Submitted:
22 June 2023
Posted:
23 June 2023
Read the latest preprint version here
Abstract
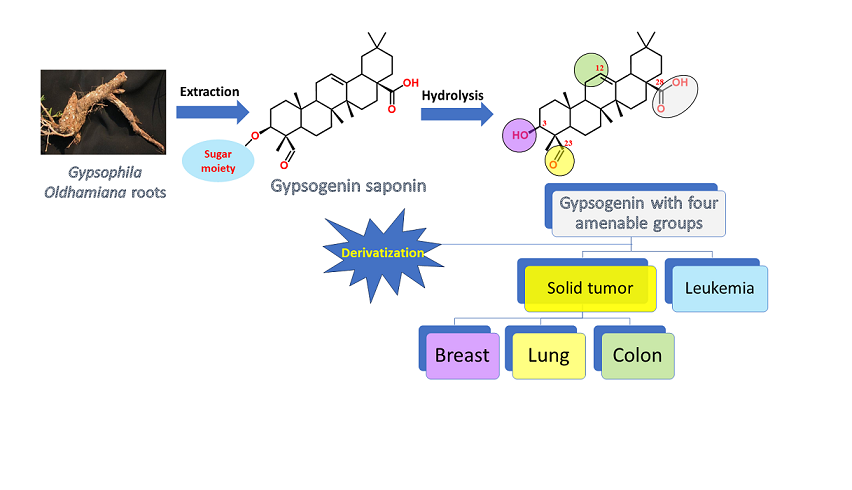
Keywords:
1. Introduction
2. Anti-cancer effect of gypsogenin, gypsogenic acid, and their semisynthetic derivatives
2.1. Anti-leukemic effect
2.2. Anti-breast cancer activity
2.3. Anti-lung cancer activity
2.4. Other anti-cancer activities
3. Conclusion and Future Directions
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: a global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Y.; Weng, S.; Xu, H.; Li, L.; Han, X. A New Trend in Cancer Treatment: The Combination of Epigenetics and Immunotherapy. Front. Immunol. 2022, 13, 809761. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Billaud, M.; Almeida, R. RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends Cancer 2017, 3, 506–528. [Google Scholar] [CrossRef]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Radwan, M.O.; Toma, T.; Arakaki, Y.; Kamo, M.; Inoue, N.; Koga, R.; Otsuka, M.; Tateishi, H.; Fujita, M. New insight into the bioactivity of substituted benzimidazole derivatives: Repurposing from anti-HIV activity to cell migration inhibition targeting hnRNP M. Bioorganic Med. Chem. 2023, 86, 117294. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- AlQathama, A.; Umm Al-Qura University; Yonbawi, A. R.; Shao, L.; Bader, A.; Abdalla, A.N.; Gibbons, S.; Prieto, J.M.; King Abdulaziz University; University College London; et al. The in vitro cytotoxicity against human melanoma cells, tyrosinase inhibition and antioxidant activity of Grewia tenax leaves extracts. Boletin Latinoam. Caribe Plantas Med. Aromat. 2022, 22, 268–276. [Google Scholar] [CrossRef]
- Bader, A.; Abdalla, A.N.; Obaid, N.A.; Youssef, L.; Naffadi, H.M.; Elzubier, M.E.; Almaimani, R.A.; Flamini, G.; Pieracci, Y.; El-Readi, M.Z. In Vitro Anticancer and Antibacterial Activities of the Essential Oil of Forsskal’s Basil Growing in Extreme Environmental Conditions. Life 2023, 13, 651. [Google Scholar] [CrossRef]
- Abo-Elghiet, F.; Ibrahim, M.H.; El Hassab, M.A.; Bader, A.; Abdallah, Q.M.; Temraz, A. LC/MS analysis of Viscum cruciatum Sieber ex Boiss. extract with anti-proliferative activity against MCF-7 cell line via G0/G1 cell cycle arrest: An in-silico and in-vitro study. J. Ethnopharmacol. 2022, 295, 115439. [Google Scholar] [CrossRef]
- Bader, A.; Bkhaitan, M.M.; Abdalla, A.N.; Abdallah, Q.M.A.; Ali, H.I.; Sabbah, D.A.; Albadawi, G.; Abushaikha, G.M. Design and Synthesis of 4-O-Podophyllotoxin Sulfamate Derivatives as Potential Cytotoxic Agents. Evidence-Based Complement. Altern. Med. 2021, 2021, e6672807. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Siordia-Reyes, A.G.; Meckes-Fischer, M.; Jiménez-Arellanes, A. Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage. Asian Pac. J. Trop. Med. 2016, 9, 644–651. [Google Scholar] [CrossRef]
- Xu, G.-B.; Xiao, Y.-H.; Zhang, Q.-Y.; Zhou, M.; Liao, S.-G. Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Aly, A.M.; Al-Alousi, L.; Salem, H.A. Licorice: A possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech 2005, 6, E74–E82. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Yin, M.-C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef]
- Radwan, M.O.; Ismail, M.A.; El-Mekkawy, S.; Ismail, N.S.; Hanna, A.G. Synthesis and biological activity of new 18β-glycyrrhetinic acid derivatives. Arab. J. Chem. 2016, 9, 390–399. [Google Scholar] [CrossRef]
- Somova, L.; Shode, F.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef]
- Somova, L.; Nadar, A.; Rammanan, P.; Shode, F. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive activity of oleanolic acid is mediated via downregulation of secretory phospholipase A2 and fatty acid synthase in spontaneously hypertensive rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef]
- Pompei, R.; Laconi, S.; Ingianni, A. Antiviral properties of glycyrrhizic acid and its semisynthetic derivatives. Mini-Reviews Med. Chem. 2012, 9, 996–1001. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Zhao, T.-T.; Lu, N.; Yang, Y.-A.; Zhu, H.-L. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mini-Reviews Med. Chem. 2019, 19, 826–832. [Google Scholar] [CrossRef]
- Tohmé, M.; Giménez, M.; Peralta, A.; Colombo, M.; Delgui, L. Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents 2019, 54, 601–609. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Huang, C.; Lv, X.; Liu, L.; Wang, Y.; Li, J. Antifibrosis effects of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf in a rat model of bleomycin-induced pulmonary fibrosis. J. Pharm. Pharmacol. 2012, 64, 1751–1760. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, K.Y.; Jeon, H.Y.; Sung, S.H.; Kim, Y.C. Antifibrotic activity of triterpenoids from the aerial parts ofEuscaphis japonicaon hepatic stellate cells. J. Enzym. Inhib. Med. Chem. 2009, 24, 1276–1279. [Google Scholar] [CrossRef]
- Xiang, H.; Han, Y.; Zhang, Y.; Yan, W.; Xu, B.; Chu, F.; Xie, T.; Jia, M.; Yan, M.; Zhao, R.; et al. A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats. Int. J. Mol. Sci. 2017, 18, 553. [Google Scholar] [CrossRef]
- Farina, C.; Pinza, M.; Pifferi, G. Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Il Farm. 1998, 53, 22–32. [Google Scholar] [CrossRef]
- Somensi, L.B.; Costa, P.; Boeing, T.; Mariano, L.N.B.; Longo, B.; Magalhães, C.G.; Duarte, L.P.; e Silva, A.T.M.; de Souza, P.; de Andrade, S.F.; et al. Gastroprotective properties of Lupeol-derived ester: Pre-clinical evidences of Lupeol-stearate as a potent antiulcer agent. Chem. Interactions 2020, 321, 108964. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Medica 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Shaheen, U.; Ragab, E.A.; Abdalla, A.N.; Bader, A. Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry 2018, 145, 168–178. [Google Scholar] [CrossRef]
- Liese, J.; Abhari, B.A.; Fulda, S. Smac mimetic and oleanolic acid synergize to induce cell death in human hepatocellular carcinoma cells. Cancer Lett. 2015, 365, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK–p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinog. 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Mu, D.-W.; Guo, H.-Q.; Zhou, G.-B.; Li, J.-Y.; Su, B. Oleanolic acid suppresses the proliferation of human bladder cancer by Akt/mTOR/S6K and ERK1/2 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 13864–13870. [Google Scholar]
- Amara, S.; Zheng, M.; Tiriveedhi, V. Oleanolic Acid Inhibits High Salt-Induced Exaggeration of Warburg-like Metabolism in Breast Cancer Cells. Cell Biochem. Biophys. 2016, 74, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Bid, H.K.; Rajendran, S.; Yadav, P.P.; Konwar, R. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: Role of pro-apoptotic effects of oleanolic acid and urosolic acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, M.; Li, D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol. Cell. Biochem. 2015, 400, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.A.; Rodrigues. P.; Araújo, F.R.R.; Oliveira, W.L.; Furtado, M.A.; Castro, M.B.; Cunha, W.R.; Tavares, D.C. Ursolic Acid and Oleanolic Acid Suppress Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon. Toxicol. Pathol. 2008, 36, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Indranie, C.; Malisetty, S.V.; Jagan, P.; Steele, V.E.; Rao, C.V. Chemoprevention of Colon Carcinogenesis by Oleanolic Acid and Its Analog in Male F344 Rats and Modulation of COX-2 and Apoptosis in Human Colon HT-29 Cancer Cells. Pharm. Res. 2008, 25, 2151–2157. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef]
- Borella, R.; Forti, L.; Gibellini, L.; De Gaetano, A.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids. Molecules 2019, 24, 4097. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Hsieh, W.-C.; Chen, S.-H.; Li, Y.-Z.; Liao, H.-F.; Lin, M.-Y.; Sheu, S.-M. 18β-glycyrrhetinic Acid Modulated Autophagy is Cytotoxic to Breast Cancer Cells. Int. J. Med Sci. 2023, 20, 444–454. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.-Q.; Song, J.; Liu, Q.-M.; Wang, C.; Huang, Z.; Chu, L.; Liang, H.-F.; Zhang, B.-X.; Chen, X.-P. 18β-Glycyrrhetinic-acid-mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma. Sci. Rep. 2018, 8, 9365. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, C.; Yin, M.; Lu, J.; Hu, H.; Chen, D. Hepatocellular carcinoma-targeted effect of configurations and groups of glycyrrhetinic acid by evaluation of its derivative-modified liposomes. Int. J. Nanomed. 2018, ume 13, 1621–1632. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, Y.J.; Lee, M.S.; Han, E.S.; Lee, S.J. 18β-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Noshita, T.; Yu, T.; Kidachi, Y.; Kamiie, K.; Umetsu, H.; Ryoyama, K. Novel effects of glycyrrhetinic acid on the central nervous system tumorigenic progenitor cells: Induction of actin disruption and tumor cell-selective toxicity. Eur. J. Med. Chem. 2010, 45, 2943–2948. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Iranshahy, M.; Iranshahi, M. Glycyrrhetinic Acid and Its Derivatives: Anti-Cancer and Cancer Chemopreventive Properties, Mechanisms of Action and Structure- Cytotoxic Activity Relationship. Curr. Med. Chem. 2016, 23, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Khan, K.; Hafeez, A.; Irfan, M.; Armaghan, M.; Rahman, A.U.; Gürer, E.S.; Sharifi-Rad, J.; Butnariu, M.; Bagiu, I.-C.; et al. Ursolic acid: a natural modulator of signaling networks in different cancers. Cancer Cell Int. 2022, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Raphael, T.; Kuttan, G. Effect of Naturally Occurring Triterpenoids Ursolic Acid and Glycyrrhizic Acid on the Cell-Mediated Immune Responses of Metastatic Tumor-Bearing Animals. Immunopharmacol. Immunotoxicol. 2008, 30, 243–255. [Google Scholar] [CrossRef]
- Kim, D.K.; Baek, J.H.; Kang, C.M.; A Yoo, M.; Sung, J.W.; Chung, H.Y.; Kim, N.D.; Choi, Y.H.; Lee, S.H.; Kim, K.W. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int. J. Cancer 2000, 87. [Google Scholar] [CrossRef]
- Liu, X.-S.; Jiang, J. Induction of Apoptosis and Regulation of the MAPK Pathway by Ursolic Acid in Human Leukemia K562 Cells. Planta Medica 2007, 73, 1192–1194. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic acid: A natural product with anticancer activity. Mol. Nutr. Food Res. 2009, 53, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Selzer, E.; Pimentel, E.; Wacheck, V.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of Betulinic Acid Alone and in Combination with Irradiation in Human Melanoma Cells. J. Investig. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.-M. Sensitization for Anticancer Drug-Induced Apoptosis by Betulinic Acid. Neoplasia 2005, 7, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Ong, P.S.; Wang, L.; Goel, A.; Ding, L.; Wong, A.L.-A.; Ho, P.C.-L.; Sethi, G.; Xiang, X.; Goh, B.C. Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Lett. 2021, 521, 252–267. [Google Scholar] [CrossRef]
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Dou, Q.P. Celastrol, a Triterpene Extracted from the Chinese “Thunder of God Vine,” Is a Potent Proteasome Inhibitor and Suppresses Human Prostate Cancer Growth in Nude Mice. Cancer Res 2006, 66, 4758–4765. [Google Scholar] [CrossRef]
- Nagase, M.; Oto, J.; Sugiyama, S.; Yube, K.; Takaishi, Y.; Sakato, N. Apoptosis Induction in HL-60 Cells and Inhibition of Topoisomerase II by Triterpene Celastrol. Biosci. Biotechnol. Biochem. 2003, 67, 1883–1887. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis Int. J. Program. Cell Death 2011, 16, 1028–1041. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, Y.; Wang, F.; Song, L.; Sun, J.; Liu, Y.; Sun, J. Synthesis of gypsogenin derivatives with capabilities to arrest cell cycle and induce apoptosis in human cancer cells. R. Soc. Open Sci. 2018, 5, 171510. [Google Scholar] [CrossRef]
- Gampe, C.; Verma, V.A. Curse or Cure? A Perspective on the Developability of Aldehydes as Active Pharmaceutical Ingredients. J. Med. Chem. 2020, 63, 14357–14381. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef]
- Lee, I.; Yoo, J.K.; Na, M.; Min, B.S.; Lee, J.; Yun, B.S.; Jin, W.; Kim, H.; Youn, U.; Chen, Q.C.; et al. Cytotoxicity of Triterpenes Isolated from Aceriphyllum rossii. Chem. Pharm. Bull. 2007, 55, 1376–1378. [Google Scholar] [CrossRef]
- Krasteva, I.; Yotova, M.; Yosifov, D.; Benbassat, N.; Jenett-Siems, K.; Konstantinov, S. Cytotoxicity of gypsogenic acid isolated from Gypsophila trichotoma. Pharmacogn. Mag. 2014, 10, 430–S433. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Radwan, M.O.; Ozturk, S.E.; Ulusoy, N.G.; Sozer, E.; Ellakwa, D.E.; Ocak, Z.; Can, M.; Ali, T.F.; Abd-Alla, H.I.; et al. Design, Synthesis and Biological Evaluation of Pentacyclic Triterpene Derivatives: Optimization of Anti-ABL Kinase Activity. Molecules 2019, 24, 3535. [Google Scholar] [CrossRef] [PubMed]
- Emirdağ-Öztürk, S.; Karayıldırım, T.; Çapcı-Karagöz, A.; Alankuş-Çalışkan, Ö.; Özmen, A.; Poyrazoğlu-Çoban, E. Synthesis, antimicrobial and cytotoxic activities, and structure–activity relationships of gypsogenin derivatives against human cancer cells. Eur. J. Med. Chem. 2014, 82, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Emirdağ-Öztürk, S.; Babahan, I.; Özmen, A. Synthesis, characterization and in vitro anti-neoplastic activity of gypsogenin derivatives. Bioorganic Chem. 2014, 53, 15–23. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Ozturk, S.E.; Ali, T.F.S.; Radwan, M.O.; Tateishi, H.; Koga, R.; Ocak, Z.; Can, M.; Otsuka, M.; Fujita, M. The First Pentacyclic Triterpenoid Gypsogenin Derivative Exhibiting Anti-ABL1 Kinase and Anti-chronic Myelogenous Leukemia Activities. Biol. Pharm. Bull. 2018, 41, 570–574. [Google Scholar] [CrossRef]
- Ulusoy, N.G.; Emirdağ, S.; Sözer, E.; Radwan, M.O.; Çiftçi, H.; Aksel, M.; Bölükbaşı, S. .; Özmen, A.; Yaylı, N.; Karayıldırım, T.; et al. Design, semi-synthesis and examination of new gypsogenin derivatives against leukemia via Abl tyrosine kinase inhibition and apoptosis induction. Int. J. Biol. Macromol. 2022, 222, 1487–1499. [Google Scholar] [CrossRef]
- Wu, G.; Chu, H.; Wang, J.; Mu, Y.; Sun, J. Synthesis of gypsogenin and gypsogenic acid derivatives with antitumor activity by damaging cell membranes. New J. Chem. 2019, 43, 18898–18914. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, T.; Liu, L.; Mu, X.; Sun, J. Anticancer Structure-activity Relationships and Potential Target Exploration of the Natural Product Gypsogenin. Chemistryselect 2023, 8, e202300072. [Google Scholar] [CrossRef]
- Tian, G.; Zhou, L.; Zhong, Y.; Xu, W.; Bai, H.; Liu, L.; Cui, S. Experimental studies of the therapeutic effect of Gypsophila oldhamiana gypsogenin on Lewis lung cancer in mice. Chin. J. Clin. Oncol. 2008, 5, 206–210. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Radwan, M.O.; Sever, B.; Hamdy, A.K.; Emirdağ, S.; Ulusoy, N.G.; Sozer, E.; Can, M.; Yayli, N.; Araki, N.; et al. EGFR-Targeted Pentacyclic Triterpene Analogues for Glioma Therapy. Int. J. Mol. Sci. 2021, 22, 10945. [Google Scholar] [CrossRef] [PubMed]
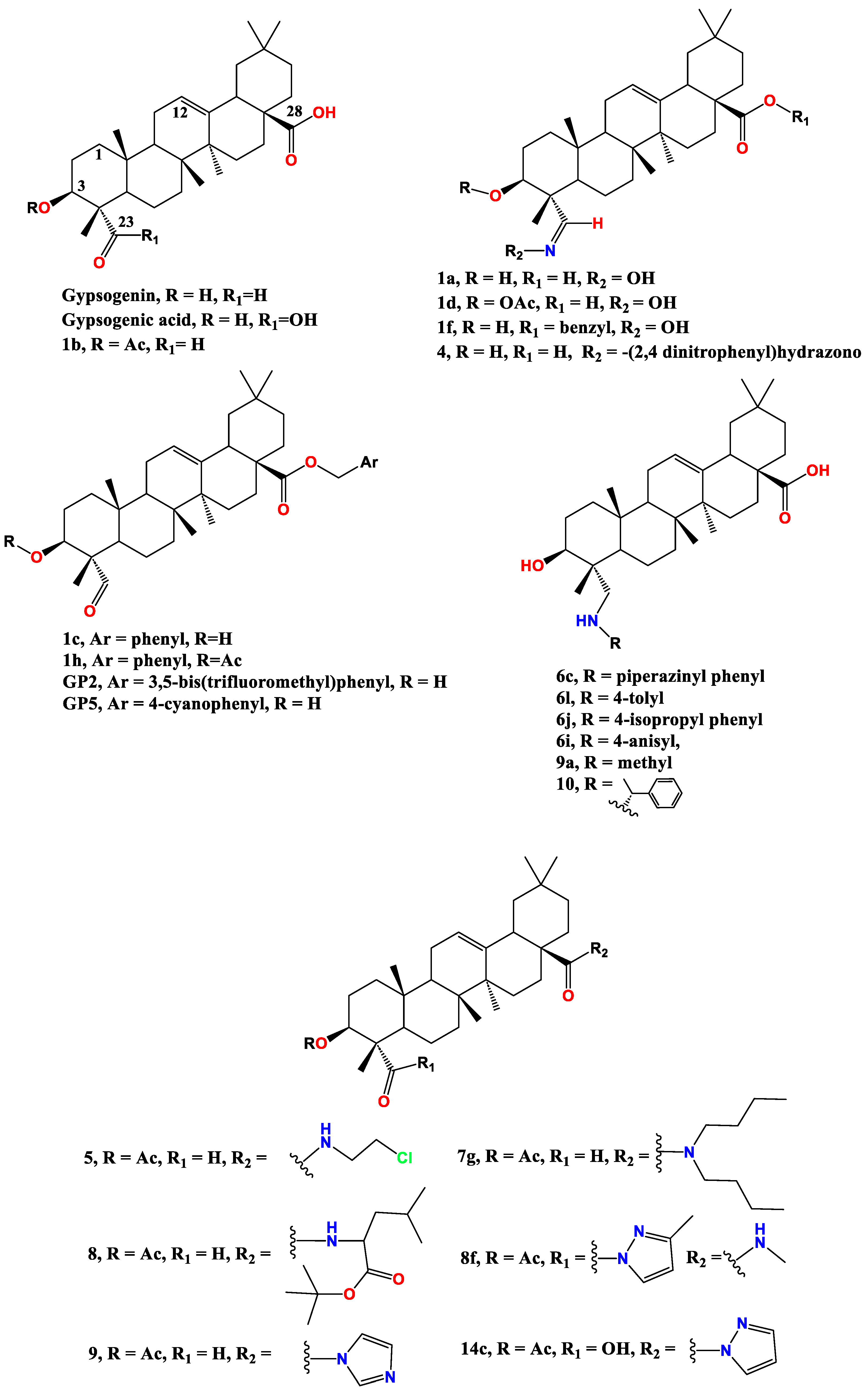
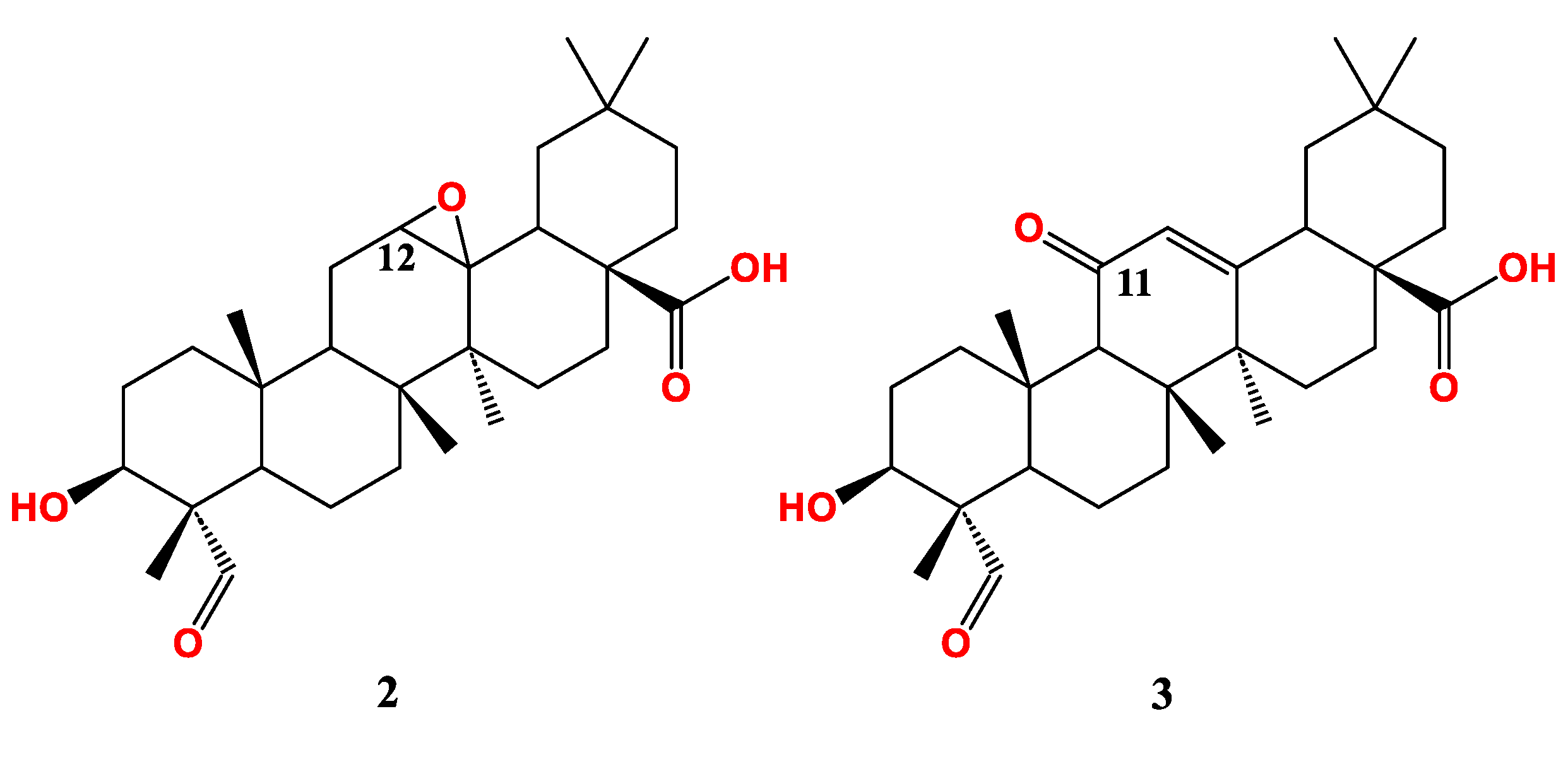
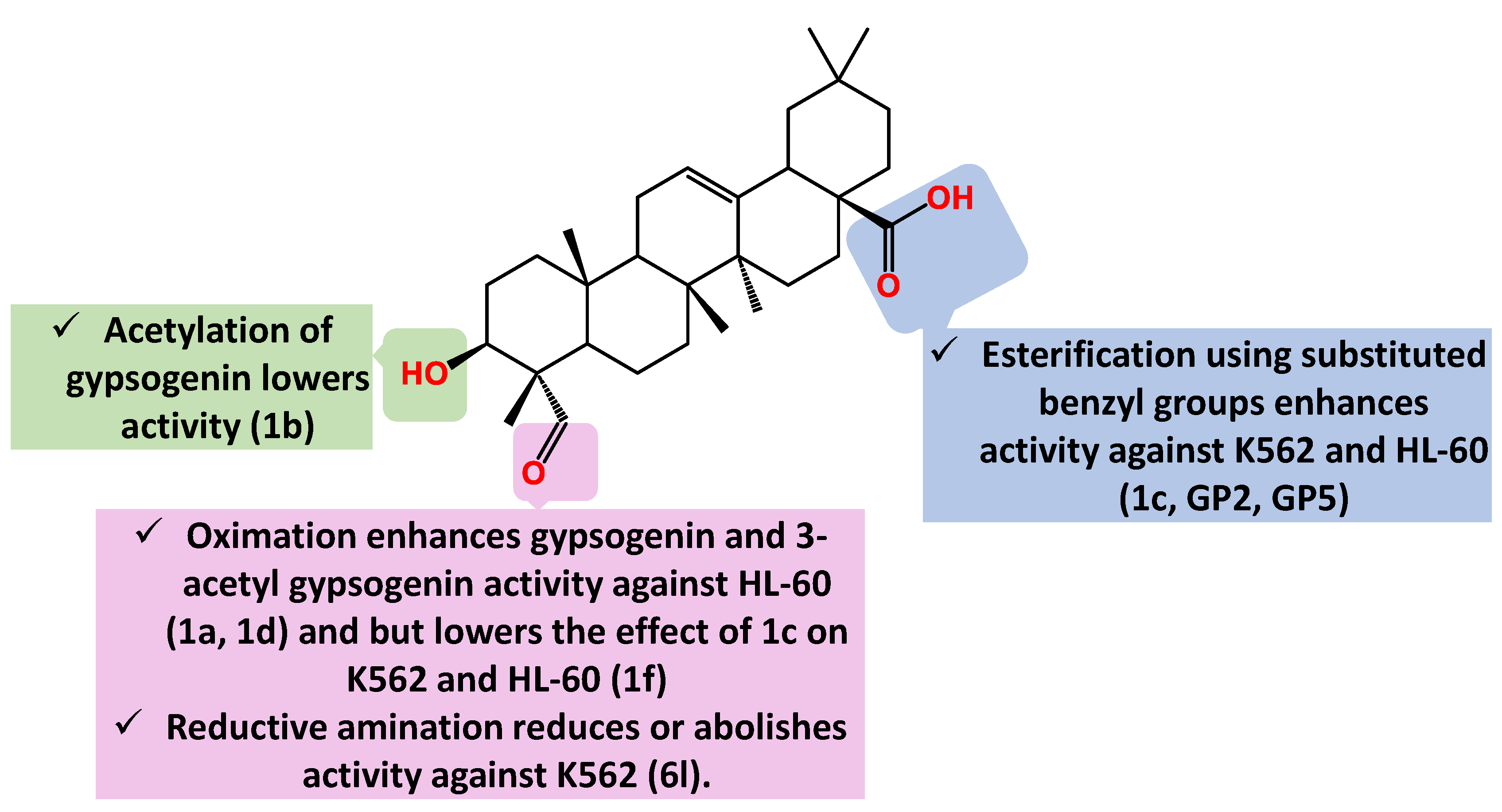
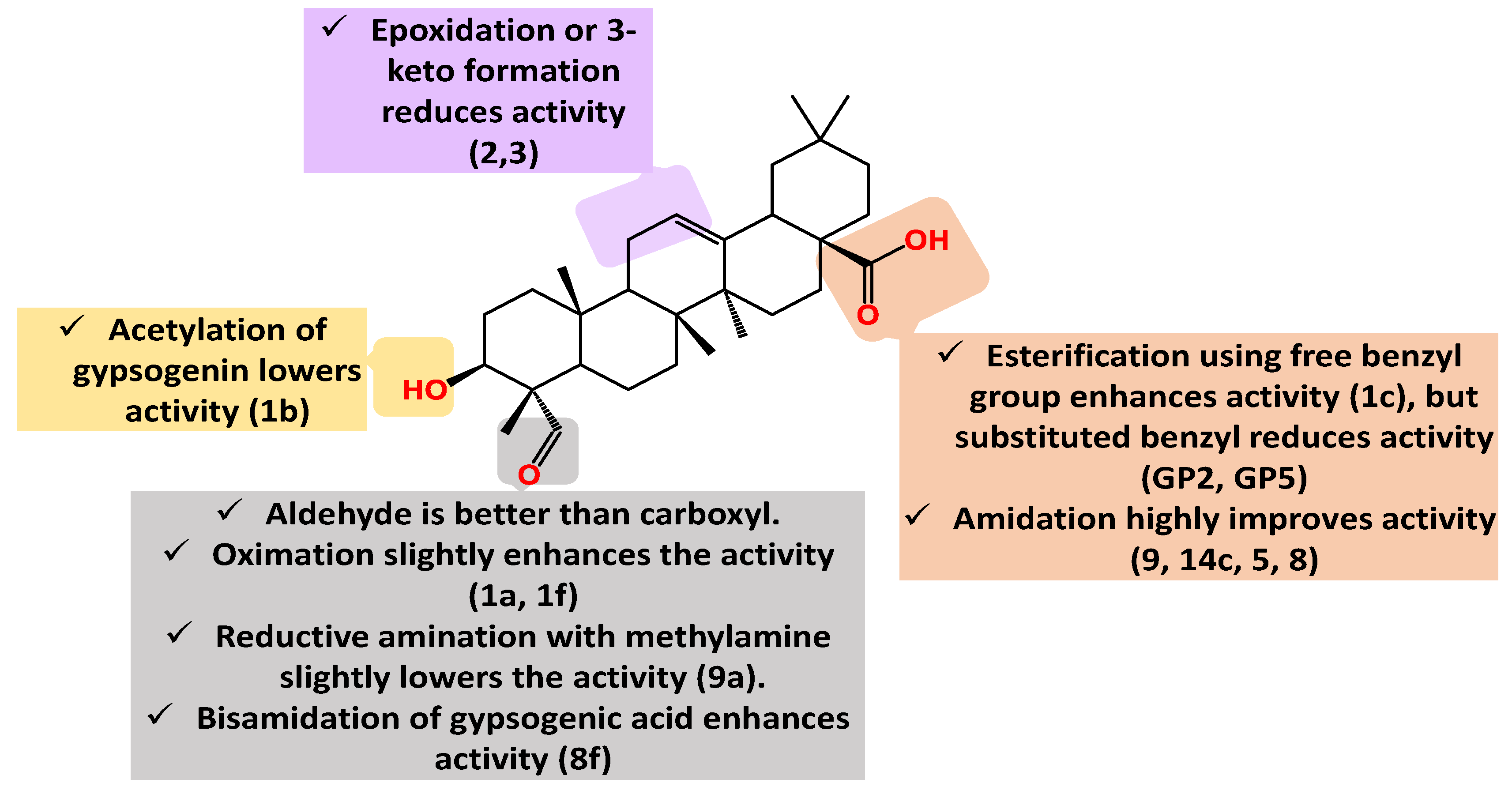
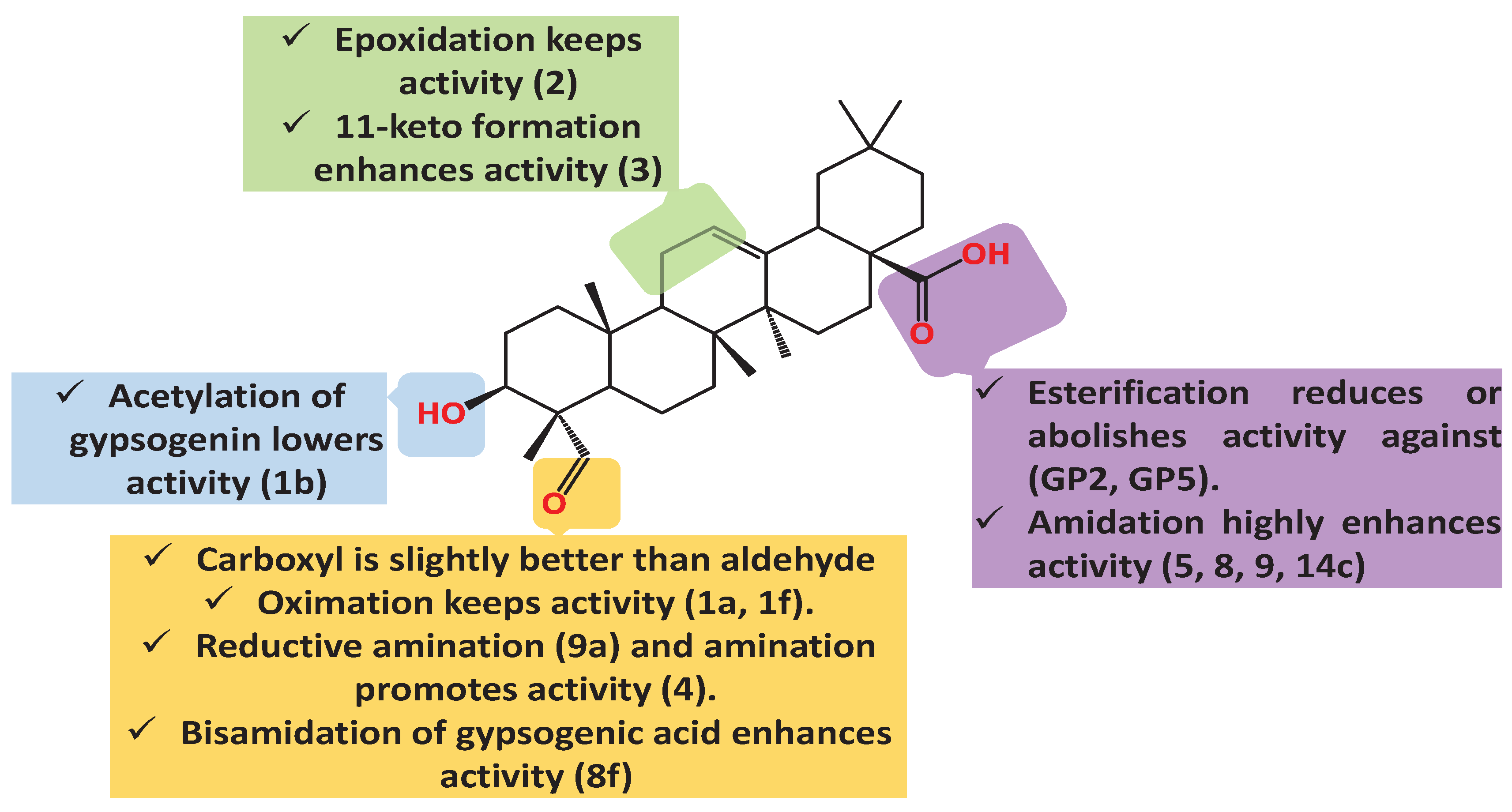
| Compound Cell line name and IC50 µM | |||
| HT-29 [74] | Saos-2 [75] | HeLa [74] | |
| Gypsogenin | 10.4 | 7.8 | 22.4 |
| 1a | 10.8 | 7.9 | 8.7 |
| 1b | 11.1 | 8.2 | 35.0 |
| 1d | 6.7 | 8.9 | >100 |
| LOVO [79] | |||
| 2 | > 30 | ||
| 3 | 17.8 | ||
| 5 | 7.2 | ||
| 8 | 0.8 | ||
| 9a | 5.8 | ||
| LOVO [68] | HePG2 [68] | SKOV3 [68] | |
| 4 | 2.9 | 10.0 | 9.7 |
| 7g | 3.5 | 12.5 | 13.1 |
| HepG2 [78] | TE-1[78] | MC3-8 [78] | |
| 8f | 3.6 | 5.4 | 4.8 |
| 9 | 4.0 | 4.7 | 2.9 |
| 14c | 2.2 | 4.2 | 2.6 |
| HeLa [73] | |||
| GP2 | 35.2 | ||
| GP5 | 5.6 | ||
| U251 | T98G | U87 | |
| 10 [81] | 5.8 | 8.1 | 17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





