Significance (p<0.05) |
| One dose |
467 |
117.93 |
113.38 |
4.55 |
Significant |
| Two doses |
429 |
116.64 |
103.59 |
13.05 |
Significant |
| More than two doses |
115 |
113.89 |
95.25 |
18.64 |
Significant |
Table 13.
Symptomatic improvements correlated with improved brain metabolism observed on Brain PET CT scan.
Table 13.
Symptomatic improvements correlated with improved brain metabolism observed on Brain PET CT scan.
| Brain regions |
Correlated Symptomatic improvements |
| Amygdala |
Aggressiveness, emotional responsiveness, Self-injurious behavior |
| Hippocampus |
Attention/Concentration, learning and memory |
| Parahippocampal gyrus |
Cognition and memory |
| Caudate nucleus |
Social Interaction, Stereotypical behavior |
| Cerebellum |
Social interaction, command following, Speech, Communication, Cognition |
| Medial temporal lobe |
Memory, Hyperactivity, Sitting tolerance |
| Thalamus |
Eye contact, Sensory issues |
| Superior and middle Temporal poles |
Social Interaction, Hyperactivity, Speech, Communication |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).