Submitted:
01 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of MnO2@NiCo-LDH Nanosheet Arrays
2.2. Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
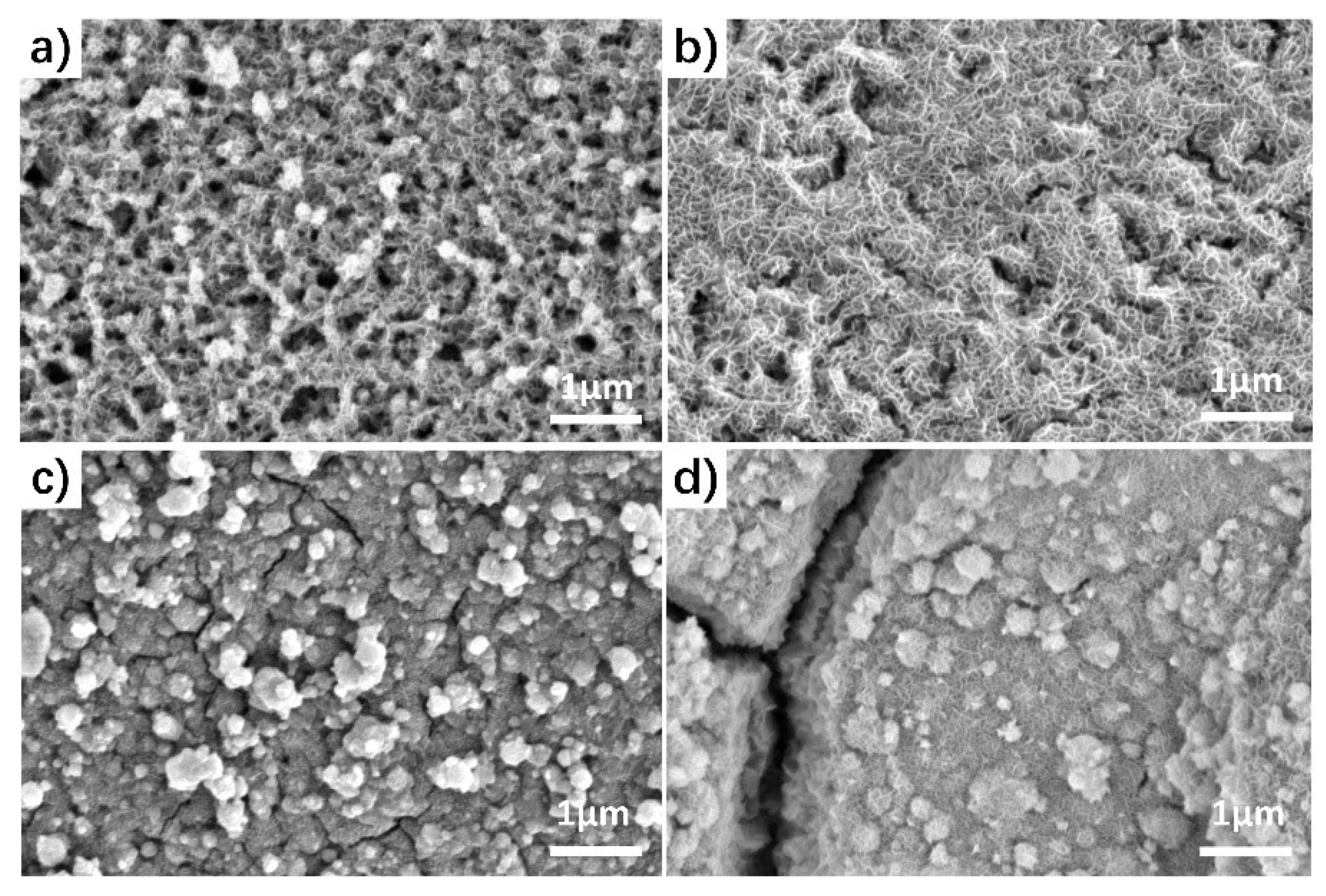
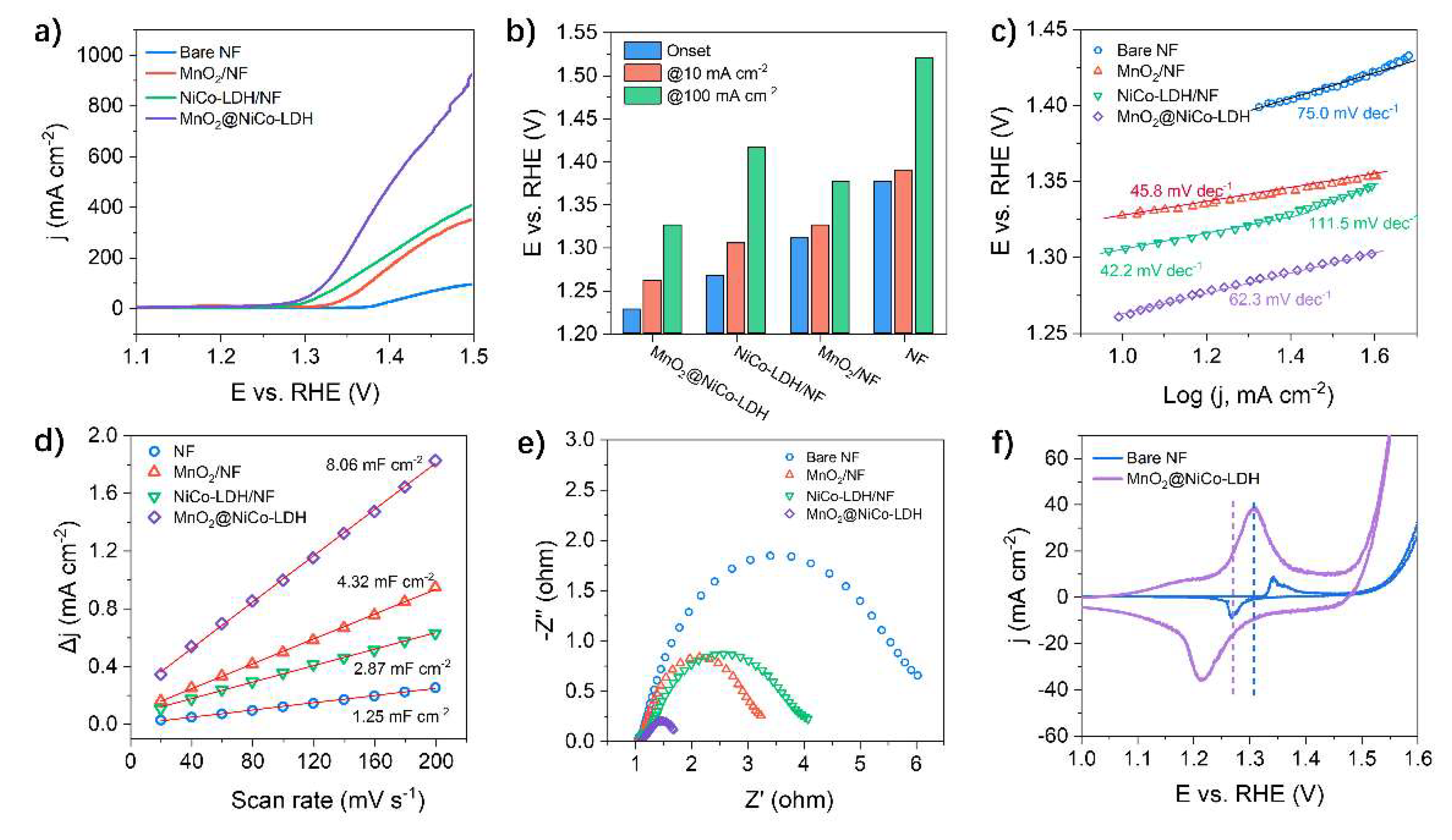
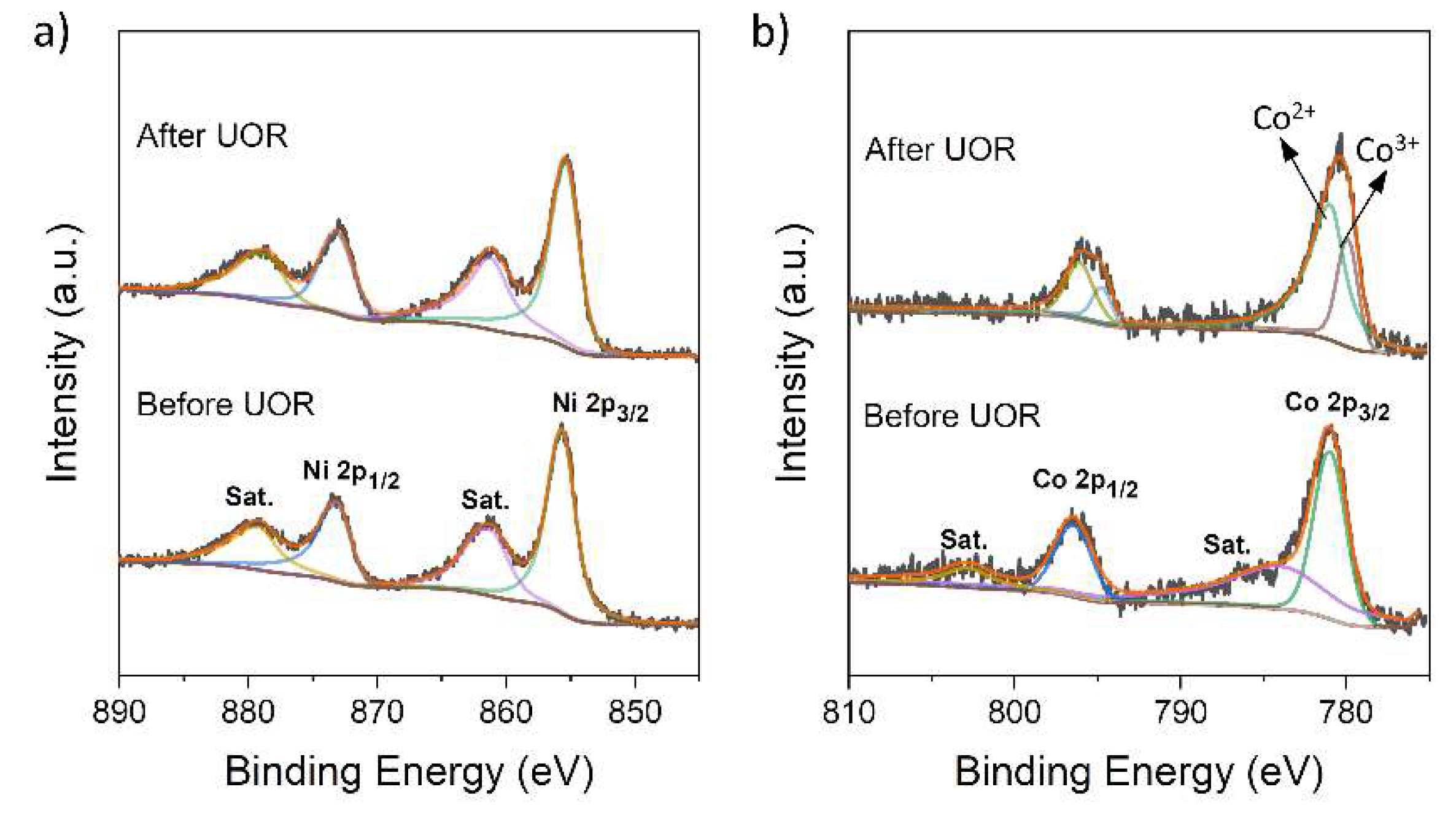
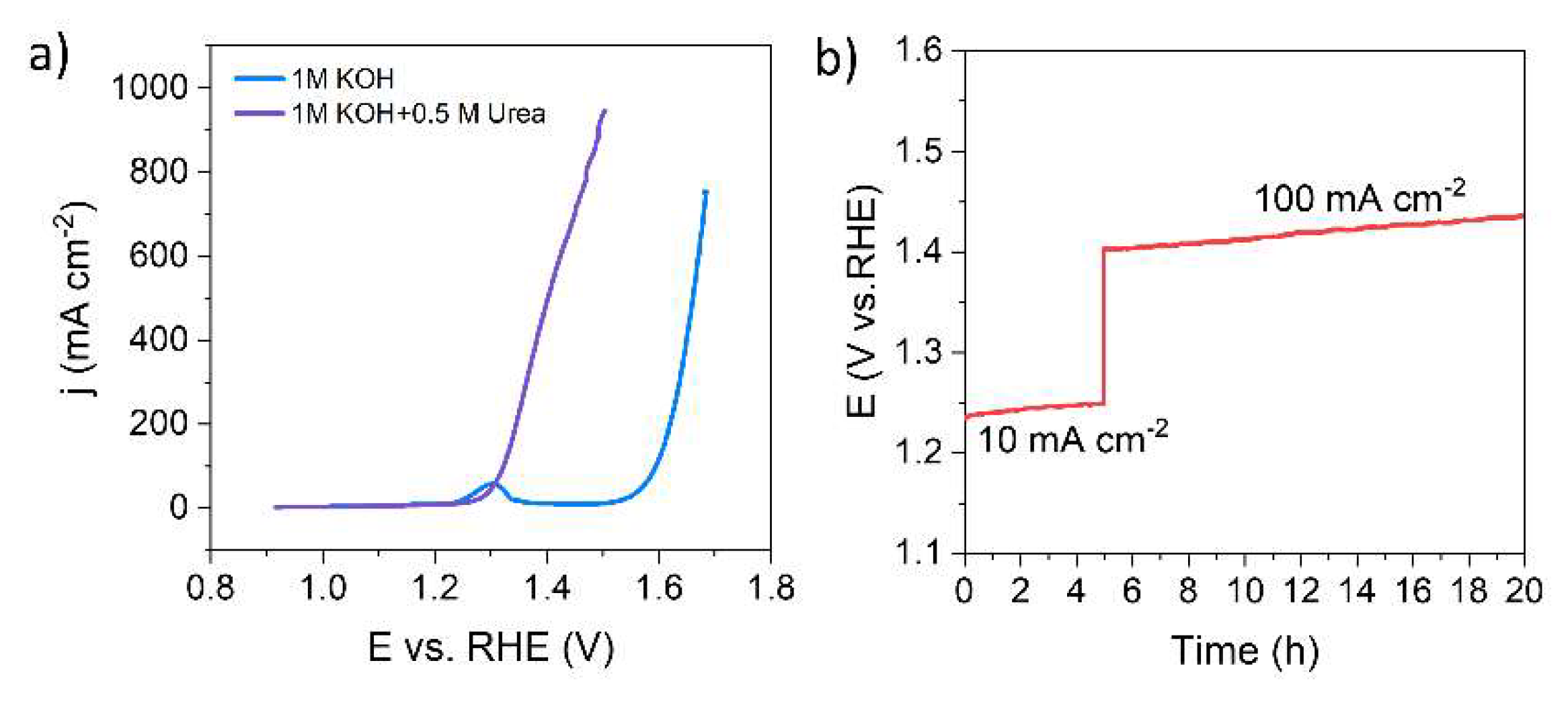
3.1. Fabrication and Electrocatalytic Performance of MnO2/NF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Taibi, E.; Miranda, R.; Vanhoudt, W.; Winkel, T.; Lanoix, J.-C.; Barth, F. Hydrogen from Renewable Power: Technology Outlook for the Energy Transition Available online: https://www.h2knowledgecentre.com/content/policypaper1306.
- Shih, A.J.; Monteiro, M.C.O.; Dattila, F.; Pavesi, D.; Philips, M.; da Silva, A.H.M.; Vos, R.E.; Ojha, K.; Park, S.; van der Heijden, O.; et al. Water Electrolysis. Nat. Rev. Methods Primer 2022, 2, 1–19. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Duan, Y.; Feng, X.-Y.; Yu, X.; Gao, M.-R.; Yu, S.-H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-Hydrogen Energy Conversion Based on Water Splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Xiao, Z.; Qian, Y.; Tan, T.; Lu, H.; Liu, C.; Wang, B.; Zhang, Q.; Sarwar, M.T.; Gao, R.; Tang, A.; et al. Energy-Saving Hydrogen Production by Water Splitting Coupling Urea Decomposition and Oxidation Reactions. J. Mater. Chem. A 2022, 11, 259–267. [Google Scholar] [CrossRef]
- Geng, S.-K.; Zheng, Y.; Li, S.-Q.; Su, H.; Zhao, X.; Hu, J.; Shu, H.-B.; Jaroniec, M.; Chen, P.; Liu, Q.-H.; et al. Nickel Ferrocyanide as a High-Performance Urea Oxidation Electrocatalyst. Nat. Energy 2021, 6, 904–912. [Google Scholar] [CrossRef]
- Qin, H.; Ye, Y.; Li, J.; Jia, W.; Zheng, S.; Cao, X.; Lin, G.; Jiao, L. Synergistic Engineering of Doping and Vacancy in Ni(OH)2 to Boost Urea Electrooxidation. Adv. Funct. Mater. 2023, 33, 2209698. [Google Scholar] [CrossRef]
- Ji, Z.; Song, Y.; Zhao, S.; Li, Y.; Liu, J.; Hu, W. Pathway Manipulation via Ni, Co, and V Ternary Synergism to Realize High Efficiency for Urea Electrocatalytic Oxidation. ACS Catal. 2022, 12, 569–579. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, Q.; Wang, X.; Shao, Z.; Yao, L.; Zeng, H.; Wu, X.; Chen, J.; Huang, K.; Feng, S. Formation and Stabilization of NiOOH by Introducing α-FeOOH in LDH: Composite Electrocatalyst for Oxygen Evolution and Urea Oxidation Reactions. Adv. Mater. 2023, 35, 2209338. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J.; Kim, H.; Song, S.; Fei, L.; Hu, Z.; Lin, H.-J.; Chen, C.-T.; Ciucci, F.; Jung, W. Ni-Doped CuO Nanoarrays Activate Urea Adsorption and Stabilizes Reaction Intermediates to Achieve High-Performance Urea Oxidation Catalysts. Adv. Sci. 2022, 9, 2204800. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Xu, W.-B.; Han, S.; Cao, P.; Xu, W.; Zhu, D.; Lu, Y.; Liu, W. Enhanced Urea Oxidization Electrocatalysis on Spinel Cobalt Oxide Nanowires via on-Site Electrochemical Defect Engineering. Mater. Chem. Front. 2021, 5, 3717–3724. [Google Scholar] [CrossRef]
- Fang, M.; Xu, W.-B.; Shen, Y.; Cao, P.; Han, S.; Xu, W.; Zhu, D.; Lu, Y.; Liu, W. Electrochemical Tuning of Nickel Molybdate Nanorod Arrays towards Promoted Electrocatalytic Urea Oxidization. Appl. Catal. Gen. 2021, 622, 118220. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, P.; Zhang, M.; Zhou, T.; Zhang, L.; Chu, W.; Wu, C.; Xie, Y. Oxygen Vacancies Confined in Nickel Molybdenum Oxide Porous Nanosheets for Promoted Electrocatalytic Urea Oxidation. ACS Catal. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Wang, X.-H.; Hong, Q.-L.; Zhang, Z.-N.; Ge, Z.-X.; Zhai, Q.-G.; Jiang, Y.-C.; Chen, Y.; Li, S.-N. Two-Dimensional Nickel–Cobalt Bimetallic Hydroxides towards Urea Electrooxidation. Appl. Surf. Sci. 2022, 604, 154484. [Google Scholar] [CrossRef]
- Li, D.; Zhou, X.; Liu, L.; Ruan, Q.; Zhang, X.; Wang, B.; Xiong, F.; Huang, C.; Chu, P.K. Reduced Anodic Energy Depletion in Electrolysis by Urea and Water Co-Oxidization on NiFe-LDH: Activity Origin and Plasma Functionalized Strategy. Appl. Catal. B Environ. 2023, 324, 122240. [Google Scholar] [CrossRef]
- Song, W.; Xu, M.; Teng, X.; Niu, Y.; Gong, S.; Liu, X.; He, X.; Chen, Z. Construction of Self-Supporting, Hierarchically Structured Caterpillar-like NiCo2S4 Arrays as an Efficient Trifunctional Electrocatalyst for Water and Urea Electrolysis. Nanoscale 2021, 13, 1680–1688. [Google Scholar] [CrossRef]
- Wang, P.; Bai, X.; Jin, H.; Gao, X.; Davey, K.; Zheng, Y.; Jiao, Y.; Qiao, S.-Z. Directed Urea-to-Nitrite Electrooxidation via Tuning Intermediate Adsorption on Co, Ge Co-Doped Ni Sites. Adv. Funct. Mater. 2023, 33, 2300687. [Google Scholar] [CrossRef]
- Fang, M.; Han, D.; Xu, W.-B.; Shen, Y.; Lu, Y.; Cao, P.; Han, S.; Xu, W.; Zhu, D.; Liu, W.; et al. Surface-Guided Formation of Amorphous Mixed-Metal Oxyhydroxides on Ultrathin MnO2 Nanosheet Arrays for Efficient Electrocatalytic Oxygen Evolution. Adv. Energy Mater. 2020, 10, 2001059. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Hang, X.; Zhang, X.; Xie, J.; Pan, B.; Xie, Y. Half-Metallicity in Single-Layered Manganese Dioxide Nanosheets by Defect Engineering. Angew. Chem. Int. Ed. 2015, 54, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Vedharathinam, V.; Botte, G.G. Direct Evidence of the Mechanism for the Electro-Oxidation of Urea on Ni(OH)2 Catalyst in Alkaline Medium. Electrochimica Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the Electro-Catalytic Oxidation Mechanism of Urea on Nickel Electrodes in Alkaline Medium. Electrochimica Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




