Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
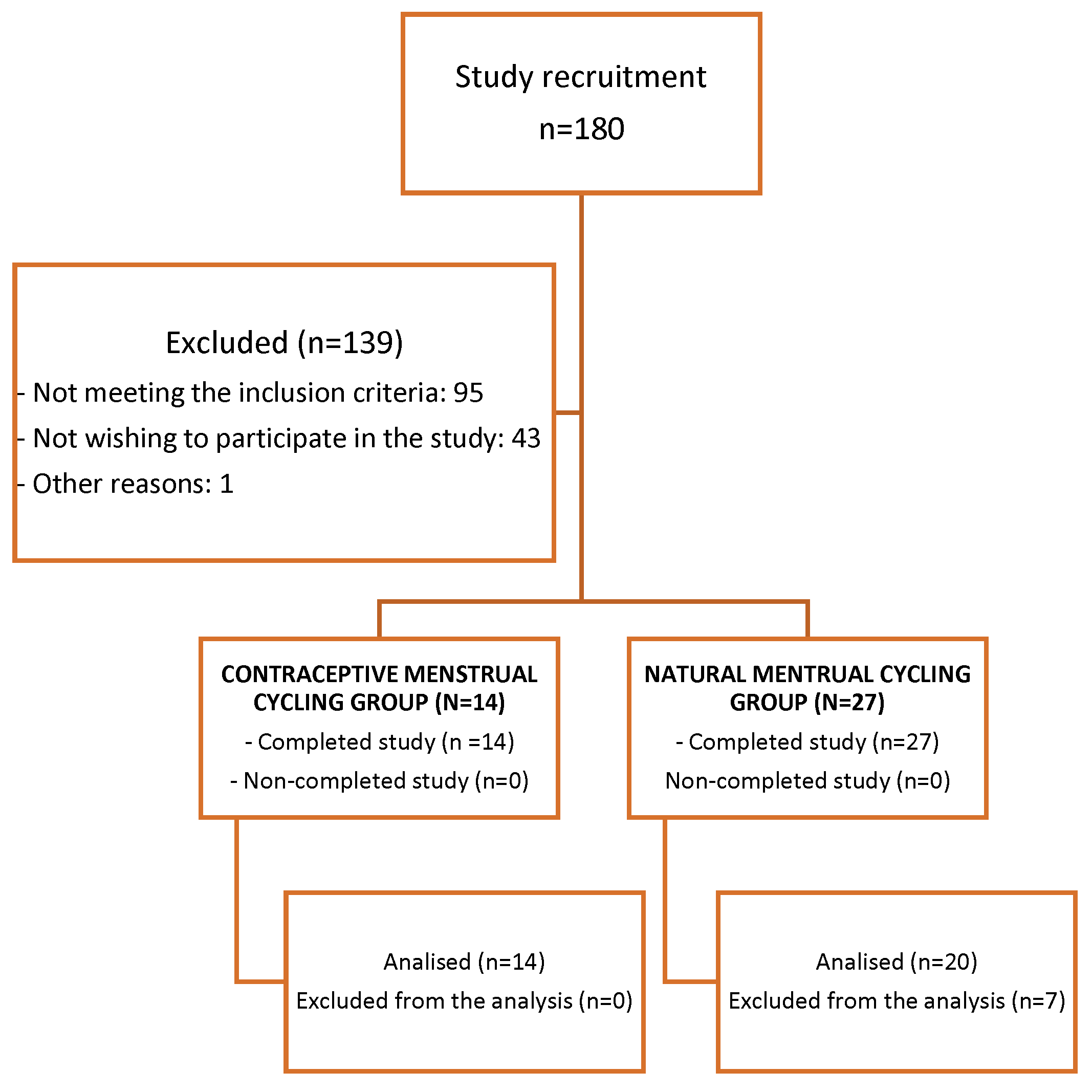
2. Materials and Methods
Experimental design.
Determination of the phases of the natural MC.
Phases of the contraceptive MC
Study variables
- Cardiorespiratory fitness: O2max (mL·kg-1·min-1) (indirectly calculated using the Course Navette test formula, explained later), and total meters achieved in the Course Navette test (m).
- High-speed strength: squat jump 50% additional body weight (cm), squat jump (cm), counter movement jump (cm), abalakov jump (cm), and drop jump from 40 cm (cm).
- Hand grip strength: dominant hand grip (kg).
- Flexibility: Seat and Reach test (cm).
Participants
Test protocol
Statistical analysis
3. Results.
3.1. Sex hormones
3.2. Fitness level and mc phases
3.3. Body composition
3.4. Sensory threshold and pain threshold
3.4.1. Multiple regression analysis
4. Discussion.
Influence of natural MC on cardiorespiratory fitness
Influence of natural MC on high-speed strength, hand grip strength and flexibility
Influence of natural MC on the pain and sensory thresholds
Influence of contraceptive menstrual cycling phases on the level of cardiorespiratory fitness, high-speed strength, hand grip strength and flexibility
STUDY LIMITATIONS
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sport Med. 2010;40(3):207–27.
- National Strength & Conditioning Association. Essentials of Strength Training and Conditioning. 4th ed. Haff GG, Triplett NT, editors. 2016.
- Elliott-Sale, K.J.; McNulty, K.L.; Ansdell, P.; Goodall, S.; Hicks, K.M.; Thomas, K.; Swinton, P.A.; Dolan, E. The Effects of Oral Contraceptives on Exercise Performance in Women: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1785–1812. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Conde, E. The reconciliation of sporting life and training of top-level sportsmen and women in Spain: A quantitative overview. 2013;447.
- Findlay, R.J.; Macrae, E.H.R.; Whyte, I.Y.; Easton, C.; Forrest, L.J. How the menstrual cycle and menstruation affect sporting performance: experiences and perceptions of elite female rugby players. Br. J. Sports Med. 2020, 54, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Ansdell, P.; Brownstein, C.G.; Škarabot, J.; Hicks, K.M.; Simoes, D.C.M.; Thomas, K.; Howatson, G.; Hunter, S.K.; Goodall, S. Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J. Appl. Physiol. 2019, 126, 1701–1712. [Google Scholar] [CrossRef]
- Hackney, AC. Sex Hormones, Exercise and Women. 1st ed. Sex Hormones, Exercise and Women. 2017. 315 p.
- Lemmer, J.T.; Ivey, F.M.; Ryan, A.S.; Martel, G.F.; Hurlbut, D.E.; Metter, J.E.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med. Sci. Sports Exerc. 2001, 33, 532–541. [Google Scholar] [CrossRef]
- Julian, R.; Hecksteden, A.; Fullagar, H.H.K.; Meyer, T. The effects of menstrual cycle phase on physical performance in female soccer players. PLoS ONE 2017, 12, e0173951. [Google Scholar] [CrossRef]
- Forsyth, J.J.; Reilly, T. The effect of menstrual cycle on 2000-m rowing ergometry performance. Eur. J. Sport Sci. 2008, 8, 351–357. [Google Scholar] [CrossRef]
- Fazil KN, Fatyh K, Guleda B, Murat T, Yakup P, Fulya E. Some Performance Parameter Changes During Menstrual Cycle Periods of Athletes and Non-Athletes. Ovidius Univ Ann Ser Phys Educ Sport Mov Heal [Internet]. 2010;10(1):46–9. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=53995267&lang=pt-br&site=ehost-live.
- Štefanovský, M.; Péterová, A.; Vanderka, M.; Lengvarský, L. Influence of selected phases of the menstrual cycle on performance in Special judo fitness test and Wingate test. Acta Gymnica 2016, 46, 136–142. [Google Scholar] [CrossRef]
- De Souza, M.J.; Miller, B.E.; Loucks, A.B.; Luciano, A.A.; Pescatello, L.S.; Campbell, C.G.; Lasley, B.L. High Frequency of Luteal Phase Deficiency and Anovulation in Recreational Women Runners: Blunted Elevation in Follicle-Stimulating Hormone Observed during Luteal-Follicular Transition1. J. Clin. Endocrinol. Metab. 1998, 83, 4220–4232. [Google Scholar] [CrossRef]
- Janse De Jonge XAK. Effects of the menstrual cycle on exercise performance. Sport Med. 2003;33(11):833–51.
- Schaumberg, M.A.; Jenkins, D.G.; de Jonge, X.A.J.; Emmerton, L.M.; Skinner, T.L. Three-step method for menstrual and oral contraceptive cycle verification. J. Sci. Med. Sport 2017, 20, 965–969. [Google Scholar] [CrossRef]
- Egan, B.; X, J.D.J.; B, T.; A, H. Faculty Opinions recommendation of Methodological recommendations for menstrual cycle research in sports and exercise. . 2020, 51. [Google Scholar] [CrossRef]
- Bermon, S.; Garnier, P.Y.; Hirschberg, A.L.; Robinson, N.; Giraud, S.; Nicoli, R.; Baume, N.; Saugy, M.; Fénichel, P.; Bruce, S.J.; et al. Serum Androgen Levels in Elite Female Athletes. J. Clin. Endocrinol. Metab. 2014, 99, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- De Crée, C. Sex Steroid Metabolism and Menstrual Irregularities in the Exercising Female. Sports Med. 1998, 25, 369–406. [Google Scholar] [CrossRef]
- Schaumberg, M.A.; Jenkins, D.G.; DE Jonge, X.A.K.J.; Emmerton, L.M.; Skinner, T.L. Oral Contraceptive Use Dampens Physiological Adaptations to Sprint Interval Training. Med. Sci. Sports Exerc. 2017, 49, 717–727. [Google Scholar] [CrossRef]
- Burrows, M.; Bird, S. The Physiology of the Highly Trained Female Endurance Runner. Sports Med. 2000, 30, 281–300. [Google Scholar] [CrossRef]
- Keller, M.F.; Harrison, M.L.; Lalande, S. Impact of Menstrual Blood Loss and Oral Contraceptive Use on Oxygen-carrying Capacity. Med. Sci. Sports Exerc. 2019, 52, 1414–1419. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Women as Participants: A Working Guide for Standards of Practice for Research on Women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef]
- Russell AM, Benton D, Kingsley M. Period Prevalence and Perceived Side Effects of Hormonal Contraceptive Use and the Menstrual Cycle in Elite Athletes. Int J. 2011.
- Reilly, T. The Menstrual Cycle and Human Performance : An Overview The Menstrual Cycle and Human Performance : 2010;1016(200002):37–41.
- Spona, J.; Elstein, M.; Feichtinger, W.; Sullivan, H.; Lüdicke, F.; Müller, U.; Düsterberg, B. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception 1996, 54, 71–77. [Google Scholar] [CrossRef]
- Blode, H.; Kowal, K.; Roth, K.; Reif, S. Pharmacokinetics of drospirenone and ethinylestradiol in Caucasian and Japanese women. Eur. J. Contracept. Reprod. Heal. Care 2012, 17, 284–297. [Google Scholar] [CrossRef]
- Endrikat J, Blode H, Gerlinger C, Rosenbaum P, Kuhnz W. A pharmacokinetic study with a low-dose oral contraceptive containing 20 μg ethinylestradiol plus 100 μg levonorgestrel. Eur J Contracept Reprod Heal Care. 2002;7(2):79–90.
- Sociedad Española de Ginecología. Combined Oral, Transdermal and Vaginal Hormonal Contraception. 2006;(28036):1–20.
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Byrne, N.M.; Hills, A.P.; Hunter, G.R.; Weinsier, R.L.; Schutz, Y. Metabolic equivalent: one size does not fit all. J. Appl. Physiol. 2005, 99, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Digitimer. Digitimer Operator’s Manual. 2013.
- Current DC, Voltage H. Digitimer. 2014;510.
- Barbosa, M.d.B.; Guirro, E.C.d.O.; Nunes, F.R. Evaluation of sensitivity, motor and pain thresholds across the menstrual cycle through medium-frequency transcutaneous electrical nerve stimulation. Clinics 2013, 68, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, M.A.; Berkley, K.J.; Iezzi, S.; de Bigontina, P.; Vecchiet, L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain 1997, 71, 187–197. [Google Scholar] [CrossRef] [PubMed]
- López-Miñarro PÁ, Vaquero-Cristóbal R, Muyor JM, Espejo-Antúnez L. Criterion-related validity of sit-and-reach test as a measure of hamstring extensibility in older women. Nutr Hosp. 2015;32(1):312–7.
- Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. Force-Velocity Characteristics, Muscle Strength, and Flexibility in Female Recreational Marathon Runners. Front. Physiol. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Ruiz JR, España Romero V, Castro Piñero J, Artero EG, Ortega FB, Cuenca García M, et al. alpha-fitness battery: Field test for the assessment of health-related physical fitness in children and adolescents. Nutr Hosp. 2011;26(6):1210–4.
- National Strength & Conditioning Association. High-speed strength with a jump battery. In: Haff GG, Triplett NT, editors. Essentials of Strength Training and Conditioning. 4th ed. 2016.
- Centeno Prada, RA. REFERENCE VALUES FOR JUMPS ON A DYNAMOMETRIC PLATFORM IN A POPULATION OF ANDALUSIAN SPORTSMEN AND WOMEN. Universidad Pablo de Olavide; 2013.
- De Blas X, Padullés JM, Del Amo JLL, Guerra-Balic M. Creación y validación de Chronojump-Boscosystem: un instrumento libre para la medición de saltos verticales. RICYDE Rev Int Ciencias del Deport. 2012;8(30):334–56.
- de Blas, X. Chronojump-Boscosystem project. Free software tool for the kinematic study of vertical jumping: time measurement, markerless bending angle detection and percentile tables. TDX (Tesis Dr en Xarxa) [Internet]. 2012; Available from: http://www.tdx.cat/handle/10803/83302. 1080. [Google Scholar]
- Xu, J.; Turner, A.; Comfort, P.; Harry, J.R.; McMahon, J.J.; Chavda, S.; Bishop, C. A Systematic Review of the Different Calculation Methods for Measuring Jump Height During the Countermovement and Drop Jump Tests. Sports Med. 2023, 53, 1055–1072. [Google Scholar] [CrossRef] [PubMed]
- de Blas Foix X, Padull X. Manual de Chronojump. 2021;1–116. Available from: http://creativecommons.org/licenses/by-sa/3.0/deed.esLaúltimaversióndeestedocumentoseencuentraenlaúltimaversióndeChronojumpyaquí:http://www.chronojump.org/documents_es.html.
- Léger LA, Lambert J. A maximal multistage 20-m shuttle run test to predict VO2 max. Eur J Appl Physiol Occup Physiol. 1982;49(1):1–12.
- Léger, L.A.; Mercier, D.; Gadoury, C.; Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sports Sci. 1988, 6, 93–101. [Google Scholar] [CrossRef]
- García GC, Secchi JD. 20-metre test course navette with one-minute stages. An original idea that has been around for 30 years. Apunt Sport Med [Internet]. 2014;49(183):93–103. Available from: https://www.apunts.org/es-test-course-navette-20metros-con-articulo-X0213371714492019.
- Dokumacı, B.; Hazır, T. Effects of the Menstrual Cycle on Running Economy: Oxygen Cost Versus Caloric Cost. Res. Q. Exerc. Sport 2019, 90, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Farrel PA, Wilmor JH, Coyl EF, Billin JE, Costil DL. Plasma lactate accumulation and distance running performance. Med Sci Sports Exerc. 1979;25(10):1091–7.
- Sawilowsky, SS. Very large and huge effect sizes. J Mod Appl Stat Methods. 2009;8(2):597–9.
- Cohen, J. F Tests on Means in the Analysis of Variance and Covariance. 1977, 273–406. [Google Scholar] [CrossRef]
- Janse De Jonge, X.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef]
- Vaiksaar, S.; Jürimäe, J.; Mäestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jürimäe, T. No Effect of Menstrual Cycle Phase and Oral Contraceptive Use on Endurance Performance in Rowers. J. Strength Cond. Res. 2011, 25, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Barba-Moreno, L.; Cupeiro, R.; Romero-Parra, N.; Janse de Jonge, X.A.K.; Peinado, A.B. Cardiorespiratory Responses to Endurance Exercise Over the Menstrual Cycle and With Oral Contraceptive Use. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Baltgalvis, K.A.; Greising, S.M.; Warren, G.L.; Lowe, D.A. Estrogen Regulates Estrogen Receptors and Antioxidant Gene Expression in Mouse Skeletal Muscle. PLOS ONE 2010, 5, e10164. [Google Scholar] [CrossRef]
- Lowe, D.A.; Baltgalvis, K.A.; Greising, S.M. Mechanisms Behind Estrogen's Beneficial Effect on Muscle Strength in Females. Exerc. Sport Sci. Rev. 2010, 38, 61–67. [Google Scholar] [CrossRef]
- Isacco, L.; Boisseau, N. Sex Hormones and Substrate Metabolism During Endurance Exercise. In: Sex Hormones, Exercise and Women. 2016, 35–58. [Google Scholar] [CrossRef]
- Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: The up-stream regulatory elements. Vol. 40, Sports Medicine. Springer; 2010. p. 1037–53.
- Collado-Boira, E.; Baliño, P.; Boldo-Roda, A.; Martínez-Navarro, I.; Hernando, B.; Recacha-Ponce, P.; Hernando, C.; Muriach, M. Influence of Female Sex Hormones on Ultra-Running Performance and Post-Race Recovery: Role of Testosterone. Int. J. Environ. Res. Public Heal. 2021, 18, 10403. [Google Scholar] [CrossRef]
- Martinez-Navarro, I.; Montoya-Vieco, A.; Collado, E.; Hernando, B.; Hernando, C. Ultra Trail Performance is Differently Predicted by Endurance Variables in Men and Women. Int. J. Sports Med. 2020, 43, 600–607. [Google Scholar] [CrossRef]
- Thompson, B.M.; Drover, K.B.; Stellmaker, R.J.; Sculley, D.V.; de Jonge, X.A.K.J. The Effect of the Menstrual Cycle and Oral Contraceptive Cycle on Muscle Performance and Perceptual Measures. Int. J. Environ. Res. Public Heal. 2021, 18, 10565. [Google Scholar] [CrossRef]
- Dam, T.V.; Dalgaard, L.B.; Sevdalis, V.; Bibby, B.M.; DE Jonge, X.J.; Gravholt, C.H.; Hansen, M. Muscle Performance during the Menstrual Cycle Correlates with Psychological Well-Being, but Not Fluctuations in Sex Hormones. Med. Sci. Sports Exerc. 2022, 54, 1678–1689. [Google Scholar] [CrossRef]
- Eiling, E.; Bryant, A.L.; Petersen, W.; Murphy, A.; Hohmann, E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surgery, Sports Traumatol. Arthrosc. 2006, 15, 126–132. [Google Scholar] [CrossRef]
- Bell, D.R.; Blackburn, J.T.; Hackney, A.C.; Marshall, S.W.; Beutler, A.I.; Padua, D.A. Hamstring Stiffness and Knee Laxity Across the Menstrual Cycle in Females with ACL Reconstruction. Med. Sci. Sports Exerc. 2011, 43, 805. [Google Scholar] [CrossRef]
- Campa, F.; Micheli, M.L.; Pompignoli, M.; Cannataro, R.; Gulisano, M.; Toselli, S.; Greco, G.; Coratella, G. The Influence of Menstrual Cycle on Bioimpedance Vector Patterns, Performance, and Flexibility in Elite Soccer Players. Int. J. Sports Physiol. Perform. 2022, 17, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sambanis, M.; Kofotolis, N.; Kalogeropoulou, E.; Noussios, G.; Sambanis, P.; Kalogeropoulos, J. A study of the effects on the ovarian cycle of athletic training in different sports. J. sports Med. Phys. Fit. 2003, 43. [Google Scholar]
- Romero-Parra, N.; Alfaro-Magallanes, V.M.; Rael, B.; Cupeiro, R.; Rojo-Tirado, M.A.; Benito, P.J.; Peinado, A.B. Indirect Markers of Muscle Damage Throughout the Menstrual Cycle. Int. J. Sports Physiol. Perform. 2021, 16, 190–198. [Google Scholar] [CrossRef]
| Days of the standard menstrual cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases of the natural cycle | Phase I | Phase II | Phase III | |||||||||||||||||||||||||
| Phases of the contraceptive cycle | Active HC | Inactive HC | ||||||||||||||||||||||||||
| Interview | x | x | y | x | y | |||||||||||||||||||||||
| Body composition | x | x | y | x | y | |||||||||||||||||||||||
| Sensory threshold | x | x | y | x | y | |||||||||||||||||||||||
| Pain threshold | x | x | y | x | y | |||||||||||||||||||||||
| Blood collection | x | x | y | x | y | |||||||||||||||||||||||
| Warm up | x | x | y | x | y | |||||||||||||||||||||||
| Sit and Reach | x | x | y | x | y | |||||||||||||||||||||||
| Hand grip | x | x | y | x | y | |||||||||||||||||||||||
| SJ with 50% body weight | x | x | y | x | y | |||||||||||||||||||||||
| SJ | x | x | y | x | y | |||||||||||||||||||||||
| CMJ | x | x | y | x | y | |||||||||||||||||||||||
| Abalakov jump | x | x | y | x | y | |||||||||||||||||||||||
| DJ from 40 cms | x | x | y | x | y | |||||||||||||||||||||||
| Ovulation test(Natural MC) | From day 8 of the cycle to positive test | |||||||||||||||||||||||||||
| Measurement | Natural MC (n=20) | Contraceptive MC (n=14) | P value |
|---|---|---|---|
| Age (yr) | 26.55 ± 5.880 | 26.86 ± 5.187 | 0.769 |
| Height (cm) | 165.21 ± 6.529 | 162.11 ± 5.088 | 0.231 |
| BMI (kg·m-2) | 23.06 ± 2.419 | 22.79 ± 3.087 | 0.545 |
| Age at first menstruation (yr) | 12.15 ± 1.137 | 12. 62 ± 1.557 | 0.501 |
| Duration of cycles | 27.90 ± 2.732 | 27. 23 ± 1.739 | 0.598 |
| Duration of bleedings | 4.41 ± 0.795 | 3. 83 ± 0.937 | 0.166 |
| Years practicing sport | 13.75 ± 8.22 | 14.14 ± 8.55 | 0.894 |
| Values are presented as mean ± SD. P values less than 0.05 indicating group differences are reported in bold. The data shown refer to phase I of both groups. | |||
| Measurement Natural cycle |
PHASE I | P value / d de Cohen | PHASE II | P value / d de Cohen | PHASE III | P value / d de Cohen |
|---|---|---|---|---|---|---|
| Progesterone (nmol/L) | 1.98± 1.09 (1.47 to 2.49) b | 0.022/0.80 | 9.21±8.84 (5.07 to 13.35) c | 0.005/1.66 | 33.89± 9.80 (29.30 to 38.48) a | 0.001/3.28 |
| Estrogen (pmol/L) | 140.39± 84.50 (100.84 to 179.94)b | 0.001/1.05 | 493.34± 326.31 (340.62 to 646.06) | 519.26 ± 192.54 (429.14 to 609.38) a | 0.001/2.05 | |
| P/E ratio | 20.94±19.87 (26.84-9.59) b | 29.07±29.52 (39.73-4.75) c | 0.001/1.01 | 72.20±29.30 (94.79-50.64) a | 0.001/2.16 | |
| FSH (mIU/mL) | 6.01± 1.69 (5.21 to 6.80) b | 5.88± 3.53 (4.22 to 7.54)c | 0.001/0.83 | 2.75± 0.90 (2.32 to 3.17) a | 0.001/1.79 | |
| LH (mIU/mL) | 4.07± 1.83 (3.21 to 4.92) b | 0.001/0.61 | 15.07± 18.10 (6.59 to 23.54) c | 0.001/0.62 | 3.80± 2.41 (2.67 to 4.93) | |
| Total testosterone (nmol/L) | 1.21± 0.37 (1.03 to 1.38) b | 0.02/0.54 | 1.38 ± 0.41(1.18 to 1.57) c | 0.02/0.84 | 1.13 ± 0.24 (1.01 to 1.24) | |
| SHBG (nmol/L) | 75.69 (63.41 to 87.96) | 81.13 (66.32 to 95.93) | 83.98 (68.12 to 99.84) | |||
| Free Androgen Index (nmol/L) | 1.68± 26.22 (1.45 to 1.91) | 1.92 ± 31.63 (1.49 to 2.35) c | 0.011/0.46 | 1.54 ± 33.88 (1.22 to 1.86) a | 0.027/0.30 |
| Measurement Contraceptive MC |
Inactive HC phase | Active HC Phase | P value | d de Cohen |
|---|---|---|---|---|
| Progesterone (nmol/L) | 2.22± 0.95 (1.67 to 2.77) | 2.95± 1.78 (1.60 to 3.60)) | 0.470 | 0.28 |
| Estrogen (pmol/L) | 103.14± 130.53 (27.77 to 178.51) | 30.05± 58.29 (-3.60 to 63.71) | 0.016 | 0.49 |
| P/E ratio | 63.90±79.03 (18.27 to 109.54) | 200.44±211.18 (78.50 to 322.37) | 0.026 | 0.70 |
| FSH (mIU/mL) | 5.05± 3.42 (3.33 to 7.29) | 1.07± 1.27 (0.34 to 1.81) | 0.001 | 1.2 |
| LH (mIU/mL) | 2.72± 2.41 (1.33 to 4.12) | 0.59± 0.02 (0.06 to 1.12) | 0.003 | 0.8 |
| Total testosterone (nmol/L) | 1.34± 0.41(1.10 to 1.58) | 1.01± 0.25(0.86 to 1.16) | 0.003 | 1.09 |
| SHBG (nmol/L) | 217.91± 92.81 (164.32 to 271.50) | 361.59± 142.64 (279.23 to 443.95) | 0.002 | 1.51 |
| Free Androgen Index (nmol/L) | 0.84±0.76 (0.39 to 1.28) | 0.36±0.24 (0.22 to 0.50) | 0.005 | 0.60 |
| Measurement Natural cycle |
PHASE I | P value/ d de Cohen (I vs II) |
PHASE II | P value/ d de Cohen (II vs III) |
PHASE III | P value/ d de Cohen (I vs III) |
|---|---|---|---|---|---|---|
| Body mass (kg) | 63.23 ± 10.05 (58.52 to 67.94) c |
62.64 ± 9.66 (58.11 to 67.16) |
62.48 ± 9.74 (57.92 to 67.04) a |
0.006/ 0,60 | ||
| Total body water (L) | 36.50 ± 5.02 (34.15 to 38.84) |
36.39 ± 4.90 (34.09 to 38.68) |
36.42 ± 4.66 (34.23 to 38.60) |
|||
| Body fat Percent (%) | 20.61 ± 6,691 (17.48 to 23.74) c |
20.18 ± 6,603 (17.09 to 23.27) |
19.85 ± 6,583 (16.76 to 22.93) a |
0.011/0.54 | ||
| Skeletal muscle mass | 27.84 ± 4.06 (25.93 to 29.73) |
27.75 ± 3.9 (25.87 to 29.62) |
27.82 ± 3.86 (26.01 to 29.62) |
|||
| BMI (kg·m-2) | 23.05 ± 2.41 (21.92 to 24.18) b,c |
0.003/ 1.15 | 22.84 ± 2.35 (21.73 to 23.94) a |
22.79 ± 2.37 (21.67 to 23.90) a |
0.040/0.68 | |
| Course Navette (meters) | 1100 ± 332.96 (944 to 1255) b,c |
0.005/1.15 | 1207 ± 316.91 (1058 to 1355) a |
1176 ± 396.91 (990 to 1361) a |
0.034 / 0.40 | |
| O2max (mL·kg-1·min-1) | 41.75 ± 5.28 (39.27 to 44.22) b,c |
0.004/ 1.45 | 43.85 ± 5.13 (41.44 to 46.25) a |
43.25 ± 6.19 (40.35 to 46.14) a |
0.043 / 0.49 | |
| Hand grip dominant hand (Kg) | 32.15 ± 6.93 (28.90 to 35.40) |
32.87 ± 7.80 (29.22 to 36.52) |
33.51 ± 6.53 (30.45 to 36.56) |
|||
| Seat and reach (cm) | 11.15 ± 7.41 (7.68 to 14.62) |
11.28 ± 7.38 (7.82 to 14.74) |
11.97 ± 6.70 (8.83 to 15.11) |
|||
| SJ 50% additional body weight (cm) | 14.08 ± 3.74 (12.33 to 15.84) |
14.40 ± 3.76 (12.67 to 16.20) |
14.85 ± 4.04 (12.96 to 16.74) |
|||
| SJ (cm) | 26.49 ± 5.19 (24.06 to 28.92) |
25.97 ± 5.52 (23.39 to 28.56) |
26.74 ± 5.91 (23.74 to 29.51) |
|||
| CMJ (cm) | 27.80 ± 5.40 (25.27 to 30.33) |
27.38 ± 5.00 (27.38 to 29.73) |
28.58 ± 6.10 (25.72 to 31.44) |
|||
| ABK jump (cm) | 30.23 ± 5.19 (29.28 to 34.63) b |
0.001 / 0.71 | 29.15 ± 5.45 (27.77 to 32.77) a |
30.21 ± 6.00 (28.47 to 34.87) |
||
| DJ 40cm (cm) | 24.32 ± 6.25 (21.39 to 27.24) |
25.39 ± 6.42 (22.38 to 28.40) |
25.45 ± 7.72 (21.84 to 29.07) |
|||
| Sensory threshold (mA) | 0.64 ± 0.22 (0.53 to 0.74) b |
0.017/0.50 | 0.76 ± 0.29 (0.62 to 0.89) a |
0.75 ± 0.29 (0.61 to 0.89) |
||
| First pain threshold (mA) | 1.34± 1.05 (0.85 to 1.83) b,c |
0.027/0.40 | 1.69 ± 1.60 (0.94 to 2.44) a |
1.59 ± 1.31 (0.97 to 2.20) a |
0.011/0.31 |
| Measurement Contraceptive menstrual cycling |
Inactive HC phase | Active HC Phase | P value |
|---|---|---|---|
| Body mass (kg) | 59.75 ± 7.67 (55.32 to 64.18) | 59.02 ± 7.68 (54.58 to 63.45) | 0.027/0.87 |
| Total body water (L) | 33.49 ± 2.70 (31.93 to 35.05) | 33.56 ±2.54 (32.08 to 35.02) | |
| Body fat Percent (%) | 22.64 ± 7.88 (18.08 to 27.19) | 21.54 ± 7.96 (16.94 to 26.14) | 0.014/0.76 |
| Skeletal muscle mass | 25.34 ± 2.23 (24.04 to 26.62) | 25.39 ± 2.10 (24.17 to 26.60) | |
| BMI (kg·m-2) | 22.79 ± 3.08 (21.00 to 24.56) | 22.49 ± 3.04 (20.73 to 24.25) | 0.017/0.66 |
| Course Navette (meters) | 1110.00 ± 305.81 (933 to 1285) | 1185.71 ± 307.58 (1008 to 1363) | 0.040/0.59 |
| O2max (mL·kg-1·min-1) | 42.02 ± 4.53 (39.41 to 44.64) | 42.60 ± 6.34 (38.93 to 46.26) | |
| Hand grip hand dominant (kg) | 27.82 ± 3.32 (25.89 to 29.74) | 29.31 ± 3.89 (27.06 to 31.56) | |
| Seat and reach (cm) | 10.95 ± 6.68 (7.09 to 14.81) | 11.51 ± 7.68 (7.07 to 15.95) | 0.041/0.22 |
| SJ WITH 50% additional body weight (cms) | 12.15 ± 5.84 (8.77 to 15.52) | 13.48 ± 5.85 (10.10 to 16.86) | 0.009/0.40 |
| SJ (cms) | 23.26 ± 6.81 (19.32 to 27.19) | 23.80 ± 7.29 (19.58 to 28.01) | |
| CMJ (cms) | 24.20 ± 7.10 (20.10 to 28.31) | 25.07 ± 7.83 (20.55 to 29.59) | |
| ABK jump (cms) | 27.71 ± 8.03 (23.07 to 32.35) | 28.52 ± 8.05 (23.88 to 33.17) | |
| DJ (cms) | 23.42 ± 9.87 (17.17 to 29.12) | 22.02 ± 8.77 (16.95 to 27.09) | |
| Sensory threshold (mA) | 0.57 ± 0.26 (0.42 to 0.73) | 0.62 ± 0.19 (0.51 to 0.73) | |
| First pain threshold (mA) | 1.43 ± 0.98 (0.86 to 2.00) | 1.67 ± 1.14 (1.01 to 2.33) |
| Model | R2 Adjusted | Standardized Coefficients Beta |
Standard Error | F (p) |
|---|---|---|---|---|
|
Dependent Variable:O2max Covariates: First pain threshold. |
0.319 | 0.595 | 4.3619 | 9.885 (0.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





