Submitted:
05 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
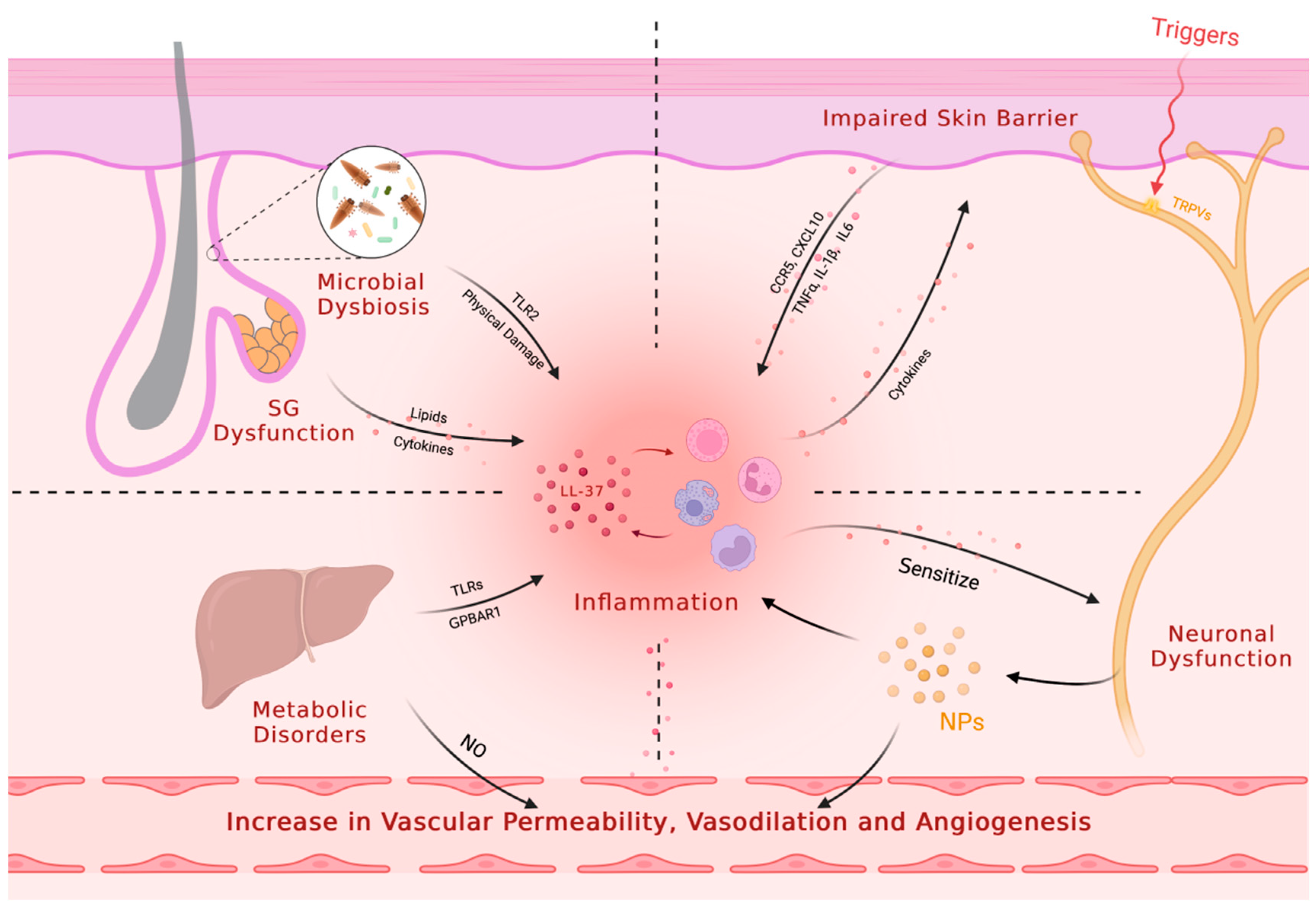
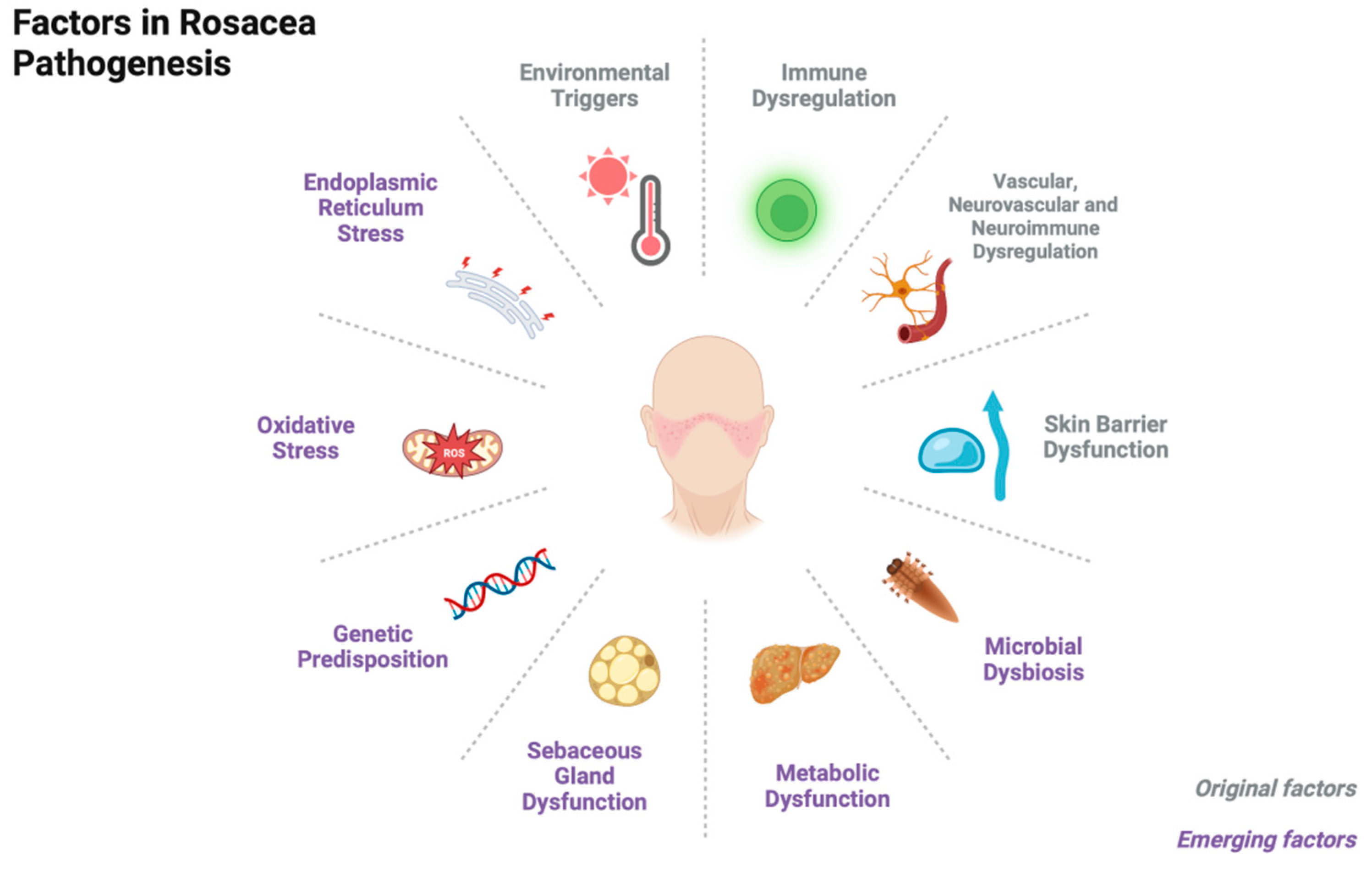
2. Pathogenesis
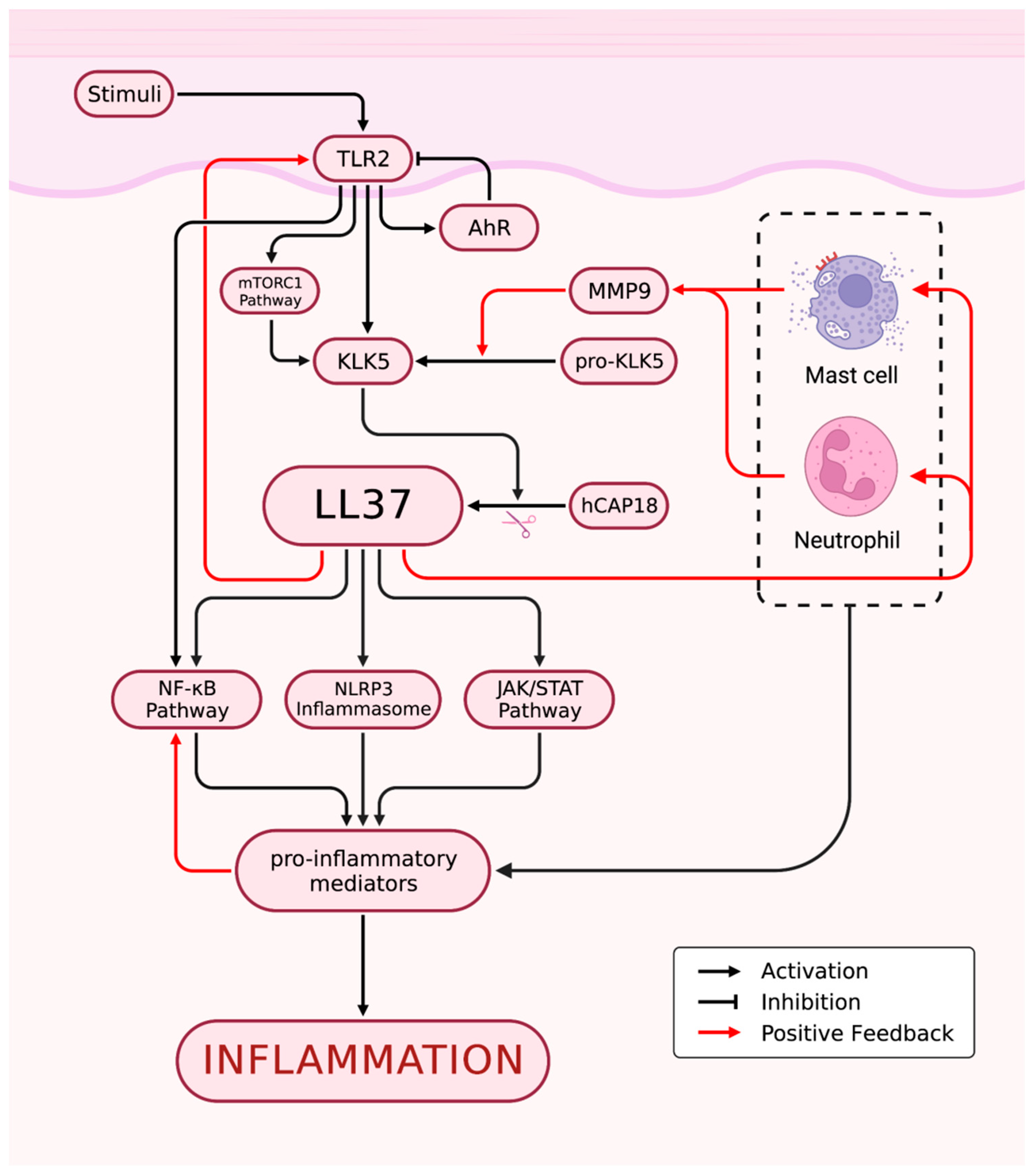
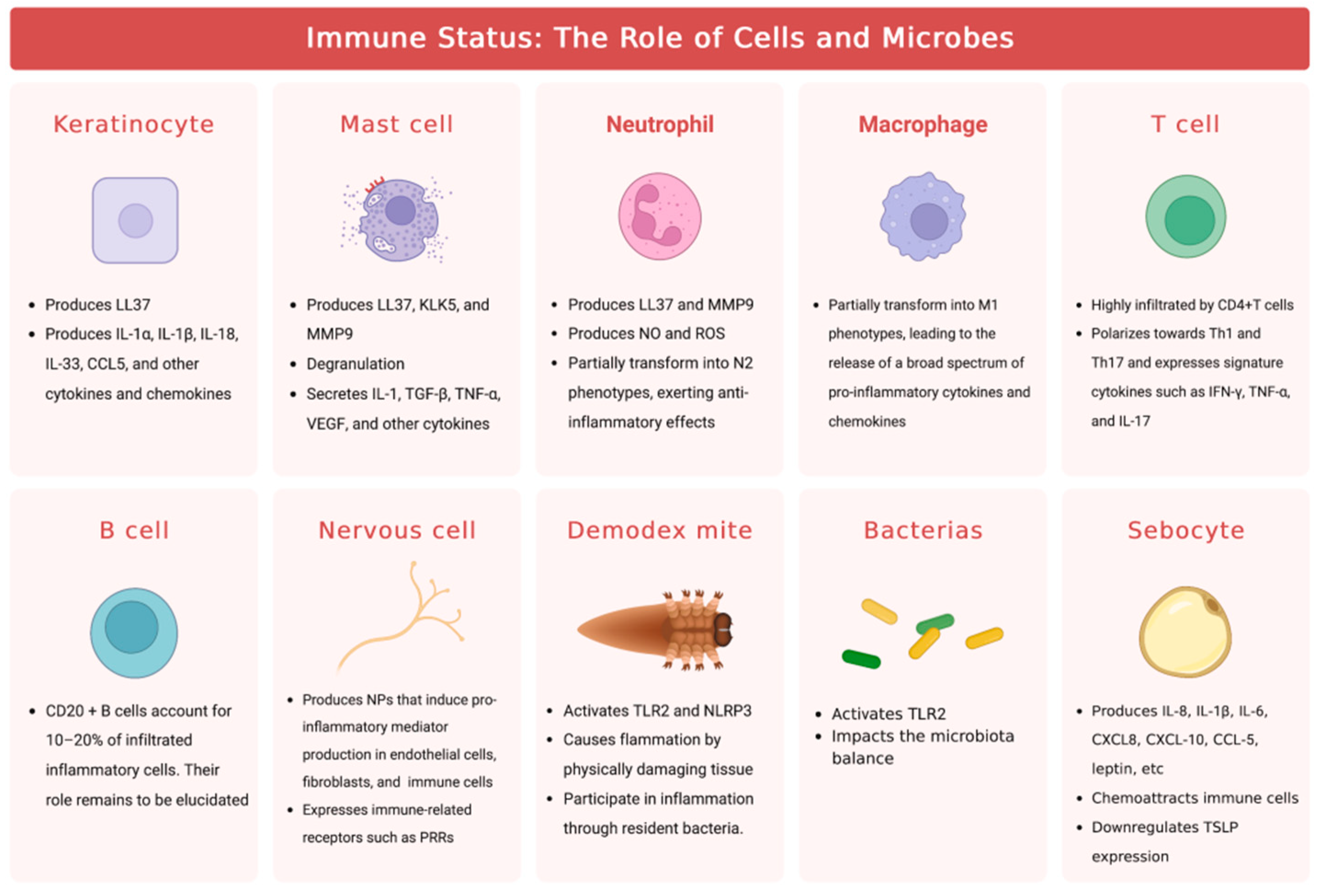
2.1. Immune Dysregulation
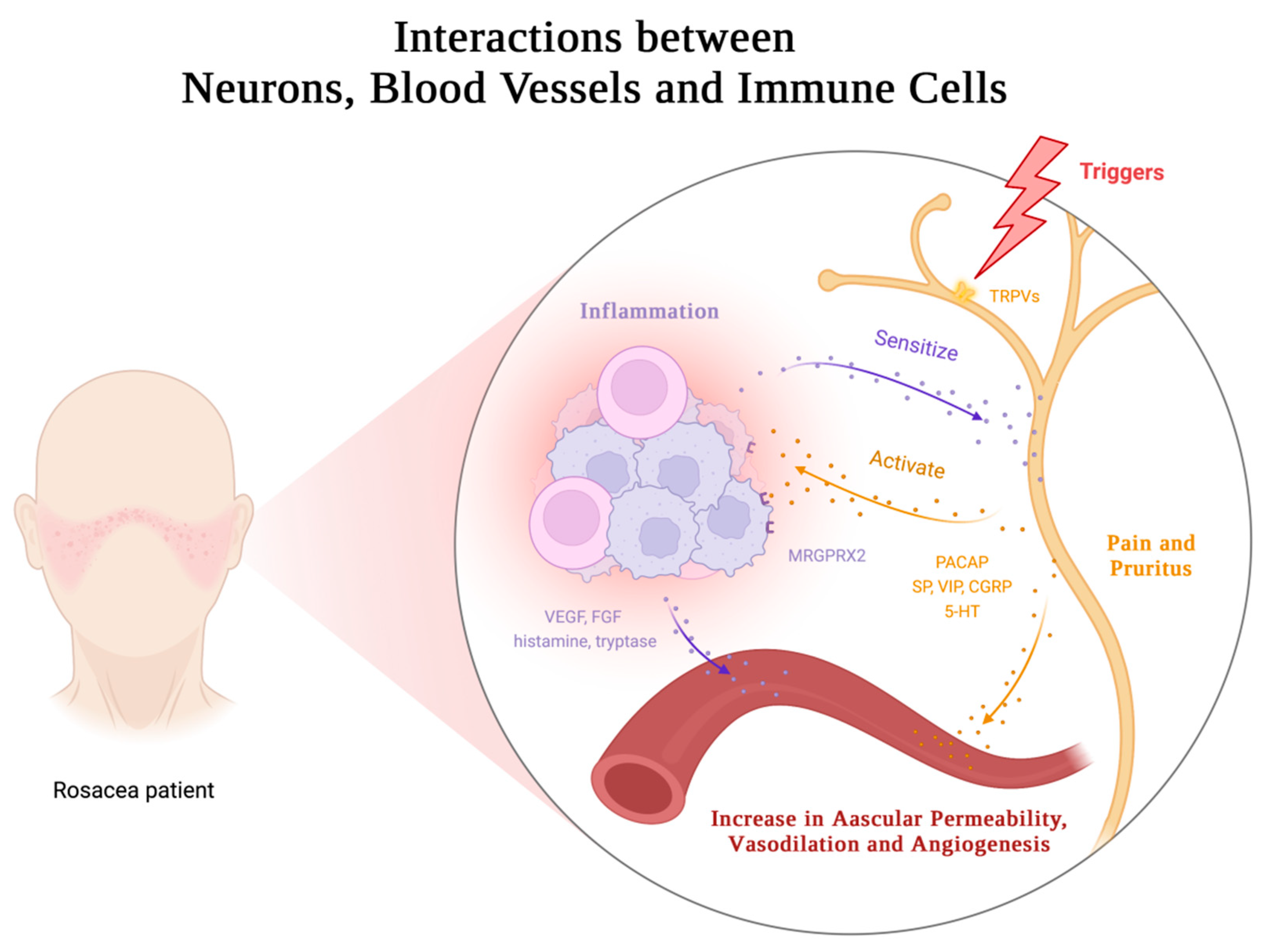
2.2. Vascular, Neurovascular and Neuroimmune Dysregulation
2.3. Skin Barrier Dysfunction
2.4. Microbial Dysbiosis
2.5. Metabolic Dysfunction
2.6. Sebaceous Gland Dysfunction
2.7. Miscellaneous
3. Treatments
3.1. Anti-inflammatory Strategies
3.2. Vascular Targeted Strategies
3.3. Targeting Neurological and Psychological Factors
3.4. Antimicrobial Strategies
3.5. Physical Therapy
3.6. Miscellaneous
| Target | Management Options | Pharmacological Effects | Current Clinical Trials |
|---|---|---|---|
| Immune Dysregulation | Azelaic acid | Suppresses expression of KLK5 and cathelicidin, activates PPARγ to exhibit anti-inflammatory properties, and curbs expression of IL-1, IL-6, and TNF-α | FDA-approved |
| ε-aminocaproic acid | Inhibits KLK5 | Shows beneficial impact on the severity of rosacea in a small, randomized pilot trial [190] | |
| Doxycycline (sub-antibiotic doses) | Inhibits chemotaxis and ROS production in neutrophils, suppresses several MMPs and subsequent antimicrobial peptide production, targets abnormal amino acid metabolism and sebaceous gland cells | FDA-approved | |
| Isotretinoin | Modulates TLR2 expression negatively in keratinocytes, reduces sebum production and sebaceous gland size | Supported by guidelines or expert consensus [2,5,7,195] | |
| Pimecrolimus | Inhibits T cell and mast cell activation by blocking calcineurin action | Supported by guidelines or expert consensus [2] | |
| Tacrolimus | Inhibits calcineurin | Clinical trials conducted with varying numbers of participants (1 to 200) in a systematic review of 28 articles [197] | |
| Hydroxychloroquine | Attenuates LL37-induced mast cell activation partly by inhibiting calcium influx | Small-sample, multicenter randomized controlled trial comparing hydroxychloroquine to standard doxycycline treatment showed similar efficacy and safety[198] | |
| Artemether | Suppresses expression of inflammatory biomarkers induced by LL37 via inhibition of transcription factors NF-κB, mTOR, and STAT | Randomized pilot study including 130 subjects evaluated efficacy of artemether emulsion[202] | |
| Tranexamic acid | Suppresses expression of KLK5, Camp, and TLR2, suppresses expression of cytokines and chemokines, inhibits angiogenesis induced by LL37, inhibits serine protease and physical interaction between urokinase-type plasminogen activator and the stratum corneum | Small-sample clinical trials and case studies have shown effectiveness of tranexamic acid, administered via different routes[205,206,207,210] | |
| ACU-D1 | Inhibits activation of NF-κB | Shows efficacy in double-blind, randomized, placebo-controlled study involving 40 patients[211] | |
| Secukinumab | Blocks IL-17 | Small open-label study conducted[213] | |
| Vascular Dysregulation | Brimonidine tartrate | α2-adrenergic receptor agonist, promotes contraction of vascular smooth muscle cells | FDA-approved |
| Oxymetazoline | α1-adrenergic receptor agonist, promotes contraction of vascular smooth muscle cells | FDA-approved | |
| Timolol | Nonselective β-adrenergic receptor blocker, induces vasoconstriction, inhibits inflammatory mediators such as MMPs and IL-6, inhibits angiogenesis by downregulating VEGF, promotes migration and re-epithelialization of keratinocytes, affects the secretion of lamellar bodies mediating repair of the skin barrier | Pilot trial found long-term topical use improved erythema, but rebound occurred after discontinuation; small trial showed significant improvement in erythema with nightly use for 28 days; larger trial found improvement in clinical parameters, but did not reach statistical significance[219,220] | |
| Neurological and Psychological Factors | Botulinum toxin | Inhibits release of vasodilating acetylcholine, regulates NPs such as SP, CGRP, and VIP, reduces mast cell count and degranulation, decreases expression of certain MMPs, reduces sebum production and increase skin hydration | Limited clinical trials with small sample sizes, imperfect study designs, and short follow-up times suggest potential efficacy and safety for rosacea treatment[226,227,228,229] |
| Paroxetine | Inhibits the reuptake of 5-HT | Demonstrated efficacy in a multicenter randomized controlled trial[232] | |
| Sumatriptan | 5-HT1B/1D receptor agonist, inhibits degranulation of macrophages, reduces PACAP levels | Alleviates features of rosacea in double-blind, randomized, placebo-controlled, cross-over trial and successful treatment of severe and painful flushing in a single case report[233,234] | |
| Propranolol | β-adrenergic receptor blocker, reduces sympathetic activity and alleviates anxiety symptoms | Beneficial impact in some small-sample studies and case reports[235,236] | |
| Carvedilol | Has both α1 receptor blocking and non-selective β receptor blocking effects, slows heart rate by acting on cardiac β1-adrenergic receptors to reduce patient tension and anxiety, and exerts anti-inflammatory effects by inhibiting NLRP3 inflammasome and the expression of TLR2 in macrophages. | A large-scale randomized controlled trial showed that oral carvedilol exhibited better efficacy than topical brimonidine[240,241] | |
| Microbial Dysbiosis | Metronidazole | Exerts acaricidal effects via its active metabolites, reduces ROS production and scavenges reactive species, impairs IL-17 induction | FDA-approved |
| Ivermectin | Eliminates Demodex mites, reduces neutrophil response, stimulates production of anti-inflammatory cytokines such as IL-10, inhibits pro-inflammatory cytokines like IL-1b and TNF-α | FDA-approved | |
| Omiganan | Rapidly kills bacteria and fungi | Phase III clinical trial shows effectiveness and safety in severe papulopustular rosacea[248] | |
| Rifaximin | Treats SIBO by inhibiting bacterial RNA synthesis | Several clinical trials and case reports have shown that rifaximin effectively improves rosacea characteristics in SIBO patients[113,114,234,249,250,251] | |
| Physical Therapy | IPL, Nd:YAG, PDL, and KTP | Primarily targets sebaceous glands, hemoglobin, and pigmentation | Supported by guidelines or expert consensus[2,5,7,195,244] |
| Ablative laser resurfacing | Targets water, causes vaporization and ablation effects | Supported by guidelines or expert consensus[2,5,7,195,244] | |
| Photodynamic therapy | Activates photosensitizers with light to generate ROS, modulates immunity and pilosebaceous units, targets Demodex mites and exhibits antimicrobial effects | Systematic review of nine Level 4 studies suggests PDT may be a safe and effective treatment option. Findings from ongoing and smaller-scale trials indicate that PDT may offer efficacy comparable to that of first-line therapies in addressing PPR. Results from larger randomized controlled trials combining PDT with other modalities indicate improved efficacy and milder adverse reactions[256,257,258,261,262,263] | |
| Pro-yellow laser | Emits laser with a wavelength of 577nm, demonstrating preferential absorption by hemoglobin | Demonstrates efficacy in select case reports and small sample trials; a retrospective study identifies reduction of mite density[265,266,267,268,269] | |
| Radiofrequency | Generates thermal energy, has positive effects on the nervous system, cardiovascular system, immune system, and reduces burning sensations by decreasing TRPV1 expression | Randomized, controlled, split-face study showed radiofrequency and PDL equally effective in treating ETR; radiofrequency treatment showed greater improvement in PPR[273] | |
| Short-wave Radiofrequency | Enhances local blood oxygen supply, repairs skin barriers, and reduces chronic inflammation | Prospective, single-arm, open-label pilot study reported rapid and sustained improvement in mild to moderate ETR patients[271] | |
| Fractional Microneedling Radiofrequency | Delivers thermal energy through targeted microneedles. Reduces dermal inflammation, mast cell count, and the expression of TLR2, LL37, VEGF, NF-κB, IL-8, and TRPVs | Prospective, randomized, split-face clinical trial showed modest but statistically significant improvement in rosacea[274] | |
| Ultrasound | Restores skin barrier function by inhibiting MMPs | Both retrospective and prospective studies have reported significant improvements in patient self-assessment and clinical measures[276,277,278,279] |
4. Conclusions
Funding
Authors’ Contributions
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETR | erythematotelangiectatic rosacea |
| PPR | papulopustular rosacea |
| PhR | phymatous rosacea |
| OR | ocular rosacea |
| TLRs | Toll-like receptors |
| KCs | keratinocytes |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| MCs | mast cells |
| NF-κB | nuclear factor-kappa B |
| TNF | tumor necrosis factor |
| IL | interleukin |
| AhR | arylhydrocarbon receptor |
| CCL | chemokine (C-C motif) ligand |
| KLK5 | kallikrein 5 |
| JAK | janus kinase |
| STAT | signal transducer and activator of transcription |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MMP9 | matrix metalloproteinase 9 |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| PRRs | pattern recognition receptors |
| NPs | neuropeptides |
| MRGPRX2 | Mas-related G protein-coupled receptor member X2 |
| VEGF | vascular endothelial growth factor |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| ADAMDEC1 | ADAM-like Decysin-1 |
| GBP5 | guanylate-binding protein 5 |
| NEAT1 | nuclear paraspeckle assembly transcript 1 |
| FPR1 | formyl peptide receptor 1 |
| EGFR | epidermal growth factor receptors |
| FGF | fibroblast growth factor |
| UV | ultraviolet |
| TRP | transient receptor potential |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| SP | substance P |
| VIP | vasoactive intestinal peptide |
| CGRP | calcitonin gene-related peptide |
| 5-HT | serotonin |
| PAR2 | protease-activated receptor 2 |
| TEWL | transepidermal water loss |
| CLDNs | claudins |
| SIBO | small intestinal bacterial overgrowth |
| CXCL | C-X-C motif chemokine ligand |
| GPBAR1 | G protein-coupled bile acid receptor 1 |
| TSLP | thymic stromal lymphopoietin |
| GST | glutathione S-transferase |
| HLA | human leukocyte antigen |
| TACR3 | tachykinin 3 receptor |
| VDR | vitamin D receptor |
| ER | endoplasmic reticulum |
| S1P | sphingosine-1-phosphate |
| HPA | hypothalamic-pituitary-adrenal |
| FDA | Food and Drug Administration |
| IGA | Investigator Global Assessment |
| CEA | Clinician Erythema Assessment |
| PSA | Patient Self-Assessment |
| IPL | Intense pulsed light |
| Nd:YAG | neodymium:yttrium-aluminum-garnet laser |
| PDL | pulsed dye laser |
| KTP | potassium titanyl phosphate laser |
| PDT | Photodynamic therapy |
| siRNA | small RNA interference |
References
- Gether, L.; et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol 2018, 179, 282–289. [Google Scholar] [CrossRef]
- Anzengruber, F.; et al. Swiss S1 guideline for the treatment of rosacea. J Eur Acad Dermatol Venereol 2017, 31, 1775–1791. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, J.; et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol 2002, 46, 584–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; et al. The Theranostics Role of Mast Cells in the Pathophysiology of Rosacea. Front Med (Lausanne) 2019, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.; et al. Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol 2020, 82, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol 2020, 182, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Asai, Y.; et al. Canadian Clinical Practice Guidelines for Rosacea. J Cutan Med Surg 2016, 20, 432–45. [Google Scholar] [CrossRef]
- Meylan, E., J.; Tschopp, J.; Karin, M. Intracellular pattern recognition receptors in the host response. Nature 2006, 442, 39–44. [Google Scholar]
- Kumar, V. Going, Toll-like receptors in skin inflammation and inflammatory diseases. EXCLI J 2021, 20, 52–79. [Google Scholar]
- Portou, M.J.; et al. The innate immune system, toll-like receptors and dermal wound healing: A review. Vascul Pharmacol 2015, 71, 31–6. [Google Scholar] [CrossRef]
- Yamasaki, K.; et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol 2011, 131, 688–97. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Medzhitov, Innate immune recognition. Annu Rev Immunol 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 2002, 169, 1535–41. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; et al. Aging-Conferred SIRT7 Decline Inhibits Rosacea-Like Skin Inflammation by Modulating Toll-Like Receptor 2‒NF-kappaB Signaling. J Invest Dermatol 2022, 142, 2580–2590 e6. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Braz, D.; et al. Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models. Mol Vis 2021, 27, 323–353. [Google Scholar]
- Sun, Y.; et al. Activation of aryl hydrocarbon receptor ameliorates rosacea-like eruptions in mice and suppresses the TLR signaling pathway in LL-37-induced HaCaT cells. Toxicol Appl Pharmacol 2022, 451, 116189. [Google Scholar] [CrossRef]
- Wang, L.; et al. AhR Regulates Peptidoglycan-Induced Inflammatory Gene Expression in Human Keratinocytes. J Innate Immun 2022, 14, 124–134. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 2010, 6, e1001067. [Google Scholar] [CrossRef]
- Agerberth, B.; et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–93. [Google Scholar] [CrossRef]
- Frohm Nilsson, M.; et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun 1999, 67, 2561–6. [Google Scholar] [CrossRef]
- Lau, Y.E.; et al. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun 2005, 73, 583–91. [Google Scholar] [CrossRef] [PubMed]
- Frohm, M.; et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997, 272, 15258–63. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; et al. A positive feedback loop between mTORC1 and cathelicidin promotes skin inflammation in rosacea. EMBO Mol Med 2021, 13, e13560. [Google Scholar] [CrossRef] [PubMed]
- Two, A.M.; et al. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol 2015, 72, 749–758; quiz 759–760. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; et al. Antimicrobial Peptide LL-37 Drives Rosacea-Like Skin Inflammation in an NLRP3-Dependent Manner. J Invest Dermatol 2021, 141, 2885–2894. [Google Scholar] [CrossRef]
- Yamasaki, K.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 2007, 13, 975–80. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; et al. Koebnerisin (S100A15): A novel player in the pathogenesis of rosacea. J Am Acad Dermatol 2019, 80, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol 2008, 181, 1499–506. [Google Scholar] [CrossRef]
- Hegyi, Z.; et al. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 "alarmins" psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J Invest Dermatol 2012, 132, 1416–24. [Google Scholar] [CrossRef] [PubMed]
- Batycka-Baran, A.; et al. Leukocyte-derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J Dermatol Sci 2015, 79, 214–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; et al. Long non-coding RNA NEAT1 functions as a competing endogenous RNA to regulate S100A9 expression by sponging miR-196a-5p in rosacea. J Dermatol Sci 2021, 102, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Marson, J.W.; Baldwin, H.E. Rosacea: a wholistic review and update from pathogenesis to diagnosis and therapy. Int J Dermatol 2020, 59, e175–e182. [Google Scholar]
- Cribier, B. Rosacea under the microscope: characteristic histological findings. J Eur Acad Dermatol Venereol 2013, 27, 1336–43. [Google Scholar] [CrossRef] [PubMed]
- Schwab, V.D.; et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Aroni, K.; et al. A study of the pathogenesis of rosacea: how angiogenesis and mast cells may participate in a complex multifactorial process. Arch Dermatol Res 2008, 300, 125–31. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; et al. Role of MrgprB2 in Rosacea-Like Inflammation in Mice: Modulation by beta-Arrestin 2. J Invest Dermatol 2022, 142, 2988–2997. [Google Scholar] [CrossRef]
- Li, J.; et al. Hydroxychloroquine is a novel therapeutic approach for rosacea. Int Immunopharmacol 2020, 79, 106178. [Google Scholar] [CrossRef]
- Muto, Y.; et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol 2014, 134, 2728–2736. [Google Scholar] [CrossRef]
- Agier, J.; et al. Cathelicidin LL-37 Affects Surface and Intracellular Toll-Like Receptor Expression in Tissue Mast Cells. J Immunol Res 2018, 2018, 7357162. [Google Scholar] [CrossRef]
- Agier, J.; et al. The RLR/NLR expression and pro-inflammatory activity of tissue mast cells are regulated by cathelicidin LL-37 and defensin hBD-2. Sci Rep 2018, 8, 11750. [Google Scholar] [CrossRef]
- Gupta, K.; Subramanian, H.; Ali, H. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun 2016, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; et al. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem 2011, 286, 44739–49. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.N.; et al. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front Immunol 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, A.; et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65, 2006–19. [Google Scholar] [CrossRef] [PubMed]
- Millikan, L. The proposed inflammatory pathophysiology of rosacea: implications for treatment. Skinmed 2003, 2, 43–7. [Google Scholar] [CrossRef]
- Buhl, T.; et al. Molecular and Morphological Characterization of Inflammatory Infiltrate in Rosacea Reveals Activation of Th1/Th17 Pathways. J Invest Dermatol 2015, 135, 2198–2208. [Google Scholar] [CrossRef]
- Zhao, Z.; et al. N2-Polarized Neutrophils Reduce Inflammation in Rosacea by Regulating Vascular Factors and Proliferation of CD4(+) T Cells. J Invest Dermatol 2022, 142, 1835–1844. [Google Scholar] [CrossRef]
- Zhou, L.; et al. GBP5 exacerbates rosacea-like skin inflammation by skewing macrophage polarization towards M1 phenotype through the NF-kappaB signalling pathway. J Eur Acad Dermatol Venereol 2023, 37, 796–809. [Google Scholar] [CrossRef]
- Holmes, A.D.; Steinhoff, M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol 2017, 26, 659–667. [Google Scholar] [CrossRef]
- Zhang, J.; et al. A Novel Mechanism of Carvedilol Efficacy for Rosacea Treatment: Toll-Like Receptor 2 Inhibition in Macrophages. Front Immunol 2021, 12, 609615. [Google Scholar] [CrossRef]
- Liu, T.; et al. ADAMDEC1 promotes skin inflammation in rosacea via modulating the polarization of M1 macrophages. Biochem Biophys Res Commun 2020, 521, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol 2014, 71, 100–7. [Google Scholar] [CrossRef] [PubMed]
- Rufli, T.; Buchner, S.A. T-cell subsets in acne rosacea lesions and the possible role of Demodex folliculorum. Dermatologica 1984, 169, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; et al. Elucidating the immune infiltration in acne and its comparison with rosacea by integrated bioinformatics analysis. PLoS One 2021, 16, e0248650. [Google Scholar] [CrossRef] [PubMed]
- Hayran, Y.; et al. Serum IL-17 levels in patients with rosacea. J Cosmet Dermatol 2022, 21, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- You, T.; et al. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci Rep 2017, 7, 41779. [Google Scholar] [CrossRef]
- Gazi, U.; et al. Skin-homing T-cell responses associated with Demodex infestation and rosacea. Parasite Immunol 2019, 41, e12658. [Google Scholar] [CrossRef]
- Rosina, P.; et al. Videocapillaroscopic alterations in erythematotelangiectatic rosacea. J Am Acad Dermatol 2006, 54, 100–4. [Google Scholar] [CrossRef]
- Kim, M.; et al. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp Dermatol 2015, 24, 680–5. [Google Scholar] [CrossRef]
- Neufeld, G.; et al. Vascular endothelial growth factor and its receptors. Prog Growth Factor Res 1994, 5, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.H.; et al. Lymphangiogenesis and angiogenesis in non-phymatous rosacea. J Cutan Pathol 2007, 34, 748–53. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; et al. Expression of vascular endothelial growth factor and its receptors in rosacea. Br J Ophthalmol 2007, 91, 226–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; et al. mTORC1-Mediated Angiogenesis is Required for the Development of Rosacea. Front Cell Dev Biol 2021, 9, 751785. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; et al. Inhibition of Hippo Signaling Improves Skin Lesions in a Rosacea-Like Mouse Model. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; et al. Aberrant amino acid metabolism promotes neurovascular reactivity in rosacea. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Egeberg, A.; et al. Patients with rosacea have increased risk of dementia. Ann Neurol 2016, 79, 921–8. [Google Scholar] [CrossRef]
- Wang, Z.; et al. Relationship between rosacea and sleep. J Dermatol 2020, 47, 592–600. [Google Scholar] [CrossRef]
- Chang, H.C.; et al. Association of rosacea with depression and anxiety: A systematic review and meta-analysis. J Affect Disord 2022, 299, 239–245. [Google Scholar] [CrossRef]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin Immunopathol 2018, 40, 249–259. [Google Scholar] [CrossRef]
- Helfrich, Y.R.; et al. Clinical, Histologic, and Molecular Analysis of Differences Between Erythematotelangiectatic Rosacea and Telangiectatic Photoaging. JAMA Dermatol 2015, 151, 825–36. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; et al. Rosacea: The cytokine and chemokine network. J Investig Dermatol Symp Proc 2011, 15, 40–7. [Google Scholar] [CrossRef] [PubMed]
- Sulk, M.; et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol 2012, 132, 1253–62. [Google Scholar] [CrossRef] [PubMed]
- Baylie, R.L.; Brayden, J.E. TRPV channels and vascular function. Acta Physiol (Oxf) 2011, 203, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Stearns, V.; et al. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003, 289, 2827–34. [Google Scholar] [CrossRef] [PubMed]
- Nordlind, K.; et al. Expression of serotonergic receptors in psoriatic skin. Arch Dermatol Res 2006, 298, 99–106. [Google Scholar] [CrossRef]
- Lin, M.T.; et al. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol 1998, 274, R1260–7. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; et al. Molecular Mechanisms of Neurogenic Inflammation of the Skin. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu Rev Immunol 2019, 37, 19–46. [Google Scholar] [CrossRef]
- Tamari, M.; Ver Heul, A.M.; Kim, B.S. Immunosensation: Neuroimmune Cross Talk in the Skin. Annu Rev Immunol 2021, 39, 369–393. [Google Scholar] [CrossRef]
- Klein Wolterink, R.G.J.; et al. Neuroimmune Interactions in Peripheral Organs. Annu Rev Neurosci 2022, 45, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Chen, O.; Ji, R.R. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci 2020, 43, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Endoplasmic reticulum stress: key promoter of rosacea pathogenesis. Exp Dermatol 2014, 23, 868–73. [Google Scholar] [CrossRef]
- McNeil, B.D.; et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–41. [Google Scholar] [CrossRef] [PubMed]
- Madva, E.N.; Granstein, R.D. Nerve-derived transmitters including peptides influence cutaneous immunology. Brain Behav Immun 2013, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; et al. Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J Allergy Clin Immunol 2021, 148, 293–308. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol 2016, 138, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; et al. Acute irritant threshold correlates with barrier function, skin hydration and contact hypersensitivity in atopic dermatitis and rosacea. Exp Dermatol 2013, 22, 752–3. [Google Scholar] [CrossRef]
- Addor, F.A. Skin barrier in rosacea. An Bras Dermatol 2016, 91, 59–63. [Google Scholar] [CrossRef]
- Powell, F.C.; Ni Raghallaigh, S. Interventions for 'rosacea'. Br J Dermatol 2011, 165, 707–8. [Google Scholar] [CrossRef]
- Zhou, M.; et al. Clinical characteristics and epidermal barrier function of papulopustular rosacea: A comparison study with acne vulgaris. Pak J Med Sci 2016, 32, 1344–1348. [Google Scholar] [CrossRef]
- Deng, Z.; et al. Claudin reduction may relate to an impaired skin barrier in rosacea. J Dermatol 2019, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Medgyesi, B.; et al. Rosacea Is Characterized by a Profoundly Diminished Skin Barrier. J Invest Dermatol 2020, 140, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, S.H. Skin Barrier and Calcium. Ann Dermatol 2018, 30, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Elsholz, F.; et al. Calcium--a central regulator of keratinocyte differentiation in health and disease. Eur J Dermatol 2014, 24, 650–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; et al. Multi-Transcriptomic Analysis and Experimental Validation Implicate a Central Role of STAT3 in Skin Barrier Dysfunction Induced Aggravation of Rosacea. J Inflamm Res 2022, 15, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
- Atsugi, T.; et al. Holocrine Secretion Occurs outside the Tight Junction Barrier in Multicellular Glands: Lessons from Claudin-1-Deficient Mice. J Invest Dermatol 2020, 140, 298–308. [Google Scholar] [CrossRef]
- Kim, H.S. Microbiota in Rosacea. Am J Clin Dermatol 2020, 21, 25–35. [Google Scholar] [CrossRef]
- Desch, C.; Nutting, W.B. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol 1972, 58, 169–77. [Google Scholar] [CrossRef]
- Bonnar, E.; Eustace, P.; Powell, F.C. The Demodex mite population in rosacea. J Am Acad Dermatol 1993, 28, 443–8. [Google Scholar] [CrossRef]
- Segal, R.; et al. Dermoscopy as a diagnostic tool in demodicidosis. Int J Dermatol 2010, 49, 1018–23. [Google Scholar] [CrossRef] [PubMed]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol 1993, 128, 650–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Huang, Y.C. Role of Demodex mite infestation in rosacea: A systematic review and meta-analysis. J Am Acad Dermatol 2017, 77, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.M.; Foley, R.; Powell, F.C. Demodex and rosacea revisited. Clin Dermatol 2017, 35, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Gonzalez, R.R.; et al. Innate type 2 immunity controls hair follicle commensalism by Demodex mites. Immunity 2022, 55, 1891–1908. [Google Scholar] [CrossRef]
- Ni Raghallaigh, S.; et al. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br J Dermatol 2012, 166, 279–87. [Google Scholar] [CrossRef]
- Casas, C.; et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol 2012, 21, 906–10. [Google Scholar] [CrossRef]
- Lacey, N.; et al. Demodex mites modulate sebocyte immune reaction: possible role in the pathogenesis of rosacea. Br J Dermatol 2018, 179, 420–430. [Google Scholar] [CrossRef]
- Paichitrojjana, A. Demodex: The worst enemies are the ones that used to be friends. Dermatol Reports 2022, 14, 9339. [Google Scholar] [CrossRef]
- Uberoi, A.; et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe 2021, 29, 1235–1248. [Google Scholar] [CrossRef]
- Han, J.; et al. The relationship between inflammatory bowel disease and rosacea over the lifespan: A meta-analysis. Clin Res Hepatol Gastroenterol 2019, 43, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.G.; et al. The skin microbiota as a link between rosacea and its systemic comorbidities. Int J Dermatol 2020, 59, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol 2008, 6, 759–64. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.; et al. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United European Gastroenterol J 2015, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.R.; et al. Rosacea is associated with Helicobacter pylori: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2017, 31, 2010–2015. [Google Scholar] [CrossRef]
- Chen, Y.J.; et al. An altered fecal microbial profiling in rosacea patients compared to matched controls. J Formos Med Assoc 2021, 120, 256–264. [Google Scholar] [CrossRef]
- Nam, J.H.; et al. Rosacea and its association with enteral microbiota in Korean females. Exp Dermatol 2018, 27, 37–42. [Google Scholar] [CrossRef]
- Wang, F.Y.; Chi, C.C. Rosacea, Germs, and Bowels: A Review on Gastrointestinal Comorbidities and Gut-Skin Axis of Rosacea. Adv Ther 2021, 38, 1415–1424. [Google Scholar] [CrossRef]
- El-Khalawany, M.; et al. Role of Helicobacter pylori in common rosacea subtypes: a genotypic comparative study of Egyptian patients. J Dermatol 2012, 39, 989–95. [Google Scholar] [CrossRef]
- Arck, P.; et al. Is there a 'gut-brain-skin axis'? Exp Dermatol 2010, 19, 401–5. [Google Scholar] [CrossRef]
- Utas, S.; et al. Helicobacter pylori eradication treatment reduces the severity of rosacea. J Am Acad Dermatol 1999, 40, 433–5. [Google Scholar] [CrossRef] [PubMed]
- Vera, N.; Patel, N.U.; Seminario-Vidal, L. Rosacea Comorbidities. Dermatol Clin 2018, 36, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; et al. Characterization of the Blood Microbiota in Korean Females with Rosacea. Dermatology 2019, 235, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; et al. Association between rosacea and cardiometabolic disease: A systematic review and meta-analysis. J Am Acad Dermatol 2020, 83, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Gurel, G.; Turan, Y. Noninvasive assessment of subclinical atherosclerosis in patients with rosacea. Ital J Dermatol Venerol 2021, 156, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Caf, N.; et al. Evaluation of subclinical atherosclerosis in rosacea patients by flow-mediated dilatation method. J Cosmet Dermatol 2023, 22, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, N.; Karaaslan, E.; Ozdemir Cetinkaya, P. Evaluation of serum uric acid levels in patients with rosacea. Arch Dermatol Res 2020, 312, 447–451. [Google Scholar] [CrossRef]
- Akin Belli, A.; et al. Assessment of thyroid disorders in patients with rosacea: a large case-control study. An Bras Dermatol 2021, 96, 539–543. [Google Scholar] [CrossRef]
- Gonulal, M.; et al. Investigation of thyroid blood tests and thyroid ultrasound findings of patients with rosacea. Dermatol Ther 2021, 34, e14632. [Google Scholar] [CrossRef]
- Xiao, W.; et al. Lithocholic acid promotes rosacea-like skin inflammation via G protein-coupled bile acid receptor 1. Biochim Biophys Acta Mol Basis Dis 2022, 1868, 166563. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015, 15, 104–16. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, D. Serum bilirubin and uric acid antioxidant levels in rosacea patients. J Cosmet Dermatol 2020, 19, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–8. [Google Scholar] [CrossRef] [PubMed]
- Akin Belli, A.; et al. The relationship between rosacea and insulin resistance and metabolic syndrome. Eur J Dermatol 2016, 26, 260–4. [Google Scholar] [CrossRef] [PubMed]
- Demir Pektas, S.; et al. Thiol/disulfide homeostasis and its relationship with insulin resistance in patients with rosacea. J Cosmet Dermatol 2022, 21, 2655–2661. [Google Scholar] [CrossRef]
- Toka Ozer, T.; Akyurek, O.; Durmaz, S. Association between Demodex folliculorum and Metabolic Syndrome. J Cosmet Dermatol 2020, 19, 3145–3149. [Google Scholar] [CrossRef]
- Gokce, C.; et al. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. J Int Med Res 2013, 41, 1752–8. [Google Scholar] [CrossRef]
- Keskin Kurt, R.; et al. Increased density of Demodex folliculorum mites in pregnancies with gestational diabetes. Med Princ Pract 2014, 23, 369–72. [Google Scholar] [CrossRef]
- Eroglu, S.; Cakmakliogullari, M.; Kal Cakmakliogullari, E. Is the presence of Demodex folliculorum increased with impaired glucose regulation in polycystic ovary syndrome? J Obstet Gynaecol 2020, 40, 546–550. [Google Scholar] [CrossRef]
- de Farias Pires, T.; et al. A population-based study of the stratum corneum moisture. Clin Cosmet Investig Dermatol 2016, 9, 79–87. [Google Scholar] [CrossRef]
- Loffler, H.; Aramaki, J.U.; Effendy, I. The influence of body mass index on skin susceptibility to sodium lauryl sulphate. Skin Res Technol 2002, 8, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; et al. The impact of obesity on skin disease and epidermal permeability barrier status. J Eur Acad Dermatol Venereol 2010, 24, 191–5. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.W.; et al. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br J Dermatol 2019, 181, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Deplewski, D.; Rosenfield, R.L. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology 1999, 140, 4089–94. [Google Scholar] [CrossRef] [PubMed]
- Hoting, E.; Paul, E.; Plewig, G. Treatment of rosacea with isotretinoin. Int J Dermatol 1986, 25, 660–3. [Google Scholar] [CrossRef]
- Dahlhoff, M.; et al. Sebaceous lipids are essential for water repulsion, protection against UVB-induced apoptosis and ocular integrity in mice. Development 2016, 143, 1823–31. [Google Scholar] [CrossRef]
- Kabashima, K.; et al. The immunological anatomy of the skin. Nat Rev Immunol 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Nikkari, T. Comparative chemistry of sebum. J Invest Dermatol 1974, 62, 257–67. [Google Scholar] [CrossRef]
- Marples, R.R.; Downing, D.T.; Kligman, A.M. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol 1971, 56, 127–31. [Google Scholar] [CrossRef]
- Torocsik, D.; et al. Genome wide analysis of TLR1/2- and TLR4-activated SZ95 sebocytes reveals a complex immune-competence and identifies serum amyloid A as a marker for activated sebaceous glands. PLoS One 2018, 13, e0198323. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinol 2011, 3, 41–9. [Google Scholar] [PubMed]
- Li, Z.J.; et al. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J Dermatol Sci 2013, 72, 116–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, V.Y.; et al. Role of sebaceous glands in inflammatory dermatoses. J Am Acad Dermatol 2015, 73, 856–63. [Google Scholar] [CrossRef] [PubMed]
- Forton, F.M.N.; De Maertelaer, V. Which factors influence Demodex proliferation? A retrospective pilot study highlighting a possible role of subtle immune variations and sebaceous gland status. J Dermatol 2021, 48, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Dajnoki, Z.; et al. Sebaceous Gland-Rich Skin Is Characterized by TSLP Expression and Distinct Immune Surveillance Which Is Disturbed in Rosacea. J Invest Dermatol 2017, 137, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; et al. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 2019, 176, 982–997. [Google Scholar] [CrossRef]
- Mattii, M.; et al. Sebocytes contribute to skin inflammation by promoting the differentiation of T helper 17 cells. Br J Dermatol 2018, 178, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Lovaszi, M.; et al. Sebum lipids influence macrophage polarization and activation. Br J Dermatol 2017, 177, 1671–1682. [Google Scholar] [CrossRef]
- Kovacs, D.; et al. Sebocytes differentially express and secrete adipokines. Exp Dermatol 2016, 25, 194–9. [Google Scholar] [CrossRef]
- Lee, S.H.; et al. Sebaceous glands participate in the inflammation of rosacea. J Eur Acad Dermatol Venereol 2020, 34, e144–e146. [Google Scholar] [CrossRef]
- Georgala, S.; et al. Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J Eur Acad Dermatol Venereol 2001, 15, 441–4. [Google Scholar] [CrossRef] [PubMed]
- Powell, F.C. The histopathology of rosacea: 'where's the beef? Dermatology 2004, 209, 173–4. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, N.; et al. Genetic vs Environmental Factors That Correlate With Rosacea: A Cohort-Based Survey of Twins. JAMA Dermatol 2015, 151, 1213–9. [Google Scholar] [CrossRef] [PubMed]
- Dall'Oglio, F.; Fusto, C.; Micali, G. Intrafamilial Transmission of Rosacea Spanning Six Generations: A Retrospective Observational Study. J Clin Aesthet Dermatol 2022, 15, 35–39. [Google Scholar]
- Yazici, A.C.; et al. GSTM1 and GSTT1 null genotypes as possible heritable factors of rosacea. Photodermatol Photoimmunol Photomed 2006, 22, 208–10. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol 2015, 135, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Karpouzis, A.; et al. Assessment of Tachykinin Receptor 3' Gene Polymorphism rs3733631 in Rosacea. Int Sch Res Notices 2015, 2015, 469402. [Google Scholar] [CrossRef]
- Akdogan, N.; et al. Role of serum 25-hydroxyvitamin D levels and vitamin D receptor gene polymorphisms in patients with rosacea: a case-control study. Clin Exp Dermatol 2019, 44, 397–403. [Google Scholar] [CrossRef]
- Jansen, T.; et al. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans). J Dermatol 2004, 31, 244–6. [Google Scholar] [CrossRef]
- Hayran, Y.; et al. Vascular endothelial growth factor gene polymorphisms in patients with rosacea: A case-control study. J Am Acad Dermatol 2019, 81, 348–354. [Google Scholar] [CrossRef]
- Baghad, B.; et al. Pediatric Demodicosis Associated with Gain-of-Function Variant in STAT1 Presenting as Rosacea-Type Rash. J Clin Immunol 2021, 41, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Second, J.; et al. Rosacea and demodicidosis associated with gain-of-function mutation in STAT1. J Eur Acad Dermatol Venereol 2017, 31, e542–e544. [Google Scholar] [CrossRef] [PubMed]
- Saez-de-Ocariz, M.; et al. Rosacea as a striking feature in family members with a STAT1 gain-of-function mutation. J Eur Acad Dermatol Venereol 2020, 34, e265–e267. [Google Scholar] [CrossRef] [PubMed]
- Tisma, V.S.; et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol 2009, 60, 270–6. [Google Scholar] [CrossRef] [PubMed]
- Plenkowska, J.; Gabig-Ciminska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Georgescu, S.R.; et al. Thiol-Disulfide Homeostasis in Skin Diseases. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; et al. Nav1.8 in keratinocytes contributes to ROS-mediated inflammation in inflammatory skin diseases. Redox Biol 2022, 55, 102427. [Google Scholar] [CrossRef]
- Park, K.; et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci U S A 2016, 113, E1334–E1342. [Google Scholar] [CrossRef]
- Melnik, B.C. Rosacea: The Blessing of the Celts - An Approach to Pathogenesis Through Translational Research. Acta Derm Venereol 2016, 96, 147–56. [Google Scholar] [CrossRef]
- Suhng, E.; et al. Increased expression of IL-33 in rosacea skin and UVB-irradiated and LL-37-treated HaCaT cells. Exp Dermatol 2018, 27, 1023–1029. [Google Scholar] [CrossRef]
- Brink, N.; et al. Comparative quantification of IL-1beta, IL-10, IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflamm Res 2000, 49, 290–6. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.N.; et al. Innate Immune Dysfunction in Rosacea Promotes Photosensitivity and Vascular Adhesion Molecule Expression. J Invest Dermatol 2020, 140, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; et al. Rosacea and Diet: What is New in 2021? J Clin Aesthet Dermatol 2021, 14, 49–54. [Google Scholar] [PubMed]
- Drago, F.; et al. Rosacea and alcohol intake. J Am Acad Dermatol 2018, 78, e25. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.P.; et al. The effects of alcohol and illicit drug use on the skin. Clin Dermatol 2021, 39, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Alia, E.; Feng, H. Rosacea pathogenesis, common triggers, and dietary role: The cause, the trigger, and the positive effects of different foods. Clin Dermatol 2022, 40, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Saric-Bosanac, S.; et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J 2020, 26. [Google Scholar] [CrossRef]
- Coda, A.B.; et al. Cathelicidin, kallikrein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol 2013, 69, 570–7. [Google Scholar] [CrossRef]
- Searle, T.; Ali, R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J Dermatolog Treat 2022, 33, 722–732. [Google Scholar] [CrossRef]
- Two, A.M.; et al. Reduction in serine protease activity correlates with improved rosacea severity in a small, randomized pilot study of a topical serine protease inhibitor. J Invest Dermatol 2014, 134, 1143–1145. [Google Scholar] [CrossRef]
- Gold, L.S.; et al. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials. J Am Acad Dermatol 2020, 82, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.; et al. A multicentre, randomized, double-masked, parallel group, vehicle-controlled phase IIb study to evaluate the safety and efficacy of 1% and 3% topical minocycline gel in patients with papulopustular rosacea. Br J Dermatol 2020, 183, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rosso, J.Q.; et al. Oral Sarecycline for Treatment of Papulopustular Rosacea: Results of a Pilot Study of Effectiveness and Safety. J Drugs Dermatol 2021, 20, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Stein Gold, L.; et al. Open-label Extension Study Evaluating Long-term Safety and Efficacy of FMX103 1.5% Minocycline Topical Foam for the Treatment of Moderate-to-Severe Papulopustular Rosacea. J Clin Aesthet Dermatol 2020, 13, 44–49. [Google Scholar] [PubMed]
- Del Rosso, J.Q.; et al. Update on the Management of Rosacea from the American Acne & Rosacea Society (AARS). J Clin Aesthet Dermatol 2020, 13 (6 Suppl), S17–S24. [Google Scholar]
- Koca, R.; et al. A comparison of metronidazole 1% cream and pimecrolimus 1% cream in the treatment of patients with papulopustular rosacea: a randomized open-label clinical trial. Clin Exp Dermatol 2010, 35, 251–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; et al. Topical calcineurin inhibitors as a double-edged sword in rosacea: A systematic review. J Cosmet Dermatol 2022, 21, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; et al. Efficacy and safety of hydroxychloroquine for treatment of patients with rosacea: A multicenter, randomized, double-blind, double-dummy, pilot study. J Am Acad Dermatol 2021, 84, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev 2021, 41, 3023–3061. [Google Scholar] [CrossRef]
- Yuan, X.; et al. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed Pharmacother 2019, 117, 109181. [Google Scholar] [CrossRef]
- Li, T.; et al. The therapeutic effect of artesunate on rosacea through the inhibition of the JAK/STAT signaling pathway. Mol Med Rep 2018, 17, 8385–8390. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; et al. Evaluation of the efficacy and tolerance of artemether emulsion for the treatment of papulopustular rosacea: a randomized pilot study. J Dermatolog Treat 2019, 30, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lim, H.W. The uses of tranexamic acid in dermatology: a review. Int J Dermatol 2023, 62, 589–598. [Google Scholar] [CrossRef]
- Li, Y.; et al. Tranexamic acid ameliorates rosacea symptoms through regulating immune response and angiogenesis. Int Immunopharmacol 2019, 67, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol 2013, 40, 70–1. [Google Scholar] [CrossRef] [PubMed]
- Bageorgou, F.; et al. The new therapeutic choice of tranexamic acid solution in treatment of erythematotelangiectatic rosacea. J Cosmet Dermatol 2019, 18, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Daadaa, N.; et al. Intradermal tranexamic acid microinjections: a novel treatment option for erythematotelangiectatic rosacea. J Cosmet Dermatol 2021, 20, 3324–3329. [Google Scholar] [CrossRef]
- Denda, M.; et al. trans-4-(Aminomethyl)cyclohexane carboxylic acid (T-AMCHA), an anti-fibrinolytic agent, accelerates barrier recovery and prevents the epidermal hyperplasia induced by epidermal injury in hairless mice and humans. J Invest Dermatol 1997, 109, 84–90. [Google Scholar] [CrossRef]
- Katsuta, Y.; et al. trans-4-(Aminomethyl)cyclohexane carboxylic acid methylamide (t-AMCHA methylamide) inhibits the physical interaction between urokinase-type plasminogen activator and stratum corneum, and accelerates the recovery of barrier function. J Dermatol Sci 2005, 40, 218–20. [Google Scholar] [CrossRef]
- Zhong, S.; et al. Topical tranexamic acid improves the permeability barrier in rosacea. Dermatologica Sinica 2015, 33, 112–117. [Google Scholar] [CrossRef]
- Jackson, J.M.; Coulon, R.; Arbiser, L. Evaluation of a First-in-Class Proteasome Inhibitor in Patients With Moderate to Severe Rosacea. J Drugs Dermatol 2021, 20, 660–664. [Google Scholar]
- Amir Ali, A.; Vender, R.; Vender, R. The Role of IL-17 in Papulopustular Rosacea and Future Directions. J Cutan Med Surg 2019, 23, 635–641. [Google Scholar] [CrossRef]
- Kumar, A.M.; et al. An exploratory, open-label, investigator-initiated study of interleukin-17 blockade in patients with moderate-to-severe papulopustular rosacea. Br J Dermatol 2020, 183, 942–943. [Google Scholar] [CrossRef]
- Layton, A.M. Pharmacologic treatments for rosacea. Clin Dermatol 2017, 35, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; et al. Long-term safety and efficacy of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of a 1-year open-label study. J Drugs Dermatol 2014, 13, 56–61. [Google Scholar] [PubMed]
- Al Mokadem, S.M.; Ibrahim, M; El Sayed, A.M. Efficacy of Topical Timolol 0.5% in the Treatment of Acne and Rosacea: A Multicentric Study. J Clin Aesthet Dermatol 2020, 13, 22–27. [Google Scholar] [PubMed]
- Chen, L.; Tsai, T.F. The role of beta-blockers in dermatological treatment: a review. J Eur Acad Dermatol Venereol 2018, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Pawar, M.K. Treatment of painful and deep fissures of the heel with topical timolol. J Am Acad Dermatol 2021, 85, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; et al. Topical timolol 0.5% gel-forming solution for erythema in rosacea: A quantitative, split-face, randomized, and rater-masked pilot clinical trial. J Am Acad Dermatol 2021, 85, 1044–1046. [Google Scholar] [CrossRef]
- Wei, D.; Hamblin, M.R.; Wen, X. A randomized, controlled, split-face study of topical timolol maleate 0.5% eye drops for the treatment of erythematotelangiectatic rosacea. J Cosmet Dermatol 2021, 20, 3968–3973. [Google Scholar] [CrossRef]
- Aoki, K.R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005, 26, 785–93. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; et al. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci 2007, 120 Pt 16, 2864–74. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; et al. Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J Dermatol Sci 2019, 93, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.; et al. Role of botulinum toxin A in improving facial erythema and skin quality. Arch Dermatol Res 2022, 314, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; et al. Assessment of Skin Physiology Change and Safety After Intradermal Injections With Botulinum Toxin: A Randomized, Double-Blind, Placebo-Controlled, Split-Face Pilot Study in Rosacea Patients With Facial Erythema. Dermatol Surg 2019, 45, 1155–1162. [Google Scholar] [CrossRef]
- Zhang, H.; et al. Use of Botulinum Toxin in Treating Rosacea: A Systematic Review. Clin Cosmet Investig Dermatol 2021, 14, 407–417. [Google Scholar] [CrossRef]
- Tong, Y.; et al. A randomized, controlled, split-face study of botulinum toxin and broadband light for the treatment of erythematotelangiectatic rosacea. Dermatol Ther 2022, 35, e15395. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; et al. Botulinum Toxin A Alleviates Persistent Erythema and Flushing in Patients with Erythema Telangiectasia Rosacea. Dermatol Ther (Heidelb) 2022, 12, 2285–2294. [Google Scholar] [CrossRef]
- Calvisi, L.; Diaspro, A.; Sito, G. Microbotox: A prospective evaluation of dermatological improvement in patients with mild-to-moderate acne and erythematotelangiectatic rosacea. J Cosmet Dermatol 2022, 21, 3747–3753. [Google Scholar] [CrossRef]
- Wagner, K.D.; et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry 2004, 61, 1153–62. [Google Scholar] [CrossRef]
- Stearns, V.; et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005, 23, 6919–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; et al. Paroxetine is an effective treatment for refractory erythema of rosacea: Primary results from the Prospective Rosacea Refractory Erythema Randomized Clinical Trial. J Am Acad Dermatol 2023. [Google Scholar] [CrossRef] [PubMed]
- Wienholtz, N.K.F.; et al. Infusion of Pituitary Adenylate Cyclase-Activating Polypeptide-38 in Patients with Rosacea Induces Flushing and Facial Edema that Can Be Attenuated by Sumatriptan. J Invest Dermatol 2021, 141, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Wienholtz, N.; et al. Subtype-Specific Off-Label Treatment of Rosacea. Case Rep Dermatol 2021, 13, 121–128. [Google Scholar] [CrossRef]
- Craige, H.; Cohen, J.B. Symptomatic treatment of idiopathic and rosacea-associated cutaneous flushing with propranolol. J Am Acad Dermatol 2005, 53, 881–4. [Google Scholar] [CrossRef]
- Park, J.M.; et al. Propranolol, doxycycline and combination therapy for the treatment of rosacea. J Dermatol 2015, 42, 64–9. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.F. The drug treatment of anxiety. N Engl J Med 1982, 306, 401–4. [Google Scholar] [CrossRef]
- Wilkin, J.K. Effect of nadolol on flushing reactions in rosacea. J Am Acad Dermatol 1989, 20, 202–5. [Google Scholar] [CrossRef]
- Peet, M. Beta-blockade in anxiety. Postgrad Med J 1984, 60 Suppl 2, 16–8. [Google Scholar]
- Li, J.; et al. Carvedilol ameliorates persistent erythema of erythematotelangiectatic rosacea by regulating the status of anxiety/depression. J Dermatol 2022, 49, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lee, J.Y. Carvedilol for the treatment of refractory facial flushing and persistent erythema of rosacea. Arch Dermatol 2011, 147, 1258–60. [Google Scholar]
- Wong, W.T.; et al. Repositioning of the beta-Blocker Carvedilol as a Novel Autophagy Inducer That Inhibits the NLRP3 Inflammasome. Front Immunol 2018, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.A.; et al. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin-metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int J Infect Dis 2013, 17, e343–7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; et al. Rosacea management: A comprehensive review. J Cosmet Dermatol 2022, 21, 1895–1904. [Google Scholar] [CrossRef]
- Narayanan, S.; et al. Scavenging properties of metronidazole on free oxygen radicals in a skin lipid model system. J Pharm Pharmacol 2007, 59, 1125–30. [Google Scholar] [CrossRef]
- Huang, H.P.; Hsu, K.; Lee, J.Y. Topical ivermectin-induced transient flare of rosacea as a host reaction to killed Demodex mites preventable by short-term use of topical corticosteroid. Dermatol Ther 2022, 35, e15517. [Google Scholar] [CrossRef]
- Rubinchik, E.; et al. Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int J Antimicrob Agents 2009, 34, 457–61. [Google Scholar] [CrossRef]
- Grada, A.; et al. LB1092 Topical omiganan for severe papulopustular rosacea: A randomized, vehicle-controlled, double-blind, multicenter study. Journal of Investigative Dermatology 2019. [Google Scholar] [CrossRef]
- Drago, F.; et al. The role of small intestinal bacterial overgrowth in rosacea: A 3-year follow-up. J Am Acad Dermatol 2016, 75, e113–e115. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Steinhoff, M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol 2013, 68, 875–6. [Google Scholar] [CrossRef]
- Weinstock, L.B. Rosacea in Crohn's Disease: Effect of Rifaximin. J Clin Gastroenterol 2011, 45, 295–6. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; et al. Chinese guidelines on the clinical application of 5-aminolevulinic acid-based photodynamic therapy in dermatology (2021 edition). Photodiagnosis Photodyn Ther 2021, 35, 102340. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; et al. Photodynamic therapy with methyl aminolevulinate for resistant scalp folliculitis secondary to Demodex infestation. J Eur Acad Dermatol Venereol 2009, 23, 718–9. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Pierpoint, L. Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial agent. Med Res Rev 2012, 32, 1292–327. [Google Scholar] [CrossRef]
- Li, X.; et al. Effects of 5-aminolevulinic acid-mediated photodynamic therapy on antibiotic-resistant staphylococcal biofilm: an in vitro study. J Surg Res 2013, 184, 1013–21. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; et al. Photodynamic therapy in the treatment of rosacea: A systematic review. Photodiagnosis Photodyn Ther 2022, 38, 102875. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; et al. Topical photodynamic therapy with 5-aminolevulinic acid in Chinese patients with Rosacea. J Cosmet Laser Ther 2019, 21, 196–200. [Google Scholar] [CrossRef]
- Fan, L.; et al. Photodynamic therapy for rosacea in Chinese patients. Photodiagnosis Photodyn Ther 2018, 24, 82–87. [Google Scholar] [CrossRef]
- Yang, J.; et al. 5-Aminolevulinic Acid Photodynamic Therapy Versus Minocycline for moderate to severe rosacea: A single-center, randomized, evaluator-blind controlled study. J Am Acad Dermatol 2023. [Google Scholar] [CrossRef]
- Caterina, M.J.; et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–24. [Google Scholar] [CrossRef]
- Yu, X.; et al. Evaluation of Therapeutic Effect and Prognosis of Danzhi Xiaoyao Powder Combined with Photodynamic Therapy in the Treatment of Rose Acne. Comput Math Methods Med 2022, 2022, 1636839. [Google Scholar] [CrossRef]
- Wang, H.; An, X.; Wang, Z. Effect and Safety of ALA-PDT Combined with 1550 nm Fractional Therapy Laser in Treating Rosacea. Evid Based Complement Alternat Med 2022, 2022, 3335074. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; et al. Combined therapy of 5-aminolevulinic acid photodynamic therapy and intense pulsed light for rosacea. Lasers Med Sci 2022, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; et al. Topical aminolevulinic acid-photodynamic therapy in acne. Chinese Journal of Dermatology 2009, 42, 78–80. [Google Scholar]
- Aksoy Sarac, G.; Onder, M. Evaluation of the efficacy of pro-yellow laser in the management of vascular skin disorders. J Cosmet Dermatol 2022, 21, 1018–1022. [Google Scholar] [CrossRef]
- Kapicioglu, Y.; Sarac, G.; Cenk, H. Treatment of erythematotelangiectatic rosacea, facial erythema, and facial telangiectasia with a 577-nm pro-yellow laser: a case series. Lasers Med Sci 2019, 34, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Temiz, S.A.; et al. The effect of 577-nm pro-yellow laser on demodex density in patients with rosacea. J Cosmet Dermatol 2022, 21, 242–246. [Google Scholar] [CrossRef]
- Altunisik, N.; Turkmen, D. Commentary on "The effect of 577-nm pro-yellow laser on demodex density in patients with rosacea". J Cosmet Dermatol 2022, 21, 5254. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Mohamed Tawfik, K.; Hassan Ahmad, W. Successful treatment of facial vascular skin diseases with a 577-nm pro-yellow laser. J Cosmet Dermatol 2019, 18, 1675–1679. [Google Scholar] [CrossRef]
- Son, M.; et al. Radiofrequency irradiation attenuates angiogenesis and inflammation in UVB-induced rosacea in mouse skin. Exp Dermatol 2020, 29, 659–666. [Google Scholar] [CrossRef]
- Wang, B.; et al. Efficacy and safety of non-surgical short-wave radiofrequency treatment of mild-to-moderate erythematotelangiectatic rosacea: a prospective, open-label pilot study. Arch Dermatol Res 2022, 314, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; et al. Radiofrequency Irradiation Modulates TRPV1-Related Burning Sensation in Rosacea. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; et al. Comparative Efficacy of Radiofrequency and Pulsed Dye Laser in the Treatment of Rosacea. Dermatol Surg 2017, 43, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; et al. Clinical and Histologic Effects of Fractional Microneedling Radiofrequency Treatment on Rosacea. Dermatol Surg 2016, 42, 1362–1369. [Google Scholar] [CrossRef]
- Kwon, H.H.; et al. Combined treatment of recalcitrant papulopustular rosacea involving pulsed dye laser and fractional microneedling radiofrequency with low-dose isotretinoin. J Cosmet Dermatol 2020, 19, 105–111. [Google Scholar] [CrossRef]
- Kim, Y.J.; et al. The Efficacy and Safety of Dual-Frequency Ultrasound for Improving Skin Hydration and Erythema in Patients with Rosacea and Acne. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Park, J.Y.; et al. Dual-Frequency Ultrasound as a New Treatment Modality for Refractory Rosacea: A Retrospective Study. Dermatol Surg 2018, 44, 1209–1215. [Google Scholar] [CrossRef]
- Schlessinger, J.; George, R.; Lupin, M.; Amato, D.; McDaniel, D. Evaluation of the safety and efficacy of microfocused ultrasound with visualization (MFU-V) for the treatment of signs and symptoms of erythematotelangiectatic rosacea: Final data. Journal of the American Academy of Dermatology 2017, 76 (6, Supplement 1), AB133. [Google Scholar]
- Schlessinger, J.; et al. Safety and Effectiveness of Microfocused Ultrasound for Treating Erythematotelangiectatic Rosacea. J Drugs Dermatol 2019, 18, 522. [Google Scholar]
- Berardesca, E.; et al. A Randomized, Controlled Clinical Trial of a Dermocosmetic Containing Vichy Volcanic Mineralizing Water and Probiotic Fractions in Subjects with Rosacea Associated with Erythema and Sensitive Skin and Wearing Protective Masks. Clin Cosmet Investig Dermatol 2023, 16, 71–77. [Google Scholar] [CrossRef]
- Baldwin, H.; et al. A novel moisturizer with high sun protection factor improves cutaneous barrier function and the visible appearance of rosacea-prone skin. J Cosmet Dermatol 2019, 18, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; et al. Clinical Evaluation of a Nature-Based Bakuchiol Anti-Aging Moisturizer for Sensitive Skin. J Drugs Dermatol 2020, 19, 1181–1183. [Google Scholar] [CrossRef]
- Shen, S.; et al. Dietary supplementation of n-3 PUFAs ameliorates LL37-induced rosacea-like skin inflammation via inhibition of TLR2/MyD88/NF-kappaB pathway. Biomed Pharmacother 2023, 157, 114091. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, P.; Mousdicas, *!!! REPLACE !!!*; Bednarek, R. Rosacea Fulminans Precipitated by Acute Stress: A Case Report Describing an Integrative Approach for a Patient Reluctant to Use Isotretinoin. Integr Med (Encinitas) 2016, 15, 32–35. [Google Scholar] [PubMed]
- Vaughn, A.R.; et al. Dietary supplementation with turmeric polyherbal formulation decreases facial redness: a randomized double-blind controlled pilot study. J Integr Med 2019, 17, 20–23. [Google Scholar] [CrossRef]
- Zeng, Q.; et al. Celastrol inhibits LL37-induced rosacea by inhibiting Ca(2+)/CaMKII-mTOR-NF-kappaB activation. Biomed Pharmacother 2022, 153, 113292. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; et al. Efficacy of diammonium glycyrrhizinate in the treatment of rosacea with papules and pustules: A randomized, double-blind, placebo-controlled study. Dermatol Ther 2022, 35, e15905. [Google Scholar] [CrossRef]
- Weber, T.M.; et al. Skin tolerance, efficacy, and quality of life of patients with red facial skin using a skin care regimen containing Licochalcone A. J Cosmet Dermatol 2006, 5, 227–32. [Google Scholar] [CrossRef]
- Hoffmann, J.; et al. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8. [Google Scholar] [CrossRef]
- Wang, L.; et al. Retrospective analysis of 19 papulopustular rosacea cases treated with oral minocycline and supramolecular salicylic acid 30% chemical peels. Exp Ther Med 2020, 20, 1048–1052. [Google Scholar] [CrossRef]
- Ogasawara, H.; et al. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J Leukoc Biol 2019, 106, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; et al. Exploring the potential for rosacea therapeutics of siRNA dispersion in topical emulsions. Exp Dermatol 2019, 28, 261–269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





