1. Introduction
Intramedullary spinal cord tumors (IMSCT) are rare entities and account for only 5–10% of all spinal tumors. The most frequent entities are ependymomas, astrocytomas, and hemangioblastomas [
15]. Patients with low-grade tumors usually present with mild neurological deficit as gait ataxia, hypesthesia, or pain. The optimal treatment for IMSCT depends on the tumor type. For the majority of IMSCT, the standard therapy is the surgical gross total resection (GTR), although it bears a risk for neurological deterioration [
4,
18]. The GTR is the main predictor of progression-free survival in benign or low-grade entities [
9]. The GTR results in a more prolonged progression-free survival with a good neurological long-term outcome for ependymomas, hemangioblastomas, and pilocytic astrocytomas [
18]. However for high-grade astrocytomas, surgical resection does not provide any oncological benefit, is often not feasible, and comes along with a high rate of neurological impairment; therefore, usually a biopsy with adjuvant chemo- or radiotherapy is the commonly accepted approach [
3].
The most commonly affected site of IMSCT is the cervical spine [
18]. Especially their location in the upper cervical spine, i.e., craniocervical junction downwards up to the level of C4 or C5, carries the highest risk for postoperative neurological deficits resulting in a tetraparesis - a devastating disability condition. Additionally, surgical treatment may result in respiratory failure due to the innervation of the diaphragm by the C3-5 nerve roots. Further, tumor mass extending into the medulla oblongata can aggravate respiratory dysfunction. This problem is commonly known in patients who experienced traumatic spinal cord injury [
6]. However, there is little evidence of this complication in patients who undergo surgery for IMSCT. We are hereby investigating the surgical risks of intramedullary tumor surgery in the upper spine with particular emphasis on respiratory failure.
2. Patients and methods
We conducted a retrospective cohort study of patients who underwent surgical resection of IMSCT above or at the C4 level from January 2008 to December 2022 at our tertiary care institution.
2.1. Surgery Protocol and Postoperative Management
Microsurgical techniques with continuous neuromonitoring were used in all cases. Patients were placed in a prone position with a rigid head fixation in the Mayfield clamp. The most common approach was laminoplasty (57%), followed by a durotomy and median myelotomy in the vast majority of cases. For tumors near the surface of the spinal cord, we used either the transpial method or the dorsal root entry zone technique. Tumor debulking using a cavitron ultrasound surgical aspirator (CUSA) was applied in more extensive tumors (excluding hemangioblastomas) before the final tumor removal to minimize traction damage to the spinal cord tissue.
The local multidisciplinary tumor board decided on surgical and adjuvant treatment. High-grade astrocytomas received fractional radiation therapy with a total 40-60 Gy dose in 1.8 to 2-Gy fractions with or without Temozolomide therapy. Patients with ependymomas and hemangioblastomas underwent follow-up examinations every 12 months.
2.2. Medical and surgical data
All patients had preoperative contrast-enhanced magnetic resonance imaging (MRI) of the spinal cord to evaluate the tumor location, size, and extent. The tumor volume was calculated using the formula for ellipsoid: 4/3 × π × ABC/2, where A, B, and C are the length of semi-axes. We also assessed the presence of syrinx on the preoperative MRI scan.
The surgery-specific information included the extent of resection (GTR defined as complete removal of the contrast-enhancing tumor, subtotal resection (STR) defined as removal of more than 90% of the contrast-enhancing tumor, tumor debulking, or biopsy), as well as the intraoperative changes in recordings of the intraoperative neuromonitoring. The surgical approach was subclassified as unilateral (interlaminar fenestration or hemilaminectomy) or bilateral (laminoplasty and laminectomy).
In our cases, tumor histology was defined according to the current WHO classification, congruent with the WHO 2021 classification. The preoperative neurological status was evaluated. The outcome was assessed at discharge and the most recent follow-up. We used the modified McCormick scale to determine the clinical status of the patients, which is a widely accepted tool for neurological grading function in spinal cord tumours [
13]. We also recorded any postoperative neurological worsening, such as new motor deficit, hypesthesia, aggravation of gait ataxia, or respiratory insufficiency, which required postoperative intubation, prolonged weaning, or respiratory tract infections.
The patient underwent a follow-up MRI three months after the surgery and had yearly imaging and clinical tests afterward, as long as their clinical and neurological status remained stable. In cases of hemangioblastoma, further examinations to rule out Von-Hippel-Lindau syndrome were conducted.
2.3. Statistical Analysis
Statistical analyses were performed using Microsoft Excel (Version 2019) and StatTech v. 3.1.6. (Developer – StatTech LLC, Russia). The median or mean values were compared using the Student t-test. The correlation between potential predictive factors and the transient and permanent neurologic deficit was analyzed with a chi-square test for the categorical variable as sex, the extent of resection, presence of syrinx, WHO grade, tumor entity, and preoperative McCormick scale. For the continuous variables such as tumor volume, age, and duration of symptoms, the Kruskal-Wallis test was utilized.
3. Results
3.1. Entities and presentation
We included 35 consecutive patients who underwent surgical treatment protocol, 57% of patients were male with a mean age of 45 ± 16 years. The mean duration of symptoms before the intervention was 22 ± 48 months. Patients with high-grade tumors (metastasis and high-grade astrocytomas) had a significantly shorter symptoms duration than those with low-grade counterparts (p = 0.012). The median follow-up period was 3.27 ± 3.83 years and was available for 29 patients (83%).
The most common type of tumor was ependymoma WHO°II, which affected 22 patients (62.9%), followed by astrocytoma WHO ° II-IV in 4 patients (11.4%), hemangioblastoma WHO ° I in 6 patients (17.1%), and carcinoma metastasis (adenocarcinoma of colon cancer and melanoma metastasis) in 2 patients (5.7%). Four patients (11.4%) had a clinical diagnosis of neurofibromatosis type 2. Two patients (5.7%) had recurrent tumors at the same level, and two patients (5.7%) had drop metastasis of ependymomas at different levels from the upper cervical spine (
Table 1).
The tumor extension ranged from 1 to 9 segments, with a mean of 2.8. The mean tumor volume was 1.86 ± 2.58 cm [
3]. Syringomyelia was found in 8 out of 35 patients (23%).
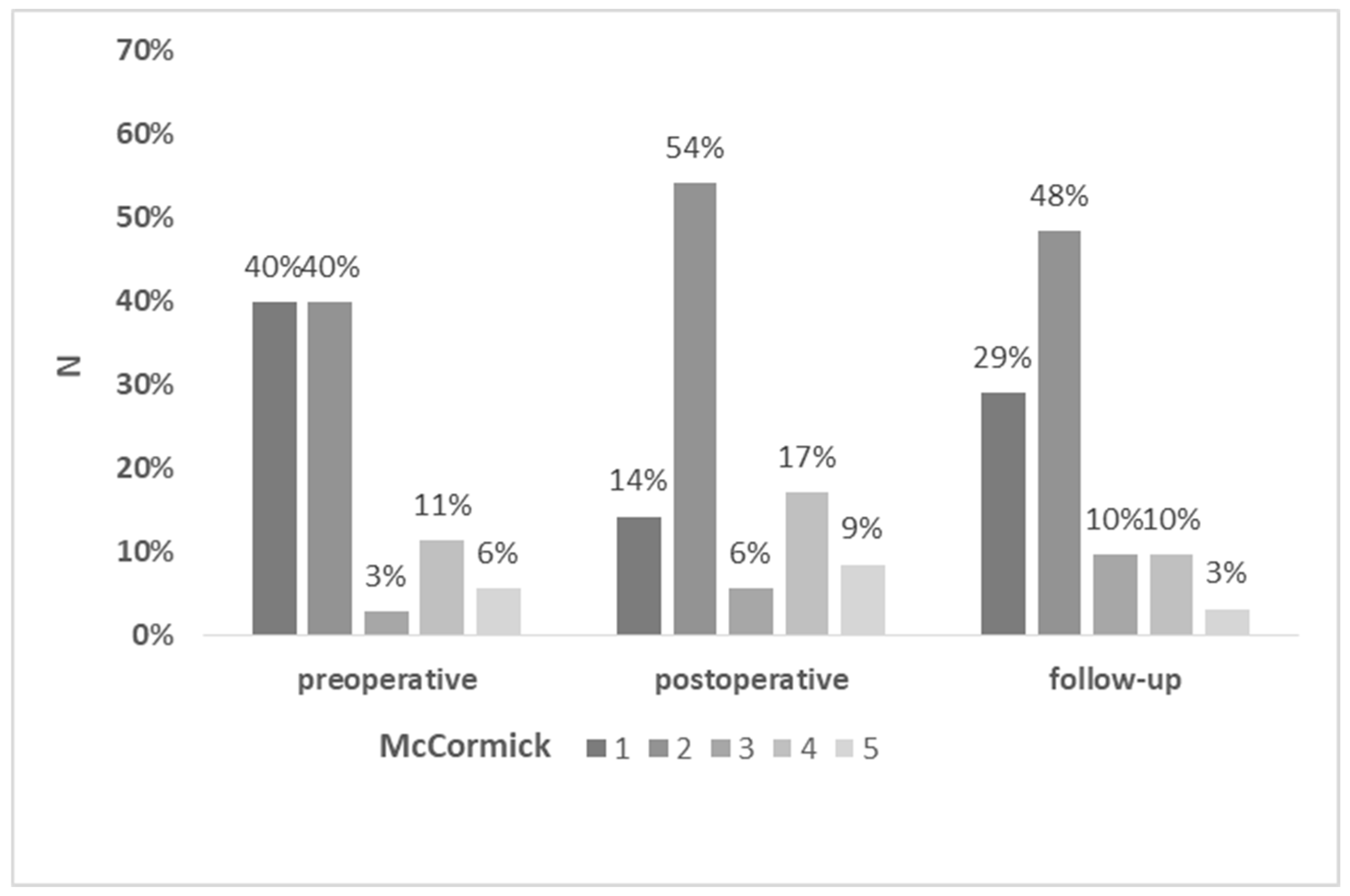
Table 2 shows the initial clinical symptoms that prompted the diagnosis. Most preoperative symptoms were mild, whereas 29 of 35 patients (83%) had a grade 1 or 2 on the modified McCormick scale rendering them functionally fully or nearly independent. Only five patients harboring high-grade astrocytomas or carcinoma metastases had a rapidly progressive neurological deficit.
3.2. Surgical and clinical outcome
GTR was achieved in 77% of patients who were candidates for this procedure because of the suspected tumor biology. In three patients, we performed a biopsy followed by resection in one case and combined radiation and chemotherapy in two cases with confirmed high-grade gliomas.
Dorsal column-related deficits, i.e. sensory loss and gait ataxia, and motor deficits occurred in 64% and 44% of patients, respectively. At follow up, 25% of patients with dorsal column-related dysfunction and 33% with motor dysfunction recovered to at least their preoperative status. Permanent motor deficits were observed in 33%, and sensory and gait dysfunction in 43% of patients, respectively. In 2 patients (5.7%) the preoperative neurological symptoms improved after surgery. Three patients had further neurologic decline during the follow-up, related to the tumor progression in one case and neurofibromatosis type 2-associated secondary ependymoma manifestation in two cases. The most common deficits were hypesthesia and gait ataxia. The results of preoperative, postoperative, and follow-up McCormick scores are summarized in
Figure 2. The median preoperative and follow-up McCormick score was 2, with no significant difference in the preoperative and follow-up functional status (p= 0,0897).
Three patients (8.6%) experienced respiratory complications in the early postoperative course. Two patients with high-grade lesions (glioblastoma WHO°IV and intramedullary metastasis) required prolonged mechanical ventilation and subsequent tracheotomy after the failed weaning. Both of these patients presented with higher-graded tetraparesis already before surgery. One of these patients was successfully decannulated during the follow-up period of 3 months; the other died during the same hospital stay as the treatment plan was switched to palliative care due to poor prognosis of the melanoma metastasis of the craniocervical junction. A third patient developed respiratory failure mainly due to a bronchial obstruction by a mucus plug. None of the remaining 32 patients (92%) developed any signs of respiratory dysfunction, including pneumonia or dyspnea.
Other surgery-related complications included postoperative hemorrhage that required surgery to remove the hematoma in one case and delayed wound healing in 2 patients who underwent revision surgery. The overall perioperative complication rate including patients with a postoperative respiratory failure was 14%. The 30-day mortality was 2.8% (n=1), whereas this case was not surgery-related.
The preoperative McCormick scale and the WHO grades were the only predictors of the transient (p < 0.001 and p = 0.045, respectively) and permanent postoperative impairment (p < 0.001 and p = 0.009, respectively) (
Table 3 and
Table 4). There were no statistically significant differences when comparing postoperative and follow-up McCormick scores on demographic parameters, duration of symptoms, tumor size, the presence of syringomyelia, or surgery-specific data.
3.3. Case presentation
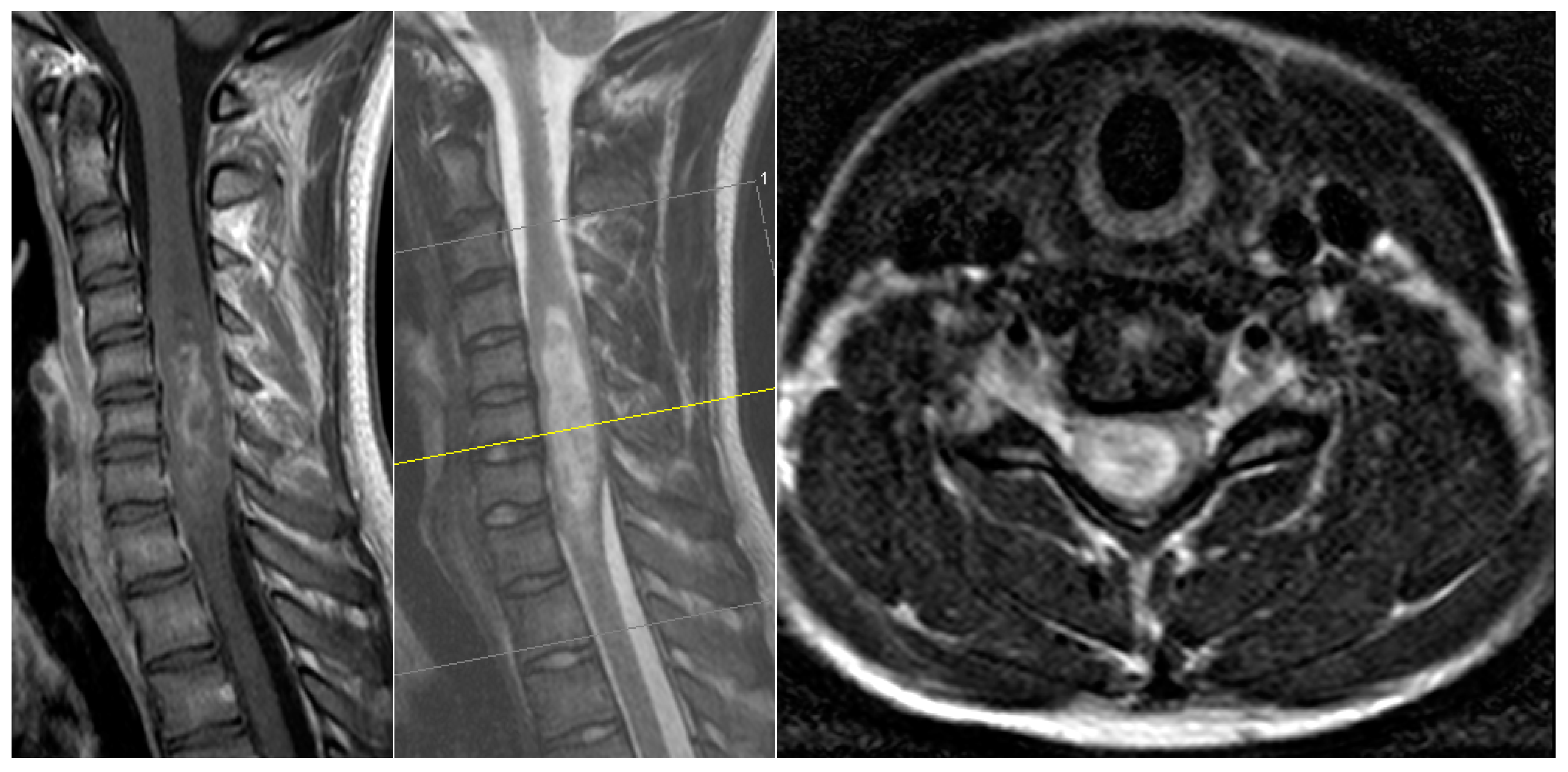
A 17-year-old male patient came to our emergency department with a subacute tetraparesis with accentuation on the right side. An MRI scan (
Figure 1) revealed a large intramedullary lesion reaching to the upper cervical spinal cord and demonstrating irregular contrast enhancement, highly suspicious for a high-grade tumor. His neurological condition worsened overnight despite intravenous dexamethasone administration, and he developed a C5-level ASIA A quadriplegia by the next morning. We performed a microsurgical GTR of the lesion on the same day. The histopathology revealed a diagnosis of a glibomastoma. The patient did not recover from the quadriplegia. Due to occurred respiratory insufficiency, early tracheotomy was performed 8 days later after the failed weaning. He was sent to neurological rehabilitation. Three months later, he was still quadriplegic, but no longer needed the tracheotomy. He passed away seven months after the surgery.
4. Discussion
4.1. Clinical outcome
Surgery or at least a biopsy is usually inevitable during the treatment course of IMSCT. GTR is considered the best treatment strategy for the vast majority, as it benefits progression-free survival.
The risk for transient postoperative impairment is known to be significantly high. Previous research on patients with spinal ependymomas reported that more than 60% of patients experience neurological decline immediately after surgery [
7,
11,
18]. In our case series, 77% of the patients developed new neurological deficits, respectively, resulting in a relevant functional decline in 40% according to a deterioration in the McCormick scale. There is current evidence that the cervical location is an adverse predictive factor for functional outcomes after the surgery of IMSCT. In a large retrospective study reporting on ependymomas of the upper cervical spinal cord, Fei et al. reported on postoperative neurological impairment in 76% of patients and observed a subsequent slow recovery during the next 56 months, still rendering 21% of patients with permanent deterioration [
5]. A similar trend of preoperative, early, and late postoperative neurological status of their patients was reported by another series of intramedullary ependymomas, with a significant deterioration of McCormick scale in the early postoperative period and a recovery to the preoperative condition at the follow-up [
16]. However, the difference in the patient´s neurological status was not accompanied by any relevant decline in functional disability as reflected by McCormick scale in our study.
4.2. Prognostic factors for neurologic deterioration
We analyzed the predictive factors of postsurgical neurologic deterioration in the early and late follow-up periods. We found that the preoperative McCormick scale grade and WHO grade were the strongest predictors of postoperative decline. This is consistent with several publications [
2,
12]. In accordance with our findings and previously conducted studies [
5] observing a solid correlation of postoperative neurological decrease with the extent of preoperative disability, we recommend early surgery when patients’ neurological deficits are mild. As observed in our study, only a small proportion of patients improved their functional status compared to the status before surgery (5.7% in our series). According to this observation, the wait-and-see strategy, which would inevitably lead to a slow deterioration due to the tumor progression, appears unreasonable: first, retrieving the initial status quo is quite unlikely if the surgery is done later on; second, the risk for further functional decline is rising during the waiting time.
The other significant criterion for poor neurologic outcomes at follow-up is the WHO grade. This could be explained by the higher neurologic deficits in patients with high-grade astrocytomas and metastases compared to those with ependymomas and hemangioblastomas at presentation. The infiltrating nature of these entities renders surgical treatment less safe. For this reason, the commonly accepted treatment strategy for these tumors is to refrain from GTR and to go for a sample collection via a biopsy to confirm the diagnosis and proceed with adjuvant treatment [
3]. Furthermore, Intramedullary higher-graded spinal cord gliomas have a poor prognosis despite surgery, radiation, and chemotherapy [
3,
10]. These treatment options can hardly prevent rapid neurological deterioration and poor overall survival of most patients. Future research on molecular genetics and targeted therapy for these tumors may bring new potential treatment options for these entities [
1,
8].
Intramedullary metastases occur mainly during the late stages of cancer and are frequently associated with leptomeningeal spread reflecting an overall poor prognosis. However, surgical therapy may result in a temporary delay of the further decline, or even improvement of neurological deficits in some cases. In contrast, the surgery's only goal is spinal cord decompression by tumor mass debulking or reducing the associated syrinx [
14,
17].
Injury to the upper cervical spine carries the potential risk of respiratory failure requiring mechanical ventilation, prolonged ICU stay, prolonged weaning, early tracheotomy, increased risk of pulmonary infections, and other associated complications [
6]. The respiratory function cannot be monitored by conventional intraoperative monitoring. Nevertheless, though patients with IMSCT seem to be at risk for this adverse clinical course, the incidence appears relatively low. In our series, only three patients (8.6%) developed respiratory failure; in two cases (5.7%), this was clearly caused by the upper spinal cord dysfunction. Although respiratory failure appears to be a relatively rare complication, we strongly recommend a close monitoring for patients who underwent surgery of IMSCT of the upper spine during the first days after surgery and at least for 24 hours due to possible life-threatening consequences.
5. Conclusion
Patients with low-grade IMSCT of the upper cervical spine can maintain a good functional status after surgery during the long-term follow-up. However, they should be informed about the high likelihood of transient neurological deficits along with functional decline and temporary disability. Poor preoperative neurological condition and tumor malignancy may contribute to new neurologic deficits. The risk of respiratory deterioration is relatively low but should be considered for postoperative management and rehabilitation.
Author Contributions
Conception and design: VB, MW. Acquisition of data: VB, KG. Analysis and interpretation of data: VB, MW, KG. Manuscript draft: VB, MW, KG, MG. Critical revision for important intellectual content: BM, VB, MW. Final approval: VB, MW, BM.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was funded by the Technical University of Munich.
Institutional Review Board Statement
The presented study meets the ethical standards outlined in the Declaration of Helsinki, ethics approval was obtained and the positive vote was registered under the Number 230/20-S.
Informed Consent Statement
Patient informed consent was not required and waived by our local ethics committee due to the retrospective design of this study.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interest
All authors report no confict of interest concerning the materials or methods used in this study or the fndings specifed in this publication.
References
- Azad, T.D.; Jiang, B.; Bettegowda, C. Molecular foundations of primary spinal tumors-implications for surgical management. Ann Transl Med 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, A.; Kanther, N.C.; Grote, A.; Bostrom, J. Management and outcome in adult intramedullary spinal cord tumours: a 20-year single institution experience. BMC Res Notes 2014, 7, 908. [Google Scholar] [CrossRef]
- Butenschoen, V.M.; Hubertus, V.; Janssen, I.K.; Onken, J.; Wipplinger, C.; Mende, K.C.; et al. Surgical treatment and neurological outcome of infiltrating intramedullary astrocytoma WHO II-IV: a multicenter retrospective case series. J Neurooncol 2021, 151, 181–191. [Google Scholar] [CrossRef]
- Butenschoen, V.M.; Schwendner, M.; Hubertus, V.; Onken, J.; Koegl, N.; Mohme, T.; et al. Preoperative angiographic considerations and neurological outcome after surgical treatment of intradural spinal hemangioblastoma: a multicenter retrospective case series. J Neurooncol 2023, 161, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Jia, W.; Gao, H.; Yang, C.; Li, D.; Qian, Z.; et al. Clinical characteristics and surgical outcomes of ependymomas in the upper cervical spinal cord: a single-center experience of 155 consecutive patients. Neurosurg Rev 2021, 44, 1665–1673. [Google Scholar] [CrossRef]
- Galeiras Vazquez, R.; Rascado Sedes, P.; Mourelo Farina, M.; Montoto Marques, A.; Ferreiro Velasco, M.E. Respiratory management in the patient with spinal cord injury. Biomed Res Int 2013, 2013, 168757. [Google Scholar] [CrossRef] [PubMed]
- Gembruch, O.; Chihi, M.; Haarmann, M.; Parlak, A.; Oppong, M.D.; Rauschenbach, L.; et al. Surgical outcome and prognostic factors in spinal cord ependymoma: a single-center, long-term follow-up study. Ther Adv Neurol Disord 2021, 14, 17562864211055694. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.; Melnick, K.; Porche, K.; Dastmalchi, F.; Hoh, D.J.; Rahman, M.; et al. Glioma Immunotherapy: Advances and Challenges for Spinal Cord Gliomas. Neurospine 2022, 19, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Antar, A.; Pennington, Z.; Aygun, N.; Patel, J.; Goldsborough, E., 3rd; et al. Predictors of survival and time to progression following operative management of intramedullary spinal cord astrocytomas. J Neurooncol 2022, 158, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Houten, J.K.; Cooper, P.R. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol 2000, 47, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Parker, W.E.; Barzilai, O.; Bilsky, M.H. Surgical Management of Intramedullary Spinal Cord Tumors. Neurosurg Clin N Am 2020, 31, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Cho, Y.E.; Kwon, Y.M. Neurological outcome after surgical treatment of intramedullary spinal cord tumors. Korean J Spine 2014, 11, 121–126. [Google Scholar] [CrossRef] [PubMed]
- McCormick, P.C.; Torres, R.; Post, K.D.; Stein, B.M. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Payer, S.; Mende, K.C.; Westphal, M.; Eicker, S.O. Intramedullary spinal cord metastases: an increasingly common diagnosis. Neurosurg Focus 2015, 39, E15. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Gillis, C.C.; Shih, P.; O'Toole, J.E.; Fessler, R.G. Intramedullary Spinal Cord Tumors: Part I-Epidemiology, Pathophysiology, and Diagnosis. Global Spine J 2015, 5, 425–435. [Google Scholar] [CrossRef]
- Takami, T.; Naito, K.; Yamagata, T.; Ohata, K. Surgical management of spinal intramedullary tumors: radical and safe strategy for benign tumors. Neurol Med Chir (Tokyo) 2015, 55, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Wostrack, M.; Pape, H.; Kreutzer, J.; Ringel, F.; Meyer, B.; Stoffel, M. Surgical treatment of spinal intradural carcinoma metastases. Acta Neurochir (Wien) 2012, 154, 349–357. [Google Scholar] [CrossRef]
- Wostrack, M.; Ringel, F.; Eicker, S.O.; Jagersberg, M.; Schaller, K.; Kerschbaumer, J.; et al. Spinal ependymoma in adults: a multicenter investigation of surgical outcome and progression-free survival. J Neurosurg Spine 2018, 28, 654–662. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).