1. Introduction
Steroid-responsive meningitis-arteritis (SRMA) describes a systemic immune-mediated inflammatory reaction affecting mostly the cervical spinal meninges leading to a suppurative leptomeningitis with severe arteritis [
1,
2,
3]. It typically occurs in young-adult dogs and clinical signs include stiff gait or reluctance to walk, pyrexia, and neck pain worsening during neck flexion or extension in acute cases [
2,
4,
5,
6,
7]. In some cases, SRMA occurs in combination with an immune-mediated polyarthritis [
5,
7,
8]. Typical findings in blood work of dogs with SRMA are leucocytosis with neutrophilia and left-shift, in cerebrospinal fluid (CSF) samples a neutrophilic pleocytosis is visible [
2,
3,
6,
7]. Additionally in dogs with SRMA elevated immunoglobulin A (IgA) levels in serum and CSF are frequently measurable [
1,
2,
9]. Immunosuppressive or anti-inflammatory therapy with prednisolone or with prednisolone in combination with other immunosuppressive drugs often has a positive outcome [
2,
6,
10,
11], but relapses can occur in up to one third of treated dogs with SRMA [
6,
10,
12,
13]. A T-helper cell 2 (Th2) mediated immune response is highly probable in SRMA [
2,
14,
15].
In one of our previous studies it could be shown, that Interleukin-31 (IL-31) levels were elevated in serum samples of dogs with suspected Th 2-helper cell mediated inflammatory response including dogs with secondary inflammation after an intervertebral disc herniation or with otitis media and interna [
16]. Interleukin-31 is a four-helix bundle cytokine from the gp130/IL-6 cytokine family [
17,
18]. It is mainly produced by activated Th2 cells, especially from CD4+ T-cells [
17,
18,
19,
20]. IL-31 develops its effect by binding to a receptor complex consisting of the IL-31 receptor A and the oncostatin M receptor β [
21,
22,
23]. IL-31 has an important role in inducing atopic dermatitis and pruritus in several species [
17,
21,
24,
25,
26,
27]. In addition, its influence on various immune and non-immune cells and regulation of immune responses and haematopoiesis was studied [
18]. IL-31 is involved in developing inflammatory bowel disease, airway hypersensitivity or dermatitis [
18].
As described above, IL-31 is part of the Interleukin-6 (IL-6) cytokine family and expressed during proinflammatory conditions. According to Maiolini et al., Interleukin-6 (IL-6) is elevated in patients with SRMA and was correlated with the degree of CSF pleocytosis [
28]. We hypothesize that IL-31 might be involved in the pathogenesis of SRMA represented by elevated IL-31 levels in serum and/or CSF of dogs with SRMA. The aim of this study is twofold: firstly, to clarify a further part of the pathogenesis of SRMA, and secondly, to enable a new therapeutic approach for a specific treatment against IL-31, provided that our hypothesis is confirmed.
2. Materials and Methods
This study was conducted as a retrospective single center study. The clinic’s biobank was searched for archived serum and CSF samples from dogs with SRMA, meningoencephalitis of unknown origin (MUO), infectious meningoencephalitis, or atopy presented between 2011-2021 at the Department of Small Animal Medicine and Surgery, University of Veterinary Medicine Hannover (Hannover, Germany). All patient samples were obtained as part of routine diagnostic examination and used with written owner’s consent. The samples from healthy beagles were residuals of previous studies performed according to the ethical guidelines of the University of Veterinary Medicine Hannover and approved by the Lower Saxony State Office for Consumer Protection and Food Safety (Lower Saxony, Germany; animal experiment number 33.12-42502-04-20/3352). All samples were stored after sampling at -80°C.
General information about breed, age, sex, onset and duration of clinical signs, previous treatment, results of examinations, and diagnostic tests including general and neurological examination, blood examination, magnetic resonance imaging (MRI) of the brain and/or cervical/thoracic spinal cord if available, CSF examinations, and IgA content in CSF and serum, were taken from the electronic medical record of each individual patient (easyVET, Veterinärmedizinisches Dienstleistungszentrum (VetZ) GmbH, Germany).
The samples of the dogs were grouped according to their underlying diseases into five groups:
Group A: Patients with SRMA (n = 26). The diagnosis of SRMA was based on typical clinical signs of SRMA including neck pain detectable by stiff gait, low head carriage, vocalisation occurring spontaneously or during the neurological examination, anamnestic pyrexia or fever during the clinical examination in combination with blood leucocytosis and a moderate to marked predominantly neutrophilic pleocytosis in the cerebrospinal fluid as described in the current literature [
2,
3,
4,
5,
6,
7].
Group B: Patients with MUO (n = 21). In 14 dogs the presumptive diagnosis of MUO was made on the basis of clinical signs of intracranial lesions in combination with MRI of the brain showing intra-axial, T2-weighted hyperintense and T1-weighted iso- to hypointense lesions with a variable degree of contrast enhancement in T1-weighted sequences [
29,
30,
31] in combination with the result of a CSF examination showing a lymphocytic or mononuclear or mixed pleocytosis with an elevated protein content [
30]. Infectious meningoencephalitis was ruled out by negative testing for frequently occurring pathogens in serum and CSF (n = 14). In seven patients the diagnosis of MUO was histopathologically confirmed and all cases were sub-classified as granulomatous meningoencephalitis (n = 7).
Group C: Patients with infectious meningoencephalitis (n = 3). Two cases revealed bacterial meningoencephalitis (n =2) and one dog a lymphoplasma-histiocytic meningoencephalomyelitis with suspected viral aetiology. The diagnosis was histopathologically confirmed in two cases.
Group D: Patients with atopic dermatitis (n = 3) served as positive control group. Diagnosis of atopic dermatitis was suspected after exclusion of possible other underlying causes for itching like cutaneous infection or ectoparasites, if the dogs showed initial itching without lesions at the age of less than three years, had an indoor lifestyle, and affected areas were feet and concave aspects of the pinnae [
32]. For these patients only serum samples were available and included in this study.
Group E: Healthy control group (n = 11). This group included samples from healthy clinic owned beagles. The general clinical examination was unremarkable in these dogs and all included dogs showed no clinical signs for atopic dermatitis.
Group A patients with SRMA were further divided into four subgroups considering pre-treatment before the sampling was performed. The pre-treatment groups were divided into no prior treatment (group A.1), pre-treatment with non-steroidal anti-inflammatory drugs (NSAIDs) (group A.2), pre-treatment with metamizole (group A.3), and pre-treatment with steroids (group A.4).
The obtained CSF and serum samples were examined for the IL-31 level using a competitive Enzyme-Linked Immunosorbent Assay (ELISA; Canine Interleukin 31 ELISA kit, MyBioSource, Inc., San Diego, USA, catalogue number: MBS740462). The frozen samples were thawed immediately before the assay was performed and brought to room temperature according to the manufacturer’s instruction. Subsequently, the ELISA was carried out according to the enclosed instructions of the manufacturer. The measurement of the optical density of the samples was carried out immediately after the addition of the stop solution from the ELISA kit in a Multi-Detection Microplate Reader (SynergyTM 2, BioTek Instruments, Inc., Winooski, Vermont, USA) at 450nm. Each sample was measured as duplicates and the coefficient of variation was determined.
Only results from samples with a coefficient of variation below 20% were considered for the statistical analysis. The coefficient of variation is used as a marker for the intraassay variation for testing in duplicates of each sample [
33]. If the coefficient of variation-values were higher than 20% for one sample, the measurement was repeated. This was not possible for all samples due to a small sample size. If the coefficient of variation-value was again higher than 20% in the second attempt or if the sample amount was too small for repeated measurements, these samples were excluded from statistical analysis. A standard reference curve was established using six standard solutions with a specified canine IL-31 level of 0 – 1000 pg/ml. If IL-31 values exceeded the upper detection range of 1050 pg/ml, values are given as > 1050 pg/ml and statistical analysis was performed with the fixed value of 1050 pg/ml.
The statistical evaluation was carried out using the GraphPad Prism 9 software (GraphPad Software (part of Dotmatics), San Diego, California, USA). After evaluating descriptive statistics, the examination for normal distribution was carried out using the Shapiro-Wilk test. The results of CSF IL-31 levels were logarithmised to achieve a normal distribution to carry out further statistical tests. Afterwards the Mann Whitney or Kruskal-Wallis test and the unpaired t-test or one-way analysis of variance were used for testing for significance with a significance level p <.05. In paired serum and CSF samples or to detect a correlation between two other parameters the statistical evaluation of correlation was performed with the Spearman correlation.
3. Results
A total of 49 serum and 52 CSF samples from 64 patients with a coefficient of variation below 20% were used for the statistical evaluation. The sample distribution was as follows:
26 patients with SRMA (group A)
21 patients with MUO (group B)
3 patients with infectious meningoencephalitis (group C)
3 patients with atopic dermatitis (group D)
11 patients within the healthy control group (group E)
As described above, IL-31 levels could not be evaluated in all samples due to an inappropriate coefficient of variation. Therefore numbers of samples included in statistical analysis differ from totally evaluated numbers. Beagle was the most frequent represented breed (n = 13/64; including eleven healthy Beagles of group E), followed by Boxer (n = 9/64), mixed breed dogs (n = 8/64), French Bulldogs (n = 4/64), Bernese Mountain Dogs and Yorkshire Terriers (each n = 3/64). Two samples from the following breeds each were included: Golden Retriever, Magyar Vizsla, Labrador Retriever, Pug Dog and Belgian Shepherd Dog. Additionally, one sample from the following breeds were included: Weimaraner, German shorthaired pointer, Nova Scotia Duck Tolling Retriever, German Pinscher, Goldendoodle, Border Collie, Chihuahua, German Shepherd, Podenco Ibicenco, Airedale Terrier, Australian Kelpie, Galgo Español, Husky and Hanoverian Scenthound. In the group of SRMA, Boxers were the most frequently occurring breed (n = 9), followed by Bernese Mountain Dogs (n = 3). 45.3% of the dogs were male (n = 29/64) and 14.1% of the dogs were male-neutered (n = 9/64). In addition 31.2% of the dogs were female (n = 20/64) and 9.4% female-spayed (n = 6/64). Median age of all dogs was 43.2 months (n = 64) with a range from 3 to 120 months.
3.1. Serum samples
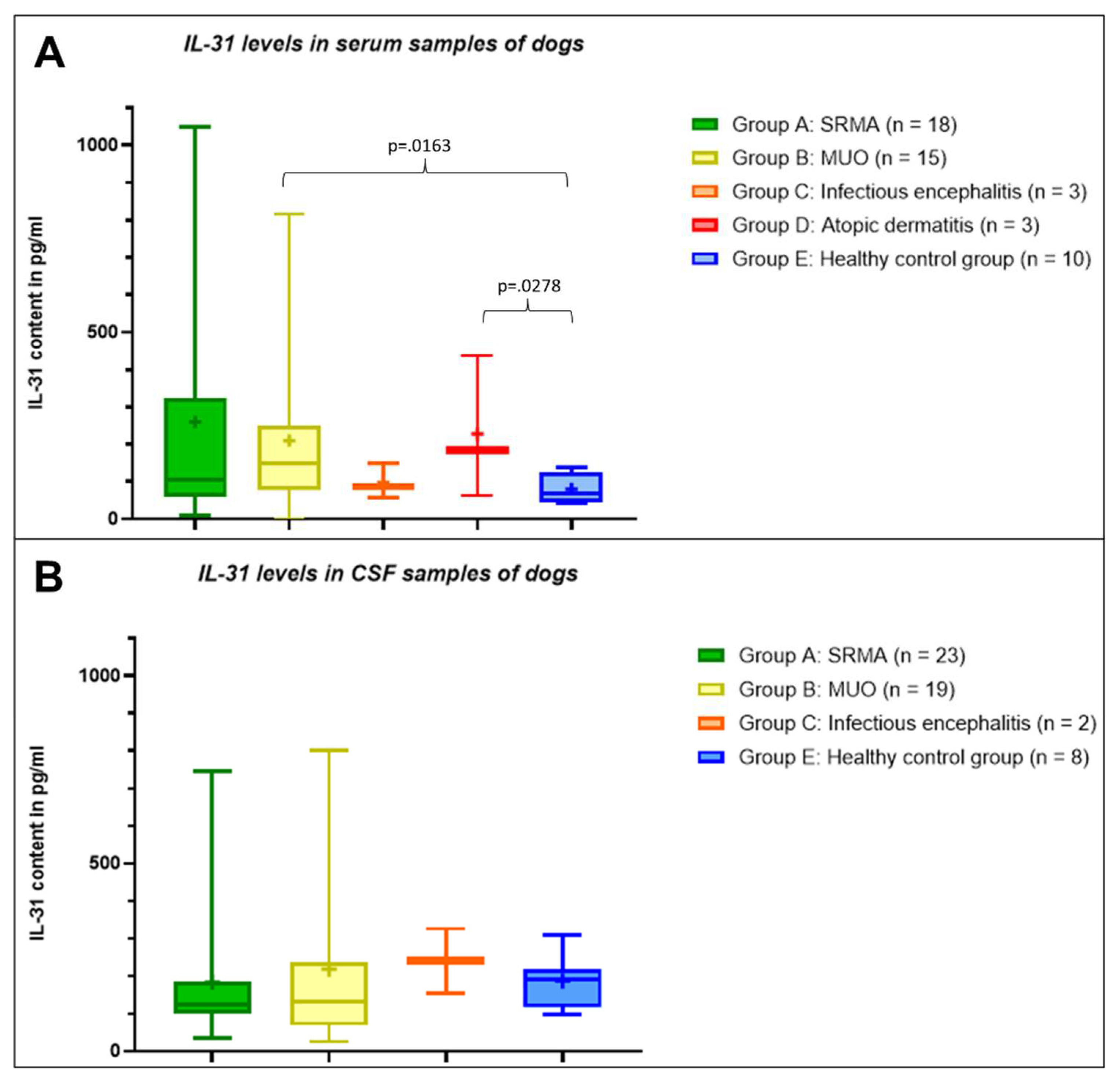
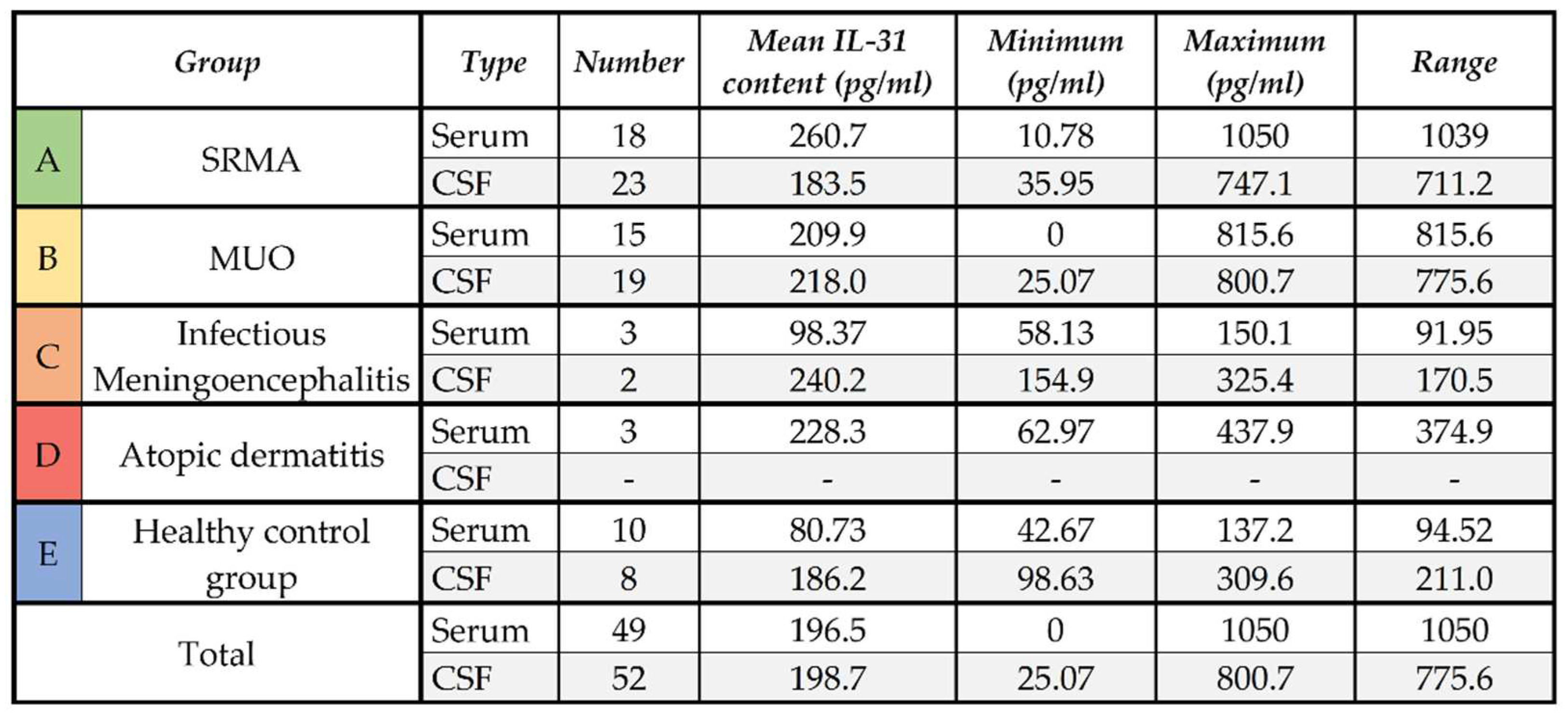
In the serum samples, healthy control dogs had IL-31 levels ≤137.2 pg/ml. The lowest mean IL-31 level was measurable in serum samples from the healthy control group. The IL-31 levels in serum samples of dogs with infectious meningoencephalitis were comparable to the samples from the healthy control group (
Table 1). The highest mean IL-31 content was measurable in samples from patients with SRMA (n = 18,
Table 1) followed by the second highest mean IL-31 level in serum samples of the dogs with atopic dermatitis (n = 3,
Table 1) and patients with MUO (n = 15,
Table 1). The mean IL-31 level in patients with SRMA was markedly higher than in patients with atopic dermatitis, although this difference was not statistically significant (p=.8135), while IL-31 levels in serum of dogs with MUO was significantly different from serum of healthy dogs (p=.0163). Complete descriptive statistics are presented in
Table 1.
IL-31 levels varied widely within the serum samples of dogs with SRMA. Four patients with SRMA had extreme high IL-31 levels above the maximum IL-31 level of dogs with atopic dermatitis (IL-31 level > 437.9 pg/ml) and six dogs with SRMA revealed relative low IL-31 levels in serum below the mean IL-31 level of the healthy control group (IL-31 level < 80.73 pg/ml). The remaining ten results were in between the range between > 80.73 pg/ml and < 437.9 pg/ml. An IL-31 level > 1050 pg/ml was measured in the serum of one patient from group A with SRMA. The exact value of this sample could not be determined as small sample size prevented repeated measurement of the diluted sample. Almost all dogs from the group with high IL-31 values > 437.9 pg/ml in serum did not receive any pre-treatment before sample collection (n = 3/4). Only one dog received pre-treatment with metamizole prior to sampling (n = 1/4). Patients with very high (n = 4) or moderately elevated serum IL-31 levels (n = 8) were predominantly patients with a peracute or acute disease process and duration of clinical signs was three days or less in these dogs (n = 8/12). In addition, SRMA patients with very high IL-31 levels in serum showed increased body temperature (n = 3/4; temperature unknown n = 1/4). In contrast, SRMA patients with low serum IL-31 levels had predominantly shown clinical signs of the disease for five days or longer (n = 5/6). In addition, the majority of dogs with SRMA and low IL-31 levels in serum (n = 6) showed a physiological body temperature or a mildly, subfebrile elevation of the body temperature below 39.3°C at the physical examination prior the sampling.
There was a wide variation of the IL-31 levels within the serum samples of dogs with MUO as well. In two patients with MUO extreme high IL-31 levels above the maximum IL-31 level of dogs with atopic dermatitis (IL-31 level > 437.9 pg/ml) were measurable, while in four dogs with MUO relative low IL-31 levels in serum below the mean IL-31 level of the healthy control group (IL-31 level < 80.73 pg/ml) could be detected. The remaining values of nine dogs were in between the range from > 80.73 pg/ml and < 437.9 pg/ml. Overall, patients with MUO and high IL-31 serum levels showed clinical signs more than nine days. Only two dogs with MUO and a duration of clinical signs more than 9 days revealed low IL-31 levels in serum (0 pg/ml and 62.22 pg/ml). Those dogs were pre-treated with either glucocorticosteroids and NSAIDs or with an unknown pain medication.
3.2. CSF samples
In the CSF, the mean IL-31 level of patients with SRMA (n = 23) and the healthy control group (n = 8) did not differ significantly (p=.4454), whereas the mean IL-31 content in CSF samples of patients with MUO was mildly higher (n = 19;
Table 1). The mean IL-31 level in CSF samples of patients with infectious meningoencephalitis (n = 3) was higher than in the other groups, but due to the low number of CSF samples from dogs with infectious meningoencephalitis, no statistical analysis was performed. No CSF samples of dogs with atopic dermatitis were included in this calculation. The whole results of the IL-31 content in the different groups A to E in serum and CSF are visualized in
Figure 1.
3.3. Testing for correlation
For 35 dogs in total and for 15 dogs with SRMA IL-31 levels of CSF and serum were available from the same sampling time point. IL-31 levels in CSF and serum were not correlated when taking dogs of all groups into consideration (n = 35; r=0.05191, p=0.7349) as well when just looking at dogs with SRMA (n = 15; r=-0.05714, p=0.8425). No correlation was detectable between the IL-31 level in the serum or CSF samples of dogs with SRMA and the amount of leukocytes in CSF (r=.1502, p=.4939). In addition, there was no correlation between the protein content in the CSF samples with the IL-31 level in serum (r=-0.03612, p=0.8869) or CSF of dogs with SRMA (r=0.06991, p=0.7513). IgA levels were available in serum of seventeen dogs with SRMA (n = 17/18) and in CSF for 18 dogs with SRMA (n = 18/23), but there was also no correlation between the IgA levels in serum respectively in CSF and the IL-31 levels in serum (r=0.07108, p=0.7874) respectively CSF (r=0.1889, p=4529).
3.4. Pre-treatment
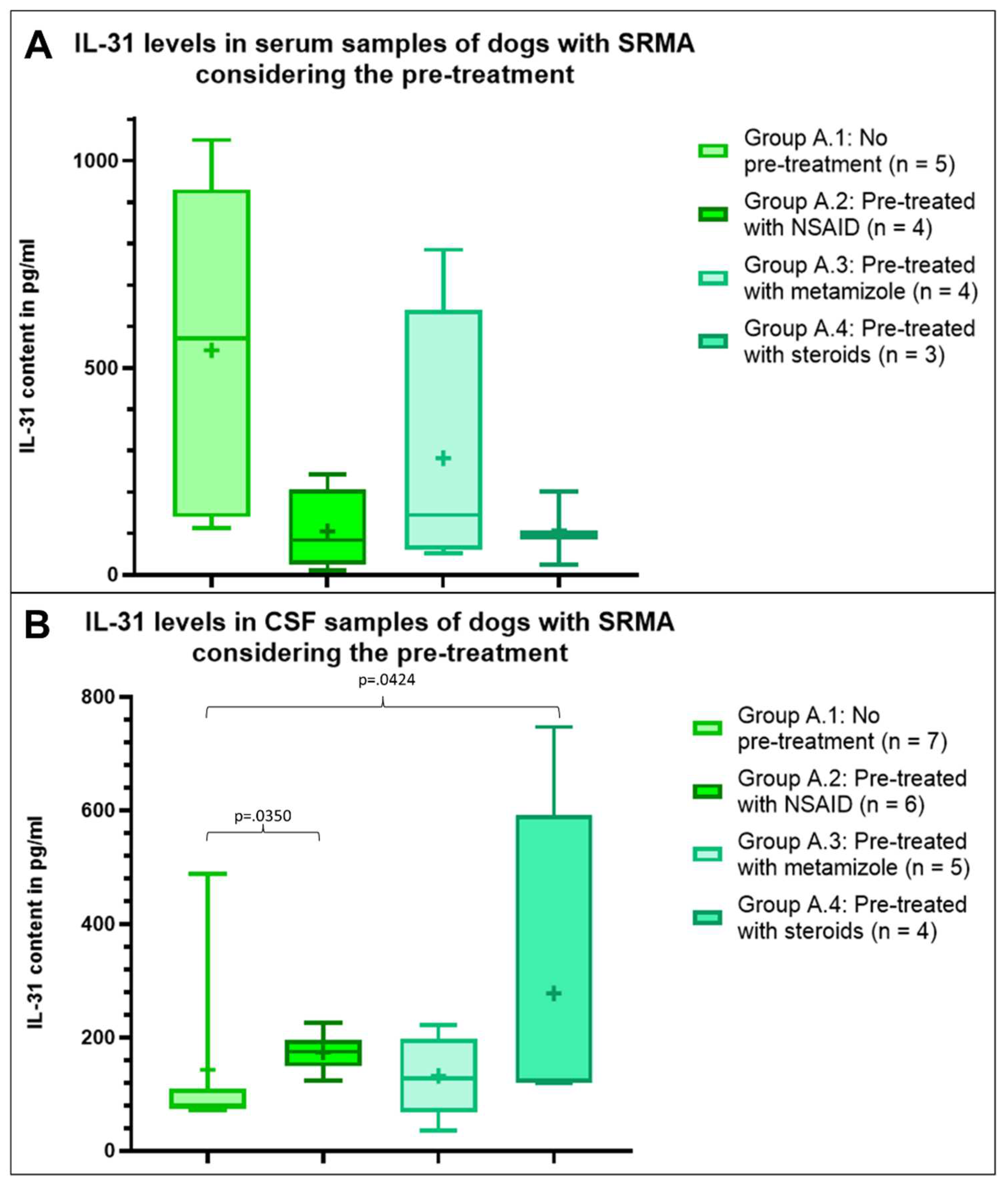
To evaluate, if IL-31 levels in dogs with SRMA (group A) might be influenced by a prior treatment, this group was divided in four subgroups depending on the pre-treatment. Seven dogs with SRMA received no prior treatment (group A.1, n = 7). Seven dogs were pre-treated with NSAID (group A.2, n = 7) including treatment with carprofen (n = 3) or meloxicam (n = 4). No information regarding the dosage was available. Six dogs were pre-treated with metamizole (group A.3, n = 6). Four dogs received steroids as a prior treatment (group A.4, n = 4). Two dogs received prednisolone, one dog an unknown glucocorticosteroid and one dog dexamethasone and prednisolone together. For two dogs the pre-treatment was unknown, so they were excluded from the statistical analysis.
The mean IL-31 content in serum was highest in dogs with SRMA without prior treatment (group A.1, n = 5), followed by dogs with SRMA pre-treated with metamizole (group A.3, n = 4), but the mean IL-31 level was less than half of the mean IL-31 level in the group without treatment (
Figure 2). In the serum of dogs with SRMA pre-treated with NSAIDs (group A. 2, n = 4) and with steroids (group A.4, n =3) the mean IL-31 content was nearly similar, but markedly lower compared to both other groups (
Figure 2). Statistically no significant differences were detectable within the serum samples (p>.05).
Regarding CSF samples the results were more homogenous between the groups and different to serum results. Highest IL-31 levels were measurable in the CSF of dogs with SRMA pre-treated with steroids (group A.4, n = 4), followed by the mean IL-31 level in dogs with SRMA pre-treated with NSAID (group A.2, n = 6) (
Figure 2). Lowest IL-31 levels were detectable in CSF samples of dogs pre-treated with metamizole (group A.3, n = 5) and dogs with SRMA without pre-treatment (group A.1, n = 7,
Figure 2). The IL-31 levels were significant higher in CSF of dogs with SRMA pre-treated with steroids (group A.4, p=.0350) and of dogs with SRMA pre-treated with NSAID (group A.2, p=.0424) compared to dogs without pre-treatment. No further significant differences were detectable within the CSF samples (p>.05).
4. Discussion
The purpose of this study was to investigate the presence of IL-31 in serum and CSF in patients with SRMA and their dependence on a possible pre-treatment. We expected that dogs with SRMA have higher levels of IL-31 in CSF and/or serum due to the Th2-mediated immune response in SRMA [
2,
15] and hypothesized that IL-31 might be involved in the pathogenesis of SRMA as a further component leading to an aberrant immune reaction in SRMA. In SRMA several acute-phase proteins are elevated indicate an inflammatory reaction [
12,
34]. Especially elevation of the C-reactive protein (CRP) can be measured in acute SRMA cases in serum and CSF [
12,
34] and a significant decrease was found after treatment prednisolone in serum and CSF [
34]. In addition, the immunoglobulin A (IgA) levels are elevated in serum and CSF in SRMA [
1,
2,
9,
35], but do not decrease after starting an immunosuppressive therapy or during remission [
2,
6,
9,
10].
IL-31 is a cytokine produced by activated Th2 cells and influences the immune response [
17,
18,
19]. In one of our previous studies it could be shown that IL-31 levels were elevated in serum samples of dogs with suspected Th2 cell mediated inflammatory response including dogs with secondary inflammation due to intervertebral disc herniation or with suspected otitis media and interna [
16].
Indeed, it could be shown that patients with SRMA had increased IL-31 levels in serum. Especially when comparing the IL-31 levels of dogs with SRMA (group A) with the healthy control group (group E), the IL-31 levels were markedly higher in serum of SRMA patients. Within CSF samples the mean IL-31 were quite similar between the groups, so our hypothesis to detect higher IL-31 levels in CSF samples of SRMA cases could not be confirmed with the measured data of this study, but there was an elevation of IL-31 levels in the CSF after steroidal treatment which will be discussed later.
Particularly high levels of IL-31 were observed in serum of SRMA patients with a peracute or acute disease process of three days or less, whereas SRMA patients with low serum IL-31 levels had predominantly shown clinical signs of disease for at least five days. In addition, patients with very high IL-31 levels in serum showed increased body temperature in the clinical examination. Fever can be caused by infectious diseases via exogenous pyrogens or neoplasms, but in nearly half of dogs fever is caused by non-infectious inflammatory diseases [
36,
37]. Interleukin-1 (IL-1) is an endogenous pyrogen, which – in addition to inducing fever – also leads to activation of B and T cells to be able to generate an effective immune response to the causative pathogen [
36]. IL-1 also stimulates the production of IL-6 [
38,
39], which is a pro-inflammatory cytokine activating mechanisms for fever in the central nervous system (CNS) [
36,
39]. In addition, IL-6 also induces the production of acute-phase proteins in the liver like CRP [
36]. As already described, IL-31 is part of the IL-6 cytokine family [
17,
18], which could explain well high IL-31 serum levels in dogs with SRMA and a higher body temperature. But in contrast to IL-6 [
28], IL-31 seems not be involved in the migration of cells into the CSF space, as IL-31 levels did not correlate with the degree of CSF pleocytosis in the current study.
The breed distribution of the group of dogs with SRMA was in line with previous studies describing a higher prevalence in Boxers and Bernese Mountain dogs amongst others [
2]. The typical findings of SRMA in CSF including a neutrophilic pleocytosis was also detectable in most of the dogs with SRMA in this study.
IL-31 levels in CSF varied in dogs with SRMA. Due to the retrospective nature of this study not for all patients the side of CSF sampling was documented. It might be possible, that in some dogs lumbar while in others suboccipital tap was performed. Sampling side can have an impact on results of CSF examination leading to false negative results in up to 7% of dogs with SRMA [
40] and might also have an impact on IL-31 levels.
We included also dogs with MUO in this study. MUO is also an immune-mediated inflammatory condition, but in this disease not only the meninges, but the brain and/or the spinal cord are affected [
29,
30,
31]. While MUO is an umbrella term it includes several histologic subtypes like granulomatous meningoencephalitis (GME) or necrotizing meningoencephalitis [
30]. In the current study, not all patients with MUO had histopathological examination. The ones that had, where classified as GME. Patients with MUO had significantly higher IL-31 levels in serum compared to the healthy control group. Although MUO is a predominantly Th1-driven immune reaction, but expressed pattern of cytokines and chemokines differ between the subtypes of MUO and even between individual patients [
41] and especially in GME increased Th17 cells indicate a contribution of an additional Th2-immune response similar to SRMA [
41,
42,
43]. Therefore our results are in line with the previous literature as higher IL-31 levels were found in the Th2-driven immune response in SRMA. Nevertheless, patients with MUO also had elevated IL-31 levels compared to the healthy control group in this study, but lower levels than the dogs with SRMA.
Three dogs with infectious meningoencephalitis were also included in this study. The IL-31 level in serum was comparable low as in the healthy control group, but in the CSF a higher IL-31 level could be detected. In contrast to SRMA, which is a systemic immune-mediated inflammatory disease [
1,
2,
3], the dogs had a more local infectious inflammatory reaction in the CNS caused by bacterial infection either secondary to ascending otitis or iatrogenic bacterial infection in two out of three dogs. Due to the blood brain barrier, which prevents uncontrolled transfer of cells from the blood into the CNS parenchyma [
44], activated immune cells and their cytokines might not be visible in the peripheral blood in case of an infectious meningoencephalitis. This could explain the low IL-31 levels in serum of dogs with infectious meningoencephalitis, but the elevated IL-31 levels within the CSF of these patients.
In the current study, we also examined the influence of different pre-treatments on the IL-31 levels at the time point of diagnosis of SRMA. The results revealed markedly higher levels of IL-31 in serum of dogs without any prior treatment. Dogs with SRMA pre-treated with NSAID or prednisolone showed markedly lower IL-31 levels in serum than dogs without any prior treatment, but the serum IL-31 levels were still above the mean levels in serum of the healthy control group. Immunosuppressive/anti-inflammatory therapy with prednisolone monotherapy is mostly successful in dogs with SRMA [
2,
6,
10], but sometimes a combination with further immunosuppressive medication is necessary [
11]. Among other mechanisms, prednisolone reduces the release of IL-1 and IL-6, which is one reason for its anti-inflammatory effect [
45]. In addition, a direct suppression of the function of T cells can be assumed [
45]. Dogs pre-treated with prednisolone therefore might have lower IL-31 levels in the serum due to the effects of prednisolone. NSAIDs are anti-inflammatory and analgetic drugs [
46]. They are not useful to treat SRMA in a long-term scheme, but as a pre-treatment they can lead to a suppression of the inflammatory reaction and might therefore lower IL-31 levels. In contrast, Metamizole is an analgetic and anti-pyretic drug, with only limited anti-inflammatory effect [
46]. Therefore it does not suppress IL-31 which might explain the higher IL-31 levels in serum of dogs with SRMA pre-treated with metamizole in comparison to dogs pre-treated with prednisolone or NSAIDs. However, due to the retrospective nature of this study the administration of prednisolone was quite variable prior to the initial sampling of serum and CSF to diagnose SRMA.
Results regarding IL-31 levels in CSF of pre-treated dogs with SRMA were unexpected. In CSF highest IL-31 levels were detectable in dogs with SRMA pre-treated with prednisolone followed by dogs with SRMA pre-treated with NSAIDs, while lowest IL-31 levels were measurable in dogs with SRMA without any pre-treatment. Due to the severe arteritis occurring with SRMA and due to an upregulation of metalloproteinases (MMP), including MMP-2 and -9, a disruption of the blood brain barrier occurs [
2,
47]. In combination with an upregulation of integrins like CD11a in the CSF an invasion of neutrophils into the subarachnoidal space leads to the marked neutrophilic pleocytosis in the CSF [
2,
48]. During this invasion into the subarachnoid space also other blood cells can move into the subarachnoid space and into the CSF such as Th2 cells producing IL-31. After treatment with prednisolone, the integrity of the blood brain barrier is increased, so that the immune cells are trapped within the CSF still producing their interleukins. It is likely that the elevated IL-31 levels in CSF of dogs with SRMA pre-treated with prednisolone represent the intrathecally produced IL-31 from Th2 cells remaining in the CSF after restoration of the blood brain barrier. The same effect but to a smaller part can be detected in dogs pre-treated with NSAIDs, due to the anti-inflammatory effect of this medication [
46].
5. Conclusions
Based on this study, an involvement of IL-31 in the pathogenesis of the Th-2 immune mediated immune response in SRMA and also in MUO can be assumed, especially the systemic changes of the disease and the proinflammatory action of the cytokine can be well explained in SRMA. In the CSF no elevated IL-31 levels in dogs with SRMA or MUO were detectable. Prospective studies examining the influence on maintenance of SRMA or the influence of treatment on the IL-31 levels with standardized treatment protocols might be helpful to clarify the course of IL-31 levels under treatment with steroids and additionally to enable a new therapeutic approach for a specific treatment against IL-31 in dogs with SRMA.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Supplement 1: Overview of the data presented and analyzed in this study shown for each dog.
Author Contributions
Conceptualization, A.T. and J.N.; methodology, R.C.; validation, R.C. and L.L.; formal analysis, L.L.; investigation, L.L and R.C.; resources, K.W., L.L., R.C., A.V. and J.N.; data curation, L.L.; writing—original draft preparation, L.L.; writing—review and editing, A.T., K.W., L.L., R.C., A.V. and J.N.; visualization, L.L.; supervision, A.T. and J.N.; project administration, J.N.; funding acquisition, J.N. All authors have read and agreed to the published version of the manuscript.”.
Funding
The present work was performed at the Department of Small Animal Medicine and Surgery of the University of Veterinary Medicine, Hannover, Germany. This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 491094227 "Open Access Publication Funding" and the University of Veterinary Medicine Hannover, Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The study was conducted according to the ethical guidelines of the University of Veterinary Medicine, Hannover, Germany and the national guidelines. All samples were remains of diagnostic tests obtained and used with owners’ written informed consent or residuals from a granted animal experiment (animal experiment reference number 33.12-42502-04-20/3352), so, with the confirmation of the animal welfare officer of the University of Veterinary Medicine, Hannover, Germany (member of the research ethics commission), the study is therefore not an animal experiment according to guideline 2010/63/EU.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tipold, A.; Vandevelde, M.; Zurbriggen, A. Neuroimmunological studies in steroid-responsive meningitis-arteritis in dogs. Res Vet Sci. 1995, 58(2), 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tipold, A.; Schatzberg, S.J. An update on steroid responsive meningitis-arteritis. J Small Anim Pract. 2010, 51(3), 150–154. [Google Scholar] [CrossRef] [PubMed]
- de Lahunta, A.; Glass, E.; Kent, M. Chapter 10 - Small Animal Spinal Cord Disease. In de Lahunta's Veterinary
Neuroanatomy and Clinical Neurology. 5th ed.; de Lahunta, A., Glass, E., Eds.; W.B. Saunders: Philadelphia,
United States of America, 2021; pp. 267-311. [CrossRef]
- Tipold, A.; Jaggy, A. Steroid responsive meningitis-arteritis in dogs: Long-term study of 32 cases. J Small Anim Pract. 1994, 35, 311–316. [Google Scholar] [CrossRef]
- Webb, A.A.; Taylor, S.M.; Muir, G.D. Steroid-responsive meningitis-arteritis in dogs with noninfectious, nonerosive, idiopathic, immune-mediated polyarthritis. J Vet Intern Med. 2002, 16(3), 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, M.; Penderis, J.; McLaughlin, M.; Eckersall, P.D.; Anderson, T.J. Steroid responsive meningitis-arteritis: a prospective study of potential disease markers, prednisolone treatment, and long-term outcome in 20 dogs (2006-2008). J Vet Intern Med. 2009a, 23(4), 862–870. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Nettifee, J.A.; Early, P.J.; Mariani, C.L.; Olby, N.J.; Muñana, K.R. Clinical characteristics, breed differences, and quality of life in North American dogs with acute steroid-responsive meningitis-arteritis. J Vet Intern Med. 2019, 33(4), 1719–1727. [Google Scholar] [CrossRef]
- Rose, J.H.; Harcourt-Brown, T.R. Screening diagnostics to identify triggers in 21 cases of steroid-responsive meningitis-arteritis. J Small Anim Pract. 2013, 54(11), 575–578. [Google Scholar] [CrossRef]
- Maiolini, A.; Carlson, R.; Schwartz, M.; Gandini, G.; Tipold, A. Determination of immunoglobulin A concentrations in the serum and cerebrospinal fluid of dogs: an estimation of its diagnostic value in canine steroid-responsive meningitis-arteritis. Vet J. 2012, 191(2), 219–224. [Google Scholar] [CrossRef]
- Cizinauskas, S.; Jaggy, A.; Tipold, A. Long-term treatment of dogs with steroid-responsive meningitis-arteritis: clinical, laboratory and therapeutic results. J Small Anim Pract. 2000, 41(7), 295–301. [Google Scholar] [CrossRef]
- Giraud, L.; Girod, M.; Cauzinille, L. Combination of Prednisolone and Azathioprine for Steroid-Responsive Meningitis-Arteritis Treatment in Dogs. J Am Anim Hosp Assoc. 2021, 57(1), 1–7. [Google Scholar] [CrossRef]
- Bathen-Noethen, A.; Carlson, R.; Menzel, D.; Mischke, R.; Tipold, A. Concentrations of acute-phase proteins in dogs with steroid responsive meningitis-arteritis. J Vet Intern Med. 2008, 22(5), 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, E.; Tipold, A.; Flegel, T. Relapses in dogs with steroid-responsive meningitis-arteritis. J Small Anim Pract. 2016, 57(2), 91–95. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Moore, P.F.; Tipold, A. Disproportionally strong increase of B cells in inflammatory cerebrospinal fluid of dogs with Steroid-responsive Meningitis-Arteritis. Vet Immunol Immunopathol. 2008, 125(3-4), 274-283. [CrossRef]
- Schwartz, M.; Puff, C.; Stein, V.M.; Baumgärtner, W.; Tipold, A. Pathogenetic factors for excessive IgA production: Th2-dominated immune response in canine steroid-responsive meningitis-arteritis. Vet J. 2011, 187(2), 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lemke, L.; Carlson, R.; Flegel, T.; Volk, A.; Volk, H.A.; Tipold, A.; Nessler, J. Interleukin-31 in serum and cerebrospinal fluid of dogs with syringomyelia. BMC Vet Res. 2023. submitted. [Google Scholar]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; Bort, S.; Mudri, S.; Kuijper, J.L.; Bukowski, T.; Shea, P.; Dong, D.L.; Dasovich, M.; Grant, F.J.; Lockwood, L.; Levin, S.D.; LeCiel, C.; Waggie, K.; Day, H.; Topouzis, S.; Kramer, J.; Kuestner, R.; Chen, Z.; Foster, D.; Parrish-Novak, J.; Gross, J.A. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004, 5(7), 752-760. [published correction appears in Nat Immunol. 2005, 6(1), 114]. [CrossRef]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19(5-6), 347-356. [CrossRef]
- Marsella, R.; Ahrens, K.; Sanford, R. Investigation of the correlation of serum IL-31 with severity of dermatitis in an experimental model of canine atopic dermatitis using beagle dogs. Vet Dermatol. 2018, 29(1), 69–e28. [Google Scholar] [CrossRef] [PubMed]
- McCandless, E.E.; Rugg ,C.A.; Fici, G.J.; Messamore, J.E.; Aleo, MM, Gonzales AJ. Allergen-induced production of IL-31 by canine Th2 cells and identification of immune, skin, and neuronal target cells. Vet Immunol Immunopathol. 2014, 157(1-2), 42-48. [CrossRef]
- Gonzales, A.J.; Humphrey, W.R.; Messamore, J.E.; Fleck, T.J.; Fici, G.J.; Shelly, J.A.; Teel, J.F.; Bammert, G.F.; Dunham, S.A.; Fuller, T.E.; McCall, R.B. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013, 24(1), 48-53.e11-2. [CrossRef]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy. 2018, 73(1), 29–36. [Google Scholar] [CrossRef]
- Nakashima, C.; Otsuka, A.; Kabashima, K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp Dermatol. 2018, 27(4), 327–331. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; Steinhoff, M.; Hoffmann, T.K.; Ruzicka, T.; Zlotnik, A.; Homey, B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006, 117(2), 411–417. [Google Scholar] [CrossRef]
- Gonzales, A.J.; Fleck, T.J.; Humphrey, W.R.; Galvan, B.A.; Aleo, M.M.; Mahabir, S.P.; Tena, J.K.; Greenwood, K.G.; McCall, R.B. IL-31-induced pruritus in dogs: a novel experimental model to evaluate anti-pruritic effects of canine therapeutics. Vet Dermatol. 2016, 27(1), 34–e10. [Google Scholar] [CrossRef]
- Lewis, K.E.; Holdren, M.S.; Maurer, M.F.; Underwood, S.; Meengs, B.; Julien, S.H.; Byrnes-Blake, K.A.; Freeman, J.A.; Bukowski, T.R.; Wolf, A.C.; Hamacher, N.B.; Rixon, M.W.; Dillon, S.R. Interleukin (IL) 31 induces in cynomolgus monkeys a rapid and intense itch response that can be inhibited by an IL-31 neutralizing antibody. J Eur Acad Dermatol Venereol. 2017, 31(1), 142–150. [Google Scholar] [CrossRef]
- Oyama, S.; Kitamura, H.; Kuramochi, T.; Higuchi, Y.; Matsushita, H.; Suzuki, T.; Goto, M.; Adachi, H.; Kasutani, K.; Sakamoto, A.; Iwayanagi, Y.; Kaneko, A.; Nanami, M.; Fujii, E.; Esaki, K.; Takashima, Y.; Shimaoka, S.; Hattori, K.; Kawabe, Y. Cynomolgus monkey model of interleukin-31-induced scratching depicts blockade of human interleukin-31 receptor A by a humanized monoclonal antibody. Exp Dermatol. 2018, 27(1), 14–21. [Google Scholar] [CrossRef] [PubMed]
- Maiolini, A.; Otten, M.; Hewicker-Trautwein, M.; Carlson, R.; Tipold, A. Interleukin-6, vascular endothelial growth factor and transforming growth factor beta 1 in canine steroid responsive meningitis-arteritis. BMC Vet Res. 2013, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, G.B.; Platt, S.R.; Anderson, T.J.; Rusbridge, C.; Lorenzo, V.; Mantis, P.; Cappello, R. Characteristics of magnetic resonance images of granulomatous meningoencephalomyelitis in 11 dogs. Vet Rec. 2006, 159(4), 110–115. [Google Scholar] [CrossRef] [PubMed]
- Granger, N.; Smith, P.M.; Jeffery, N.D. Clinical findings and treatment of non-infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Vet J. 2010, 184(3), 290–297. [Google Scholar] [CrossRef] [PubMed]
- Paušová, T.K.; Tomek, A.; Šrenk, P.; Belašková, S. Clinical Presentation, Diagnostic Findings, and Long-term Survival Time in 182 Dogs With Meningoencephalitis of Unknown Origin From Central Europe That Were Administered Glucocorticosteroid Monotherapy. Top Companion Anim Med. 2021, 44: 100539. [CrossRef]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. 2019, 254(11), 1291–1300. [Google Scholar] [CrossRef]
- Kelley, M.; DeSilva, B. Key elements of bioanalytical method validation for macromolecules. AAPS J. 2007, 9(2), E156–E163. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, M.; Penderis, J.; Eckersall, P.D.; McLaughlin, M.; Mellor, D.; Anderson, T.J. The role of acute phase proteins in diagnosis and management of steroid-responsive meningitis arteritis in dogs. Vet J. 2009b, 182(1), 125–130. [Google Scholar] [CrossRef]
- Felsburg, P.J.; HogenEsch, H.; Somberg, R.L.; Snyder, P.W.; Glickman, L.T. Immunologic abnormalities in canine juvenile polyarteritis syndrome: a naturally occurring animal model of Kawasaki disease. Clin Immunol Immunopathol. 1992, 65(2), 110–118. [Google Scholar] [CrossRef]
- El-Radhi, A.S. Pathogenesis of Fever. In Clinical Manual of Fever in Children., 2nd ed.; El-Radhi, A.S., Carroll, J., Klein, N., Eds.; Springer: Berlin, Heidelberg, Germany, 2009; pp. 53–68. [Google Scholar] [CrossRef]
- Chervier, C.; Chabanne, L.; Godde, M.; Rodriguez-Piñeiro, M.I.; Deputte, B.L.; Cadoré, J.L. Causes, diagnostic signs, and the utility of investigations of fever in dogs: 50 cases. Can Vet J. 2012, 53(5), 525–530. [Google Scholar]
- Sironi, M.; Breviario, F.; Proserpio, P.; Biondi, A.; Vecchi, A.; Van Damme, J.; Dejana, E.; Mantovani, A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989, 142(2), 549–553. [Google Scholar] [CrossRef]
- Cartmell, T.; Poole, S.; Turnbull, A.V.; Rothwell, N.J.; Luheshi, G.N. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000, 526 Pt 3(Pt 3), 653–661. [Google Scholar] [CrossRef]
- Carletti, B.E.; De Decker, S.; Rose, J.; Sanchez-Masian, D.; Bersan, E.; Cooper, C.; Szladovits, B.; Walmsley, G.; Gonçalves, R. Evaluation of concurrent analysis of cerebrospinal fluid samples collected from the cerebellomedullary cistern and lumbar subarachnoid space for the diagnosis of steroid-responsive meningitis arteritis in dogs. J Am Vet Med Assoc. 2019, 255(9), 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Uchida, K.; Nakayama, H. Th1-, Th2-, and Th17-related cytokine and chemokine receptor mRNA and protein expression in the brain tissues, T cells, and macrophages of dogs with necrotizing and granulomatous meningoencephalitis. Vet Pathol. 2013, 50(6), 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Freundt-Revilla, J.; Maiolini, A.; Carlson, R.; Beyerbach, M.; Rentmeister, K.; Flegel, T.; Fischer, A.; Tipold, A. Th17-skewed immune response and cluster of differentiation 40 ligand expression in canine steroid-responsive meningitis-arteritis, a large animal model for neutrophilic meningitis. J Neuroinflammation. 2017, 14(1), 20. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.; Barber, J. Differential T-cell responses in dogs with meningoencephalomyelitis of unknown origin compared to healthy controls. Front Vet Sci. 2022, 9:, 925770. [CrossRef]
- de Lahunta, A.; Glass, E.; Kent, M. Chapter 4 - Cerebrospinal Fluid and Hydrocephalus. In de Lahunta's Veterinary Neuroanatomy and Clinical Neurology., 5th ed.; de Lahunta, A., Glass, E., Eds.; W.B. Saunders: Philadelphia, United States of America, 2021; pp. 79–105. [Google Scholar] [CrossRef]
- Whitley, N.T.; Day, M.J. Immunomodulatory drugs and their application to the management of canine immune-mediated disease J Small Anim Pract. 2011, 52(2), 70-85. [published correction appears in J Small Anim Pract. 2011, 52(12), 670]. [CrossRef]
- Schug, S.A.; Manopas, A. Update on the role of non-opioids for postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007, 21(1), 15–30. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Puff, C.; Stein, V.M.; Baumgärtner, W.; Tipold, A. Marked MMP-2 transcriptional up-regulation in mononuclear leukocytes invading the subarachnoidal space in aseptic suppurative steroid-responsive meningitis-arteritis in dogs. Vet Immunol Immunopathol. 2010, 133(2-4), 198-206. [CrossRef]
- Schwartz, M.; Carlson, R.; Tipold, A. Selective CD11a upregulation on neutrophils in the acute phase of steroid-responsive meningitis-arteritis in dogs. Vet Immunol Immunopathol. 2008, 126(3-4), 248-255. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).