Submitted:
04 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
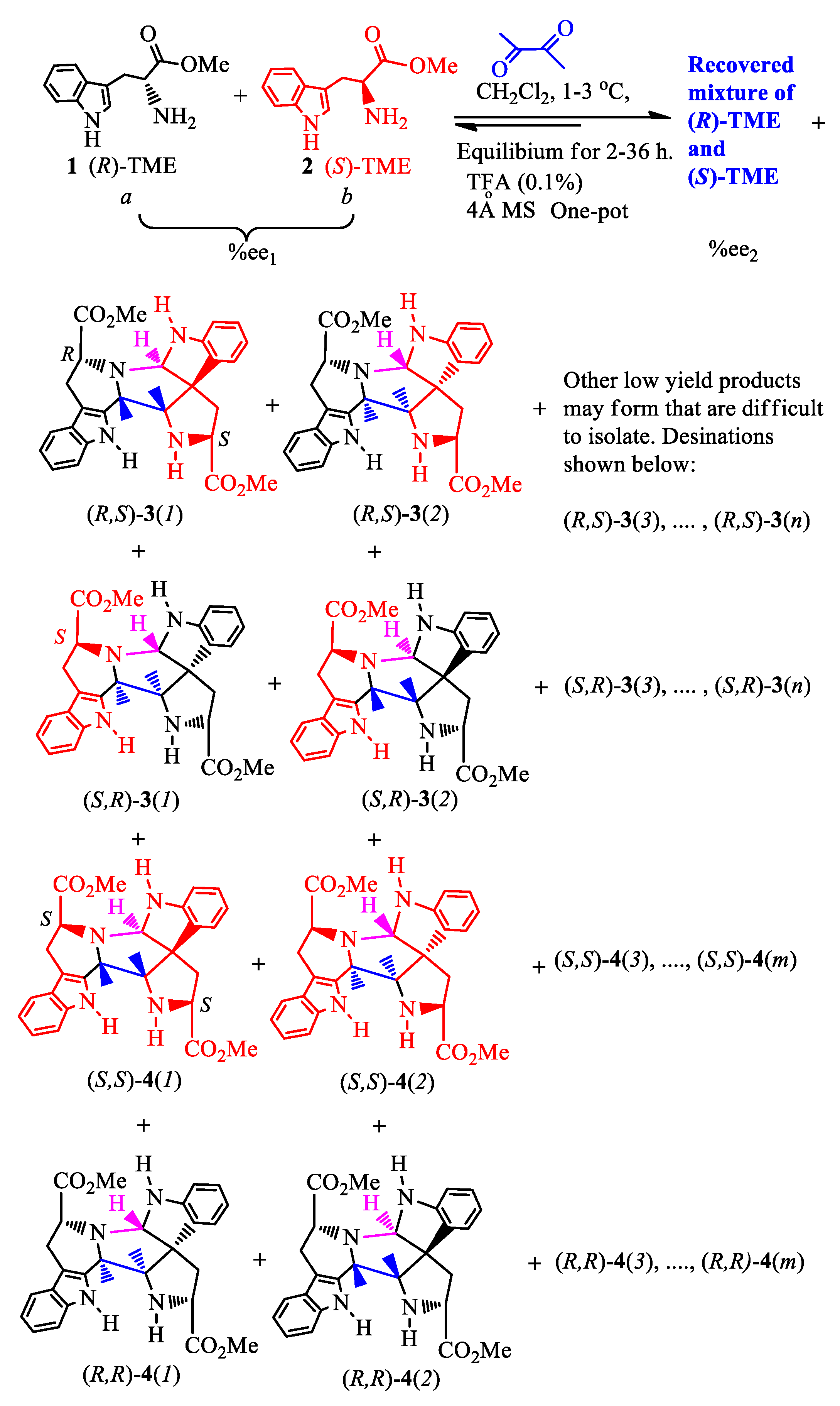
2.1. Theoretical investigation of chiral amplification without any chiral source
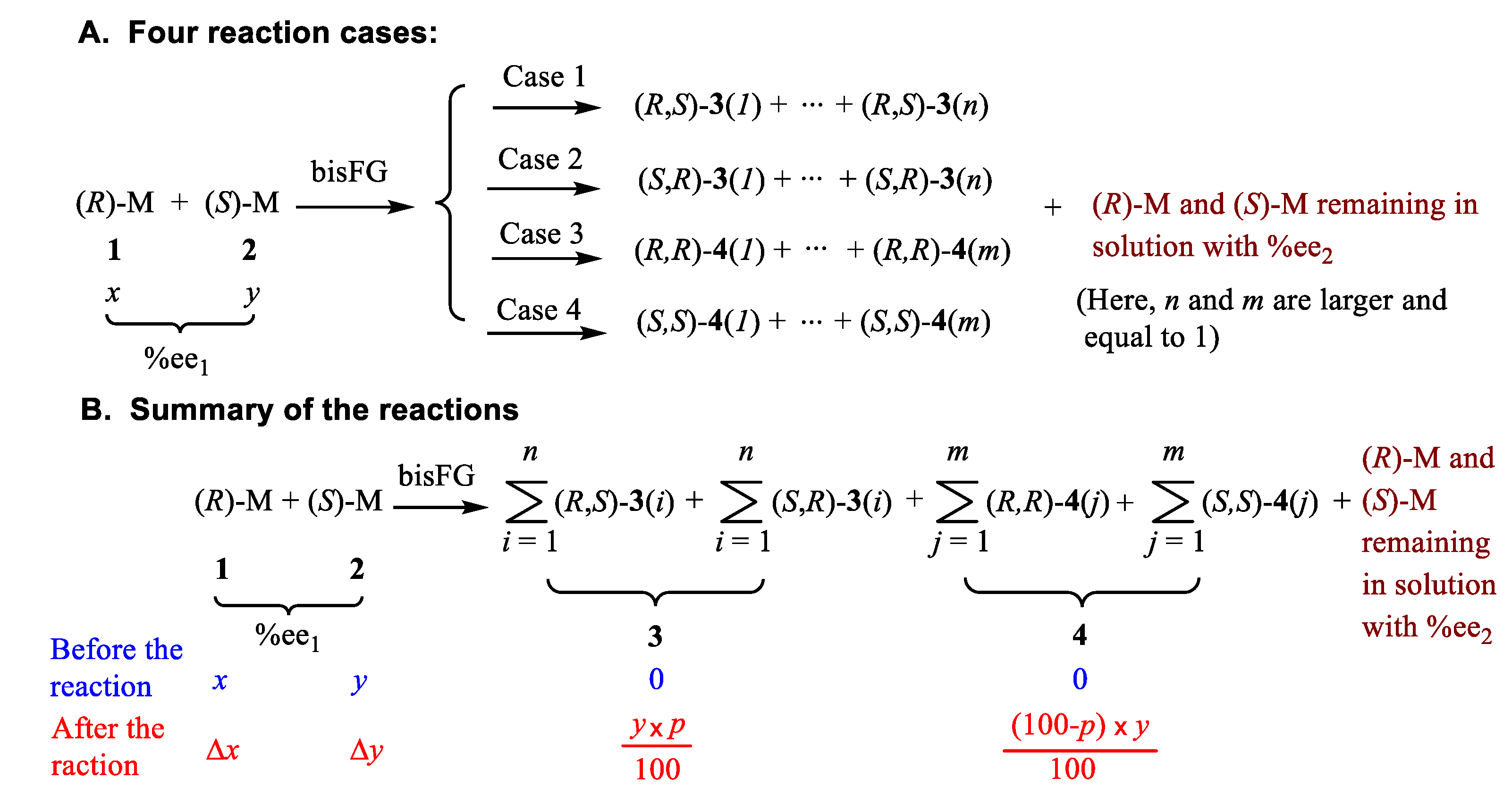
2.2. A simplified expression of the four reaction cases and amplification equations
2.3. Relationship among the x, y and p
2.4. Experimental verification of chiral amplification as predicted by equation 8.
2.4.1. Chiral amplification using TME and 2,3-diketobutane
2.4.2. Amplification of a 0.7%ee of L-TME up to 3%ee
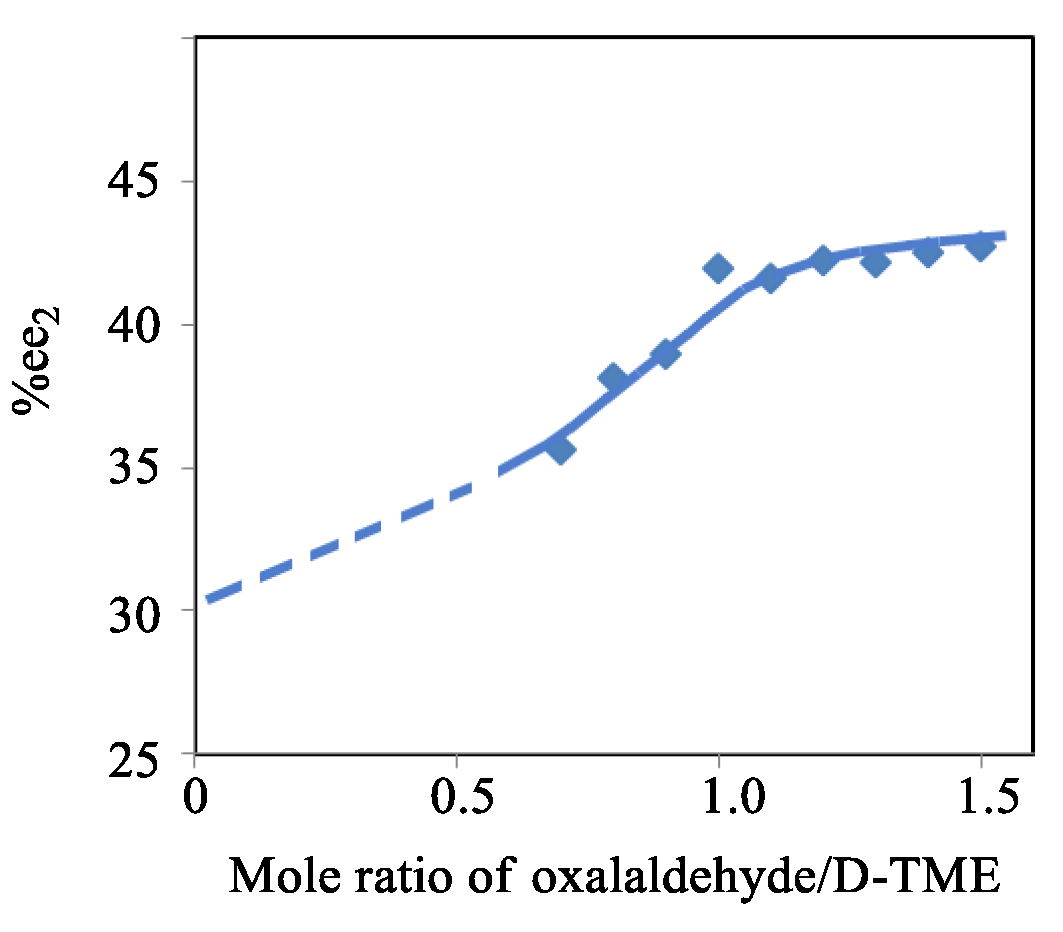
2.4.3. Amplification of high %ee of L-TME using oxalaldehyde
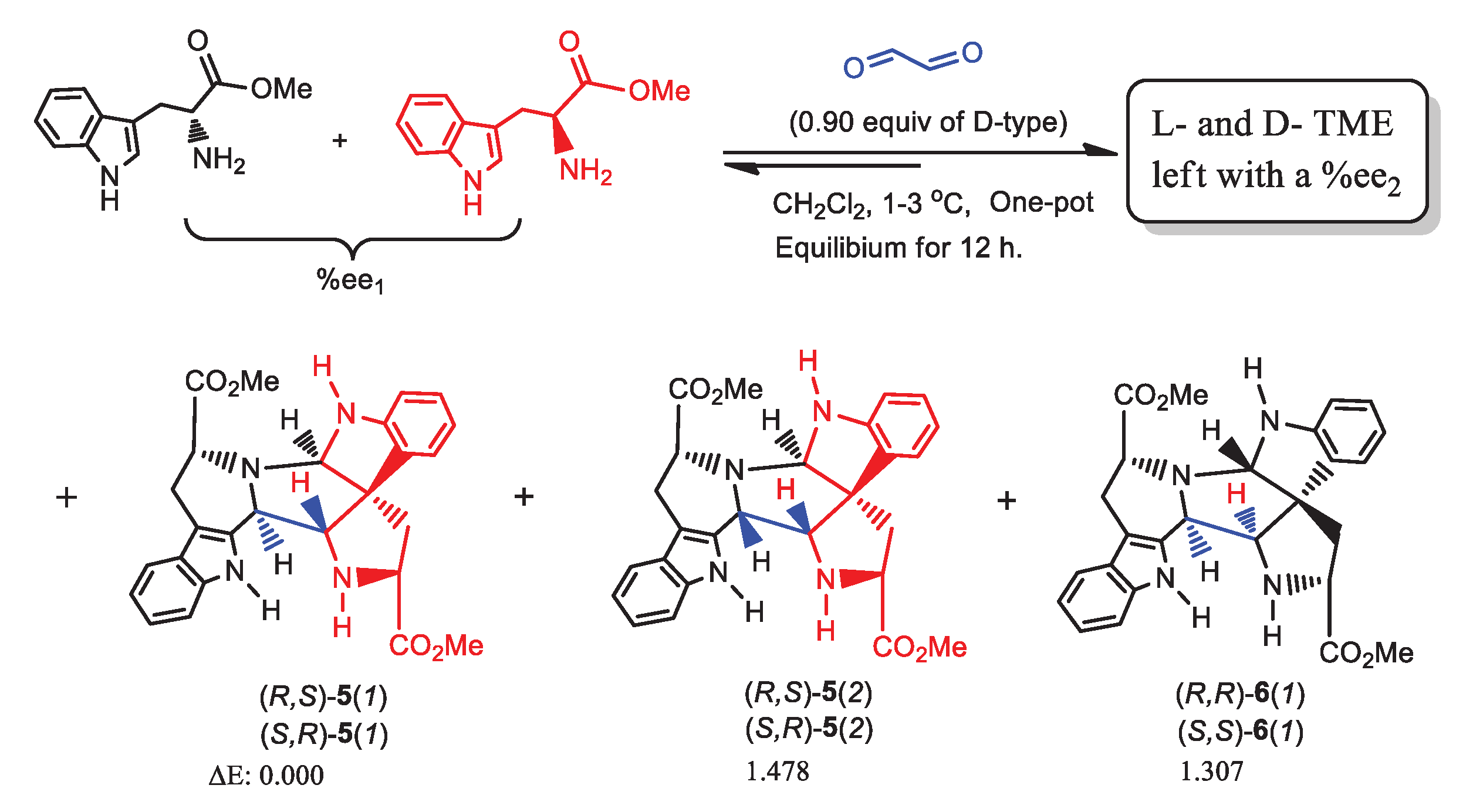
2.4.4. Chiral amplifications of L-amino acids
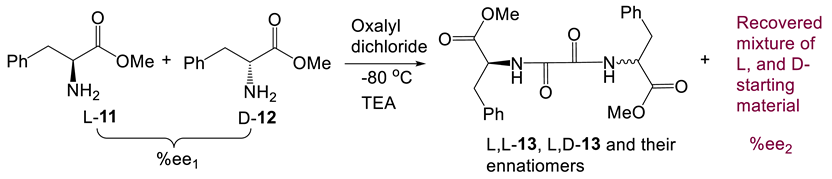
2.4.5. Possible L-amino acid amplification by dimerization to cyclic dipeptides
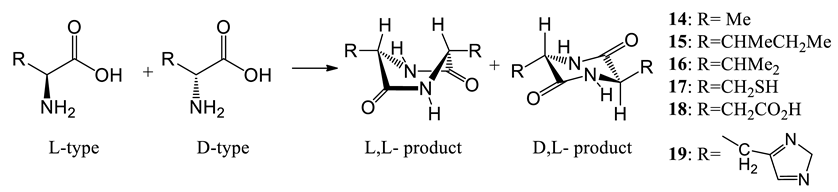
3. Materials and Methods
3.1. Experimental section
3.2. Computational section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- (a). Prebiotic Chemistry: From simple amphiphiles to model protocells; Walde, P. Ed., Topics in Curr. Chem. Vol. 259; Springer-Verlag: Berlin/Heidelberg, 2005. (b) Das, K.; Gabrielli, L.; and Prins. L. J. Chemically fueled self-assembly in biology and chemistry. Angew. Chem. Int. Ed. 2021, 60, 20120– 20143. (c) Gözen, I.; Köksal, E.S.; Põldsalu, I.; Xue, L.; Spustova, K.; Pedrueza-Villalmanzo, E.; Ryskulov, R.; Meng, F.; and Jesorka, A. Protocells: milestones and recent advances. Small, 2022, 2106624. (c). Davankov, V.A. Chance and necessity in the evolution of matter to life: a comprehensive hypothesis. Symmetry 2021, 13, 1918. (d) Kolodiazhnyi, O.I. Phosphorus compounds of natural origin: prebiotic, stereochemistry, application. Symmetry 2021, 13, 889. (e) Chen, Y.; Ma, W. The origin of biological homochirality along with the origin of life. PLoS Comput Biol 2020, 16, e1007592. (f) Jheeta, S.; Chatzitheodoridis, E.; Devine, K.; and Block, J. The way forward for the origin of life: prions and prion-like molecules first hypothesis. Life 2021, 11, 872. (g) Smith, H.H.; Hyde, A.S.; Simkus, D.N.; Libby, E.; Maurer, S.E.; Graham, H.V.; Kempes, C.P.; Lollar, B.S.; Chou, L.; Ellington, A.D.; Fricke, G.M.; Girguis, P.R.; Grefenstette, N.M.; Pozarycki, C.I.; House, C.H. and Johnson, S.S. The grayness of the origin of life. Life, 2021, 11, 498. (h) Seitz, C.; Eisenreich, W.; Huber, C. The abiotic formation of pyrrole under volcanic, hydrothermal conditions—an initial step towards life’s first breath? Life, 2021, 11, 980. (i) Devínsky, F.. Chirality and the origin of life. Symmetry 2021, 13, 2277. (j) Jia, T.Z.; Nishikawa, S.; Fujishima, K. Sequencing the origins of life. BBA Advances 2022, 2, 100049. (k) Baum, D.A. The origin and early evolution of life in chemical composition space. J. Theo. Biology 2018, 456, 295–304. (K) Liu, B.; Charalampos, G.; Pappas, J.; Schaeffer, G.; Jurissek, C.; Pieters, P.F.; Altay, M.; Marić, I.; Stuart, M.C.A. and Otto, S. Spontaneous emergence of self-replicating molecules containing nucleobases and amino acids. J. Am. Chem. Soc. 2020 142, 4184–4192.
- Pizzarello, S.; Groy, T.L. Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochim. Cosmochim. Acta. 2011, 75, 645–656. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature. 1970, 228, 923−926. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science. 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Amino acids of the Murchison meteorite: II. Five carbon acyclic primary β-, γ-, and δ-amino alkanoic acids. Geochim. Cosmochim. Acta. 1985, 49, 2259–2265. [Google Scholar] [CrossRef]
- Pizzarello, S.; Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R. Isotopic analyses of amino acids from the Murchison meteorite. Geochim. Cosmochim. Acta. 1991, 55, 905–910. [Google Scholar] [CrossRef]
- Pizzarello, S.; Weber, A.L. Prebiotic amino acids as asymmetric catalysts. Science. 2004, 303, 1151–1151. [Google Scholar] [CrossRef]
- Levine, M.; Kenesky, C.S.; Mazori, D.; Breslow, R. Enantioselective synthesis and enantiomeric amplification of amino acids under prebiotic conditions. Org. Lett. 2008, 10, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- (a) Breslow, R. Likely possible origin of homochirality in amino acids and sugars on prebiotic earth. Tetrahedron Lett. 2011, 52, 2028–2032. (b). Siegel, J.S. Homochiral imperative of molecular evolution. Chirality 1998, 10, 24–27.
- (a) J. Greenwald, W. Kwiatkowski and R. Riek, Peptide Amyloids in the Origin of Life, J Mol Biol, 2018, 430, 3735–3750. (b) G. Kostyrka. What roles for viruses in origin of life scenarios? Stud. Hist. & Phil. Biolog. Biomed. Sci.. 2016, http://dx.doi.org/10.1016/j.shpsc.2016.02.014. (c) C. Huber, W. Eisenreich, G. Wächtershäuser. Tetrahedron Lett. 51 (2010) 1069–1071. (d) S. Toxvaerd, The role of the peptides at the origin of life. J. Theoretical Biology. 2017, doi: 10.1016/j.jtbi.2017.06.023.
- Flores, J.J.; Bonner, W.A.; Massey, G.A. Asymmetric photolysis of (R,S)-leucine with circularly polarized ultraviolet light. J. Am. Chem. Soc. 1977, 99, 3622–3625. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Menard, F.; Tamura, M. Circular polarization in star-formation regions: implications for biomolecular homochirality. Science, 1998, 281, 672–674. [Google Scholar] [CrossRef]
- Rubenstein, E.; Bonner, W.A.; Noyes, H.P.; Brown, G.S. Supernovae and life. Nature, 1983, 306, 118–121. [Google Scholar] [CrossRef]
- Mauksch, M.; Tsogoeva, S.B.; Wei, S.W.; Martynova, I.M. Demonstration of spontaneous chiral symmetry breaking in asymmetric Mannich and Aldol reactions. Chirality 2007, 19, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C. Chiral symmetry breaking and complete chiral purity by thermodynamic-kinetic feedback near equilibrium: implications for the origin of biochirality. Astrobiology 2007, 7, 312–319. [Google Scholar] [CrossRef]
- Weissbuch, I.; Leiserowitz, L.; Lahav, M. Stochastic “mirror symmetry breaking” via self-assembly, reactivity and amplification of chirality: relevance to abiotic conditions. Top Curr Chem 2005, 259, 123–165. [Google Scholar]
- (a). Kitamura, A.M.; Suga, S.; Kawai, K.; Noyori, R. Catalytic Asymmetric Induction. Highly Enantioselective Addition of Dialkylzincs to Aldehydes. J. Am. Chem. Soc. 1986, 108, 6071–6072. (b). Andrew S. Tupper, Paul G. Higgs. Rolling-circle and strand-displacement mechanisms for non-enzymatic RNA replication at the time of the origin of life J. Theor. Biology, 2021, 527, 110822. (c) Michaelian, K.. Non-Equilibrium Thermodynamic Foundations of the Origin of Life. Foundations, 2022, 2, 308–337. (c) Vanchurin, V.; Wolf, Y.I.; Koonin, E.V.; Katsnelson, M.I. Thermodynamics of evolution and the origin of life. PNAS 2022, 119, e2120042119. (d) Ghosh, B.; Bose R. and Tang, T.Y. D. Curr. Opin. in Coll. & Interface Sci., 2021, 52, 101415. (e) Krishnamurthy, R.; Chemical Origins of Life: Its Engageme with Society. Trends in Chem. https://doi.org/10.1016/j.trechm.2020.02.011. (f) Slijepcevic, P.. Serial Endosymbiosis Theory: From biology to astronomy and back to the origin of life BioSystems 2021, 202, 104353. (g) Maruyama, S.; Kurokawa, K.; Ebisuzaki, T.; Sawaki, Y.; Suda, K.; Santosh, M. Nine requirements for the origin of Earth’s life: Not at the hydrothermal vent, but in a nuclear geyser system. Geosci. Front. 2019, 10, 1337–1357.
- (a) Puchot, B.C.; Samuel, O.; Duiiach, E.; Zhao, S.; Agami, C.; Kagan, H.B. Nonlinear effects in asymmetric synthesis, Examples in asymmetric oxidations and aldolization reactions. J. Am. Chem. Soc. 1986, 108, 2353–2357. (b) V. N. Obridko, M.V. Ragulskaya, E.G. Khramova. Young Sun, galactic processes, and origin of life. J. Atmosph. Solar-Terr. Phys. 2020, 208, 105395.
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature. 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Blackmond, D.G. Spoilt for choice: assessing phase behavior models for the evolution of homochirality. Chem. Eur. J. 2007, 13, 3290–3295. [Google Scholar] [CrossRef]
- Blackmond, D.G.; Matar, O.K. Re-examination of reversibility in reaction models for the spontaneous emergence of homochirality. J. Phys. Chem. B. 2008, 112, 5098–5104. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 5487–5492. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C. and de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285–366. [CrossRef]
- Mundy, B.P.; Ellerd, M.G.; Favaloro, F.G. Name reactions and reagents in organic synthesis. John Wiley & Sons. 2005.
- Bailey, P.D.; Collier, L.D.; Hollinshead, S.P.; Moore, M.H.; Morgan, K.M.; Smith, D.I.; Vernon, J.M. A new asymmetric route to bridged indole alkaloids: formal syntheses of (–)-suaveoline,(–)-raumacline and (–)-Nb-methylraumacline. J. Chem. Soc., Chem. Commun. 1994, 25, 1559–1560. [Google Scholar] [CrossRef]
- Bai, B.; Li, D.-S.; Huang, S.-Z.; Ren, J.; Zhu, H.-J. Chirality Paring Recognition, A Unique Reaction Forming Spiral Alkaloids from Amino Acids Stereoselectively in One-Pot. Nat. Prod. Bioprospect. 2012, 2, 53–58. [Google Scholar] [CrossRef]
- Oxalaldehyde was used in the Pictet-Spengler reactions with tryptophan methyl ester first and similar structures were obtained. However, commercially available oxalaldehyde contains about 0.5% formaldehyde, which could react with tryptophan methyl ester to afford tetrahydro-β-carboline analogue. Its polarity is extremely close to the tryptophan methyl ester and is hard to isolate it from tryptophan methyl ester from the tetrahydro-β-carboline analogue. Therefore, the recovered L-tryptophane methyl ester must contain the tetrahydro-β-carboline analogue with unknown quantity. It brings the difficulty too to determine the quantity of the recovered L-tryptophan methyl ester that must be used in the second Pictet-Spengler cycle reactions. Thus, it is not suitable to use the L-tryptophan methyl ester with small %ee value as a starting material to examine %ee amplification in the reaction. Finally, biacetyl was used for the reaction to see how much the %ee1 value increased from small values to relative big values.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, Jr. J. A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratemann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuk, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03 User’s Reference Gaussian Inc: Carnegie, PA, USA, 2003.
- Berova, N.; Polavarapu, P.L.; Nakanish, K.; Woody, R.W. Comprehensive chiroptical spectroscopy, John Wiley & Sons, Hoboken, New Jersey, 2011.
- Zhu, H.J. Organic Stereochemistry---Experimental and computational methods, Wiley-VCH, Verlag GmbH & Co. KGaA, 2015.
- Halgren, T.A. MMFF VI. MMFF94s Option for Energy Minimization Studies. J. Comput. Chem. 1999, 20, 720–729. [Google Scholar] [CrossRef]
- Jørgensen, P.; Jensen, H.J.A. and Olsen, J. Linear response calculations for large scale multiconfiguration self-consistent field wave functions. J. Chem. Phys. 1988, 89, 3654–3661. [CrossRef]
- Autschbach, J.; Ziegler, T.; van Gisbergen, S.J.A.; Baerends, E.J. Chiroptical properties from time-dependent density functional theory. I. Circular dichroism spectra of organic molecules. J. Chem. Phys. 2002, 116, 6930–6940. [Google Scholar] [CrossRef]
- Bak, K.L.; Hansen, A.E.; Ruud, K.; Helgaker, T.; Olsen, J.; Jøgensen, P. Ab initio calculation of electronic circular dichroism for trans-cyclooctene using London atomic orbitals. Theor. Chem. Acc. 1995, 90, 441–58. [Google Scholar]
- Kondru, P.K.; Wipf, P.; Beratan, D.N. Theory-Assisted Determination of Absolute Stereochemistry for Complex Natural Products via Computation of Molar Rotation Angles. J. Am. Chem. Soc. 1998, 120, 2204–2205. [Google Scholar] [CrossRef]
- Generally, to determine the ratio of 3 and 4 in the reaction, the energy difference of various conformations of intermediates should be computed before 3 and 4 formations. However, there are many conformations need to be computed for each intermediate, and intermediates have several different configuration. Therefore, a simple but not very accurate method is used to compute the energy difference between the products 3 and 4. All possible configurations of all 64 were investigated. Conformational searches were performed first using FFMM94S force field. Then, three different quantum methods were used for all for the computations on all the geometries to ensure the geometries with the lowest and second lowest energies were correctly identified. For details see ESI.
- Zhao, D.; Zhao, Q.Q.; Zhu, H.J.; Liu, L. Theoretical investigation of the relationship between four-carbon D-sugars and five L-amino acids. Tetrahedron 2016, 72, 5558–5562. [Google Scholar] [CrossRef]
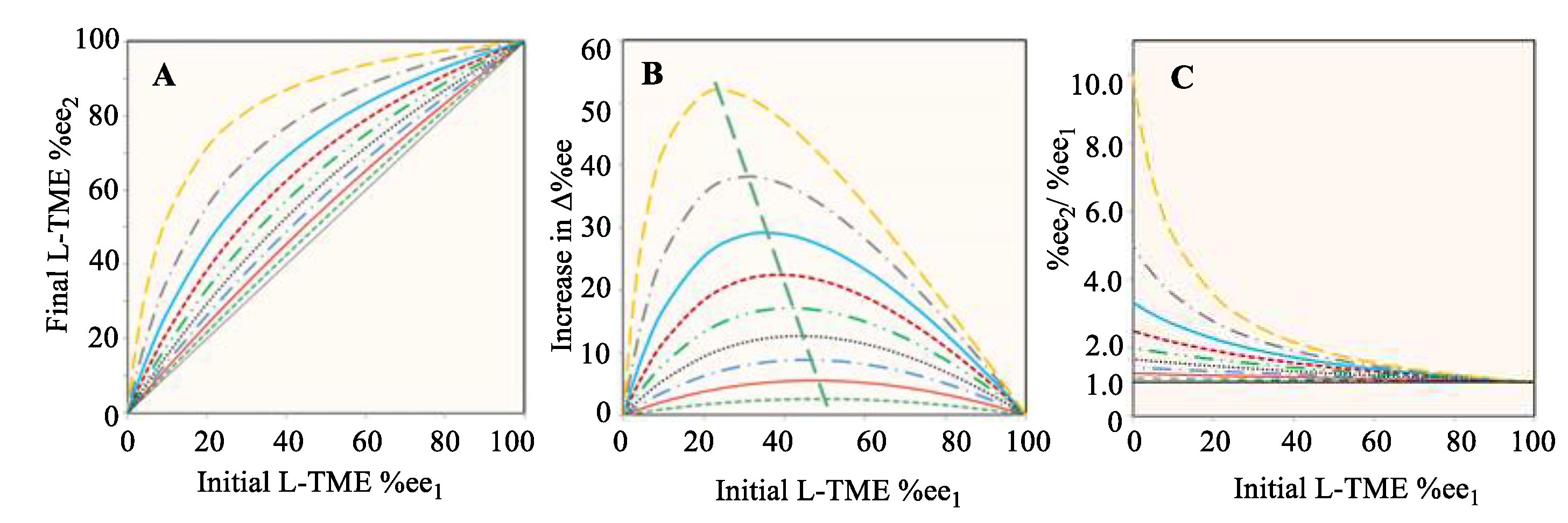
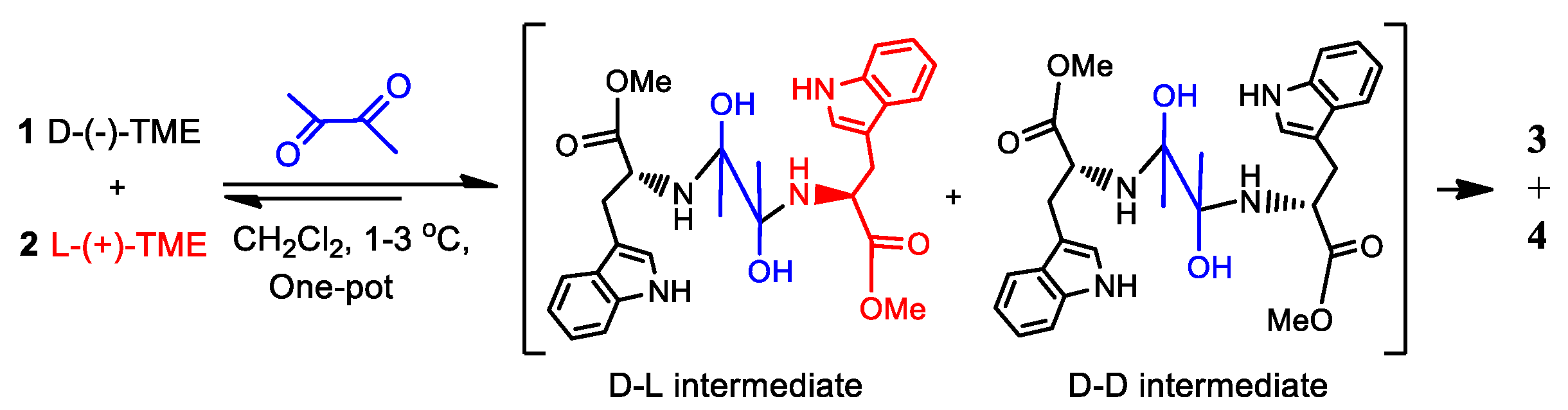
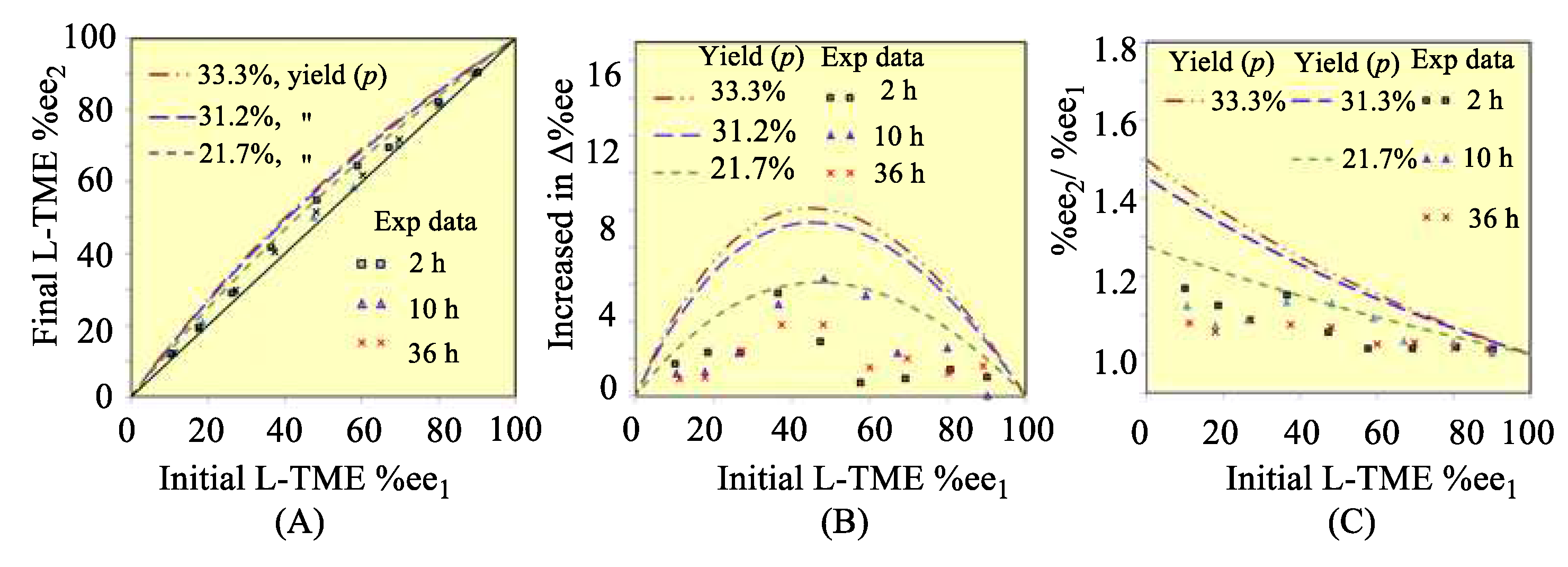
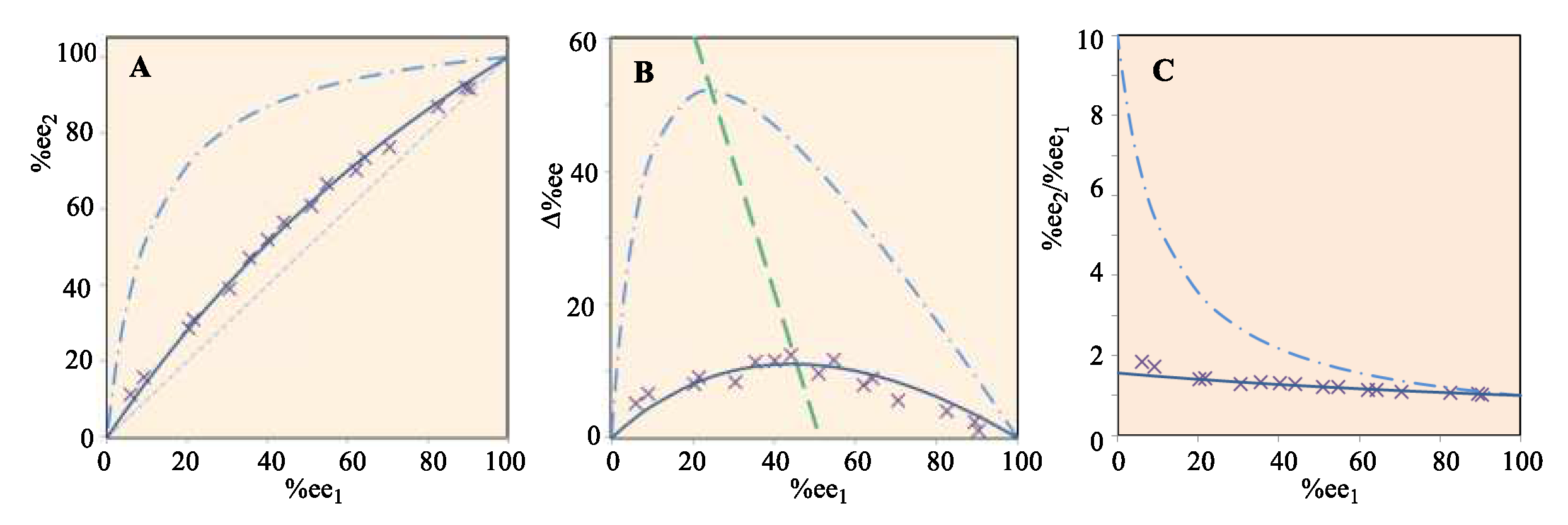
| Entry | %ee1 of L-TME initially used | %ee2 of L- TME recovered | Δ%ee (%ee2-%ee1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st series | 2nd series |
3rd series |
1st series (yield) b |
2nd series |
3rd series |
1st series |
2nd series |
3rd series |
|
| 1 | 10.3 | 10.6 | 11.3 | 11.8(62%) | 11.8(61%) | 12.2(55%) | 1.5 | 1.2 | 0.9 |
| 2 | 18.7 | 17.9 | 17.9 | 20.9(60%) | 19.0 (56%) | 18.8 (53%) | 2.2 | 1.1 | 0.9 |
| 3 | 27.1 | 26.5 | 27.4 | 29.4(58%) | 28.8 (55%) | 29.7 (55%) | 2.3 | 2.3 | 2.3 |
| 4 | 36.6 | 36.7 | 37.6 | 42.2(59%) | 41.5 (55%) | 40.7 (51%) | 5.6 | 4.8 | 3.1 |
| 5 | 47.7 | 48.4 | 47.9 | 50.2(56%) | 54.2 (56%) | 51.4 (52%) | 2.5 | 5.8 | 3.5 |
| 6 | 57.8 | 59.0 | 60.2 | 58.4(60%) | 64.4 (62%) | 61.7 (53%) | 0.6 | 5.4 | 1.5 |
| 7 | 69.4 | 67.2 | 69.8 | 70.3(64%) | 69.5 (61%) | 71.8 (53%) | 0.9 | 2.3 | 2.0 |
| 8 | 80.5 | 79.9 | 80.1 | 82.1(62%) | 82.5 (63%) | 81.2(56%) | 1.6 | 2.6 | 1.1 |
| 9 | 90.4 | 90.18 | 89.1 | 91.2(60%) | 90.22(56%) | 90.6 (52%) | 0.8 | 0.04 | 1.5 |
| Entry | Cpd | ΔE a | ΔE b | p D,L-prod (%) | %ee2 f | Δ%ee | %ee2/%ee1 |
|---|---|---|---|---|---|---|---|
| 1 | 14 | -0.194 | -0.101 | 58.1 d / 54.2 e | 1.19 | 0.69 | 2.37 |
| 2 | 15 | -0.485 | -0.469 | 69.4 / 68.8 | 1.64 | 1.12 | 3.23 |
| 3 | 16 | -0.438 | -0.458 | 67.3 / 68.4 | 1.53 | 1.01 | 3.02 |
| 4 | 17 | +0.018 | -0.028 | 49.2 / 51.2 | 0.98 | 0.48 | 1.96 |
| 5 | 18 | -0.185 -0.547 c |
+0.198 -0.650 c |
57.7 / 41.7 60.3 / 75.0 |
1.18 1.26 |
0.68 0.75 |
2.34 2.50 |
| 6 | 19 | +0.626 -1.026 c |
+0.796 -1.569 c |
25.8 / 20.7 85.0 / 93.4 |
0.68 3.26 |
0.18 2.76 |
1.34 6.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




