Submitted:
11 July 2023
Posted:
11 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
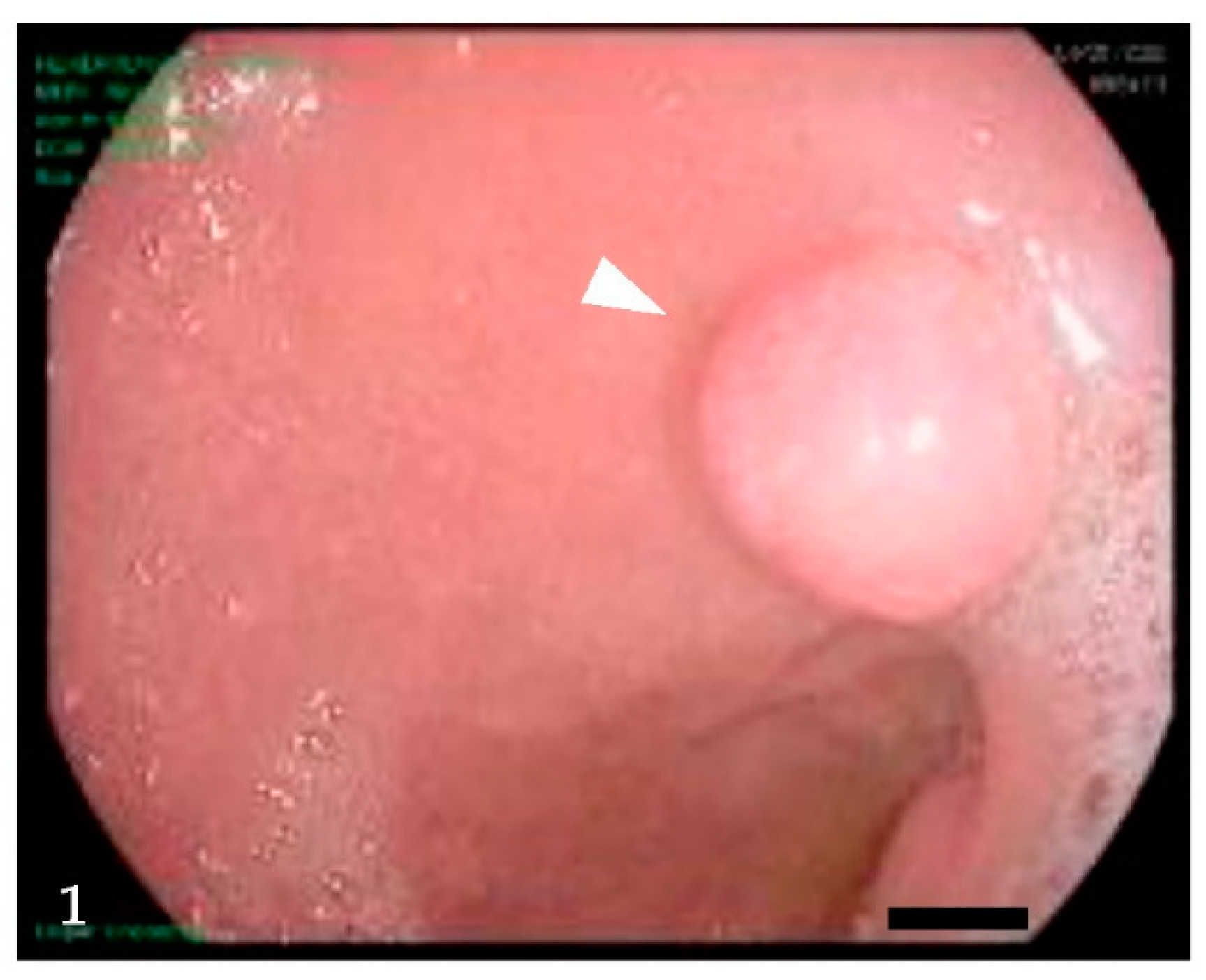
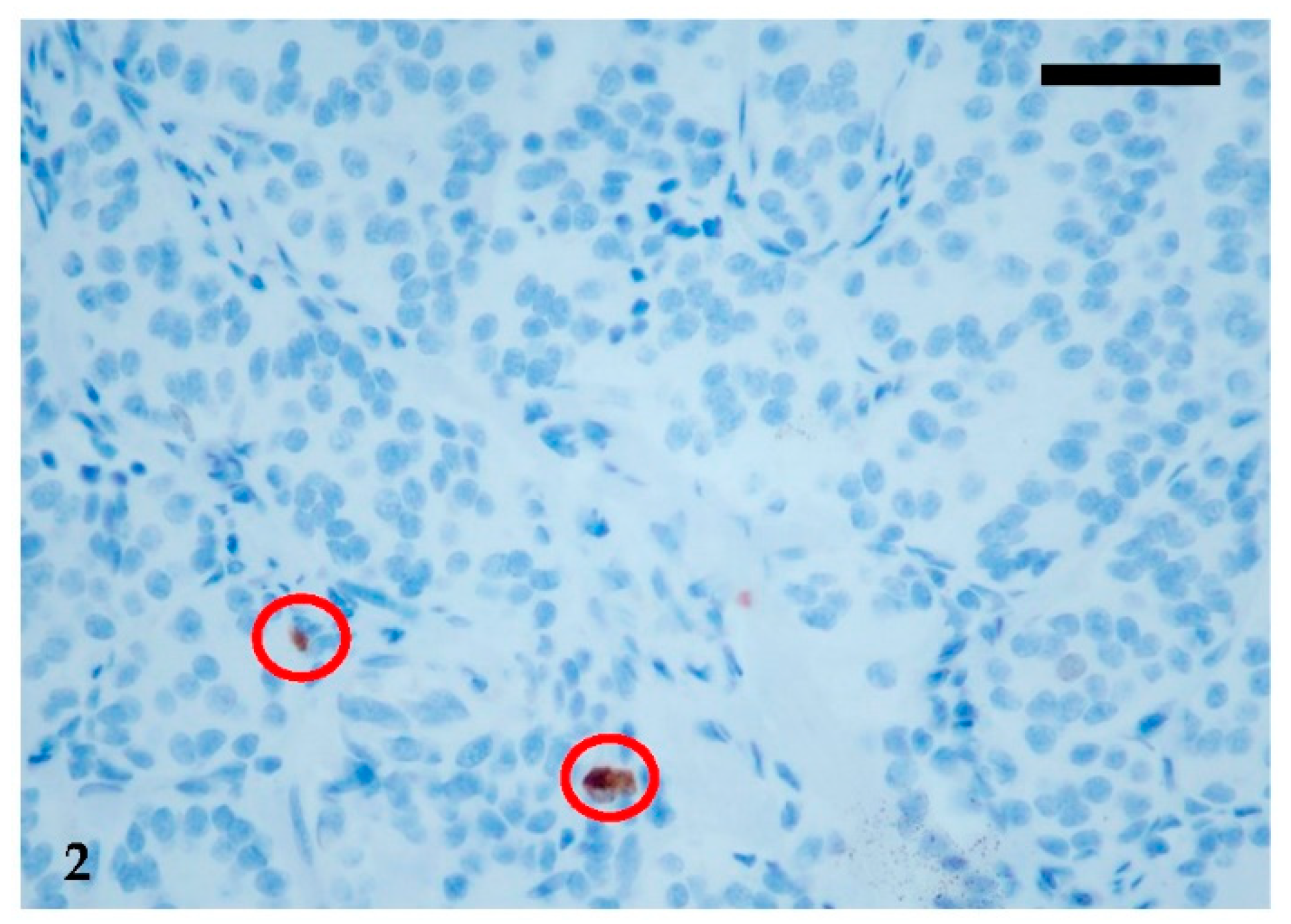
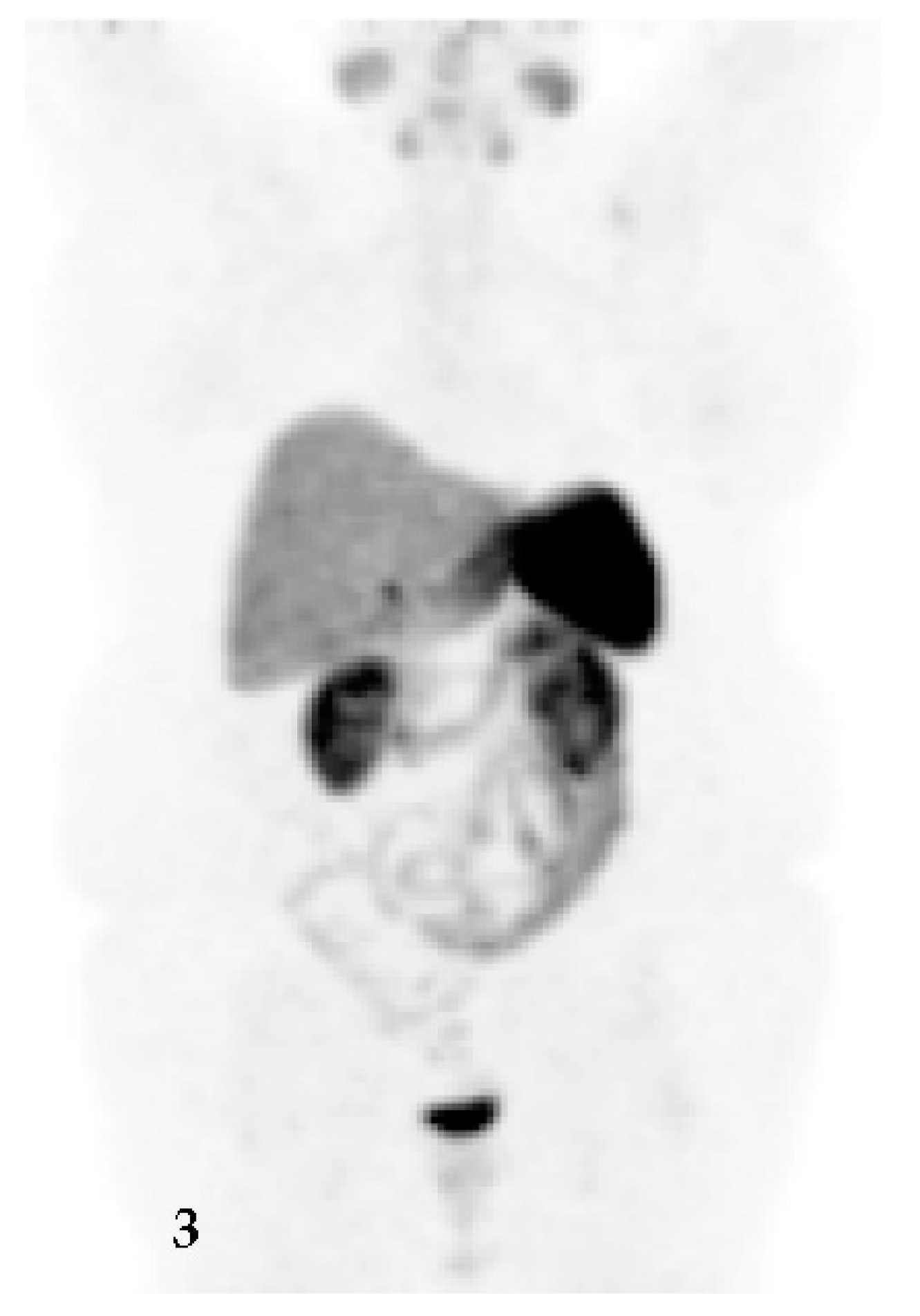
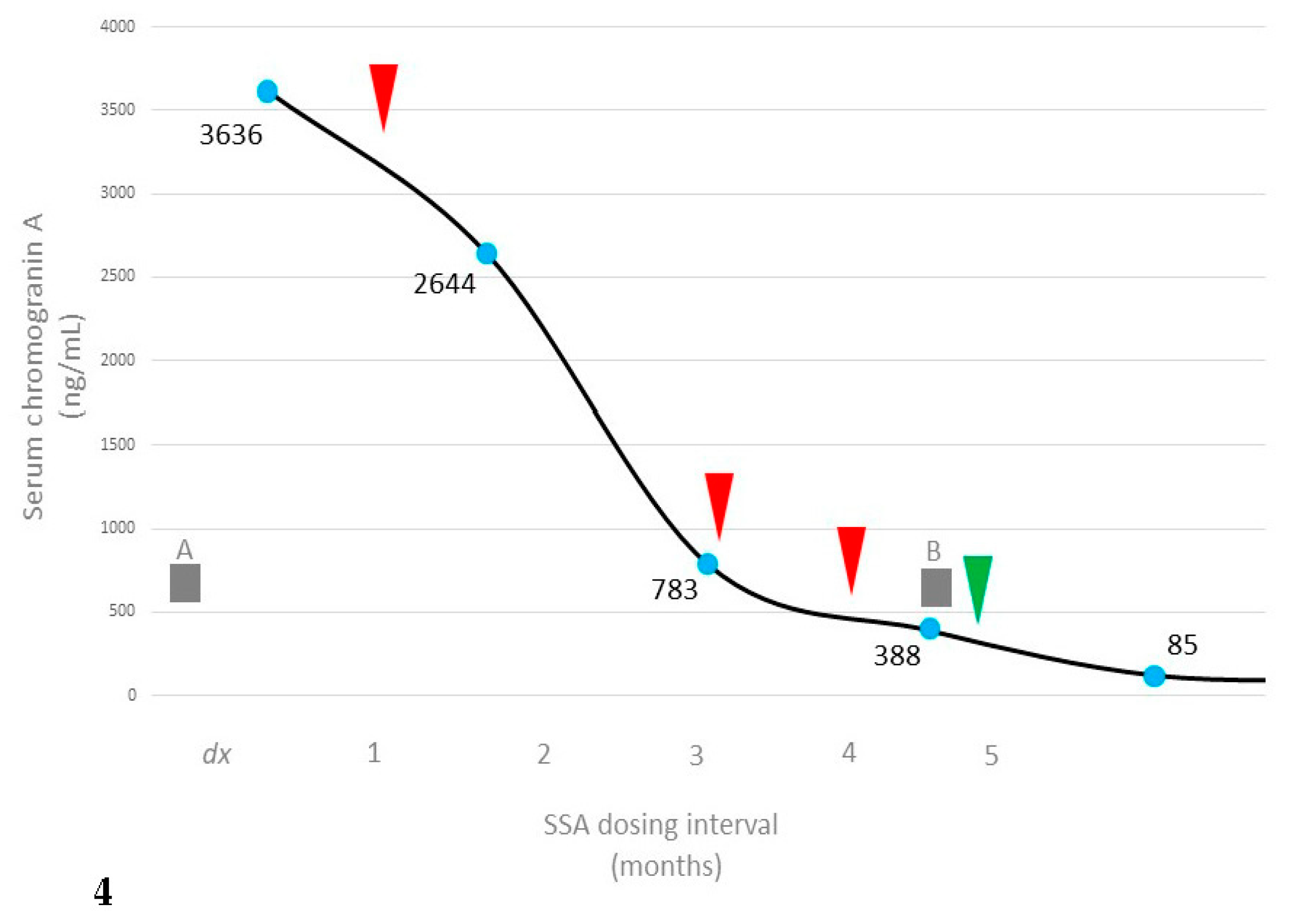
2. Clinical Presentation
3. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Armentano DPD, Monteiro MR, Aguiar PN Jr, Tsukamoto JS, Pio RB, Arakelian R, et al. Laboratory variables as predictors of progression in gastroenteropancreatic neuroendocrine tumors in different lines of antineoplastic treatments. Einstein (Sao Paulo) 2022;20:eAO6985. [CrossRef]
- Zobel EH, Ripa RS, von Scholten BJ, Curovic VR, Diaz LJ, Hansen TW, et al. Effect of liraglutide on vascular inflammation evaluated by [64Cu]DOTATATE. Diagnostics (Basel) 2021;11(8):1431. [CrossRef]
- Aoyama N, Wada M, Taniguchi Y, Inokuma T, Nakanishi Y, Fukuda A, et al. A case of neuroendocrine neoplasm of the minor duodenal papilla. Clin J Gastroenterol 2022;Dec 21. [CrossRef]
- Wang X, Wu Y, Cao X, Zhang X, Cheng Y, Kong L. Duodenal neuroendocrine tumor: A rare case report. Medicine (Baltimore) 2021;100(6):e24635. [CrossRef]
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26(18):3063-72. [CrossRef]
- Khatri W, Spiro E, Henderson A, Rowe SP, Solnes LB (2023). Gastroenteropancreatic tumors: FDG positron emission tomography/computed tomography. PET Clin 2023;S1556-8598(22)00094-3. [CrossRef]
- Paulson S, Ray D, Aranha S, Scales A, Wang Y, Liu E. Lanreotide depot to treat gastroenteropancreatic neuroendocrine tumors in a U.S. community oncology setting: A prospective, observational study. Oncol Ther 2022;10(2):463-79. [CrossRef]
- Gaiani F, de’Angelis N, Minelli R, Kayali S, Carra MC, de’Angelis GL. Pediatric gastroenteropancreatic neuroendocrine tumor: A case report and review of the literature. Medicine (Baltimore) 2019;98(37):e17154. [CrossRef]
- Al-Risi ES, Al-Essry FS, Mula-Abed WS (2017). Chromogranin A as a biochemical marker for neuroendocrine tumors: A single center experience at Royal Hospital, Oman. Oman Med J 2017;32(5):365-70. [CrossRef]
- Qiao XW, Qiu L, Chen YJ, Meng CT, Sun Z, Bai CM, et al. Chromogranin A is a reliable serum diagnostic biomarker for pancreatic neuroendocrine tumors but not for insulinomas. BMC Endocr Disord 2014;14:64. [CrossRef]
- Loft M, Carlsen EA, Johnbeck CB, Johannesen HH, Binderup T, Pfeifer A, et al. 64Cu-DOTATATE PET in patients with neuroendocrine neoplasms: Prospective, head-to-head comparison of imaging at 1 hour and 3 hours after injection. J Nucl Med 2021;62(1):73-80. [CrossRef]
- Delpassand ES, Ranganathan D, Wagh N, Shafie A, Gaber A, Abbasi A, et al. 64Cu-Dotatate PET/CT for imaging patients with known or suspected somatostatin receptor-positive neuroendocrine tumors: Results of the first U.S. prospective, reader-masked clinical trial. J Nucl Med 2020;61(6):890-6. [CrossRef]
- Simonenko VB, Dulin PA, Makanin MA. Somatostatin analogues in treatment of gastrointestinal and pancreatic neuroendocrine tumors. Klin Med (Mosk) 2006;84(4):4-8.
- Kehoe, TE. NDA approval notification [letter to InvaGen Pharmaceuticals]. U.S. FDA Reference ID: 4906945; Silver Spring MD, 17 December 2021:1-5.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



