Case presentation:
A two-month-old Saudi boy a known case of Osteopetrosis.(The carrier status of the CA2 variant is confirmed by genetic study), a product of Full term, spontaneous vaginal delivery, no neonatal intensive care admission, birth weight was 2.5 kg, current weight 2.5 kg.
Presented to the emergency department with a history of abdominal distention and frequent progressive vomiting, projectile and non-projectile, milk content non-bilious for the last three weeks
His bowel habit is constipation, but he started to have a one-day history of diarrhoea.
Voiding was normal. No fever
Family history:
-History of pyloric stenosis in three of his elder brother, operated on in their first month of life; both were diagnosed with Osteopetrosis with a genetic study on follow-up.
-Consanguineous parents
-Variant of Osteopetrosis initially identified in sister.
-Three of his cousin had an Osteopetrosis diagnosis confirmed by a generic study
On examination:
No dysmorphic features, Looks fare dehydrated
Vital signs were normal
Abdomen: Mild Distended, soft, lax, no tenderness, no hepatosplenomegaly or masses
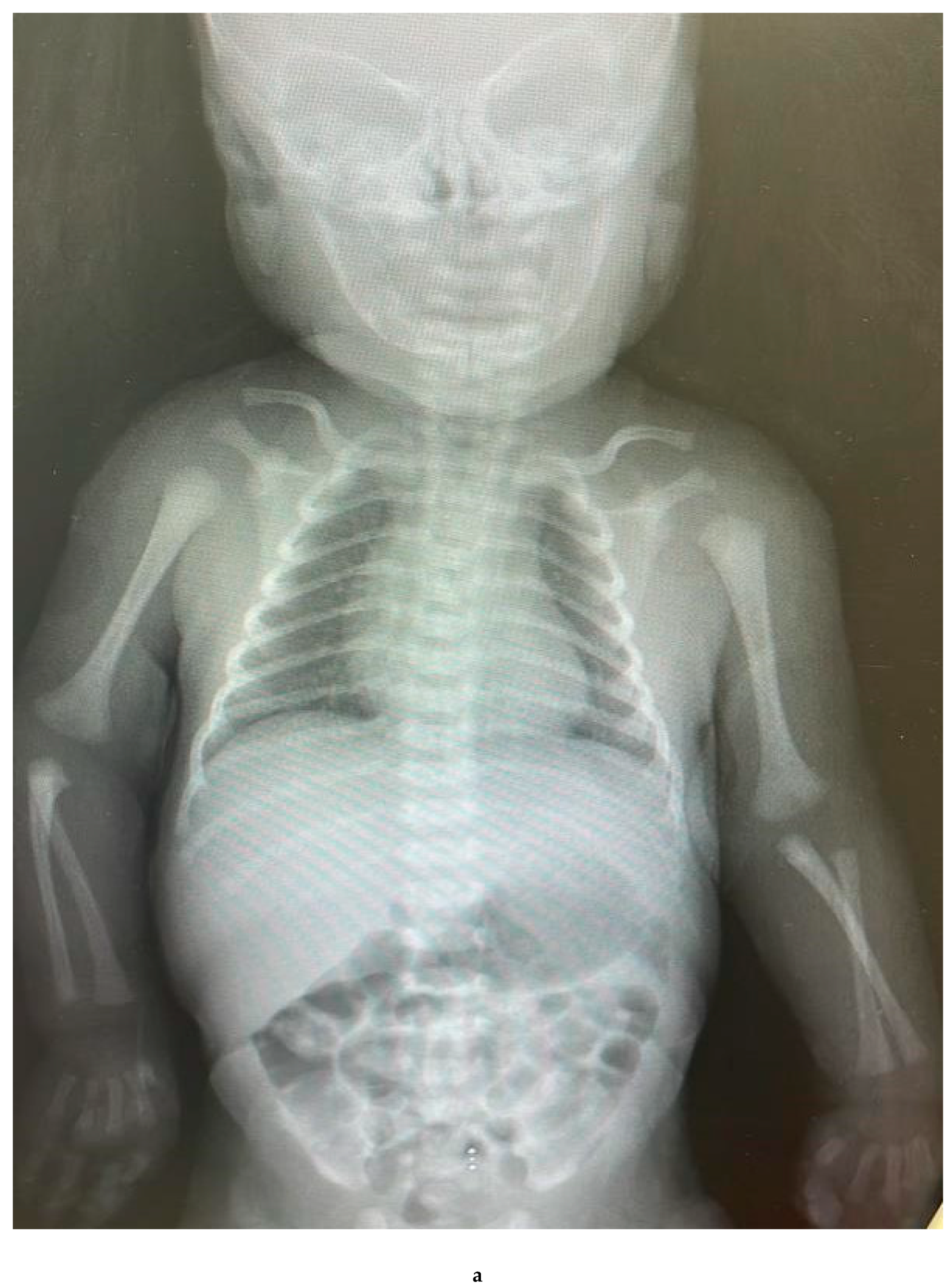
Cauterization marks in the abdominal wall.
Systemic examination was normal.
Investigations:
Complete Blood Count: Normal
C-Reactive Protein: 1.3 mg/L
Renal function: normal
Electrolytes: Na 136 mmol/L, K 3.9 mmol/L, CL 95 mmol/L, Mg 0.9 mmol/L
Liver function: ALT 62 U/L, AST 81 U/L, GGT 33 U/L, Alkaline phosphatase 158 U/L, Bilirubin Total
19 umol/L
Venous Blood Gas: PH 7.64, PCO 41, PO 50, HCO 29.4
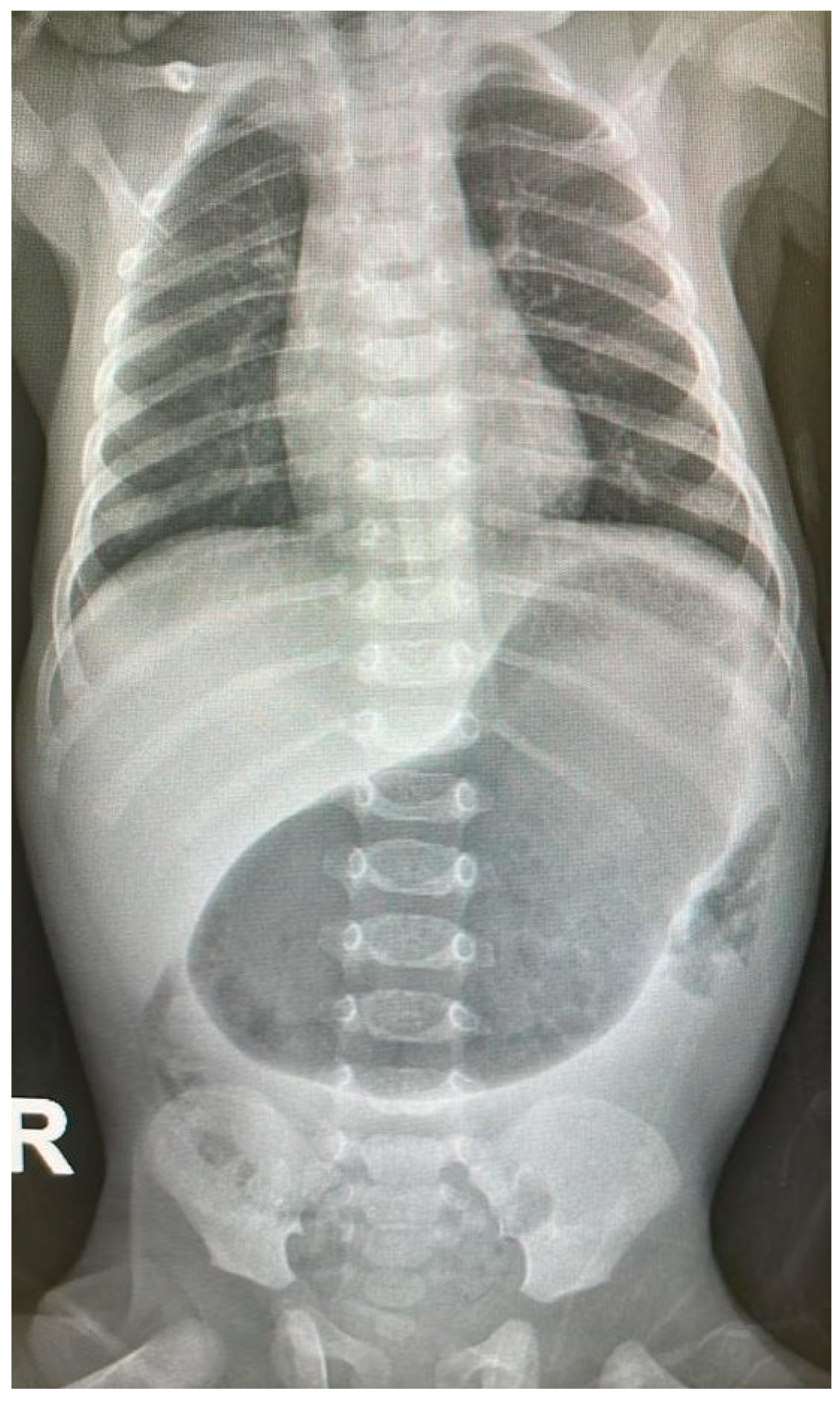
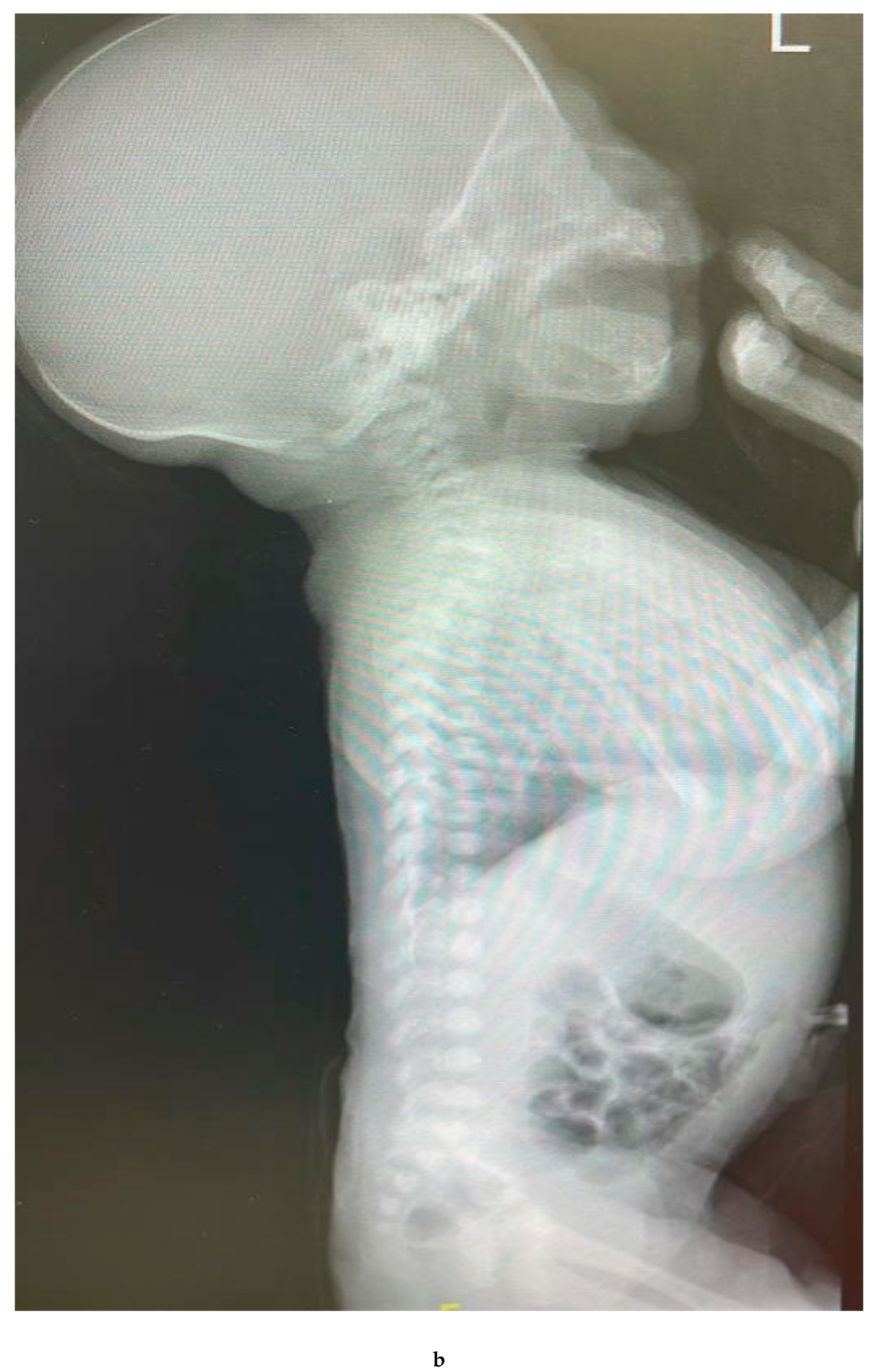
ABDOMINAL X-RAY [
Figure 1]: Marked distended stomach with air.
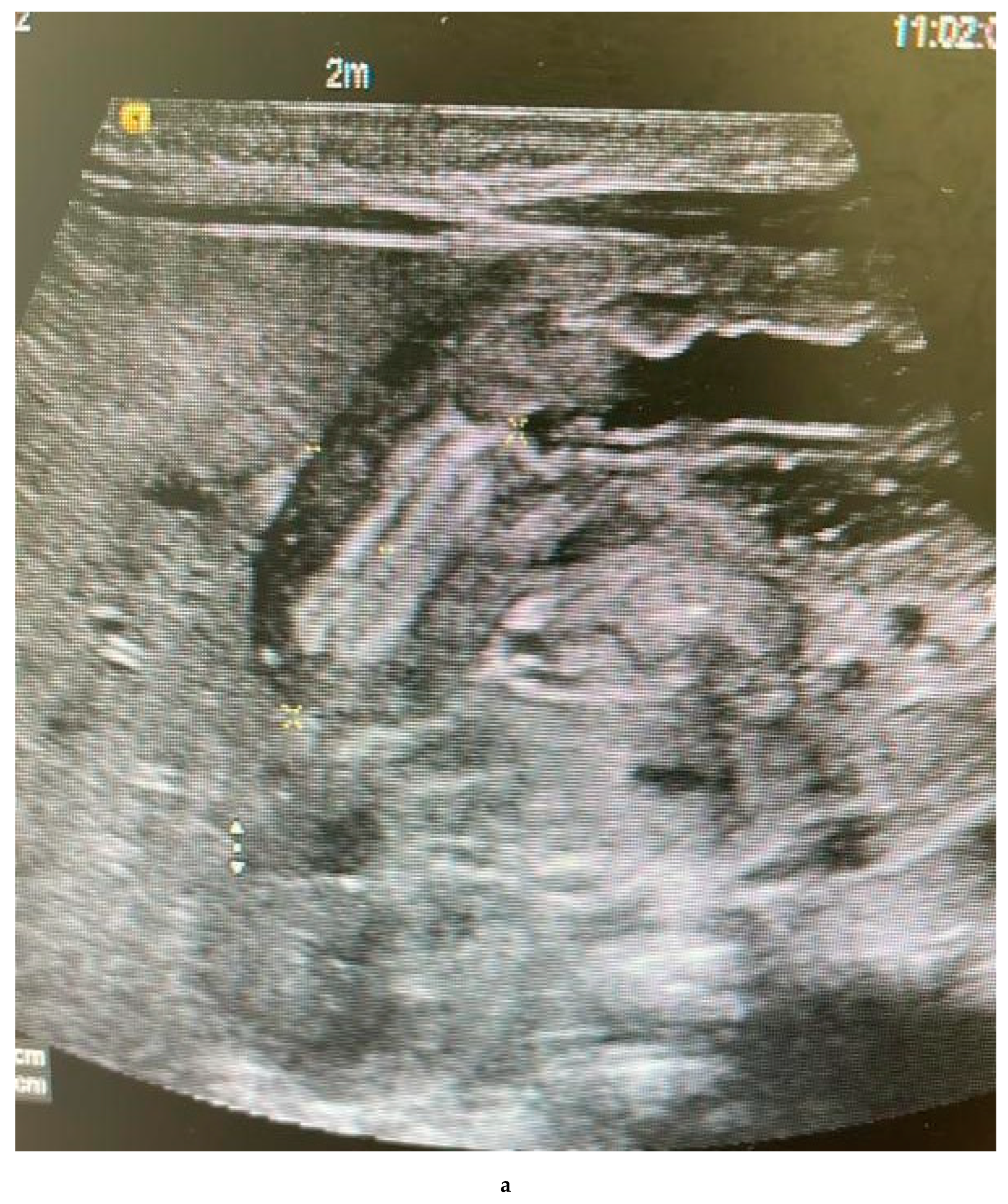
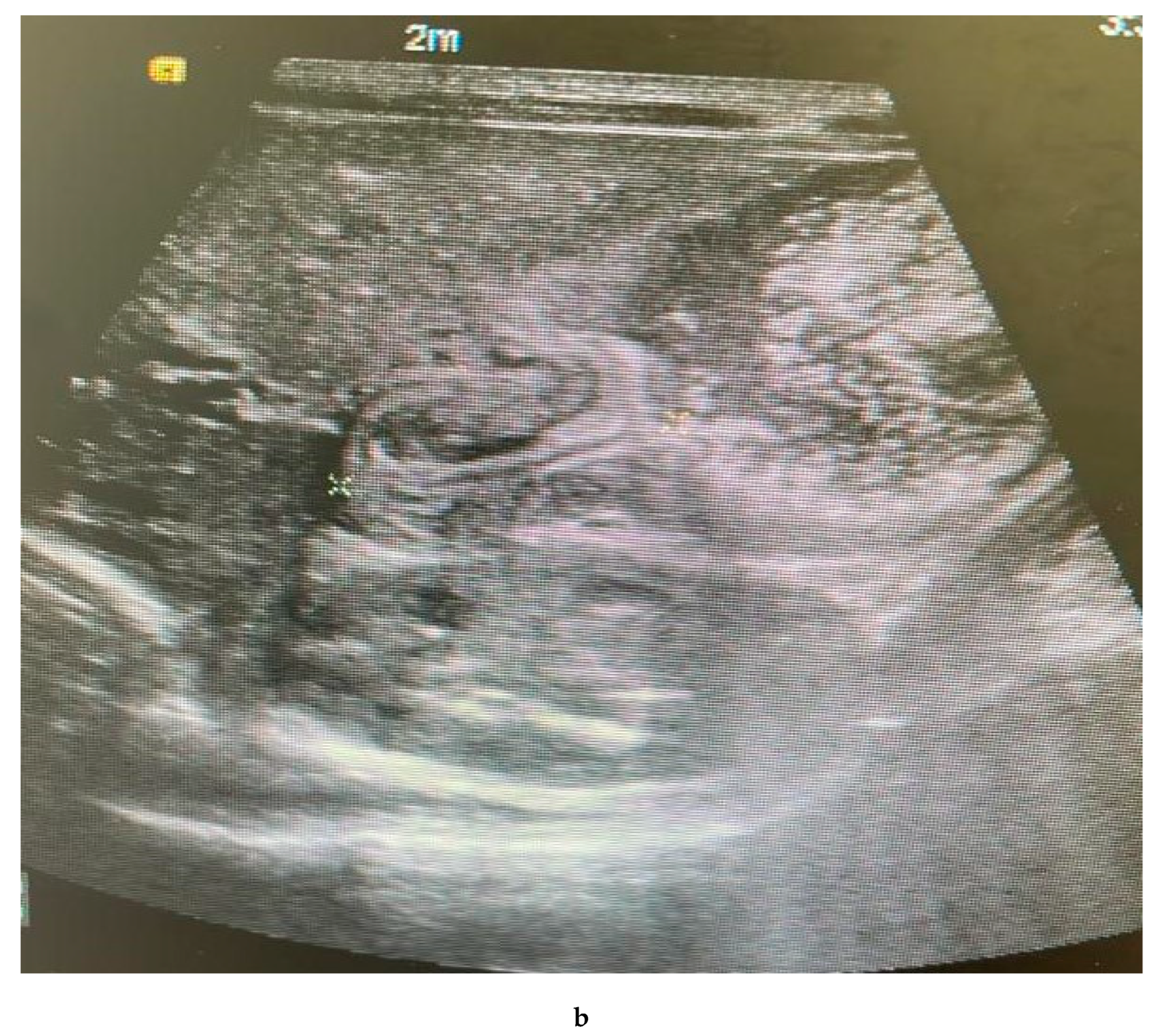
Findings:
The pylorus demonstrates a thickened wall measuring approximately 0.7 cm, with an elongated channel measuring about 2.5 cm. Features suggestive of pyloric stenosis
Innumerable tiny diffusely scattered echogenic foci are seen at the periphery of the liver and within the portal vein. Findings are suggestive of portal vein gas. The portal and hepatic veins are patents.
No intrahepatic biliary dilatation.
The Common Bile Duct is not dilated, measuring 0.1 cm.
The spleen measures approximately 4.8 cm, with no focal lesions.
Impression:
1) Findings are in keeping with hypertrophic pyloric stenosis.
2) Scattered echogenic foci seen in the periphery of the liver and within the portal vein; concerning portal venous gas, follow-up with an abdominal radiograph and surgical consultation is recommended.
GENETIC STUDY: The targeted familial pathogenic variant was identified in the CA2 gene in the heterozygous state. Biallelic pathogenic variants in this gene are related to autosomal recessive Osteopetrosis. The carrier status of the CA2 variant is confirmed.
Introduction
Osteopetrosis incidence is less than 1:200,000 birth in most populations (Worth, 1963). It's more common in consanguineous people as it's unusual in two members of the same family (Van Hul, 2001).Osteopewasosis is derived from the Greek 'osteo', meaning bone and 'petros', stone. Osteopetrosis was described in 1904 by a German radiologist, also known as 'marble bone disease' and 'Albers-Schönberg disease'. (Sajjan, 2020).
There are four types of Osteopetrosis:
i) autosomalrecessive infantile 'malignant' osteopetrosis, ii) autosomalrecessive 'mild' osteopetrosis iii) autosomal dominant osteopetrosis and iv) osteopetrosis due to carbonic anhydrase deficiency. (Othman, 2009)
Osteopetrosis results from the impaired function of osteoclasts (Albers-Schonberg, 1904). The osteoclast function disorder results in clinical variants for the disease in humans: infantile, intermediate, and adult (Shapiro, 1993).
The defined treatment for Osteopetrosis is Hematopoietic stem cell transplantation (HSCT). With a 73% five years disease-free survival in donors. (Driessen, 2003).
Interferon-gamma 1b (IFNg1b) treatment was used in those who didn't respond to HSCT; IFNg1 b leads to improved immune function, increased bone resorption, and increased bone marrow space. (Key Jr, 1992). Medication advised to be used in Osteopetrosis include Vitamin-D supplements and corticosteroids-stimulate bone resorption. While some may be asymptomatic, many of these patients require orthopaedic surgery for fractures at some point in their lives. (Das, 2022).
The incidence of Hypertrophic pyloric stenosis is 1 in 300–900 newborns. Hypertrophic pyloric stenosis is due to hypertrophy of the smooth muscle of the pyloric sphincter. The classic age of occurrence is the first few months of life, and the classic presentation is non-bilious projectile vomiting after feeding. (Sabiston, 2001).
The theory behind pyloric stenosis is the pyloric sphincter swelling from hypertrophy, and the growth-enhancing effect of high gastrin levels aids in repeated sphincter contraction. There is no evidence that pyloric stenosis results from an abnormal collection of growth factors within the sphincter or any genetic connection other than the association with Blood group O. (Rogers, 2023)
The diagnosis of hypertrophic pyloric stenosis (PS) in infants mainly depends on the clinical picture as the patient is the firstborn male child with a family history of Pyloric Stenosis, and presenting with projectile vomiting at four weeks of age, with the pathognomonic finding a palpable "olive" mass in the upper abdomen. An upper-Gastrointestinal series or US if the diagnosis is unclear. (Olson, 1998).
Discussion
In 1972, an association between Osteopetrosis and renal tubular acidosis (RTA) was first described, so it was called Guibaud– Vainsel syndrome or marble brain disease. (Guibaud, 1972), (Vainsel, 1972)
Three Saudi Arabian families were reported by Ohlsson et al. with first-cousin marriages who were born with Osteopetrosis (Renal Tubular Acidosis)RTA and cerebral calcification, and the name 'marble brain disease' was given. (Ohlsson, 1980) In 1988, Al Rajeh et al. reported two sisters in Saudi Arabia who had a marble brain disease. (Al Rajeh, 1988) Marble brain disease was reported in Turkey in 2001 in a family. (Ocal, 2001).
In 1943, it was suggested that a single recessive gene, with penetrance dependent on sex and birth order, was responsible for pyloric stenosis. (Cockayne, 1943) later many authors (Lalouel, 1977), (Mitchell LE, 1993) concluded that no single gene was found, and the gene expressed required some environmental component for their expression (Ismail, 2020).
Other than the male sex, the most commonly reported risk factors for pyloric stenosis are a family history of pyloric stenosis and the firstborn child. (MacMahon, 2006), (Schechter, 1997), (Velaoras, 2005), (RASMUSSEN, 1989), (Jedd, 1988).
Many studies (Peeters, 2012) believed multifactorial factors are the leading causes of pyloric stenosis. Some reported other risk factors for pyloric stenosis
include; prematurity, caesarean delivery, and maternal smoking (Svenningsson, 2014), a hereditary factor involved in the prevalence of pyloric stenosis was documented (Krogh, 2010).
In Denmark, between 1977-2008, they reported maternal smoking, male sex, premature birth, small for gestational age (SGA) newborns, caesarean delivery, and firstborn children were the main risk factors for pyloric stenosis (Krogh C. G., 2012). The same study in Denmark supported the presence of decisive genetic factors in the pathophysiology of pyloric stenosis, with a high incidence rate in monozygotic (200-fold higher rate) and dizygotic twins (20-fold) (Krogh C. F., 2010).
The possible increased risk of pyloric stenosis among cousins of pyloric stenosis cases has been conflicting (Carter, 1969), (McKeown, 1951) with a statistically significant 3-fold increased risk in cousins of both sexes. Krogh's study found a 60% increased risk in half-cousins; although this increase was not significant, it suggests aggregation in even distant relatives. (Krogh C. F., 2010).
Conclusion
Osteopetrosis had variable Clinical manifestations, ranging from asymptomatic to fatal course. To our knowledge, the association of Osteopetrosis with pyloric stenosis was not reported.
A familial cascade carrier testing and Genetic counselling is recommended for the extended family.
References
- Al Rajeh, S.E. The syndrome of osteopetrosis, renal acidosis and cerebral calcification in two sisters. Neuropediatrics 1988, 19, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Albers-Schonberg, H. Rntgenbilder einer seltenen Knochenkrankung. Munchen Med Wochenschr 1904, 51, 365. [Google Scholar]
- Carter, C.O. Inheritance of congenital pyloric stenosis. Journal of Medical Genetics 1969, 6, 233. [Google Scholar] [CrossRef]
- Cockayne, E.A. The genetics of congenital pyloric stenosis. THE OHIO JOURNAL OF SCIENCE 1943, XLIII, 1–16. [Google Scholar]
- Das, N.S. Osteopetrosis: A rare case. Annals of Medical Science & Research 2022, 1, 93–96. [Google Scholar]
- Driessen, G.J. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone marrow transplantation 2003, 32, 657–663. [Google Scholar] [CrossRef]
- Guibaud, P.L. Osteopetrosis and renal tubular acidosis. 2 cases of this association in a sibship. Archives francaises de pediatrie 1972, 29, 269–286. [Google Scholar]
- Ismail, I.E. Laparoscopic vs. open pyloromyotomy in treatment of infantile hypertrophic pyloric stenosis. Frontiers in Pediatrics 2020, 8, 426. [Google Scholar]
- Jedd, M.B. Factors associated with infantile hypertrophic pyloric stenosis. American Journal of Diseases of Children 1988, 142, 334–337. [Google Scholar] [CrossRef]
- Key Jr, L.L. Recombinant human interferon gamma therapy for osteopetrosis. The Journal of pediatrics 1992, 121, 119–124. [Google Scholar]
- Krogh, C.F. Familial aggregation and heritability of pyloric stenosis. Jama 2010, 303, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Krogh, C.G. Pre-and perinatal risk factors for pyloric stenosis and their influence on the male predominance. American journal of epidemiology 2012, 176, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lalouel, J.M. Recurrence risks in complex inheritance with special regard to pyloric stenosis. Journal of Medical Genetics 1977, 14, 408–414. [Google Scholar] [CrossRef]
- MacMahon, B. The continuing enigma of pyloric stenosis of infancy a review. Epidemiology 2006, 195–201. [Google Scholar] [CrossRef]
- McKeown, T.M. The incidence of congenital pyloric stenosis related to birth rank and maternal age. Annals of Eugenics 1951, 16, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Mitchell LE, R.N. he Genetics of Infantile Hypertrophic Pyloric Stenosis: A Reanalysis. Am J Dis Child 1993, 147, 1203–1211. [Google Scholar] [CrossRef]
- Ocal, G.B. Osteopetrosis, renal tubular acidosis without urinary concentration abnormality, cerebral calcification and severe mental retardation in three Turkish brothers. Journal of Pediatric Endocrinology and Metabolism 2001, 14, 1671–1678. [Google Scholar] [CrossRef]
- Ohlsson, A.S. Marble brain disease: recessive osteopetrosis, renal tubular acidosis and cerebral calcification in three Saudi Arabian families. Developmental Medicine & Child Neurology 1980, 1, 72–84. [Google Scholar]
- Olson, A.D. The role of ultrasonography in the diagnosis of pyloric stenosis: a decision analysis. Journal of pediatric surgery 1998, 33, 676–681. [Google Scholar] [CrossRef]
- Othman, I.S. Mild autosomal recessive osteopetrosis: successful treatment with bone marrow transplant. The Medical journal of Malaysia 2009, 64, 325–326. [Google Scholar]
- Peeters, B.B. Infantile hypertrophic pyloric stenosis—genetics and syndromes. Nature Reviews Gastroenterology & Hepatology 2012, 9, 646–660. [Google Scholar]
- RASMUSSEN, L.G. The epidemiology of infantile hypertrophic pyloric stenosis in a Danish population. nternational journal of epidemiology, 1989, 18, 413–417. [Google Scholar]
- Rogers, I.M. Pyloric Stenosis of Infancy (PS): Discovering the Cause. Journal of Pediatric Surgery 2023, S0022–3468. [Google Scholar] [CrossRef] [PubMed]
- Sabiston, D.C. Sabiston textbook of surgery: the biological basis of modern surgical practice. Philadelphia: Wb Saunders.
- Sajjan 2001, P.G. Infantile Osteopetrosis-A Case Series. Medica 2020, 9, 119. [Google Scholar]
- Schechter, R.T. The epidemiology of infantile hypertrophic pyloric stenosis. Paediatric and perinatal epidemiology 1997, 11, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F. Osteopetrosis. Current clinical considerations. Clinical orthopaedics and related research 1993, 294, 34–44. [Google Scholar] [CrossRef]
- Svenningsson, A.S. Maternal and pregnancy characteristics and risk of infantile hypertrophic pyloric stenosis. Journal of pediatric surgery 2014, 49, 1226–1231. [Google Scholar] [CrossRef]
- Vainsel, M.F. Osteopetrosis associated with proximal and distal tubular acidosis. Acta Paediatrica 1972, 61, 429–434. [Google Scholar] [CrossRef]
- Van Hul, W.V. Molecular and radiological diagnosis of sclerosing bone dysplasias. European journal of radiology 2001, 40, 198–207. [Google Scholar] [CrossRef]
- Velaoras, K.B. Hypertrophic pyloric stenosis in twins: same genes or same environments? . Pediatric Surgery International 2005, 21, 669–671. [Google Scholar] [CrossRef]
- Worth, H.M. Principles and practice of oral radiologic interpretation. Year Book Medical Publishers. 1963.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).