Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
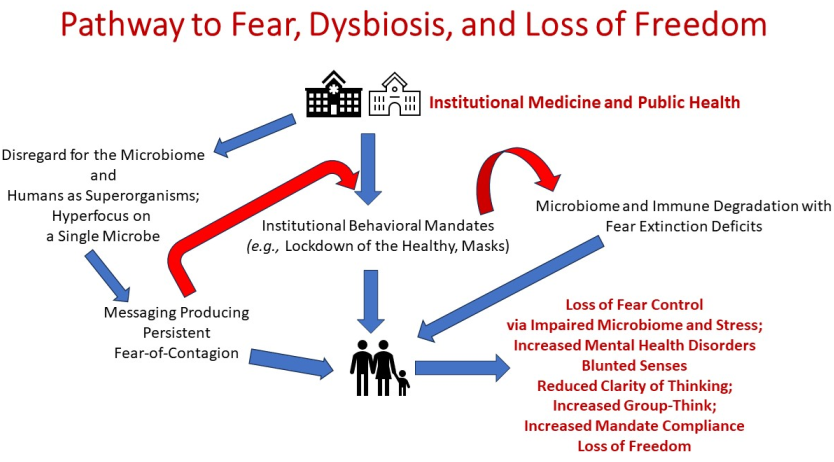
Keywords:
1. Introduction
2. Clear Risk-Benefit Communication with the Public for Self-Empowerment and Fear Reduction
2.1. Microbiota and Risk of Viral and/or Bacterial Pathogenesis
2.2. History of Secondary Bacterial Pneumonia Deaths in Human Pandemics and Animal Coronavirus Infections
2.3. The Covid-19 Example with Bacterial and/or Fungal Infections Causing Death
2.4. Adverse Risk Communication Regarding of Mask Mandates
2.5. Risk of Microbiome and Immune (Microimmunosome) Degradation
3. The Covid-19 Pandemic, Fear-of-Contagion and Compliance
4. Regulation of Fear Extinction by the Microbiome and Public Health Implications
5. Defective Fear Extinction and Mental Health Implications
6. Lockdown of the Healthy: Sensory Dulling, Microbiota, and Mental Health
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bell, K.; Bordenstein, S.R. , 2022. A margulian view of symbiosis and speciation: the Nasonia wasp system. Symbiosis, 2022, 87, 3–10. [Google Scholar] [CrossRef]
- Menditto, E.; Gimeno Miguel, A.; Moreno Juste, A.; Poblador Plou, B.; Aza Pascual-Salcedo, M.; Orlando, V.; González Rubio, F.; Prados Torres, A. Patterns of multimorbidity and polypharmacy in young and adult population: Systematic associations among chronic diseases and drugs using factor analysis. PLoS One 2019, 14, e0210701. [Google Scholar] [CrossRef] [PubMed]
- Almodóvar, A.S.; Nahata, M.C. Associations between chronic disease, polypharmacy, and medication-related problems among medicare beneficiaries. J. Manag. Care Special. Phar. 2019 25, 573–577. [CrossRef]
- Dietert, R.R. The microbiome-immune-host defense barrier complex (microimmunosome) and developmental programming of noncommunicable diseases. Reprod. Toxicol. 2017, 68, 49–58. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: a narrative review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nat. Rev. Microbiol. 2023, 21, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.A. , Satchell, L.P., Fido, D. et al. Functional Fear Predicts Public Health Compliance in the COVID-19 Pandemic. Int. J. Ment. Health Addiction 2021, 19, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.P.; Rebelo, J.S.; Dionisio, F.; Botelho, A.; Nogueira, T. 2020. The social distancing imposed to contain COVID-19 can affect our microbiome: a double-edged sword in human health. Msphere 2020, 5, e00716–20. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, HS.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. The most important challenges ahead of microbiome pattern in the post era of the COVID-19 pandemic. J. Diabetes Metab. Disord. 2020, 19, 2031–2033. [Google Scholar] [CrossRef]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N.; Gros, P. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. U S A. 2021, 118, e2010217118. [Google Scholar] [CrossRef]
- Bacorn, M.; Romero-Soto, H.N.; Levy, S.; Chen, Q.; Hourigan, S.K. The Gut Microbiome of Children during the COVID-19 Pandemic. Microorganisms 2022, 10, 2460. [Google Scholar] [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front. Cell. Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef] [PubMed]
- k Ghezzi, L.; Cantoni., C.; Rotondo, E.; Galimberti, D. The Gut Microbiome-Brain Crosstalk in Neurodegenerative Diseases. Biomedicines 2022, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Hashish, S.; Salama, M. The Role of an Altered Gut Microbiome in Parkinson’s Disease: A Narrative Review. Appl. Microbiol. 2023, 3, 429–447. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Coleman, M.E.; Dietert, R.R.; North, D.W.; Stephenson, M.M. Enhancing Human Superorganism Ecosystem Resilience by Holistically ‘Managing Our Microbes’. Appl. Microbiol. 2021, 1, 471–497. [Google Scholar] [CrossRef]
- Nirmalkar, K.; Qureshi, F.; Kang, D.W.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Shotgun Metagenomics Study Suggests Alteration in Sulfur Metabolism and Oxidative Stress in Children with Autism and Improvement after Microbiota Transfer Therapy. Int. J. Mol. Sci. 2022, 23, 13481. [Google Scholar] [CrossRef]
- Thirion, F.; Speyer, H.; Hansen, T.H.; Nielsen, T.; Fan, Y.; Le Chatelier, E.; Fromentin, S.; Berland, M.; Plaza Oñate, F.; Pons, N.; et al. Alteration of Gut Microbiome in Patients With Schizophrenia Indicates Links Between Bacterial Tyrosine Biosynthesis and Cognitive Dysfunction. Biol. Psychiatry Glob. Open Sci. 2022, 3, 283–291. [Google Scholar] [CrossRef]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Guijarro, L.G.; Lahera, G.; Monserrat, J.; Valls, P.; Mora, F.; Rodríguez-Jiménez, R.; et al. Gut Microbiota Metabolites in Major Depressive Disorder-Deep Insights into Their Pathophysiological Role and Potential Translational Applications. Metabolites 2022, 12, 50. [Google Scholar] [CrossRef]
- Gkougka, D.; Mitropoulos, K.; Tzanakaki, G.; Panagouli, E.; Psaltopoulou, T.; Thomaidis, L.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Gut microbiome and attention deficit/hyperactivity disorder: a systematic review. Pediatr. Res. 2022, 92, 1507–1519. [Google Scholar] [CrossRef]
- Dietert, R.R. 2021. The microbiological basis of human superorganism freedom. Am. J. Biomed. Sci. Res. 2021 13, 653-662. Available online: https://biomedgrid.com/fulltext/volume13/the-microbiological-basis-of-human-superorganism-freedom.001933.php.
- Dietert, R.R.; Dietert, J.M. Strategies For Protecting Your Child's Immune System: Tools for Parents and Parents-To-Be. 1st ed.; World Scientific Publishing. Singapore, Singapore, 2010; pp. 1–26. Available online: https://www.worldscientific.com/worldscibooks/10.1142/7444#t=aboutBook.
- Dietert, R.R.; Dietert, J.M. The Completed Self: An Immunological View of the Human-Microbiome Superorganism and Risk of Chronic Diseases. Entropy 2012, 14, 2036–2065. [Google Scholar] [CrossRef]
- Dietert, R.R. A Focus on Microbiome Completeness and Optimized Colonization Resistance in Neonatology. NeoReviews 2018, 19, e78–e88. [Google Scholar] [CrossRef]
- Dietert, R.R. Microbiome First Medicine in Health and Safety. Biomedicines 2021, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Rossini, V.; Tolosa-Enguis, V.; Frances-Cuesta, C. Sanz, Y. Gut microbiome and anti-viral immunity in COVID-19. Crit. Rev. Food Sci. Nutr. 2022, 16, 1–16. [Google Scholar] [CrossRef]
- Merra, G.; Capacci, A.; Cenname, G.; Esposito, E.; Dri, M.; Di Renzo, L.; Marchetti. M. The "Microbiome": A Protagonist in COVID-19 Era. Microorganisms 2022, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Mercader Rubio, I.; Sánchez-López, P.; Ángel, N.G.; Ruiz, N.F.O. Psychological Consequences of Fear of COVID-19: Symptom Analysis of Triggered Anxiety and Depression Disorders in Adolescents and Young Adults. Int. J. Environ. Res. Public Health 2022, 19, 14171. [Google Scholar] [CrossRef] [PubMed]
- Rania, N.; Coppola, I. The fear of contagion and the attitude toward the restrictive measures imposed to face COVID-19 in Italy: The psychological consequences caused by the pandemic one year after it began. Front. Psychol. 2022, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Savastano, S. 2021. Fear of contagion: one of the most devious enemies to fight during the COVID-19 pandemic. Disaster Med. Public Health Prep. 2021, 15, e8–e9. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.L.; Gewirtz, A.T. Microbiota as a potentially-modifiable factor influencing COVID-19. Curr. Opin. Virol. 2021, 49, 21–26. [Google Scholar] [CrossRef]
- Di Stadio, A.; Costantini, C.; Renga, G.; Pariano, M.; Ricci, G.; Romani, L. The microbiota/host immune system interaction in the nose to protect from COVID-19. Life 2020, 10, 345. [Google Scholar] [CrossRef]
- Smith, A.P.; Williams, E.P.; Plunkett, T.R.; Selvaraj, M.; Lane, L.C.; Zalduondo, L.; Xue, Y.; Vogel, P.; Channappanavar, R.; Jonsson, C.B.; et al. Time-Dependent Increase in Susceptibility and Severity of Secondary Bacterial Infections During SARS-CoV-2. Front. Immunol. 2022, 13, 894534. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.P.; Ferreira, D.M.; Smits, H.H. Microbiota and compartment matter in the COVID-19 response. Nat. Immunol. 2021, 22, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Weinberger, D.M.; Satzke, C. 2023. Thematic issue on bacterial–viral co-infections. FEMS Microbes 2023, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Schippa, S.; Frassanito, A.; Marazzato, M.; Nenna, R.; Petrarca, L.; Neroni, B.; Bonfiglio, G.; Guerrieri, F.; Frasca, F.; Oliveto, G.; et al. Nasal microbiota in RSV bronchiolitis. Microorganisms 2020, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. 2017. Secondary bacterial infections associated with influenza pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef]
- Prasso, J.E.; Deng, J.C. Postviral complications: bacterial pneumonia. Clin. Chest Med. 2017, 38, 127–138. [Google Scholar] [CrossRef]
- Tilocca, B.; Soggiu, A.; Musella, V.; Britti, D.; Sanguinetti, M.; Urbani, A.; Roncada, P. Molecular basis of COVID-19 relationships in different species: a one health perspective. Microbes Infect. 22, 218–220. [CrossRef]
- Fahkrajang, W.; Sudaryatma, P.E.; Mekata, H.; Hamabe, S.; Saito. A.; Okabayashi, T. Bovine respiratory coronavirus enhances bacterial adherence by upregulating expression of cellular receptors on bovine respiratory epithelial cells. Vet. Microbiol. 2021, 255, 109017. [Google Scholar] [CrossRef]
- Frucchi, A.P.S.; Dall Agnol, A.M.; Bronkhorst, D.E.; Beuttemmuller, E.A.; Alfieri, A.A.; Alfieri, A.F. Bovine coronavirus co-infection and molecular characterization in dairy calves with or without clinical respiratory disease. Front. Vet. Sci. 2022, 9, 895492. [Google Scholar] [CrossRef]
- Nefedova, E.; Koptev, V.; Bobikova, A.S.; Cherepushkina, V.; Mironova, T.; Afonyushkin, V.; Shkil, N.; Donchenko, N.; Kozlova, Y.; Sigareva, N.; Davidova, N. The infectious bronchitis coronavirus pneumonia model presenting a novel insight for the SARS-CoV-2 dissemination route. Vet. Sci. 2021, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.H.; Cook, J.K.; Parsell, Z.E. The experimental infection of chickens with mixtures of infectious bronchitis virus and Escherichia coli. J. Gen. Virol. 1985, 66, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Cook, J.K.; Frazier, J.A.; Narita, M. Escherichia coli multiplication and lesions in the respiratory tract of chickens inoculated with infectious bronchitis virus and/or E. coli. Avian Dis. 1992, 881–890. [Google Scholar] [CrossRef]
- Hoerr, F.J. The pathology of infectious bronchitis. Avian Dis. 2021, 65, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Dudoignon, E.; Caméléna, F.; Deniau, B.; Habay, A.; Coutrot, M.; Ressaire, Q.; Plaud, B.; Berçot, B.; Dépret, F. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin. Infect. Dis. 2021, 72, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Dela Cruz, C.S.; Sharma, L. Coronaviruses, Lysosomes, and Secondary Bacterial Infections: Coronaviruses Outsmart the Host. DNA Cell Biol. 2023, 42, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Finn, T.; Babushkin, F.; Geller, K.; Alexander, H.; Shapiro, M.; Uda, M.; Mostrchy, A.R.; Amash, R.; Shimoni, Z.; Paikin, S. High rate of bacterial respiratory tract co-infections upon admission amongst moderate to severe COVID-19 patients. Infect. Dis. 2022, 54, 134–144. [Google Scholar] [CrossRef]
- Weidmann, M.D.; Berry, G.J.; Zucker, J.E.; Huang, S.; Sobieszczyk, M.E.; Green, D.A. Bacterial Pneumonia and Respiratory Culture Utilization among Hospitalized Patients with and without COVID-19 in a New York City Hospital. J. Clin. Microbiol. 2022, 60, e00174–22. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Stessel, B.; and Dubois, J. Secondary infection in COVID-19 critically ill patients: a retrospective single-center evaluation. BMC Infect. Dis. 2022, 22, 207. [Google Scholar] [CrossRef]
- Velásquez-Garcia, L.; Mejia-Sanjuanelo, A.; Viasus, D.; Carratalà, J. Causative Agents of Ventilator-Associated Pneumonia and Resistance to Antibiotics in COVID-19 Patients: A Systematic Review. Biomedicines 2022, 10, 1226. [Google Scholar] [CrossRef]
- Ortega-Peña, S.; Rodríguez-Martínez, S.; Cancino-Diaz, M.E.; Cancino-Diaz, J.C. Staphylococcus epidermidis Controls Opportunistic Pathogens in the Nose, Could It Help to Regulate SARS-CoV-2 (COVID-19) Infection? Life 2022, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Raineri, E.J.; Altulea, D.; van Dijl, J.M. Staphylococcal trafficking and infection—from ‘nose to gut’and back. FEMS Microbiol. Rev. 2022, 46, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Khadka, S.; Sato, F.; Omura, S.; Fujita, M.; Hashiwaki, K.; Tsunoda, I. Bacterial and fungal isolation from face masks under the COVID-19 pandemic. Sci. Rep. 2022, 12, 11361. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, P., Masiuk, H., Kulig, P., Skoryk, A., Wcisłek, A., Jursa-Kulesza, J., Sarna, A., Sławiński, M., Kotowski, M., Tejchman, K. and Kotfis, K. Medical Face Masks Do Not Affect Acid–Base Balance Yet Might Facilitate the Transmission of Staphylococcus aureus in Hospital Settings during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2023, 20, 2474. [CrossRef]
- Kwon, M.; Yang, W. Effects of face masks and acoustical environments on speech recognition by preschool children in an auralised classroom. Appl. Acoust. 2023, 202, 109149. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Using microbiome-based approaches to deprogram chronic disorders and extend the healthspan following adverse childhood experiences. Microorganisms 2022, 10, 229. [Google Scholar] [CrossRef]
- Beckers, T.; Hermans, D.; Lange, I.; Luyten, L.; Scheveneels, S.; Vervliet, B. Understanding clinical fear and anxiety through the lens of human fear conditioning. Nat. Rev. Psychol. 2023, 2, 233–245. [Google Scholar] [CrossRef]
- Mertens, G.; Lodder, P.; Smeets, T.; Duijndam, S. Pandemic panic? Results of a 14-month longitudinal study on fear of COVID-19. J. Affect. Disord. 2023, 322, 15–23. [Google Scholar] [CrossRef]
- Hauck, A.; Michael, T.; Ferreira de Sá, D.S. Fear learning and generalization during pandemic fear: How COVID-19-related anxiety affects classical fear conditioning with traumatic film clips. J. Psychiatr. Res. 2022, 155, 90–99. [Google Scholar] [CrossRef]
- Cannito, L.; Di Crosta, A.; Palumbo, R.; Ceccato, I.; Anzani, S.; La Malva, P.; Palumbo, R.; Di Domenico, A. Health anxiety and attentional bias toward virus-related stimuli during the COVID-19 pandemic. Sci. Rep. 2020, 10, 16476. [Google Scholar] [CrossRef]
- Meaklim, H.; Saunders, W.J.; Byrne, M.L.; Junge, M.F.; Varma, P.; Finck, W.A.; Jackson, M.L. Insomnia is a key risk factor for persistent anxiety and depressive symptoms: A 12-month longitudinal cohort study during the COVID-19 pandemic. J. Affect. Disord. 2023, 322, 52–62. [Google Scholar] [CrossRef]
- Dietert, R.R.; DeWitt, J.C.; Germolec, D.R.; Zelikoff., J.T. Breaking patterns of environmentally influenced disease for health risk reduction: immune perspectives. Environ. Health Perspect. 2010, 118, 1091–1099. [Google Scholar] [CrossRef]
- Lerouge, R., Lema, M.D. and Arnaboldi, M., 2023. The role played by government communication on the level of public fear in social media: An investigation into the Covid-19 crisis in Italy. Government Information Quarterly 2023, p.101798. [CrossRef]
- Feng, P.; Chen, Z.; Becker, B.; Liu, X.; Zhou, F.; He, Q.; Qiu, J.; Lei, X.; Chen, H.; Feng, T. Predisposing Variations in Fear-Related Brain Networks Prospectively Predict Fearful Feelings during the 2019 Coronavirus (COVID-19) Pandemic. Cereb. Cortex. 2022. 32. 540-553. [CrossRef]
- Józefacka, N.M., Karpiński, E.A., Superson, B., Kołek, M.F., Skrzypczak, A.R. and Kania, G., 2023. Potential Factors Conditioning the Compliance to Mandatory Face Covering in the Public Space Due to SARS-CoV-2 Pandemic. Int. J. Environ. Res. Public Health 2023, 20, 726. [CrossRef]
- Altintas, E., El Haj, M., Boudoukha, A.H., Olivier, C., Lizio, A., Luyat, M. and Gallouj, K., 2022. Emotional exhaustion and fear of COVID-19 in geriatric facilities during the COVID-19 pandemic. Int. J. Geriatr. Psychiatry 2022, 37. [CrossRef]
- Baldi, E.; Savastano, S. Fear of Contagion: One of the Most Devious Enemies to Fight During the COVID-19 Pandemic. Disaster Med. Public Health. Prep. 2021, 15, e8–e9. [Google Scholar] [CrossRef]
- Muñoz-Vela, F.J.; Rodríguez-Díaz, L.; Gómez-Salgado, J.; Fernández-Carrasco, F.J.; Allande-Cussó, R.; Vázquez-Lara, J.M.; Fagundo-Rivera, J. Fear and Anxiety in Pregnant Women During the COVID-19 Pandemic: A Systematic Review. Int. J. Public Health 2023, 68, 1605587. [Google Scholar] [CrossRef]
- Albikawi, Z.F. Fear Related to COVID-19, Mental Health Issues, and Predictors of Insomnia among Female Nursing College Students during the Pandemic. Healthcare 2023, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Kerker, B.D.; Willheim, E.; Weis, J.R. The COVID-19 pandemic: implications for maternal mental health and early childhood development. Am. J. Health Promot. 2023, 37, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Shechner, T.; Hong, M.; Britton, J.C.; Pine, D.S.; Fox, N.A. Fear conditioning and extinction across development: evidence from human studies and animal models. Biological Psychol. 2014, 100, 1–12. [Google Scholar] [CrossRef]
- Salinas-Hernández, X.I.; and Duvarci, S. 2021. Dopamine in fear extinction. Front. Synaptic Neurosci. 2021, 13, 635879. [Google Scholar] [CrossRef] [PubMed]
- Furini, C.; Myskiw, J.; Izquierdo, I. The learning of fear extinction. Neurosci. Biobehav. Rev. 47, 670–683. [CrossRef] [PubMed]
- Leblanc, H.; Ramirez, S. Linking Social Cognition to Learning and Memory. J. Neurosci. 2020, 40, 8782–8798. [Google Scholar] [CrossRef]
- Donato, F.; Alberini, C.M.; Amso, D.; Dragoim, G.; Dranovskym, A.; Newcombe, N.S. The Ontogeny of Hippocampus-Dependent Memories. J. Neurosci. 2021, 41, 920–926. [Google Scholar] [CrossRef]
- Sosa, M.; Giocomo, L.M. Navigating for reward. Nat. Rev. Neurosci. 2021, 22, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Takehara-Nishiuchi, K. Neurobiology of systems memory consolidation. Eur. J. Neurosci. 2021, 54, 6850–6863. [Google Scholar] [CrossRef]
- Banquet, J.P.; Gaussier, P.; Cuperlier, N.; Hok, V.; Save, E.; Poucet, B.; Quoy, M.; Wiener, S.I. Time as the fourth dimension in the hippocampus. Prog. Neurobiol. 2021, 199, 101920. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.Z. From structure to behavior in basolateral amygdala-hippocampus circuits. Front. Neural Circuits 2017, 11, 86. [Google Scholar] [CrossRef]
- Gage, N.M.; Baars, B.J. 2018. Humans Are Social Beings. Fundamentals of Cognitive Neuroscience: A Beginners Guide, 2nd ed.; Academic Press: Cambridge, Massachusetts, USA, 2018; pp. 321–356. [Google Scholar]
- Tang, W.; Meng, Z.; Li, N.; Liu, Y.; Li, L.; Chen, D.; Yang, Y. 2021. Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and hippocampus-dependent behaviors. Front. Cell. Infect. Microbiol. 2021, 10, 611014. [Google Scholar] [CrossRef]
- Sharvin, B.L.; Aburto, M.R.; Cryan, J.F. Decoding the neurocircuitry of gut feelings: Region-specific microbiome-mediated brain alterations. Neurobiol. Dis. 2023, 179, 106033. [Google Scholar] [CrossRef]
- Laudani, S.; Torrisi, S.A.; Alboni, S.; Bastiaanssen, T.F.; Benatti, C.; Rivi, V.; Moloney, R.D.; Fuochi, V.; Furneri, P.M.; Drago, F.; Salomone, S. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav. Immun. 2023, 107, 385–396. [Google Scholar] [CrossRef]
- Swer, N.M.; Venkidesh, B.; Murali, T.S.; et al. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef]
- Gacias, M.; Gaspari, S.; Santos, P.M.G.; Tamburini, S.; Andrade, M.; Zhang, F.; Shen, N.; Tolstikov, V.; Kiebish, M.A.; Dupree, J.L.; and Zachariou, V. 2016. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife 2016, 5, e13442. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; and Cong, Y. 2021. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Garcia-Reyero, N.; Martyniuk, C.J.; Tubbs, C.W.; and Bisesi Jr, J.H. Regulation of endocrine systems by the microbiome: perspectives from comparative animal models. Gen. Comp. Endocrinol. 2020, 2020 292, 113437. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Ho, Y.S.; and Chang, R.C.C. Linking circadian rhythms to microbiome-gut-brain axis in aging-associated neurodegenerative diseases. Ageing Res. Rev. 2022, 78, 101620. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022, 35, e00338–20. [Google Scholar] [CrossRef]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef]
- Fox, M.; Lee, S.M.; Wiley, K.S.; Lagishetty, V.; Sandman, C.A.; Jacobs, J.P.; Glynn, L.M. Development of the infant gut microbiome predicts temperament across the first year of life. Dev. Psychopathol. 2022, 34, 1914–1925. [Google Scholar] [CrossRef]
- Lynch, C.M.; Cowan, C.S.; Bastiaanssen, T.F.; Moloney, G.M.; Theune, N.; van de Wouw, M.; Zanuy, E.F.; Ventura-Silva, A.P.; Codagnone, M.G.; Villalobos-Manríquez, F.; et al. Critical windows of early-life microbiota disruption on behaviour, neuroimmune function, and neurodevelopment. Bran, Behav. Immun. 2023, 108, 309–327. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Deepika, A.K. Shukla; Kumari, A.; and Kumar, A. Gut brain regulation using psychobiotics for improved neuropsychological illness. Developmental Psychobiology, 2023, 65, e22404. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Bastiaanssen, T.F.; Moloney, G.M.; Boscaini, S.; Strain, C.R.; Anesi, A.; Long-Smith, C.; Mattivi, F.; Stanton, C.; Clarke, G.; Dinan, T.G. Feed your microbes to deal with stress: A psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol. Psychiatry 2023, 28, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Karbowiak, M.; and Brzezicka, A. The Role of Psychobiotics to Ensure Mental Health during the COVID-19 Pandemic—A Current State of Knowledge. International Journal of Environmental Research and Public Health, 19(17), p.11022. Int. J. Environ. Res. Public Health 2022, 19, 11022. [Google Scholar] [CrossRef] [PubMed]
- Dursun, P.; Alyagut, P.; Yılmaz, I. Meaning in life, psychological hardiness and death anxiety: individuals with or without generalized anxiety disorder (GAD). Curr. Psychol. 2022, 41, 3299–3317. [Google Scholar] [CrossRef] [PubMed]
- Sangha, S.; Diehl, M.M.; Bergstrom, H.C.; Drew, M.R. 2020. Know safety, no fear. Neurosci. Biobehav. Rev. 2020, 108, 218–230. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, Z.S.; Milad, M.R. Fear extinction learning modulates large-scale brain connectivity. Neuroimage 2021, 238, 118261. [Google Scholar] [CrossRef]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Lach, G.; Fülling, C.; Bastiaanssen, T.F.; Fouhy, F.; Donovan, A.N.O.; Ventura-Silva, A.P.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry 2020, 10, 382. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, S.; Xia, G.; Chen, J.; Jiang, L.; Huang, J.; and Tong, J. A multispecies probiotic accelerates fear extinction and inhibits relapse in mice: role of microglia. Neuropharmacology 2021, 193, 108613. [Google Scholar] [CrossRef]
- Chu, C.; Murdock, M.H.; Jing, D.; Won, T.H.; Chung, H.; Kressel, A.M.; Tsaava, T.; Addorisio, M.E.; Putzel, G.G.; Zhou, L.; Bessman, N.J. The microbiota regulate neuronal function and fear extinction learning. Nature 2019, 574, 543–548. [Google Scholar] [CrossRef]
- Cowan, C.S.; Stylianakis, A.A.; Richardson, R. Early-life stress, microbiota, and brain development: probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev. Cogn. Neurosci. 2019, 37, 100627. [Google Scholar] [CrossRef]
- Fox, J.H.; Hassell Jr, J.E.; Siebler, P.H.; Arnold, M.R.; Lamb, A.K.; Smith, D.G.; Day, H.E.; Smith, T.M.; Simmerman, E.M.; Outzen, A.A.; et al. Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain Behave. Immun. 2017, 66, 70–84. [Google Scholar] [CrossRef]
- Maeng, L.Y.; and Beumer, A. Never fear, the gut bacteria are here: Estrogen and gut microbiome-brain axis interactions in fear extinction. Int. J. Psychophysiol. 2023, 189, 66–75. [Google Scholar] [CrossRef]
- Chbeir, S.; Carrión, V. 2023. Resilience by design: How nature, nurture, environment, and microbiome mitigate stress and allostatic load. World J. Psychiatry 2023, 13, 144. [Google Scholar] [CrossRef]
- Tyagi, P.; Tasleem, M.; Prakash, S.; Chouhan, G. Intermingling of gut microbiota with brain: Exploring the role of probiotics in battle against depressive disorders. Food Research International 2020, 137, 109489. [Google Scholar] [CrossRef]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef]
- Kumar, A.; Pramanik, J.; Goyal, N.; Chauhan, D.; Sivamaruthi, B.S.; Prajapati, B.G.; Chaiyasut, C. 2023. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals 2023, 16, 565. [Google Scholar] [CrossRef]
- Lebois, L.A.; Seligowski, A.V.; Wolff, J.D.; Hill, S.B.; Ressler, K.J. Augmentation of extinction and inhibitory learning in anxiety and trauma-related disorders. Annu. Rev. Clin. Psychol. 2019, 15, 257–284. [Google Scholar] [CrossRef]
- Berding, K.; and Cryan, J.F. Microbiota-targeted interventions for mental health. Curr. Opin. Psychiatry 2022, 35, 3. [Google Scholar] [CrossRef]
- Sherwin, E., Bordenstein, S.R., Quinn, J.L., Dinan, T.G. and Cryan, J.F., 2019. Microbiota and the social brain. Science 2019, 366, eaar2016. [CrossRef]
- Maren, S.; Holmes, A. Stress and Fear Extinction. Neuropsychopharmacol. 2016, 41, 58–79. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Seo, J.; Bottary, R. The influence of sleep on fear extinction in trauma-related disorders. Neurobiol. Stress 2023, 22, 100500. [Google Scholar] [CrossRef]
- Wilson, D.R.; Binford, L.; Hickson, S. The Gut Microbiome and Mental Health. J. Holist Nurs. 2023, Apr 20:8980101231170487. [CrossRef]
- Cooper, S.E.; Dunsmoor, J.E. 2021. Fear conditioning and extinction in obsessive-compulsive disorder: a systematic review. Neurosci. Biobehav. Rev. 2021, 129, 75–94. [Google Scholar] [CrossRef]
- Zuj, D.V.; Palmer, M.A.; Lommen, M.J.; Felmingham, K.L. The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neurosci. Biobehav. Rev. 2016, 69, 15–35. [Google Scholar] [CrossRef]
- Cohn, M.D.; van Lith, K.; Kindt, M.; Pape, L.E.; Doreleijers, T.A.; van den Brink, W.; Veltman, D.J.; Popma, A. Fear extinction, persistent disruptive behavior and psychopathic traits: fMRI in late adolescence. Soc. Cogn. Affect. Neurosci. 2016, 11, 1027–1035. [Google Scholar] [CrossRef]
- Muench, C.l.; Charlet, K.; Balderston, N.L.; Grillon, C.; Heilig, M.; Cortes, C.R.; Momenan, R.; Lohoff, F.W. Fear conditioning and extinction in alcohol dependence: evidence for abnormal amygdala reactivity. Addict. Biol. 2021, 26, e12835. [Google Scholar] [CrossRef]
- Spencer, A.E.; Marin, M.F.; Milad, M.R.; Spencer, T.J.; Bogucki, O.E.; Pope, A.L.; Plasencia, N.; Hughes, B.; Pace-Schott, E.F.; Fitzgerald, M.; et al. Abnormal fear circuitry in attention deficit hyperactivity disorder: a controlled magnetic resonance imaging study. Psychiatry Research: Neuroimaging 2017, 262, 55–62. [Google Scholar] [CrossRef]
- Craske, M.G.; Sandman, C.F.; Stein, M.B. 2022. How can neurobiology of fear extinction inform treatment? Neurosci. Biobehav. Rev. 2022, 143, 104923. [Google Scholar] [CrossRef]
- Luijten, M.A.; van Muilekom, M.M.; Teela, L.; Polderman, T.J.; Terwee, C.B.; Zijlmans, J.; Klaufus, L.; Popma, A.; Oostrom, K.J.; van Oers, H.A.; Haverman, L. The impact of lockdown during the COVID-19 pandemic on mental and social health of children and adolescents. Qual. Life Res. 30, 2795–2804. [CrossRef]
- Panchal, U.; Salazar de Pablo, G.; Franco, M.; Moreno, C.; Parellada, M.; Arango, C.; Fusar-Poli, P. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur. Child Adolesc. Psychiatry 2021, 1–27. [Google Scholar] [CrossRef]
- Cellini, N.; Di Giorgio, E.; Mioni, G.; Di Riso, D. Sleep and psychological difficulties in Italian school-age children during COVID-19 lockdown. J. Pediatr. Psychol. 2021, 46, 153–167. [Google Scholar] [CrossRef]
- Christner, N.; Essler, S.; Hazzam, A.; Paulus, M. Children’s psychological well-being and problem behavior during the COVID-19 pandemic: An online study during the lockdown period in Germany. PloS One 2021, 16, e0253473. [Google Scholar] [CrossRef]
- Amicucci, G.; Salfi, F.; D’Atri, A.; Viselli, L.; Ferrara, M. The differential impact of COVID-19 lockdown on sleep quality, insomnia, depression, stress, and anxiety among late adolescents and elderly in Italy. Brain Sci. 2021, 11, 1336. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Using the Internet of Microbes to Survive the Assault on the Human Microbiome. Am. J. Biomed. Biol. Res. 2023, 19, 71–76. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Ford, M.; Bonomo, R.A.; Gamal, A.; McCormick, T.S. A microbiome-driven approach to combating depression during the COVID-19 pandemic. Front. Nutr. 2021, 8, 576. [Google Scholar] [CrossRef]
- Athanassi, A.; Dorado Doncel, R.; Bath, K.G.; Mandairon, N. Relationship between depression and olfactory sensory function: a review. Chem. Senses 2021, 46, 1–12. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Jayakody, D.M.; Bennett, R.J.; Eikelboom, R.H.; Gasson, N.; Friedland, P.L. Hearing loss and depression in older adults: a systematic review and meta-analysis. Gerontologist 2020, 60, e137–e154. [Google Scholar] [CrossRef]
- Hur, K.; Choi, J.S.; Zheng, M.; Shen, J.; Wrobel, B. Association of alterations in smell and taste with depression in older adults. Laryngoscope Investing. Otolaryngol. 2018, 3, 94–99. [Google Scholar] [CrossRef]
- Vithoulkas, G.; Muresanu, D.F. Conscience and consciousness: a definition. J. Med. Life 2014, 7, 104–108. [Google Scholar]
- Dietert, R.R. Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering. Biomedicines 2021, 9, 1581. [Google Scholar] [CrossRef]
- Werber, Y.; Natan, E.; Lavner, Y.; and Vortman, Y. Antibiotics affect migratory restlessness orientation. J. Ethol. 2022, 40, 175–180. [Google Scholar] [CrossRef]
- Simon, R.A.; Ranasinghe, P.D.; Barazanji, N.; Jungeström, M.B.; Xu, J.; Bednarska, O.; Serrander, L.; Engström, M.; Bazylinski, D.A.; Keita, Å.V.; Walter, S. Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain. J. of Oceanol. Limnol. 2021, 39, 2044–2052. [Google Scholar] [CrossRef]
- Schamarek, I.; Anders, L.; Chakaroun, R.M.; Kovacs, P.; Rohde-Zimmermann, K. The role of the oral microbiome in obesity and metabolic disease: potential systemic implications and effects on taste perception. Nutr. J. 2023, 22, 1–13. [Google Scholar] [CrossRef]
- Leung, R.; and Covasa, M. Do gut microbes taste? Nutrients 2021, 13, 2581. [Google Scholar] [CrossRef]
- Doyle, M.E.; Premathilake, H.U.; Yao, Q.; Mazucanti, C.H.; and Egan, J.M. 2023. Physiology of the tongue with emphasis on taste transduction. Physiol. Rev. 2023, 103, 1193–1246. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Chaiyasut, C. Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review. Microorganisms 2022, 10, 1405. [Google Scholar] [CrossRef]
- López-Dávalos, P.C.; Requena, T.; Pozo-Bayón, M.Á.; and Muñoz-González, C. Decreased retronasal olfaction and taste perception in obesity are related to saliva biochemical and microbiota composition. Food Research International 2023, 167, 112660. [Google Scholar] [CrossRef]
- Kociszewska, D.; Chan, J.; Thorne, P.R.; and Vlajkovic, S.M. The link between gut dysbiosis caused by a high-fat diet and hearing loss. Int. J. of Mol. Sci. 2021, 22, 13177. [Google Scholar] [CrossRef]
- Kociszewska, D.; and Vlajkovic, S. 2022. Age-related hearing loss: the link between inflammaging, immunosenescence, and gut dysbiosis. International Journal of Molecular Sciences 2022, 23, 7348. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.M.; Son, H.S.; Yoon, Y.N.; Shin, J.E.; Sul, W.J.; Yu, D.; Choe, Y.B.; Lee, Y.W. Analysis of the Microbiome of the Ear Canal in Normal Individuals and Patients with Chronic Otitis Externa. Ann. Dermatol. 2022, 34, 461–471. [Google Scholar] [CrossRef]
- Jörissen, J.; van den Broek, M.F.; De Boeck, I.; Van Beeck, W.; Wittouck, S.; Boudewyns, A.; Van de Heyning, P.; Topsakal, V.; Van Rompaey, V.; Wouters, I.; and Van Heirstraeten, L. Case-Control Microbiome Study of Chronic Otitis Media with Effusion in Children Points at Streptococcus salivarius as a Pathobiont-Inhibiting Species. Msystems 2021, 6, e00056–21. [Google Scholar] [CrossRef]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 1–16. [Google Scholar] [CrossRef]
- Keum, H.L.; Kim, H.; Kim, H.J.; Park, T.; Kim, S.; An, S.; Sul, W.J. Structures of the skin microbiome and mycobiome depending on skin sensitivity. Microorganisms 2020, 8, 1032. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Olunoiki, E.; Rehner, J.; Bischoff, M.; Koshel, E.; Vogt, T.; Reichrath, J.; Becker, S.L. Characteristics of the Skin Microbiome in Selected Dermatological Conditions: A Narrative Review. Life 2022, 12, 1420. [Google Scholar] [CrossRef]
- Zysset-Burri, D.C.; Morandi, S.; Herzog, E.L.; Berger, L.E.; Zinkernagel, M.S. The role of the gut microbiome in eye diseases. Progr. Retin. Eye Res. 2023, 92, 101117. [Google Scholar] [CrossRef]
- Peter, V.G.; Morandi, S.C.; Herzog, E.L.; Zinkernagel, M.S.; Zysset-Burri, D.C. Investigating the Ocular Surface Microbiome: What Can It Tell Us? Clin. Ophthalmol. 2023, 17, 259–271. [Google Scholar] [CrossRef]
- Aragona, P.; Baudouin, C.; Del Castillo, J.M.B.; Messmer, E.; Barabino, S.; Merayo-Lloves, J.; Brignole-Baudouin, F.; Inferrera, L.; Rolando, M.; Mencucci, R.; Rescigno, M. The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders. Surv. Ophthalmol. 2021, 66, 907–925. [Google Scholar] [CrossRef]
- Borroni, D.; Paytuví-Gallart, A.; Sanseverino, W.; Gómez-Huertas, C.; Bonci, P.; Romano, V.; Giannaccare, G.; Rechichi, M.; Meduri, A.; Oliverio, G.W.; et al. Exploring the healthy eye microbiota niche in a multicenter study. Int. J. Mol. Sci. 2022, 23, 10229. [Google Scholar] [CrossRef]
- An, Q.; Zou, H. Ocular surface microbiota dysbiosis contributes to the high prevalence of dry eye disease in diabetic patients. Crit. Rev. Microbiol. 2022, 1–10. [Google Scholar] [CrossRef]
- Deng, Y.; Ge, X.; Li, Y.; Zou, B.; Wen, X.; Chen, W.; Lu, L.; Zhang, M.; Zhang, X.; Li, C.; Zhao, C. Identification of an intraocular microbiota. Cell Discov. 2021, 7, 13. [Google Scholar] [CrossRef]
| Group Studied | Findings | Reference(s) |
|---|---|---|
| Italian government and media |
Messaging emphasizing life-changing scenarios produced the greatest fear among the public. The researchers emphasized that government must balance the level of fear with proper causation or risk public psychological harm. | [65] |
|
Study of international population with the majority being European young adults. |
Found that those who consulted regular media, professional websites, and social media for additional pandemic information experienced the greatest fear. |
[60] |
| Study of Chinese population using pre-pandemic MRIs compared with later pandemic fear responses. |
Study revealed severe impact resulting from fear of contagion during the pandemic. The pre-pandemic neural connectome pattern could predict those who would experience the most pandemic fear. | [66] |
| An online study with majority female participants. |
Researchers observed a significant moderate positive correlation between anxiety around Covid-19 and adherence to the mandates. | [67] |
| Study of young adult university students in Germany | Researchers found that those students with high COVID-Anxiety exhibited poorer discrimination performance between fear and safety cues. |
[61] |
| International study with 2,069 majority female participants |
Pre-existing or new-onset insomnia elevated the risk of affective disorder outcomes (anxiety and/or depression symptoms) during the pandemic. |
[63] |
| Study included 132 Italian individuals with the majority female. |
Researchers found that higher health anxiety in general predicted attentional bias toward a fear of Covid-19 contagion. | [62] |
| Study of 118 healthcare workers in French geriatric facilities | Researchers found a significant increase in emotional exhaustion among the workers and this was related to both increased demands at work and increased fear of contagion. | [68] |
| Review concerning Milan Italy region fear of hospital contagion and reduction in available emergency services for cardiac events |
Discussion of the high rate of acute coronary syndrome observed out of the hospital with low hospital emergency service use | [69] |
| Review of research studies on fear and anxiety among pregnant women during the Covid-19 pandemic |
The researchers reported a high prevalence of fear and anxiety with significant impact on mental health. Intolerance of uncertainty was identified as one of the risk factors. | [70] |
| A study of Covid-19 fear and mental health issues among 154 female nursing students in Saudi Arabia |
Fear of Covid-19 contagion, depression, anxiety and insomnia were highly prevalent among the students with fear of contagion the most prevalent (79.3%). | [71] |
| Review article focused on highly vulnerable populations for pandemic fear-based mental health challenges | Women and young children were identified as highly vulnerable with mandates such as social isolation and school closures exacerbating fear-based anxiety and depression. | [72] |
| Study | Significant Findings | References(s) |
|---|---|---|
| Early study on germ-free and specific pathogen free rats | Absence of a microbiome produced HPA axis disruption, brain function changes and significantly heightened anxiety behavior and lack of cognitive flexibility. | [105] |
| Research on mice concerning the neurobehavioral effects of transitory microbiome depletion in different age groups. |
Adolescent mice were more sensitive to transitory microbiome depletion than adults. Short-term depletion produced long-lasting shifts in fear-based learning, heightened anxiety-like behaviors and changes in amygdala gene expression in adolescents. | [106] |
| Study examined the effects of multi-species probiotic (Bifidobacterium longum Lactobacillus acidophilus and Enterococcus faecalis) administration on recovery of fear memory after fear conditioning |
Probiotic administration in mice modulated fear conditioning-induced microbial dysbiosis, promoted long-term fear extinction, alleviated hippocampal synapse loss induced by fear conditioning, and limited microglia activation |
[107] |
| Study in mice examined the role of microbiota in fear extinction |
Researchers found that antibiotic treatment of adult mice resulting in microbiome dysbiosis produced impaired fear extinction. They also found that extinction learning and learning-related plasticity require microbiota-derived signals. Microbial deficits in early life produced deficits in fear extinction learning in adulthood. | [108] |
| Study of maternal separation stress in rats and protective effects of probiotics |
Researchers found that maternally-separated male rat pups experienced inappropriate, accelerated development of fear circuitry/behavior and that probiotics protected against unbalanced fear. | [109] |
| Study of the effects of the environmental/soil bacterium, Mycobacterium vaccae, on fear extinction in adult rats |
Researchers found that injection with M. vaccae preparations produced long-lasting enhancement of the rate of within-session fear extinction. | [110] |
| Review of the relationship between gut microbiota, fear extinction, and mental illness with an emphasis on sex-based differences in microbiota. |
Researchers stressed that female stress-related, mental illness is significantly more prevalent than that in men. They emphasized the importance of sex hormones in the gut microbiota-brain regulation, gut microbiota differences based on sex and the need for female focused studies. | [111] |
| Review of gut microbiota functionality of brain regions | Effects of antibiotics and probiotics on fear extinction is included in this recent review | [84] |
| Review concerning how nature, nurture, and microbiota mitigate stress. |
This review includes information on microbiota and fear extinction within the broader subject | [112] |
| Review of probiotics as anti-anxiety and anti-depression psychobiotics |
This review emphasizes specific beneficial microbial metabolism | [113] |
| Review of the microbiota-gut-brain axis in depression | This review emphasizes pathophysiological mechanisms and processes through which microbiota have an anti-depressant function. | [114] |
| Comprehensive review of gut microbiota in anxiety and depression |
This review considers a variety of strategies for gut rebiosis as an anti-anxiety, anti-depression tool. | [115] |
| Title 1 | Title 2 | Title 3 |
|---|---|---|
| Obsessive-Compulsive Disorder | Systematic review revealing importance of defective fear extinction | [122] |
| Posttraumatic Stress Disorder | Review of fear extinction as a predictor of PTSD | [123] |
| Disruptive Behavior Disorder | Fear extinction deficits reported to be involved in late adolescent endotype. | [124] |
| Alcohol Dependency | Fear conditioning and extinction with a focus on the role of amygdalain FCE-involved addiction | [125] |
| Attention Deficit Hyperactivity Disorder | Abnormal circuits for fear extinction detected in adults with ADHD | [126] |
| Anxiety Disorders | Focus on neurobiology of fear extinction for treatment of anxiety disorders | [127] |
| Sense | Findings | Reference(s) |
|---|---|---|
| Taste | Review including the role of the microbiome in regulation of taste | [139] |
| Taste | Recent review of the role of oral microbiota in taste perception | [142] |
| Taste | A review detailing specific bacteria and their metabolism as it influences taste perception. The review also covered gut microbiome dysbiosis and linked pathologies. | [143] |
| Taste | Review of the tongue including the role of the tongue microbiome in taste perception. | [144] |
| Smell | Review including the role of the microbiome in regulation of smell | [139] |
| Smell | Recent review describing the pivotal role of nasal microbiota in olfactory development, function, and dysfunctions. | [145] |
| Taste and Smell | Human study reported that obese population displayed reductions in odor and taste preferences some of which were related to oral microbiota difference. | [146] |
| Hearing |
Reviews provide evidence supporting a link between gut microbiome dysbiosis, inner ear inflammation, and sensorineural hearing loss. | [147,148] |
| Hearing | Study of the ear canal microbiota from healthy individuals vs. those with chronic otitis externa | [149] |
| Hearing | Study of 70 otitis media effusion children and two control groups revealed Streptococcus salivarius as a commensal with effective colonization resistance capacity against several key pathobionts. | [150] |
| Neuropathic Pain (including |
Review of microbiome regulation of neuropathic pain | [151] |
| Somatosensory function) | ||
| Skin Sensitivity Syndrome |
Study examining the skin microbiome/mycobiome of 23 patients with sensitivity syndrome compared against control groups. | [152] |
| Skin (sensitivity) | Review covering most aspects of the skin microbiome including both intrinsic and extrinsic factors. | [153] |
| Skin (sensitivity) | Review of skin microbiome differences among healthy vs. diseased/sensitive skin | [154] |
| Sight | Review of dysbiotic gut microbiome-driven eye diseases (age-related macular degeneration, retinal artery occlusion, central serous chorioretinopathy and uveitis) via microbial metabolites and the immune system |
[155] |
| Sight | Introduction to the recently discovered ocular surface microbiome. | [156] |
| Sight | Review of the role of the ocular microbiome in eye disease | [157] |
| Sight | Study of the characterization of healthy eye microbiomes | [158] |
| Sight | Study describes the role of specific ocular surface microbiota in contributing to dry eye disease in diabetic patients. | [159] |
| Sight | Review of intraocular microbiota | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




