1. Introduction
Volatile Organic Compounds (VOCs) are toxic gases present in ambient air because of natural resources and human activities, such as the transportation sector or industrial processes.
Several materials have been synthetized for VOCs detection such as activated carbons [
1], zeolites [
2] and metal oxide materials [
3]. Activated carbons are useful materials for VOCs detection due to their microporous structure, large surface area and fast adsorption capability [
1,
4]. However, these materials reveal experimental problems such as their high flammability [
5] and difficulty of regeneration [
6] that restrain their use in such applications. Alternatively, zeolite materials seems to be useful candidates for VOCs detection thanks to their high specific area, non-toxic behavior and high thermal stability [
7,
8]. Nevertheless, zeolite are hydrophilic and thus need a surface treatment to turn hydrophobic [
9]. Furthermore, the detection of VOCs using zeolite materials requires a compact Gas Chromatograph (GC) system to analyze the gaseous effluent desorbed from the zeolite by thermal heating [
10]. Numerous other studies focused on the sorption of toluene and benzene on metal oxide materials such as ZnO and WO
3 [
11,
12]. A good sensitivity has been obtained towards benzene and toluene detection with a fast response. Nevertheless, these materials have shown poor selectivity against other VOCs such as acetone and ethanol, except for high operating temperatures [
13,
14]. On the other hand, metal oxide materials show an important sensitivity to water vapor that highly reduce their sensitivity towards VOCs [
15].
Finally, organic polymers represent a very competitive class of materials for VOCs detection, for instance polyisobutylene (PIB) and polybutadiene (PBD), or organosilicon (SiOCH) polymers like polydimethylsiloxane (PDMS) and poly(methylphenylsiloxane) [
16,
17,
18]. Organosilicon polymers present a high affinity to VOCs and especially to benzene, toluene, ethylbenzene and xylene denoted as BTEX. These polymers are widely used in chromatographic columns for VOCs detection. They have received a remarkable attention for VOCs sensing applications due to their swelling effect upon VOCs absorption [
19,
20]. In addition, they are non-toxic [
21], thermally and chemically stable [
22] with high surface hydrophobicity, which is important for the detection of non-polar molecules such as BTEX [
23]. More particularly, organosilicon materials prepared by Plasma-Enhanced Chemical Vapor Deposition (PECVD) are currently used in NEMS (Nano Electro Mechanical Devices) devices for organic vapor detection not only because of their high affinity towards the target molecules, but also because they equilibrate more rapidly and reversibly with the sorbed species than bulk polymers due to their micrometric thickness [
24]. Sabahy
et al. [
25] have studied PECVD-deposited SiOCH thin films for BTEX sensing application. By varying the precursors and plasma deposition conditions i.e. plasma power, monomer flux and O
2 flow rate, different SiOCH chemical compositions have been synthesized and the effects of the chemical bonds on toluene sorption (as BTEX representative) has been investigated. The authors have shown that SiOSi bonds play the major role in toluene adsorption due to the free volumes created by these bonds in the films, either issued from the SiOSi chains flexibility or directly related to the high methyl bonds proportion. In addition, they have concluded that Si-OH bonds are detrimental to toluene sorption. This result can be related to water vapor adsorption on the films surface after deposition favored by the polarity of the Si-OH bonds preventing toluene adsorption. The impact of Si-(CH
3) bonds has also been studied. High concentration of Si-CH
3 bonds is detrimental to toluene adsorption, however a compromise between low Si-CH
3 concentration and films hydrophobicity is necessary. An optimized composition of the plasma-SiOCH thin film could be obtained with a higher affinity towards BTEX than conventional polymers. Finally, Boutamine
et al. [
26] have elaborated plasma polymerized thin films from HMDSO and investigated VOCs sorption using a quartz crystal microbalance (QCM). The affinity toward VOCs such as ethanol, methanol and chloroform has been correlated to the material surface hydrophobicity, the molecules size and to the free volumes within the films network.
In the present work, we aim at elaborating performant materials for VOCs detection that can be used in the manufacturing of portable gravimetric sensors being competitive in term of compactness when compared to gas chromatograph system (GC). As previously mentioned, due to their well-known low thickness, hydrophobicity, non-toxicity, thermal stability, and high affinity to VOCs, plasma polymerized organosilicon thin films appear as perfect candidates for the targeted application.
In a recent paper [
27] we have thoroughly investigated morphological and structural characterizations of plasma polymerized (PP-) organosilicon materials prepared in a microwave plasma discharge from HMDSO. The characterization of the bulk chemical composition studied by FTIR spectroscopy and quantitative 29Si solid state NMR has revealed a high amount of SiOC
3 termination in the films deposited under soft plasma conditions (low plasma energy) due to the low monomer fragmentation. Moreover, linear SiO
2C
2 chains that are close to PDMS chains were present in the material bulk deposited under soft plasma conditions. However, under hard plasma conditions (high plasma energy), the high monomer fragmentation has reduced the amount of SiOC
3 terminations and favored the introduction of carbon/hydrogen atoms into the material bulk leading to the formation of new chemical bonds such as Si-O-C, Si-CH
2-Si and Si-H. Furthermore, the amount of the linear SiO
2C
2 chains has decreased in the material bulk at hard plasma conditions. These major evolutions have introduced chemical and structural disorder in the bulk of the elaborated films. The surface chemical composition detected using a combination between X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) calculations has shown also a chemical modification while increasing the plasma energy. In fact, the number of SiOC
3 unit have increased and the amount of the SiO
2C
2 units have decreased. In addition, the formation of new chemical bonds on the surface such as Si-CH
2-Si, Si-H, Si-O-C and Si-OH, has been confirmed leading to some disorder in the chains formation. Furthermore, the hydrophobicity of the films has been investigated; the PP-HMDSO films have shown a hydrophobic character especially at low plasma energy (close to 103°). As a conclusion of this previous paper, the films deposited under soft plasma conditions have shown a quite similarity to conventional PDMS. On the contrary, the films elaborated under hard plasma conditions have lost their PDMS-like character and changed to more hybrid structures due to the new bond formation and enhancement of the structural disorder in the films.
In the present study, some selected films among the previously prepared SiOCH plasma polymerized thin films are more deeply characterized by Energy dispersive X-ray spectroscopy (EDX) for the elemental composition, X-ray reflectometry (XRR) for the density, and ellipsometry for the film thickness and refractive index. We have decided to select films synthesized in a finer W/F range (5 W/sccm to 20 W/sccm) for which the influence of the chemical composition modification on sorption properties is more pronounced. The deposition conditions of the three samples WF5, WF10 and WF20 are reproduced from [
27] and presented in
Table 1. The thicknesses of the samples are different from those presented in the previous work due to a different deposition duration. In order to study the affinity of the synthetized films towards VOCs, quartz crystal microbalance (QCM) and ellipsometry, both coupled with gas sorption techniques, are used. Finally, the impact of the chemical composition on the affinity towards VOCs is analyzed leading to the identification of an optimized SiOCH film for VOCs gravimetric sensing applications.
2. Materials and Methods
EDX (Silicon Drift Detector (SDD), X-MaxN, Oxford Instrument) analyses were performed in order to obtain the elemental composition of the PP-HMDSO films.
X-ray reflectometry analysis (XRR) were carried out using a Bruker D8 diffractometer with Cu Kα1 (λ = 0.154 nm) radiation. Standard θ−2θ scans for the data collections were taken from 2θ = 0 to 4° with an angular resolution of 0.002°. XRR measurements allowed the determination of the electronic density (ρ
e) from the limit angle of total reflection (critical angle) Ɵ
c, using (1):
where
λ is the wavenumber and
r0 is the electron radius.
The elemental composition determined by EDX and the electronic density calculated by XRR analyses lead to the mass density (ρ
m) of the PP-HMDSO films using (2):
where
Cj is the percentage of an element j in the PP-HMDSO chemical composition,
Zj its atomic number,
Aj its atomic mass and
NA the Avogadro number.
The error on the density value is estimated to be ± 0.03 g/cm3. This error is due to the zero-point error (sample height and/or goniometer) and the unknown hydrogen content which is needed to convert the electron density to the mass density.
Thin films refractive indexes and thicknesses were determined by spectroscopic ellipsometry (Semilab GES5E) at atmospheric pressure. The angle of incidence was fixed at 70° and the collected data range was from E = 1.24 eV to E = 4.25 eV. In the article, the refractive index was given at λ = 633 nm but the data were fitted in the full light range using a composition of Cauchy transparent dispersion law and a Lorentz absorption term towards the UV range. The fit quality exceeds 0.99 in the full range for all analyses.
In situ ellipsometry coupled to sorption measurements [
28] were carried out by exposing PP-HMDSO thin films to toluene or ethanol at controlled vapor pressures. Samples were placed in a homemade optical cell equipped with pressure and temperature control systems. The cell was initially pumped down to 0.05 mbar before any adsorbate introduction. Afterwards, toluene or ethanol gas vapors were introduced inside the cell gradually up to its saturation pressure to get the refractive index isotherm.
QCM measurements for gas sorption and desorption were performed using an Inficon STM2 thin film oscillator monitor driven by a lab-made data collection software in order to get mass uptake sorption isotherms. PP-HMDSO thin films were deposited on an AT-cut gold-covered quartz (6 MHz). The coated quartz was placed in a chamber under vacuum in which gas vapors were introduced systematically up to its saturated vapor pressure value at room temperature. For all the measurements, the quartz was thermally stabilized at 25°C using a water circulation and bath. The frequency variation (Δ
f) is proportionally linked to the mass variation (
∆m) due to gas vapor sorption or desorption according to Sauerbrey’s equation [
29] (3):
where
fq = 6 MHz is the frequency of the uncoated quartz, depending on the quartz size.
S = 0.5 cm
2 represents the surface area of the deposited film,
ρq = 2.65 g/cm
3 is the density of the quartz and
C = 1.67 × 10
5 cm/s is an apparatus constant.
The mass of the deposited film can be neglected compared to the mass of the quartz. Thus, the density of the coated quartz is equal to the density of the pristine quartz (
ρq). Since the mass of both the quartz and the films are constant,
Δm corresponds to the sorbed vapor mass uptake
(msorbed) by the thin films. Therefore,
msorbed of the deposited thin films was determined using (4):
The collected data were converted into mass uptake and isotherms of
msorbed as a function of relative pressure (
P/P0) were plotted,
P0 being the saturated pressure of the used vapors (
Table 2). For isotherms under air, the maximal collected absolute pressure is 1 bar.
3. Results
In this section, the elemental composition and physical properties such as density and refractive index of the PP-HMDSO thin films as well as their response towards several VOCs vapors separately are presented.
The elemental composition determined by EDX reveals a quite similar composition of the three films. The carbon, oxygen and silicon percentages are at% 52.2±0.8, 22.4±0.8 and 25.4±0.8 respectively, revealing a composition close to that of conventional PDMS, i.e. C:50at%, O:25at% and Si:25at%.
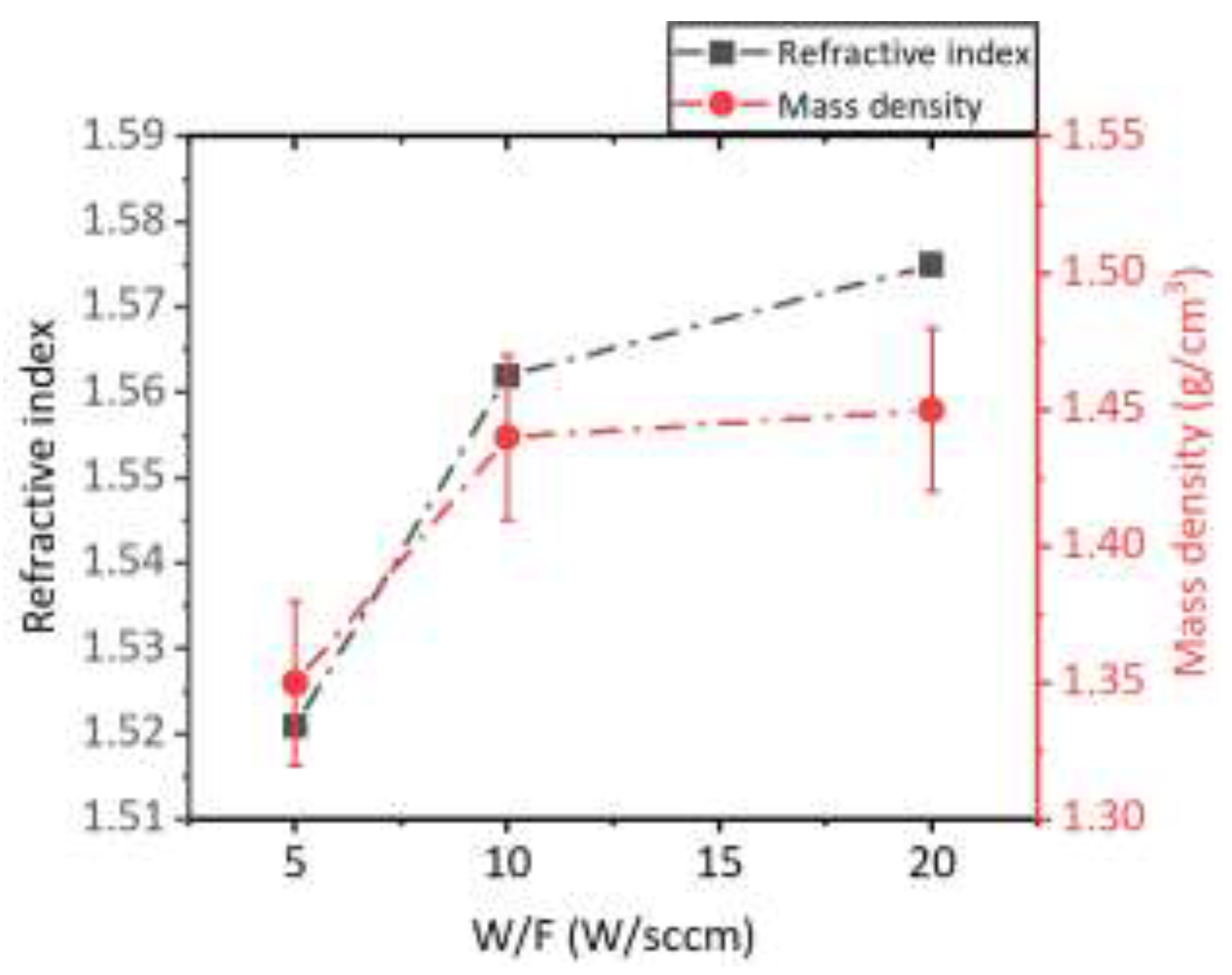
The calculated density and the refractive index of the PP-HMDSO thin films are presented in
Figure 1. The film deposited at 5 W/sccm reveals a low density (1.35 g/cm
3) compared to the films deposited at 10 W/sccm and 20 W/sccm (values close to 1.45 g/cm3). In addition, the refractive index of the SiOCH films increases from 1.52 for the films deposited at 5 W/sccm to values close to 1.57 for the films deposited at 10 W/sccm and 20 W/sccm. In fact, the variation of the films density generates a variation of the deposited films refractive index while increasing the W/F parameter. Indeed, the well-known Clausius-Mosotti equation (5) reveals the influence of the material density to the dielectric constant or the refractive index (n), since
n = ε2 in the visible optical range:
where
ρm is the density of the elaborated SiOCH films,
ε0 is the vacuum permittivity,
ε is the dielectric constant,
Ni is the number of atoms i per cm
3, and
αi is the polarizability of atom i.
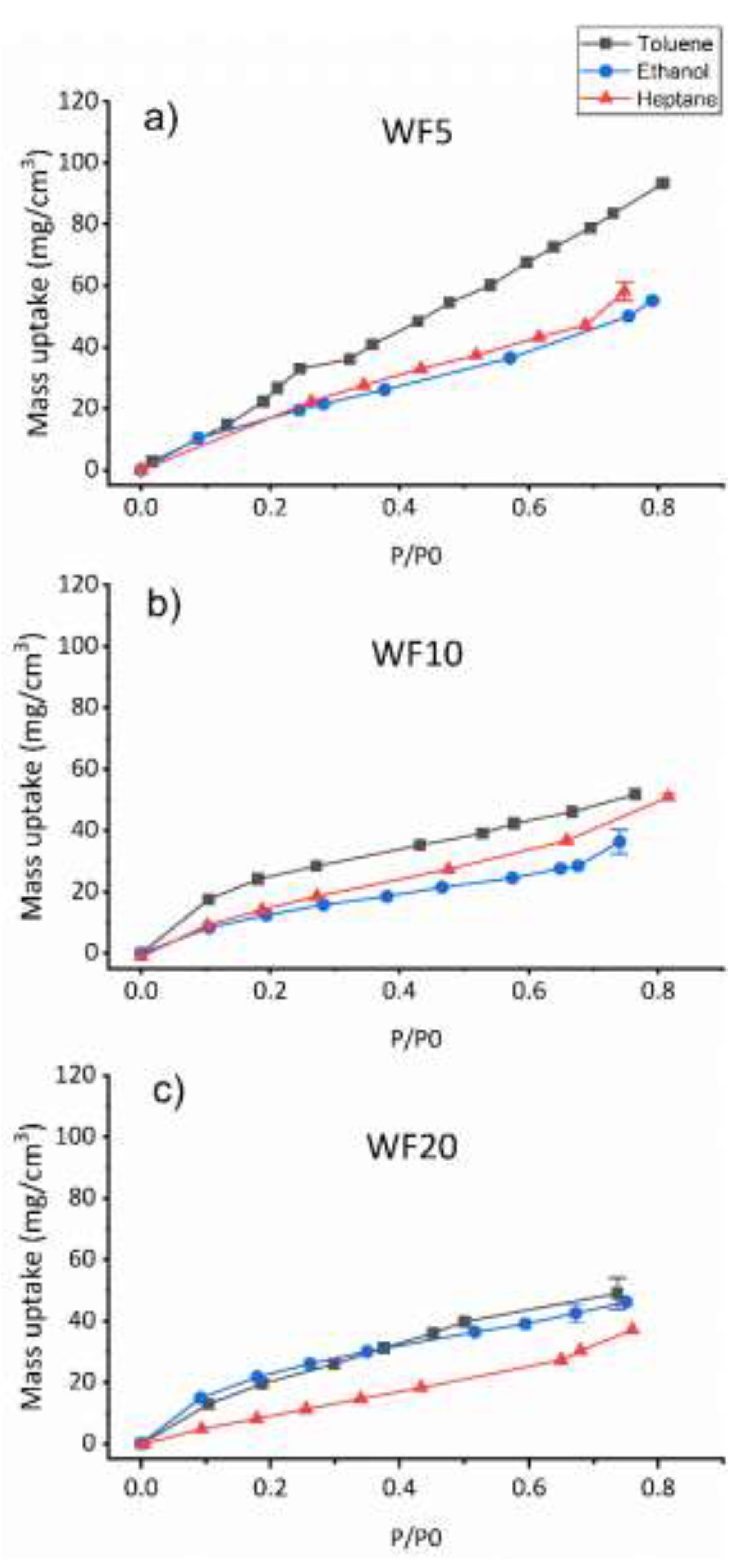
The responses of the PP-HMDSO thin films towards hydrocarbons and ethanol vapors sorption studied using QCM are presented in
Figure 2. In this study, heptane and ethanol vapors were used as representatives for COVs and toluene vapor as representative for BTEX gases. Ethanol vapor was used as reference in order to compare its sorption to hydrocarbons. The calculated mass uptake value represents the mass of the vapor sorbed by the thin film. Moreover as the mass uptake increases proportionally with the film thickness, it can be concluded that the molecules are not only adsorbed on the film surface but are absorbed in the bulk also. Therefore, the mass uptake was simply divided by the film thickness value to get the concentration of absorbed molecules per material volume unity even if no conclusion can be written on how the absorbed molecules are diluted in the material matrix, homogeneously or in a gradient concentration from the surface to the bulk. The uncertainty on the mass uptake value is taken as the standard deviation over three repeated QCM measurements.
As observed in
Figure 2, the mass uptake of the gas vapors increases with increasing the relative pressure P/P0 for all the PP-HMDSO thin films, P0 being the saturated vapor pressure at the temperature of the quartz, stabilized at 25°C. The WF5 film shows a linear increase of the toluene uptake mass up to 100 mg/cm
3 at a relative pressure equal to 0.8. Moreover, heptane and ethanol uptake masses reveal also a linear increase but to lower values (close to 60 mg/cm3) compared to toluene. Thus, the WF5 deposit shows a better affinity to toluene vapor than the two other vapors. The WF10 deposit shown in
Figure 2b illustrates weaker responses to the three vapors compared to the WF5 deposit especially to toluene vapor. Besides, the toluene isotherm of the WF10 film shows a non-linear increase. However, the final mass uptake for toluene vapor is higher than the two other vapors masses especially than ethanol uptake mass indicating a better affinity for WF10 towards toluene vapor. Besides, WF5 and WF10 deposits reveal a slightly better affinity towards heptane vapor compared to ethanol vapor. On the other hand, the deposit with the higher W/F (WF20) shows similar isotherms for toluene and ethanol. The toluene and ethanol isotherms shows a non-linear uptake mass increase showing a fast sorption between 0 and 0.2 P/P
0 followed by a slower sorption between 0.2 and 0.8 P/P
0. Furthermore, heptane vapor isotherm reveal mass uptakes lower than that of toluene and ethanol, suggesting a weaker affinity towards heptane vapor compared to toluene and ethanol vapors.
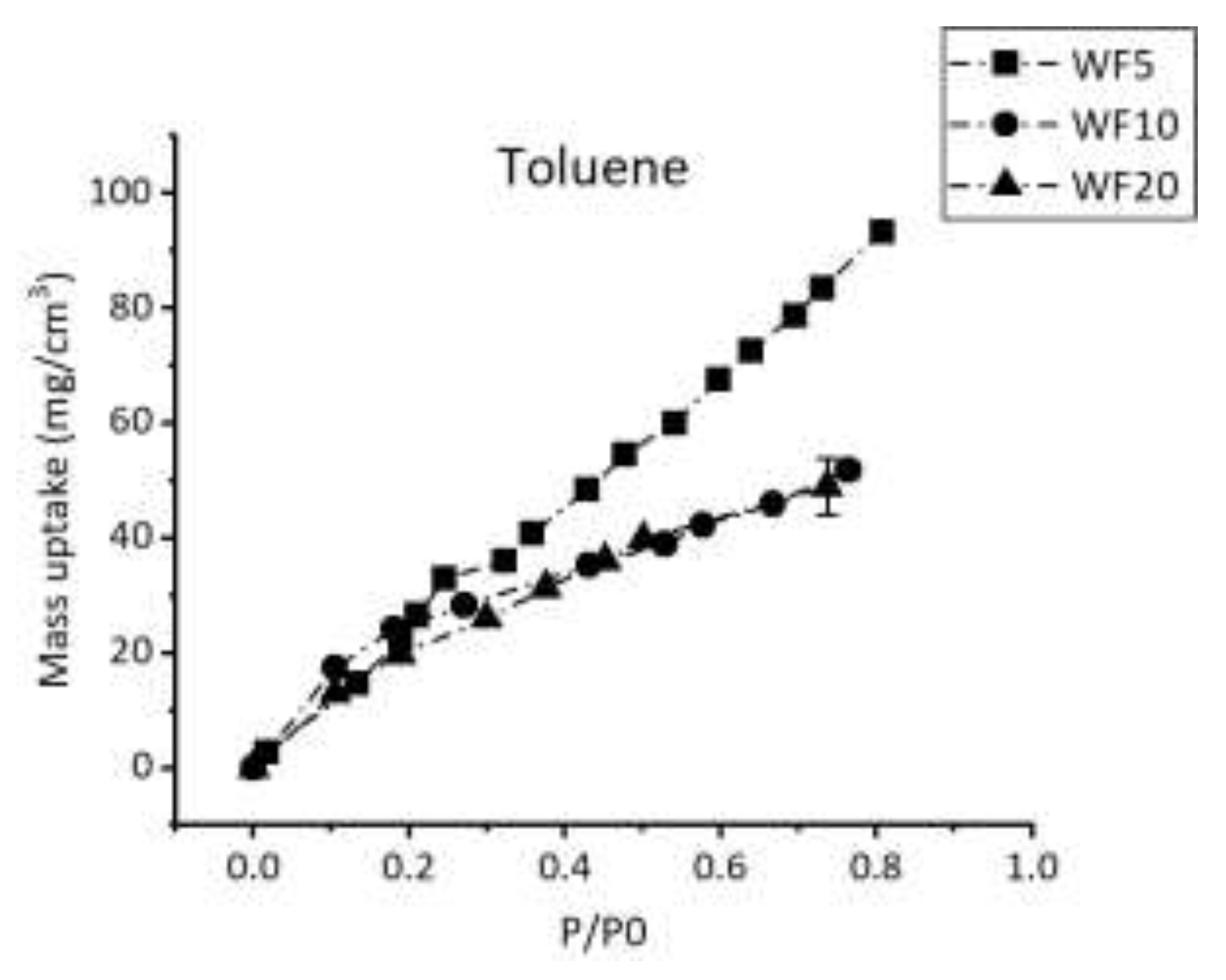
Figure 3 focuses on the comparison between the toluene sorption isotherms of the three PP-HMDSO thin films. A remarkable change in the sorption isotherms is shown while increasing W/F from 5 W/sccm to 10 W/sccm. However, identical isotherms are observed for the films elaborated at 10 W/sccm and 20 W/sccm.
In fact, the toluene isotherm loses its linearity while increasing W/F from 5 W/sccm to 10 W/sccm and the final uptake mass value decreases from 100 mg/cm3 to 50 mg/cm3 approximately. These variations indicates a slower sorption process especially at lower P/P0 values, typically between 0 and ~ 0.2, and a weaker affinity for WF10 and WF20 deposits towards toluene compared to WF5.
The responses of the PP-HMDSO thin films towards toluene and ethanol vapors was also studied using ellipsometry coupled to vapor sorption.
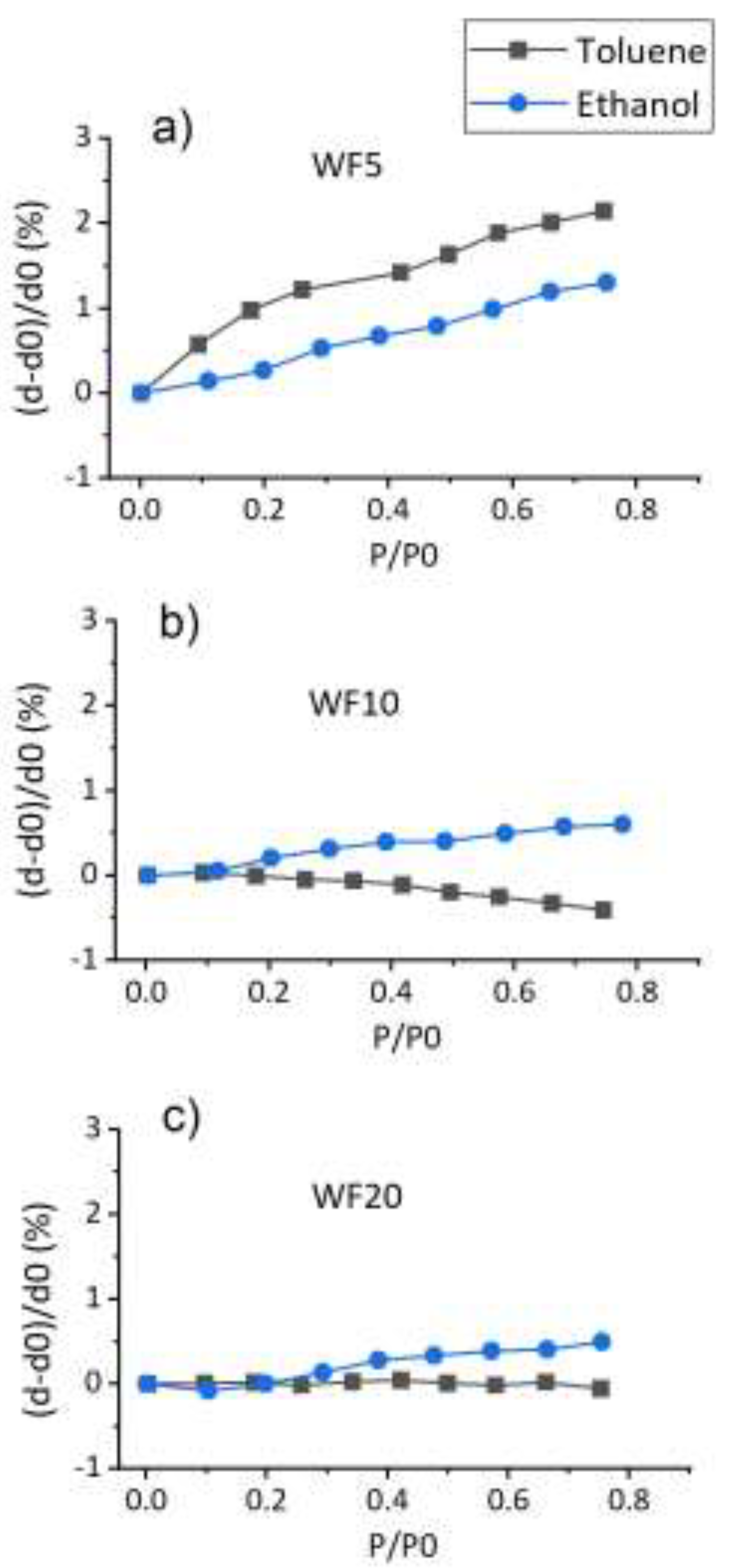
Figure 4 shows the swelling of the PP-HMDSO thin films along the normal to the film when exposed to toluene or ethanol vapors. We assume in this study that the swelling is homogenous despite the mechanical constraints present between the film and the silicon support. This model induces that the COVs concentration should vary along the depth of the film from the surface. d0 is the thickness measured under vacuum and d is the thickness measured as a function of the stabilized environment pressure, i.e. after enough time to attend the equilibrium between the gaseous molecules pressure and the absorbed molecules pressure inside the material bulk.
The swelling phenomenon of the SiOCH films when exposed to VOCs is due to the penetration of the gas vapors into the material bulk through the free volumes present in the polymer network.
Figure 4 reveals that the thickness of WF5 thin film improves while increasing toluene or ethanol vapor relative pressure. However, WF5 reveals a higher swelling rate for toluene compared to ethanol. Therefore, WF5 shows a better absorption of toluene molecules compared to ethanol molecules. On the other hand, WF10 and WF20 isotherms indicate a low film-swelling rate while exposed to both vapors. Nevertheless, WF10 swelling rate is slightly better when exposed to ethanol compared to the swelling rate in the presence of toluene for which a slight film contraction is observed.
Since the WF5 deposit revealed the better affinity towards toluene, it might be interesting to study the affinity of the WF5 deposit towards humidity.
Figure 5 shows a comparison between the WF5 deposit responses towards toluene and water vapor. The water vapor isotherm reveals extremely low mass uptakes compared to the toluene indicating a very weak sensitivity of WF5 towards water vapor.
5. Conclusions
In a previous work, we determined the effect of the plasma energy on the chemical composition and structure of the PP-HMDSO thin films. In the present article, physical properties such as refractive index and density of the films were presented. In addition, the effects of the materials chemical composition and the physical properties on the sorption of VOCs vapors were also investigated.
In conclusion, the plasma energy governs the density of the films In fact, the films free volumes proportion decreases while increasing the plasma energy leading to a denser material due to the formation new bonds such as Si-CH2-Si and Si-O-C in the film structure. The chemical composition modification shown previously and the density variation with the plasma energy affect both the sensitivity and the sorption selectivity of the materials towards the VOCs. In fact, the PP-HMDSO thin films elaborated in this study, showed different responses towards tested VOCs vapors (ethanol, heptane, toluene) despite the small plasma conditions variation between WF5, WF10 and W20 (5 W/sccm to 20 W/sccm). The WF5 showed good sensitivity towards toluene and good sorption selectivity between toluene and the other tested VOCs compared to the WF10 and WF20 deposits. This is due to a low density and high hydrophobicity (≈ 103° detected previously in [
27]) of the WF5 material as well as a high SiOSi bonds amount in the PP-HMDSO network.
Finally, chemical and physical properties play important roles in the vapors sorption into the organosilicon films. A combination of surface hydrophobicity and free volumes size (density) should be optimized in order to achieve a good sensitivity/selectivity to toluene (BTEX) vapor compared to other VOCs.