Submitted:
20 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Search strategy
2.3. Data collection
2.4. Assessment of the quality of included studies
2.5. Data synthesis
3. Results
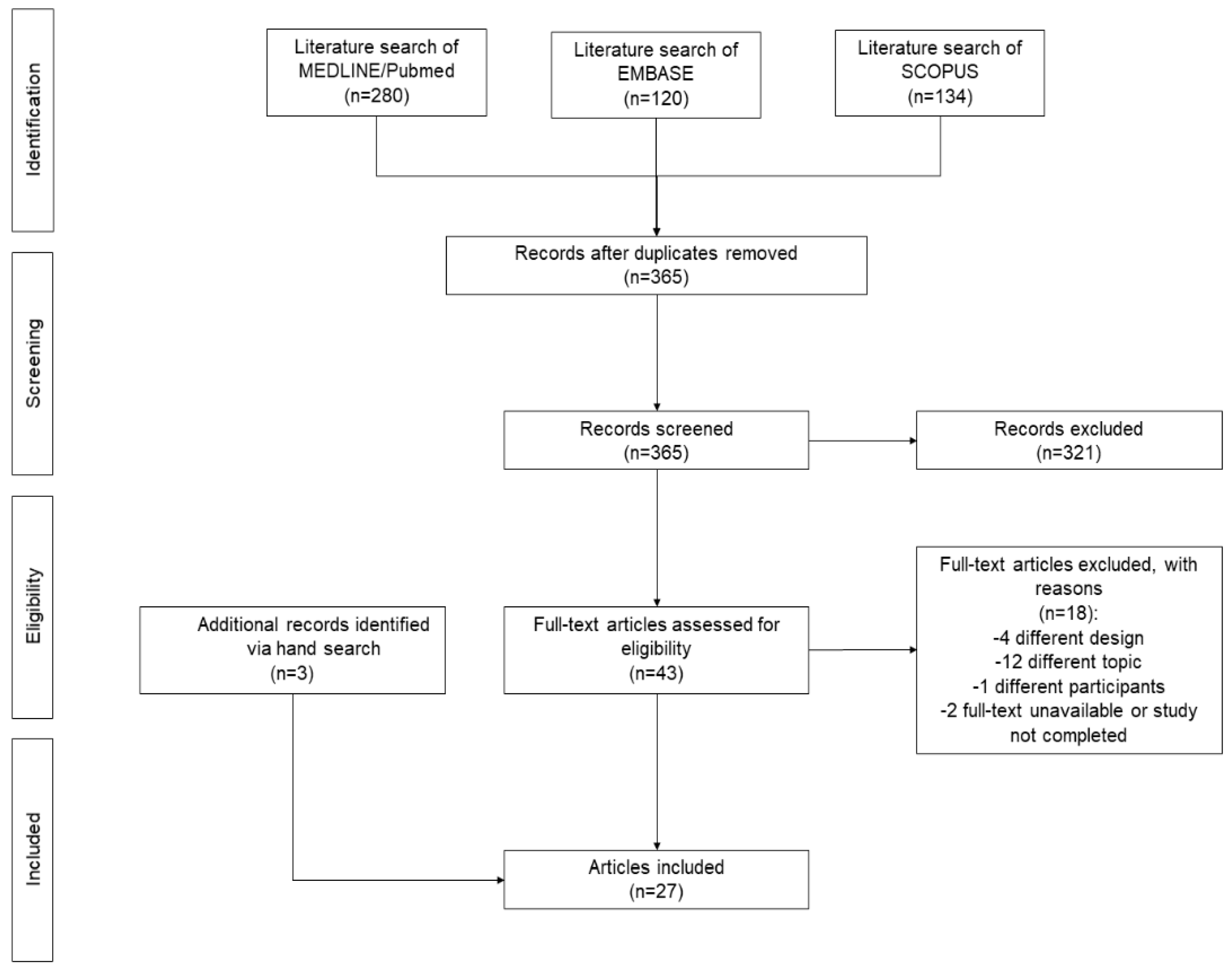
3.1. Search results
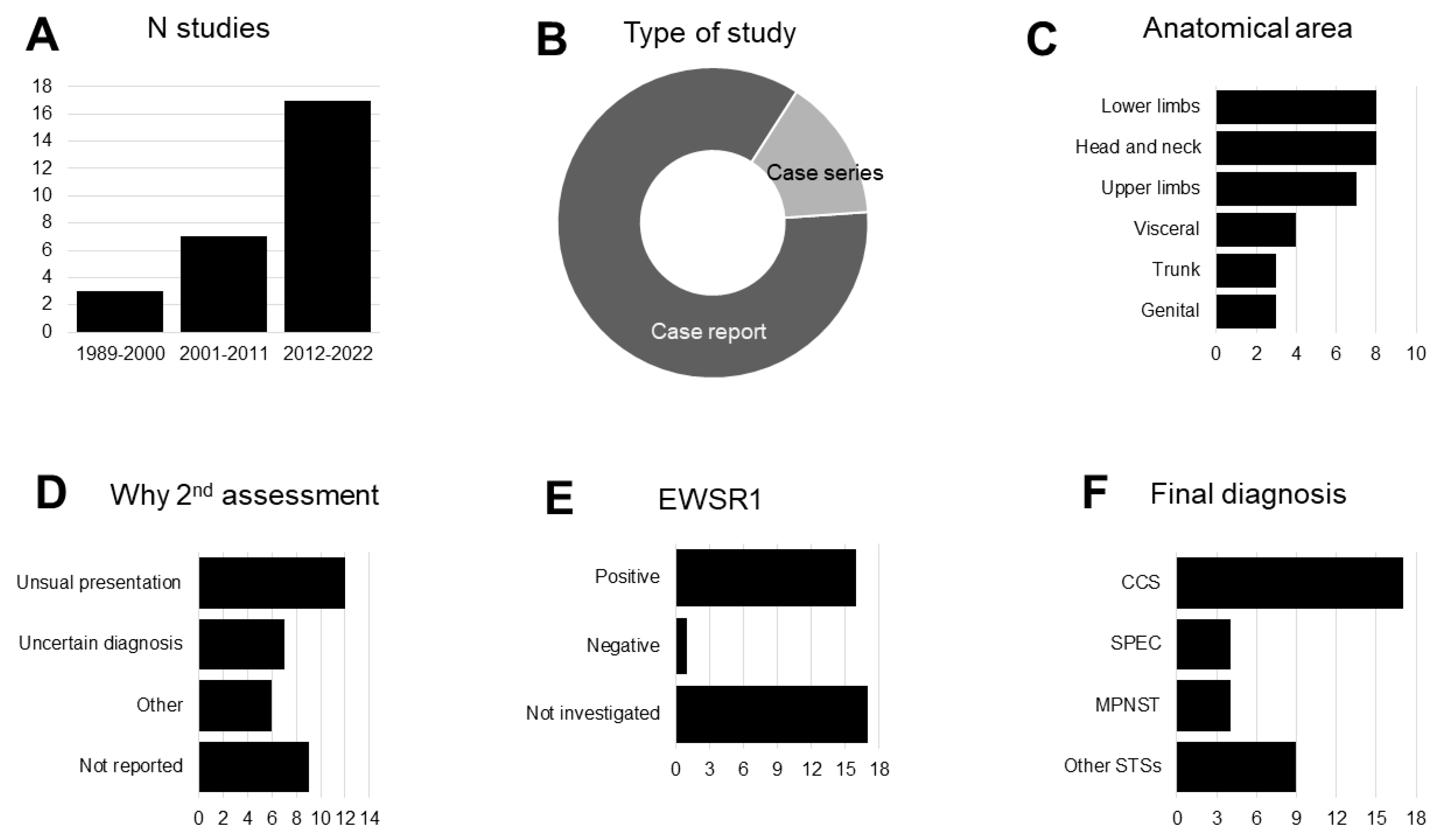
3.2. Narrative synthesis of the findings
3.3. Critical appraisal of the quality of included studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatta G, van der Zwan JM, Casali PG, et al.; RARECARE working group. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493-511. [CrossRef]
- Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021 Apr;113(2):70-84. [CrossRef]
- American Cancer Society Key statistics for soft tissue sarcomas (https://www.cancer.org/cancer/soft-tissue-sarcoma/about/key-statistics.html. (Accessed April 10, 2023).
- Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K, Kuroda H, Inayama Y, Oshiro H, Kobayashi H, Nakajima T, Fukuda T, Ae K, Hashimoto H. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol. 2008;32(3):452-60. [CrossRef]
- Panagopoulos I, Mertens F, Isaksson M, Mandahl N. Absence of mutations of the BRAF gene in malignant melanoma of soft parts (clear cell sarcoma of tendons and aponeuroses) Cancer Genet Cytogenet. 2005;156:74–76. [CrossRef]
- Patel RM, Downs-Kelly E, Weiss SW, et al. Dual-color, break-apart fluorescence in situ hybridization for EWS gene rearrangement distinguishes clear cell sarcoma of soft tissue from malignant melanoma. Mod Pathol. 2005;18:1585–1590. [CrossRef]
- Dim DC, Cooley LD, Miranda RN. Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med. 2007; 131:152–156. [CrossRef]
- Pavlidis NA, Fisher C, Wiltshaw E. Clear-cell sarcoma of tendons and aponeuroses: a clinicopathologic study. Presentation of six additional cases with review of the literature. Cancer. 1984; 54:1412–1417.
- Biglow LR, Cuda J, Dotson J. A Rare Case of Epithelioid Malignant Peripheral Nerve Sheath Tumor Mimicking Malignant Melanoma. Cureus. 2021;13(2):e13424. [CrossRef]
- Gaspard M, Lamant L, Tournier E, et al. Evaluation of eight melanocytic and neural crest-associated markers in well-characterized series of 124 malignant peripheral nerve sheath tumours (MPNST): useful to distinguish MPNST from melanoma? Histopathology. 2018;73:969–982. [CrossRef]
- Zoufaly, A., Schmiedel, S., Lohse, A., & van Lunzen, J. (2007). Intestinal Kaposi's sarcoma may mimic gastrointestinal stromal tumor in HIV infection. World journal of gastroenterology, 13(33), 4514–4516. [CrossRef]
- Shafiq MB Sr, Rafi I, Shoaib A, Ali S, Iqbal F, Latif T, Mushtaq U. The Outcome of Extremity Soft Tissue Sarcomas in Terms of Resection Margins: A Study From a Cancer Dedicated Center. Cureus. 2022;14(6):e26086. [CrossRef]
- Kollender R, Merimsky O, Sternheim A, Gortzak Y, Dadia S, Doron A, Novikov I, Kollender Y, Soyfer V. Radiation Therapy Before Repeat Wide Resection for Unplanned Surgery of Soft Tissue Sarcoma ("Oops" Operation) Results in Improved Disease-Free Survival. Adv Radiat Oncol. 2022;7(6):101007. [CrossRef]
- Keung EZ, Krause KJ, Maxwell J, Morris CD, Crago AM, Houdek MT, Kane J, Lewis V, Callegaro D, Miller B, Lazar AJ, Gladdy R, Raut CP, Fabbri N, Al-Refaie W, Fairweather M, Wong SL, Roland CL. Sentinel Lymph Node Biopsy for Extremity and Truncal Soft Tissue Sarcomas: A Systematic Review of the Literature. Ann Surg Oncol. 2023;30(2):958-967. [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. [CrossRef]
- Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. [CrossRef]
- Potter AJ, Dimitriou F, Karim RZ, Mahar A, Chan C, Long GV, Scolyer RA. Cutaneous clear cell sarcoma with an epidermal component mimicking melanoma. Pathology. 2022;54(3):369-371. [CrossRef]
- Tahiri Elousrouti L, Hammas N, Elmernissi FZ, Elfatemi H, Chbani L. Clear-Cell Sarcoma With an Unusual Presentation Mimicking Metastatic Melanoma. Cureus. 2022;14(11):e32010. [CrossRef]
- Zhang X, Zhang PJ, Sussman R, Litzky LA, Kucharczuk JC, Deshpande C. Atypical clear cell sarcoma of the pleura presenting as large pleural effusion with 22q12 abnormality: A challenging case with twists and turns. Human Pathology: Case Reports. 2021;24:200489. [CrossRef]
- Nawrocki S, Fitzhugh VA, Groisberg R, Aviv HA, Maghari A. A rare case of primary dermal clear cell sarcoma with focal epidermotropism: An entity difficult to distinguish from melanoma. J Cutan Pathol. 2020;47(7):621-624. [CrossRef]
- Obiorah IE, Ozdemirli M. Clear cell sarcoma in unusual sites mimicking metastatic melanoma. World J Clin Oncol. 2019;10(5):213-221. [CrossRef]
- Donzel M, Zidane-Marinnes M, Paindavoine S, Breheret R, de la Fouchardière A. Clear cell sarcoma of the soft palate mimicking unclassified melanoma. Pathology. 2019;51(3):331-334. [CrossRef]
- Obiorah IE, Brenholz P, Özdemirli M. Primary Clear Cell Sarcoma of the Dermis Mimicking Malignant Melanoma. Balkan Med J. 2018;35(2):203-207. [CrossRef]
- Curry JL, Tetzlaff MT, Wang SA, Landon G, Alouch N, Patel SP, Nagarajan P, Gupta S, Aung PP, Devine CE, Khoury JD, Loghavi S, Prieto VG, DiNardo CD, Gershenwald JE. Case Report of Myeloid Sarcoma Masquerading as In-Transit Metastasis at a Previous Melanoma Site: Avoiding a Diagnostic Pitfall. Am J Dermatopathol. 2018;40(11):831-835. [CrossRef]
- Leon-Castillo A, Chrisinger JSA, Panse G, Samdani RT, Ingram DR, Ravi V, Prieto VG, Wang WL, Lazar AJ. Index report of cutaneous angiosarcomas with strong positivity for tyrosinase mimicking melanoma with further evaluation of melanocytic markers in a large angiosarcoma series. J Cutan Pathol. 2017;44(8):692-697. [CrossRef]
- Zivanovic M, Luzar B, Bacchi CE, Calonje JE. Cutaneous clear cell sarcoma with intraepidermal component mimicking spitzoid melanoma. Virchows Arch. 2017:471(Suppl 1):S1–S352.
- Jackson CR, Minca EC, Kapil JP, Smith SC, Billings SD. Superficial malignant peripheral nerve sheath tumor with overlying intradermal melanocytic nevus mimicking spindle cell melanoma. J Cutan Pathol. 2016;43(12):1220-1225. [CrossRef]
- Castriconi M, Antropoli M, Grillo M, Andreano M, Santoro M, Villamania E. Unusual bleeding of a giant cell fibroblastoma: a soft tissue sarcoma of the skin mimicking metastatic melanoma. Ann Ital Chir. 2015;86(ePub):S2239253X15023701.
- Sayah M, Hammer S. Colonic Clear Cell Sarcoma Associated With Neurofibromatosis Type 2. American Journal of Clinical Pathology. 2015;144 (Issue suppl_2):A409. [CrossRef]
- Liu C, Ren Y, Li X, Cao Y, Chen Y, Cui X, Li L, Li F. Absence of 19 known hotspot oncogenic mutations in soft tissue clear cell sarcoma: two cases report with review of the literature. Int J Clin Exp Pathol. 2014;7(8):5242-9.
- Sidiropoulos M, Busam K, Guitart J, Laskin WB, Wagner AM, Gerami P. Superficial paramucosal clear cell sarcoma of the soft parts resembling melanoma in a 13-year-old boy. J Cutan Pathol. 2013;40(2):265-8. [CrossRef]
- Falconieri G, Bacchi CE, Luzar B. Cutaneous clear cell sarcoma: report of three cases of a potentially underestimated mimicker of spindle cell melanoma. Am J Dermatopathol. 2012;34(6):619-25. [CrossRef]
- Rodríguez-Martín M, Sáez-Rodríguez M, Esquivel B, Gonzáalez RS, Cabrera AN, Herrera AM. Clear cell sarcoma: a case mimicking primary cutaneous malignant melanoma. Indian J Dermatol. 2009;54(2):168-72. [CrossRef]
- Tanas MR, Rubin BP. Malignant neuroectodermal tumor with melanocytic and rhabdomyoblastic differentiation. Rare Tumors. 2009;1(2):e26. [CrossRef]
- Brightman LA, Demierre MF, Byers HR. Macrophage-rich epithelioid angiosarcoma mimicking malignant melanoma. J Cutan Pathol. 2006;33(1):38-42. [CrossRef]
- Matsuda Y, Saoo K, Hosokawa K, Yamakawa K, Yokohira M, Zeng Y, Takeuchi H, Iwai J, Shirai T, Obika K, Imaida K. Epithelioid malignant peripheral nerve sheath tumor. Report of a case with inflammatory infiltration. Pathol Res Pract. 2005;201(4):355-60. [CrossRef]
- Demir Y, Tokyol C. Superficial malignant schwannoma of the scalp. Dermatol Surg. 2003;29(8):879-81. [CrossRef]
- Bonetti F, Martignoni G, Colato C, Manfrin E, Gambacorta M, Faleri M, Bacchi C, Sin VC, Wong NL, Coady M, Chan JK. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Mod Pathol. 2001;14(6):563-8. [CrossRef]
- Ferreiro JA, Nascimento AG. Hyaline-cell rich chondroid syringoma. A tumor mimicking malignancy. Am J Surg Pathol. 1995;19(8):912-7. [CrossRef]
- Honma K, Watanabe H, Ohnishi Y, Tachikawa S, Tachikawa K. Epithelioid malignant schwannoma. A case report. Acta Pathol Jpn. 1989 Mar;39(3):195-202. [CrossRef]
- Gould E, Albores-Saavedra J, Rothe M, Mnaymneh W, Menendez-Aponte S. Malignant giant cell tumor of soft parts presenting as a skin tumor. Am J Dermatopathol. 1989;11(3):197-201. [CrossRef]
| First author | Year | Type of study | N pts | Sex | Age (years) | Initial diagnosis a | Anatomical region | Site | Clinical presentation | IHC positivity |
|---|---|---|---|---|---|---|---|---|---|---|
| Potter AJ | 2022 | Case report | 1 | M | 30 | Melanoma | Lower limbs | Toe | Ulcerated nodular cutaneous lesion on the distal third toe, which had been present for several years | S100, HMB45, SOX10 |
| Tahiri EL | 2022 | Case report | 1 | M | 31 | Melanoma | Lower limbs | Heel | Heel mass nodule | S100, HMB45, MelanA |
| Biglow LR | 2021 | Case report | 1 | F | 47 | Melanoma | Upper limbs | Finger | Subcutaneous nodule at the finger without any obvious nevus or skin color changes | S100, SOX10, vimentina, BCL2 |
| Zhang X | 2021 | Case report | 1 | M | 68 | Melanoma | Visceral | Pleura | Dyspnea, cough following a dental abscess that was treated with root canal procedure; imaging studies revealed a large right pleural effusion, raising the concern of an empyema | SOX10, S100, HMB45, Melan-A |
| Nawrocki S | 2020 | Case report | 1 | M | 25 | Melanoma | Lower limbs | Left inguinal region | Raised blood blister that changed colors | S100, HMB45, Melan A |
| Obiorah IE |
2019 |
Case series |
2 |
F | 37 | Melanoma | Head and neck | Left neck | Complaint of left jaw pain and swelling. | HMB45, S100, CD31, CD34, CD68 |
| M | 33 | Melanoma | Trunk | Back | Mid back pain radiating to the flanks, and leg weakness and numbness, with gait abnormalities | HMB45, S100, Vimentine | ||||
| Donzel M | 2019 | Case report | 1 | M | 27 | Melanoma | Head and neck | Palate | Palatal ulcerations. Ill-defined and erythematous, with a friable centre, superficial erosions, and irregular, raised edges | HMB45, SOX10, Melan A |
| Obiorah IE | 2018 | Case report | 1 | F | 43 | Melanoma | Head and neck | Right neck | Mall nodule on the right side of her neck | S100, HMB45, vimentina |
| Curry JL | 2018 | Case report | 1 | M | 68 | Melanoma | Upper limbs | Left upper harm | Primary tumor not told; recurrence: new, slightly tender, 1.0 cm purpuric cutaneous nodule within the lymphatic drainage field of his previous primary melanoma of his left upper arm | CD11, CD43, CD68 |
| Leon-Castillo A | 2017 | Case report | 1 | M | 65 | Melanoma | Head and neck | Occipital scalp | Large multi-nodular cutaneous occipital scalp lesion with erythematous background | CD31, ERG, D2-40, factor VIII–related antigen, Tyrosinase, HMB45, melan A |
| Zivanovic M | 2017 | Case report | 1 | M | 20 | Melanoma | Lower limbs | Foot | N/A | S100, melanA, HMB45 |
| Jackson CR | 2016 | Case report | 1 | M | 56 | Melanoma | Trunk | Chest | Flesh-colored chest lesion for 7 years | S100, SOX10, CD34 |
| Castriconi M | 2015 | Case report | 1 | M | 56 | Melanoma | Upper limbs | Right axilla | Giant mass located on the right axilla. | N/A |
| Sayah M | 2015 | Case report | 1 | F | 54 | Suspect of melanoma | Visceral | Cecum | Severe iron deficiency anemia and hematochezia | S100, cytokeratins, HMB45 |
| Liu C | 2014 |
Case series |
2 | M | 29 | Suspect of melanoma | Upper limbs | Left thumb | Solid gray-white tumor | HMB45, Melan A, CD56, S100, vimentine, NSE |
| M | 76 | Suspect of melanoma | Visceral | Jejunum | Complaining of bowel obstruction, macroscopic examination: tumor (2.5 cm×2.2 cm×1.5 cm) with a whitish-grey surface. | S100, vimentine, NSE | ||||
| Sidiropoulos M | 2012 | Case report | 1 | M | 13 | Suspect of melanoma | Head and neck | Lower lip | Symptomatic papule on the lower lip that was suggestive of a mucocele. | S100, CD99, sinaptofisina, HMB45, MiTF |
| Falconieri G | 2012 |
Case series |
3 | M | 12 | Melanoma | Lower limbs | Left foot | Lesion in the dorsal aspect of foot | S100, Melan A |
| M | 60 | Melanoma | Lower limbs | Upper thigh | Slowly growing pigmented nodular lesion | S100 | ||||
| F | 29 | Melanoma | Lower limbs | Right foot | Lesion in the sole of the foot | S100, Melan A, HMB45 | ||||
| Rodríguez MM | 2009 | Case report | 1 | M | 53 | Melanoma | Upper limbs | Right harm | Painful lesion erythematous, dome-shaped, nodular lesion, 1.3 cm in diameter, firm to palpation and movable with a serohemorragic crust on its surface | S100, HMB45 |
| Tanas MR | 2009 | Case report | 1 | M | 67 | Melanoma | Trunk | Abdomen | Abdominal mass | S100, HMB45, MiFT, Melan A |
| Zoufaly A | 2007 | Case report | 1 | M | 69 | Melanoma | N/A | N/A | N/A | N/A |
| Brightman LA | 2006 | Case report | 1 | M | 86 | Melanoma | Head and neck | Scalp | Large irregular dark grey-blue plaque with an adjacent speckled tan nodule | S100, CD31, CD34 |
| Matsuda Y | 2005 | Case report | 1 | M | 75 | Suspect of melanoma | Lower limbs | Left thight | Oval-shaped mass, elastic soft, and adherent to the left thigh on palpation | S-100, NSE, GFAP, MBP, cromogranina A e sinaptofisina, |
| Demir Y | 2003 | Case report | 1 | M | 80 | Suspect of melanoma | Head and neck | Scalp | Painless ulceration on his scalp | S100 |
| Bonetti F | 2001 |
Case series |
4 | F | 28 | Suspect of melanoma | Visceral | Ileum | Abdominal pain | HMB45, MART 1 |
| F | 19 | Suspect of melanoma | Genital | Uterus | Abdominal pain | HMB45 | ||||
| F | 40 | Suspect of melanoma | Genital | Uterus | Surgery because of uterine leiomyomas; during the operation, a2.5x12x1.5 cm pelvic nodule was accidentally found and thought to represent endometriosis | HMB45 | ||||
| F | 41 | Suspect of melanoma | Genital | Myometrium | Presumed fibroids in uterus | HMB45, MART 1 | ||||
| Ferreiro JA | 1995 | Case report* | 1 | M | 75 | Melanoma | Head and neck | Face | Not painful mass of the face | keratin, S100, vimentine |
| Honma K | 1989 | Case report | 1 | M | 65 | Melanoma | Upper limbs | Left axillary fossa | N/A | Leu7, NSE |
| Gould E | 1989 | Case report b | 1 | M | 78 | Melanoma | Upper limbs | Left arm | Black nodule above the elbow | a1 anti-chymotrypsin (AACT), a1 antitrypsin (AAC) |
| First author | Year | Why second assessment and/or diagnostic re-evaluation | EWSR1 translocation | Final diagnosis |
|---|---|---|---|---|
| Potter AJ | 2022 | Unresponsive to treatment | Positive | Clear cell sarcoma |
| Tahiri EL | 2022 | Unusual presentation | Positive | Clear cell sarcoma |
| Biglow LR | 2021 | Unusual presentation | Negative | Malignant peripheral nerve sheath tumor |
| Zhang X | 2021 | Uncertain diagnosis | Positive | Clear cell sarcoma |
| Nawrocki S | 2020 | Uncertain diagnosis | Positive | Clear cell sarcoma |
| Obiorah IE | 2019 |
Review after death | Positive | Clear cell sarcoma |
| Uncertain diagnosis | Positive | Clear cell sarcoma | ||
| Donzel M | 2019 | Uncertain diagnosis | Positive | Clear cell sarcoma |
| Obiorah IE | 2018 | Uncertain diagnosis | N/A | Clear cell sarcoma |
| Curry JL | 2018 | Review after surgery | N/A | Myeloid sarcoma |
| Leon-Castillo A | 2017 | N/A | N/A | Cutaneous angiosarcoma |
| Zivanovic M | 2017 | N/A | Positive | Clear cell sarcoma |
| Jackson CR | 2016 | Expert opinion | N/A | MPNST - malignant peripheral nerve sheath tumors |
| Castriconi M | 2015 | N/A | N/A | Pleomorphic sarcoma |
| Sayah M | 2015 | Unusual presentation | Positive | Clear cell sarcoma |
| Liu C | 2014 |
Unusual presentation | Positive | Clear cell sarcoma |
| Unusual presentation | Positive | Clear cell sarcoma | ||
| Sidiropoulos M | 2012 | Research for EWSR1 translocation | Positive | Clear cell sarcoma |
| Falconieri G | 2012 |
Unusual presentation | Positive | Clear cell sarcoma |
| Unusual presentation | Positive | Clear cell sarcoma | ||
| Unusual presentation | Positive | Clear cell sarcoma | ||
| Rodríguez MM | 2009 | Uncertain diagnosis | Positive | Clear cell sarcoma |
| Tanas MR | 2009 | N/A | N/A | Malignant peripheral nerve sheath tumor |
| Zoufaly A | 2007 | N/A | N/A | Kaposi's sarcoma |
| Brightman LA | 2006 | Uncertain diagnosis | N/A | Cutaneous epithelioid angiosarcoma |
| Matsuda Y | 2005 | N/A | N/A | Malignant peripheral nerve sheath tumor |
| Demir Y | 2003 | Expert opinion | N/A | Malignant schwannoma |
| Bonetti F | 2001 |
Unusual presentation | N/A | Sarcoma of perivascular epithelioid cells |
| Unusual presentation | N/A | Sarcoma of perivascular epithelioid cells | ||
| Unusual presentation | N/A | Sarcoma of perivascular epithelioid cells | ||
| Unusual presentation | N/A | Sarcoma of perivascular epithelioid cells | ||
| Ferreiro JA | 1995 | N/A | N/A | Chondroid syringoma |
| Honma K | 1989 | N/A | N/A | Epithlioid malignant Schwannoma |
| Gould E | 1989 | N/A | N/A | Malignant Giant Cell Tumor of soft tissue |
| First author | Year | Clear criteria for inclusion | Valid methods for the identification of the initial condition | Valid methods for identification of the final condition | In a case series, consecutive inclusion of participant | Clear reporting of demographics | Clear reporting of clinical information | Reporting of time of second assessment | Reporting of reason for second assessment |
|---|---|---|---|---|---|---|---|---|---|
| Potter AJ | 2022 | Yes | Yes | Yes | N/A | Yes | Yes | No | Yes |
| Tahiri EL | 2022 | Yes | Yes | yes | N/A | Yes | Yes | No | Yes |
| Biglow LR | 2021 | Yes | Yes | Unclear | N/A | Yes | Yes | No | Yes |
| Zhang X | 2021 | Yes | Yes | Yes | N/A | Yes | Yes | Unclear | Yes |
| Nawrocki S | 2020 | Yes | Yes | Yes | N/A | Yes | Yes | No | Yes |
| Obiorah IE | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Donzel M | 2019 | Yes | Yes | Yes | N/A | Yes | Yes | No | Yes |
| Obiorah IE | 2018 | Yes | Yes | Yes | N/A | Yes | Yes | No | Yes |
| Curry JL | 2018 | Yes | Yes | Yes | N/A | Yes | Yes | No | Yes |
| Leon-Castillo A | 2017 | Yes | Yes | Yes | N/A | Yes | Yes | No | No |
| Zivanovic M | 2017 | Yes | Yes | Yes | N/A | Yes | No | No | No |
| Jackson CR | 2016 | Yes | Yes | Unclear | N/A | Yes | Yes | No | Yes |
| Castriconi M | 2015 | Yes | Yes | Unclear | N/A | Yes | Yes | No | No |
| Sayah M | 2015 | Yes | Unclear | Yes | N/A | Yes | Yes | No | Yes |
| Liu C | 2014 | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes |
| Sidiropoulos M | 2012 | Yes | Unclear | Yes | N/A | Yes | Yes | No | Yes |
| Falconieri G | 2012 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Rodríguez MM | 2009 | Yes | Unclear | Yes | N/A | Yes | Yes | No | Unclear |
| Zoufaly A | 2007 | Yes | Yes | No | N/A | Yes | No | No | No |
| Tanas MR | 2009 | Yes | Unclear | Unclear | N/A | Yes | Yes | No | No |
| Brightman LA | 2006 | Yes | Yes | Yes | N/A | Yes | Yes | No | Yes |
| Matsuda Y | 2005 | Yes | Unclear | Unclear | N/A | Yes | Yes | No | No |
| Demir Y | 2003 | Yes | Unclear | Yes | N/A | Yes | Yes | No | Yes |
| Bonetti F | 2001 | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes |
| Ferreiro JA | 1995 | Yes | Unclear | Unclear | N/A | Yes | Yes | No | No |
| Honma K | 1989 | Yes | Unclear | Unclear | N/A | Yes | No | No | No |
| Gould E | 1989 | Yes | Unclear | unclear | N/A | Yes | Yes | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





