1. Introduction
ATC is a rare cancer with an incidence far below six new cases per 100,000 person- years. Indeed, recent epidemiological studies have confirmed an age-adjusted incidence in the US of 0.12 per 100,000 person-years and 0.1 to 0.3 per 100,000 person- years in Europe. There seems to be an average annual percent increase of respectively 3.0% and 1.3% per year over a 30–40-year period of time. Incidence rates are consistent across the different studies in Europe and US. The increase in incidence is unlikely to be related to better screening/diagnosis because all ATC patients end up being diagnosed with cervical compressive symptoms [
1].
ATC is rare but serious with a poor prognosis that has improved over the last twenty years by a better understanding of its molecular mechanisms and the development of targeted therapies. However, the delay of treatment determines the prognosis and it is important to diagnose it and refer the patient to an expert center as soon as possible.
It usually presents as a rapidly enlarging goiter with compressive signs but may sometimes be accompanied by symptoms of thyroiditis mimicking subacute thyroiditis (SAT), leading to a delay in final diagnosis that may be detrimental to prognosis [
2].
In addition, the advent of the COVID-19 pandemic has been accompanied by new clinical entities and atypical clinical presentations of common pathologies, increasing the likelihood of diagnostic delays.
We present herein the case of a 60-year-old male patient who, one month after a COVID 19 infection, developed a clinical presentation of SAT that turned out to be a rapidly fatal ATC. We report how the presence of some atypia allowed a management that we considered optimal despite the lethal outcome.
2. Case Report
We report the case of a 60-years -old male patient who had previous history of type 2 diabetes mellitus and who presented with a right thyroid nodule, which was scored EU-TIRADS 4 , and Bethesda 2 by using two FNABs (in 2016 and 2017). This nodule was identified later on as an autonomously functioning nodule on a I-123 scintigraphy performed , due to a low TSH serum level (in 2019).
He contracted COVID-19 acute infection in April 2021. One month later, he presented with fever, palpitations, a loss of 2 kg and a firm and painful goiter. Blood tests showed inflammatory syndrome with elevated CRP and hyperleukocytosis. TSH was 0.13 U/L, increased FT4 was increased at 24.4 pmol/L (N<21.9) and FT3 was at the upper range of 6.3 pmol/L (N<6.4). The FT4/FT3> 3 molar ratio was in favor of a cytolytic process and the main hypothesis was subacute thyroiditis (SAT) triggered by a COVID-19 infection as anti-TPO and antithyroglobulin autoantibodies were negative.
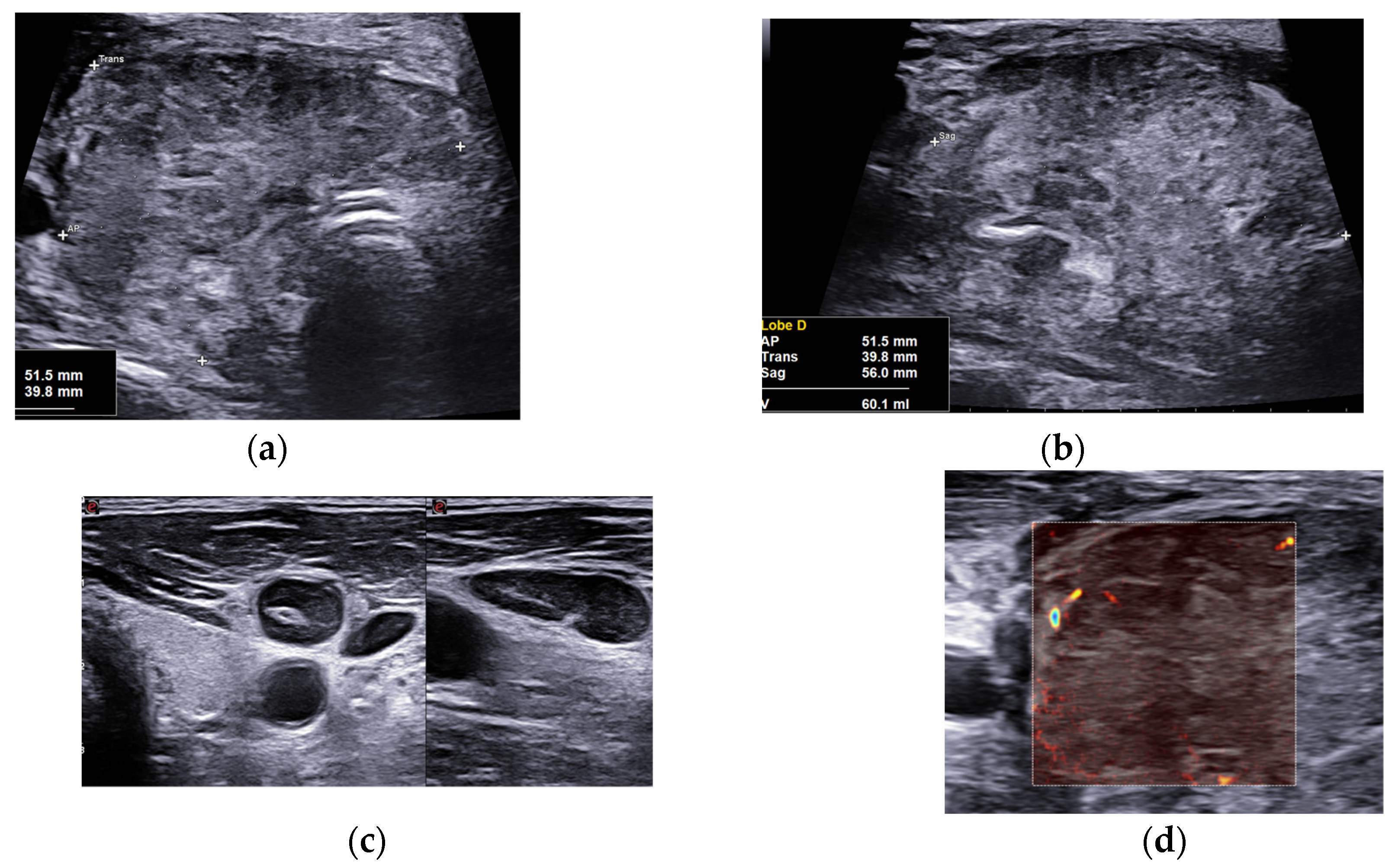
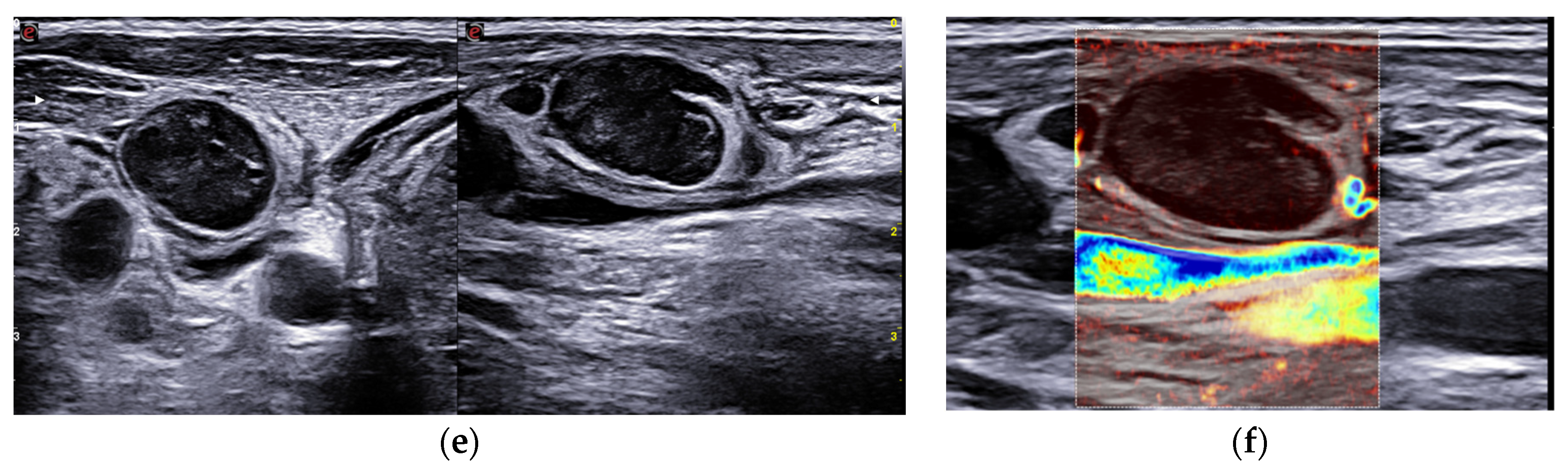
We performed, as usual in a thyroidology consultation, a cervical ultrasound which found some elements suggestive of SAT: normal thyroid parenchyma with a normoechoic and homogeneous left lobe estimated at 5.2 cm3 and a right lobe which volume was increased at 60.1 cm3, uniformly hard in elastography (about 220 KPa). However, it showed atypical features such as a very heterogeneous echostructure, with hypoechoic and normoechoic spots, and irregular and spiculated margins without extrathyroidal spread (
Figure 1). It also showed large, round and homogeneous lymphadenopathies with preserved hilum and central vascularization in right and left sectors III and IV (
Figure 1).
The hypothesis of atypical lymphoma and poorly differentiated carcinoma was also evoked and a core-needle biopsy (CNB) of the right-lobe mass and a fine-needle aspiration biopsy (FNAB) of the largest lymphadenopathy was performed for cytological and biochemical study (in situ thyroglobulin wash out). A cervico-thoracic CT-Scan looked for chest lymphadenopathies and lung parenchymal nodules. The patient was prescribed beta-blockers for his thyrotoxicosis and NSAI in order to manage pain (corticosteroids were ruled out due to diabetes). A surgical biopsy was thus planned.
At day 15, the mass volume and aspect were unchanged. Thyroid status spontaneously improved. LDH was increased to 239 (N<213).
The CT scan revealed an infiltration of the thyroid right lobe and isthmus with respect of the left lobe. It also showed an infiltration of the para-tracheo-esophageal and pre-vertebral spaces, lymphadenopathies of the right sectors III, IV and Vb and of the left sectors III and IV. CT disclosed a 7mm lateral peripheral solid macronodule of the right lower lobe, and another 7mm nodule, discretely spiculated in the left inferior lobe.
Core-needle US-guided microbiopsy of the mass revealed complete rearrangement of thyroid tissue with fibrosis, necrotic spots and inflammatory infiltrate of heterogeneous density including neutrophils, lymphoplasmacytic elements and some pseudo-epithelioid cells. Rare thyroid dystrophic vesicles were visible and marked with Cytokeratin AE1/AE3 and TTF1 (Cell Marque). Ki67 showed moderate marking. BCL6 showed nuclear marking. CD10, CD20, CD 79 were negative. CD3 showed highlighted T-cells and CD30 highlighted some activated cells. In some necrotic spots, there were slicks composed of medium-sized cells with more or less nucleoli and a moderately abundant eosinophilic cytoplasm, associated with rare mitosis. The first conclusion was in favor of aspecific reactive thyroiditis, however, final proof reading in expert center (Léon Berard, Lyon) was requested and concluded that there was no argument for lymphoma but there was indeed an unclassifiable tumor proliferation.
Cytological study of the right lymphadenopathy showed similar features with the absence of thyroglobulin and suspicious cells, misleading first to a falsely reassuring cytological diagnosis of aspecific adenitis.
At day 17, the patient was hospitalized for further investigations and, during his stay, a compressive syndrome appeared with dyspnea, dysphagia and general status alteration due to the severe dysphagia. The patient was intubated and a new CT scan found a progression of the mass with macroscopical invasion of the trachea, larynx and esophagus, a voluminous conglomerate of bilateral necrotic lymphadenopathies predominant on the right neck side., thrombosis of the right jugular vein and progression of the lung secondary lesions. Surgical biopsies of the thyroid mass were performed as well as a concomitant surgical tracheostomy. Trans-tumor drilling was mandatory to access the trachea. Antibiotics and curative anticoagulation were started to treat the internal jugular thrombosis
Surgical biopsies found a poorly differentiated malignant tumor proliferation of massive architecture, consisting of cells with poorly represented cytoplasms, poorly limited cell contours and polymorphic nuclei, large size, with an atypical and prominent nucleoli. Mitotic activity was increased with the presence of several atypical mitosis. The proliferation index with KI67 was 90% with the presence of a few foci of necrosis. Tumor cells were negative for anti CD10, thyroglobulin, INSM1, P53, TTF1, CKA1-AE3, PAX8, BCL2, Chromogranin, CD45, C-MYC, CD5, CD20, MUM1, BCL6, OCT3-4, CD34, AML, EMA, SALL4, Desmine, Calcitonin, SOX10, CD30, CD138, CD79a, HMB45, CD56, GATA3, NUT, MelanA, anti-CD3, CD43, OCT2, PAX5, Myeloperoxidase, ALK1A4, CD68 and CD4 antibodies. Labeling with anti-BRGA and INI1 antibodies were retained by tumor cells. EBER ISH was also negative. NGS analysis found no mutation. It was concluded to a malignant poorly differentiated tumor proliferation of difficult typing.
Despite palliative chemotherapy, the patient died on day 58.
Given the clinical presentation, topography and morphology of disease, and the histopathological uncertainty, our final retained diagnosis was malignant pseudothyroiditis revealing a poorly-cellular variant of anaplastic thyroid carcinoma (ATC).
3. Discussion
The concept of “malignant pseudothyroiditis” (MPT) was first introduced in 1978 by Rosen et al [
2]. Two cases of anaplastic carcinoma revealed by thyrotoxicosis mimicking subacute thyroiditis had already been described in the literature in 1966 and 1967 by Rosen et al [
3] and Mangla et al [
4] at a time when neither ultrasound nor thyroid hormone testing were available.
If we define MPT as a clinical presentation of SAT revealing infiltration of the thyroid by malignant cells, we can distinguish three categories: (a) MPT revealing a solid cancer, seventeen cases were described in the review of Jonklaas et al[
5] most of which are of breast or lung cancer origin (b) MPT revealing hematological malignancies: seven cases were described in the same review, most of which are lymphomas (c) MPT revealing anaplastic carcinoma with 12 cases described in the literature including ten in the publication of Daroszewski [
6] and one, published in 2020, by Fatula et al [
7].
One the one hand, there are many similarities between our patient and the reported cases: an age always greater than 55 years-old, an initial clinical presentation combining a clinical and biological inflammatory syndrome, a firm, painful, sometimes compressive progressively enlarging goiter, a thyrotoxicosis of clinical variable intensity and a concomitant biological profile with a high FT4/FT3 ratio in favor of a cytolytic mechanism. In all cases, anti-TPO antibodies were negative. Anti-thyroglobulin antibodies were sometimes positive but we know the limit of the pathological significance of this marker. Including our patient, the sex ratio is 8 female to 5 male, consistent with the epidemiology of ATC [
1] Mean survival was variable, depending of therapeutic response in categories (a) and (b) but still severely low in category (c) [3-7;10] In all cases, FNAB or CNB immediately confirmed the diagnosis and made it possible to adopt an adapted therapeutic strategy [3-7;10].
On the other hand, three peculiarities distinguish our patient from the data of the literature.
First, this is, to our best knowledge, the first reported case of MPT occurring in the immediate aftermath of COVID-19 infection.
In addition, the cytological and histological diagnosis was difficult despite an acute analysis by three experts. It is noted that on the first analyzes, the inflammatory contingent was so important that the hypothesis of adenitis and aspecific thyroiditis on both biopsy of the mass and on the lymph node cytology was retained. Moreover, despite the use of a panel of markers that have only been available for a few years, the diagnosis of histological certainty could not be formally made; thus full final diagnosis was made on a bundle of clinical, radiological and histological arguments.
Finally, our patient had a known EU-TIRADS 4 nodule whose benignity had been established by two FNABs performed and analyzed by expert practitionners . Moreover the diagnosis had been reinforced by the autonomous nature of the nodule established 3 years before.
This brings us to some reflexions.
Regarding the thyrotoxicosis: most authors agree that massive infiltration of the thyroid gland by malignant cells leads to cytolysis of thyrocytes with the same underlying mechanism as thyroiditis [2-7;10] thus causing the release of thyroid hormones into the blood, leading to a predominance of serum T4 level explaining the high FT4/FT3 ratio as described in SATs and painless autoimmune thyroiditis [8-9].
Some authors have hypothesized hormonal hypersecretion by malignant cells [
10] but this mechanism cannot be considered in PMT of extrathyroid origin . Moreover, anaplastic carcinoma cells are too dedifferentiated to have the appropriate enzyme equipment to do so; In our patient, all the elements we had clearly pointed out were in favor of a cytolytic process.
Regarding the genesis of this ATC: we wonder about the nature of the nodule previously described in this patient. Indeed, it has been established that ATC can result from the dedifferentiation of a neglected papillary carcinoma left in place [1;11-12]. Given the workup previously performed on this nodule, the probability of malignancy was negligible enough not to incriminate it. The chronology of events has also prompted us to question the role played by COVID-19 infection: no link has ever been described or proposed between thyroid carcinogenesis and viral infection (with COVID-19 or any other virus) [13-16] and the time to onset does not seem consistent to support the hypothesis of an initiating role. We can also raise the hypothesis of a promoting role of thyroiditis: is it thyroiditis that triggered rapid growth or is it cancer that caused cytolysis? The first hypothesis implies that the malignant cells were already present, and it has been shown that the presence of chronic thyroiditis is not associated with a different behavior of tumor cells when cancer and inflammation were diagnosed at the same time [
17] In addition, although this is a rare situation, no experimental or epidemiological data in the literature supports the role of a thyroiditis flare-up as a factor triggering the rapid growth of a thyroid tumor mass.
It is also legitimate to question the role of COVID-19 in the atypical nature of ultrasound and histological presentation. Many authors have reported cases of SATs triggered by COVID-19 infection. From the first clinical case of SAT published in May 2020 [
18], a large number of SAT cases have been progressively reported [
14] Indeed the evolution and some biological features seem different and a novel entity called COVID-19 painless SAT was described: among 6.8% of patients with COVID-19 developing SAT, those with painless SAT (n = 5) presented earlier, had more severe thyrotoxic manifestations and exhibited higher CRP, interleukin-6 (IL-6) and neutrophil-lymphocyte ratio (NLR) and lower absolute lymphocyte count than those with painful SAT [
19]. Besides, the authors reported significant correlations of IL-6 with free and total T4 and free and total T3, suggesting that destructive effects of cytokines may play a key role in painless SAT [
19].
Nevertheless, except painless SAT (which did not concern our patient), the clinical and biological presentation appears like non-COVID 19 SAT and unusual ultrasound features were not described or highlighted in the different case reports that were studied.
The histopathological findings of the thyroid gland in patients with SARS-CoV-2 infection have been published in only two studies [
20,
21]. Both studies reported lymphocytic infiltration in the interstitium. In the two patients from Hanley et al. article [
21], follicular epithelial cell disruption was also noted. However, the significance of these histopathological data regarding the thyroid gland in patients with COVID-19 remains uncertain.
4. Conclusions
In conclusion, this is the first ever reported case of MPT mimicking COVID-19 SAT. We emphasize that MPT is similar to SAT: both entities include similar medical history, clinical, biology, scintigraphy features . Indeed, only ultrasound and especially histology assessment may help to reach the correct diagnosis. In the present case, ultrasound showed atypical features that alerted the thyroidologist and led to quickly perform histological samples and accelerate patient’s management.
Some guidelines, such as in France, do not recommend to perform ultrasound in the presence of a typical SAT on the argument that MPT is a rare situation. Noteworthy, any delay in the management of such patients may seriously worsen the prognosis. This argues for the systematic realization of neck ultrasound when facing any cervical symptom or thyrotoxicosis storm. Ideally, neck US should be performed during the thyroidology consultation , and moreover FNAC/ FNAB samples should be promptly performed at the same time as well as body CT imaging prescribed, thus optimizing the patient’care.
Fristly, in front of any painful goiter, it is necessary to evoke SAT, lymphoma, hematocele and MPT and, helped by ultrasound, do not hesitate to perform an emergency microbiopsy, to provide an emergency cervico-thoracic CT scan and to send the sample and refer the patient to an expert center
Secondly, when facing patients with COVID-19 infection context, we have little perspective on its impact on the semiology of the diseases we encounter and we should be very wary of the slightest clinical, biological, ultrasound or histological atypical features in order to make the full proper diagnosis.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, methodology ,EG PYM; writing—original draft preparation, EG.; writing—review and editing, EG, PYM.; HM, TC, RG, VC contributed to data collection and patient’s management. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study.
Informed Consent Statement
Informed consent was obtained from the patient’s family.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
this article was written on the behalf of AFTHY (French Thyroid Association).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, Deschamps F, Lamartina L, Lacroix L, Dupuy C, Baudin E, Do Cao C, Hadoux J. Anaplastic Thyroid Carcinoma: An Update. Cancers (Basel). 2022 Feb 19;14(4):1061. [CrossRef] [PubMed] [PubMed Central]
- Rosen IB, Strawbridge HG, Walfish PG, Bain J. Malignant pseudothyroiditis: a new clinical entity. Am J Surg. 1978 Oct;136(4):445-9. [CrossRef] [PubMed]
- Rosen F, Row VV, Volpe R, Ezrin C. Anaplastic carcinoma of the thyroid with abnormal circulating iodoprotein: a case simulating subacute thyroiditis. Can Med Assoc J. 1966 Nov 12;95(20):1039-41.
- Mangla JC, Rastogi GK, Pathak IC. Anaplastic carcinoma of the thyroid complicating severe thyrotoxicosis. J Indian Med Assoc. 1967; 49(6), 1967.
- Jonklaas, J. Infiltration of the thyroid gland by non-thyroid malignancy: A literature review reveals this to be an unusual cause of hyperthyroidism. J Clin Transl Endocrinol. 2020 Feb 19;20:100221. [CrossRef] [PubMed Central]
- [Daroszewski J, Paczkowska K, Jawiarczyk-Przybyłowska A, Bolanowski M, Jeleń M. Anaplastic thyroid carcinoma with rapid thyrotoxicosis - a case report and the literature review. Endokrynol Pol. 2018;69(1):28-31. [CrossRef] [PubMed]
- Fatula L, Snyder RA. Rare Presentation of Anaplastic Thyroid Cancer With Thyrotoxicosis. Am Surg. 2020 May;86(5):516-518. [CrossRef] [PubMed]
- Tura Bahadır Ç, Yılmaz M, Kılıçkan E. Free triiodothyronine to free thyroxine ratio in the differential diagnosis of thyrotoxicosis and hyperthyroidism: A retrospective study. Int J Clin Pract. 2021 May;75(5):e14003. [CrossRef] [PubMed]
- Chen X, Zhou Y, Zhou M, Yin Q, Wang S. Diagnostic Values of Free Triiodothyronine and Free Thyroxine and the Ratio of Free Triiodothyronine to Free Thyroxine in Thyrotoxicosis. Int J Endocrinol. 2018 Jun 4;2018:4836736. [CrossRef] [PubMed] [PubMed Central]
- Phillips JS, Pledger DR, Hilger AW. Rapid thyrotoxicosis in anaplastic thyroid carcinoma. J Laryngol Otol. 2007 Jul;121(7):695-7. [CrossRef] [PubMed]
- Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006 Apr;13(4):453-64. [CrossRef] [PubMed]
- Ngo TNM, Le TTB, Le T, Bychkov A, Oishi N, Jung CK, Hassell L, Kakudo K, Vuong HG. Primary Versus Secondary Anaplastic Thyroid Carcinoma: Perspectives from Multi-institutional and Population-Level Data. Endocr Pathol. 2021 Dec;32(4):489-500. [CrossRef] [PubMed]
- Naguib, R. Potential relationships between COVID-19 and the thyroid gland: an update. J Int Med Res. 2022 Feb;50(2):3000605221082898. [CrossRef] [PubMed] [PubMed Central]
- Gorini F, Vassalle C. A Literature Review on SARS-CoV-2 and Other Viruses in Thyroid Disorders: Environmental Triggers or No-Guilty Bystanders? Int J Environ Res Public Health. 2023 Jan 29;20(3):2389. [CrossRef] [PubMed] [PubMed Central]
- Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2021 Dec;22(4):803-815. [CrossRef] [PubMed] [PubMed Central]
- et al. Subacute THYROiditis Related to SARS-CoV-2 VAccine and Covid-19 (THYROVAC Study): A Multicenter Nationwide Study. J Clin Endocrinol Metab. 2023 Apr 25:dgad235. [CrossRef] [PubMed]
- Grigerova M, Kasko M, Mojtova E, Takacsova E, Kralik R, Waczulikova I, Podoba J. Influence of autoimmune thyroiditis on the prognosis of papillary thyroid carcinoma. Bratisl Lek Listy. 2022;123(12):885-890. [CrossRef] [PubMed]
- Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute Thyroiditis After Sars-COV-2 Infection. J Clin Endocrinol Metab. 2020 Jul 1;105(7):dgaa276. [CrossRef] [PubMed] [PubMed Central]
- Mondal S, DasGupta R, Lodh M, Ganguly A. Subacute thyroiditis following recovery from COVID-19 infection: novel clinical findings from an Eastern Indian cohort. Postgrad Med J. 2023 Jun 15;99(1172):558-565. [CrossRef] [PubMed]
- Yao XH, TY L, ZC H, et al. Histopathological study of new coronavirus pneumonia (COVID-19) in three patients. Chin J Pathol 2020;49.
- Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 Oct;1(6):e245-e253. [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).