Submitted:
04 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Lipofuscin: formation, composition, and potential deleterious effects
3. Lipofuscin fluorescence
4. Lipofuscin in the retina
4.1. Retinal pigment epithelium (RPE) is the major site of lipofuscin accumulation in the retina
4.2. Oxidative stress, lysosomal dysfunction, and vitamin A derivatives as contributors to the accumulation of RPE lipofuscin
4.3. Structure and composition of RPE lipofuscin
4.4. Distribution of lipofuscin in the human RPE
4.5. Effects of RPE lipofuscin on the function and viability of RPE cells and photoreceptors
5. Fluorescence of RPE lipofuscin
6. Fluorescence of the retina:
6.1. Sources of fluorescence in the retina
6.2. Imaging of fluorescence in the retina
6.3. Age-related changes in retinal fluorescence
| Study on normal human eyes in vivo and ex vivo | Excitation (nm) | Emission (nm) | Age-related changes in fluorescence intensity |
| Cross-sections from 19 White donors, 2 weeks-88 years of age, and 19 Black donors, 6.5-90 years old [148] | 365 | 470 | Age-related increase for Whites No correlation with age for Blacks |
| Cross-sections from 44 human eyes from 35 donors, 6-week premature newborn - 88 years [147] | 380 | 460-480 | A fast increase in the 1st and 2nd decades of life, then slowing down followed by an increase in people above the age of 60 years; about a 40% increase in fluorescence emission intensity in the oldest age group 61-88 in comparison with 31-60 years group |
| 30 participants, 21-67 years of age [278] | 430 | 620 | No significant correlation with age |
| RPE-Bruch’s membrane flat-mounts from 20 donors divided into two age groups: 16-51 years of age (10 donors, average age of 40 years), and 82-90 years of age (10 donors, average age of 85 years) [336] | 460-490 | >505 | Increased in the 82-90 years-old group in comparison with the 16-51 years-old group |
| 33 white participants 6-78 years of age [284] | 488 | >521 | linear increase with age from 6 to about 60 years, above 60 the emission appeared to plateau |
| Formalin-fixed 8 mm in diameter circles centred on the fovea of 88 donors ranging in age from 1-98 years [337] | 450-490 | >520 | a linear increase up to the age of 60 years, followed by a plateau; supported by TEM quantification of LF |
| 277 participants of different ethnicities from 5-60 years of age [338] | 488 | 500-680 | The age-related increase in fundus fluorescence was the greatest for Whites, followed by Indians, Hispanics, Blacks, and Asians |
| 145 participants, 15-80 years of age [335] | 470 | >520 | Intensities reached a maximum for the age group in their 7th decade and remained at the same level in the 8th decade |
| 30 participants, 21-67 years of age [278] | 470, 510 or 550 | 620 | Positive correlation with age |
| 145 participants, 15-80 years of age [335] | 550 | 650-750 | A linear increase in fluorescence occurred up to the age of 70 years, followed by a steep decrease |
| 44 participants below 40 years of age (average age of 24 years) and 18 participants above 40 years of age (average age of 67.5 years); calculated emission maxima based on emission of fluorescence in two spectral channels [340] | 473 | 498-560 and 560-720 | For the young group, the emission maxima were at 602±16, 614±12, and 621±11 nm for the fovea, inner and outer ring, respectively. For the elderly group the emission maxima were at 599±17, 611±11, and 614±11, respectively |
6.3. Fundus autofluorescence in age-related macular degeneration (AMD)
6.4.1. Sources of fluorescence in the AMD retina examined ex vivo
6.4.2. Fluorescence characteristics of AMD retina in vivo
6.4.3. Current evidence for the prognostic value of fundus fluorescence characteristics for AMD progression
7. Retinal spectral fluorescence characteristics as a potential in vivo biomarker of oxidative damage and efficacy of potential antioxidant therapies
7.1. Current evidence for photooxidation of lipofuscin in vivo
7.2. Current evidence for increased oxidative stress and oxidative damage in AMD retina
7.3. RPE lipofuscin fluorescence: intensity and spectral characteristics as a potential biomarker of oxidative damage to the retina in vivo.
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic Biol Med 1996, 21, 871–888. [Google Scholar] [CrossRef]
- Terman, A.; Gustafsson, B.; Brunk, U.T. Autophagy, organelles and ageing. J Pathol 2007, 211, 134–143. [Google Scholar] [CrossRef]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 2007, 1119, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Kurz, T.; Navratil, M.; Arriaga, E.A.; Brunk, U.T. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal 2010, 12, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Wang, Q.; Hu, H.; Shen, Y.; Fan, C.; Chen, P.; Ma, Y.; Wu, H.; Xiang, M. Restoring autophagic flux attenuates cochlear spiral ganglion neuron degeneration by promoting TFEB nuclear translocation via inhibiting MTOR. Autophagy 2019, 15, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Gugiu, B.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Rozanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics 2008, 7, 1397–1405. [Google Scholar] [CrossRef]
- Krog, S.; Ludvigsen, T.P.; Nielsen, O.L.; Kirk, R.K.; Lykkegaard, K.; Wulff, E.M.; Moller, J.E.; Pedersen, H.D.; Olsen, L.H. Myocardial Changes in Diabetic and Nondiabetic Nonhuman Primates. Vet Pathol 2020, 57, 332–343. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci Rep 2019, 9, 3304. [Google Scholar] [CrossRef]
- Moreno-Garcia, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front Neurosci 2018, 12, 464. [Google Scholar] [CrossRef]
- Couve, E.; Schmachtenberg, O. Autophagic activity and aging in human odontoblasts. J Dent Res 2011, 90, 523–528. [Google Scholar] [CrossRef]
- Sulzer, D.; Mosharov, E.; Talloczy, Z.; Zucca, F.A.; Simon, J.D.; Zecca, L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem 2008, 106, 24–36. [Google Scholar] [CrossRef]
- Jung, T.; Hohn, A.; Grune, T. Lipofuscin: detection and quantification by microscopic techniques. Methods Mol Biol 2010, 594, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Simonati, A.; Williams, R.E. Neuronal Ceroid Lipofuscinosis: The Multifaceted Approach to the Clinical Issues, an Overview. Front Neurol 2022, 13, 811686. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nelvagal, H.R.; Lange, J.; Cooper, J.D. Glial Dysfunction and Its Contribution to the Pathogenesis of the Neuronal Ceroid Lipofuscinoses. Front Neurol 2022, 13, 886567. [Google Scholar] [CrossRef]
- Nelvagal, H.R.; Lange, J.; Takahashi, K.; Tarczyluk-Wells, M.A.; Cooper, J.D. Pathomechanisms in the neuronal ceroid lipofuscinoses. Biochim Biophys Acta Mol Basis Dis 2020, 1866, 165570. [Google Scholar] [CrossRef]
- Valdez, C.; Wong, Y.C.; Schwake, M.; Bu, G.; Wszolek, Z.K.; Krainc, D. Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients. Hum Mol Genet 2017, 26, 4861–4872. [Google Scholar] [CrossRef]
- Pan, C.; Banerjee, K.; Lehmann, G.L.; Almeida, D.; Hajjar, K.A.; Benedicto, I.; Jiang, Z.; Radu, R.A.; Thompson, D.H.; Rodriguez-Boulan, E.; et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Klein, Z.A.; Takahashi, H.; Ma, M.; Stagi, M.; Zhou, M.; Lam, T.T.; Strittmatter, S.M. Loss of TMEM106B Ameliorates Lysosomal and Frontotemporal Dementia-Related Phenotypes in Progranulin-Deficient Mice. Neuron 2017, 95, 281–296 e286. [Google Scholar] [CrossRef]
- Kohlschutter, A.; Schulz, A. CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis). Pediatr Endocrinol Rev 2016, 13 Suppl 1, 682–688. [Google Scholar]
- Ach, T.; Tolstik, E.; Messinger, J.D.; Zarubina, A.V.; Heintzmann, R.; Curcio, C.A. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci 2015, 56, 3242–3252. [Google Scholar] [CrossRef]
- Nilsson, M.I.; MacNeil, L.G.; Kitaoka, Y.; Suri, R.; Young, S.P.; Kaczor, J.J.; Nates, N.J.; Ansari, M.U.; Wong, T.; Ahktar, M.; et al. Combined aerobic exercise and enzyme replacement therapy rejuvenates the mitochondrial-lysosomal axis and alleviates autophagic blockage in Pompe disease. Free Radic Biol Med 2015, 87, 98–112. [Google Scholar] [CrossRef]
- Simonati, A.; Pezzini, F.; Moro, F.; Santorelli, F.M. Neuronal Ceroid Lipofuscinosis: The Increasing Spectrum of an Old Disease. Curr Mol Med 2014, 14, 1043–1051. [Google Scholar] [CrossRef]
- Vidal-Donet, J.M.; Carcel-Trullols, J.; Casanova, B.; Aguado, C.; Knecht, E. Alterations in ROS activity and lysosomal pH account for distinct patterns of macroautophagy in LINCL and JNCL fibroblasts. PLoS One 2013, 8, e55526. [Google Scholar] [CrossRef] [PubMed]
- Piyanova, A.; Albayram, O.; Rossi, C.A.; Farwanah, H.; Michel, K.; Nicotera, P.; Sandhoff, K.; Bilkei-Gorzo, A. Loss of CB1 receptors leads to decreased cathepsin D levels and accelerated lipofuscin accumulation in the hippocampus. Mech Ageing Dev 2013, 134, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Baltazar, G.C.; Coffey, E.E.; Tu, L.A.; Lim, J.C.; Beckel, J.M.; Patel, S.; Eysteinsson, T.; Lu, W.; O’Brien-Jenkins, A.; et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J 2013, 27, 4500–4509. [Google Scholar] [CrossRef] [PubMed]
- Kohan, R.; Cismondi, I.A.; Oller-Ramirez, A.M.; Guelbert, N.; Anzolini, T.V.; Alonso, G.; Mole, S.E.; de Kremer, D.R.; de Halac, N.I. Therapeutic approaches to the challenge of neuronal ceroid lipofuscinoses. Curr Pharm Biotechnol 2011, 12, 867–883. [Google Scholar] [CrossRef] [PubMed]
- 10.2174/138920111795542633.
- Katz, M.L.; Shanker, M.J. Development of lipofuscin-like fluorescence in the retinal pigment epithelium in response to protease inhibitor treatment. Mech Ageing Dev 1989, 49, 23–40. [Google Scholar] [CrossRef]
- Ivy, G.O.; Schottler, F.; Wenzel, J.; Baudry, M.; Lynch, G. Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science 1984, 226, 985–987. [Google Scholar] [CrossRef]
- Kang, H.T.; Lee, K.B.; Kim, S.Y.; Choi, H.R.; Park, S.C. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One 2011, 6, e23367. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, M.H.; Kim, H.J.; Koh, J.Y. Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia 2010, 58, 1186–1196. [Google Scholar] [CrossRef]
- Stroikin, Y.; Dalen, H.; Loof, S.; Terman, A. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur J Cell Biol 2004, 83, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Sittig, A.; Jung, T.; Grimm, S.; Grune, T. Lipofuscin is formed independently of macroautophagy and lysosomal activity in stress-induced prematurely senescent human fibroblasts. Free Radic Biol Med 2012, 53, 1760–1769. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Nagy, M.; Fenton, W.A.; Horwich, A.L. Absence of lipofuscin in motor neurons of SOD1-linked ALS mice. Proc Natl Acad Sci U S A 2014, 111, 11055–11060. [Google Scholar] [CrossRef]
- Martinez-Cisuelo, V.; Gomez, J.; Garcia-Junceda, I.; Naudi, A.; Cabre, R.; Mota-Martorell, N.; Lopez-Torres, M.; Gonzalez-Sanchez, M.; Pamplona, R.; Barja, G. Rapamycin reverses age-related increases in mitochondrial ROS production at complex I, oxidative stress, accumulation of mtDNA fragments inside nuclear DNA, and lipofuscin level, and increases autophagy, in the liver of middle-aged mice. Exp Gerontol 2016, 83, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Tzekov, R.; Li, H.; McDowell, J.H.; Gao, G.; Smith, W.C.; Tang, S.; Kaushal, S. Inhibition or Stimulation of Autophagy Affects Early Formation of Lipofuscin-Like Autofluorescence in the Retinal Pigment Epithelium Cell. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Rao, S.; Fliesler, S.J. Monitoring basal autophagy in the retina utilizing CAG-mRFP-EGFP-MAP1LC3B reporter mouse: technical and biological considerations. Autophagy 2022, 18, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Yu, M.; Liu, Y.; Weh, E.; Pawar, M.; Li, L.; Besirli, C.G.; Schwendeman, A.A. Synthetic high-density lipoprotein nanoparticles delivering rapamycin for the treatment of age-related macular degeneration. Nanomedicine 2022, 44, 102571. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2022, 1–13. [Google Scholar] [CrossRef]
- Li, W.W.; Wang, H.J.; Tan, Y.Z.; Wang, Y.L.; Yu, S.N.; Li, Z.H. Reducing lipofuscin accumulation and cardiomyocytic senescence of aging heart by enhancing autophagy. Exp Cell Res 2021, 403, 112585. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Bajaj, L.; Lotfi, P.; Pal, R.; Ronza, A.D.; Sharma, J.; Sardiello, M. Lysosome biogenesis in health and disease. J Neurochem 2019, 148, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, K.; Otto, L.; Haeren, R.; Hoogland, G.; Schijns, O.; Vink, H.; Klein, D.; van Zandvoort, M.; Rijkers, K. Shedding light on human cerebral lipofuscin: An explorative study on identification and quantification. J Comp Neurol 2021, 529, 605–615. [Google Scholar] [CrossRef]
- McElnea, E.M.; Hughes, E.; McGoldrick, A.; McCann, A.; Quill, B.; Docherty, N.; Irnaten, M.; Farrell, M.; Clark, A.F.; O’Brien, C.J.; et al. Lipofuscin accumulation and autophagy in glaucomatous human lamina cribrosa cells. BMC Ophthalmol 2014, 14, 153. [Google Scholar] [CrossRef]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta 2008, 1780, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.B.; Lin, Y.; Luna, C.; Li, G.; Gonzalez, P.; Epstein, D.L. Cultured porcine trabecular meshwork cells display altered lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci 2008, 49, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Marzabadi, M.R.; Sohal, R.S.; Brunk, U.T. Effect of alpha-tocopherol and some metal chelators on lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Anal Cell Pathol 1990, 2, 333–346. [Google Scholar]
- Marzabadi, M.R.; Sohal, R.S.; Brunk, U.T. Effect of ferric iron and desferrioxamine on lipofuscin accumulation in cultured rat heart myocytes. Mech Ageing Dev 1988, 46, 145–157. [Google Scholar] [CrossRef]
- Brunk, U.T.; Jones, C.B.; Sohal, R.S. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res 1992, 275, 395–403. [Google Scholar] [CrossRef]
- Shevtsova, Z.; Garrido, M.; Weishaupt, J.; Saftig, P.; Bahr, M.; Luhder, F.; Kugler, S. CNS-expressed cathepsin D prevents lymphopenia in a murine model of congenital neuronal ceroid lipofuscinosis. Am J Pathol 2010, 177, 271–279. [Google Scholar] [CrossRef]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid Redox Signal 2015, 23, 239–255. [Google Scholar] [CrossRef]
- Stroikin, Y.; Dalen, H.; Brunk, U.T.; Terman, A. Testing the “garbage” accumulation theory of ageing: mitotic activity protects cells from death induced by inhibition of autophagy. Biogerontology 2005, 6, 39–47. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T.; Nilsson, E.; Docke, W.D.; Brunk, U.T. Lipofuscin accumulation and ageing of fibroblasts. Gerontology 1995, 41 Suppl 2, 95–108. [Google Scholar] [CrossRef]
- Terman, A.; Kurz, T.; Gustafsson, B.; Brunk, U.T. The involvement of lysosomes in myocardial aging and disease. Curr Cardiol Rev 2008, 4, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Epstein, D.L.; Liton, P.B. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Invest Ophthalmol Vis Sci 2010, 51, 6483–6495. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gomez, N.M.; Lim, J.C.; Guha, S.; O’Brien-Jenkins, A.; Coffey, E.E.; Campagno, K.E.; McCaughey, S.A.; Laties, A.M.; Carlsson, L.G.; et al. The P2Y12 Receptor Antagonist Ticagrelor Reduces Lysosomal pH and Autofluorescence in Retinal Pigmented Epithelial Cells From the ABCA4(-/-) Mouse Model of Retinal Degeneration. Front Pharmacol 2018, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Kravic, B.; Meyer, H. Repair or Lysophagy: Dealing with Damaged Lysosomes. J Mol Biol 2020, 432, 231–239. [Google Scholar] [CrossRef]
- Kurz, T.; Eaton, J.W.; Brunk, U.T. Redox activity within the lysosomal compartment: implications for aging and apoptosis. Antioxid Redox Signal 2010, 13, 511–523. [Google Scholar] [CrossRef]
- Hohn, A.; Jung, T.; Grimm, S.; Grune, T. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic Biol Med 2010, 48, 1100–1108. [Google Scholar] [CrossRef]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol 2008, 129, 389–406. [Google Scholar] [CrossRef]
- Grubman, A.; Pollari, E.; Duncan, C.; Caragounis, A.; Blom, T.; Volitakis, I.; Wong, A.; Cooper, J.; Crouch, P.J.; Koistinaho, J.; et al. Deregulation of biometal homeostasis: the missing link for neuronal ceroid lipofuscinoses? Metallomics 2014, 6, 932–943. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Park, M.K.; Mori, T.; Kawashima, S. The difference in autofluorescence features of lipofuscin between brain and adrenal. Zoolog Sci 1995, 12, 283–288. [Google Scholar] [CrossRef]
- Katz, M.L.; Robison, W.G., Jr.; Herrmann, R.K.; Groome, A.B.; Bieri, J.G. Lipofuscin accumulation resulting from senescence and vitamin E deficiency: spectral properties and tissue distribution. Mech Ageing Dev 1984, 25, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Docchio, F.; Dayhaw-Barker, P.; Ramponi, R.; Cubeddu, R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res 1990, 30, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Beppu, M.; Sato, A.; Kasai, H. Separation of multiple yellow fluorescent lipofuscin components in rat kidney and their characterization. Mech Ageing Dev 1997, 97, 93–107. [Google Scholar] [CrossRef]
- Eldred, G.E.; Miller, G.V.; Stark, W.S.; Feeney-Burns, L. Lipofuscin: resolution of discrepant fluorescence data. Science 1982, 216, 757–759. [Google Scholar] [CrossRef]
- Warburton, S.; Southwick, K.; Hardman, R.M.; Secrest, A.M.; Grow, R.K.; Xin, H.; Woolley, A.T.; Burton, G.F.; Thulin, C.D. Examining the proteins of functional retinal lipofuscin using proteomic analysis as a guide for understanding its origin. Mol Vis 2005, 11, 1122–1134. [Google Scholar] [PubMed]
- Kikugawa, K.; Beppu, M.; Kato, T.; Yamaki, S.; Kasai, H. Accumulation of autofluorescent yellow lipofuscin in rat tissues estimated by sodium dodecylsulfate extraction. Mech Ageing Dev 1994, 74, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Beppu, M. Involvement of lipid oxidation products in the formation of fluorescent and cross-linked proteins. Chem Phys Lipids 1987, 44, 277–296. [Google Scholar] [CrossRef]
- Kikugawa, K.; Kato, T.; Beppu, M.; Hayasaka, A. Fluorescent and cross-linked proteins formed by free radical and aldehyde species generated during lipid oxidation. Adv Exp Med Biol 1989, 266, 345–356; discussion 357. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Modification of bovine serum albumin structure following reaction with 4,5(E)-epoxy-2(E)-heptenal. Chem Res Toxicol 2000, 13, 501–508. [Google Scholar] [CrossRef]
- d’Ischia, M.; Costantini, C.; Prota, G. Lipofuscin-like pigments by autoxidation of polyunsaturated fatty acids in the presence of amine neurotransmitters: the role of malondialdehyde. Biochim Biophys Acta 1996, 1290, 319–326. [Google Scholar] [CrossRef]
- Eldred, G.E.; Katz, M.L. The autofluorescent products of lipid peroxidation may not be lipofuscin-like. Free Radic Biol Med 1989, 7, 157–163. [Google Scholar] [CrossRef]
- Yin, D.Z.; Brunk, U.T. Microfluorometric and fluorometric lipofuscin spectral discrepancies: a concentration-dependent metachromatic effect? Mech Ageing Dev 1991, 59, 95–109. [Google Scholar] [CrossRef]
- Rozanowska, M.B.; Rozanowski, B. Photodegradation of Lipofuscin in Suspension and in ARPE-19 Cells and the Similarity of Fluorescence of the Photodegradation Product with Oxidized Docosahexaenoate. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci 1984, 25, 195–200. [Google Scholar]
- Hayasaka, S. Aging changes in lipofuscin, lysosomes and melanin in the macular area of human retina and choroid. Jpn J Ophthalmol 1989, 33, 36–42. [Google Scholar] [PubMed]
- Rozanowska, M. Properties and Functions of Ocular Melanins and Melanosomes. In Melanins and Melanosomes; Biosynthesis, Biogenesis, Physiological and Pathological Functions, Borovansky, J., Riley, P.A., Eds.; Wiley-Blackwell: Singapore, 2011; pp. 187-224.
- Yanoff, M.; Duker, J.S. Ophthalmology: Expert Consult: Online and Print; Elsevier Health Sciences: 2013.
- Lewandowski, D.; Sander, C.L.; Tworak, A.; Gao, F.; Xu, Q.; Skowronska-Krawczyk, D. Dynamic lipid turnover in photoreceptors and retinal pigment epithelium throughout life. Prog Retin Eye Res 2022, 89, 101037. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; McKechnie, N.M.; Breda, J.; Bayly, M.; Marshall, J. The formation of autofluorescent granules in cultured human RPE. Invest Ophthalmol Vis Sci 1989, 30, 82–89. [Google Scholar]
- Katz, M.L.; Drea, C.M.; Eldred, G.E.; Hess, H.H.; Robison, W.G., Jr. Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp Eye Res 1986, 43, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Eldred, G.E. The fate of the phagosome: conversion to ‘age pigment’ and impact in human retinal pigment epithelium. Trans Ophthalmol Soc U K (1962) 1983, 103 ( Pt 4), 416–421. [Google Scholar]
- Feeney, L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci 1978, 17, 583–600. [Google Scholar]
- Pugh, E.N., Jr.; Lamb, T.D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1993, 1141, 111–149. [Google Scholar] [CrossRef]
- Rodieck, R.W. The first steps in seeing; Sinauer Associates Inc.: Sunderland, Massachusetts, 1998. [Google Scholar]
- de Araujo, M.E.G.; Liebscher, G.; Hess, M.W.; Huber, L.A. Lysosomal size matters. Traffic 2020, 21, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Snodderly, D.M.; Sandstrom, M.M.; Leung, I.Y.; Zucker, C.L.; Neuringer, M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci 2002, 43, 2815–2818. [Google Scholar]
- Rozanowska, M.; Rozanowski, B.; Boulton, M. Photobiology of the retina: Light damage to the retina. (accessed on 23 March 2023). In Photobiological Sciences Online: http://www.photobiology.info, Smith, K.C., Ed.; American Society for Photobiology: Herndon, VA, USA, 2009.
- Katz, M.L.; Rice, L.M.; Gao, C.L. Reversible accumulation of lipofuscin-like inclusions in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1999, 40, 175–181. [Google Scholar]
- Ivy, G.O.; Ihara, Y.; Kitani, K. The protease inhibitor leupeptin induces several signs of aging in brain, retina and internal organs of young rats. Arch Gerontol Geriatr 1991, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Stientjes, H.J.; Gao, C.L.; Christianson, J.S. Iron-induced accumulation of lipofuscin-like fluorescent pigment in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1993, 34, 3161–3171. [Google Scholar] [PubMed]
- Katz, M.L.; Christianson, J.S.; Gao, C.L.; Handelman, G.J. Iron-induced fluorescence in the retina: dependence on vitamin A. Invest Ophthalmol Vis Sci 1994, 35, 3613–3624. [Google Scholar] [PubMed]
- Liu, Y.; Bell, B.A.; Song, Y.; Kim, H.J.; Sterling, J.K.; Kim, B.J.; Poli, M.; Guo, M.; Zhang, K.; Rao, A.; et al. Intraocular iron injection induces oxidative stress followed by elements of geographic atrophy and sympathetic ophthalmia. Aging Cell 2021, 20, e13490. [Google Scholar] [CrossRef]
- Rozanowska, M.B.; Czuba-Pelech, B.; Rozanowski, B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Rozanowska, M.B.; Czuba-Pelech, B.; Landrum, J.T.; Rozanowski, B. Comparison of Antioxidant Properties of Dehydrolutein with Lutein and Zeaxanthin, and their Effects on Cultured Retinal Pigment Epithelial Cells. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Rozanowska, M.; Handzel, K.; Boulton, M.E.; Rozanowski, B. Cytotoxicity of all-trans-retinal increases upon photodegradation. Photochem Photobiol 2012, 88, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Sarna, T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol 2005, 81, 1305–1330. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Rozanowska, M.; Rozanowski, B. Retinal photodamage. J Photochem Photobiol B 2001, 64, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.E.; Bazan, N.G.; Feeney-Burns, L.; Berman, E.R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Invest Ophthalmol Vis Sci 1990, 31, 1433–1443. [Google Scholar]
- Sun, H.; Nathans, J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem 2001, 276, 11766–11774. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Garces, F.A.; Scortecci, J.F.; Molday, L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2022, 89. [Google Scholar] [CrossRef]
- Lenis, T.L.; Hu, J.; Ng, S.Y.; Jiang, Z.; Sarfare, S.; Lloyd, M.B.; Esposito, N.J.; Samuel, W.; Jaworski, C.; Bok, D.; et al. Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc Natl Acad Sci U S A 2018, 115, E11120–E11127. [Google Scholar] [CrossRef]
- Hong, J.D.; Salom, D.; Kochman, M.A.; Kubas, A.; Kiser, P.D.; Palczewski, K. Chromophore hydrolysis and release from photoactivated rhodopsin in native membranes. Proc Natl Acad Sci U S A 2022, 119, e2213911119. [Google Scholar] [CrossRef]
- Zhao, J.; Kim, H.J.; Ueda, K.; Zhang, K.; Montenegro, D.; Dunaief, J.L.; Sparrow, J.R. A vicious cycle of bisretinoid formation and oxidation relevant to recessive Stargardt disease. J Biol Chem 2021, 296, 100259. [Google Scholar] [CrossRef]
- Kim, H.J.; Sparrow, J.R. Bisretinoid phospholipid and vitamin A aldehyde: shining a light. J Lipid Res 2021, 62, 100042. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Montenegro, D.; Zhao, J.; Sparrow, J.R. Bisretinoids of the Retina: Photo-Oxidation, Iron-Catalyzed Oxidation, and Disease Consequences. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Elliott, M.H.; Floor, E.; Truscott, T.G.; Zareba, M.; Sarna, T.; Shamsi, F.A.; Boulton, M.E. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med 2001, 31, 256–265. [Google Scholar] [CrossRef]
- Avalle, L.B.; Wang, Z.; Dillon, J.P.; Gaillard, E.R. Observation of A2E oxidation products in human retinal lipofuscin. Exp Eye Res 2004, 78, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.P.; Matsuda, H.; Itagaki, Y.; Nakanishi, K.; Sparrow, J.R. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J Biol Chem 2005, 280, 39732–39739. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Higbee, D.; Anderson, D.M.; Dahrouj, M.; Grey, A.C.; Gutierrez, D.; Koutalos, Y.; Schey, K.L.; Hanneken, A.; Crouch, R.K. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci 2013, 54, 5535–5542. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.t.; Boyer, N.P.; Anderson, D.M.; Spraggins, J.M.; Schey, K.L.; Hanneken, A.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Determination of N-retinylidene-N-retinylethanolamine (A2E) levels in central and peripheral areas of human retinal pigment epithelium. Photochem Photobiol Sci 2015, 14, 1983–1990. [Google Scholar] [CrossRef]
- Kotnala, A.; Senthilkumari, S.; Wu, G.; Stewart, T.G.; Curcio, C.A.; Halder, N.; Singh, S.B.; Kumar, A.; Velpandian, T. Retinal Pigment Epithelium in Human Donor Eyes Contains Higher Levels of Bisretinoids Including A2E in Periphery than Macula. Invest Ophthalmol Vis Sci 2022, 63, 6. [Google Scholar] [CrossRef]
- Fite, K.V.; Bengston, L.; Donaghey, B. Experimental light damage increases lipofuscin in the retinal pigment epithelium of Japanese quail (Coturnix coturnix japonica). Exp Eye Res 1993, 57, 449–460. [Google Scholar] [CrossRef]
- Boyer, N.P.; Thompson, D.A.; Koutalos, Y. Relative Contributions of All-Trans and 11-Cis Retinal to Formation of Lipofuscin and A2E Accumulating in Mouse Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci 2021, 62, 1. [Google Scholar] [CrossRef]
- Adler, L.t.; Boyer, N.P.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. The 11-cis Retinal Origins of Lipofuscin in the Retina. Prog Mol Biol Transl Sci 2015, 134, e1–e12. [Google Scholar] [CrossRef]
- Boyer, N.P.; Higbee, D.; Currin, M.B.; Blakeley, L.R.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal. J Biol Chem 2012, 287, 22276–22286. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Golczak, M.; Maeda, T.; Palczewski, K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci 2009, 50, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem 2008, 283, 26684–26693. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, T.; Sun, W.; Zhang, H.; Baehr, W.; Palczewski, K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A 2007, 104, 19565–19570. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Imanishi, Y.; Kuksa, V.; Alekseev, A.; Bronson, J.D.; Zhang, H.; Zhu, L.; Sun, W.; Saperstein, D.A.; et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem 2005, 280, 18822–18832. [Google Scholar] [CrossRef] [PubMed]
- Mata, N.L.; Tzekov, R.T.; Liu, X.; Weng, J.; Birch, D.G.; Travis, G.H. Delayed dark-adaptation and lipofuscin accumulation in abcr+/- mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci 2001, 42, 1685–1690. [Google Scholar]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef]
- Eldred, G.E. Vitamins A and E in RPE lipofuscin formation and implications for age-related macular degeneration. Prog Clin Biol Res 1989, 314, 113–129. [Google Scholar]
- Katz, M.L.; Drea, C.M.; Robison, W.G., Jr. Relationship between dietary retinol and lipofuscin in the retinal pigment epithelium. Mech Ageing Dev 1986, 35, 291–305. [Google Scholar] [CrossRef]
- Katz, M.L.; Eldred, G.E.; Robison, W.G., Jr. Lipofuscin autofluorescence: evidence for vitamin A involvement in the retina. Mech Ageing Dev 1987, 39, 81–90. [Google Scholar] [CrossRef]
- Katz, M.L.; Norberg, M. Influence of dietary vitamin A on autofluorescence of leupeptin-induced inclusions in the retinal pigment epithelium. Exp Eye Res 1992, 54, 239–246. [Google Scholar] [CrossRef]
- Katz, M.L.; Norberg, M.; Stientjes, H.J. Reduced phagosomal content of the retinal pigment epithelium in response to retinoid deprivation. Invest Ophthalmol Vis Sci 1992, 33, 2612–2618. [Google Scholar]
- Dobri, N.; Qin, Q.; Kong, J.; Yamamoto, K.; Liu, Z.; Moiseyev, G.; Ma, J.X.; Allikmets, R.; Sparrow, J.R.; Petrukhin, K. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Invest Ophthalmol Vis Sci 2013, 54, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Golczak, M.; Chen, Y.; Okano, K.; Kohno, H.; Shiose, S.; Ishikawa, K.; Harte, W.; Palczewska, G.; Maeda, T.; et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol 2011, 8, 170–178. [Google Scholar] [CrossRef]
- Maeda, T.; Maeda, A.; Matosky, M.; Okano, K.; Roos, S.; Tang, J.; Palczewski, K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci 2009, 50, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Golczak, M.; Maeda, A.; Bereta, G.; Maeda, T.; Kiser, P.D.; Hunzelmann, S.; von Lintig, J.; Blaner, W.S.; Palczewski, K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem 2008, 283, 9543–9554. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Han, Y.; Bui, T.V.; Nusinowitz, S.; Bok, D.; Lichter, J.; Widder, K.; Travis, G.H.; Mata, N.L. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci 2005, 46, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Golczak, M.; Kuksa, V.; Maeda, T.; Moise, A.R.; Palczewski, K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci U S A 2005, 102, 8162–8167. [Google Scholar] [CrossRef]
- Radu, R.A.; Mata, N.L.; Nusinowitz, S.; Liu, X.; Sieving, P.A.; Travis, G.H. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc Natl Acad Sci U S A 2003, 100, 4742–4747. [Google Scholar] [CrossRef]
- Radu, R.A.; Yuan, Q.; Hu, J.; Peng, J.H.; Lloyd, M.; Nusinowitz, S.; Bok, D.; Travis, G.H. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci 2008, 49, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; O’Neil, J.; Miyagi, M.; West, K.; Hoff, H.F. Hydroxynonenal inactivates cathepsin B by forming Michael adducts with active site residues. Protein Sci 2002, 11, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Bermann, M.; Schutt, F.; Holz, F.G.; Kopitz, J. Does A2E, a retinoid component of lipofuscin and inhibitor of lysosomal degradative functions, directly affect the activity of lysosomal hydrolases? Exp Eye Res 2001, 72, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Schutt, F.; Kopitz, J.; Eldred, G.E.; Kruse, F.E.; Volcker, H.E.; Cantz, M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci 1999, 40, 737–743. [Google Scholar]
- Warburton, S.; Davis, W.E.; Southwick, K.; Xin, H.; Woolley, A.T.; Burton, G.F.; Thulin, C.D. Proteomic and phototoxic characterization of melanolipofuscin: correlation to disease and model for its origin. Mol Vis 2007, 13, 318–329. [Google Scholar]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci 2003, 44, 3663–3668. [Google Scholar] [CrossRef]
- Schutt, F.; Ueberle, B.; Schnolzer, M.; Holz, F.G.; Kopitz, J. Proteome analysis of lipofuscin in human retinal pigment epithelial cells. FEBS Lett 2002, 528, 217–221. [Google Scholar] [CrossRef]
- Clancy, C.M.R.; Krogmeier, J.R.; Pawlak, A.; Rozanowska, M.; Sarna, T.; Dunn, R.C.; Simon, J.D. Atomic force microscopy and near-field scanning optical microscopy measurements of single human retinal lipofuscin granules. J. Phys. Chem. B 2000, 104, 12098–12101. [Google Scholar] [CrossRef]
- Krogmeier, J.R.; Clancy, C.M.; Pawlak, A.; Rozanowska, M.; Sarna, T.; Simon, J.D.; Dunn, R.C. Mapping the distribution of emissive molecules in human ocular lipofuscin granules with near-field scanning optical microscopy. J Microsc 2001, 202, 386–390. [Google Scholar] [CrossRef]
- Gouras, P.; Brown, K.R.; Mattison, J.A.; Neuringer, M.; Nagasaki, T.; Ivert, L. The Ultrastructure, Spatial Distribution, and Osmium Tetroxide Binding of Lipofuscin and Melanosomes in Aging Monkey Retinal Epithelium. Curr Eye Res 2018, 43, 1019–1023. [Google Scholar] [CrossRef]
- Haralampus-Grynaviski, N.M.; Lamb, L.E.; Clancy, C.M.; Skumatz, C.; Burke, J.M.; Sarna, T.; Simon, J.D. Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc Natl Acad Sci U S A 2003, 100, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Petrukhin, A.N.; Astaf’ev, A.A.; Zolotavin, P.N.; Fel’dman, T.B.; Dontsov, A.E.; Sarkisov, O.M.; Ostrovsky, M.A. Heterogeneity of structure and fluorescence of single lipofuscin granule from retinal pigment epithelium of human donor eyes: study with the use of atomic force microscopy and near-field microscopy. Dokl Biochem Biophys 2005, 405, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1978, 17, 601–607. [Google Scholar] [PubMed]
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci 1986, 27, 145–152. [Google Scholar]
- Artigas, J.M.; Felipe, A.; Navea, A.; Fandino, A.; Artigas, C. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci 2012, 53, 4076–4084. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J Comp Neurol 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Gliem, M.; Muller, P.L.; Birtel, J.; Herrmann, P.; McGuinness, M.B.; Holz, F.G.; Charbel Issa, P. Quantitative Fundus Autofluorescence and Genetic Associations in Macular, Cone, and Cone-Rod Dystrophies. Ophthalmol Retina 2020, 4, 737–749. [Google Scholar] [CrossRef]
- Bakall, B.; Radu, R.A.; Stanton, J.B.; Burke, J.M.; McKay, B.S.; Wadelius, C.; Mullins, R.F.; Stone, E.M.; Travis, G.H.; Marmorstein, A.D. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Exp Eye Res 2007, 85, 34–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Small, K.W.; Grossniklaus, H.E. Clinicopathologic findings in Best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol 2011, 249, 745–751. [Google Scholar] [CrossRef]
- Jauregui, R.; Chan, L.; Oh, J.K.; Cho, A.; Sparrow, J.R.; Tsang, S.H. Disease asymmetry and hyperautofluorescent ring shape in retinitis pigmentosa patients. Sci Rep 2020, 10, 3364. [Google Scholar] [CrossRef]
- Jauregui, R.; Park, K.S.; Duong, J.K.; Sparrow, J.R.; Tsang, S.H. Quantitative Comparison of Near-infrared Versus Short-wave Autofluorescence Imaging in Monitoring Progression of Retinitis Pigmentosa. Am J Ophthalmol 2018, 194, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gliem, M.; Muller, P.L.; Finger, R.P.; McGuinness, M.B.; Holz, F.G.; Charbel Issa, P. Quantitative Fundus Autofluorescence in Early and Intermediate Age-Related Macular Degeneration. JAMA Ophthalmol 2016, 134, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Bermond, K.; von der Emde, L.; Tarau, I.S.; Bourauel, L.; Heintzmann, R.; Holz, F.G.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent Organelles Within the Retinal Pigment Epithelium in Human Donor Eyes With and Without Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2022, 63, 23. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K.; Wu, G.; Ebenstein, D.; Garsd, A.; Weiter, J.J. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 1989, 30, 1691–1699. [Google Scholar]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye (Lond) 2001, 15, 376–383. [Google Scholar] [CrossRef]
- De, S.; Sakmar, T.P. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J Gen Physiol 2002, 120, 147–157. [Google Scholar] [CrossRef]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Isolation of intact lysosomes from human RPE cells and effects of A2-E on the integrity of the lysosomal and other cellular membranes. Graefes Arch Clin Exp Ophthalmol 2002, 240, 983–988. [Google Scholar] [CrossRef]
- Bergmann, M.; Schutt, F.; Holz, F.G.; Kopitz, J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J 2004, 18, 562–564. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Boulton, M.; Marshall, J. Repigmentation of human retinal pigment epithelial cells in vitro. Exp Eye Res 1985, 41, 209–218. [Google Scholar] [CrossRef]
- Rakoczy, P.; Kennedy, C.; Thompson-Wallis, D.; Mann, K.; Constable, I. Changes in retinal pigment epithelial cell autofluorescence and protein expression associated with phagocytosis of rod outer segments in vitro. Biol Cell 1992, 76, 49–54. [Google Scholar] [CrossRef]
- Brunk, U.T.; Wihlmark, U.; Wrigstad, A.; Roberg, K.; Nilsson, S.E. Accumulation of lipofuscin within retinal pigment epithelial cells results in enhanced sensitivity to photo-oxidation. Gerontology 1995, 41 Suppl 2, 201–212. [Google Scholar] [CrossRef]
- Wassell, J.; Ellis, S.; Burke, J.; Boulton, M. Fluorescence properties of autofluorescent granules generated by cultured human RPE cells. Invest Ophthalmol Vis Sci 1998, 39, 1487–1492. [Google Scholar]
- Boulton, M.E. Studying melanin and lipofuscin in RPE cell culture models. Exp Eye Res 2014, 126, 61–67. [Google Scholar] [CrossRef]
- Zhang, Q.; Presswalla, F.; Calton, M.; Charniga, C.; Stern, J.; Temple, S.; Vollrath, D.; Zacks, D.N.; Ali, R.R.; Thompson, D.A.; et al. Highly Differentiated Human Fetal RPE Cultures Are Resistant to the Accumulation and Toxicity of Lipofuscin-Like Material. Invest Ophthalmol Vis Sci 2019, 60, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O. Phagocytosis of light- and dark-adapted rod outer segments by cultured pigment epithelium. Science 1978, 202, 526–528. [Google Scholar] [CrossRef]
- Nilsson, S.E.; Sundelin, S.P.; Wihlmark, U.; Brunk, U.T. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol 2003, 106, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Tzekov, R.; McDowell, J.H.; Smith, W.C.; Tang, S.; Kaushal, S. Formation of lipofuscin-like material in the RPE Cell by different components of rod outer segments. Exp Eye Res 2013, 112, 57–67. [Google Scholar] [CrossRef]
- Kaemmerer, E.; Schutt, F.; Krohne, T.U.; Holz, F.G.; Kopitz, J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest Ophthalmol Vis Sci 2007, 48, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Stratmann, N.K.; Kopitz, J.; Holz, F.G. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res 2010, 90, 465–471. [Google Scholar] [CrossRef]
- Rakoczy, P.E.; Baines, M.; Kennedy, C.J.; Constable, I.J. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp Eye Res 1996, 63, 159–167. [Google Scholar] [CrossRef]
- Escrevente, C.; Falcao, A.S.; Hall, M.J.; Lopes-da-Silva, M.; Antas, P.; Mesquita, M.M.; Ferreira, I.S.; Cardoso, M.H.; Oliveira, D.; Fradinho, A.C.; et al. Formation of Lipofuscin-Like Autofluorescent Granules in the Retinal Pigment Epithelium Requires Lysosome Dysfunction. Invest Ophthalmol Vis Sci 2021, 62, 39. [Google Scholar] [CrossRef]
- Zhang, L.; Hui, Y.N.; Wang, Y.S.; Ma, J.X.; Wang, J.B.; Ma, L.N. Calcium overload is associated with lipofuscin formation in human retinal pigment epithelial cells fed with photoreceptor outer segments. Eye (Lond) 2011, 25, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Chou, S.; Desai, A.; Hoppel, C.L.; Matsuyama, S.; Palczewski, K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem 2009, 284, 15173–15183. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Sarna, M.; Wnuk, D.; Sarna, T. Lipofuscin-mediated photodynamic stress induces adverse changes in nanomechanical properties of retinal pigment epithelium cells. Sci Rep 2018, 8, 17929. [Google Scholar] [CrossRef] [PubMed]
- Olchawa, M.M.; Furso, J.A.; Szewczyk, G.M.; Sarna, T.J. Lipofuscin-mediated photic stress inhibits phagocytic activity of ARPE-19 cells; effect of donors’ age and antioxidants. Free Radic Res 2017, 51, 799–811. [Google Scholar] [CrossRef]
- Zareba, M.; Skumatz, C.M.; Sarna, T.J.; Burke, J.M. Photic injury to cultured RPE varies among individual cells in proportion to their endogenous lipofuscin content as modulated by their melanosome content. Invest Ophthalmol Vis Sci 2014, 55, 4982–4990. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem 2005, 280, 21061–21066. [Google Scholar] [CrossRef]
- Shamsi, F.A.; Boulton, M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest Ophthalmol Vis Sci 2001, 42, 3041–3046. [Google Scholar]
- Rozanowska, M.; Jarvis-Evans, J.; Korytowski, W.; Boulton, M.E.; Burke, J.M.; Sarna, T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem 1995, 270, 18825–18830. [Google Scholar] [CrossRef]
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B 1993, 19, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Korytowski, W.; Rozanowski, B.; Skumatz, C.; Boulton, M.E.; Burke, J.M.; Sarna, T. Photoreactivity of aged human RPE melanosomes: a comparison with lipofuscin. Invest Ophthalmol Vis Sci 2002, 43, 2088–2096. [Google Scholar] [PubMed]
- Rozanowska, M.; Pawlak, A.; Rozanowski, B.; Skumatz, C.; Zareba, M.; Boulton, M.E.; Burke, J.M.; Sarna, T.; Simon, J.D. Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components. Invest Ophthalmol Vis Sci 2004, 45, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Rózanowska, M.B.; Pawlak, A.; Rózanowski, B. Products of ˙docosahexaenoate oxidation as contributors to photosensitising properties of retinal lipofuscin. Int. J. Mol. Sci. 2021, 22, 3525. [Google Scholar] [CrossRef]
- Rozanowska, M.; Wessels, J.; Boulton, M.; Burke, J.M.; Rodgers, M.A.J.; Truscott, T.G.; Sarna, T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic. Biol. Med. 1998, 24, 1107–1112. [Google Scholar] [CrossRef]
- Gaillard, E.R.; Atherton, S.J.; Eldred, G.; Dillon, J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol 1995, 61, 448–453. [Google Scholar] [CrossRef]
- Pawlak, A.; Wrona, M.; Rozanowska, M.; Zareba, M.; Lamb, L.E.; Roberts, J.E.; Simon, J.D.; Sarna, T. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem Photobiol 2003, 77, 253–258. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res 2005, 80, 595–606. [Google Scholar] [CrossRef]
- Różanowska, M.; Różanowski, B. Visual transduction and age-related changes in lipofuscin. In Ophthalmology Research: The Visual Transduction Cascade, Tombran-Tink, J., Barnstable, C.J., Eds.; The Humana Press Inc.: Totowa, NJ, 2008; pp. 405–446.
- Zhou, J.; Jang, Y.P.; Kim, S.R.; Sparrow, J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A 2006, 103, 16182–16187. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Itagaki, Y.; Jockusch, S.; Sparrow, J.R.; Turro, N.J.; Nakanishi, K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed Engl 2002, 41, 814–817. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Vollmer-Snarr, H.R.; Zhou, J.; Jang, Y.P.; Jockusch, S.; Itagaki, Y.; Nakanishi, K. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J Biol Chem 2003, 278, 18207–18213. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Mata, N.L.; Bagla, A.; Travis, G.H. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc Natl Acad Sci U S A 2004, 101, 5928–5933. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Sakina, N.L.; Kononikhin, A.S.; Feldman, T.B.; Nikolaev, E.N.; Dontsov, A.E.; Ostrovsky, M.A. Detection and study of the products of photooxidation of N-retinylidene-N-retinylethanolamine (A2E), the fluorophore of lipofuscin granules from retinal pigment epithelium of human donor eyes. Dokl Biochem Biophys 2006, 409, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Keller, L.M.; Dillon, J.; Gaillard, E.R. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem Photobiol 2006, 82, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, E.R.; Avalle, L.B.; Keller, L.M.; Wang, Z.; Reszka, K.J.; Dillon, J.P. A mechanistic study of the photooxidation of A2E, a component of human retinal lipofuscin. Exp Eye Res 2004, 79, 313–319. [Google Scholar] [CrossRef]
- Dillon, J.; Wang, Z.; Avalle, L.B.; Gaillard, E.R. The photochemical oxidation of A2E results in the formation of a 5,8,5’,8’-bis-furanoid oxide. Exp Eye Res 2004, 79, 537–542. [Google Scholar] [CrossRef]
- Kim, H.J.; Sparrow, J.R. Bisretinoids: More than Meets the Eye. Adv Exp Med Biol 2019, 1185, 341–346. [Google Scholar] [CrossRef]
- Ueda, K.; Kim, H.J.; Zhao, J.; Sparrow, J.R. Bisretinoid Photodegradation Is Likely Not a Good Thing. Adv Exp Med Biol 2018, 1074, 395–401. [Google Scholar] [CrossRef]
- Murdaugh, L.S.; Avalle, L.B.; Mandal, S.; Dill, A.E.; Dillon, J.; Simon, J.D.; Gaillard, E.R. Compositional studies of human RPE lipofuscin. J Mass Spectrom 2010, 45, 1139–1147. [Google Scholar] [CrossRef]
- Murdaugh, L.S.; Mandal, S.; Dill, A.E.; Dillon, J.; Simon, J.D.; Gaillard, E.R. Compositional studies of human RPE lipofuscin: mechanisms of molecular modifications. J Mass Spectrom 2011, 46, 90–95. [Google Scholar] [CrossRef]
- Ahmed, J.; Braun, R.D.; Dunn, R., Jr.; Linsenmeier, R.A. Oxygen distribution in the macaque retina. Invest Ophthalmol Vis Sci 1993, 34, 516–521. [Google Scholar]
- Organisciak, D.T.; Darrow, R.M.; Barsalou, L.; Darrow, R.A.; Kutty, R.K.; Kutty, G.; Wiggert, B. Light history and age-related changes in retinal light damage. Invest Ophthalmol Vis Sci 1998, 39, 1107–1116. [Google Scholar] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Imanishi, Y.; Leahy, P.; Kubota, R.; Palczewski, K. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol 2006, 70, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Imanishi, Y.; Sun, W.; Jastrzebska, B.; Hatala, D.A.; Winkens, H.J.; Hofmann, K.P.; Janssen, J.J.; Baehr, W.; et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem 2006, 281, 37697–37704. [Google Scholar] [CrossRef] [PubMed]
- Shiose, S.; Chen, Y.; Okano, K.; Roy, S.; Kohno, H.; Tang, J.; Pearlman, E.; Maeda, T.; Palczewski, K.; Maeda, A. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem 2011, 286, 15543–15555. [Google Scholar] [CrossRef]
- Chen, Y.; Okano, K.; Maeda, T.; Chauhan, V.; Golczak, M.; Maeda, A.; Palczewski, K. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J Biol Chem 2012, 287, 5059–5069. [Google Scholar] [CrossRef]
- Okano, K.; Maeda, A.; Chen, Y.; Chauhan, V.; Tang, J.; Palczewska, G.; Sakai, T.; Tsuneoka, H.; Palczewski, K.; Maeda, T. Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light-induced damage. J Neurochem 2012, 121, 146–156. [Google Scholar] [CrossRef]
- Maeda, T.; Golczak, M.; Maeda, A. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol 2012, 88, 1309–1319. [Google Scholar] [CrossRef]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy protects the retina from light-induced degeneration. J Biol Chem 2013, 288, 7506–7518. [Google Scholar] [CrossRef]
- Chen, Y.; Palczewska, G.; Mustafi, D.; Golczak, M.; Dong, Z.; Sawada, O.; Maeda, T.; Maeda, A.; Palczewski, K. Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J Clin Invest 2013, 123, 5119–5134. [Google Scholar] [CrossRef]
- Kohno, H.; Chen, Y.; Kevany, B.M.; Pearlman, E.; Miyagi, M.; Maeda, T.; Palczewski, K.; Maeda, A. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem 2013, 288, 15326–15341. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Palczewska, G.; Golczak, M.; Kohno, H.; Dong, Z.; Maeda, T.; Palczewski, K. Two-photon microscopy reveals early rod photoreceptor cell damage in light-exposed mutant mice. Proc Natl Acad Sci U S A 2014, 111, E1428–E1437. [Google Scholar] [CrossRef] [PubMed]
- Sawada, O.; Perusek, L.; Kohno, H.; Howell, S.J.; Maeda, A.; Matsuyama, S.; Maeda, T. All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis. Exp Eye Res 2014, 123, 27–36. [Google Scholar] [CrossRef]
- Wu, X.; Yu, G.; Luo, C.; Maeda, A.; Zhang, N.; Sun, D.; Zhou, Z.; Puntel, A.; Palczewski, K.; Lu, Z.R. Synthesis and evaluation of a nanoglobular dendrimer 5-aminosalicylic Acid conjugate with a hydrolyzable schiff base spacer for treating retinal degeneration. ACS Nano 2014, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, X.; Ayat, N.; Maeda, A.; Gao, S.Q.; Golczak, M.; Palczewski, K.; Lu, Z.R. Multifunctional PEG retinylamine conjugate provides prolonged protection against retinal degeneration in mice. Biomacromolecules 2014, 15, 4570–4578. [Google Scholar] [CrossRef]
- Puntel, A.; Maeda, A.; Golczak, M.; Gao, S.Q.; Yu, G.; Palczewski, K.; Lu, Z.R. Prolonged prevention of retinal degeneration with retinylamine loaded nanoparticles. Biomaterials 2015, 44, 103–110. [Google Scholar] [CrossRef]
- Parmar, T.; Parmar, V.M.; Arai, E.; Sahu, B.; Perusek, L.; Maeda, A. Acute Stress Responses Are Early Molecular Events of Retinal Degeneration in Abca4-/-Rdh8-/- Mice After Light Exposure. Invest Ophthalmol Vis Sci 2016, 57, 3257–3267. [Google Scholar] [CrossRef]
- Schur, R.M.; Gao, S.; Yu, G.; Chen, Y.; Maeda, A.; Palczewski, K.; Lu, Z.R. New GABA modulators protect photoreceptor cells from light-induced degeneration in mouse models. FASEB J 2018, 32, 3289–3300. [Google Scholar] [CrossRef]
- Parmar, T.; Parmar, V.M.; Perusek, L.; Georges, A.; Takahashi, M.; Crabb, J.W.; Maeda, A. Lipocalin 2 Plays an Important Role in Regulating Inflammation in Retinal Degeneration. J Immunol 2018, 200, 3128–3141. [Google Scholar] [CrossRef]
- Fang, Y.; Tschulakow, A.; Taubitz, T.; Illing, B.; Biesemeier, A.; Julien-Schraermeyer, S.; Radu, R.A.; Jiang, Z.; Schraermeyer, U. Fundus autofluorescence, spectral-domain optical coherence tomography, and histology correlations in a Stargardt disease mouse model. FASEB J 2020, 34, 3693–3714. [Google Scholar] [CrossRef]
- Yu, G.; Gao, S.Q.; Dong, Z.; Sheng, L.; Sun, D.; Zhang, N.; Zhang, J.; Margeivicus, S.; Fu, P.; Golczak, M.; et al. Peptide Derivatives of Retinylamine Prevent Retinal Degeneration with Minimal Side Effects on Vision in Mice. Bioconjug Chem 2021, 32, 572–583. [Google Scholar] [CrossRef]
- Fang, Y.; Taubitz, T.; Tschulakow, A.V.; Heiduschka, P.; Szewczyk, G.; Burnet, M.; Peters, T.; Biesemeier, A.; Sarna, T.; Schraermeyer, U.; et al. Removal of RPE lipofuscin results in rescue from retinal degeneration in a mouse model of advanced Stargardt disease: Role of reactive oxygen species. Free Radical Biology and Medicine 2022, 182, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Ham, W.T., Jr.; Ruffolo, J.J., Jr.; Mueller, H.A.; Clarke, A.M.; Moon, M.E. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci 1978, 17, 1029–1035. [Google Scholar]
- Morgan, J.I.; Hunter, J.J.; Masella, B.; Wolfe, R.; Gray, D.C.; Merigan, W.H.; Delori, F.C.; Williams, D.R. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Invest Ophthalmol Vis Sci 2008, 49, 3715–3729. [Google Scholar] [CrossRef]
- Morgan, J.I.; Hunter, J.J.; Merigan, W.H.; Williams, D.R. The reduction of retinal autofluorescence caused by light exposure. Invest Ophthalmol Vis Sci 2009, 50, 6015–6022. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog Retin Eye Res 2012, 31, 28–42. [Google Scholar] [CrossRef]
- Zhang, J.; Sabarinathan, R.; Bubel, T.; Williams, D.R.; Hunter, J.J. Action spectrum for photochemical retinal pigment epithelium (RPE) disruption in an in vivo monkey model. Optical Interactions with Tissue and Cells Xxvii 2016, 9706. [Google Scholar] [CrossRef]
- Ham, W.T., Jr.; Mueller, H.A.; Ruffolo, J.J., Jr.; Guerry, D., 3rd; Guerry, R.K. Action spectrum for retinal injury from near-ultraviolet radiation in the aphakic monkey. Am J Ophthalmol 1982, 93, 299–306. [Google Scholar] [CrossRef]
- Wu, L.; Ueda, K.; Nagasaki, T.; Sparrow, J.R. Light damage in Abca4 and Rpe65rd12 mice. Invest Ophthalmol Vis Sci 2014, 55, 1910–1918. [Google Scholar] [CrossRef]
- Teussink, M.M.; Lee, M.D.; Smith, R.T.; van Huet, R.A.; Klaver, C.C.; Klevering, B.J.; Theelen, T.; Hoyng, C.B. The effect of light deprivation in patients with Stargardt disease. Am J Ophthalmol 2015, 159, 964–972 e962. [Google Scholar] [CrossRef]
- Aits, S.; Kricker, J.; Liu, B.; Ellegaard, A.M.; Hamalisto, S.; Tvingsholm, S.; Corcelle-Termeau, E.; Hogh, S.; Farkas, T.; Holm Jonassen, A.; et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 2015, 11, 1408–1424. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kovalenko, A.; Bogdanov, K.; Wallach, D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017, 47, 51–65 e57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Robinson, K.; Washington, I. C20D3-Vitamin A Prevents Retinal Pigment Epithelium Atrophic Changes in a Mouse Model. Transl Vis Sci Technol 2021, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Barnard, A.R.; Herrmann, P.; Washington, I.; MacLaren, R.E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Natl Acad Sci U S A 2015, 112, 8415–8420. [Google Scholar] [CrossRef]
- Allingham, M.J.; Loksztejn, A.; Cousins, S.W.; Mettu, P.S. Immunological Aspects of Age-Related Macular Degeneration. In Age-related Macular Degeneration,, Chew, E.Y., Swaroop, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1256, pp. 143–190. [Google Scholar]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Haralampus-Grynaviski, N.M.; Lamb, L.E.; Simon, J.D.; Krogmeier, J.R.; Dunn, R.C.; Pawlak, A.; Rozanowska, M.; Sarna, T.; Burke, J.M. Probing the spatial dependence of the emission spectrum of single human retinal lipofuscin granules using near-field scanning optical microscopy. Photochem Photobiol 2001, 74, 364–368. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res 2012, 31, 121–135. [Google Scholar] [CrossRef]
- Eldred, G.E.; Lasky, M.R. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature 1993, 361, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Ragauskaite, L.; Heckathorn, R.C.; Gaillard, E.R. Environmental effects on the photochemistry of A2-E, a component of human retinal lipofuscin. Photochem Photobiol 2001, 74, 483–488. [Google Scholar] [CrossRef]
- Lamb, L.E.; Ye, T.; Haralampus-Grynaviski, N.M.; Williams, T.R.; Pawlak, A.; Sarna, T.; Simon, J.D. Primary photophysical properties of A2E in solution. J. Phys. Chem. B 2001, 105, 11507–11512. [Google Scholar] [CrossRef]
- Reszka, K.; Eldred, G.E.; Wang, R.H.; Chignell, C.; Dillon, J. The photochemistry of human retinal lipofuscin as studied by EPR. Photochem Photobiol 1995, 62, 1005–1008. [Google Scholar] [CrossRef]
- Kopitz, J.; Holz, F.G.; Kaemmerer, E.; Schutt, F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie 2004, 86, 825–831. [Google Scholar] [CrossRef]
- Dontsov, A.E.; Sakina, N.L.; Golubkov, A.M.; Ostrovsky, M.A. Light-induced release of A2E photooxidation toxic products from lipofuscin granules of human retinal pigment epithelium. Dokl Biochem Biophys 2009, 425, 98–101. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci U S A 1992, 89, 1271–1275. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 320–337. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; Vairetti, M. Autofluorescence-based optical biopsy: An effective diagnostic tool in hepatology. Liver Int 2018, 38, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Layer, G.; Reichelt, J.; Jahn, D.; Heinz, D.W. Structure and function of enzymes in heme biosynthesis. Protein Sci 2010, 19, 1137–1161. [Google Scholar] [CrossRef]
- Palczewska, G.; Wojtkowski, M.; Palczewski, K. From mouse to human: Accessing the biochemistry of vision in vivo by two-photon excitation. Prog Retin Eye Res 2023, 93, 101170. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Maeda, T.; Imanishi, Y.; Sun, W.; Chen, Y.; Williams, D.R.; Piston, D.W.; Maeda, A.; Palczewski, K. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat Med 2010, 16, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Szweda, L.I. Age-related increase in liver retinyl palmitate. Relationship to lipofuscin. J Biol Chem 1994, 269, 8712–8715. [Google Scholar] [CrossRef]
- Docchio, F.; Boulton, M.; Cubeddu, R.; Ramponi, R.; Barker, P.D. Age-related changes in the fluorescence of melanin and lipofuscin granules of the retinal pigment epithelium: a time-resolved fluorescence spectroscopy study. Photochem Photobiol 1991, 54, 247–253. [Google Scholar] [CrossRef]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp Eye Res 2018, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Bermond, K.; Wobbe, C.; Tarau, I.S.; Heintzmann, R.; Hillenkamp, J.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent Granules of the Human Retinal Pigment Epithelium: Phenotypes, Intracellular Distribution, and Age-Related Topography. Invest Ophthalmol Vis Sci 2020, 61, 35. [Google Scholar] [CrossRef]
- Taubitz, T.; Fang, Y.; Biesemeier, A.; Julien-Schraermeyer, S.; Schraermeyer, U. Age, lipofuscin and melanin oxidation affect fundus near-infrared autofluorescence. EBioMedicine 2019, 48, 592–604. [Google Scholar] [CrossRef]
- Kayatz, P.; Thumann, G.; Luther, T.T.; Jordan, J.F.; Bartz-Schmidt, K.U.; Esser, P.J.; Schraermeyer, U. Oxidation causes melanin fluorescence. Invest Ophthalmol Vis Sci 2001, 42, 241–246. [Google Scholar]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hamm, G.; Maglennon, G.; Williamson, B.; Macdonald, R.; Doherty, A.; Jones, S.; Harris, J.; Blades, J.; Harmer, A.R.; Barton, P.; et al. Pharmacological inhibition of MERTK induces in vivo retinal degeneration: a multimodal imaging ocular safety assessment. Arch Toxicol 2022, 96, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Georgiadis, A.; Ramsden, C.M.; Sanchez-Heras, E.; Haas, A.J.; Nommiste, B.; Semenyuk, O.; Bainbridge, J.W.B.; Coffey, P.J.; Smith, A.J.; et al. Spatiotemporal control of actomyosin contractility by MRCKbeta signaling drives phagocytosis. J Cell Biol 2022, 221. [Google Scholar] [CrossRef]
- Zheng, L.; Jia, J.; Chen, Y.; Liu, R.; Cao, R.; Duan, M.; Zhang, M.; Xu, Y. Pentoxifylline alleviates ischemic white matter injury through up-regulating Mertk-mediated myelin clearance. J Neuroinflammation 2022, 19, 128. [Google Scholar] [CrossRef]
- Audo, I.; Mohand-Said, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Demontant, V.; Boyard, F.; Antonio, A.; Mejecase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum Mutat 2018, 39, 887–913. [Google Scholar] [CrossRef]
- Kaga, T.; Fonseca, R.A.; Dantas, M.A.; Yannuzzi, L.A.; Spaide, R.F. Optical coherence tomography of bleb-like subretinal lesions after retinal reattachment surgery. Am J Ophthalmol 2001, 132, 120–121. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005, 112, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Noble, K.; Morgan, A.; Freund, K.B. Vitelliform macular dystrophy. Ophthalmology 2006, 113, 1392–1400. [Google Scholar] [CrossRef]
- Laud, K.; Visaetsilpanonta, S.; Yannuzzi, L.A.; Spaide, R.F. Autofluorescence imaging of optic pit maculopathy. Retina 2007, 27, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Fathi, P.; Roslend, A.; Mehta, K.; Moitra, P.; Zhang, K.; Pan, D. UV-trained and metal-enhanced fluorescence of biliverdin and biliverdin nanoparticles. Nanoscale 2021, 13, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Ober, M.D.; Spaide, R.F. Autofluorescence and retinal pigment epithelial atrophy after subretinal hemorrhage. Retina 2006, 26, 119–120. [Google Scholar] [CrossRef]
- Piccolino, F.C.; Borgia, L.; Zinicola, E.; Iester, M.; Torrielli, S. Pre-injection fluorescence in indocyanine green angiography. Ophthalmology 1996, 103, 1837–1845. [Google Scholar] [CrossRef]
- Glushko, V.; Thaler, M.; Ros, M. The fluorescence of bilirubin upon interaction with human erythrocyte ghosts. Biochim Biophys Acta 1982, 719, 65–73. [Google Scholar] [CrossRef]
- Marmorstein, A.D.; Marmorstein, L.Y.; Sakaguchi, H.; Hollyfield, J.G. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol Vis Sci 2002, 43, 2435–2441. [Google Scholar]
- Hammer, M.; Konigsdorffer, E.; Liebermann, C.; Framme, C.; Schuch, G.; Schweitzer, D.; Strobel, J. Ocular fundus auto-fluorescence observations at different wavelengths in patients with age-related macular degeneration and diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2008, 246, 105–114. [Google Scholar] [CrossRef]
- Schultz, R.; Gamage, K.; Messinger, J.D.; Curcio, C.A.; Hammer, M. Fluorescence Lifetimes and Spectra of RPE and Sub-RPE Deposits in Histology of Control and AMD Eyes. Invest Ophthalmol Vis Sci 2020, 61, 9. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 1995, 36, 718–729. [Google Scholar] [PubMed]
- Sarna, T.; Burke, J.M.; Korytowski, W.; Rozanowska, M.; Skumatz, C.M.; Zareba, A.; Zareba, M. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res 2003, 76, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Post, R.; Johri, A.; Lee, M.D.; Ablonczy, Z.; Curcio, C.A.; Ach, T.; Sajda, P. Simultaneous decomposition of multiple hyperspectral data sets: signal recovery of unknown fluorophores in the retinal pigment epithelium. Biomed Opt Express 2014, 5, 4171–4185. [Google Scholar] [CrossRef]
- Bermond, K.; Berlin, A.; Tarau, I.S.; Wobbe, C.; Heintzmann, R.; Curcio, C.A.; Sloan, K.R.; Ach, T. Characteristics of normal human retinal pigment epithelium cells with extremes of autofluorescence or intracellular granule count. Ann Eye Sci 2021, 6. [Google Scholar] [CrossRef]
- Webb, R.H.; Hughes, G.W.; Delori, F.C. Confocal scanning laser ophthalmoscope. Appl Opt 1987, 26, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- von Ruckmann, A.; Fitzke, F.W.; Bird, A.C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol 1995, 79, 407–412. [Google Scholar] [CrossRef]
- von Ruckmann, A.; Fitzke, F.W.; Bird, A.C. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci 1997, 38, 478–486. [Google Scholar]
- von Ruckmann, A.; Fitzke, F.W.; Bird, A.C. Distribution of pigment epithelium autofluorescence in retinal disease state recorded in vivo and its change over time. Graefes Arch Clin Exp Ophthalmol 1999, 237, 1–9. [Google Scholar] [CrossRef]
- Reiter, G.S.; Told, R.; Baratsits, M.; Hecht, A.; Schlanitz, F.G.; Sacu, S.; Schmidt-Erfurth, U. Repeatability and reliability of quantitative fundus autofluorescence imaging in patients with early and intermediate age-related macular degeneration. Acta Ophthalmol. 2019, 97, E526–E532. [Google Scholar] [CrossRef]
- Fischer, J.; Otto, T.; Delori, F.; Pace, L.; Staurenghi, G. Scanning Laser Ophthalmoscopy (SLO). In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics, Bille, J.F., Ed.; Cham (CH), 2019; pp. 35–57.
- Orellana-Rios, J.; Yokoyama, S.; Bhuiyan, A.; Gao, L.; Otero-Marquez, O.; Smith, R.T. Translational Retinal Imaging. Asia Pac J Ophthalmol (Phila) 2020, 9, 269–277. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog Retin Eye Res 2021, 81, 100893. [Google Scholar] [CrossRef]
- Borrelli, E.; Battista, M.; Zuccaro, B.; Sacconi, R.; Brambati, M.; Querques, L.; Prascina, F.; Sadda, S.R.; Bandello, F.; Querques, G. Spectrally Resolved Fundus Autofluorescence in Healthy Eyes: Repeatability and Topographical Analysis of the Green-Emitting Fluorophores. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Borrelli, E.; Lei, J.; Balasubramanian, S.; Uji, A.; Cozzi, M.; Sarao, V.; Lanzetta, P.; Staurenghi, G.; Sadda, S.R. Green emission fluorophores in eyes with atrophic age-related macular degeneration: a colour fundus autofluorescence pilot study. Br J Ophthalmol 2018, 102, 827–832. [Google Scholar] [CrossRef]
- Dysli, C.; Muller, P.L.; Birtel, J.; Holz, F.G.; Herrmann, P. Spectrally Resolved Fundus Autofluorescence in ABCA4-Related Retinopathy. Invest Ophthalmol Vis Sci 2019, 60, 274–281. [Google Scholar] [CrossRef]
- Muller, P.L.; Dysli, C.; Hess, K.; Holz, F.G.; Herrmann, P. Spectral Fundus Autofluorescence Excitation and Emission in Abca4-Related Retinopathy. Retina 2020, 40, 2332–2342. [Google Scholar] [CrossRef]
- Gao, L.; Smith, R.T.; Tkaczyk, T.S. Snapshot hyperspectral retinal camera with the Image Mapping Spectrometer (IMS). Biomed Opt Express 2012, 3, 48–54. [Google Scholar] [CrossRef]
- Boguslawski, J.; Palczewska, G.; Tomczewski, S.; Milkiewicz, J.; Kasprzycki, P.; Stachowiak, D.; Komar, K.; Marzejon, M.J.; Sikorski, B.L.; Hudzikowski, A.; et al. In vivo imaging of the human eye using a 2-photon-excited fluorescence scanning laser ophthalmoscope. J Clin Invest 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ach, T.; Curcio, C.A.; Smith, R.T. Hyperspectral autofluorescence characterization of drusen and sub-RPE deposits in age-related macular degeneration. Ann Eye Sci 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human Retinal Pigment Epithelium: In Vivo Cell Morphometry, Multispectral Autofluorescence, and Relationship to Cone Mosaic. Invest Ophthalmol Vis Sci 2018, 59, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.; Hasan, S.; Curcio, C.A.; Smith, R.T.; Meller, D.; Hammer, M. Spectral and lifetime resolution of fundus autofluorescence in advanced age-related macular degeneration revealing different signal sources. Acta Ophthalmol 2022, 100, e841–e846. [Google Scholar] [CrossRef]
- Semenov, A.N.; Maksimov, E.G.; Moysenovich, A.M.; Yakovleva, M.A.; Tsoraev, G.V.; Ramonova, A.A.; Shirshin, E.A.; Sluchanko, N.N.; Feldman, T.B.; Rubin, A.B.; et al. Protein-Mediated Carotenoid Delivery Suppresses the Photoinducible Oxidation of Lipofuscin in Retinal Pigment Epithelial Cells. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Guan, Z.; Li, Y.; Jiao, S.; Yeasmin, N.; Rosenfeld, P.J.; Dubovy, S.R.; Lam, B.L.; Wen, R. A2E Distribution in RPE Granules in Human Eyes. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res 2017, 60, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Boguslawski, J.; Stremplewski, P.; Kornaszewski, L.; Zhang, J.; Dong, Z.; Liang, X.X.; Gratton, E.; Vogel, A.; Wojtkowski, M.; et al. Noninvasive two-photon optical biopsy of retinal fluorophores. Proc Natl Acad Sci U S A 2020, 117, 22532–22543. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Brauer, J.L.; Meller, D.; Hammer, M. Impact of cataract on the spectral measurement of fundus autofluorescence. Graefes Arch Clin Exp Ophthalmol 2022, 260, 2057–2059. [Google Scholar] [CrossRef]
- Berlin, A.; Clark, M.E.; Swain, T.A.; Fischer, N.A.; McGwin, G., Jr.; Sloan, K.R.; Owsley, C.; Curcio, C.A. Impact of the Aging Lens and Posterior Capsular Opacification on Quantitative Autofluorescence Imaging in Age-Related Macular Degeneration. Transl Vis Sci Technol 2022, 11, 23. [Google Scholar] [CrossRef]
- Reiter, G.S.; Schwarzenbacher, L.; Schartmuller, D.; Roggla, V.; Leydolt, C.; Menapace, R.; Schmidt-Erfurth, U.; Sacu, S. Influence of lens opacities and cataract severity on quantitative fundus autofluorescence as a secondary outcome of a randomized clinical trial. Scientific Reports 2021, 11. [Google Scholar] [CrossRef]
- Brauer, J.L.; Schultz, R.; Klemm, M.; Hammer, M. Influence of Lens Fluorescence on Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Fundus Imaging and Strategies for Its Compensation. Transl Vis Sci Technol 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- van de Kraats, J.; van Norren, D. Optical density of the aging human ocular media in the visible and the UV. J Opt Soc Am A Opt Image Sci Vis 2007, 24, 1842–1857. [Google Scholar] [CrossRef]
- Hunter, J.J.; Masella, B.; Dubra, A.; Sharma, R.; Yin, L.; Merigan, W.H.; Palczewska, G.; Palczewski, K.; Williams, D.R. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed Opt Express 2010, 2, 139–148. [Google Scholar] [CrossRef]
- Palczewska, G.; Golczak, M.; Williams, D.R.; Hunter, J.J.; Palczewski, K. Endogenous fluorophores enable two-photon imaging of the primate eye. Invest Ophthalmol Vis Sci 2014, 55, 4438–4447. [Google Scholar] [CrossRef]
- Palczewska, G.; Dong, Z.; Golczak, M.; Hunter, J.J.; Williams, D.R.; Alexander, N.S.; Palczewski, K. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat Med 2014, 20, 785–789. [Google Scholar] [CrossRef]
- Sharma, R.; Yin, L.; Geng, Y.; Merigan, W.H.; Palczewska, G.; Palczewski, K.; Williams, D.R.; Hunter, J.J. In vivo two-photon imaging of the mouse retina. Biomed Opt Express 2013, 4, 1285–1293. [Google Scholar] [CrossRef]
- Sharma, R.; Schwarz, C.; Williams, D.R.; Palczewska, G.; Palczewski, K.; Hunter, J.J. In Vivo Two-Photon Fluorescence Kinetics of Primate Rods and Cones. Invest Ophthalmol Vis Sci 2016, 57, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Sharma, R.; Fischer, W.S.; Chung, M.; Palczewska, G.; Palczewski, K.; Williams, D.R.; Hunter, J.J. Safety assessment in macaques of light exposures for functional two-photon ophthalmoscopy in humans. Biomed Opt Express 2016, 7, 5148–5169. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Williams, D.R.; Palczewska, G.; Palczewski, K.; Hunter, J.J. Two-Photon Autofluorescence Imaging Reveals Cellular Structures Throughout the Retina of the Living Primate Eye. Invest Ophthalmol Vis Sci 2016, 57, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Marcos, S.; Werner, J.S.; Burns, S.A.; Merigan, W.H.; Artal, P.; Atchison, D.A.; Hampson, K.M.; Legras, R.; Lundstrom, L.; Yoon, G.; et al. Vision science and adaptive optics, the state of the field. Vision Res 2017, 132, 3–33. [Google Scholar] [CrossRef]
- Sharma, R.; Schwarz, C.; Hunter, J.J.; Palczewska, G.; Palczewski, K.; Williams, D.R. Formation and Clearance of All-Trans-Retinol in Rods Investigated in the Living Primate Eye With Two-Photon Ophthalmoscopy. Invest Ophthalmol Vis Sci 2017, 58, 604–613. [Google Scholar] [CrossRef]
- Murashova, G.A.; Mancuso, C.A.; Sakami, S.; Palczewski, K.; Palczewska, G.; Dantus, M. Epi-direction detected multimodal imaging of an unstained mouse retina with a Yb-fiber laser. Proc SPIE Int Soc Opt Eng 2017, 10069. [Google Scholar] [CrossRef]
- Murashova, G.A.; Mancuso, C.A.; Canfield, J.L.; Sakami, S.; Palczewski, K.; Palczewska, G.; Dantus, M. Multimodal nonlinear optical imaging of unstained retinas in the epi-direction with a sub-40 fs Yb-fiber laser. Biomed Opt Express 2017, 8, 5228–5242. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Stremplewski, P.; Suh, S.; Alexander, N.; Salom, D.; Dong, Z.; Ruminski, D.; Choi, E.H.; Sears, A.E.; Kern, T.S.; et al. Two-photon imaging of the mammalian retina with ultrafast pulsing laser. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Kern, T.S.; Palczewski, K. Noninvasive Two-Photon Microscopy Imaging of Mouse Retina and Retinal Pigment Epithelium. Methods Mol Biol 2019, 1834, 333–343. [Google Scholar] [CrossRef]
- Tang, J.A.H.; Granger, C.E.; Kunala, K.; Parkins, K.; Huynh, K.T.; Bowles-Johnson, K.; Yang, Q.; Hunter, J.J. Adaptive optics fluorescence lifetime imaging ophthalmoscopy of in vivo human retinal pigment epithelium. Biomed Opt Express 2022, 13, 1737–1754. [Google Scholar] [CrossRef]
- Boguslawski, J.; Tomczewski, S.; Dabrowski, M.; Komar, K.; Milkiewicz, J.; Palczewska, G.; Palczewski, K.; Wojtkowski, M. In vivo imaging of the human retina using a two-photon excited fluorescence ophthalmoscope. STAR Protoc 2023, 4, 102225. [Google Scholar] [CrossRef]
- Wolf-Schnurrbusch, U.E.; Wittwer, V.V.; Ghanem, R.; Niederhaeuser, M.; Enzmann, V.; Framme, C.; Wolf, S. Blue-light versus green-light autofluorescence: lesion size of areas of geographic atrophy. Invest Ophthalmol Vis Sci 2011, 52, 9497–9502. [Google Scholar] [CrossRef]
- Burke, T.R.; Duncker, T.; Woods, R.L.; Greenberg, J.P.; Zernant, J.; Tsang, S.H.; Smith, R.T.; Allikmets, R.; Sparrow, J.R.; Delori, F.C. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci 2014, 55, 2841–2852. [Google Scholar] [CrossRef]
- Duncker, T.; Greenberg, J.P.; Ramachandran, R.; Hood, D.C.; Smith, R.T.; Hirose, T.; Woods, R.L.; Tsang, S.H.; Delori, F.C.; Sparrow, J.R. Quantitative fundus autofluorescence and optical coherence tomography in best vitelliform macular dystrophy. Invest Ophthalmol Vis Sci 2014, 55, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Duncker, T.; Stein, G.E.; Lee, W.; Tsang, S.H.; Zernant, J.; Bearelly, S.; Hood, D.C.; Greenstein, V.C.; Delori, F.C.; Allikmets, R.; et al. Quantitative Fundus Autofluorescence and Optical Coherence Tomography in ABCA4 Carriers. Invest Ophthalmol Vis Sci 2015, 56, 7274–7285. [Google Scholar] [CrossRef]
- Duncker, T.; Tsang, S.H.; Lee, W.; Zernant, J.; Allikmets, R.; Delori, F.C.; Sparrow, J.R. Quantitative fundus autofluorescence distinguishes ABCA4-associated and non-ABCA4-associated bull’s-eye maculopathy. Ophthalmology 2015, 122, 345–355. [Google Scholar] [CrossRef]
- Duncker, T.; Tsang, S.H.; Woods, R.L.; Lee, W.; Zernant, J.; Allikmets, R.; Delori, F.C.; Sparrow, J.R. Quantitative Fundus Autofluorescence and Optical Coherence Tomography in PRPH2/RDS- and ABCA4-Associated Disease Exhibiting Phenotypic Overlap. Invest Ophthalmol Vis Sci 2015, 56, 3159–3170. [Google Scholar] [CrossRef]
- Marsiglia, M.; Lee, W.; Mahajan, V.B.; Zernant, J.; Delori, F.C.; Tsang, S.H.; Sparrow, J.R. Quantitative autofluorescence as a clinical tool for expedited differential diagnosis of retinal degeneration. JAMA Ophthalmol 2015, 133, 219–220. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Duncker, T.; Woods, R.; Delori, F.C. Quantitative Fundus Autofluorescence in Best Vitelliform Macular Dystrophy: RPE Lipofuscin is not Increased in Non-Lesion Areas of Retina. Adv Exp Med Biol 2016, 854, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; Goerdt, L.; Schmitz-Valckenberg, S.; Mauschitz, M.M.; Mishra, D.K.; Holz, F.G.; Lindner, M.; Fleckenstein, M. Green-Light Autofluorescence Versus Combined Blue-Light Autofluorescence and Near-Infrared Reflectance Imaging in Geographic Atrophy Secondary to Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2017, 58, BIO121–BIO130. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, K.; Woods, R.L.; Lee, W.; Duncker, T.; Delori, F.C.; Allikmets, R.; Tsang, S.H.; Sparrow, J.R. Quantifying Fundus Autofluorescence in Patients With Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 2017, 58, 1843–1855. [Google Scholar] [CrossRef]
- Chen, L.; Messinger, J.D.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Fundus Autofluorescence in Neovascular Age-Related Macular Degeneration: A Clinicopathologic Correlation Relevant to Macular Atrophy. Ophthalmol Retina 2021, 5, 1085–1096. [Google Scholar] [CrossRef]
- Salcedo-Villanueva, G.; Lopez-Contreras, Y.; Gonzalez, H.L.A.; Romo-Aguas, J.C.; Garcia-Aguirre, G.; Cernichiaro-Espinosa, L.A.; Martinez-Castellanos, M.A.; Quiroz-Mercado, H. Fundus autofluorescence in premature infants. Sci Rep 2021, 11, 8823. [Google Scholar] [CrossRef]
- Delori, F.C.; Goger, D.G.; Dorey, C.K. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci 2001, 42, 1855–1866. [Google Scholar] [PubMed]
- Ach, T.; Huisingh, C.; McGwin, G., Jr.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci 2014, 55, 4832–4841. [Google Scholar] [CrossRef]
- Okubo, A.; Rosa, R.H., Jr.; Bunce, C.V.; Alexander, R.A.; Fan, J.T.; Bird, A.C.; Luthert, P.J. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Invest Ophthalmol Vis Sci 1999, 40, 443–449. [Google Scholar] [PubMed]
- Greenberg, J.P.; Duncker, T.; Woods, R.L.; Smith, R.T.; Sparrow, J.R.; Delori, F.C. Quantitative fundus autofluorescence in healthy eyes. Invest Ophthalmol Vis Sci 2013, 54, 5684–5693. [Google Scholar] [CrossRef] [PubMed]
- Ben Ami, T.; Tong, Y.; Bhuiyan, A.; Huisingh, C.; Ablonczy, Z.; Ach, T.; Curcio, C.A.; Smith, R.T. Spatial and Spectral Characterization of Human Retinal Pigment Epithelium Fluorophore Families by Ex Vivo Hyperspectral Autofluorescence Imaging. Transl Vis Sci Technol 2016, 5, 5. [Google Scholar] [CrossRef]
- Schultz, R.; Schwanengel, L.; Klemm, M.; Meller, D.; Hammer, M. Spectral fundus autofluorescence peak emission wavelength in ageing and AMD. Acta Ophthalmol 2022, 100, e1223–e1231. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.; Klemm, M.; Meller, D.; Hammer, M. Spectral calibration of fluorescence lifetime imaging ophthalmoscopy. Acta Ophthalmol 2022, 100, e612–e613. [Google Scholar] [CrossRef]
- Zuclich, J.A.; Previc, F.H.; Novar, B.J.; Edsall, P.R. Near-UV/blue light-induced fluorescence in the human lens: potential interference with visual function. J Biomed Opt 2005, 10, 44021. [Google Scholar] [CrossRef]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol 2022. [CrossRef]
- Creuzot-Garcher, C.P.; Srour, M.; Baudin, F.; Daien, V.; Dot, C.; Nghiem-Buffet, S.; Girmens, J.F.; Coulombel, N.; Ponthieux, A.; Delcourt, C. Incidence and Prevalence of Neovascular Age-Related Macular Degeneration in France between 2008 and 2018: The LANDSCAPE Study. Ophthalmol Sci 2022, 2, 100114. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-Related Macular Degeneration: Epidemiology and Clinical Aspects. In Age-related Macular Degeneration, Advances in Experimental Medicine and Biology, Chew, E.Y., Swaroop, A., Eds.; Advances in Experimental Medicine and Biology; Springer Nature Switzerland AG: Cham, Switzerland, 2021; Volume 1256, pp. 1–31.
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 2016, 3, 34. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Agron, E.; Domalpally, A.; Cukras, C.A.; Clemons, T.E.; Chen, Q.; Lu, Z.; Chew, E.Y.; Keenan, T.D.L.; Areds; Groups, A.R. Reticular Pseudodrusen: The Third Macular Risk Feature for Progression to Late Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report 30. Ophthalmology 2022, 129, 1107–1119. [CrossRef]
- Rudolf, M.; Vogt, S.D.; Curcio, C.A.; Huisingh, C.; McGwin, G., Jr.; Wagner, A.; Grisanti, S.; Read, R.W. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology 2013, 120, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zanzottera, E.C.; Ach, T.; Huisingh, C.; Messinger, J.D.; Freund, K.B.; Curcio, C.A. Visualizing Retinal Pigment Epithelium Phenotypes in the Transition to Atrophy in Neovascular Age-Related Macular Degeneration. Retina 2016, 36 Suppl 1, S26–S39. [Google Scholar] [CrossRef]
- Bonilha, V.L.; Bell, B.A.; Hu, J.; Milliner, C.; Pauer, G.J.; Hagstrom, S.A.; Radu, R.A.; Hollyfield, J.G. Geographic Atrophy: Confocal Scanning Laser Ophthalmoscopy, Histology, and Inflammation in the Region of Expanding Lesions. Invest Ophthalmol Vis Sci 2020, 61, 15. [Google Scholar] [CrossRef] [PubMed]
- Zanzottera, E.C.; Messinger, J.D.; Ach, T.; Smith, R.T.; Freund, K.B.; Curcio, C.A. The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2015, 56, 3253–3268. [Google Scholar] [CrossRef]
- Gambril, J.A.; Sloan, K.R.; Swain, T.A.; Huisingh, C.; Zarubina, A.V.; Messinger, J.D.; Ach, T.; Curcio, C.A. Quantifying Retinal Pigment Epithelium Dysmorphia and Loss of Histologic Autofluorescence in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2019, 60, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ben Ami, T.; Hong, S.; Heintzmann, R.; Gerig, G.; Ablonczy, Z.; Curcio, C.A.; Ach, T.; Smith, R.T. Hyperspectral Autofluorescence Imaging of Drusen and Retinal Pigment Epithelium in Donor Eyes with Age-Related Macular Degeneration. Retina 2016, 36 Suppl 1, S127–S136. [Google Scholar] [CrossRef]
- Mohammed, T.; Tong, Y.; Agee, J.; Challa, N.; Heintzmann, R.; Hammer, M.; Curcio, C.A.; Ach, T.; Ablonczy, Z.; Smith, R.T. Ex Vivo Hyperspectral Autofluorescence Imaging and Localization of Fluorophores in Human Eyes with Age-Related Macular Degeneration. Vision (Basel) 2018, 2. [Google Scholar] [CrossRef]
- Rozanowski, B.; Cuenco, J.; Davies, S.; Shamsi, F.A.; Zadlo, A.; Dayhaw-Barker, P.; Rozanowska, M.; Sarna, T.; Boulton, M.E. The phototoxicity of aged human retinal melanosomes. Photochemistry and photobiology 2008, 84, 650–657. [Google Scholar] [CrossRef]
- Rozanowski, B.; Burke, J.M.; Boulton, M.E.; Sarna, T.; Rozanowska, M. Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Invest Ophthalmol Vis Sci 2008, 49, 2838–2847. [Google Scholar] [CrossRef]
- Teussink, M.M.; Lambertus, S.; de Mul, F.F.; Rozanowska, M.B.; Hoyng, C.B.; Klevering, B.J.; Theelen, T. Lipofuscin-associated photo-oxidative stress during fundus autofluorescence imaging. PLoS One 2017, 12, e0172635. [Google Scholar] [CrossRef] [PubMed]
- Arend, O.; Weiter, J.J.; Goger, D.G.; Delori, F.C. [In vivo fundus fluorescence measurements in patients with age related macular degeneration]. Ophthalmologe 1995, 92, 647–653. [Google Scholar]
- Delori, F.C.; Fleckner, M.R.; Goger, D.G.; Weiter, J.J.; Dorey, C.K. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000, 41, 496–504. [Google Scholar]
- Hammer, M.; Schultz, R.; Hasan, S.; Sauer, L.; Klemm, M.; Kreilkamp, L.; Zweifel, L.; Augsten, R.; Meller, D. Fundus Autofluorescence Lifetimes and Spectral Features of Soft Drusen and Hyperpigmentation in Age-Related Macular Degeneration. Transl Vis Sci Technol 2020, 9, 20. [Google Scholar] [CrossRef]
- Weber, S.; Simon, R.; Schwanengel, L.S.; Curcio, C.A.; Augsten, R.; Meller, D.; Hammer, M. Fluorescence Lifetime and Spectral Characteristics of Subretinal Drusenoid Deposits and Their Predictive Value for Progression of Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2022, 63, 23. [Google Scholar] [CrossRef]
- Borrelli, E.; Nittala, M.G.; Abdelfattah, N.S.; Lei, J.; Hariri, A.H.; Shi, Y.; Fan, W.; Cozzi, M.; Sarao, V.; Lanzetta, P.; et al. Comparison of short-wavelength blue-light autofluorescence and conventional blue-light autofluorescence in geographic atrophy. Br J Ophthalmol 2018, 103, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Quick, S.; Klemm, M.; Schenke, S.; Mata, N.; Eitner, A.; Schweitzer, D. In-vivo and in-vitro investigations of retinal fluorophores in age - related macular degeneration by fluorescence lifetime imaging. Multiphoton Microscopy in the Biomedical Sciences Ix 2009, 7183. [Google Scholar] [CrossRef]
- Schweitzer, D.; Quick, S.; Schenke, S.; Klemm, M.; Gehlert, S.; Hammer, M.; Jentsch, S.; Fischer, J. [Comparison of parameters of time-resolved autofluorescence between healthy subjects and patients suffering from early AMD]. Ophthalmologe 2009, 106, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.S.; Hacker, V.; Told, R.; Schranz, M.; Krotka, P.; Schlanitz, F.G.; Sacu, S.; Pollreisz, A.; Schmidt-Erfurth, U. Longitudinal Changes in Quantitative Autofluorescence during Progression from Intermediate to Late Age-Related Macular Degeneration. Retin.-J. Retin. Vitr. Dis. 2021, 41, 1236–1241. [Google Scholar] [CrossRef]
- Reiter, G.S.; Told, R.; Baumann, L.; Sacu, S.; Schmidt-Erfurth, U.; Pollreisz, A. Investigating a Growth Prediction Model in Advanced Age-Related Macular Degeneration with Solitary Geographic Atrophy Using Quantitative Autofluorescence. Retin.-J. Retin. Vitr. Dis. 2020, 40, 1657–1664. [Google Scholar] [CrossRef]
- Schwanengel, L.S.; Weber, S.; Simon, R.; Lehmann, T.; Augsten, R.; Meller, D.; Hammer, M. Changes in drusen-associated autofluorescence over time observed by fluorescence lifetime imaging ophthalmoscopy in age-related macular degeneration. Acta Ophthalmol 2022. [CrossRef]
- Chen, L.; Yang, P.; Curcio, C.A. Visualizing lipid behind the retina in aging and age-related macular degeneration, via indocyanine green angiography (ASHS-LIA). Eye (Lond) 2022, 36, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Myers, C.E.; Lee, K.E.; Gangnon, R.E.; Sivakumaran, T.A.; Iyengar, S.K.; Klein, B.E. Small Drusen and Age-Related Macular Degeneration: The Beaver Dam Eye Study. J Clin Med 2015, 4, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.B.; Yakovleva, M.A.; Arbukhanova, P.M.; Borzenok, S.A.; Kononikhin, A.S.; Popov, I.A.; Nikolaev, E.N.; Ostrovsky, M.A. Changes in spectral properties and composition of lipofuscin fluorophores from human-retinal-pigment epithelium with age and pathology. Anal Bioanal Chem 2015, 407, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Feldman, T.B.; Arbukhanova, P.M.; Borzenok, S.A.; Kuzmin, V.A.; Ostrovsky, M.A. The fluorescence lifetime of lipofuscin granule fluorophores contained in the retinal pigment epithelium cells from human cadaver eyes in normal state and in the case of visualized pathology. Dokl Biochem Biophys 2017, 474, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.B.; Yakovleva, M.A.; Larichev, A.V.; Arbukhanova, P.M.; Radchenko, A.S.; Borzenok, S.A.; Kuzmin, V.A.; Ostrovsky, M.A. Spectral analysis of fundus autofluorescence pattern as a tool to detect early stages of degeneration in the retina and retinal pigment epithelium. Eye (Lond) 2018, 32, 1440–1448. [Google Scholar] [CrossRef]
- Yakovleva, M.A.; Radchenko, A.S.; Feldman, T.B.; Kostyukov, A.A.; Arbukhanova, P.M.; Borzenok, S.A.; Kuzmin, V.A.; Ostrovsky, M.A. Fluorescence characteristics of lipofuscin fluorophores from human retinal pigment epithelium. Photochem Photobiol Sci 2020, 19, 920–930. [Google Scholar] [CrossRef]
- Mata, N.L.; Weng, J.; Travis, G.H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A 2000, 97, 7154–7159. [Google Scholar] [CrossRef]
- Ueda, K.; Zhao, J.; Kim, H.J.; Sparrow, J.R. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc Natl Acad Sci U S A 2016, 113, 6904–6909. [Google Scholar] [CrossRef]
- Ueda, K.; Kim, H.J.; Zhao, J.; Song, Y.; Dunaief, J.L.; Sparrow, J.R. Iron promotes oxidative cell death caused by bisretinoids of retina. Proc Natl Acad Sci U S A 2018, 115, 4963–4968. [Google Scholar] [CrossRef]
- Pandya, V.B.; Ho, I.V.; Hunyor, A.P. Does unintentional macular translocation after retinal detachment repair influence visual outcome? Clin Exp Ophthalmol 2012, 40, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Shiragami, C.; Shiraga, F.; Yamaji, H.; Fukuda, K.; Takagishi, M.; Morita, M.; Kishikami, T. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology 2010, 117, 86–92 e81. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci 2010, 51, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim Biophys Acta Mol Cell Biol Lipids 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Datta, S.; Wang, L.; Pegany, R.; Cano, M.; Handa, J.T. The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp Eye Res 2019, 181, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Karunadharma, P.P.; Kapphahn, R.J.; Stahl, M.R.; Olsen, T.W.; Ferrington, D.A. Dissecting Regulators of Aging and Age-Related Macular Degeneration in the Retinal Pigment Epithelium. Oxid Med Cell Longev 2022, 2022, 6009787. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Milam, A.H.; Dunaief, J.L. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch Ophthalmol 2003, 121, 1099–1105. [Google Scholar] [CrossRef]
- Liu, Y.; Bell, B.A.; Song, Y.; Zhang, K.; Anderson, B.; Axelsen, P.H.; Bohannan, W.; Agbaga, M.P.; Park, H.G.; James, G.; et al. Deuterated docosahexaenoic acid protects against oxidative stress and geographic atrophy-like retinal degeneration in a mouse model with iron overload. Aging Cell 2022, 21, e13579. [Google Scholar] [CrossRef] [PubMed]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.M.; Dupont, M.; et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol 2023. [CrossRef]
- Shchepinov, M.S. Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends Pharmacol Sci 2020, 41, 236–248. [Google Scholar] [CrossRef]
- Firsov, A.M.; Fomich, M.A.; Bekish, A.V.; Sharko, O.L.; Kotova, E.A.; Saal, H.J.; Vidovic, D.; Shmanai, V.V.; Pratt, D.A.; Antonenko, Y.N.; et al. Threshold protective effect of deuterated polyunsaturated fatty acids on peroxidation of lipid bilayers. FEBS J 2019, 286, 2099–2117. [Google Scholar] [CrossRef]
- Rosell, M.; Giera, M.; Brabet, P.; Shchepinov, M.S.; Guichardant, M.; Durand, T.; Vercauteren, J.; Galano, J.M.; Crauste, C. Bis-allylic Deuterated DHA Alleviates Oxidative Stress in Retinal Epithelial Cells. Antioxidants (Basel) 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.K.; Baumann, B.; Foshe, S.; Voigt, A.; Guttha, S.; Alnemri, A.; McCright, S.J.; Li, M.; Zauhar, R.J.; Montezuma, S.R.; et al. Inflammatory adipose activates a nutritional immunity pathway leading to retinal dysfunction. Cell Rep 2022, 39, 110942. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Mukherjee, P.K.; Cui, J.G.; Bazan, N.G. A2E selectively induces cox-2 in ARPE-19 and human neural cells. Curr Eye Res 2006, 31, 259–263. [Google Scholar] [CrossRef]
- Chahal, S.; Rani, P.; Kiran; Sindhu, J.; Joshi, G.; Ganesan, A.; Kalyaanamoorthy, S.; Mayank; Kumar, P.; Singh, R.; et al. Design and Development of COX-II Inhibitors: Current Scenario and Future Perspective. ACS Omega 2023, 8, 17446–17498. [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Gu, X.; Meer, S.G.; Miyagi, M.; Rayborn, M.E.; Hollyfield, J.G.; Crabb, J.W.; Salomon, R.G. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem 2003, 278, 42027–42035. [Google Scholar] [CrossRef] [PubMed]
- Renganathan, K.; Ebrahem, Q.; Vasanji, A.; Gu, X.; Lu, L.; Sears, J.; Salomon, R.G.; Anand-Apte, B.; Crabb, J.W. Carboxyethylpyrrole adducts, age-related macular degeneration and neovascularization. Adv Exp Med Biol 2008, 613, 261–267. [Google Scholar] [CrossRef]
- Ebrahem, Q.; Renganathan, K.; Sears, J.; Vasanji, A.; Gu, X.; Lu, L.; Salomon, R.G.; Crabb, J.W.; Anand-Apte, B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc Natl Acad Sci U S A 2006, 103, 13480–13484. [Google Scholar] [CrossRef] [PubMed]
- Ayalasomayajula, S.P.; Kompella, U.B. Induction of vascular endothelial growth factor by 4-hydroxynonenal and its prevention by glutathione precursors in retinal pigment epithelial cells. Eur J Pharmacol 2002, 449, 213–220. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, B.; Jang, Y.P.; Pachydaki, S.; Schmidt, A.M.; Sparrow, J.R. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp Eye Res 2005, 80, 567–580. [Google Scholar] [CrossRef]
- Yanagi, Y.; Inoue, Y.; Iriyama, A.; Jang, W.D. Effects of yellow intraocular lenses on light-induced upregulation of vascular endothelial growth factor. J Cataract Refract Surg 2006, 32, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Ung, C.; Lains, I.; Miller, J.W.; Kim, I.K. Current Management of Age-Related Macular Degeneration. In Age-related Macular Degeneration From Clinic to Genes and Back to Patient Management. Advances in Experimental Medicine and Biology, Chew, E.Y., Swaroop, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 1256, pp. 295–314.
- Higgins, G.T.; Wang, J.H.; Dockery, P.; Cleary, P.E.; Redmond, H.P. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci 2003, 44, 1775–1782. [Google Scholar] [CrossRef]
- Hernandez, C.; Segura, R.M.; Fonollosa, A.; Carrasco, E.; Francisco, G.; Simo, R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med 2005, 22, 719–722. [Google Scholar] [CrossRef]
- Wang, L.; Cano, M.; Datta, S.; Wei, H.; Ebrahimi, K.B.; Gorashi, Y.; Garlanda, C.; Handa, J.T. Pentraxin 3 recruits complement factor H to protect against oxidative stress-induced complement and inflammasome overactivation. J Pathol 2016, 240, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Ng, E.S.Y.; Kady, N.; Hu, J.; Dave, A.; Jiang, Z.; Pei, J.; Gorin, M.B.; Matynia, A.; Radu, R.A. Membrane Attack Complex Mediates Retinal Pigment Epithelium Cell Death in Stargardt Macular Degeneration. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pauer, G.J.; Hagstrom, S.A.; Bok, D.; DeBenedictis, M.J.; Bonilha, V.L.; Hollyfield, J.G.; Radu, R.A. Evidence of complement dysregulation in outer retina of Stargardt disease donor eyes. Redox Biol 2020, 37, 101787. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Fisher, C.R.; Kowluru, R.A. Mitochondrial Defects Drive Degenerative Retinal Diseases. Trends Mol Med 2020, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, M.C.; Polanco, J.R.; Qu, J.; Tu, C.; Montezuma, S.R.; Ferrington, D.A. Improving retinal mitochondrial function as a treatment for age-related macular degeneration. Redox Biol 2020, 34, 101552. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog Retin Eye Res 2020, 79, 100858. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Kapphahn, R.J.; Leary, M.M.; Atilano, S.R.; Terluk, M.R.; Karunadharma, P.; Chen, G.K.; Ratnapriya, R.; Swaroop, A.; Montezuma, S.R.; et al. Increased retinal mtDNA damage in the CFH variant associated with age-related macular degeneration. Exp Eye Res 2016, 145, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating mitochondria as a target for treating age-related macular degeneration. J Neurosci 2015, 35, 7304–7311. [Google Scholar] [CrossRef]
- Lin, H.; Xu, H.; Liang, F.Q.; Liang, H.; Gupta, P.; Havey, A.N.; Boulton, M.E.; Godley, B.F. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci 2011, 52, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci 2010, 51, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Kapphahn, R.J.; Zhang, M.; Qian, S.; Montezuma, S.R.; Shang, P.; Ferrington, D.A.; Qu, J. Quantitative Proteomics of Human Retinal Pigment Epithelium Reveals Key Regulators for the Pathogenesis of Age-Related Macular Degeneration. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agron, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; Group, A.R. Long-term Outcomes of Adding Lutein/Zeaxanthin and omega-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol 2022, 140, 692–698. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 2017, 7, CD000254. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research, G.; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Bassetto, M.; Zaluski, J.; Li, B.; Zhang, J.; Badiee, M.; Kiser, P.D.; Tochtrop, G.P. Tuning the Metabolic Stability of Visual Cycle Modulators through Modification of an RPE65 Recognition Motif. J Med Chem 2023, 66, 8140–8158. [Google Scholar] [CrossRef] [PubMed]
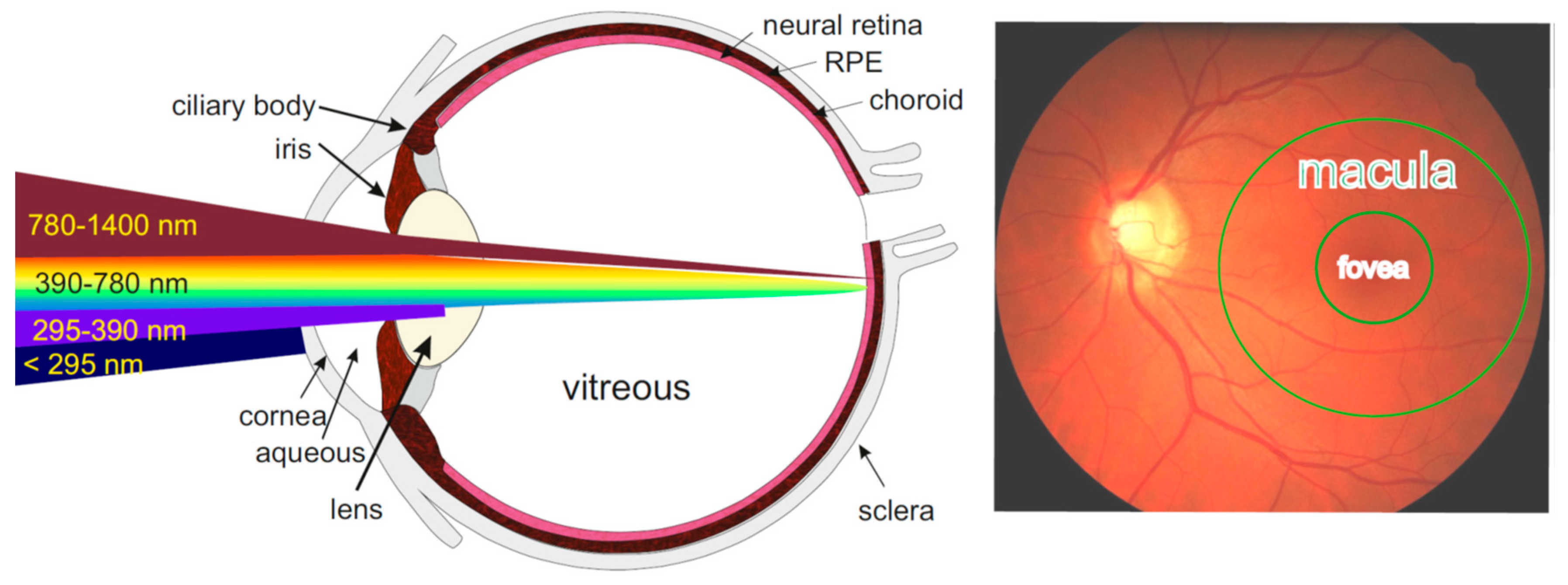
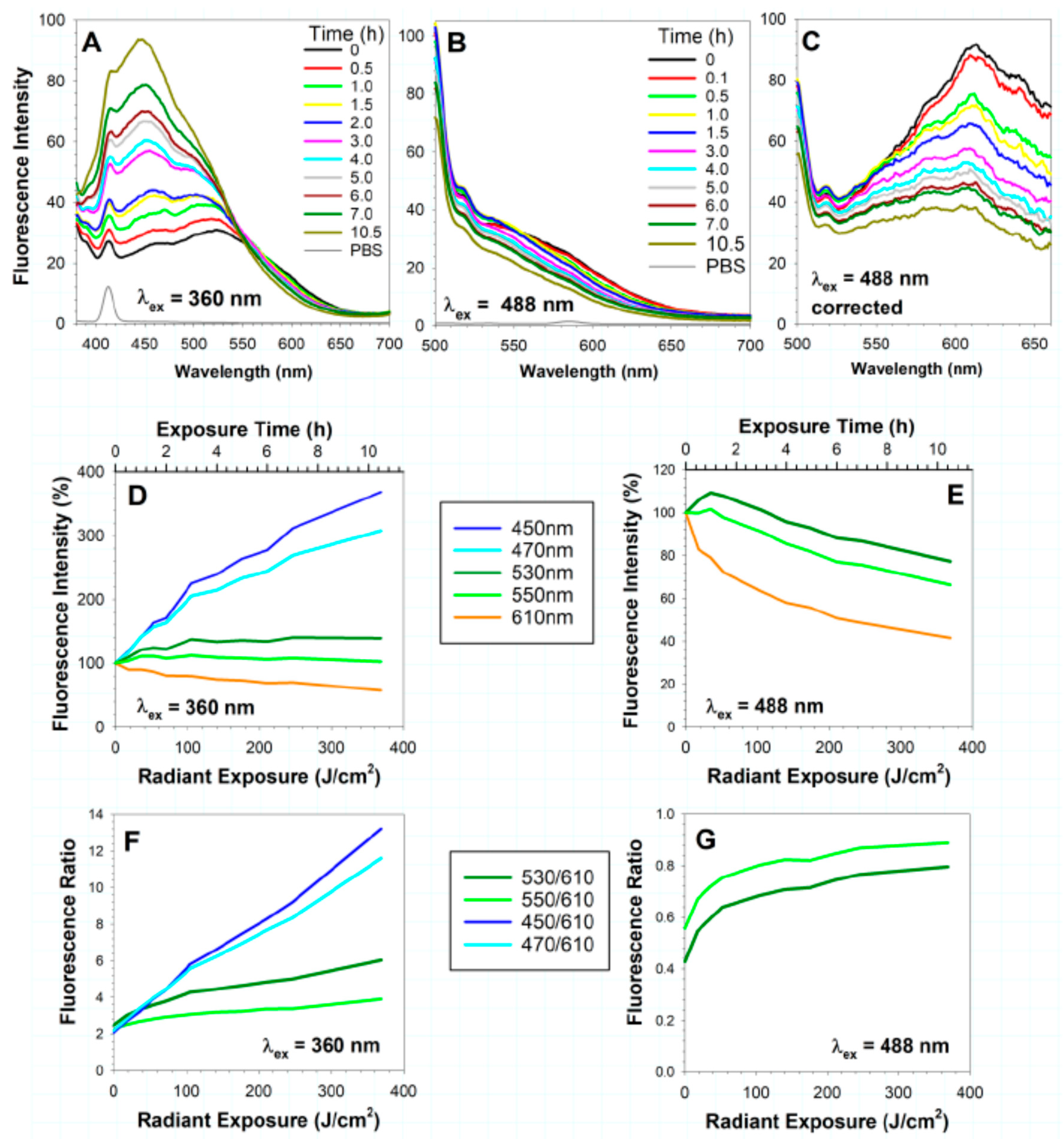
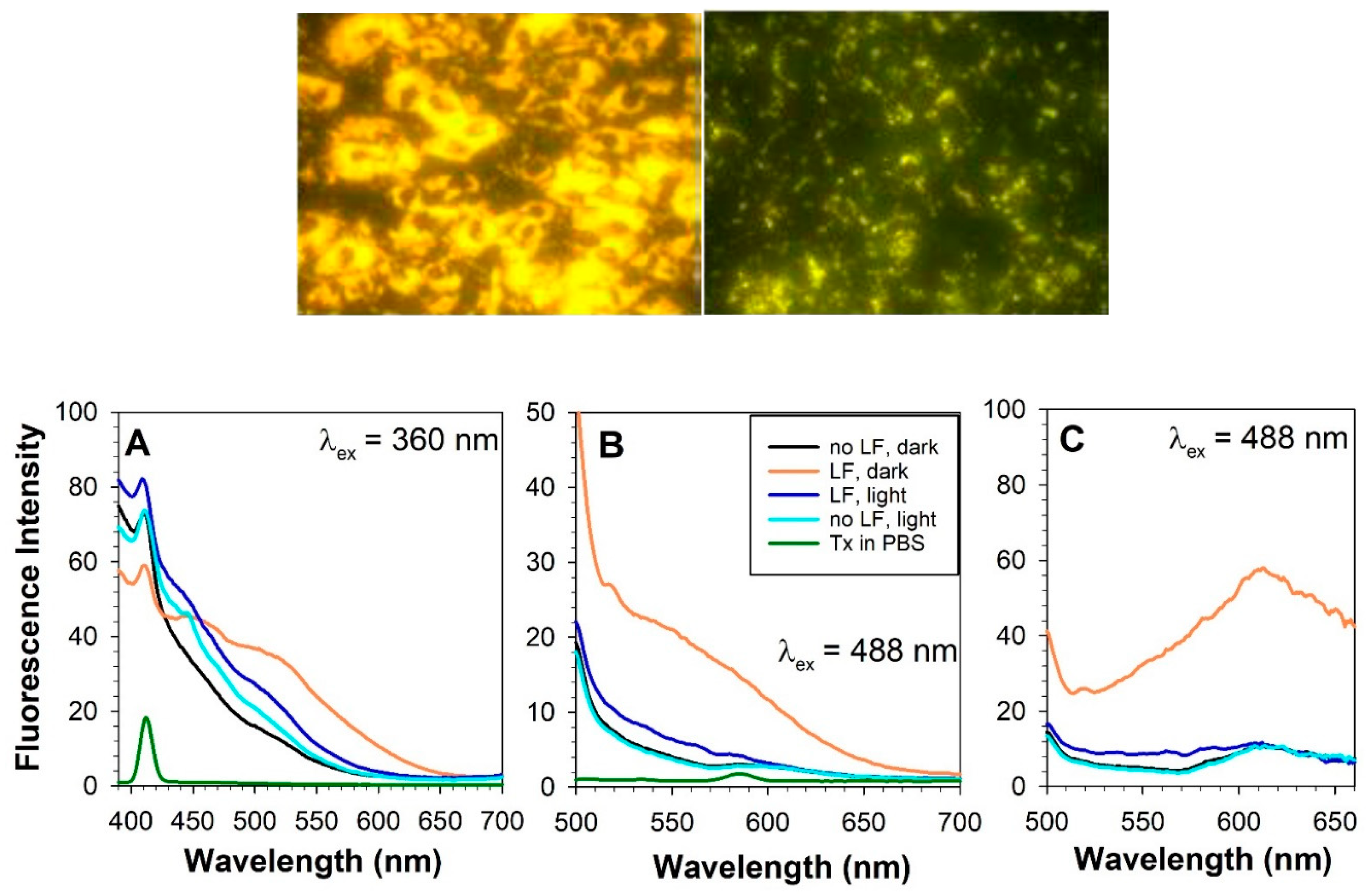
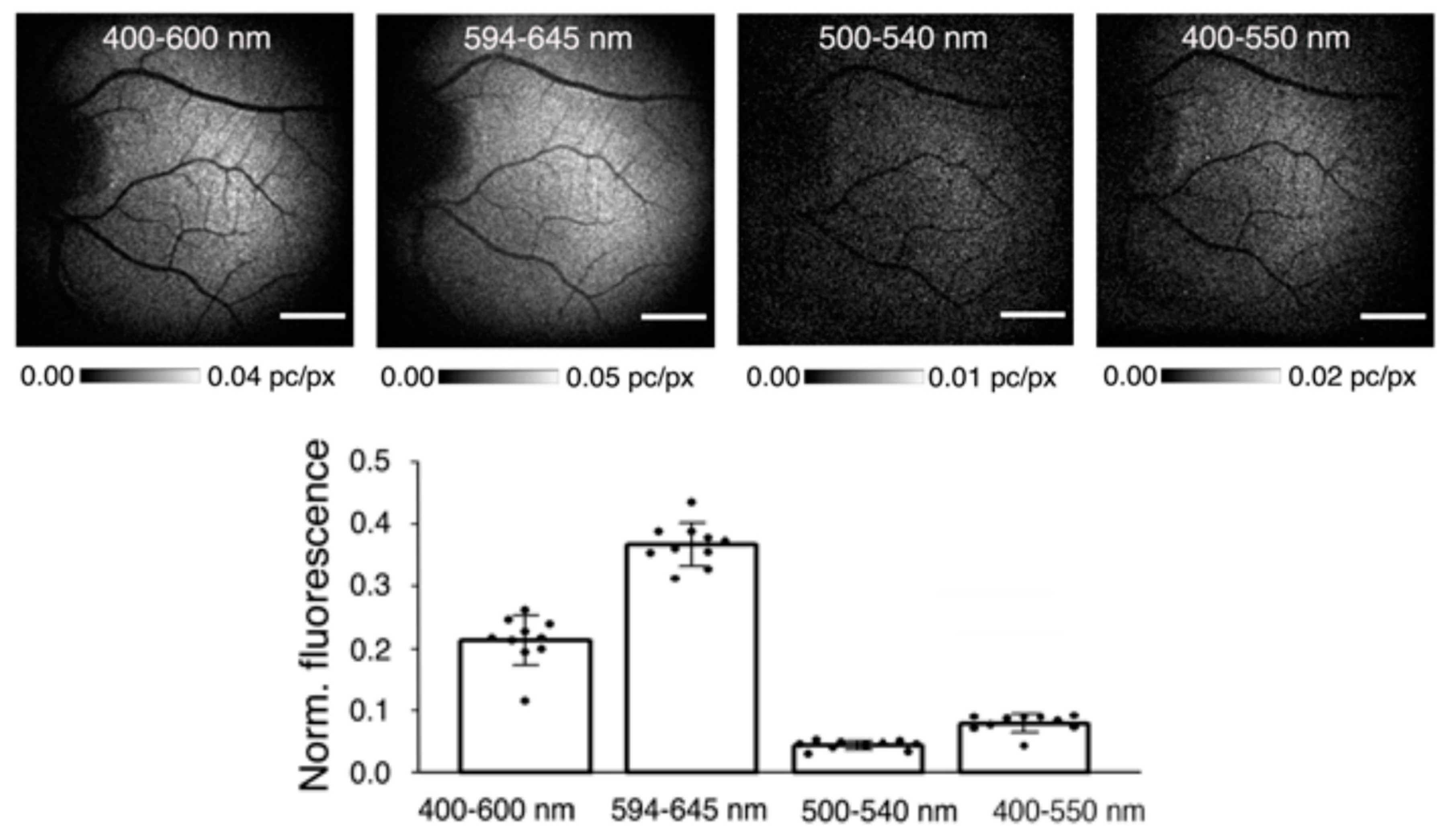
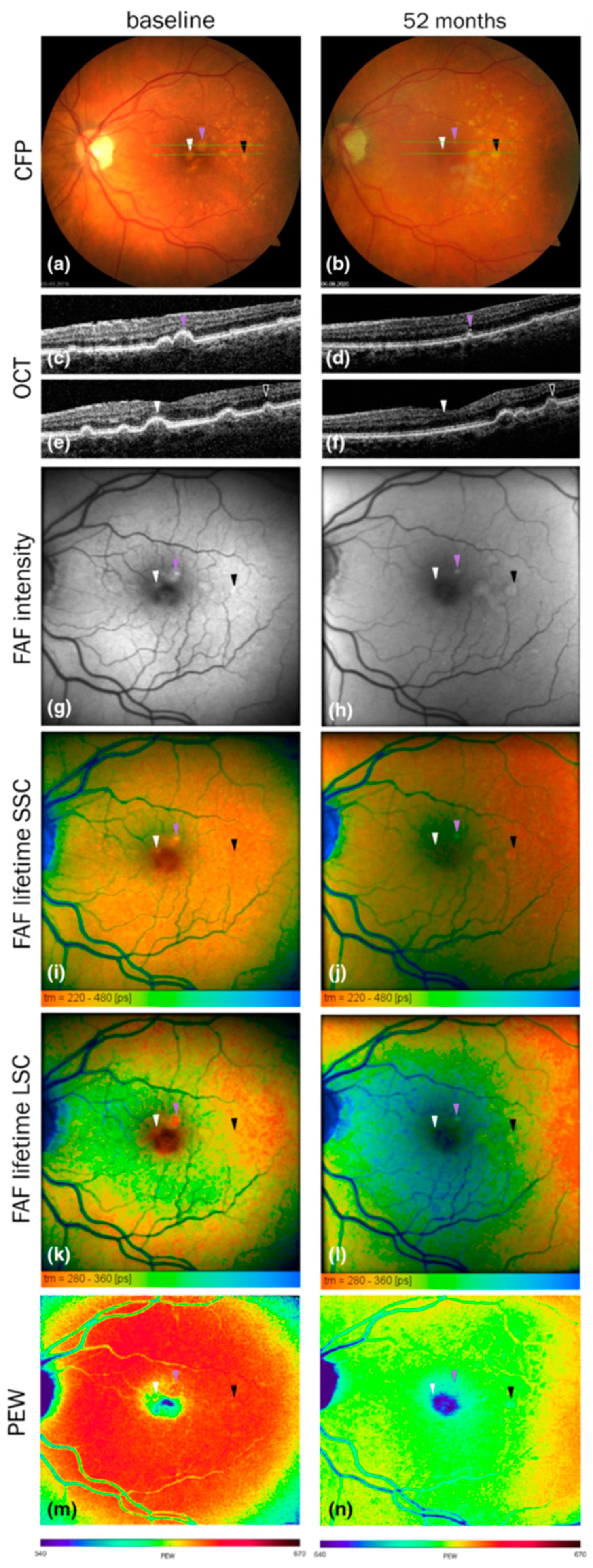
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




