Submitted:
04 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
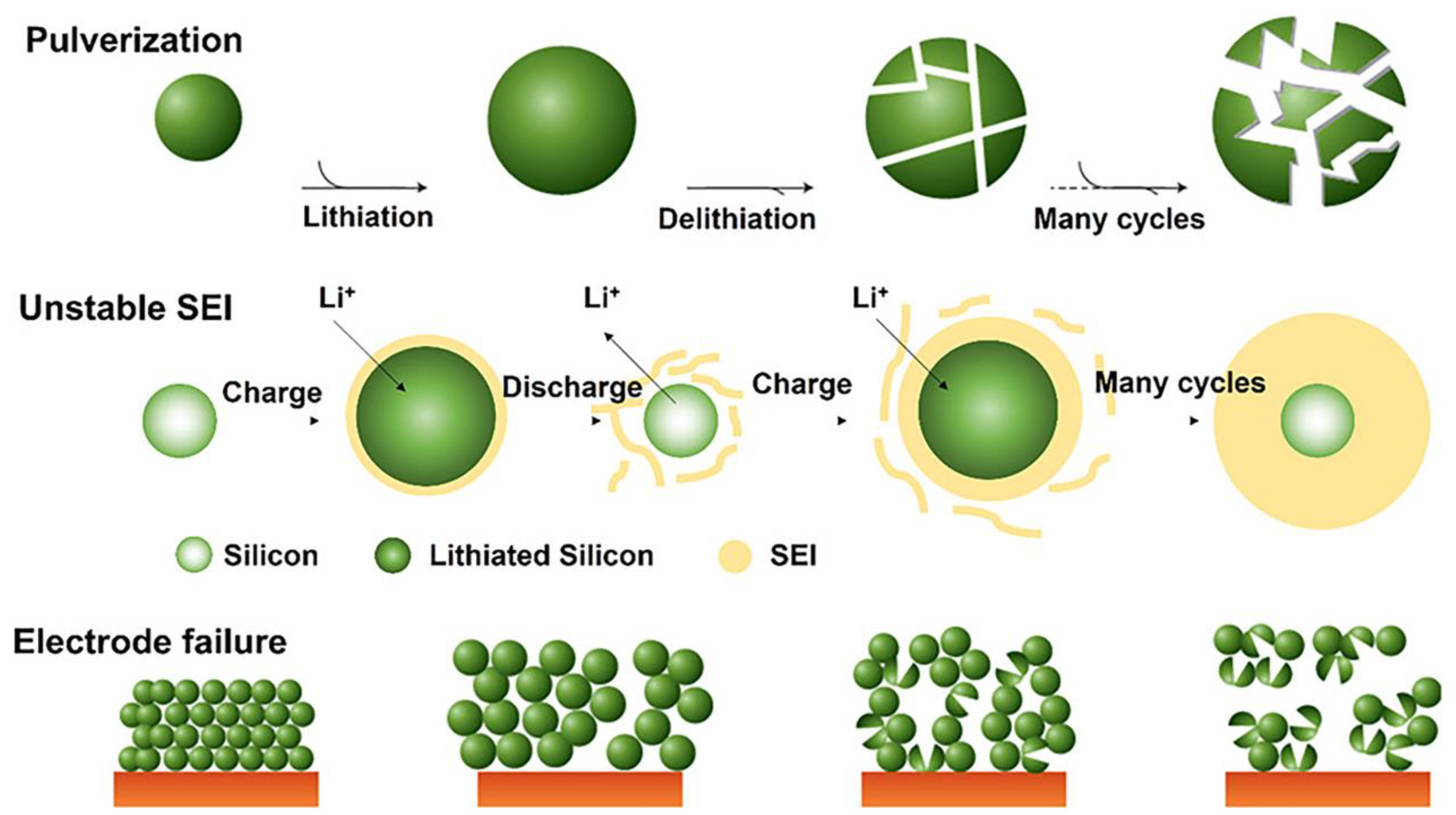
2. Problems with silicon anodes
3. Silicon anode structure optimization
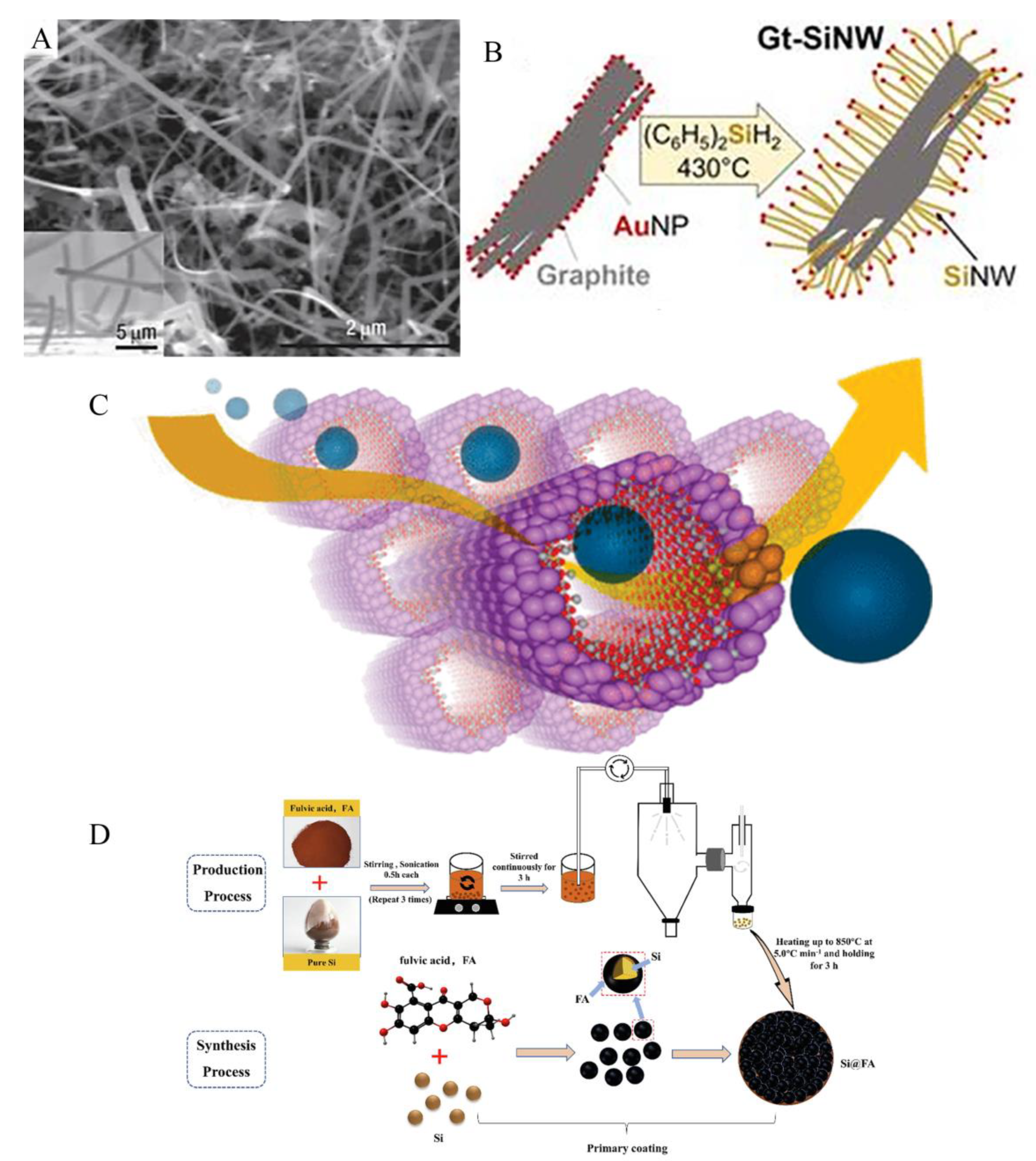
3.1. Size reduction
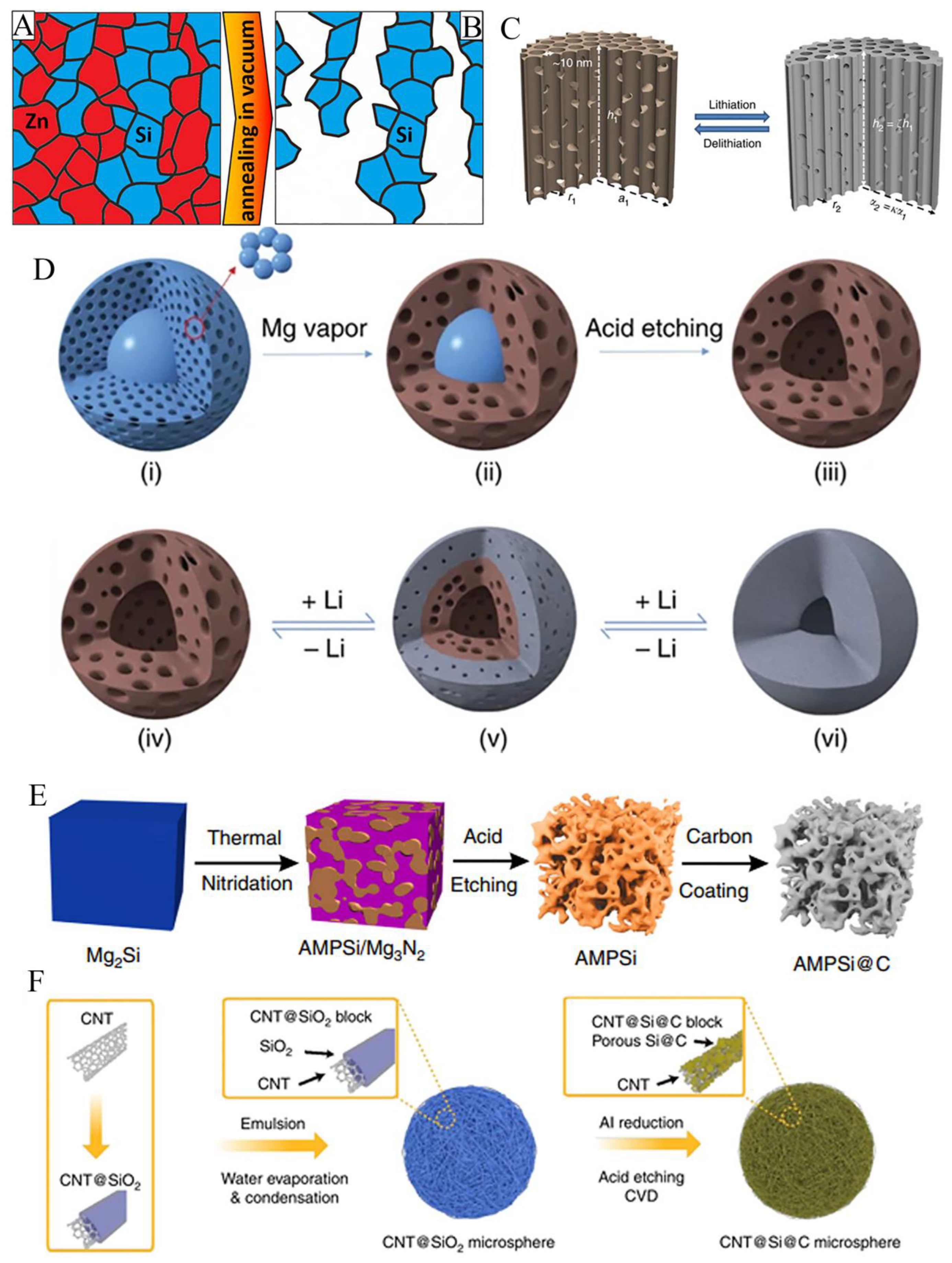
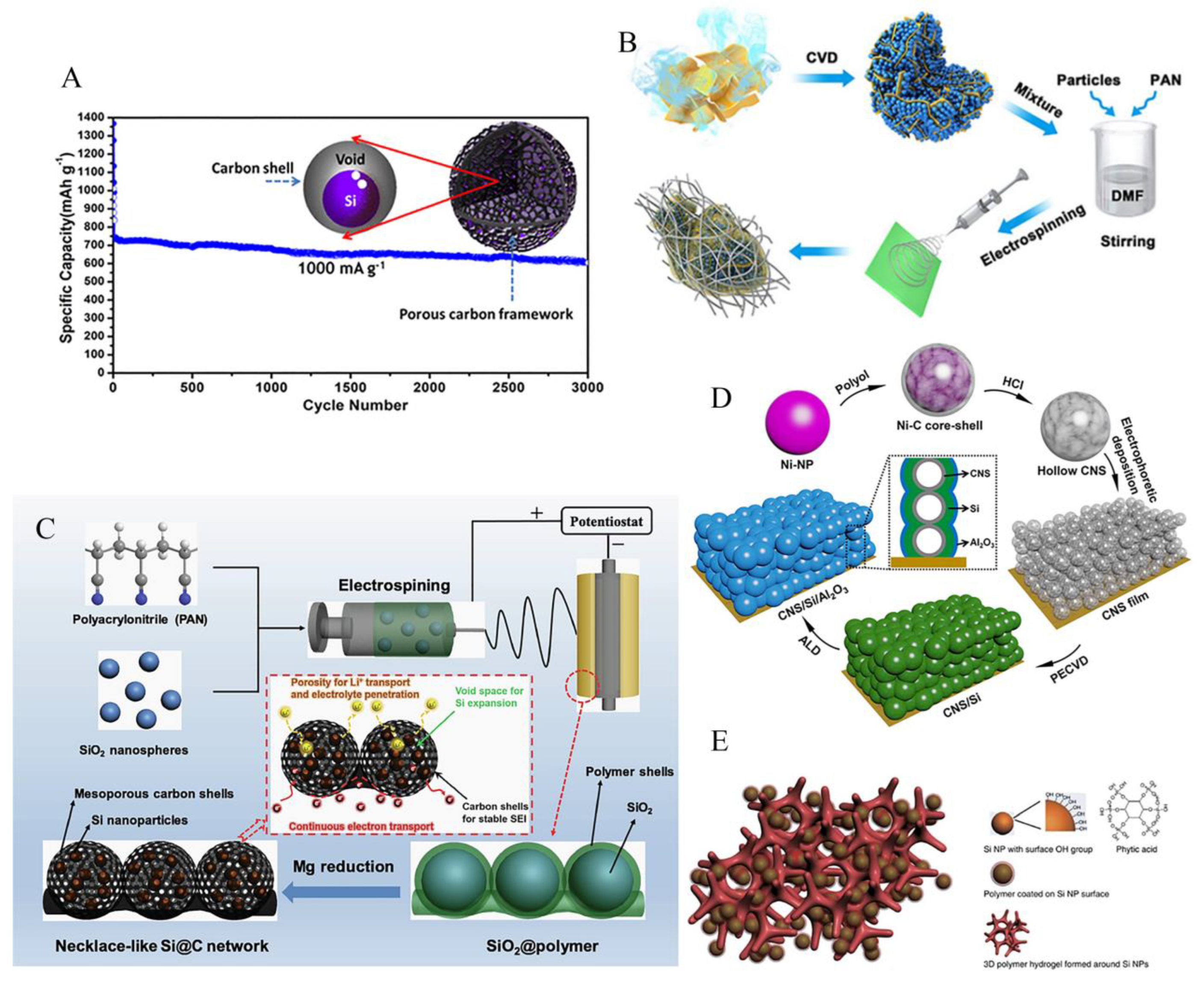
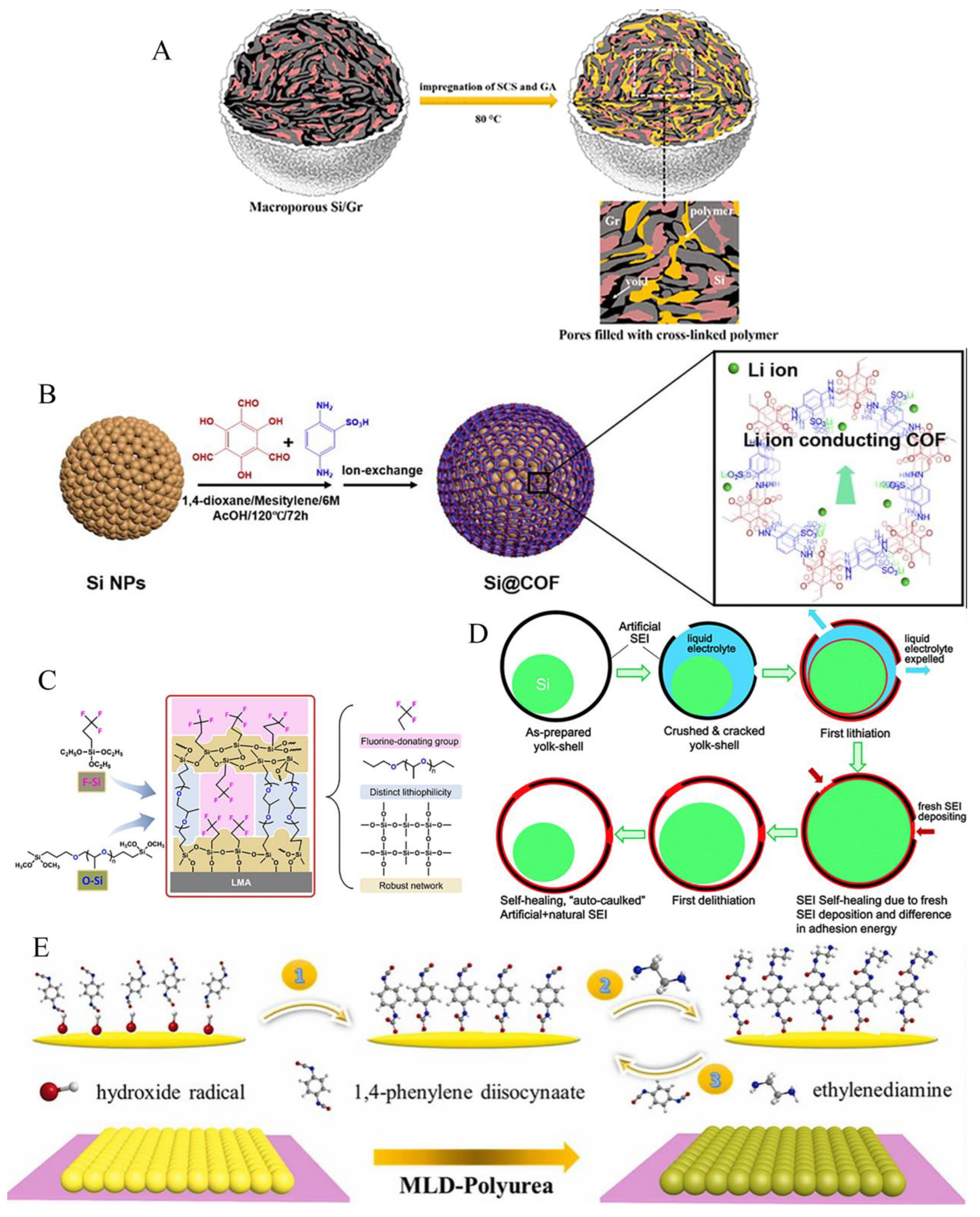
3.2. Porous structure
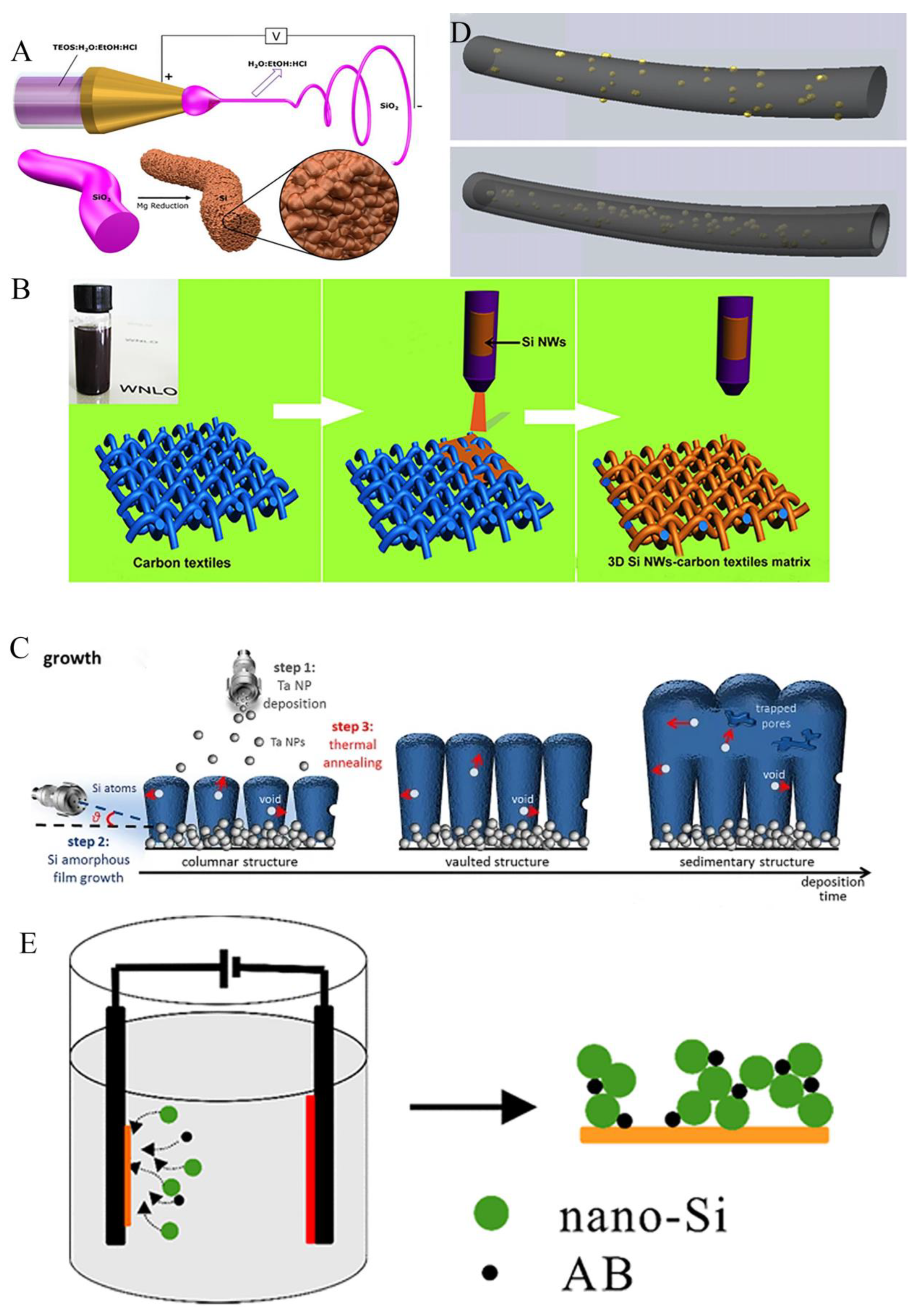
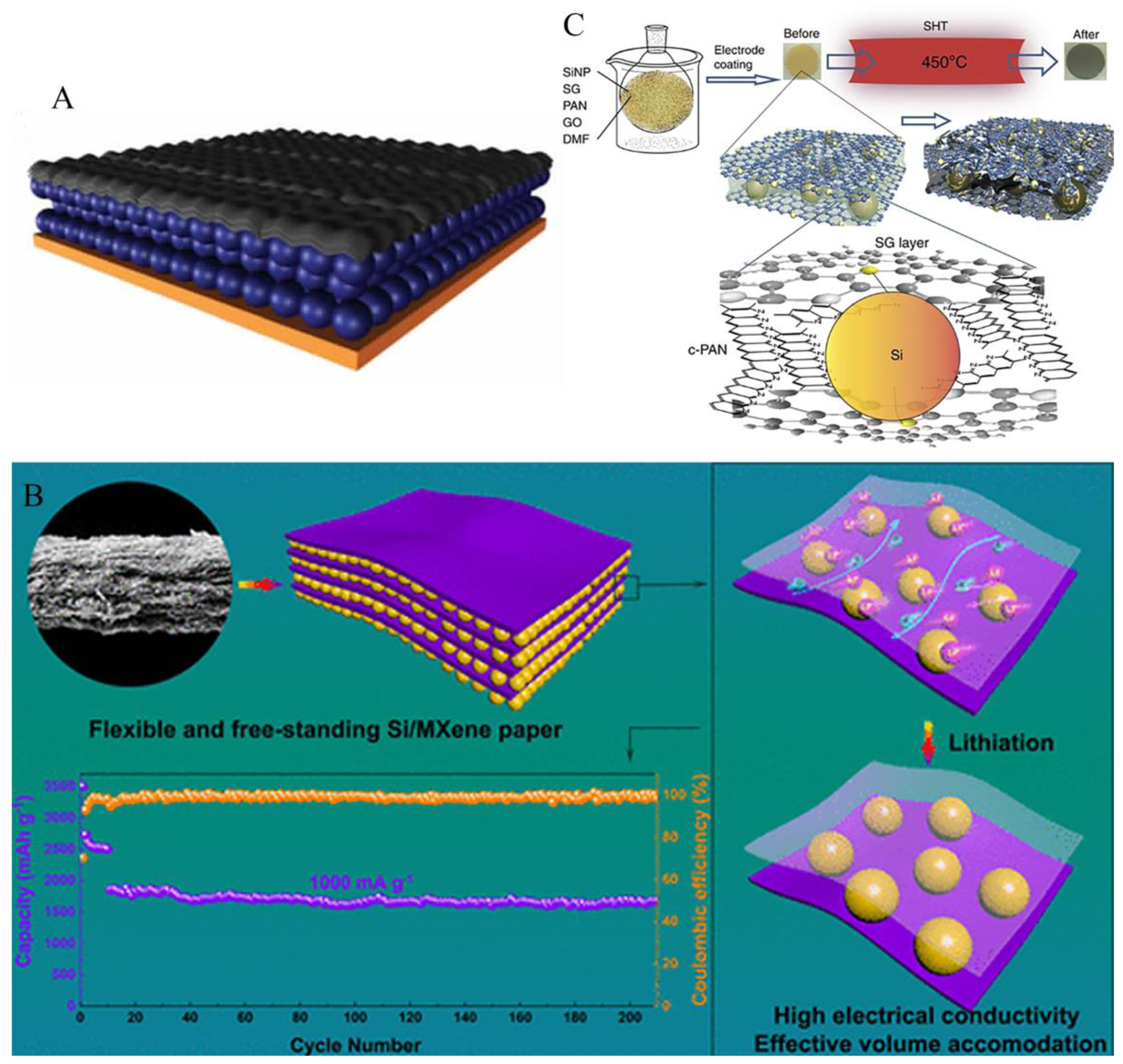
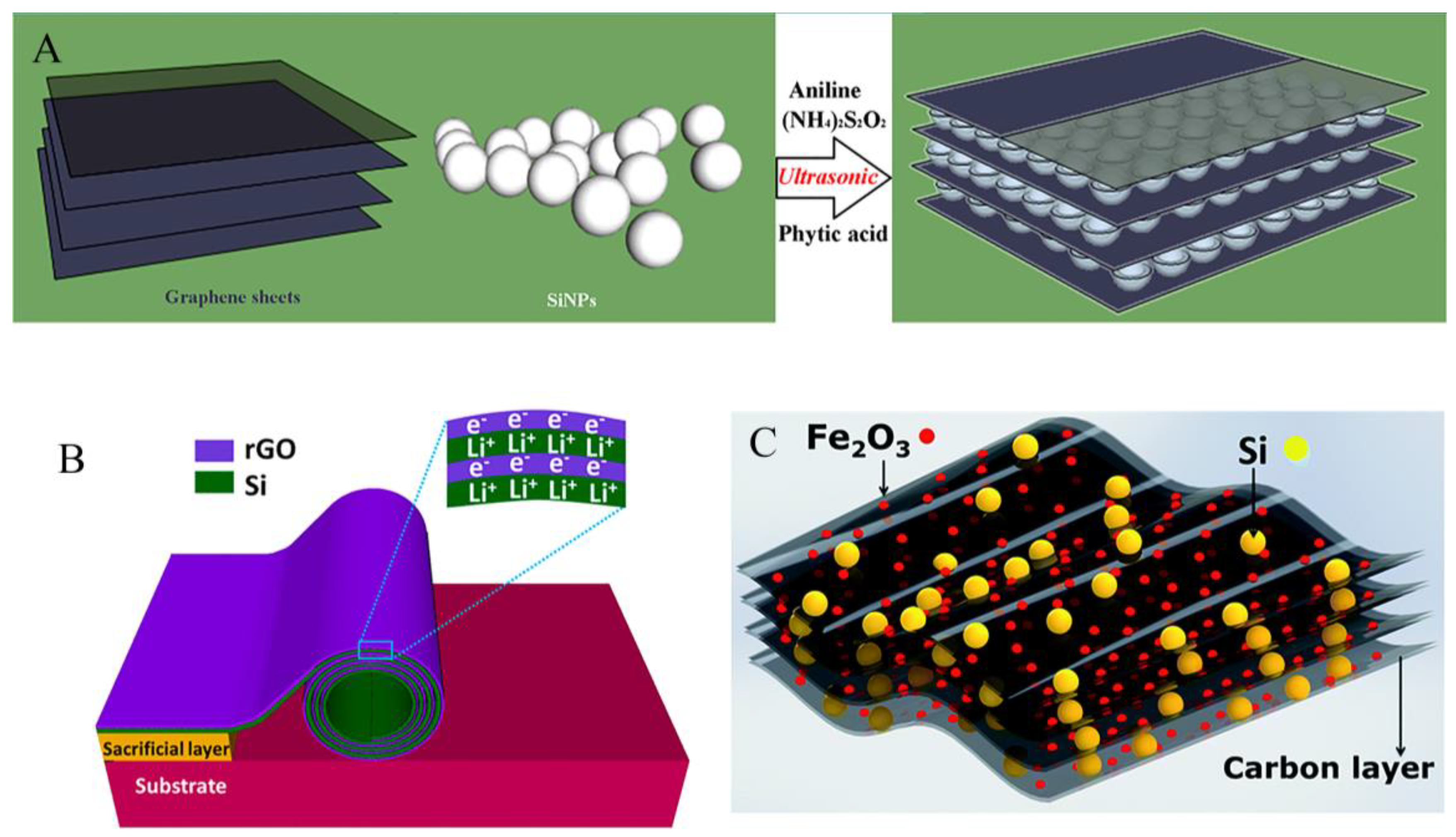
3.3. Free binder or current collector of silicon anode
4. Optimization of surface structure of silicon anode
4.1. Coating structure
4.2. Sandwich structure
5. Artificial SEI
6. Summary and Outlook
Declaration of competing interest:
Acknowledgments
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science. 2011, 334, 928–935. [Google Scholar]
- Brandt, K. Historical development of secondary lithium batteries. Solid State Ionics. 1994, 69, 173–183. [Google Scholar]
- Jeong, G.; Kim, Y.-U.; Kim, H.; Kim, Y.-J.; Sohn, H.-J. Prospective materials and applications for Li secondary batteries. Energy Environ. Sci. 2011, 4, 1986. [Google Scholar]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001, 414, 359–367. [Google Scholar]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.M. Prospects and Limits of energy storage in batteries. The Journal of Physical Chemistry Letters. 2015, 6, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Xu, J.; Yao, Y.; Wegner, G.; Lieberwirth, I.; Chen, C. Improvement of cyclability of Si as anode for Li-ion batteries. J. Power Sources. 2009, 192, 644–651. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources. 2014, 257, 421–443. [Google Scholar]
- Du, F.-H.; Wang, K.-X.; Chen, J.-S. Strategies to succeed in improving the lithium-ion storage properties of silicon nanomaterials. J. Mater. Chem. A. 2016, 4, 32–50. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small. 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nature Energy. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering empty space between Si nanoparticles for lithium-ion battery anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef]
- Demirkan, M.T.; Yurukcu, M.; Dursun, B.; Demir-Cakan, R.; Karabacak, T. Evaluation of double-layer density modulated Si thin films as Li-ion battery anodes. Materials Research Express. 2017, 4. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.H.; Woo, S.P.; Kim, H.S.; Yoon, Y.S. Fabrication of amorphous Si and C anode films via co-sputtering for an all-solid-state battery. Thin Solid Films. 2014, 564, 58–64. [Google Scholar] [CrossRef]
- Chen, Z.H.; Jia, H.; Hoeppener, S.; Friebe, C.; Wang, J.D.; Chanteux, G.; Xie, D.J.; Lu, Y.; Vlad, A.; Schubert, U.S.; Gohy, J.F. Hollow porous silicon nanospheres with 3D SiC@C coating as high-performance anodes. Mater. Design. 2023, 226. [Google Scholar] [CrossRef]
- Park, M.S.; Park, E.; Lee, J.; Jeong, G.; Kim, K.J.; Kim, J.H.; Kim, Y.J.; Kim, H. Hydrogen silsequioxane-derived Si/SiOx nanospheres for high-capacity lithium storage materials. ACS Appl. Mater. Interfaces. 2014, 6, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.P.; Hawari, N.H.; Prakoso, B. Vertically aligned n-type silicon nanowire array as a free-standing anode for lithium-ion batteries. Nanomaterials 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Peng, L.L.; Ding, Y.; Yu, G.H. Amorphous silicon honeycombs as a binder/carbon-free, thin-film Li-ion battery anode. Chem. Commun. 2014, 50, 12959–12962. [Google Scholar] [CrossRef]
- Bang, B.M.; Kim, H.; Lee, J.P.; Cho, J.; Park, S. Mass production of uniform-sized nanoporous silicon nanowire anodes via block copolymer lithography. Energy Environ. Sci. 2011, 4, 3395–3399. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tamirat, A.G.; Lui, Y.; Dong, X.; Wang, C.; Wang, Y.; Xia, Y. Ultrathin silicon nanolayer implanted NixSi/Ni nanoparticles as superlong-cycle lithium-ion anode material. Small Structures. 2021, 2, 2000126. [Google Scholar] [CrossRef]
- Sakabe, J.; Ohta, N.; Ohnishi, T.; Mitsuishi, K.; Takada, K. Porous amorphous silicon film anodes for high-capacity and stable all-solid-state lithium batteries. Communications Chemistry. 2018, 1, 24. [Google Scholar] [CrossRef]
- Wang, M.X.; Geng, Z.R. Facile synthesis of multilayer-like Si thin film as high-performance anode materials for lithium-ion batteries. Appl. Phys. A-mater. 2016, 122. [Google Scholar] [CrossRef]
- Chen, L.B.; Xie, J.Y.; Yu, H.C.; Wang, T.H. An amorphous Si thin film anode with high capacity and long cycling life for lithium ion batteries. J. Appl. Electrochem. 2009, 39, 1157–1162. [Google Scholar] [CrossRef]
- Sung, J.; Kim, N.; Ma, J.; Lee, J.H.; Joo, S.H.; Lee, T.; Chae, S.; Yoon, M.; Lee, Y.; Hwang, J.; Kwak, S.K.; Cho, J. Subnano-sized silicon anode via crystal growth inhibition mechanism and its application in a prototype battery pack. Nature Energy. 2021, 6, 1164–1175. [Google Scholar] [CrossRef]
- Karuppiah, S.; Keller, C.; Kumar, P.; Jouneau, P.-H.; Aldakov, D.; Ducros, J.-B.; Lapertot, G.; Chenevier, P.; Haon, C. A Scalable silicon nanowires-grown-on-graphite composite for high-energy lithium batteries. ACS Nano. 2020, 14, 12006–12015. [Google Scholar] [CrossRef]
- Wen, Z.H.; Lu, G.H.; Mao, S.; Kim, H.; Cui, S.M.; Yu, K.H.; Huang, X.K.; Hurley, P.T.; Mao, O.; Chen, J.H. Silicon nanotube anode for lithium-ion batteries. Electrochem. Commun. 2013, 29, 67–70. [Google Scholar] [CrossRef]
- Park, M.-H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.R.; Liu, Y.Z.; Zhang, K.; Emani, S.; Sawicki, M.S.; Shamie, J.S.; Shaw, L.L. Hollow silicon nanospheres encapsulated with a thin carbon shell: an electrochemical study. Electrochim. Acta. 2016, 215, 126–141. [Google Scholar] [CrossRef]
- Liang, J.W.; Li, X.N.; Zhu, Y.C.; Guo, C.; Qian, Y.T. Hydrothermal synthesis of nano-silicon from a silica sol and its use in lithium ion batteries. Nano Research. 2015, 8, 1497–1504. [Google Scholar] [CrossRef]
- Pu, J.; Qin, J.; Wang, Y.; Qiao, Z.; Yu, X.; Xu, J.; Zhang, X. Ruan, D. Synthesis of micro-nano sphere structure silicon–carbon composite as anode material for lithium-ion batteries. Chemical Physics Letters. 2022, 806, 140006. [Google Scholar]
- Tesfaye, A.T.; Gonzalez, R.; Coffer, J.L.; Djenizian, T. Porous silicon nanotube arrays as anode material for Li-Ion batteries. ACS Appl. Mater. Interfaces. 2015, 7, 20495–20498. [Google Scholar] [CrossRef] [PubMed]
- Saager, S.; Scheffel, B.; Zywitzki, O.; Modes, T.; Piwko, M.; Doerfler, S.; Althues, H.; Metzner, C. Porous silicon thin films as anodes for lithium ion batteries deposited by co-evaporation of silicon and zinc. Surface and Coatings Technology. 2019, 358, 586–593. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ahn, J.; Yu, S.H.; Chung, D.Y.; Lee, K.J.; Lee, J.K.; Sung, Y.E. Titanium silicide coated porous silicon nanospheres as anode materials for lithium ion batteries. Electrochim. Acta. 2015, 151, 256–262. [Google Scholar] [CrossRef]
- Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.-G.; Liu, J. Mesoporous silicon sponge as an anti-pulverization structure for high-performance lithium-ion battery anodes. Nat. Commun. 2014, 5, 4105. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, J.; Zhang, Y.; Yang, D.; Du, N. Scalable and low-cost synthesis of porous silicon nanoparticles as high-performance lithium-ion battery anode. Materials Today Nano. 2022, 18. [Google Scholar] [CrossRef]
- Ge, M.Y.; Rong, J.P.; Fang, X.; Zhou, C.W. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef]
- Xiao, Q.; Gu, M.; Yang, H.; Li, B.; Zhang, C.; Liu, Y.; Liu, F.; Dai, F.; Yang, L.; Liu, Z.; Xiao, X.; Liu, G.; Zhao, P.; Zhang, S.; Wang, C.; Lu, Y.; Cai, M. Inward lithium-ion breathing of hierarchically porous silicon anodes. Nat. Commun. 2015, 6, 8844. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Gao, B.; Mei, S.; Xiang, B.; Fu, J.; Wang, L.; Zhang, Q.; Chu, P.K.; Huo, K. Scalable synthesis of ant-nest-like bulk porous silicon for high-performance lithium-ion battery anodes. Nat. Commun. 2019, 10, 1447. [Google Scholar] [CrossRef]
- Dong, H.; Fu, X.L.; Wang, J.; Wang, P.; Ding, H.; Song, R.; Wang, S.M.; Li, R.R.; Li, S.Y. In-situ construction of porous Si@C composites with LiCl template to provide silicon anode expansion buffer. Carbon. 2021, 173, 687–695. [Google Scholar] [CrossRef]
- Hu, Y.S.; Adelhelm, P.; Smarsly, B.M.; Maier, J. Highly stable lithium storage performance in a porous carbon/silicon nanocomposite. Chemsuschem. 2010, 3, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; Wang, C.; Rosso, K.M.; Yi, R.; Patel, R.; Zhang, J.-G. Hierarchical porous silicon structures with extraordinary mechanical strength as high-performance lithium-ion battery anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef]
- Guo, J.C.; Chen, X.L.; Wang, C.S. Carbon scaffold structured silicon anodes for lithium-ion batteries. J. Mater. Chem. 2010, 20, 5035–5040. [Google Scholar] [CrossRef]
- Dong, Z.; Du, W.; Gu, H.; Long, Y.; Zhang, C.; Chen, G.; Feng, Z.; Sun, W.; Jiang, Y.; Liu, Y.; Yang, Y.; Gan, J.; Gao, M.; Pan, H. A unique structural highly compacted binder-free silicon-based anode with high electronic conductivity for high-performance lithium-ion batteries. Small Structures. 2022, 3, 2100174. [Google Scholar] [CrossRef]
- Favors, Z.; Bay, H.H.; Mutlu, Z.; Ahmed, K.; Ionescu, R.; Ye, R.; Ozkan, M.; Ozkan, C.S. Towards scalable binderless electrodes: carbon coated silicon nanofiber paper via Mg reduction of electrospun SiO2 nanofibers. Sci. Rep. 2015, 5, 8246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.Y.; Wang, Z.H.; Hu, X.J.; Liu, X.J.; Wang, H. Silicon in hollow carbon nanospheres assembled microspheres cross-linked with N-doped carbon fibers toward a binder free, high performance, and flexible anode for lithium-ion batteries. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, P.J.; Chen, C.; Ma, Y.L.; Cheng, X.Q.; Du, C.Y.; Gao, Y.Z.; Yin, G.P. Facile synthesis of binder-free reduced graphene oxide/silicon anode for high-performance lithium ion batteries. J. Power Sources. 2016, 312, 216–222. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Chen, H.; Wang, Z.; Chen, D.; Cheng, Y.B.; Zhou, C.; Shen, G. Hierarchical silicon nanowires-carbon textiles matrix as a binder-free anode for high-performance advanced lithium-ion batteries. Sci. Rep. 2013, 3, 1622. [Google Scholar] [CrossRef]
- Wang, W.; Epur, R.; Kumta, P.N. Vertically aligned silicon/carbon nanotube (VASCNT) arrays: Hierarchical anodes for lithium-ion battery. Electrochem. Commun. 2011, 13, 429–432. [Google Scholar] [CrossRef]
- Haro, M.; Kumar, P.; Zhao, J.; Koutsogiannis, P.; Porkovich, A.J.; Ziadi, Z.; Bouloumis, T.; Singh, V.; Juarez-Perez, E.J.; Toulkeridou, E.; Nordlund, K.; Djurabekova, F.; Sowwan, M.; Grammatikopoulos, P. Nano-vault architecture mitigates stress in silicon-based anodes for lithium-ion batteries. Communications Materials. 2021, 2, 16. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.L.; Zhang, X.F.; Luo, B.; Jin, M.H.; Liang, M.H.; Dayeh, S.A.; Picraux, S.T.; Zhi, L.J. Adaptable silicon-carbon nanocables sandwiched between reduced graphene oxide sheets as lithium ion battery anodes. ACS Nano. 2013, 7, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, G.; Yao, Y.; Xue, L.; Yanilmaz, M.; Lee, H.; Zhang, X. Coaxial electrospun Si/C–C core–shell composite nanofibers as binder-free anodes for lithium-ion batteries. Solid State Ionics. 2014, 258, 67–73. [Google Scholar] [CrossRef]
- Shao, F.; Li, H.; Yao, L.; Xu, S.W.; Li, G.; Li, B.; Zou, C.; Yang, Z.; Su, Y.J.; Hu, N.T.; Zhang, Y.F. Binder-free, flexible, and self-standing non-woven fabric anodes based on graphene/Si hybrid fibers for high-performance Li-ion batteries. ACS Appl. Mater. Interfaces. 2021, 13, 27270–27277. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; He, P.; Yan, C.; Gao, Y.; Gao, J.; Lu, H.; Yang, Z. Facile fabrication of hollow structured Si-Ni-C nanofabric anode for Li-ion battery. Materials Letters. 2018, 231, 205–208. [Google Scholar] [CrossRef]
- Osaka, T.; Nara, H.; Momma, T.; Yokoshima, T. New Si-O-C composite film anode materials for LIB by electrodeposition. J. Mater. Chem. A. 2014, 2, 883–896. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Liu, B.; Zhao, J. Binder-free Si nanoparticle electrode with 3D porous structure prepared by electrophoretic deposition for lithium-ion batteries. ACS Appl. Mater. Interfaces. 2015, 7, 7497–7504. [Google Scholar] [CrossRef]
- Song, S.G.; Li, J.C.; Zheng, A.Q.; Yang, Y.Q.; Yin, K.B. Facile synthesis of sponge-like porous nano carbon-coated silicon anode with tunable pore structure for high-stability lithium-ion batteries. Molecules. 2021, 26. [Google Scholar] [CrossRef]
- Tan, W.; Yang, F.; Lu, Z.G.; Xu, Z.H. A design strategy of carbon coatings on silicon nanoparticles as anodes of high-performance lithium-ion batteries. ACS Applied Energy Materials. 2022, 5, 12143–12150. [Google Scholar] [CrossRef]
- Guan, P.; Li, J.; Lu, T.; Guan, T.; Ma, Z.; Peng, Z.; Zhu, X.; Zhang, L. Facile and scalable approach to fabricate granadilla-like porous-structured silicon-based anode for lithium ion batteries. ACS Appl. Mater. Interfaces. 2018, 10, 34283–34290. [Google Scholar] [CrossRef]
- Ma, X.; Hou, G.; Ai, Q.; Zhang, L.; Si, P.; Feng, J.; Ci, L. A heart-coronary arteries structure of carbon nanofibers/graphene/silicon composite anode for high performance lithium ion batteries. Sci. Rep. 2017, 7, 9642. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Chen, D.Q.; Li, K.; Wang, J.; Zhao, J.B. Electrostatic self-assembly bmSi@C/rGO composite as anode material for lithium ion battery. Electrochim. Acta. 2016, 202, 140–146. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Song, Y.; Li, P.; Liu, J. Binary carbon modification promoting the electrochemical performance of silicon anode for lithium-ion batteries. ChemistrySelect. 2023, 8, e202204086. [Google Scholar] [CrossRef]
- Chen, H.X.; Xiao, Y.; Wang, L.; Yang, Y. Silicon nanowires coated with copper layer as anode materials for lithium-ion batteries. J. Power Sources. 2011, 196, 6657–6662. [Google Scholar] [CrossRef]
- Baek, S.H.; Park, J.S.; Jeong, Y.M.; Kim, J.H. Facile synthesis of Ag-coated silicon nanowires as anode materials for high-performance rechargeable lithium battery. J. Alloy. Compd. 2016, 660, 387–391. [Google Scholar] [CrossRef]
- Chan, C.K.; Patel, R.N.; O’Connell, M.J.; Korgel, B.A.; Cui, Y. Solution-grown silicon nanowires for lithium-ion battery anodes. ACS Nano. 2010, 4, 1443–1450. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chang, C.-C.; Duh, J.-G. Silicon nitride coated silicon thin film on three dimensions current collector for lithium ion battery anode. J. Power Sources. 2016, 325, 64–70. [Google Scholar] [CrossRef]
- He, Y.; Yu, X.Q.; Wang, Y.H.; Li, H.; Huang, X.J. Alumina-coated patterned amorphous silicon as the anode for a lithium-ion battery with high coulombic efficiency. Adv. Mater. 2011, 23, 4938–4941. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, F.S.; Chen, X.H.; Li, S.Y.; Ma, W.H.; Ding, Z.; Qu, T.; Dai, Y.N.; Deng, R. Improved lithium-ion batteries with coral-like anodes made of recycled spherical porous silicon coated with nitrogen-doped carbon. Environmental Chemistry Letters. 2022, 20, 3377–3385. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, Y.; Wang, Y.; Liang, S.; Cao, G.; Pan, A. Necklace-like Si@C nanofibers as robust anode materials for high performance lithium ion batteries. Science Bulletin. 2019, 64, 261–269. [Google Scholar] [CrossRef]
- Song, J.X.; Chen, S.R.; Zhou, M.J.; Xu, T.; Lv, D.P.; Gordin, M.L.; Long, T.J.; Melnyk, M.; Wang, D.H. Micro-sized silicon-carbon composites composed of carbon-coated sub-10 nm Si primary particles as high-performance anode materials for lithium-ion batteries. J. Mater. Chem. A. 2014, 2, 1257–1262. [Google Scholar] [CrossRef]
- Li, B.; Yao, F.; Bae, J.J.; Chang, J.; Zamfir, M.R.; Le, D.T.; Pham, D.T.; Yue, H.; Lee, Y.H. Hollow carbon nanospheres/silicon/alumina core-shell film as an anode for lithium-ion batteries. Sci. Rep. 2015, 5, 7659. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef]
- Xu, Y.H.; Yin, G.P.; Cheng, X.Q.; Zuo, P.J. Enhanced lithium storage performance of silicon anode via fabricating into sandwich electrode. Electrochim. Acta. 2011, 56, 4403–4407. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, X.; Chen, C.; Wu, H. Sandwich electrode designed for high performance lithium-ion battery. Nanoscale. 2016, 8, 9511–9516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Z.R.; Wang, C.; Feng, A.L.; Wang, K.K.; Hou, T.Q.; Liu, J.J.; Zhang, Y.; Wu, G.L. Sandwich-like silicon/Ti3C2Tx MXene composite by electrostatic self-assembly for high performance lithium ion battery. Energy. 2020, 195. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and freestanding silicon/MXene composite papers for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces. 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Sun, Z.X.; Wang, G.J.; Cai, T.W.; Ying, H.J.; Han, W.Q. Sandwich-structured graphite-metallic silicon@C nanocomposites for Li-ion batteries. Electrochim. Acta. 2016, 191, 299–306. [Google Scholar] [CrossRef]
- Hassan, F.M.; Batmaz, R.; Li, J.; Wang, X.; Xiao, X.; Yu, A.; Chen, Z. Evidence of covalent synergy in silicon–sulfur–graphene yielding highly efficient and long-life lithium-ion batteries. Nat. Commun. 2015, 6, 8597. [Google Scholar] [CrossRef]
- Huang, Z.D.; Zhang, K.; Zhang, T.T.; Liu, R.Q.; Lin, X.J.; Li, Y.; Feng, X.M.; Mei, Q.B.; Masese, T.; Ma, Y.W.; Huang, W. Binder-free graphene/carbon nanotube/silicon hybrid grid as freestanding anode for high capacity lithium ion batteries. Compos. Part. A-appl. S. 2016, 84, 386–392. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, D.H.; Zhang, S.Y.; Li, X.L.; Zhi, L.J. A hierarchical layering design for stable, self-restrained and high volumetric binder-free lithium storage. Nanoscale. 2019, 11, 21728–21732. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-a.; Guo, Y.; Chen, Z.; Zhang, X.; Wang, J.; Yang, B. An easy and scalable approach to synthesize three-dimensional sandwich-like Si/Polyaniline/Graphene nanoarchitecture anode for lithium ion batteries. Ceram. Int. 2018, 44, 4282–4286. [Google Scholar] [CrossRef]
- Wei, D.H.; Gao, X.; Zeng, S.Y.; Li, H.B.; Li, H.Y.; Li, W.Z.; Tao, X.Q.; Xu, L.L.; Chen, P. Improving the performance of micro-silicon anodes in lithium-ion batteries with a functional carbon nanotube interlayer. Chemelectrochem. 2018, 5, 3143–3149. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Si, W.; Xi, L.; Eichler, B.; Yan, C.; Schmidt, O.G. Sandwich nanoarchitecture of Si/reduced graphene oxide bilayer nanomembranes for Li-ion batteries with long cycle life. ACS Nano. 2015, 9, 1198–1205. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhang, F.; Cao, Y.Z.; Huang, Q.A. Symmetrical sandwich-structured SiN/Si/SiN composite for lithium-ion battery anode with improved cyclability and rate capacity. J. Electrochem. Soc. 2018, 165, A3397–A3402. [Google Scholar] [CrossRef]
- Jia, D.L.; Li, X.; Huang, J.G. Bio-inspired sandwich-structured carbon/silicon/titanium-oxide nanofibers composite as an anode material for lithium-ion batteries. Compos. Part. A-appl. S. 2017, 101, 273–282. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, H.; Rajagopalan, R.; Hu, X.; Huang, Y.; Dou, S.X.; Liu, H.K. One-step synthesis of a silicon/hematite@carbon hybrid nanosheet/silicon sandwich-like composite as an anode material for Li-ion batteries. J. Mater. Chem. A. 2016, 4, 4056–4061. [Google Scholar] [CrossRef]
- Chen, W.L.; Chen, K.Y.; Zeng, R.; Wan, M.; Guo, Y.X.; Liao, Y.Q.; Peng, J.Y.; Zhang, W.X.; Huang, Y.H. In situ construction of S-based artificial solid electrolyte interphases layer for stable silicon anode in lithium-ion batteries. ACS Applied Energy Materials. 2022, 5, 14136–14143. [Google Scholar] [CrossRef]
- Li, J.; Dudney, N.J.; Nanda, J.; Liang, C. Artificial solid electrolyte interphase to address the electrochemical degradation of silicon electrodes. ACS Appl. Mater. Interfaces. 2014, 6, 10083–10088. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Yuan, Y.; Wang, Z.; Zhang, H.; Li, X. N-rich solid electrolyte interface constructed in situ via a binder strategy for highly stable silicon anode. Adv. Funct. Mater. 2301716. [CrossRef]
- Bolloju, S.; Abdollahifar, M.; Parthasarathi, S.-K.; Chen, Y.-C.; Weng, Y.-T.; Chao, C.-Y.; Wu, N.-L. Efficient utilization of macropores as artificial solid–electrolyte interphase channels for high-capacity silicon/graphite anode materials. ACS Sustainable Chemistry & Engineering. 2023, 11, 2623–2633. [Google Scholar]
- Ronneburg, A.; Silvi, L.; Cooper, J.; Harbauer, K.; Ballauff, M. Solid electrolyte interphase layer formation during lithiation of single-crystal silicon electrodes with a protective aluminum oxide coating. 2021, 13, 21241–21249.
- Ai, Q.; Fang, Q.; Liang, J.; Xu, X.; Zhai, T.; Gao, G.; Guo, H.; Han, G.; Ci, L.; Lou, J. Lithium-conducting covalent-organic-frameworks as artificial solid-electrolyte-interphase on silicon anode for high performance lithium ion batteries. Nano Energy. 2020, 72, 104657. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.Y.; Wang, Y.; Huang, W.B.; Li, Y.C.; Huang, Y.H.; Zheng, H.H. Tailoring a multifunctional, boron and fluoride-enriched solid-electrolyte interphase precursor towards high-rate and stable-cycling silicon anodes. Nano Energy. 2022, 93. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Gong, X.Z.; Feng, Y.K.; Lu, J.J.; Fu, Y.L.; Wang, Z.; Liu, J.H. Constructing an artificial boundary to regulate solid electrolyte interface formation and synergistically enhance stability of nano-Si anodes. J. Colloid Interf. Sci. 2022, 619, 158–167. [Google Scholar] [CrossRef]
- Chen, L.; Lai, J.; Li, Z.; Zou, H.; Yang, J.; Ding, K.; Cai, Y.-P.; Zheng, Q. A jigsaw-structured artificial solid electrolyte interphase for high-voltage lithium metal batteries. Communications Materials. 2023, 4, 18. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Kushima, A.; Zheng, X.; Sun, Y.; Xie, J.; Sun, J.; Xue, W.; Zhou, G.; Wu, J.; Shi, F.; Zhang, R.; Zhu, Z.; So, K.; Cui, Y.; Li, J. Self-healing SEI enables full-cell cycling of a silicon-majority anode with a coulombic efficiency exceeding 99. 9%. Energy Environ. Sci. 2017, 10, 580–592. [Google Scholar] [CrossRef]
- Harpak, N.; Davidi, G.; Patolsky, F. Breathing parylene-based nanothin artificial SEI for highly-stable long life three-dimensional silicon lithium-ion batteries. Chem. Eng. J. 2022, 429. [Google Scholar] [CrossRef]
- Mu, T.; Sun, Y.; Wang, C.; Zhao, Y.; Doyle-Davis, K.; Liang, J.; Sui, X.; Li, R.; Du, C.; Zuo, P.; Yin, G.; Sun, X. Long-life silicon anodes by conformal molecular-deposited polyurea interface for lithium ion batteries. Nano Energy. 2022, 103, 107829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




