Submitted:
22 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
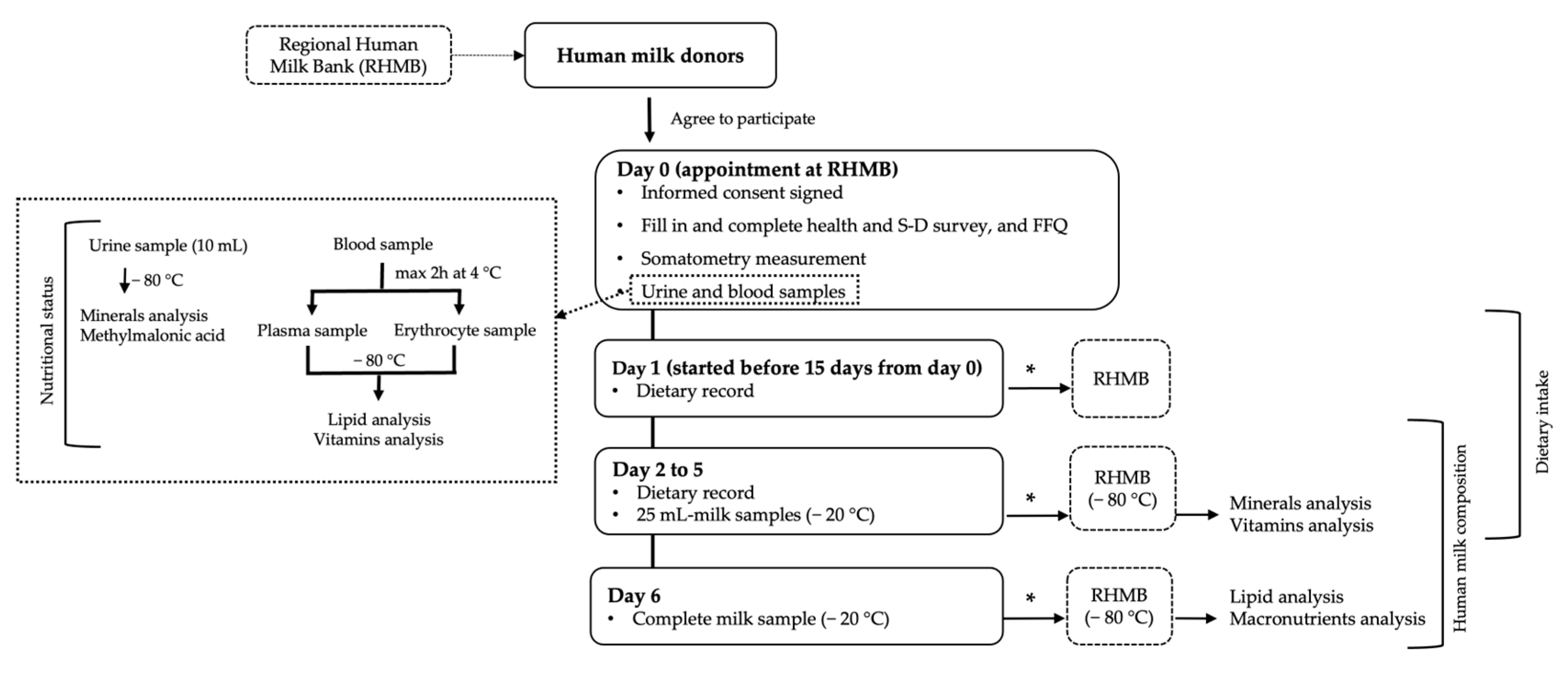
2.1. Study Design and Subjects
2.2. Protocol Study
2.3. Nutrients Analysis
2.3.1. Fatty Acids Analysis
2.3.2. Vitamins and Minerals Analysis
Water-Soluble Vitamins and Vitamin-B12-Associated Biomarkers
Fat-Soluble Vitamins
Minerals
2.3.3. Blood Biochemistry, Hemoglobin, and Urine Creatinine
2.4. Statistics
3. Results
3.1. Population Studied
3.2. Diet Survey and Nutritional Status
3.3. Donor Human Milk Composition
3.4. Associations between Clinical Characterisitics, Donors’ Daily Nutrient Intake, Their Plasma and Erythrocyte Nutrient Levels, and Nutrient Levels in DHM: Multivariate Analysis
3.4.1. Lipid Associations in DHM
3.4.2. Vitamins, Minerals, and Trace Elements Associations in DHM
Water-Soluble Vitamins (Table 9)
Fat-Soluble Vitamins (Table 9)
Minerals and Trace Elements
3.4.3. Macronutrients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; Hojsak, I.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef]
- Moro, G.E.; Billeaud, C.; Rachel, B.; Calvo, J.; Cavallarin, L.; Christen, L.; Escuder-Vieco, D.; Gaya, A.; Lembo, D.; Wesolowska, A.; et al. Processing of Donor Human Milk: Update and Recommendations From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the establishment and operation of Human Milk Banks in Europe: A consensus statement from the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- AAP Comittee on Nutrition, AAP Section on breastfeeding, AAP Comittee on fetus and newbornborn, D. Donor human milk for the high- risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139, e20163440. [CrossRef]
- Martini, S.; Beghetti, I.; Annunziata, M.; Aceti, A.; Galletti, S.; Ragni, L.; Donti, A.; Corvaglia, L. Enteral Nutrition in Term Infants with Congenital Heart Disease: Knowledge Gaps and Future Directions to Improve Clinical Practice. Nutrients 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences; Engineering; and Medicine; Health and Medicine Division; Food and Nutrition Board Nutrition During Pregnancy and Lactation: Exploring New Evidence: Proceedings of a Workshop; The National Academies Press (US): Washington (DC), 2020; ISBN 9780309679244.
- Dror, D.K.; Allen, L.H. Overview of nutrients in human milk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12. [Google Scholar] [CrossRef]
- Allen, L.H. B Vitamins in Breast Milk: Relative Importance of Maternal Status and Intake, and Effects on Infant Status and Function. Adv. Nutr. An Int. Rev. J. 2012, 3, 362–369. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Allen, L.H.; Dallas, D.C.; McManaman, J.; Raiten, D.J.; Rozga, M.; Sela, D.A.; Seppo, A.; Williams, J.E.; Young, B.E.; et al. Ecologies, synergies, and biological systems shaping human milk composition—a report from “Breastmilk Ecology: Genesis of Infant Nutrition (BEGIN)” Working Group 2. Am. J. Clin. Nutr. 2023, 117, S28–S42. [Google Scholar] [CrossRef]
- Allen, L.H. Multiple micronutrients in pregnancy and lactation: An overview. Am. J. Clin. Nutr. 2005, 81, 1206–1212. [Google Scholar] [CrossRef]
- Lima, M.S.R.; Dimenstein, R.; Ribeiro, K.D.S. Vitamin e concentration in human milk and associated factors: A literature review. J. Pediatr. (Rio. J). 2014, 90, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascuñán, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.P.; Puigrredon, C.; Valenzuela, A. The impact of maternal diet during pregnancy and lactation on the fatty acid composition of erythrocytes and breast milk of chilean women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Tang, D.; Liu, Y.; Chen, D. Trends in Dietary Patterns and Diet-related Behaviors in China. Am. J. Health Behav. 2021, 45, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; Rangan, A.; Allman-Farinelli, M. Macronutrient Composition of the Australian Population’s Diet; Trends from Three National Nutrition Surveys 1983, 1995 and 2012. Nutrients 2018, 10, 1045. [Google Scholar] [CrossRef]
- Fulgoni, K.; Fulgoni, V.L. Trends in total, added, and natural phosphorus intake in adult americans, nhanes 1988–1994 to nhanes 2015–2016. Nutrients 2021, 13, 2249. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J. Nutr. 2017, 147. [Google Scholar] [CrossRef]
- Dawodu, A.; Tsang, R.C. Maternal Vitamin D Status: Effect on Milk Vitamin D Content and Vitamin D Status of Breastfeeding Infants. Adv. Nutr. An Int. Rev. J. 2012, 3, 353–361. [Google Scholar] [CrossRef]
- Ortega, R.M.; López-Sobaler, A.M.; Martínez, R.M.; Andrés, P.; Elena Quintas, M. Influence of smoking on vitamin E status during the third trimester of pregnancy and on breast-milk tocopherol concentrations in Spanish women. Am J Clin Nutr 1998, 68, 662–667. [Google Scholar] [CrossRef]
- Perrin, M.T.; Belfort, M.B.; Hagadorn, J.I.; McGrath, J.M.; Taylor, S.N.; Tosi, L.M.; Brownell, E.A. The nutritional composition and energy content of donor human milk: A systematic review. Adv. Nutr. 2020, 11, 960–970. [Google Scholar] [CrossRef]
- Sierra-Colomina, G.; García-Lara, N.R.; Escuder-Vieco, D.; Alonso-Díaz, C.; Andrés Esteban, E.M.; Pallás-Alonso, C.R. Donor milk volume and characteristics of donors and their children. Early Hum. Dev. 2014, 90, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Valentine, C.J.; Morrow, G.; Pennell, M.; Morrow, A.L.; Hodge, A.; Haban-Bartz, A.; Collins, K.; Rogers, L.K. Randomized Controlled Trial of Docosahexaenoic Acid Supplementation in Midwestern U. S. Human Milk Donors. Breastfeed Med 2013, 8, 86–91. [Google Scholar] [CrossRef]
- Ureta-Velasco, N.; Keller, K.; Escuder-Vieco, D.; Serrano, J.C.E.; García-Lara, N.R.; Pallás-Alonso, C.R. Assessment of Iodine Concentration in Human Milk from Donors: Implications for Preterm Infants. Nutrients 2022, 14, 4304. [Google Scholar] [CrossRef] [PubMed]
- Ureta-Velasco, N.; Keller, K.; Escuder-Vieco, D.; Fontecha, J.; Calvo, M. V.; Megino-Tello, J.; Serrano, J.C.E.; Romero Ferreiro, C.; García-Lara, N.R.; Pallás-Alonso, C.R. Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers. Nutrients 2023, 15, 1855. [Google Scholar] [CrossRef]
- Ortega, R.; López-Sobaler, A.; Andrés, P.; Requejo, A.; Aparicio, A.; Molinero, L. DIAL software for assessing diets and food calculations 2013.
- Boutin, M.; Presse, N.; Martineau, T.; Perreault, A.; Gaudreau, P.; Auray-Blais, C. Mass spectrometry analysis of urinary methylmalonic acid to screen for metabolic vitamin B12 deficiency in older adults. Bioanalysis 2020, 12, 693–705. [Google Scholar]
- Olsen, I.E.; Groveman, S.A.; Lawson, M.L.; Clark, R.H.; Zemel, B.S. New intrauterine growth curves based on United States data. Pediatrics 2010, 125, 214–224. [Google Scholar] [CrossRef]
- World Health Organization WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development; World Health Organization: Geneva, 2006; ISBN 92 4 154693 X.
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed Harmonized Nutrient Reference Values for Populations. Adv Nutr 2020, 11, 469–483. [Google Scholar] [CrossRef]
- Ortega Anta, R.; Requejo Marcos, A. Nutriguía. Manual de nutrición clínica; Editorial Médica Panamericana: Madrid, 2015; ISBN 9788498358674. [Google Scholar]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Dapcich, V.; Salvador Castell, G.; Ribas Barba, L.; Pérez Rodrigo, C.; Aranceta-Bartrina, J.; Serra Majem, L. Embarazo y lactancia. Necesidades especiales. In Guía de la alimentación saludable; Sociedad Española de Nutrición Comunitaria, Ed.; Madrid, 2004; p. 82 ISBN https://www.nutricioncomunitaria.org/es/otras-publicaciones (accessed on Feb 02, 2023).
- Giuffrida, F.; Fleith, M.; Goyer, A.; Samuel, T.M.; Elmelegy-Masserey, I.; Fontannaz, P.; Cruz-Hernandez, C.; Thakkar, S.K.; Monnard, C.; De Castro, C.A.; et al. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe. Eur. J. Nutr. 2022, 61, 2167–2182. [Google Scholar] [CrossRef]
- Graham, J.; Peerson, J.; Haskell, M.; Shrestha, R.; Brown, K.; Allen, L. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin. Chem. 2005, 51, 2162–2165. [Google Scholar] [CrossRef]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, 1998; ISBN 9780309064118.
- Ehsanian, R.; Anderson, S.; Schneider, B.; Kennedy, D.; Mansourian, V. Prevalence of low plasma vitamin B1 in the stroke population admitted to acute inpatient rehabilitation. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Petteys, B.J.; Frank, E.L. Rapid determination of vitamin B2 (riboflavin) in plasma by HPLC. Clin. Chim. Acta 2011, 412, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Andraos, S.; Jones, B.; Wall, C.; Thorstensen, E.; Kussmann, M.; Smith, D.C.; Lange, K.; Clifford, S.; Saffery, R.; Burgner, D.; et al. Plasma B vitamers: Population epidemiology and parent-child concordance in children and adults. Nutrients 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Allen, L.H.; Miller, J.W.; De Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef]
- Sobczyńska-Malefora, A.; Harrington, D.J. Laboratory assessment of folate (Vitamin B9) status. J. Clin. Pathol. 2018, 71, 949–956. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory III, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of nutrition for development-Folate review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Institute of Medicine Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Press, T.N.A., Ed.; Washington, DC, 2000; ISBN 9780309069496.
- De Pee, S.; Dary, O. Biochemical indicators of vitamin A deficiency: Serum retinol and serum retinol binding protein. J. Nutr. 2002, 132, 2895–2901. [Google Scholar] [CrossRef]
- World Health Organization Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations; World Health Organization: Geneva, 2011; ISBN (WHO/NMH/NHD/MNM/11.3), (http://www.who.int/vmnis/indicators/retinol.pdf, accessed on Feb 28, 2023).
- Institute of Medicine Dietary reference intakes for calcium and vitamin D; Ross, C. A., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Medicine, I. of, Eds.; National Academies Press: Washington, D.C, 2011; ISBN 978-0-309-16395-8. [Google Scholar]
- U.S. Centers for Disease Control and Prevention Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population; Atlanta, GA, 2012; ISBN 1499234783.
- Dietary Guidelines Advisory Committee Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; Washington, D.C. 2015.
- Dror, D.K.; Allen, L.H. Vitamin E deficiency in developing countries. Food Nutr. Bull. 2011, 32, 124–143. [Google Scholar] [CrossRef]
- Henjum, S.; Manger, M.; Hampel, D.; Brantsæter, A.L.; Shahab-Ferdows, S.; Bastani, N.E.; Strand, T.A.; Refsum, H.; Allen, L.H. Vitamin B12 concentrations in milk from Norwegian women during the six first months of lactation. Eur. J. Clin. Nutr. 2020, 74, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Norman, E.J.; Morrison, J.A. Screening elderly populations for cobalamin (vitamin B12) deficiency using the urinary methylmalonic acid assay by gas chromatography mass spectrometry. Am. J. Med. 1993, 94, 589–594. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Urinary iodine concentrations for determining iodine status deficiency in populations. Vitamin and Mineral Nutrition Information System. Available online: https://apps.who.int/iris/bitstream/handle/10665/85972/WHO_NMH_NHD_EPG_13.1_eng.pdf (accessed on Mar 8, 2023).
- Ahn, J.; Lee, J.H.; Lee, J.; Baek, J.Y.; Song, E.; Oh, H.S.; Kim, M.; Park, S.; Jeon, M.J.; Kim, T.Y.; et al. Association between urinary sodium levels and iodine status in Korea. Korean J. Intern. Med. 2020, 35, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.F.; Boccuzzi, L. Urine calcium: Laboratory measurement and clinical utility. Lab. Med. 2010, 41, 683–686. [Google Scholar] [CrossRef]
- Fernández-Ruiz, L.; Rodelo Haad, C.; Rodríguez-Portillo, M.; Santamaría-Olmo, R. Variation of phosphaturia according to phosphorus intake. Actual. Medica 2020, 105, 18–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yang, X.; Cheng, Y.; Zhang, H.; Xu, X.; Zhou, J.; Chen, H.; Su, M.; Yang, Y.; et al. Human Milk Lipid Profiles around the World: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 2519–2536. [Google Scholar] [CrossRef]
- Gibson, R.S.; Rahmannia, S.; Diana, A.; Leong, C.; Haszard, J.J.; Hampel, D.; Reid, M.; Erhardt, J.; Suryanto, A.H.; Sofiah, W.N.; et al. Association of maternal diet, micronutrient status, and milk volume with milk micronutrient concentrations in Indonesian mothers at 2 and 5 months postpartum. Am. J. Clin. Nutr. 2020, 112, 1039–1050. [Google Scholar] [CrossRef]
- EFSA NDA Panel; Turck, D. ; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Dietary reference values for thiamin. EFSA J. 2016, 14, 4653. [CrossRef]
- EFSA NDA Panel; Turck, D. ; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.; Mangelsdorf, I.; McArdle, H.; et al. Dietary Reference Values for riboflavin. EFSA J. 2017, 15, 4919. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014, 12, 3759. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for pantothenic acid. EFSA J. 2014, 12, 3581. [CrossRef]
- EFSA NDA Panel Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013, 11, 3408. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for vitamin A. EFSA J. 2015, 13, 4028. [CrossRef]
- EFSA NDA Panel Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [CrossRef]
- Semba, R.D.; Delange, F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001, 59, 269–278. [Google Scholar] [CrossRef]
- Andersson, M.; Braegger, C.P. The role of iodine for thyroid function in lactating women and infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015, 13, 4101. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for phosphorus. EFSA J. 2015, 13, 4185. [CrossRef]
- EFSA NDA Panel Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [CrossRef]
- LASER Analytica Comprehensive literature search and review of breast milk composition as preparatory work for the setting of dietary reference values for vitamins and minerals. EFSA Support. Publ. 2014, 11, EN–629; 154 pp. [CrossRef]
- Castillo, F.; Castillo-Ferrer, F.J.; Cordobilla, B.; Domingo, J.C. Inadequate content of docosahexaenoic acid (DHA) of donor human milk for feeding preterm infants: A comparison with mother’s own milk at different stages of lactation. Nutrients 2021, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Castillo Salinas, F.; Montaner Ramón, A.; Castillo Ferrer, F.-J.; Domingo-Carnice, A.; Cordobilla, B.; Domingo, J.C. Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term. Nutrients 2022, 14, 4956. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.; Gibson, R. Effect of maternal docosahexaenoic (DHA) supplementation on breast milk composition. Eur. J. Clin. Nutr. 1996, 50, 352–357. [Google Scholar] [PubMed]
- Valentine, C.J.; Morrow, G.; Fernandez, S.; Gulati, P.; Bartholomew, D.; Long, D.; Welty, S.E.; Morrow, A.L.; Rogers, L.K. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J. Pediatr. 2010, 157, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Baack, M.L.; Norris, A.W.; Yao, J.; Colaizy, T. Long Chain Polyunsaturated Fatty Acid Levels in U. S. Donor Human Milk: Meeting the Needs of Premature Infants? J Perinatol 2012, 32, 598–603. [Google Scholar] [CrossRef]
- Valentine, C.J. Maternal Dietary DHA Supplementation to Improve Inflammatory Outcomes in the Preterm Infant. Adv. Nutr. 2012, 3, 370–376. [Google Scholar] [CrossRef]
- Francois, C.A.; Connor, S.L.; Bolewicz, L.C.; Connor, W.E. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am. J. Clin. Nutr. 2003, 77, 226–233. [Google Scholar] [CrossRef]
- Scopesi, F.; Ciangherotti, S.; Lantieri, P.B.; Risso, D.; Bertini, I.; Campone, F.; Pedrotti, A.; Bonacci, W.; Serra, G. Maternal dietary PUFAs intake and human milk content relationships during the first month of lactation. Clin Nutr 2001, 20, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Kodentsova, V.M.; Vrzhesinskaya, O.A. Evaluation of the vitamin status in nursing women by vitamin content in breast milk. Bull. Exp. Biol. Med. 2006, 141, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Vitamin B-12 in humanmilk: A systematic review. Adv. Nutr. 2018, 9, 358S–366S. [Google Scholar] [CrossRef] [PubMed]
- Salmenperä, L. Vitamin C nutrition during prolonged lactation: optimal in infants while marginal in some mothers. Am. J. Clin. Nutr. 1984, 40, 1050–1056. [Google Scholar] [CrossRef]
- Hoppu, U.; Rinne, M.; Salo-Väänänen, P.; Lampi, A.-M.; Piironen, V.; Isolauri, E. Vitamin C in breast milk may reduce the risk of atopy in the infant. Eur. J. Clin. Nutr. 2005, 59, 123–128. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Iodine in humanmilk: A systematic review. Adv. Nutr. 2018, 9, 347S–357S. [Google Scholar] [CrossRef]
- Dorea, J.G. Selenium and breast-feeding. Br. J. Nutr. 2002, 88, 443–461. [Google Scholar] [CrossRef]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients 2016, 8, 1–19. [Google Scholar] [CrossRef]
- Escuder-Vieco, D.; Rodríguez, J.M.; Espinosa-Martos, I.; Corzo, N.; Montilla, A.; García-Serrano, A.; Calvo, M.V.; Fontecha, J.; Serrano, J.; Fernández, L.; et al. High-Temperature Short-Time and Holder Pasteurization of Donor Milk: Impact on Milk Composition. Life 2021, 11, 114. [Google Scholar] [CrossRef]
- Nessel, I.; Khashu, M.; Dyall, S.C. The effects of storage conditions on long-chain polyunsaturated fatty acids, lipid mediators, and antioxidants in donor human milk — A review. Prostaglandins Leukot. Essent. Fat. Acids 2019, 149, 8–17. [Google Scholar] [CrossRef]
- Leaf, A.; Lansdowne, Z. Vitamins--conventional uses and new insights. World Rev. Nutr. Diet. 2014, 110, 152–166. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | |
|---|---|
| Age (years) | 35.6 (32.9, 38.7) |
| Weight (kg) | 60.5 (55.2, 70.6) |
| Height (cm) | 164.1 (6.5) |
| Pre-pregnancy BMI (kg/m2) | 22.1 (20.6, 24.8) |
| Pre-pregnancy BMI (kg/m2) category | |
| Underweight (<18.5) | 4 (3.5%) |
| Normal (18.5-24.9) | 84 (73.7%) |
| Overweight (25-29.9) | 15 (13.2%) |
| Obese (≥30) | 11 (9.6%) |
| Current BMI (kg/m2) | 22.9 (21.1, 25.1) |
| Current BMI (kg/m2) category | |
| Underweight (<18.5) | 4 (3.5%) |
| Normal (18.5-24.9) | 81 (71.1%) |
| Overweight (25-29.9) | 16 (14.0%) |
| Obese (≥30) | 13 (11.4%) |
| Gestational weight gain (kg) | 11.3 (9.0, 14.0) |
| Postpartum weight retention (kg) | 1.0 (−0.6, 2.5) |
| Number of living children | |
| 0 a–1 | 63 (55.3%) |
| 2 | 39 (34.2%) |
| ≥3 | 12 (10.5%) |
| Country of origin: Spain | 102 (89.5%) |
| Education level | |
| Secondary studies | 2 (1.8%) |
| Technical studies | 14 (12.3%) |
| University studies | 98 (86.0%) |
| Currently working | 50 (43.9%) |
| Physical activity | |
| Sedentary | 26 (22.8%) |
| Low active | 60 (52.6%) |
| Active/very active | 28 (24.6%) |
| Tobacco consumption | |
| Previously | 28 (24.6%) |
| Currently | 1 (0.9%) |
| Passive smoking | 24 (21.1%) |
| Active smoking | 1 (0.9%) |
| Alcohol consumption | |
| Prior to pregnancy | 55 (48.2%) |
| During pregnancy | 1 (0.9%) |
| Currently | 4 (3.5%) |
| Season during the study | |
| Spring | 30 (26.3%) |
| n (%) | ||
|---|---|---|
| Diseases 1 | 41 (36.0%) | |
| Endocrinological and metabolic diseases* | 10 (8.8%) | |
| Cardiovascular diseases (hypertension) | 1 (0.9%) | |
| Respiratory diseases (asthma) | 6 (5.3%) | |
| Immune diseases (allergy, psoriasis, atopia) | 7 (6.1%) | |
| Spinal/medullary pathology | 6 (5.3%) | |
| Miscellaneous ** | 13 (11.4%) | |
| Medication intake 1 | 12 (10.5%) | |
| Oral contraceptives | 4 (3.5%) | |
| Thyroid hormone replacement therapy | 4 (3.5%) | |
| Other medicines *** | 5 (4.4%) | |
| Twin pregnancy | 4 (3.5%) | |
| Problems in the last pregnancy 1 | 36 (31.6%) | |
| Thyroid disorders | 17 (14.9%) | |
| Preeclampsia | 2 (1.8%) | |
| Gestational diabetes | 2 (1.8%) | |
| Intrauterine fetal growth restriction | 6 (5.3%) | |
| Intrauterine fetal death | 3 (2.6%) | |
| Other problems **** | 11 (9.6%) |
| Characteristic | n* | |
|---|---|---|
| Gestational age (weeks) | 114 | 39+4 (38+2, 40+2); 22+6–42+3 |
| Boy | 116 | 55 (47.4%) |
| Birth weight (grams) | 3195.0 (2795.0, 3472.5); 450.0-4640.0 | |
| Birth weight percentile 1 | ||
| ≤25 | 32 (27.6%) | |
| 25–75 | 73 (62.9%) | |
| ≥75 | 11 (9.5%) | |
| Age of infant (months) | ||
| 0–6 | 45 (39.5%) | |
| 6–12 | 43 (37.7%) | |
| 12–50 | 26 (22.8%) | |
| Postmenstrual age of preterm infants (weeks) | 19 | 50.3 (38.6, 78.2) |
| Weight percentile of breastfed child 2 | 113 | |
| ≤15 | 17 (15.0%) | |
| 15–85 | 77 (68.1%) | |
| ≥85 | 19 (16.8%) |
| Characteristic | n | n (%) |
|---|---|---|
| Donor previously | 114 | 20 (17.5%) |
| Duration of lactation of the previous child (months) |
51 a |
|
| 0 | 1(2.0%) | |
| 3-6 | 2 (3.9%) | |
| 6-12 | 10 (19.6%) | |
| 12-24 | 22 (43.1%) | |
| ≥24 | 16 (3.3%) | |
| Current lactation stage (months) | 114 | 7.0 (4.8, 12.0); 1.8-50 |
| Type of lactation |
113 b |
|
| Exclusive | 52 (46.0%) | |
| Partial | 61 (54.0%) | |
| Sum of child direct breastfeeding times plus daily pumped sessions <5 5–10 >10 Missing data |
114 |
12 (10.5%) 66 (57.9%) 33 (28.9%) 3 (2.6%) |
| Tandem breastfeeding | 114 | 5 (4.4%) |
| Breastfeeding twins | 114 | 1 (0.9%) |
| Type of milk expression * Manual Mechanical breast pump Simple electric breast pump Double electric breast pump |
114 |
7 (6.1%) 12 (10.5%) 82 (71.9%) 15 (13.2%) |
| Nutrient | Donors | |
|---|---|---|
| n | Mean (SE) | |
| Macronutrients (g/100 mL milk) | ||
| Lipids | 103 | 3.13 (0.17) |
| Carbohydrates | 7.73 (0.03) | |
| Proteins | 1.17 (0.03) | |
| Lipid classes (g/100 g fat) | ||
| Triacylglycerols | 20 | 96.19 (93.87, 97.26) |
| Diacylglycerols | 3.43 (2.47, 5.50) | |
| Monoacylglycerols | 0.03 (0.02, 0.07) | |
| Free fatty acids + cholesterol | 0.31 (0.22, 0.51) | |
| Polar lipids | 0.05 (0.01) | |
| Phospholipids (g/100 g of polar lipids) | ||
| Phosphatidylethanolamine | 20 | 24.63 (7.88) |
| Phosphatidylcholine | 30.95 (5.00) | |
| Sphingomyelin | 44.43 (11.09) | |
| Triacylglycerols (g/100 g fat) | ||
| CN24 | 20 | 0.01 (0.01, 0.02) |
| CN26 | 0.10 (0.03) | |
| CN28 | 0.07 (0.05, 0.12) | |
| CN30 | 0.19 (0.14, 0.27) | |
| CN32 | 0.26 (0.19, 0.41) | |
| CN34 | 0.33 (0.13, 0.44) | |
| CN36 | 0.36 (0.22, 0.65) | |
| CN38 | 1.57 (0.68) | |
| CN40 | 2.02 (0.54) | |
| CN42 | 2.70 (0.91) | |
| CN44 | 5.02 (1.37) | |
| CN46 | 7.51 (1.50) | |
| CN48 | 10.72 (1.43) | |
| CN50 | 14.71 (2.16) | |
| CN52 | 36.89 (4.83) | |
| CN54 | 17.30 (5.10) | |
| Fatty Acid (%) | Common Name | Donors (n = 108) | Reference Values | |
|---|---|---|---|---|
| European [35] 1 | World [59] 2 | |||
| Saturated Fatty Acids (SFAs) | ||||
| C6:0 | Caproic | 0.11 (0.02) | 0.08 ± 0.02 | 0.13 ± 0.47 |
| C8:0 | Caprylic | 0.18 (0.16, 0.21) | 0.22 ± 0.06 | 0.21 ± 0.22 |
| C10:0 | Capric | 1.20 (0.29) | 1.44 ± 0.34 | 1.37 ± 0.86 |
| C12:0 | Lauric | 5.42 (1.58) | 5.46 ± 1.84 | 5.7 ± 2.81 |
| C14:0 | Myristic | 5.88 (4.91, 7.75) | 6.19 ± 1.93 | 6.56 ± 3.05 |
| C15:0 | Pentadecanoic | 0.18 (0.13, 0.25) | ||
| C15:0 ai | C15:0 anteiso | 0.02 (0.02, 0.03) | ||
| C15:0 i | C15:0 iso | 0.03 (0.02, 0.05) | ||
| C16:0 i | C16:0 iso | 0.02 (0.01, 0.03) | ||
| C16:0 | Palmitic | 19.61 (2.45) | 21.94 ± 2.92 | 21.5 ± 4.82 |
| C17:0 ai | C17:0 anteiso | 0.04 (0.03, 0.06) | ||
| C17:0 i | C17:0 iso | 0.27 (0.06) | ||
| C17:0 | Margaric | 0.19 (0.15, 0.23) | 0.31 ± 0.15 | |
| C18:0 | Stearic | 5.72 (4.96, 6.49) | 6.68 ± 1.59 | 6.36 ± 2.07 |
| C20:0 | Arachidic | 0.16 (0.11, 0.21) | 0.17 ± 0.04 | 0.23 ± 0.17 |
| Monounsaturated Fatty Acids (MUFAs) | ||||
| C14:1 cis-9 (n5) | Myristoleic | 0.07 (0.04, 0.11) | ||
| C16:1 cis-9 (n7) | Palmitoleic | 1.47 (1.19, 1.76) | 2.21 ± 0.64 | 2.3 ± 0.92 |
| C17:1 | Margaroleic | 0.07 (0.03) | ||
| ∑ C18:1 trans | 0.22 (0.13, 0.34) | 0.66 ± 0.35 | ||
| C18:1 cis-9 (n9) | Oleic | 38.23 (4.98) | 35.59 ± 4.17 | 32.6 ± 5.84 |
| C18:1 cis-11 (n7) | Cis vaccenic | 1.61 (0.28) | 2.38 ± 0.53 | |
| C20:1 (n9) | Gondoic | 0.53 (0.36, 0.82) | 0.38 ± 0.12 | 0.46 ± 0.28 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||
| C18:2 (n6) | Linoleic (LA) | 14.79 (12.37, 17.19) | 14.00 ± 4.95 | 15.7 ± 7.15 |
| C20:2 (n6) | Eicosadienoic | 0.25 (0.19, 0.35) | 0.26 ± 0.07 | 0.37 ± 0.19 |
| C20:3 (n6) | Dihomo-γ-linolenic | 0.33 (0.23, 0.44) | 0.31 ± 0.09 | 0.37 ± 0.18 |
| C20:4 (n6) | Arachidonic (AA) | 0.54 (0.18) | 0.44 ± 0.12 | 0.50 ± 0.25 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||
| C18:3 (n3) | Linolenic (ALA) | 0.50 (0.40, 0.62) | 0.94 ± 0.55 | 1.11 ± 1.05 |
| C22:5 (n3) | Docosapentaenoic (DPA) | 0.07 (0.05, 0.11) | ||
| C22:6 (n3) | Docosahexaenoic (DHA) | 0.28 (0.17, 0.45) | 0.34 ± 0.35 | 0.37 ± 0.31 |
| n-7 Polyunsaturated Fatty Acids (n-7 PUFAs) | ||||
| C18:2 c9, t11 (n7) | Rumenic | 0.08 (0.04, 0.12) | ||
| Fatty acid families | ||||
| Not identified | 0.20 (0.15, 0.25) | |||
| SFAs | 39.83 (37.0, 42.1) | 42.23 ± 5.29 | 42.2 ± 7.73 | |
| MUFAs | 42.49 (5.22) | 41.34 ± 4.48 | 36.3 ± 6.46 | |
| PUFAs | 16.71 (14.83, 19.37) | 16.43 ± 5.07 | 21.2 ± 8.18 | |
| SCFAs | 0.11 (0.10, 0.12) | |||
| MCFAs (C8-C15) | 13.02 (11.13, 16.18) | |||
| LCFAs (C16-C18) | 84.0 (80.71, 86.35) | |||
| VLCFAs (C20-C24) | 2.34 (2.02, 3.16) | |||
| n-6 PUFAs | 15.77 (13.46, 18.44) | 17.8 ± 7.51 | ||
| n-3 PUFAs | 0.87 (0.72, 1.17) | 1.88 ± 2.63 | ||
| n-6 PUFAs/n-3 PUFAs | 17.21 (13.31, 24.81) | |||
| LA/ALA ratio | 28.68 (22.52, 40.31) | |||
| ARA/DHA ratio | 1.83 (1.32, 3.06) | 1.68 ± 0.89 | ||
| Nutrient 1 | Donors | Mature Milk Nutrient Concentration Reference |
|
|---|---|---|---|
| n | Concentration | ||
| Free thiamin, B1 (UPLC-MS/MS) mcg/L | 113 | 18.10 (10.03, 28.43) | Free thiamin 18.5 [60] Total thiamin 180 [61] |
| Free riboflavin, B2 (UPLC-MS/MS) mcg/L | 113 | 47.30 (23.58, 99.90) | Free riboflavin 11.2 [60] Total riboflavin 364 [62] |
| Nicotinamide, B3 (UPLC-MS/MS) mcg/L | 113 | 46.73 (28.50, 82.58) | Nicotinamide 275 [60] Total niacin 2100 [63] |
| Pantothenic acid, B5 (UPLC-MS/MS) mcg/L | 113 | 2264.90 (1864.90, 2540.00) | 2500 [64] 1304 [60] |
| Pyridoxal, B6 (UPLC-MS/MS) mcg/L | 113 | 36.73 (27.80, 53.30) | Pyridoxal 96 [60] B6 130 [65] |
| Folic acid, B9 (UPLC-MS/MS) mcg/L | 113 | 19.88 (7.02) | Folate 80 [65] |
| Cobalamin, B12 (Competitive immunoassay) | 113 | ||
| pM | 490.63 (74.30) | ||
| mcg/L | 0.66 (0.10) | 0.5 [66] | |
| Ascorbic acid (HPLC-DAD) mg/dL | 112 | 3.91 (1.71) | |
| Dehydroascorbic acid (HPLC-DAD) mg/dL | 112 | 1.91 (1.29, 3.38) | |
| Vitamin C * (HPLC-DAD) | 112 | ||
| mg/dL | 6.37 (1.41) | ||
| mg/L | 63.70 (14.10) | 35–90 [67] | |
| Retinol (HPLC with fluorescence and UV detector) | 112 | ||
| mcg/dL | 41.15 (26.80, 72.48) | ||
| mcg/L | 411.50 (268.00, 724.80) | 530 [68] | |
| Vitamin D3 (UPLC–electrospray ionization/tandem MS) | 112 | ||
| pg/mL | 1603.65 (373.83, 5279.93) | ||
| mcg/L | 1.60 (0.37, 5.28) | 0.25–2 [69] | |
| 25(OH)D3 (UPLC–electrospray ionization/tandem MS) | 112 | ||
| pg/mL | 53.90 (27.13, 109.85) | ||
| mcg/L | 0.05 (0.03, 0.11) | ||
| α-tocopherol (HPLC with fluorescence and UV detector) | 112 | ||
| mcg/dL | 463.80 (373.33, 586.76) | ||
| mg/L | 4.64 (3.73, 5.87) | 4.6 [70] | |
| γ-tocopherol (HPLC with fluorescence and UV detector) | 112 | ||
| mcg/dL | 50.19 (36.43, 67.08) | ||
| mg/L | 0.50 (0.36, 0.67) | 0.45 [60] | |
| Vitamin E (as TE) ** | 112 | ||
| mcg/dL | 488.02 (163.13) | ||
| mg/L | 4.88 (1.63) | 5.2 [60] | |
| Iodine (ICP-MS) ppb (mcg/L) | 113 | 148.45 (98.95, 204.98) | 50–100 [65] 100–200 [71,72] |
| Calcium (ICP-MS) ppm (mg/L) | 113 | 99.10 (59.70, 127.35) | 200–300 [73] |
| Phosphorous (ICP-MS) ppm (mg/L) | 113 | 132.47 (114.00, 150.40) | 120–140 [65,74] |
| Selenium (ICP-MS) ppb (mcg/L) | 113 | 10.88 (9.30, 12.68) | 18 [75] |
| Fatty Acids (% of Total Fatty Acids) |
Associated Variables | Beta | Std. Err. | t | P>|t| | 95% CI |
|---|---|---|---|---|---|---|
| SFAs a Observations = 105 R2 = 0.34 |
DMA in erythrocytes (% of fat in erythrocytes) |
2.700 | 0.795 | 3.40 | 0.001 | [1.122, 4.277] |
| Plasma C18:0 (% of fat in plasma) |
2.023 | 0.550 | 3.68 | <0.001 | [0.932, 3.114] | |
| C17:1 in erythrocytes (% of fat in erythrocytes) |
-13.556 | 3.565 | -3.80 | <0.001 | [-20.629, -6.483] | |
|
Trans fatty acids (average % intake per day during the dietary record) |
5.789 | 2.171 | 2.67 | 0.009 | [1.481, 10.097] | |
| Breastfeeding time (months) | 0.203 | 0.071 | 2.86 | 0.005 | [0.062, 0.344] | |
| Total MUFAs b Observations = 103 R2 = 0.16 |
Plasma MUFAs (% of fat in plasma) |
0.458 | 0.165 | 2.77 | 0.007 | [0.130, 0.786] |
| C17:1 in erythrocytes (% of fat in erythrocytes) |
13.523 | 4.895 | 2.76 | 0.007 | [3.810,23.235] | |
| Total PUFAs Observations = 108 R2 = 0.04 |
PUFAs (average intake g/day during the dietary record) |
0.139 | 0.062 | 2.23 | 0.028 | [0.015, .263] |
| Linoleic Acid c Observations = 106 R2 = 0.10 |
Linoleic acid in erythrocytes (% of fat in erythrocytes) |
0.633 | 0.209 | 3.02 | 0.003 | [0.217, 1.048] |
| Meat, fish, eggs (average servings/day during the dietary record) |
0.684 | 0.281 | 2.44 | 0.016 | [0.128, 1.241] | |
| DHA d Observations = 104 R2 = 0.45 |
Plasma DHA (% of fat in plasma) |
0.164 | 0.334 | 4.91 | <0.001 | [0.098, 0.230] |
| DHA (average intake g/day during the dietary record) |
0.382 | 0.075 | 5.12 | <0.001 | [0.234, 0.530] | |
| Total Omega-3 e Observations = 106 R2 = 0.29 |
DHA (average intake g/day during the diet diary) |
0.783 | 0.120 | 6.51 | <0.001 | [0.544, 1.021] |
| Vitamins | Associated Variables | Beta | Std. Err. | t | P>|t| | 95% CI |
|---|---|---|---|---|---|---|
| Free thiamin, B1 (mcg/L) Observations = 113 R2 = 0.12 |
Plasma thiamin (mcg/L) | 10.817 | 4.915 | 2.20 | 0.030 | [1.075, 20.559] |
| Milk and dairy products (average servings/day during the diet diary) | -2.880 | 1.090 | -2.64 | 0.009 | [-5.040, -0.720] | |
| Breastfeeding time (months) |
-0.494 | 0.210 | -2.35 | 0.021 | [-0.911, -0.077] | |
| Free riboflavin, VB2 (mcg/L) a Observations = 100 R2 = 0.21 |
Riboflavin intake (average mg/day during the dietary record) | 20.991 | 8.758 | 2.40 | 0.018 | [3.609, 38.373] |
| Vitamin B2 supplementation during lactation (receiving or not) | 39.742 | 15.258 | 2.60 | 0.011 | [9.459, 70.024] | |
| Pyridoxal, B6 (mcg/L) b Observations = 106 R2 = 0.17 |
Vitamin B6 supplementation during pregnancy (receiving or not) |
11.629 | 3.114 | 3.73 | <0.001 | [5.454, 17.804] |
| Breastfeeding time (months) | -0.776 | 0.273 | -2.84 | 0.005 | [-1.318, -0.234] | |
| Dehidroascorbic acid (mg/dL) Observations = 112 R2 = 0.15 |
Plasma ascorbic acid (mcM) | -0.014 | 0.005 | -2.92 | 0.004 | [-0.024, -0.005] |
| Fruits (average servings/day during the dietary record) |
0.271 | 0.128 | 2.12 | 0.036 | [0.018, 0.524] | |
| Breastfeeding time (months) | -0.062 | 0.025 | -2.47 | 0.015 | [-0.112, -0.012] | |
| Cholecalciferol (pg/mL) c Observations = 32 R2 = 0.57 |
Plasma cholecalciferol (pg/mL) | 17.342 | 4.238 | 4.09 | <0.001 | [3.178, 23.510] |
| Milk and dairy products (average servings/day during the dietary record) | 520.533 | 168.819 | 3.08 | 0.005 | [174.722, 866.343] | |
| 25 (OH) D3 (pg/mL) d Observations = 99 R2 = 0.24 |
Plasma 1,25 (OH)2 D3 (pg/mL) | 0.156 | 0.042 | 3.73 | <0.001 | [0.073, 0.238] |
| Vitamin D supplementation during lactation (receiving or not) |
35.673 | 12.569 | 2.84 | 0.006 | [10.720, 60.625] | |
| Breastfeeding time (months) | 2.225 | 1.076 | 2.07 | 0.041 | [0.089, 4.360] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





