Submitted:
11 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Catalysts Synthesis
2.2. Physicochemical Characterization
2.3 Electrochemical Experiments
2.4. Membrane Electrode Assemblies (MEAs) Preparation
3. Results
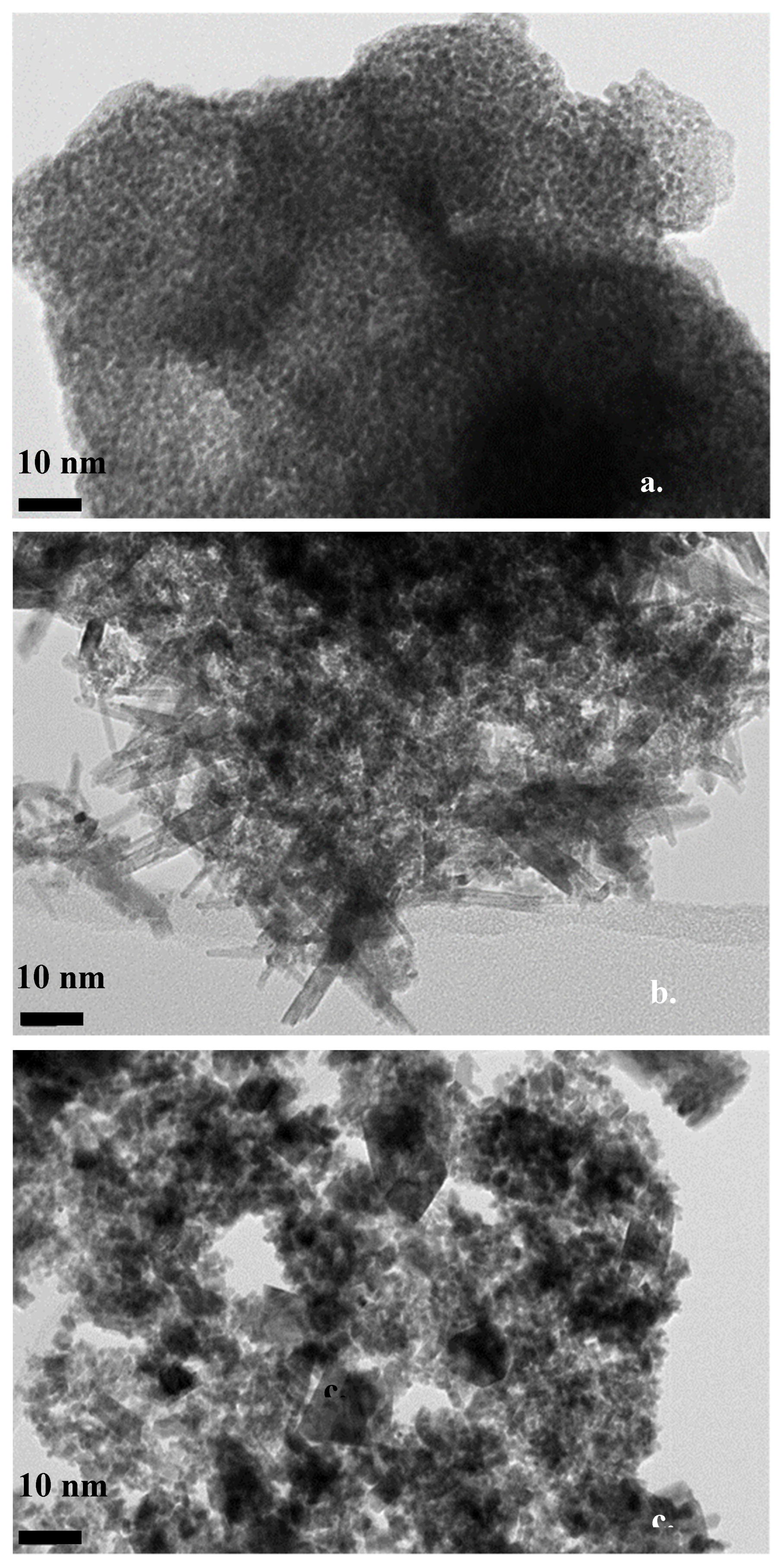
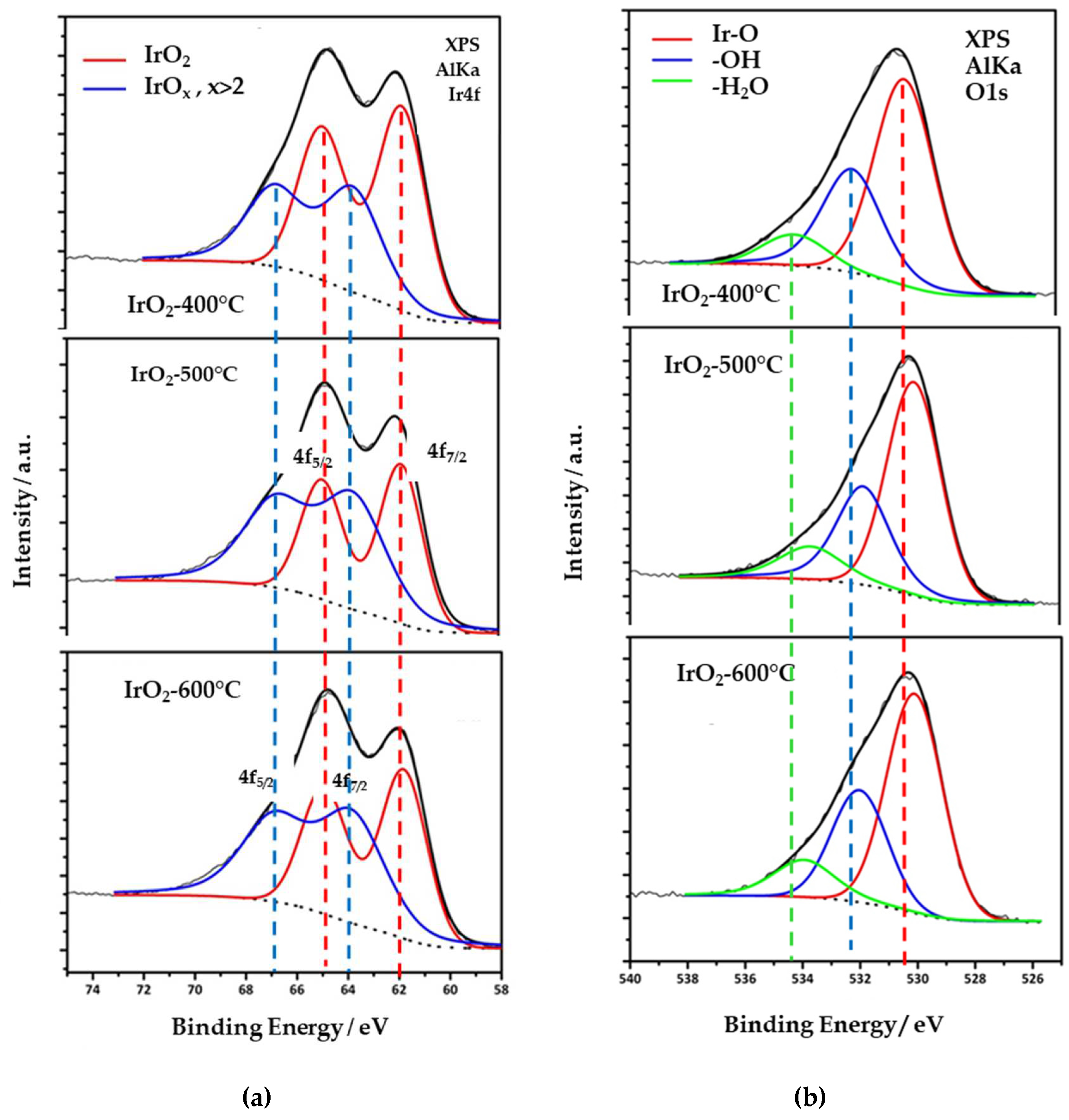
3.1. Structural Characterization of the Catalysts
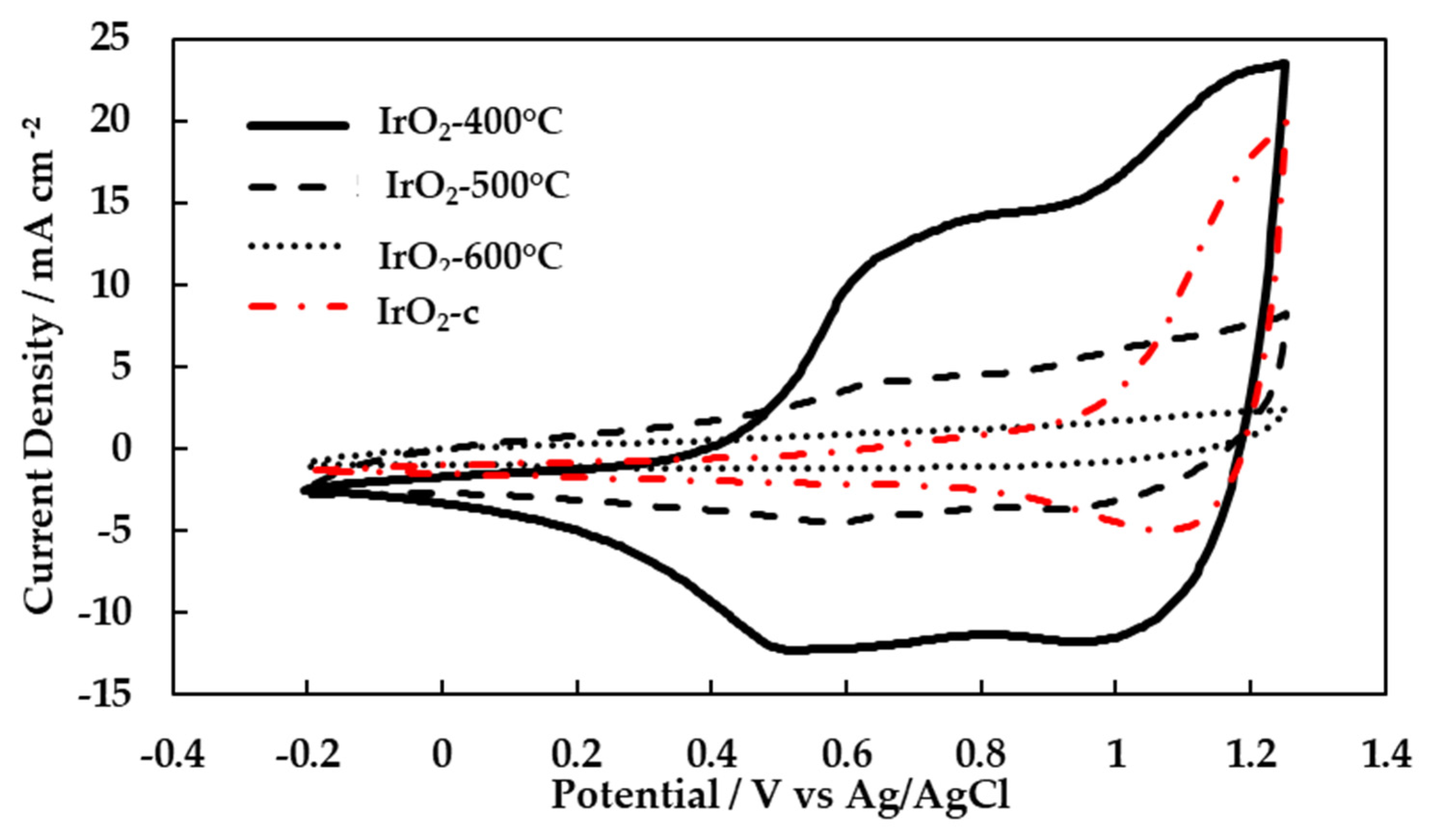
3.2. Surface Electrochemistry
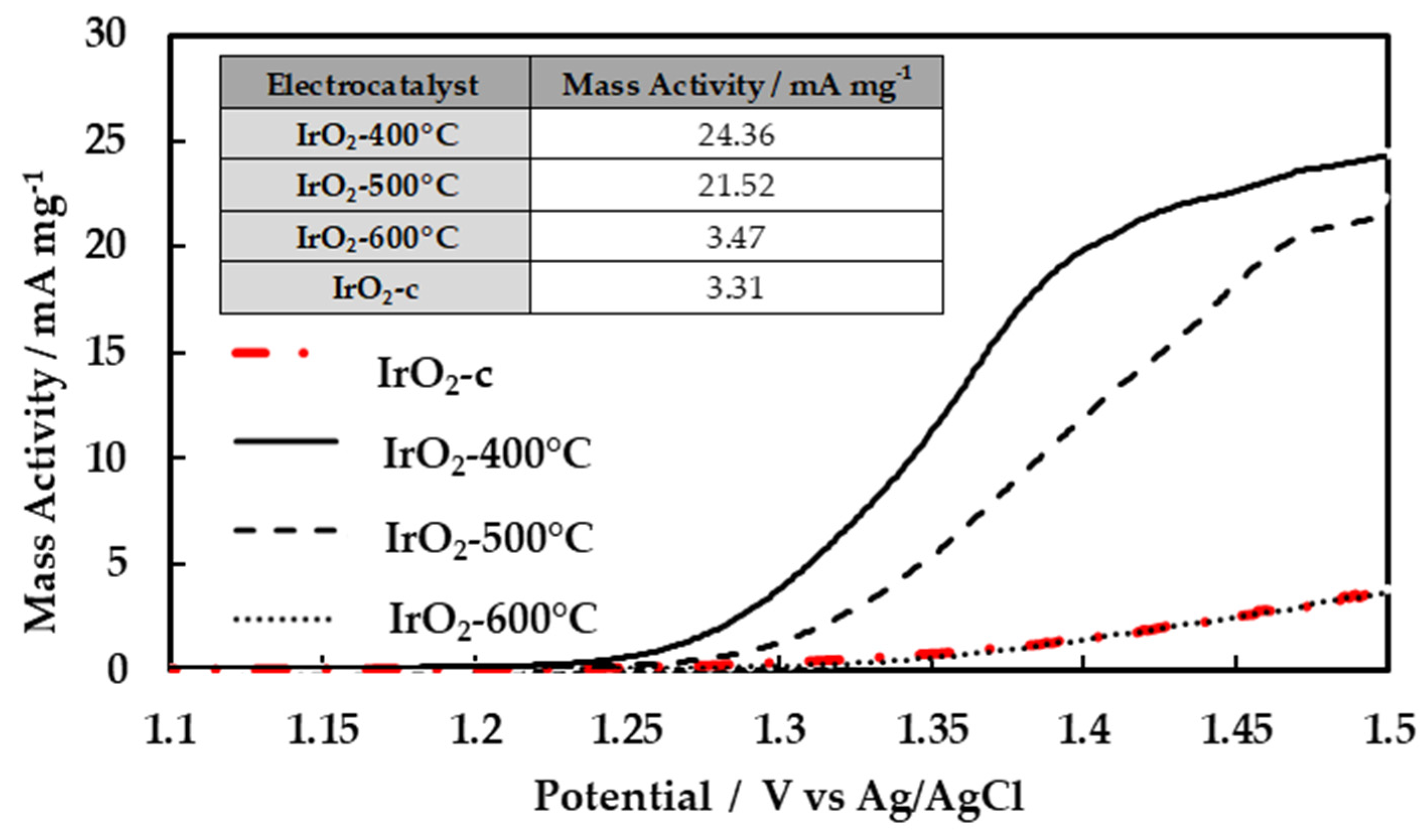
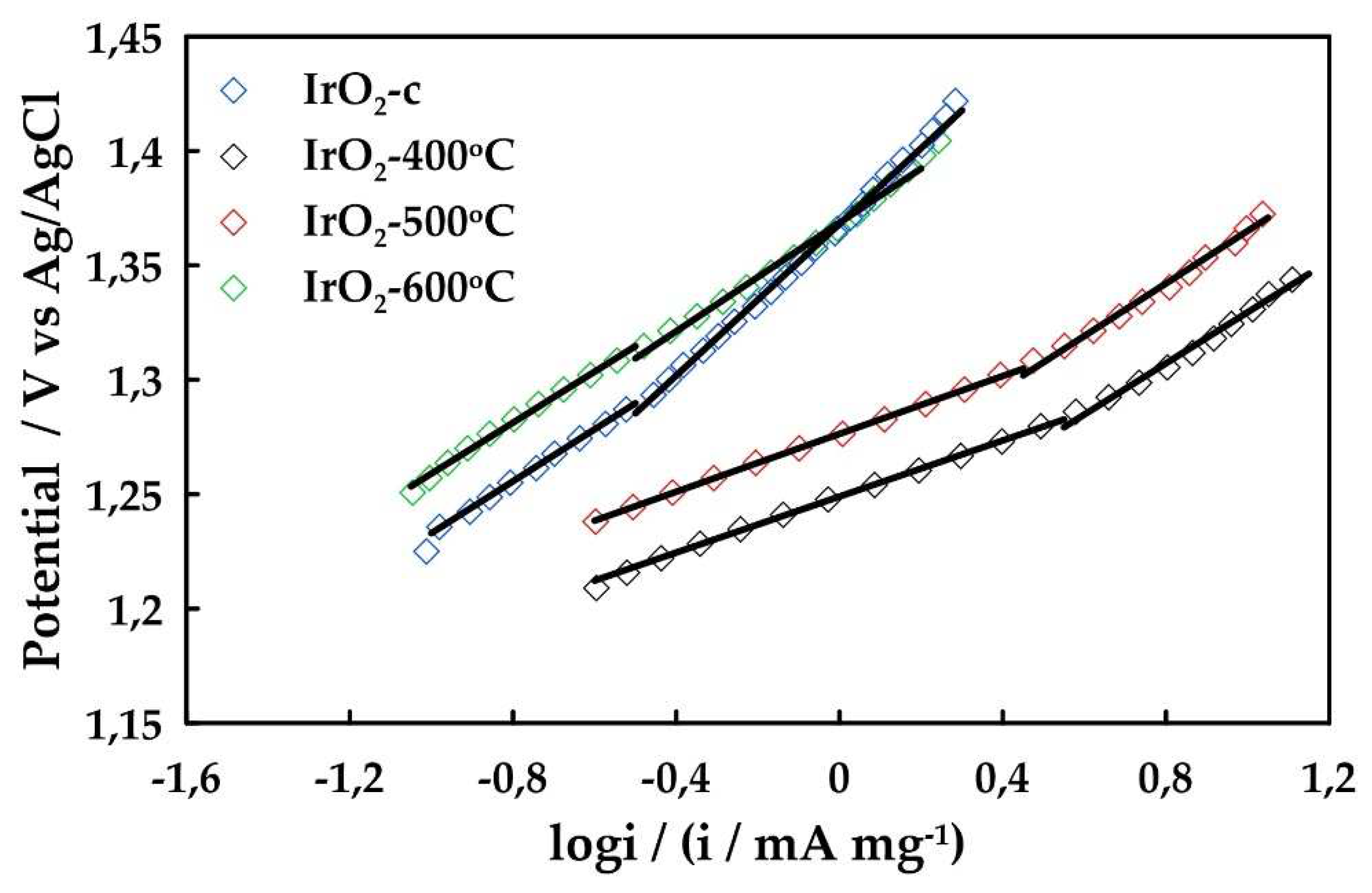
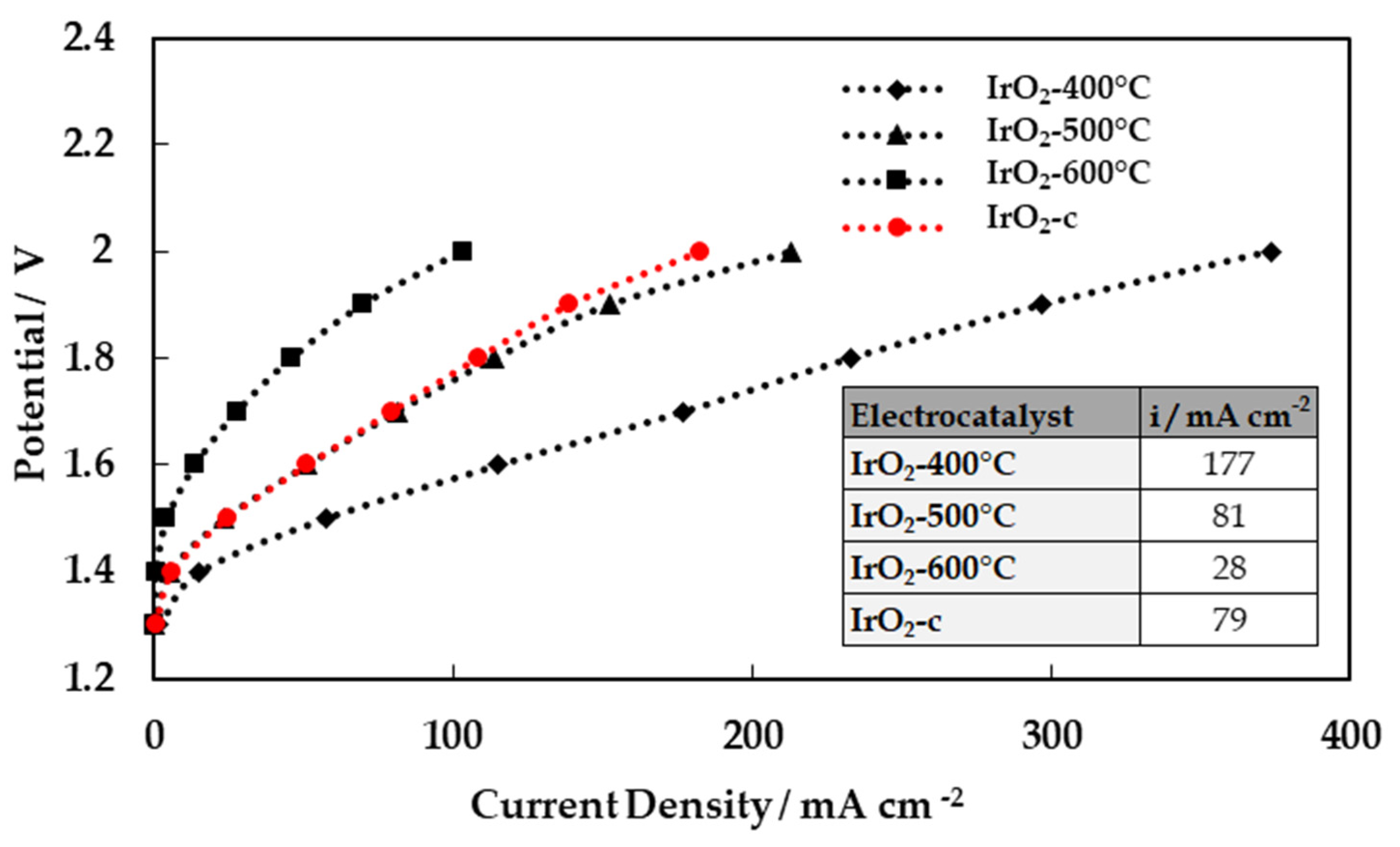
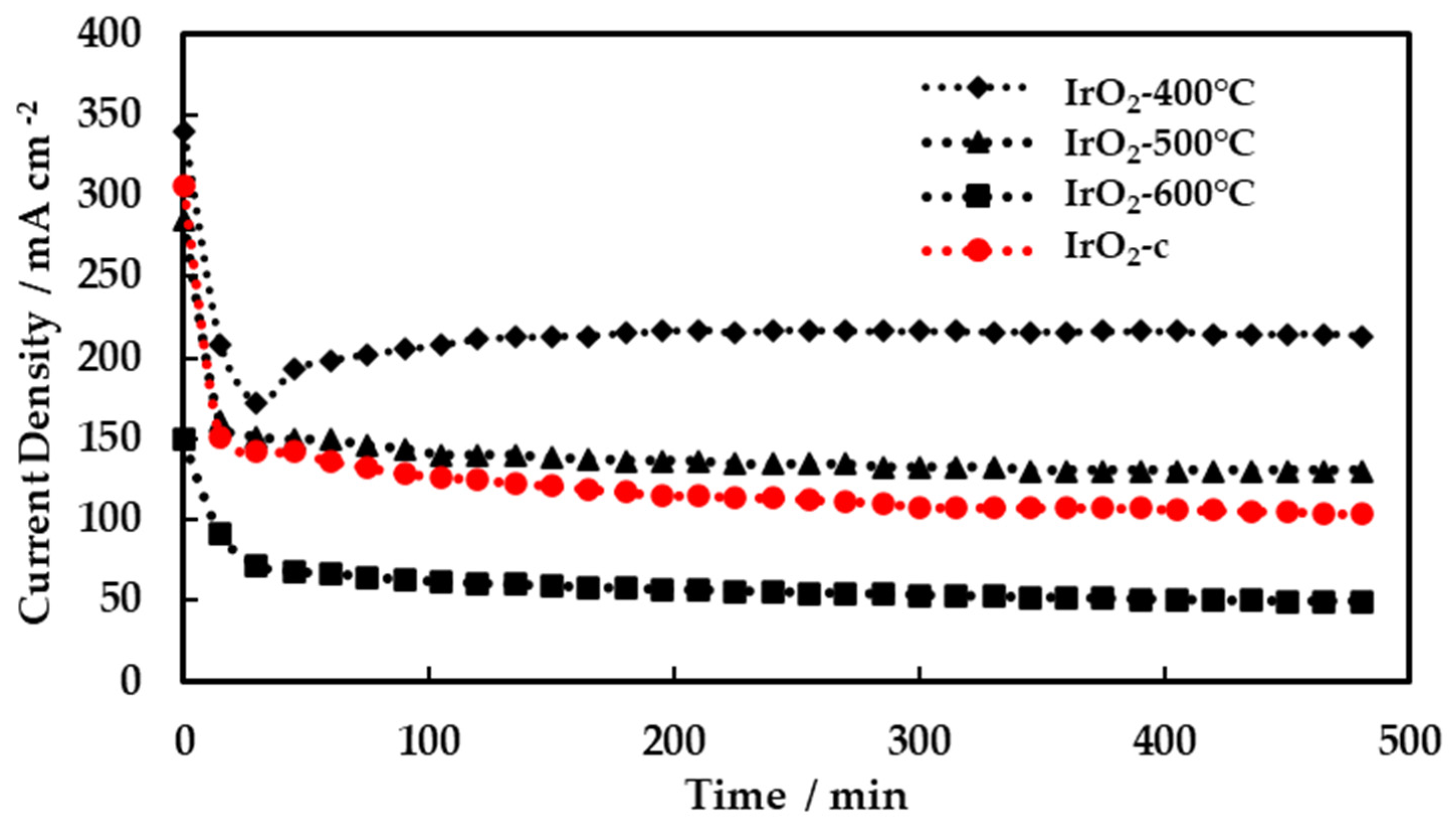
3.3. Activity for the Oxygen Evolution Reaction (OER)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Carmo, M.; Fritz, D. L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Adams, R.; Shriner, R. L. Platinum oxide as a catalyst in the reduction of organic compounds. III. Preparation and properties of the oxide of platinum obtained by the fusion of chloroplatinic acid with sodium nitrate. J. Am. Chem. Soc. 1923, 45, 2171–2179. [Google Scholar] [CrossRef]
- Papazisi, K. M.; Siokou, A.; Balomenou, S.; Tsiplakides, D. Preparation and characterization of IrxPt1−xO2 anode electrocatalysts for the oxygen evolution reaction. Int. J. Hydrog. Energy 2012, 37, 16642–16648. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Lei, Y.; Liu, F.; Tian, B.; Wang, J. Investigation of high-performance IrO2 electrocatalysts prepared by Adams method. Int. J. Hydrog. Energy 2018, 43, 19460–19467. [Google Scholar] [CrossRef]
- Silva, G. C.; Perini, N.; Ticianelli, E. A. Effect of temperature on the activities and stabilities of hydrothermally prepared IrOx nanocatalyst layers for the oxygen evolution reaction. Appl. Catal. B-Environ. 2017, 218, 287–297. [Google Scholar] [CrossRef]
- Antolini, E. Iridium as Catalyst and Cocatalyst for Oxygen Evolution/Reduction in Acidic Polymer Electrolyte Membrane Electrolyzers and Fuel Cells. ACS Catalysis 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Xia, Q. IrO2/Nb–TiO2 electrocatalyst for oxygen evolution reaction in acidic medium. Int. J. Hydrog. Energy 2014, 39, 6967–6976. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Stassi, A.; Ornelas, R.; Antonucci, V.; Aricò, A. S. Investigation of IrO2 electrocatalysts prepared by a sulfite-couplex route for the O2 evolution reaction in solid polymer electrolyte water electrolyzers. Int. J. Hydrog. Energy 2011, 36, 7822–7831. [Google Scholar] [CrossRef]
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 2007, 52, 3889–3894. [Google Scholar] [CrossRef]
- Angelucci, C. A.; D’Villa Silva, M.; Nart, F. C. Preparation of platinum–ruthenium alloys supported on carbon by a sonochemical method. Electrochim. Acta 2007, 52, 7293–7299. [Google Scholar] [CrossRef]
- Li, G.; Yu, H.; Song, W.; Dou, M.; Li, Y.; Shao, Z.; Yi, B. A Hard-Template Method for the Preparation of IrO2, and Its Performance in a Solid-Polymer-Electrolyte Water Electrolyzer. ChemSusChem 2012, 5, 858–861. [Google Scholar] [CrossRef]
- Puthiyapura, V. K.; Pasupathi, S.; Su, H.; Liu, X.; Pollet, B.; Scott, K. Investigation of supported IrO2 as electrocatalyst for the oxygen evolution reaction in proton exchange membrane water electrolyser. Int. J. Hydrog. Energy 2014, 39, 1905–1913. [Google Scholar] [CrossRef]
- Li, G.; Yu, H.; Yang, D.; Chi, J.; Wang, X.; Sun, S.; Shao, Z.; Yi, B. Iridium-Tin oxide solid-solution nanocatalysts with enhanced activity and stability for oxygen evolution. J. Power Sources 2016, 325, 15–24. [Google Scholar] [CrossRef]
- Cruz, J. C.; Baglio, V.; Siracusano, S.; Ornelas, R.; Ortiz-Frade, L.; Arriaga, L. G.; Antonucci, V.; Aricò, A.S. Nanosized IrO2 elec trocatalysts for oxygen evolution reaction in an SPE electrolyzer. J. Nanopart. Res. 2011, 13, 1639–166. [Google Scholar] [CrossRef]
- Ouattara, L.; Fierro, S.; Frey, O.; Koudelka, M.; Comninellis, C. Electrochemical comparison of IrO2 prepared by anodic oxidation of pure iridium and IrO2 prepared by thermal decomposition of H2IrCl6 precursor solution. J. Appl. Electrochem. 2009, 39, 1361–1367. [Google Scholar] [CrossRef]
- Reier, T.; Teschner, D.; Lunkenbein, T.; Bergmann, A.; Selve, S.; Kraehnert, R.; Schlogl, R.; Strasser, P. Electrocatalytic Oxygen Evolution on Iridium Oxide: Uncovering Catalyst-Substrate Interactions and Active Iridium Oxide Species. J. Electrochem. Soc. 2014, 161, F876–F882. [Google Scholar] [CrossRef]
- Cherevko, S.; Reier, T.; Zeradjanin, A. R.; Pawolek, Z.; Strasser, P.; Mayrhofer, K. J. J. Stability of nanostructured iridium oxide electrocatalysts during oxygen evolution reaction in acidic environment. Electrochem. Commun. 2014, 48, 81–85. [Google Scholar] [CrossRef]
- Ardizzone, S.; Fregonara, G.; Trasatti, S. ‘Inner’ and ‘outer’ active surface of RuO2 electrodes. Electrochim. Acta 1990, 35, 263–267. [Google Scholar] [CrossRef]
- Karimi, F.; Bazylak, A.; Peppleya, B. A. Effect of Calcination Temperature on the Morphological and Electrochemical Characteristics of Supported Iridium Hydroxyoxide Electrocatalysts for the PEM Electrolyzer Anode. J. Electrochem. Soc. 2017, 164, F464–474. [Google Scholar] [CrossRef]
- Karimi, F.; Peppley, B. A. Metal Carbide and Oxide Supports for Iridium-Based Oxygen Evolution Reaction Electrocatalysts for Polymer-Electrolyte-Membrane Water Electrolysis. Electrochim. Acta 2017, 246, 654–670. [Google Scholar] [CrossRef]
- Peuckert, M. XPS study on thermally and electrochemically prepared oxidic adlayers on iridium. Surface Science 1984, 144, 451–464. [Google Scholar] [CrossRef]
- Sanjinés, R.; Aruchamy, A.; Lévy, F. Thermal Stability of Sputtered Iridium Oxide Films. J. Electrochem. Soc. 1989, 136, 1740–1743. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Chen, G.; Zhang, Y. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Reier, T.; Weidinger, I.; Hildebrandt, P.; Kraehnert, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction on Iridium Oxide Model Film Catalysts: Influence of Oxide Type and Catalyst Substrate Interactions. ECS transactions 2013, 58, 39–51. [Google Scholar] [CrossRef]
- Fierro, S.; Kapałka, A.; Comninellis, C. Electrochemical comparison between IrO2 prepared by thermal treatment of iridium metal and IrO2 prepared by thermal decomposition of H2IrCl6 solution. Electrochem. Commun. 2010, 12, 172–174. [Google Scholar] [CrossRef]

| Electrocatalyst | Lattice Parameters | Crystallite Size | Unit Cell Volume | |
|---|---|---|---|---|
| nm | Å3 | |||
| IrO2-c | - | - | 1.24 | - |
| IrO2-400°C | - | - | 1.40 | - |
| IrO2-500°C | a=b=4.467 | c=3.110 | 5.18 | 62.0 |
| IrO2-600°C | a=b=4.457 | c=3.140 | 7.77 | 62.4 |
| Electrocatalyst | Surface Area | Pore Volume | ESCA |
|---|---|---|---|
| m2 g-1 | cm3 g-1 | m2 g-1 | |
| IrO2-c | 32 | 0.130 | 11 |
| IrO2-400°C | 185 | 0.200 | 198 |
| IrO2-500°C | 127 | 0.167 | 27 |
| IrO2-600°C | 66 | 0.145 | 21 |
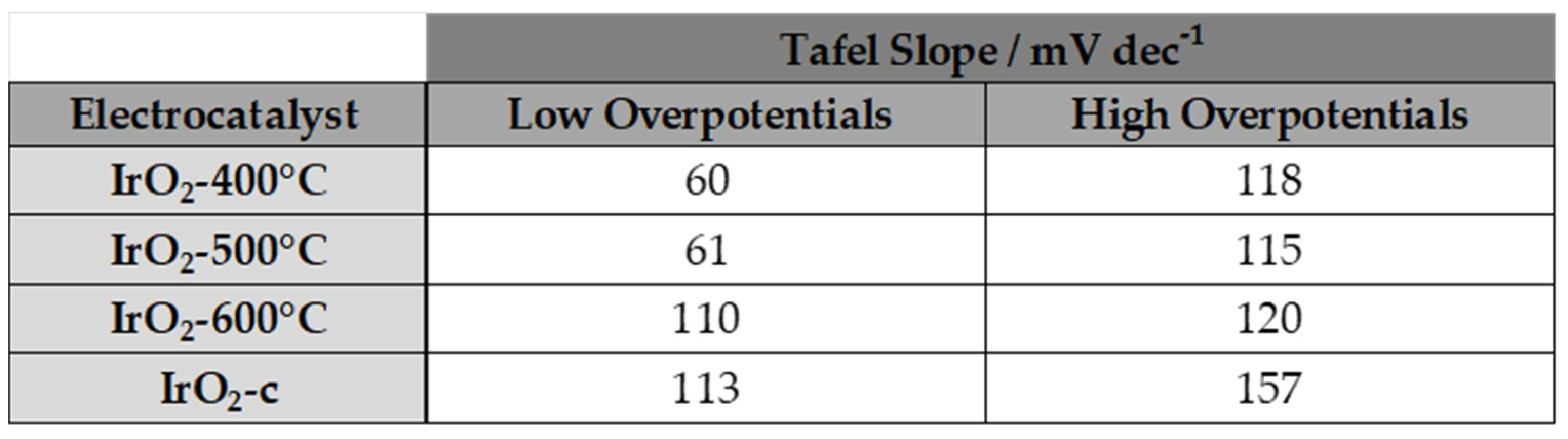 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





