Submitted:
05 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
Highlights
- Reports on diversity of jackfruits cultivation and uses in food/ diet nutrients.
- Discussed jackfruit wastes generation and also valorization methods for utility.
- Valorization techniques reported for value‐added products with zero‐wastes generation.
- Bioactive compounds and bioenergy generation from jackfruit wastes was targeted.
- Sustainable way of bioproducts generation, promotes biorefinery/ clean environment.
1. Introduction
2. Database Formation and Analysis
3. Results and Discussion
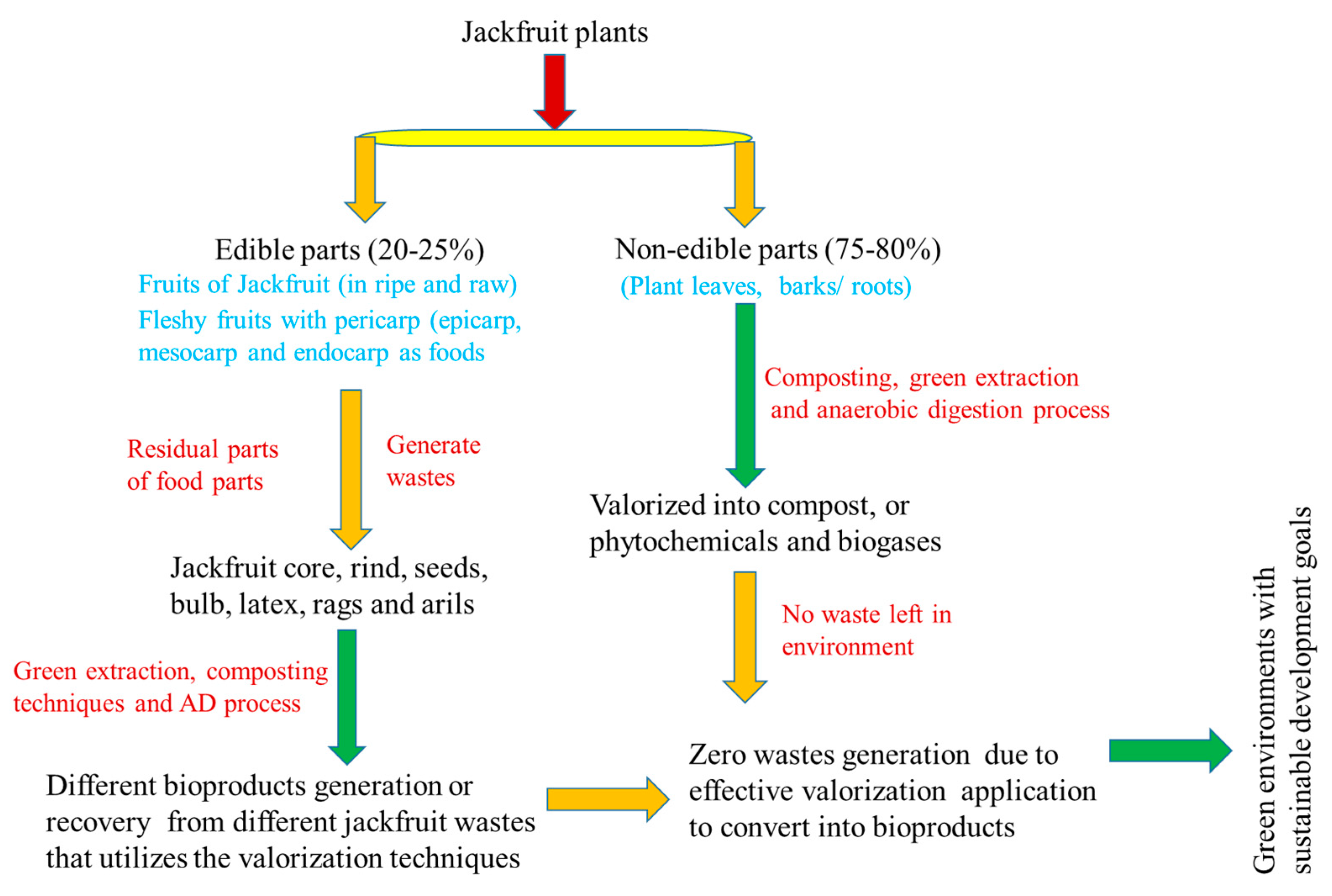
3.1. Cultivation of Jackfruits with Impact to its Nutrients
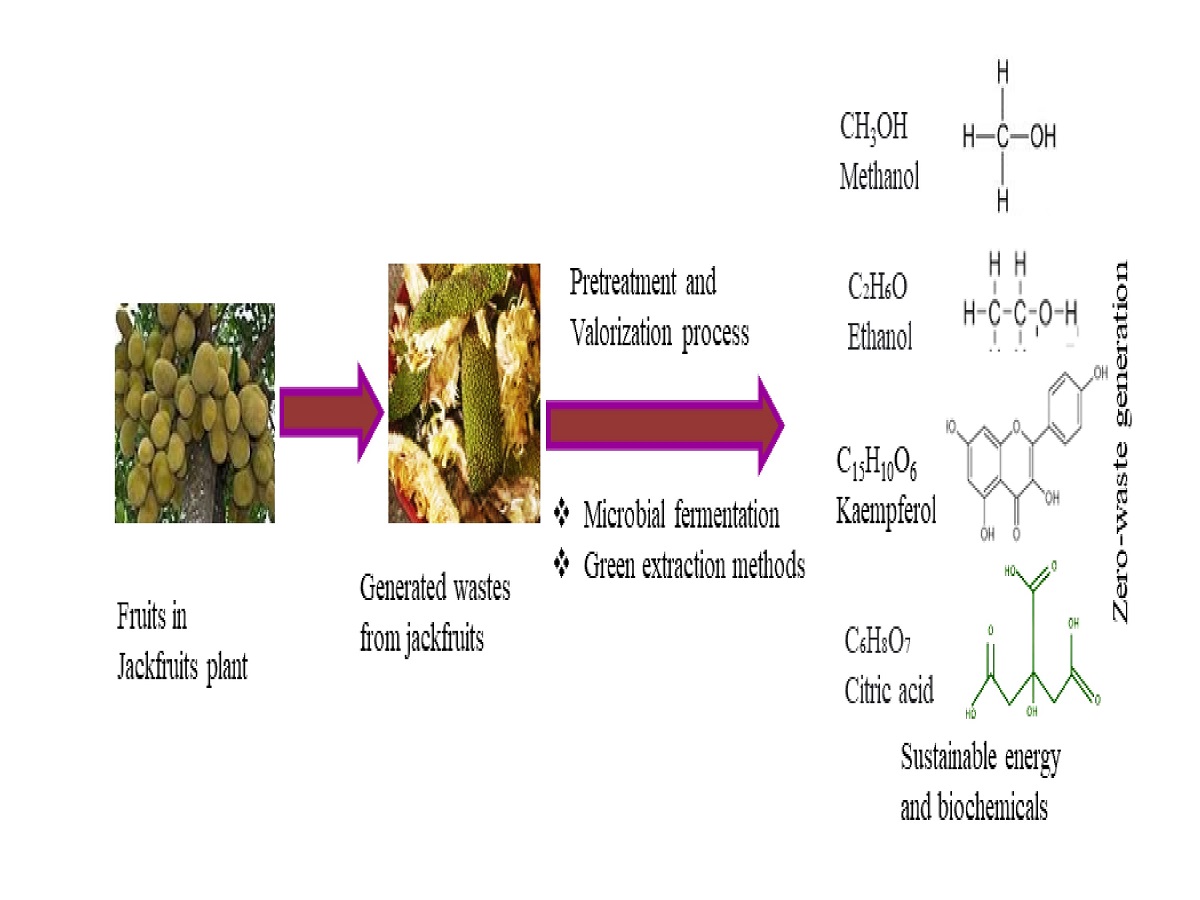
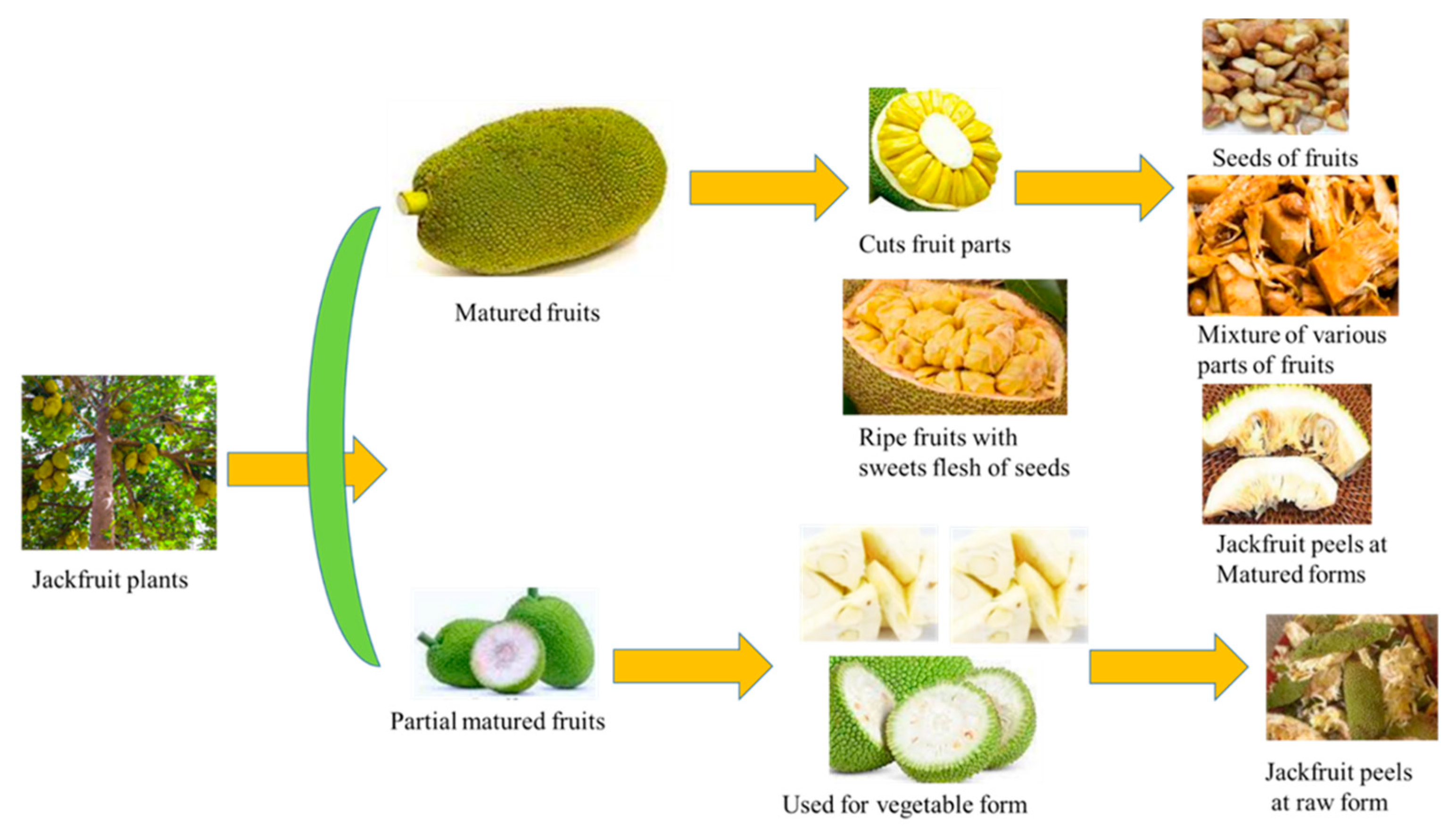
3.2. Compositions of Jackfruits Wastes
Various types of Jackfruits Wastes
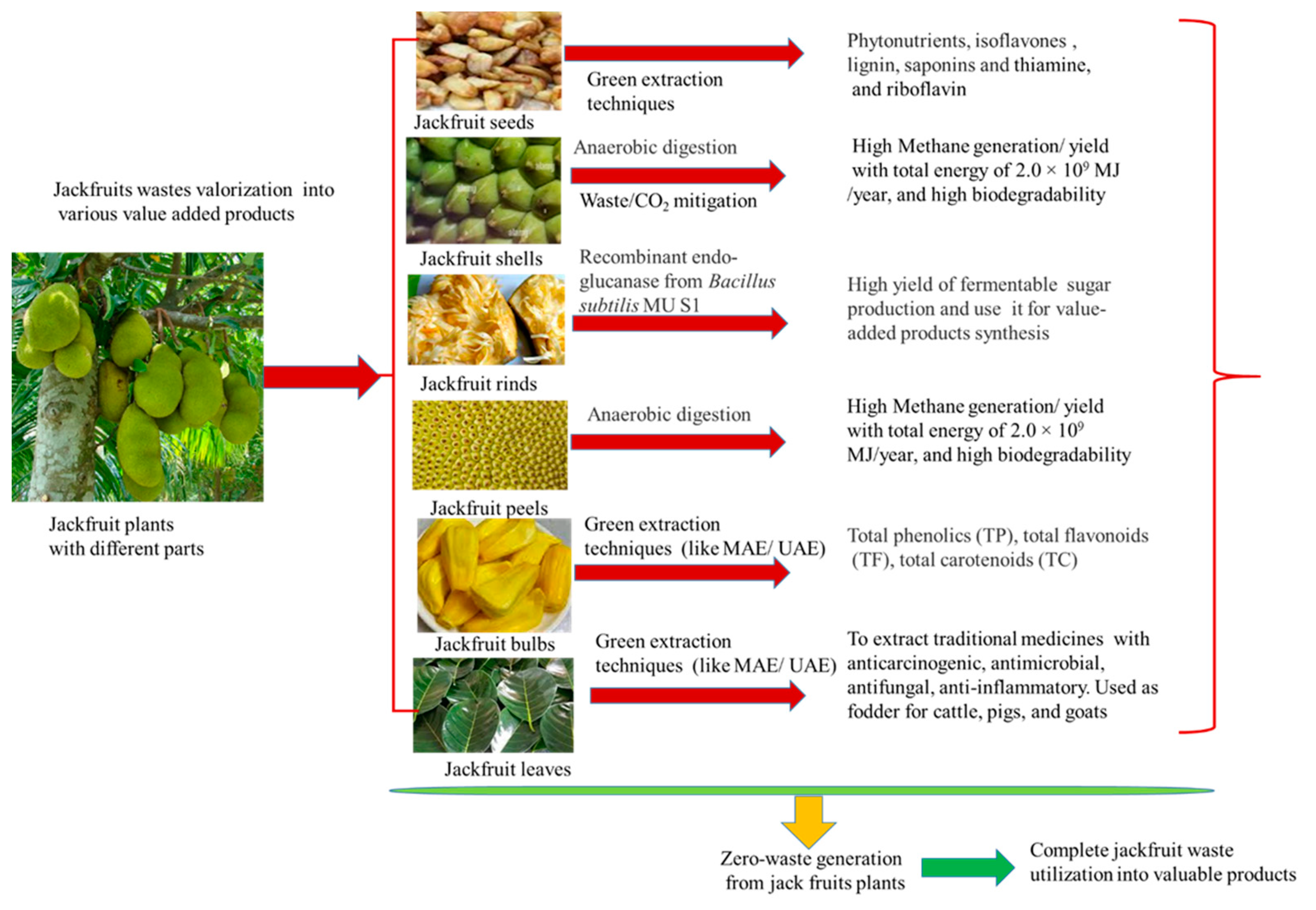
3.3. Valorization Techniques for Zero-waste Generation
3.4. Advanced/ Green Extraction Techniques for Bioactive Recovery
3.5. Microbial Fermentation for Jackfruits Waste Conversion
3.6. Value Added Products
3.7. Bioethanol
3.8. Biogas
3.9. Bioplastic
3.10. Bioactive Compounds
4. Advantages, Limitations and Drawback of Various Valorization Techniques
5. Prospect of Jackfruit Waste as a Bio-absorbant for Pollution Control
6. Conclusions and Future Perspectives
Abbreviations
References
- Thanh, L.P.; Truong, P.; Kha T.; Hang TT.T.; Jackfruit leaves can totally replace traditional grass in the diet of lactating dairy goats, J. Appl Animal Res. 50(1), (2022) ,97-102. [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [CrossRef]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.; Wong, J.W.; Drogui, P. Bioconversion of organic wastes into value-added products: A review. Bioresour. Technol. 2021, 344, 126398. [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [CrossRef]
- Sreeja Devi, P.S.; Kumar, N.S.; Sabu, K.K. Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam. (Moraceae): A review on current status of knowledge. Futur J Pharm Sci. 7 (2021), 30. [CrossRef]
- Siregar, A.B.; Bulan, R.; Yusak, Y. Antibacterial & antioxidant properties of leave & stem bark extract of Artocarpus heterophyllus as the component of peel-off mask. Int J Sci Technol Eng. 5(4), (2018), 101–106.
- Brahma, R.; Ray, S. A Comprehensive Review on the Recent Advances in the Valorization of Jackfruit Waste for the Development of Value-Added Products. J. Food Technol. Res. 2022, 9, 120–134. [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. Physicochemical characterization of Jackfruit Peel. Res J Pharm Biol Chem S. 8, (2017) 2285–95. [CrossRef]
- Begum, R.; Aziz, M.G.; Yusof, Y.A.; Saifullah; Uddin, M.B. Evaluation of gelation properties of jackfruit (Artocarpus heterophyllus) waste pectin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100160. [CrossRef]
- Lubis, M.; Gana, A.; Maysarah, S.; Ginting, M.H.S.; Harahap, M.B. Production of bioplastic from jackfruit seed starch (Artocarpus heterophyllus) reinforced with microcrystalline cellulose from cocoa pod husk (Theobroma cacao L.) using glycerol as plasticizer. IOP Conf. Series: Mater. Sci. Eng. 2018, 309, 012100. [CrossRef]
- Cagasan, C.U.; Li̇ngatong, C.A.V.; Pore, K.M.T.; Ramada, R.V.; Restor, C.D.D.; Lauzon, R.D. Production and Quality Evaluation of Wine from Jackfruit Co-Products. Int. J. Life Sci. Biotechnol. 2021, 4, 340–352. [CrossRef]
- Widjaja, C.; Djojorahardjo, Y.; Kurniawan, A Irawaty, W.; Soetaredjo, F.E. Biorefinery concept on jackfruit peel waste: bio-oil upgrading. ARPN J Eng Appl Sci. 13 (2018) 2202–7.
- George, N.; Debroy, A.; Bhat, S.; Singh, S.; Bindal, S. Biowaste to Bioplastics: An Ecofriendly Approach for A Sustainable Future. J Appl Biotechnol Rep. 8(3) (2021) 221-233. [CrossRef]
- Meera, M.; Ruckmani, A.; Saravanan, R.; Prabhu, R.L. Anti-inflammatory effect of ethanolic extract of spine, skin and rind of Jack fruit peel – A comparative study. Nat. Prod. Res. 2017, 32, 2740–2744. [CrossRef]
- Ojwang, R.A.; Muge, E.K.; Mbatia, B.; Mwanza, B.; Ogoyi, D.O. Comparative Analysis of Phytochemical Composition and Antioxidant Activities of Methanolic Extracts of Leaves, Roots and Bark of Jackfruit (Artocapus heterophyllus) from Selected Regions in Kenya and Uganda. J. Adv. Biol. Biotechnol. 2017, 16, 1–13. [CrossRef]
- Soumya, B.; Gupta, P.; Vikas, R.; Pradeep, K. .Chemoenzymatic saccharification of Artocarpus heterophyllus Lam.(jackfruit) peel waste and its utilization for bioethanol production. Indian Forest, 145(12) (2019)1204-1209.
- Kahar, A.W.M.; Lingeswarran, M.; Hulwani, M.Z.A.; Ismail, H. Plasticized jackfruit seed starch: a viable alternative for the partial replacement of petroleum-based polymer blends. Polym. Bull. 2018, 76, 747–762. [CrossRef]
- Marichelvam, M.; Manimaran, P.; Sanjay, M.; Siengchin, S.; Geetha, M.; Kandakodeeswaran, K.; Boonyasopon, P.; Gorbatyuk, S. Extraction and development of starch-based bioplastics from Prosopis Juliflora Plant: Eco-friendly and sustainability aspects. Curr. Res. Green Sustain. Chem. 2022, 5, 100296. [CrossRef]
- Ahmed, M.; Nasar, A. Utilization of Jackfruit Peel as a Low-cost Adsorbent for the Removal of Methylene Blue Dye from Synthetically Polluted Water. Curr. Anal. Chem. 2021, 17, 1016–1026. [CrossRef]
- Butool, S.; Butool, M. Nutritional quality on value addition to jack fruit seed flour. Int J Sci Res. 4, (2015) 2406–11.
- Sundarraj, A.A.; Ranganathan, T.V. Phytochemical constituents and thin-layer chromatography evaluation of the ethanolic extract of jackfruit (Artocarpus integer) peel. J Pharm Res. 12(5), (2018) 717.
- Pathak, N.; Singh, P.; Singh, P.K.; Sharma, S.; Singh, R.P.; Gupta, A.; Mishra, R.; Mishra, V.K.; Tripathi, M. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front. Nutr. 2022, 9, 963413. [CrossRef]
- Bhat, V.; Mutha, A.; Dsouza, M.R. Pharmacognostic and physiochemical studies of Artocarpus heterophyllus seeds. Int J ChemTech Res. 10(9), (2017) 525–536.
- Sundarraj, A.A.; Ranganathan, T.V. Phytochemical screening and spectroscopy analysis of jackfruit (Artocarpus integer Thumb. peel. Int Res J Pharm. 8(9) (2017) 151–159.
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [CrossRef]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [CrossRef]
- Islam, M.R.; Haque, A.R.; Kabir, M.R.; Hasan, M.M.; Khushe, K.J.; Hasan, S.M.K. Fruit by-products: the potential natural sources of antioxidants and α-glucosidase inhibitors. J. Food Sci. Technol. 2021, 58, 1715–1726. [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [CrossRef]
- Jancy, S.; Shruthy, R.; Preetha, R. Fabrication of packaging film reinforced with cellulose nanoparticles synthesised from jack fruit non-edible part using response surface methodology. Int. J. Biol. Macromol. 2019, 142, 63–72. [CrossRef]
- Kalse, S.; Swami, S. Recent application of jackfruit waste in food and material engineering: A review. Food Biosci. 2022, 48. [CrossRef]
- Mahesh, R.; Surendrababu, K.; Kumar, S.S.; Anoop, C.T.; Nuhaiz, V.P. Emission characteristics of jackfruit peel oil with three hole nozzle. IOP Conf. Series: Mater. Sci. Eng. 2020, 993, 012010. [CrossRef]
- Mardarveran, P.; Mokhtar, N.M. Analysis of Artocarpus heterophyllus peel as a natural coagulant using response surface methodology (RSM). J. Technol. 82 (4) (2020). [CrossRef]
- Brahma, R.; Ray, S. In-depth analysis on potential applications of jackfruit peel waste: A systematic approach. Food Chem. Adv. 2022, 1. [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V.; Gobikrishnan, S. Optimized extraction and characterization of pectin from jackfruit (Artocarpus integer) wastes using response surface methodology. Internat. J. Biologic. Macromolec. 106, (2018) 698-703.
- Jung, J.-M.; Kim, J.Y.; Kim, J-H.; Kim, S.M.; Jung, S.; Song, H.; Kwon, EE. Choi, Y.-E. Zero-waste strategy by means of valorization of bread waste. J Cleaner Product. 365 (2022), 132795. [CrossRef]
- Zhang, C.; Kang, X.; Wang, F.; Tian, Y.; Liu, T.; Su, Y.; Qian, T.; Zhang, Y. Valorization of food waste for cost-effective reducing sugar recovery in a two-stage enzymatic hydrolysis platform. Energy 2020, 208. [CrossRef]
- Nsubuga, D.; Banadda, N.; Kabenge, I.; Wydra, K.D. Potential of Jackfruit Waste for Biogas, Briquettes and as a Carbondioxide Sink-A Review. J. Sustain. Dev. 2020, 13. [CrossRef]
- Zhang, C.; Ling, Z.; Yang, L.; Liu, Y.; Cao, T.; Sun, Y.; Liu, W.; Huo, S.; Zhang, Z.-H.; Su, H.; et al. Efficient caproate production from ethanol and acetate in open culture system through reinforcement of chain elongation process. J. Clean. Prod. 2023, 383. [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol Adv. 37(5), (2019), 599-615. [CrossRef]
- Daud, M.N.H.; Ahmad, R.; Abdullah, N.; Jabit, M.L.; Wibowo, A. Effect of storage on antioxidant activity and bioactive compound of Artocarpus heterophyllus J33 rind extract. Adv Appl Chem Biochem. 2019,1, 68–81.
- Nuriana, W.; Wuryantoro Ethanol Synthesis from Jackfruit (Artocarpus Heterophyllus Lam.) Stone Waste as Renewable Energy Source. Energy Procedia 2015, 65, 372–377. [CrossRef]
- Ginting, M.H.S.; Irvan; Misran, E.; Maulina, S. Potential of durian, avocado and jackfruit seed as raw material of bioethanol: a review. IOP Conf. Series: Mater. Sci. Eng. 2020, 801. [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [CrossRef]
- Štambuk, P.; Tomašković, D.; Tomaz, I.; Maslov, L.; Stupić, D.; Kontić, J.K. Application of pectinases for recovery of grape seeds phenolics. 3 Biotech 2016, 6, 1–12. [CrossRef]
- Pathak, N.; Singh, S.; Singh, P.; Singh, P.K.; Singh, R.; Bala, S.; Thirumalesh, B.V.; Gaur, R.; Tripathi, M. Valorization of jackfruit waste into value added products and their potential applications. Front. Nutr. 2022, 9, 1061098. [CrossRef]
- Lal, A.N.; Prince, M.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74. [CrossRef]
- Jiang, Z.; Shi, R.; Chen, H.; Wang, Y. Ultrasonic microwave-assisted extraction coupled with macroporous resin chromatography for the purification of antioxidant phenolics from waste jackfruit (Artocarpus heterophyllus Lam.) peels. J. Food Sci. Technol. 2019, 56, 3877–3886. [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [CrossRef]
- Azooz, E.A.; Ridha, R.K.; Abdulridha, H.A. The Fundamentals and Recent Applications of Micellar System Extraction for Nanoparticles and Bioactive Molecules: A Review. Nano Biomed. Eng. 2021, 13. [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: a review. Int. J. Food Sci. Technol. 2018, 54, 1–13. [CrossRef]
- Nishad, S.; Saha, C. Kaur, Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J Food Proc Preserv. 43 (8) (2019) 1-13. [CrossRef]
- Wan, N.; Kou, P.; Pang, H.-Y.; Chang, Y.-H.; Cao, L.; Liu, C.; Zhao, C.-J.; Gu, C.-B.; Fu, Y.-J. Enzyme pretreatment combined with ultrasonic-microwave-assisted surfactant for simultaneous extraction of essential oil and flavonoids from Baeckea frutescens. Ind. Crop. Prod. 2021, 174, 114173. [CrossRef]
- Rafińska, K.; Wrona, O.; Krakowska-Sieprawska, A.; Walczak-Skierska, J.; Kiełbasa, A.; Rafiński, Z.; Pomastowski, P.; Kolankowski, M.; Buszewski, B. Enzyme-assisted extraction of plant material – New functional aspects of the process on an example of Medicago sativa L.. Ind. Crop. Prod. 2022, 187. [CrossRef]
- Li, H.-Z.; Zhang, Z.-J.; Xue, J.; Cui, L.-X.; Hou, T.-Y.; Li, X.-J.; Chen, T. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from perilla leaves using response surface methodology. Food Sci. Technol. 2016, 36, 686–693. [CrossRef]
- Shen, X.; Shao, S.; Guo, M. Ultrasound-induced changes in physical and functional properties of whey proteins. Int. J. Food Sci. Technol. 2016, 52, 381–388. [CrossRef]
- ivković J, Šavikin K, Janković T, Ćujić N, Menković N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep Purif Technol 194 (2018) 40–47.
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2018, 101, 342–350. [CrossRef]
- Júnior, M.E.S.; Araújo, M.V.R.; Santana, A.A.; Silva, F.L.H.; Maciel, M.I.S. Ultrasound-assisted extraction of bioactive compounds from ciriguela (Spondias purpurea L.) peel: Optimization and comparison with conventional extraction and microwave. Arab. J. Chem. 2021, 14, 103260. [CrossRef]
- Carpentieri, S.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds From Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [CrossRef]
- Pef-assisted Supercritical CO2 Extraction of Pigments from Microalgae Nannochloropsis Oceanica in a Continuous Flow System., 74, 97–102. [CrossRef]
- Pataro, G.; Carullo, D.; Siddique, A.B.; Falcone, M.; Donsì, F.; Ferrari, G. Improved extractability of carotenoids from tomato peels as side benefits of PEF treatment of tomato fruit for more energy-efficient steam-assisted peeling. J. Food Eng. 2018, 233, 65–73. [CrossRef]
- Pataro, G.; Bobinaitė, R.; Bobinas, Č..; Satkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viskelis, P. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their By-products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017, 10, 1595–1605. [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viskelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Obanijesu, E.O.; Alara, J.A.; Mudalip, S.K.A. Extract-rich in flavonoids from Hibiscus sabdariffa calyces: Optimizing microwave-assisted extraction method and characterization through LC-Q-TOF-MS analysis. J. Food Process. Eng. 2019, 43, e13339. [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51, 138–149. [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović. I.T. Optimization of microwave assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep Purif Technol. 160 (2016) 89–97.
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2017, 6, 189–196. [CrossRef]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and Waste Reduction as an Integral Part of a Circular Economy. Front. Environ. Sci. 2017, 5. [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: Kinetic modelling and process intensification. Ind. Crop. Prod. 2019, 137, 528–535. [CrossRef]
- Alara, O.R.; Abdurahman, N.H., Adbul Mudalip, S.K. Optimizing microwave-assisted extraction conditions to obtain Phenolic compounds-rich extract from Chromolaena odorata leaves. Chem. Eng. Technol. 42(9) (2019), 1733-1740. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018, 122, 533–544. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Alara, J.A. Optimization of microwave-assisted extraction of phenolic compounds from Ocimum gratissimum leaves and its LC–ESI–MS/MS profiling, antioxidant and antimicrobial activities. J. Food Meas. Charact. 2020, 14, 3590–3604. [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2016, 101, 1–10. [CrossRef]
- Argun, M. E.; Argun, M. Ş.; Arslan, F.N.; Nas, B.; Ates, H.; Tongur, S.; Cakmakcı, O. Recovery of valuable compounds from orange processing wastes using supercritical carbon dioxide extraction. J. Cleaner Prod. 375, (2022), 134169. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [CrossRef]
- Dey, T.K.; Banerjee, P.; Chatterjee, R.; Dhar, P. Designing of ω-3 PUFA enriched biocompatible nanoemulsion with sesame protein isolate as a natural surfactant: Focus on enhanced shelf-life stability and biocompatibility. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 538, 36–44. [CrossRef]
- de Andrade Lima, M.; Andreou, R.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Carbon Dioxide Extraction of Phenolic Compounds from Potato (Solanum tuberosum) Peels. Appl. Sci. 11, (2021), 3410. [CrossRef]
- Constance, E. C., Ifunanya, W. U., Amarachi, M. C. E., Idera, E. E. C., Ikechukwu, N., Ogechi, P. C., & Perpetua, O. C.. Evaluation of vinegar production properties of Garcina kola and Artocarpus heterophyllus. J. Appl. Chem. Sci. Internat. 12(2) (2021), 48-60.
- Nguyen, T.K.; That, N.T.T.; Nguyen, N.T.; Nguyen, H.T. Development of Starch-Based Bioplastic from Jackfruit Seed. Adv. Polym. Technol. 2022, 2022, 1–9. [CrossRef]
- Ochaikul, D.; Noiprasert, N.;Laoprasert, W.; Pookpun, S.Ethanol Production on Jackfruit Seeds by Selected Fungi and Yeast from Loog-pang. KMITL Sci. Tech. J. 2012, 12 (1). 1-5.
- Miah, R.A.; Alam, M.J.; Khatun, A.; Suhag, M.H.; Kayes, N. The Decolorization and Phytotoxic Efficiency of Jackfruit Seed on a Textile Dye Novacron Blue. J. Eng. Adv. 2022, 6–11. [CrossRef]
- Abid, M.K.; Bin Ibrahim, H.; Zulkifli, S.Z. Synthesis and Characterization of Biochar from Peel and Seed of Jackfruit plant waste for the adsorption of Copper Metal Ion from water. Res. J. Pharm. Technol. 2019, 12, 4182. [CrossRef]
- Widjaja, C.; Djojorahardjo, Y.; Kurniawan, A.; Irawaty, W.; Soetaredjo, F.E. Biorefinery concept on jackfruit peel waste: bio-oil upgrading. ARPN J Eng Appl Sci. 13 (2018) 2202–7.
- C.P., S.; Sebastian, D. Jackfruit outer rind: A sustainable feedstock for fermentable sugar production using recombinant endoglucanase from Bacillus subtilis MU S1. Environ. Technol. Innov. 2019, 16. [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass – An overview. Bioresour. Technol. 2016, 199, 76–82. [CrossRef]
- Wang, L.; Wei, B.; Cai, F.; Chen, C.; Liu, G. Recycling durian shell and jackfruit peel via anaerobic digestion. Bioresour. Technol. 2021, 343, 126032. [CrossRef]
- Nelluri, P.; Venkatesh, T.; Kothakota, A.; Pandiselvam, R.; Garg, R.; Eswaran, V.; Vaddevolu, U.B.P.; Venkatesh, R.; Khaneghah, A.M. Recent advances in non-thermal and thermal processing of jackfruit ( Artocarpus heterophyllus Lam ): An updated review. J. Food Process. Preserv. 2022, 46. [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [CrossRef]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2021, 346, 126580. [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Di Domenico, M.; Filho, V.F.d.S.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Insights into the bioenergy potential of jackfruit wastes considering their physicochemical properties, bioenergy indicators, combustion behaviors, and emission characteristics. Renew. Energy 2020, 155, 1328–1338. [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [CrossRef]
- Sreeletha, A.S.; Lini, J.J.; Dhanyalekshmi, C.S.; Sabu, K.R.; Pratap, C.R. Phytochemical, proximate, antimicrobial, antioxidant and FTIR analyses of seeds of Artocarpus heterophyllus Lam. Adv Biotechnol Microbiol. 2017, 5(1), 555–653.
- Burci, L.M.; da Silva, C.B.; de Oliveira, M.; Dalarmi, L.; Zanin, S.M.W.; Miguel, O.G.; Miguel, M.D. Determination of antioxidant, radical scavenging activity and total phenolic compounds of Artocarpus heterophyllus (Jackfruit) seeds extracts. J Med Plants Res. 2015, 9(40), 1013–1020.
- Jiang, T.-T.; Liang, Y.; Zhou, X.; Shi, Z.-W.; Xin, Z.-J. Optimization of a pretreatment and hydrolysis process for the efficient recovery of recycled sugars and unknown compounds from agricultural sweet sorghum bagasse stem pith solid waste. PeerJ 2019, 6, e6186. [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass- Convers. Biorefinery 2020, 11, 219–226. [CrossRef]
- Adewuyi, Y.G.; Deshmane, V.G. Intensification of enzymatic hydrolysis of cellulose using high-frequency ultrasound: an investigation of the effects of process parameters on glucose yield. Energy Fuel. 29 (2015) 4998–5006. [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: factors influencing particle size and colour. Int. J. Food Sci. Technol. 2017, 52, 1408–1416. [CrossRef]
- ; Sundari, C.D.D.; Sunarya, R.R.; Suryaningsih, S. Optimization of bioethanol production from jackfruit straw waste through the addition of a starter and fermentation duration. 2023; 2572, 030014. [CrossRef]
- Arif, A.R.; Natsir, H.; Rohani, H.; Karim, A. Effect of pH fermentation on production bioethanol from jackfruit seeds (Artocarpus heterophyllus) through separate fermentation hydrolysis method. J. Physics: Conf. Ser. 2018, 979, 012015. [CrossRef]
- Nikolić, S.; Lazić, V.; Veljović, Đ.; Mojović, L. Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr.polym. 164 (2017) 136-144.
- Brexó, R.P.; Sant’ana, A.S. Impact and significance of microbial contamination during fermentation for bioethanol production. Renew. Sustain. Energy Rev. 2017, 73, 423–434. [CrossRef]
- Yuvarani, M.; Immanuel, C.S. Dhas.Synthesis of Bioethanol from Artocarpus Heterophyllus Peel by Fermentation using Saccharomyces Cerevisiae at Low Cost. GRD J. Eng. 2 (12) (2017), 1-7.
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol Production from Fermentable Sugar Juice. Sci. World J. 2014, 2014, 1–11. [CrossRef]
- Kaewchana, A.; Techaparin, A.; Boonchot, N.; Thanonkeo, P.; Klanrit, P. Improved high-temperature ethanol production from sweet sorghum juice using Zymomonas mobilis overexpressing groESL genes. Appl. Microbiol. Biotechnol. 2021, 105, 9419–9431. [CrossRef]
- Rukshika, S.; Sisira, K.; Sanath, R.; Subramanium, S. Isolation and Characterization of an Ethanol Resistant Bacterium from Sap of Saccharum officinarum for Efficient Fermentation. Internat J Biotechnol Bioeng. 03(02) (2016) 15-20.
- Kularathne, I.W.; Gunathilaka, C.A.; Ratnaweera, A.C.; Kalpage, C.S.; Rajapakse, S.; Gamage, P. Optimization of Fermentation Process Parameters for Bioethanol Production from Sri Lankan Overripe Fruits. Eng. J. Inst. Eng. Sri Lanka 2021, 54, 77. [CrossRef]
- Mibulo, T.; Nsubuga, D.; Kabenge, I.; Kiggundu, N.; Wydra, K.D. Comparative Study of Biogas Production from Jackfruit Waste, Banana Peels, and Pineapple Peels Co-Digested with Cow Dung. J. Sustain. Bioenergy Syst. 2023, 13, 1–15. [CrossRef]
- Balamaze, J.; Muyonga, J.; Byaruhanga, Y. Production and utilization of jackfruit (Artocarpus heterophyllus) in Uganda. Afr. J. Food, Agric. Nutr. Dev. 2019, 19, 14289–14302. [CrossRef]
- Bijarchiyan, M.; Sahebi, H.; Mirzamohammadi, S. A sustainable biomass network design model for bioenergy production by anaerobic digestion technology: using agricultural residues and livestock manure. Energy, Sustain. Soc. 2020, 10, 1–17. [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A Circular Economy Model Based on Biomethane: What Are the Opportunities for the Municipality of Rome and Beyond? Rene Ener. 163, (2021) 1660-1672.
- Waluyo, J.; Pratiwi, Y. Analysis Proximate , Ultimate , and Thermal Gravimetric Based on Variations Dimensions of Briquettes from Waste Jackfruit Crust. Internat J Scient. Eng. Sci. 2(10), (2018) 36–39.
- Walsh, J.J.; Jones, D.L.; Chadwick, D.R.; Williams, A.P. Repeated application of anaerobic digestate, undigested cattle slurry and inorganic fertilizer N: Impacts on pasture yield and quality. Grass Forage Sci. 2018, 73, 758–763. [CrossRef]
- Westerholm, M.; Castillo, M.; Andersson, A.C.; Nilsen, P.J.; Schnürer, A. Effects of thermal hydrolytic pre-treatment on biogas process efficiency and microbial community structure in industrial- and laboratory-scale digesters. Waste Manag. 2019, 95, 150–160. [CrossRef]
- Zakaria, M.R.; Ariffin, H.; Abd-Aziz, S.; Hassan, M.A.; Shirai, Y. Improved Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Produced byComamonassp. EB172 Utilizing Volatile Fatty Acids by Regulating the Nitrogen Source. BioMed Res. Int. 2013, 2013, 1–7. [CrossRef]
- Gowda, V.; Shivakumar, S. Agrowaste-based Polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [CrossRef]
- Sehgal, R.; Gupta, R. Polyhydroxyalkanoate and its efficient production: an eco-friendly approach towards development. 3 Biotech 2020, 10, 1–14. [CrossRef]
- Nelluri, P.; Venkatesh, T.; Kothakota, A.; Pandiselvam, R.; Garg, R.; Eswaran, V.; Vaddevolu, U.B.P.; Venkatesh, R.; Khaneghah, A.M. Recent advances in non-thermal and thermal processing of jackfruit ( Artocarpus heterophyllus Lam ): An updated review. J. Food Process. Preserv. 2022, 46. [CrossRef]
- Gupta, R.; Gupta, S.K.; Gehlot, C.L.; Bahadur, I. Chemically modified jackfruit leaves as a low-cost agro-waste adsorbent for Pb(II) removal from synthetic wastewater. J. Hazard. Mater. Adv. 2023, 10. [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [CrossRef]
- Khan. A.U. A Review on Importance of Artocarpus heterophyllus L. (Jackfruit). J. Multidiscip. Appl. Nat. Sci., 2021. 1(2), 106-116.
- Zhang, L.; Lu, S.; Xiao, D.; Gu, M. Characterization of full pore size distribution and its significance to macroscopic physical parameters in tight glutenites. J. Nat. Gas Sci. Eng. 2017, 38, 434–449. [CrossRef]
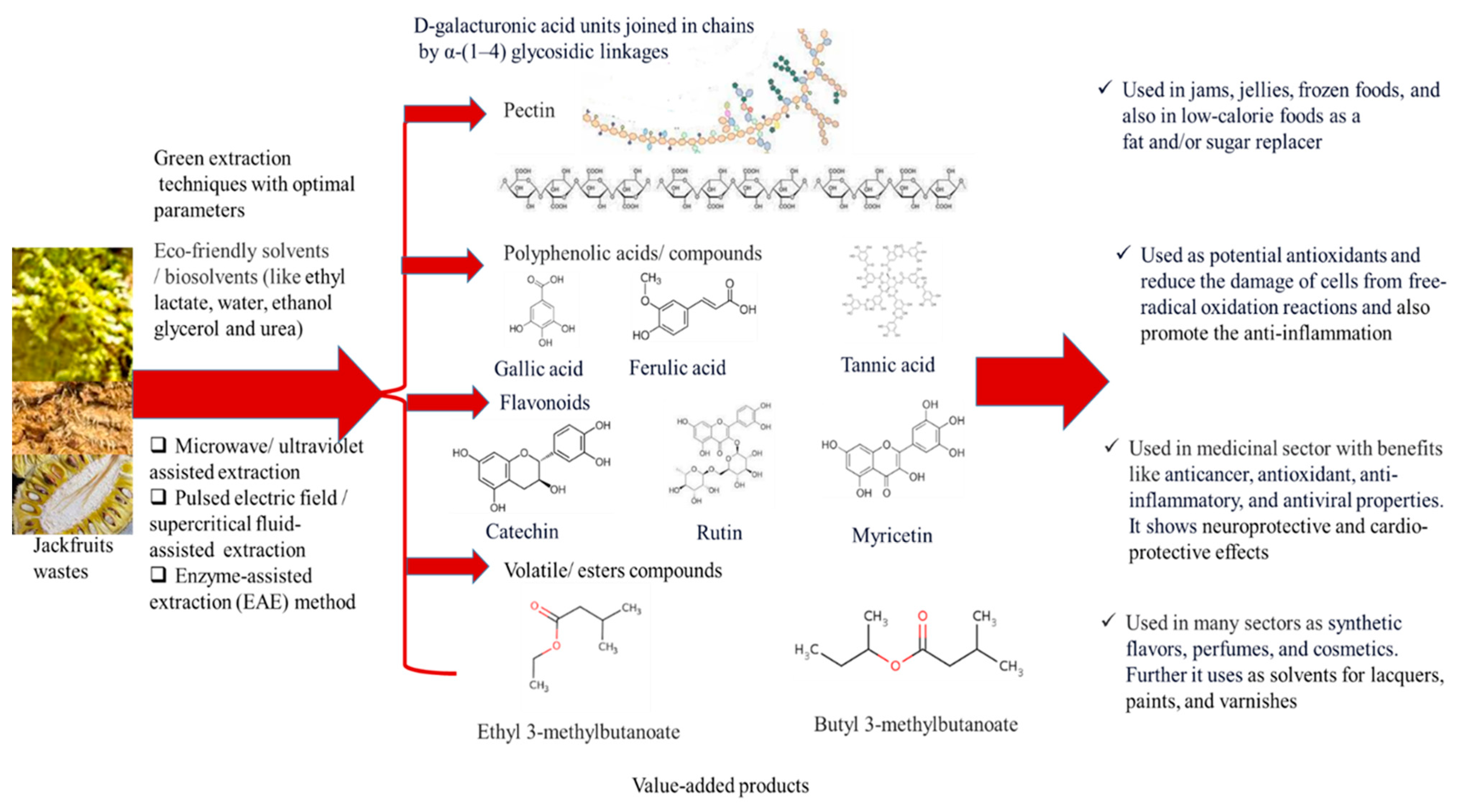
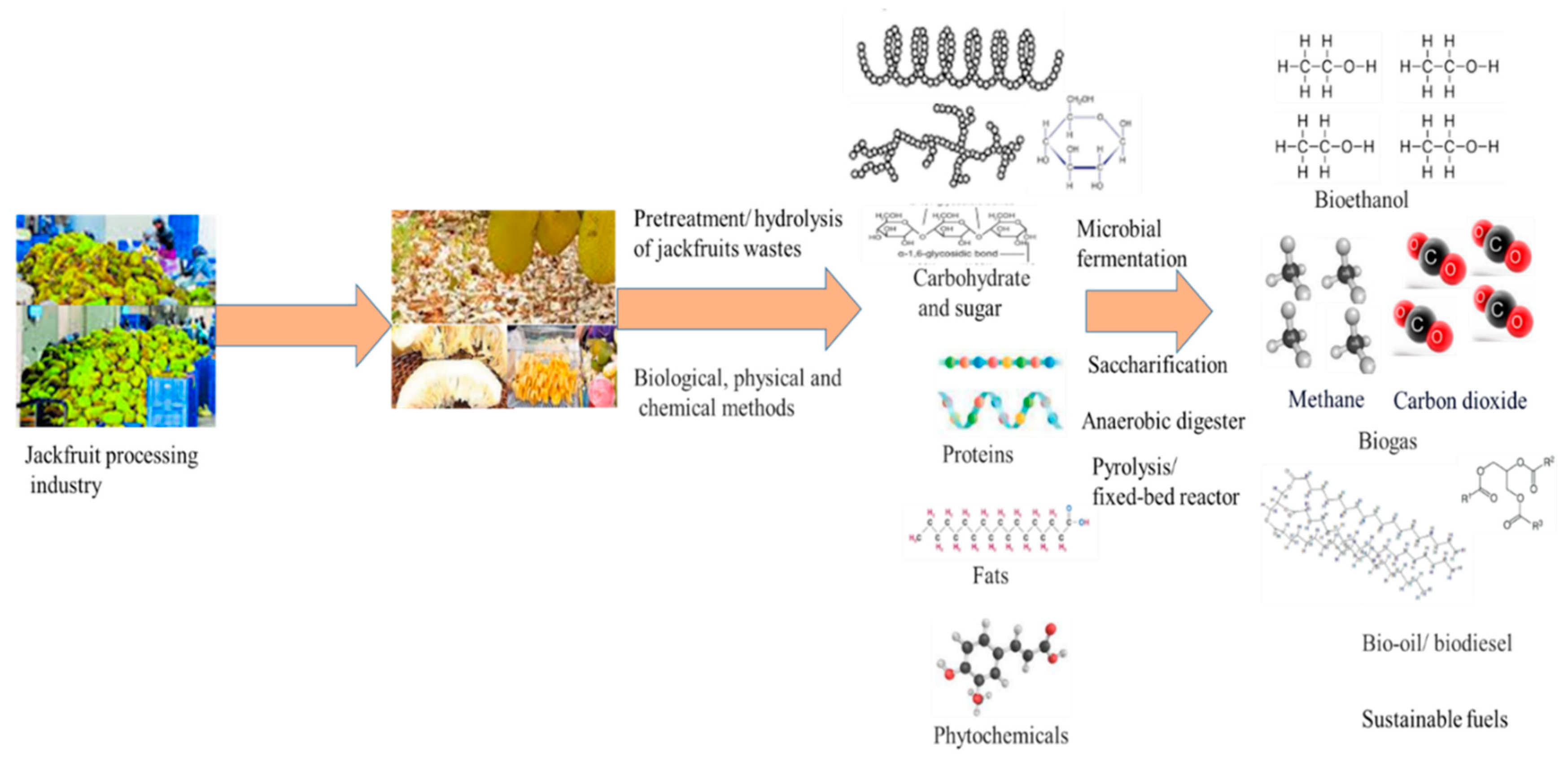
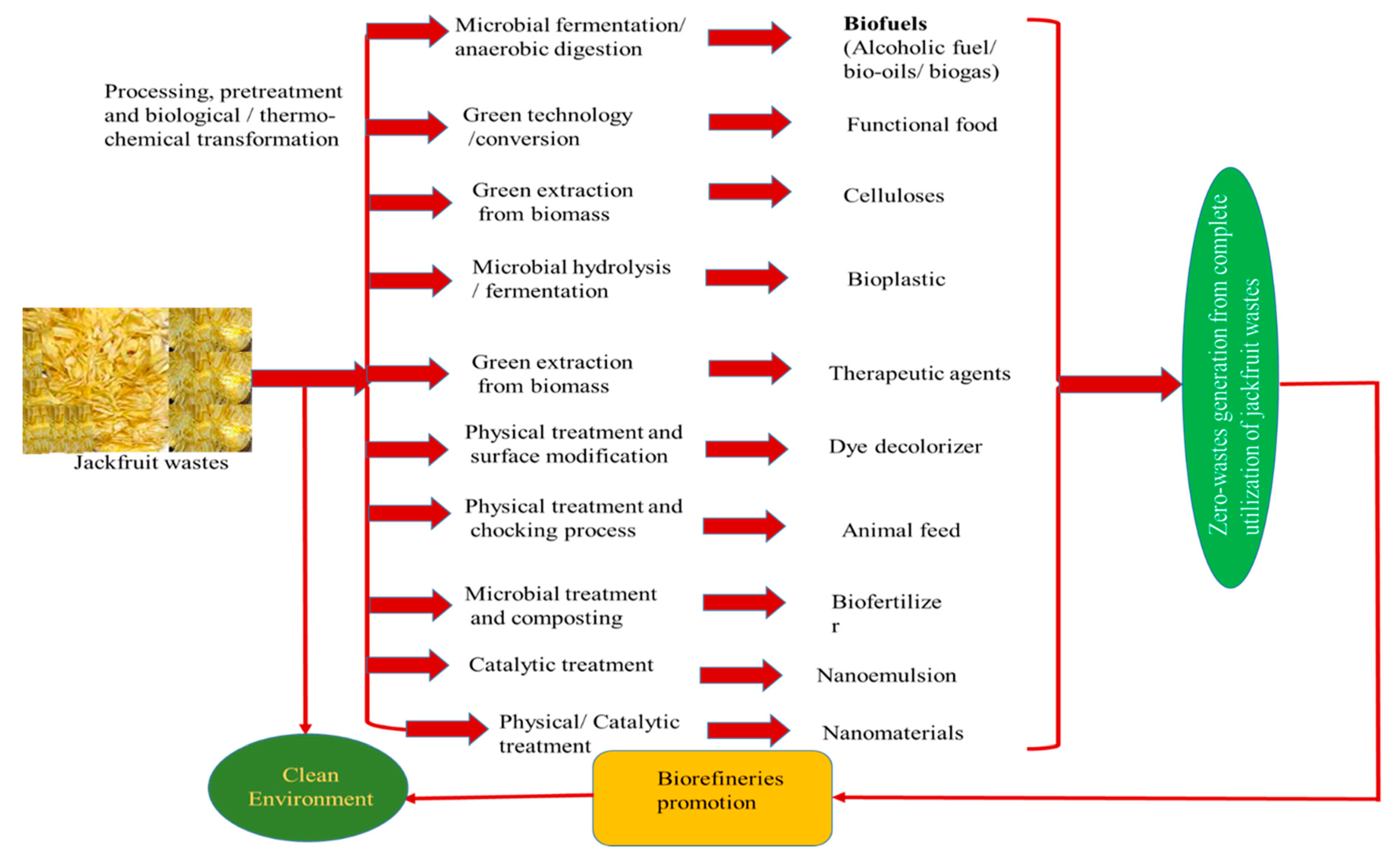
| Jackfruit wastes | Value-added products | Application | References |
|---|---|---|---|
| Jackfruit wastes with its different parts like peel/ skins | Bioethanol, biogas, bioplastics, are so on generated. | Biofuel is cheaper and greener than fossil fuels. Processed peel cleans dye-contaminated aquatic environments. | [7] |
| Durian shell and jackfruit peel | An increase of 103.8% in methane output and 69.8% in biodegradability. | Produces high levels of sustainable energy and fuel (2.0 109 MJ/year), while simultaneously decreasing coal usage (6.8 104 tons/year) and cutting emissions by 2.2 1010 particulate/year. | [9] |
| Jackfruit outer rind | Used as substrate for producing recombinant endoglucanase from Bacillus subtilis MU S1 | This enzyme helps in highest saccharification process (33.4 %) from jackfruit outer rind at 96 h of incubation | [11] |
| Jackfruit peel waste | Bio-oil upgrading is done by sub/super-critical fluids, solvent addition, and steam reforming | The application of bio=oil can substitute fuel for the commercial or industrial burner | [12] |
| Jackfruit seed starch was plasticized | Used to produce starch-based bioplastics | Four distinct kinds of bioplastics were manufactured in order to investigate the influence of the plasticizers and to characterise the features of the bioplastics that correspond to those plasticizers. | [18] |
| Utilization of jackfruit peel | There have been reports of the use of an adsorbent made from jackfruit peel. | Jackfruit peel adsorbent can biosorb maximally of232.55 mg/g of MB. | [19] |
| Artocarpus heterophyllus Lam. seed powder extract (ASPE) | Green production of silver nanoparticles from silver nitrate in water | ASPE may be utilized to synthesize AgNPs for nanomedicine in the future. It is eco-friendly and harmless. | [25] |
| Artocarpus heterophyllus peels | Green synthesis of iron nanoparticles is reported | Iron nanoparticles were highly catalytic, removing 87.5% in 20 minutes at 318 K. | [28] |
| Artocarpus heterophyllus (jackfruit) peel | Bio-based coagulating agent | The extract from the peel has the potential to serve as a bio-based coagulating agent alternative that is useful in the pre-treatment of wastewater. | [32] |
| Jackfruit seed powder (JSP) | The surface of JSP is used | Novacron blue textile dye can be decolorized using this substance. After a contact time of 60 minutes, the surface of JSP has been observed to adsorb 73% of Novacron blue. | [83] |
| Jackfruit waste feedstock | Biogas production was improved by chemical catalysts with maintaining the pH and C/N ratio. | Biogas is produced via decreasing the digestion time with improving the efficiency of digester unit by using jack fruit waste as raw material. This raw material contains significant quantity of fiber with small proportions of glucose. | [84] |
| Jackfruit waste together with peels (JP) as well as seeds (JS) | Bioenergy has optimal physicochemical, bioenergy indicators, combustion, and emission properties. | The bioenergy yields for JP and JS were 2.5 and 0.9 ha−1 yr−1 (dry basis), correspondingly. Low concentrations of CO, CO2, and SO2 may be released. | [92] |
| Jackfruit straw waste | Used as raw material for making bioethanol. | The amount of bioethanol distillate that could be produced under ideal circumstances was 30 ml. This research employed a distillation temperature range of 70 to 78 °C. | [100] |
| Jackfruit (Artocarpus heterophyllus) stone waste | Ethanol from these waste is found and it is used as renewable fuels | A quantity of Jackfruit flour was subjected to hydrolysis by the addition of 0.3 to 0.7 mL of alpha-amylase and 0.2 to 0.6 mL of glucoamylase. The resulting mixture was then subjected to a fermentation process lasting between 3 to 6 days. The yield of ethanol obtained from this process was found to be between 11 to 13%. | [41] |
| Renewable energy from jackfruit's seeds | Bioethanol (57.94%) is reported | The fermentation process for ethanol production is used with Saccharomyces cereviceae with a variation of pH values with 70 hours | [101] |
| Jackfruit wastes | Bioactive compounds | Health benefits | References |
|---|---|---|---|
| Jackfruit skins, leaves, and barks | Vitamins, minerals, and phytochemicals | The substance under consideration exhibits various properties such as anticarcinogenic, antimicrobial, antifungal, anti-inflammatory, wound healing, and hypoglycemic effects. | [2] |
| Agro-residues from jackfruit plants | Nanocapsules uses increase the bioactive efficacy | Bioactive in micro- and nanoencapsulation forms enhanced target site delivery in human body with more benefits | [4] |
| Jackfruits are known for their prickly outer bark and axis. | Flavonoids, stillbenoids, morin, artocarpin, dihydromorin and cynomacurin, | Because of their bioactivity, these chemicals have the potential to be developed into nutraceuticals with antioxidant characteristics. | [5] |
| Leave and stem bark extract of Artocarpus Heterophyllus | Extract is dominated with tannin and saponin | The methanol extract of Artocarpus stem and leaf bark has antibacterial and antioxidant properties, and the bark may be used topically as a peel-off mask. | [6] |
| Jackfruit peel | Functional food additives and pectin materials | These compounds are manipulated for food ingredient applications with providing of health benefit to gastro-intestinal tract. | [8] |
| Spine, skin and rind of jack fruit | Polyphenol as well as flavonoids | Crude ethanolic extracts undergo evaluation for their anti-inflammatory potential. | [14] |
| Fruit peel of jackfruit | Flavonoid, and also present some phenolic compounds is reported | Various phyto-constituents can use In different additives of human use with more health benefits |
[21] |
| Jackfruit seed extracts in three different solvents: methanol, hydroalcoholic, and aqueous. | Alkaloids, flavonoids, terpenoids, and so on | This extracts showed the antioxidant, anti-inflammatory and antibacterial activity | [23] |
| Artocarpus heterophyllus J33 rind parts | Proto-catechuic acid (PCA) antioxidant activity depends on its temperature: 25°C, 4°C, and -18°C. | The extract’s antioxidant activity was maintained because PCA is so stable. It has antioxidant properties and might be used in food and dietary supplements. | [40] |
| Seeds of jack fruit | The different solvent extracts showed the presence of fats, phenols and flavonoids. | Acetone extract demonstrated the greatest antibacterial activity towards Staphylococcus aureus and the greatest antifungal activity against Aspergillus flavus. | [94] |
| Jack fruit seeds | Total phenolic compounds in this seed extract confirmed by different screening tests | Total phenolic substances are identified as antioxidants with radical scavenging action. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





