Submitted:
20 June 2023
Posted:
21 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Plant material and preparation of crude extracts
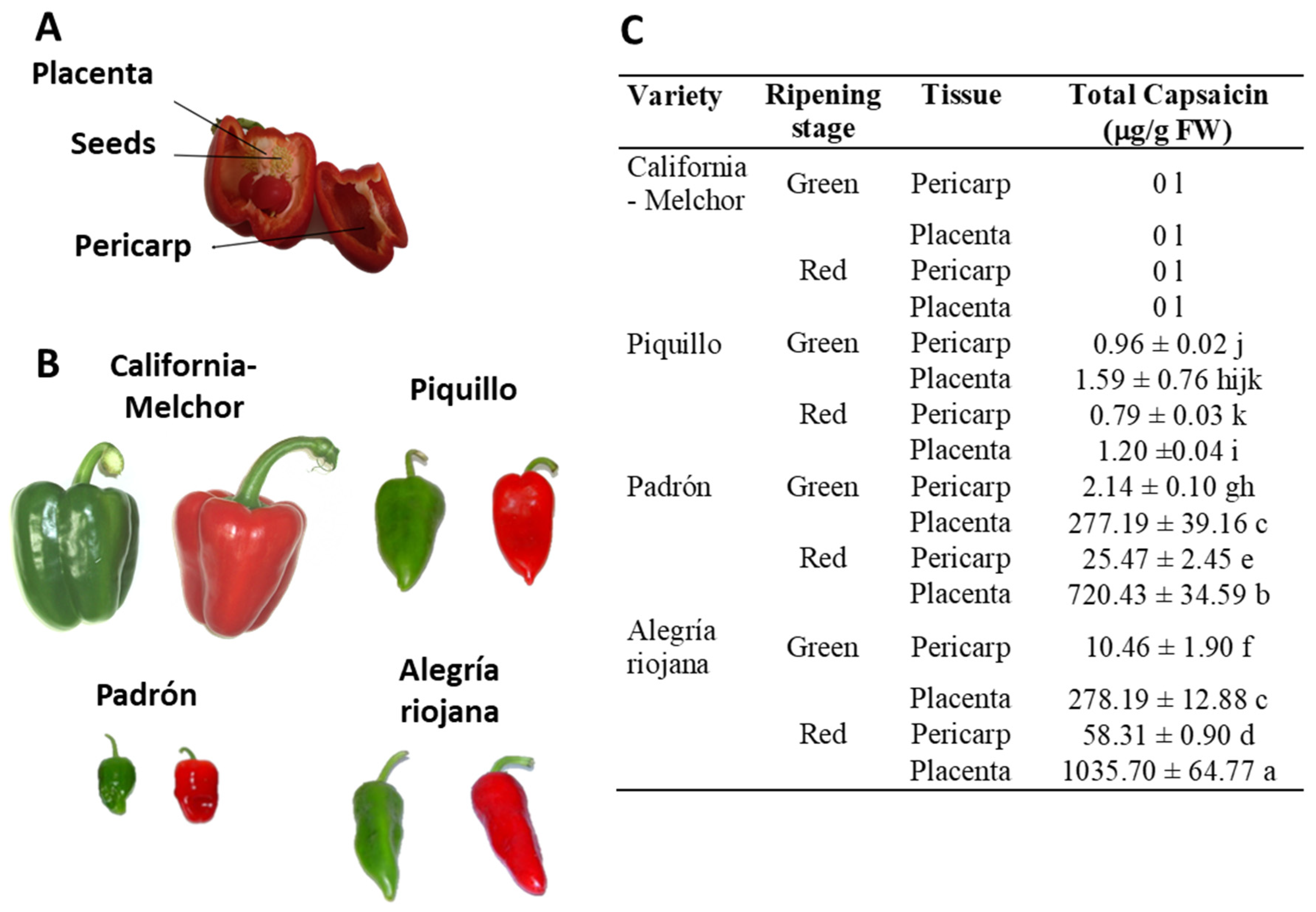
2.2. Determination of capsaicin
2.3. Tumor cell lines, anti-proliferative activity assays, and preparation of crude extracts
2.4. Enzyme activities
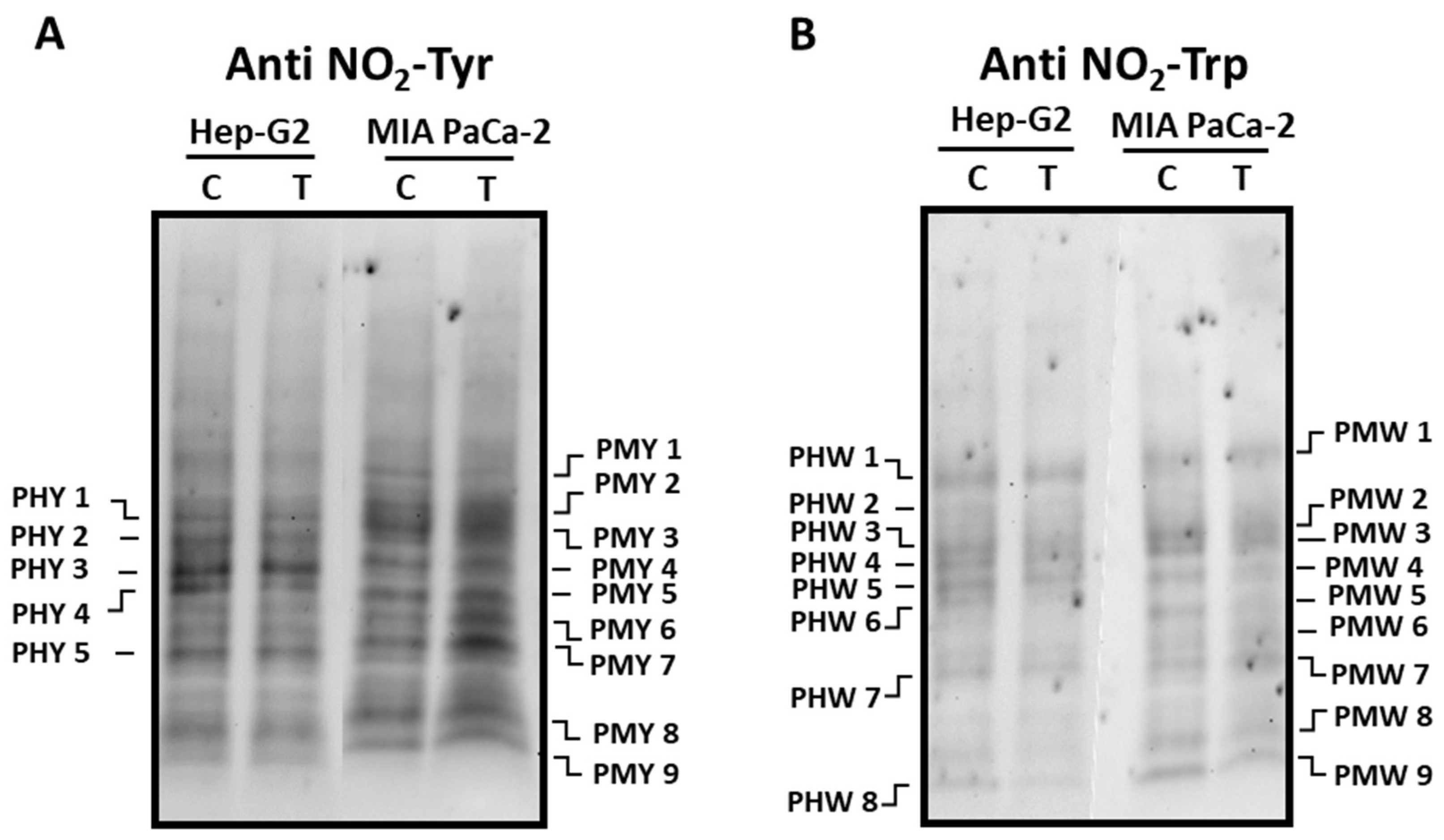
2.5. SDS-PAGE and immunoblot analyses
2.6. Antioxidant capacity assay
2.7. Statistical analysis
3. Results
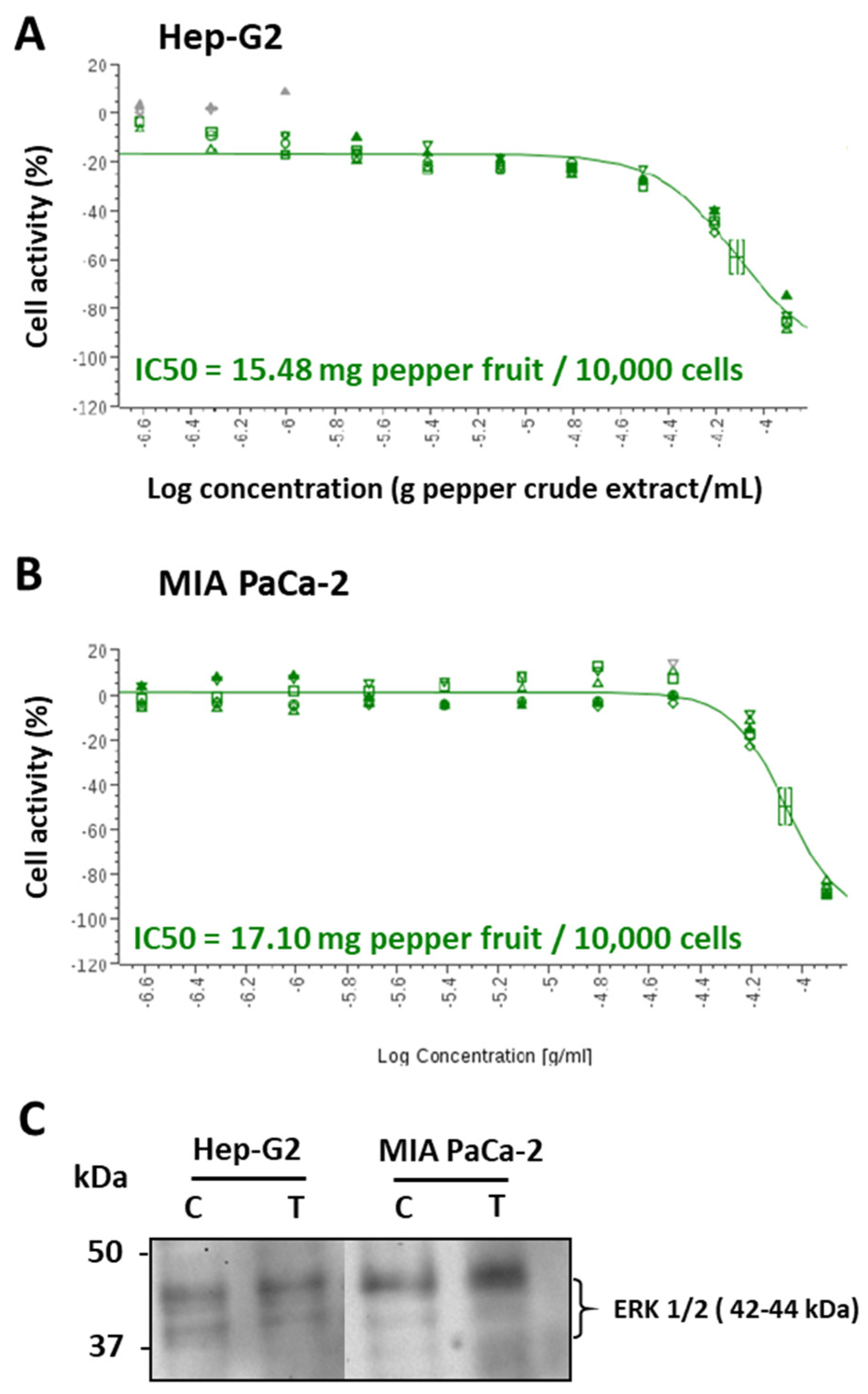
3.1. Crude extracts from pepper fruit show anti-proliferative activity against tumor cell lines
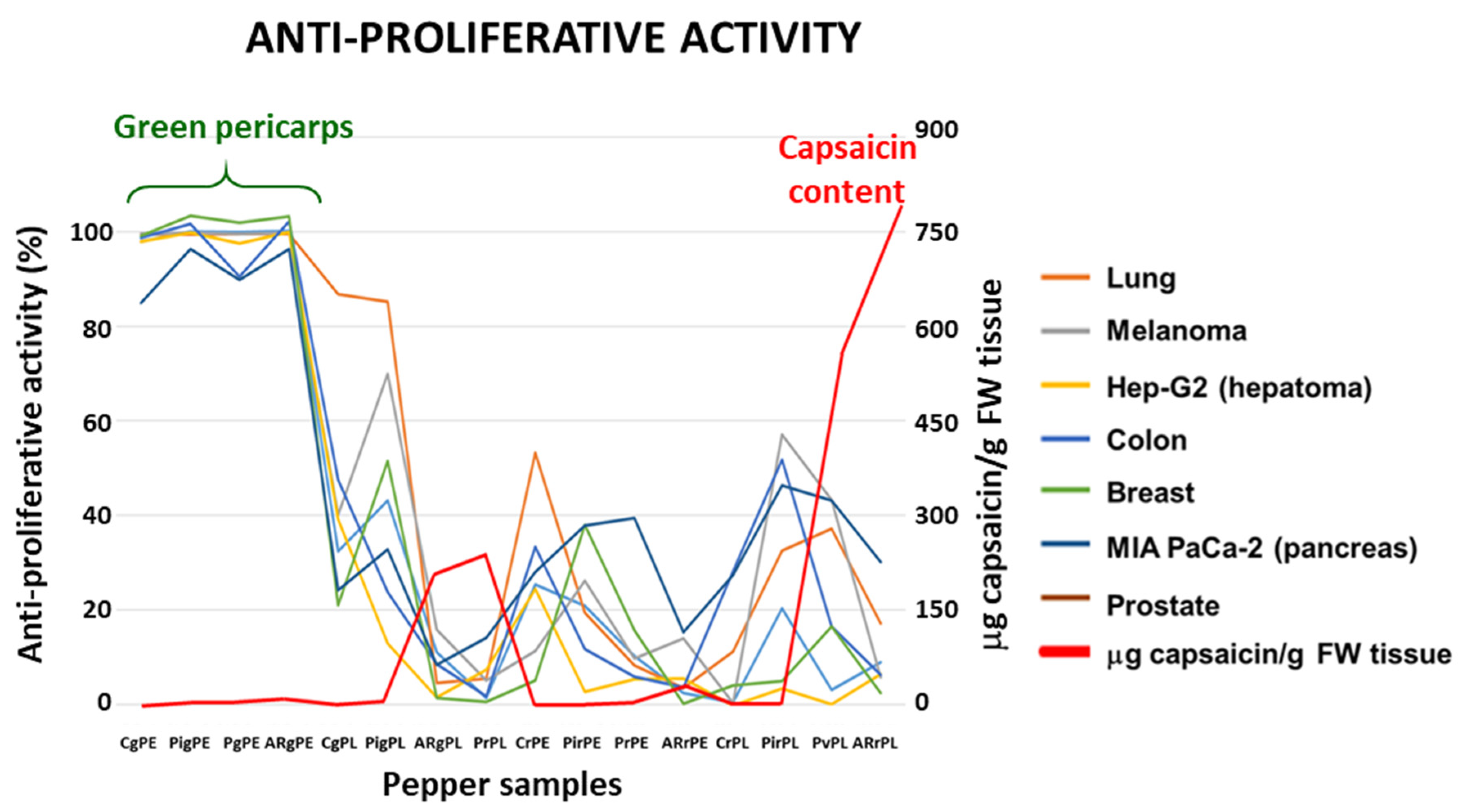
| Cell lines | IC50 (μM capsaicin) |
Capsaicin in the assay (μg/well) |
| A549 | 40.70 | 1.243 |
| A2058 | 53.00 | 1.618 |
| Hep-G2 | 28.00 | 0.855 |
| HT-29 | 51.50 | 1.573 |
| MCF-7 | 33.15 | 1.012 |
| MIA PaCa-2 | 61.25 | 1.870 |
| PC-3 | 72.20 | 2.199 |
3.2. Pepper fruit extracts slightly alter the nitro-oxidative status of tumor cells
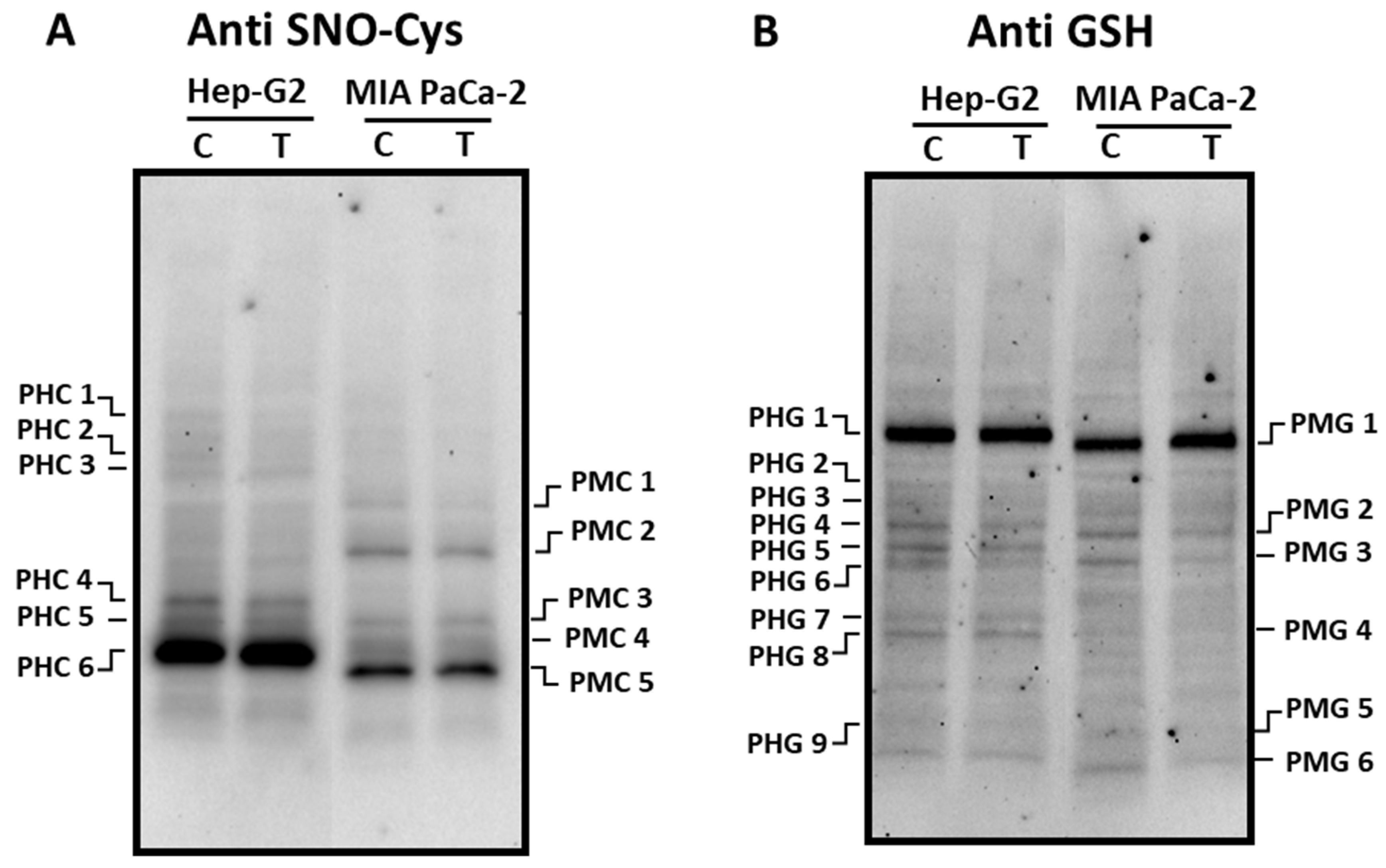
3.3. ROS metabolism in tumor cells under pepper fruit treatment
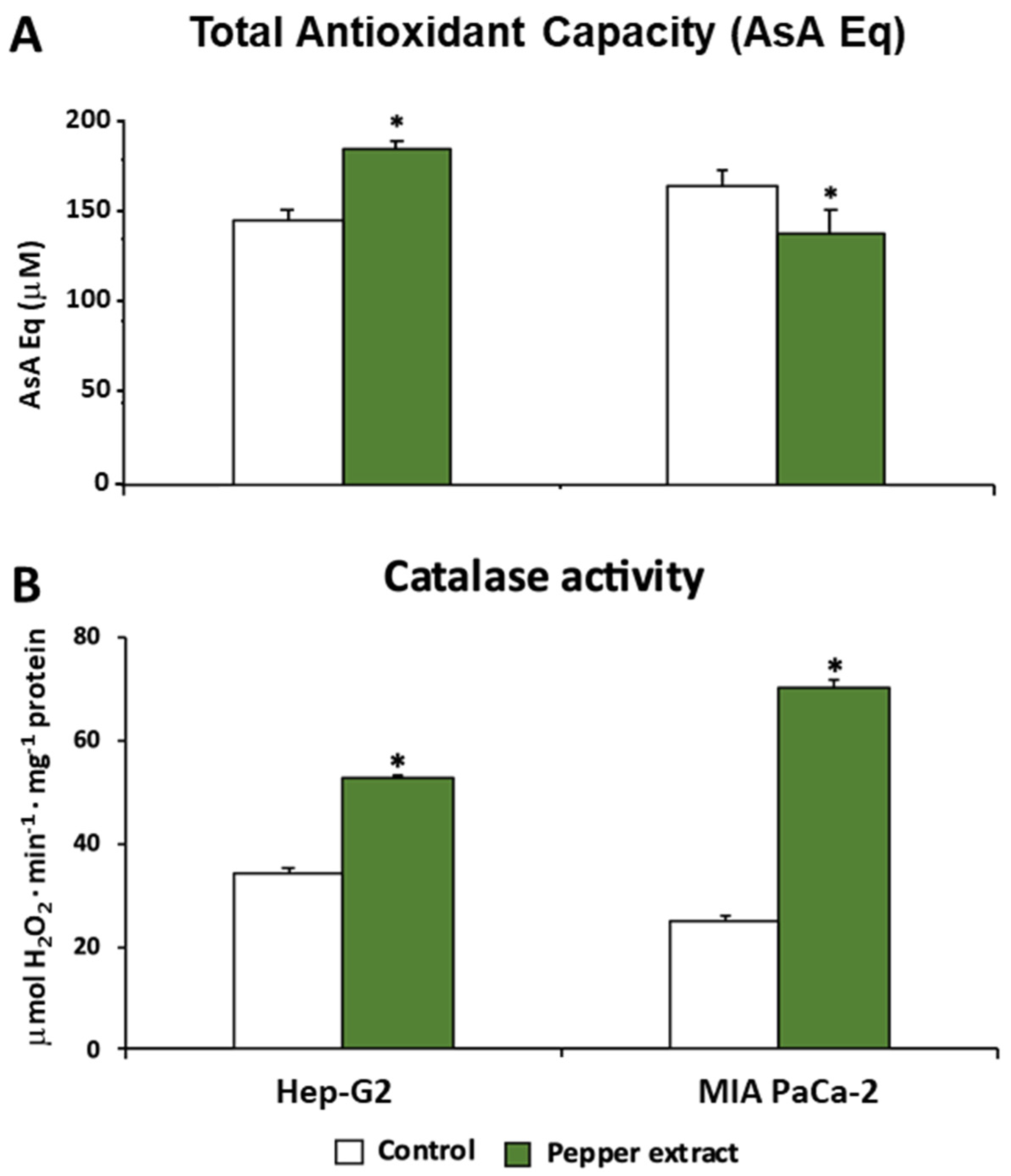
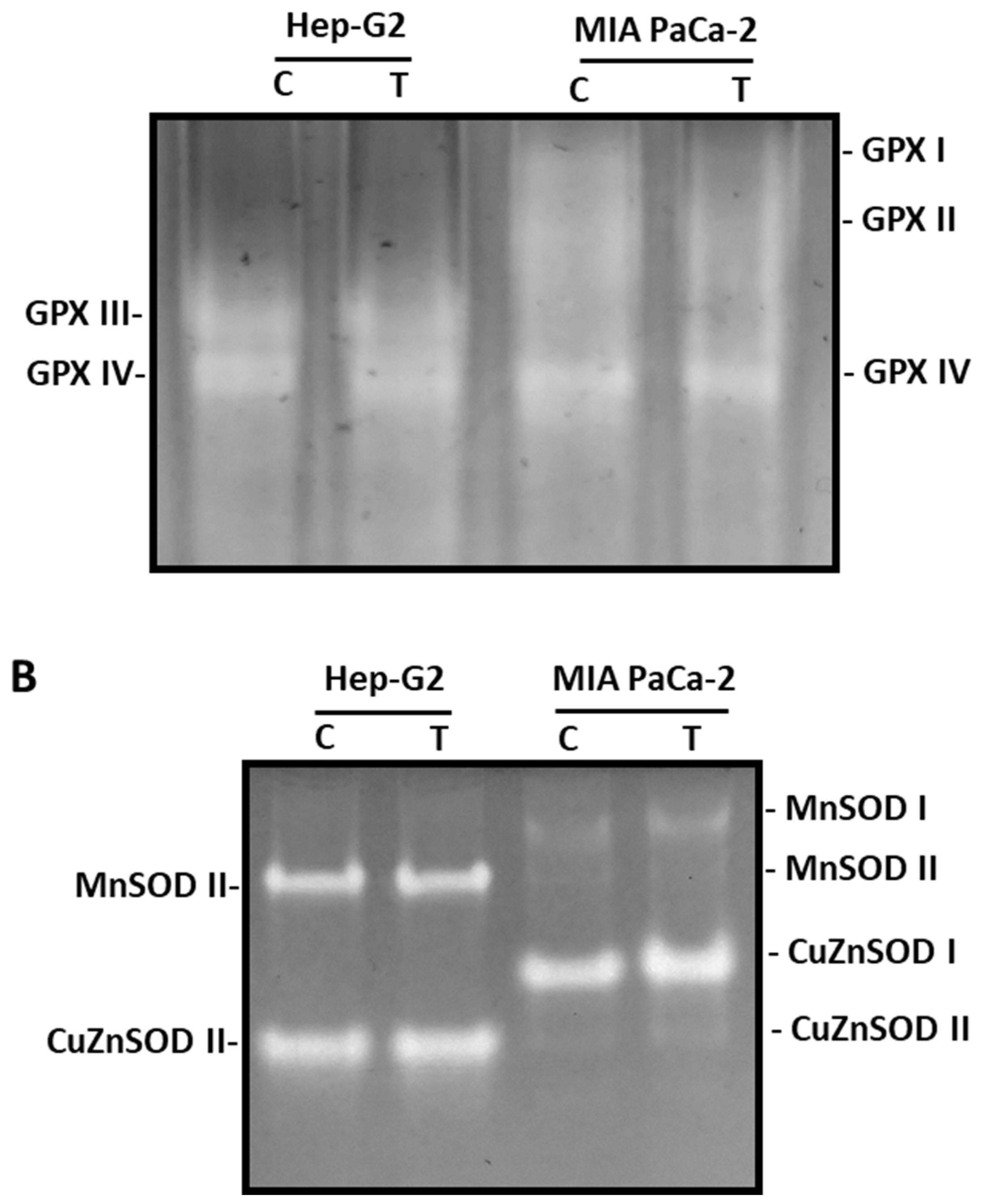
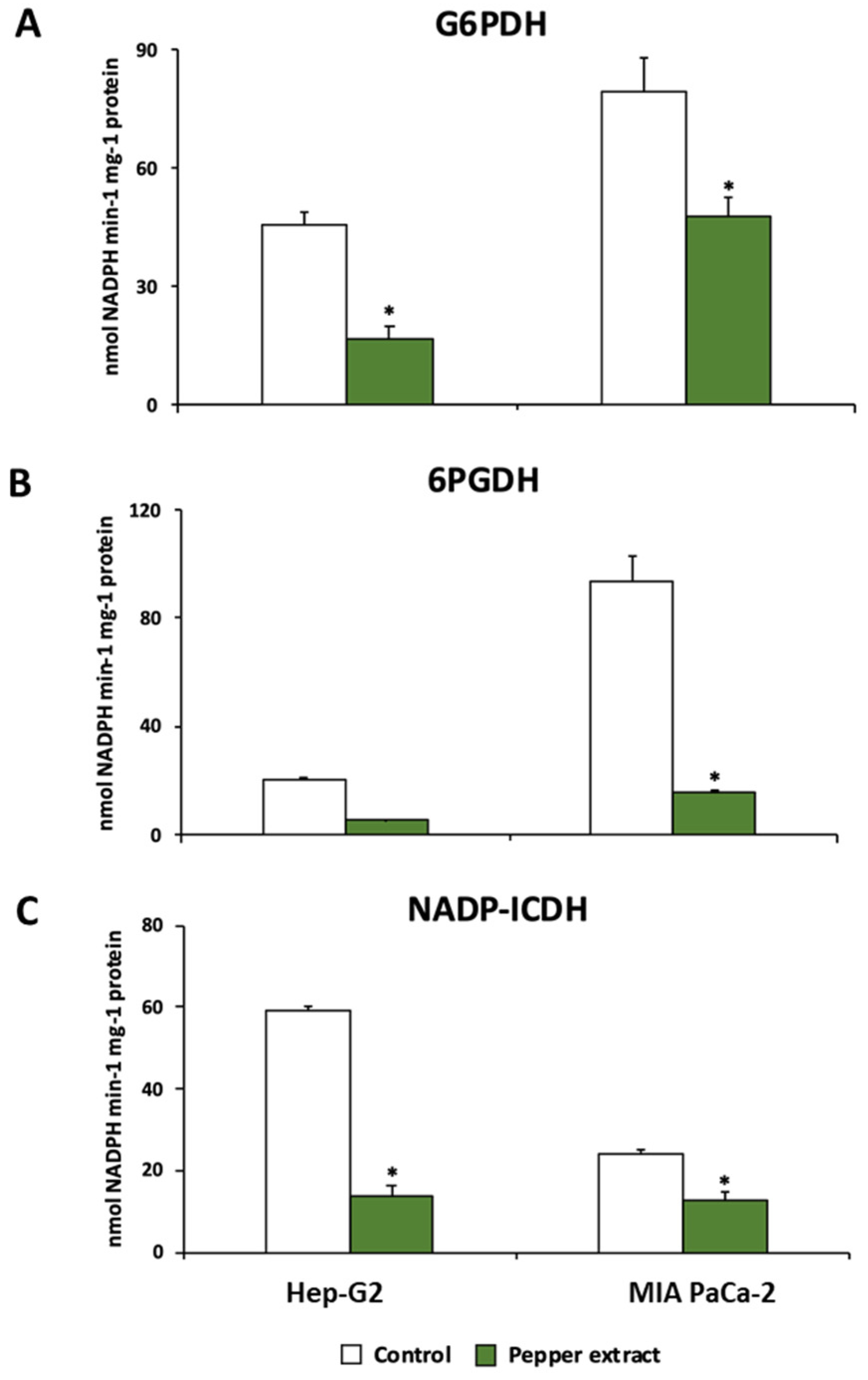
3.4. Pepper fruit treatment disturbs the NADPH-generating systems in tumor cells
4. Discussion
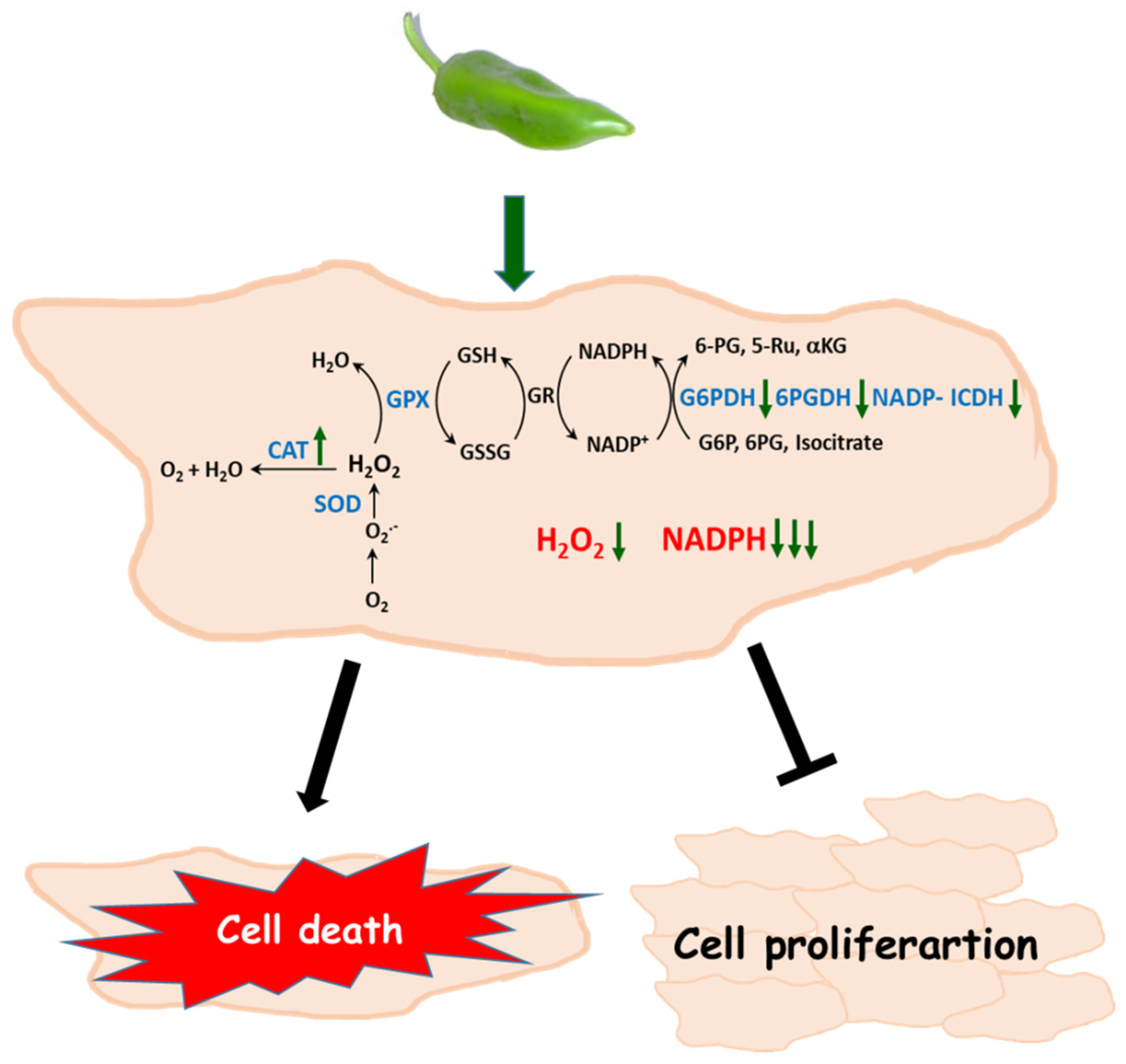
5. Conclusions
Authors’ contribution
Acknowledgments
Conflicts of Interests
References
- J.E. Klaunik, Oxidative stress and cancer, Curr. Pharm. Des. 24 (2018) 4771-4778. [CrossRef]
- F. Tas, H. Hansel, A. Belce, S. Ilvan, A. Argon, H. Camlica, E, Topuz, Oxidative stress in breast cancer, Med. Oncol. 22 (2005) 11-15. [CrossRef]
- J. Shen, M.D. Gammon, M.B. Terry, Q. Wang, P. Bradshaw, S.L. Teitelbaum, A.I. Neugut, R.M. Santella, Telomere length, oxidative damage, antioxidants and breast cancer risk, Int. J. Cancer 124 (2009) 1637-1643. 10.1002/ijc.24105.
- M.W. Lawless, K.J. O'Byrne, S.G. Gray, Targeting oxidative stress in cancer, Expert Opin. Ther. Targets 14 (2010) 1225-1245. [CrossRef]
- L.Y. Tong, C.C. Chuang, S.Y. Wu, L. Zuo, Reactive oxygen species in redox cancer therapy, Cancer Lett. 367 (2015) 18-25. [CrossRef]
- H. Forouzandeh, H. Kalantari, N. Saki, Z. Foruozandeh, E. Arefian, A. Farahani, G. Hassani, M.R. Bazrafshan, S. Rasouli, Role of oxidative stress in liver cancer, Clin. Cancer Invest. J. 6 (2017) 1-9. [CrossRef]
- M.D. Jelic, A.D. Mandic, S.M. Maricic, B.U. Srdjenovic, Oxidative stress and its role in cancer, J. Cancer Res. Ther. 17 (2021) 22-28. [CrossRef]
- E. Cecerska-Heryc, O. Surowska, R.L. Heryc, N. Serwin, S. Napiontek-Balinska, B. Dolegowska, Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients? - A review, Clin. Biochem. 93 (2021) 1-8. [CrossRef]
- T.D. Oberley, L.W. Oberley, Antioxidant enzyme levels in cancer, Histol. Histopathol. 12 (1997) 525-535.
- J.A. Cockfield, Z.T. Schafer, Antioxidant defenses: a context-specific vulnerability of cancer cells, Cancers 11 (2019) 1208. [CrossRef]
- S. George, H. Abrahamse, Redox Potential of antioxidants in cancer progression and prevention, Antioxidants 9 (2020) 1156. [CrossRef]
- E. Taucher, I. Mykoliuk, M. Fediuk, F.M. Smolle-Juettner, Autophagy, oxidative stress and cancer development, Cancers 14 (2022) 1637. [CrossRef]
- S. Banerjee, Y.W. Li, Z.W. Wang, F.H. Sarkar, Multi-targeted therapy of cancer by genistein, Cancer Lett. 269 (2008) 226-242. [CrossRef]
- H. Greenlee, M.L. Kwan, L.H. Kushi, J. Song, A. Castillo, E. Weltzien, C.P. Quesenberry Jr., B.J. Caan, Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort, Cancer 118 (2012) 2048-2058. [CrossRef]
- M. Moradi-Joo, S. Heidari, M. Seyed-Nezhad, M.E. Akbari, A. Moosavi, S.H. Davoodi, Antioxidant supplements and breast cancer: a systematic review and meta-analysis, Int. J. Cancer Manag. 11 (2018) 10082. [CrossRef]
- V. Jayaprakash, J.R. Marshall, Selenium and other antioxidants for chemoprevention of gastrointestinal cancers, Best Pract. Res. Clin. Gastroenterol. 25 (2011) 507-518. [CrossRef]
- X.S. Han, J.J. Li, T.M. Brasky, P.C. Xun, J. Stevens, E. White, M.D. Gammon, K. He, Antioxidant intake and pancreatic cancer risk: The VITamins And Lifestyle (VITAL) study, Cancer 119 (2013) 1314-1320. [CrossRef]
- S.K. Saha, S.B. Lee, J. Won, H.Y. Choi, K. Kim, G.M. Yang, A.A. Dayem, S.G. Cho, Correlation between oxidative stress, nutrition, and cancer initiation, Int. J. Mol. Sci. (2017) 1544. [CrossRef]
- M. Serafini, P. Jakszyn, L. Lujan-Barroso, A. Agudo, H.B. Bueno-de-Mesquita, F.J.B. van Duijnhoven, M. Jenab, C. Navarro, D. Palli, H. Boeing, Dietary total antioxidant capacity and gastric cancer risk in the European prospective investigation into cancer and nutrition study, Int. J. Cancer. 131 (2012) E544-E554. [CrossRef]
- K. Roy, Y.Z. Wu, J.L. Meitzler, A. Juhasz, H. Liu, G.J. Jiang, J.M. Lu, S. Antony, J.H. Doroshow, NADPH oxidases and cancer, Clin. Sci. (London) 128 (2015) 863-875. [CrossRef]
- G.M. Rather, A.A. Pramono, Z. Szekely, J.R. Bertino, P.M. Tedeschi, In cancer, all roads lead to NADPH, Pharmacol. Therap. 226 (2021) 107864. [CrossRef]
- H.Q. Ju, J.F. Lin, T. Tian, D. Xie, R.H. Xu, NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Trans. Targ. Ther. 5 (2020) 231. [CrossRef]
- H.C. Yang, Y.H. Wu, W.C. Yen, H.Y. Liu, T.L. Hwang, A. Stern, D.T.Y. Chiu, The Redox role of G6PD in cell growth, cell death, and cancer, Cells 8 (2019) 1055. [CrossRef]
- H.C. Yang, A. Stern, D.T.Y. Chiu, G6PD: A hub for metabolic reprogramming and redox signaling in cancer, Biomed. J. 44 (2021) 285-292. [CrossRef]
- K.O. Alfarouk, S.B.M. Ahmed, R.L. Elliott, A. Benoit, S.S. Alqahtani, M.E. Ibrahim, A.H.H. Bashir, S.T.S. Alhoufie, G.O. Elhassan, C.C. Wales, L.H. Schwartz, H.S. Ali, A. Ahmed, P.F. Forde, J. Devesa, R.A. Cardone, S. Fais, S. Harguindey, S.J. Reshkin, The pentose phosphate pathway dynamics in cancer and its dependency on intracellular pH, Metabolites 10 (2020) 285. [CrossRef]
- T.X. Ge, J.W. Yang, S.H. Zhou, Y.C. Wang, Y.K. Li, X.M. Tong, The role of the pentose phosphate pathway in diabetes and cancer, Front. Endocrinol. 11 (2020) 365. [CrossRef]
- R. Li, W. Wan, Y. Yang, C.Y. Gu, Exploring the role of glucose-6-phosphate dehydrogenase in cancer, Oncol. Rep. 44 (2020) 2325-2336. [CrossRef]
- S. Liu, T. Cadoux-Hudson, C.J. Schofield, Isocitrate dehydrogenase variants in cancer - Cellular consequences and therapeutic opportunities, Curr. Op. Chem. Biol. 57 (2020) 122-134. [CrossRef]
- F.A. Simmen, I. Alhallak, R.M.C. Simmen, Malic enzyme 1 (ME1) in the biology of cancer: it is not just intermediary metabolism, J. Mol. Endocrinol. 65 (2020) R77-R90. [CrossRef]
- J.M. Palma, I. Seiquer, To be or not be…an antioxidant? That is the question, Antioxidants 9 (2020) 1234. [CrossRef]
- W. Li, J. Li, J. Zhao, C. He, Evolutionary developmental genetics of fruit morphological variation within the Solanaceae, Front. Plant Sci. 6 (2015) 248. [CrossRef]
- C. Gebhardt, The historical role of species from the Solanaceae plant family in genetic research, Theor. Appl. Genet. 129 (2016) 2281–2294. [CrossRef]
- N. Baenas, N. Belović, N. Ilicb, D.A. Moreno, C. García-Viguera, Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages, Food Chem. 274 (2019) 872–885. [CrossRef]
- F.J. Corpas, L. Freschi, J.M. Palma, ROS metabolism and ripening of fleshy fruits, Adv. Bot. Res. 105 (2023) 205-238. [CrossRef]
- Y. Wahyuni, A.R. Ballester, E. Sudarmonowati, R.J. Bino, A.G. Bovy, Secondary metabolites of Capsicum species and their importance in the human diet, J. Nat. Prod. 76 (2013) 783–793. [CrossRef]
- Y. Li, J. Yao, C. Han, J. Yang, M.T. Chaudhry, S. Wang, H. Liu, Y. Yin, Quercetin, inflammation and immunity, Nutrients 8 (2016) 167. [CrossRef]
- M. Reyes-Farias, C. Carrasco-Pozo, The anti-cancer effect of quercetin: Molecular implications in cancer metabolism, Int. J. Mol. Sci. 20 (2019) 3177. [CrossRef]
- S. Metsämuuronen, H. Sirén, Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce, Phytochem. Rev. 18 (2019) 623–664. [CrossRef]
- G.E.S. Batiha, A. Alqahtani, A.O. Oluwafemi, H.M. Shaheen, L. Wasef, M. Elzeiny, M. Ismail, M. Shalaby, T. Murata, A. Zaragoza-Bastida, N. Rivero-Perez, A.M. Beshbishy, K.I. Kasozi, P. Jeandet, H.F. Hetta, Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids, Int. J. Mol. Sci. 21 (2020) 5179. [CrossRef]
- E. Dessalegn, G. Bultosa, G.D. Haki, F. Chen, H.P.V. Rupasinghe, Antioxidant and cytotoxicity to liver cancer HepG2 cells in vitro of Korarima (Aframomum corrorima (Braun) PCM Jansen) seed extracts, Int. J. Food Prop. 25 (2022) 1-10. [CrossRef]
- L. Guevara, M.A. Domínguez-Anaya, A. Ortigosa, S. González-Gordo, C. Díaz, F. Vicente, F.J. Corpas, J. Pérez del Palacio, J.M. Palma, Identification of compounds with potential therapeutic uses from sweet pepper (Capsicum annuum L.) fruits and their modulation by nitric oxide (NO), Int. J. Mol. Sci. 22 (2021) 4476. [CrossRef]
- E. Cao, J.F. Cordero-Morales, B.Y. Liu, F. Qin, D. Julis, TRPV1 Channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids, Neuron 77 (2013) 667-679. [CrossRef]
- A.M. Chapa-Oliver, L. Mejía-Teniente, Capsaicin: From plants to a cancer-suppressing agent, Molecules 21 (2016) 931. [CrossRef]
- R. Clark, S.H. Lee, Anticancer properties of capsaicin against human cancer. Antican. Res.; 36 (2016) 837-844.
- S.R. Georgescu, M.I. Sârbu, C. Matei, M.A. Ilie, C. Caruntu, C. Constantin, M. Neagu M. Tampa, Capsaicin: friend or foe in skin cancer and other related malignancies?, Nutrients 9 (2017) 1365. [CrossRef]
- M.A. Tabrizi, P.G. Baraldi, S. Baraldi, S. Gessi, S. Merighi, P.A. Borea, Medicinal chemistry, pharmacology, and clinical implications of TRPV1 receptor antagonists, Medic. Res. Rev. 37 (2017) 936-983. [CrossRef]
- F. Yang, J. Zheng, Understand spiciness: mechanism of TRPV1 channel activation by capsaicin, Protein Cell 8 (2017) 169-177. [CrossRef]
- K.H. Zhang, D. Julis, Y.F. Cheng, Structural snapshots of TRPV1 reveal mechanism of polymodal functionality, Cell 184 (2021) 5138-5150. [CrossRef]
- M.M.G. Karasawa, C. Mohan, Fruits as prospective reserves of bioactive compounds: A review, Nat. Prod. Bioprospect. 8 (2018) 335-346. [CrossRef]
- Y. Yoshida, N. Koyama, H. Tamura, Color and anthocyanin composition of strawberry fruit: Changes during fruit development and differences among cultivars, with special reference to the occurrence of pelargonidin 3-malonylglucoside, J. Jpn. Soc. Hortic. Sci. 7 (2020) 355–361. [CrossRef]
- J.M. Palma, F. Terán, A. Contreras-Ruiz, M. Rodríguez-Ruiz, F.J. Corpas, Antioxidant profile of pepper (Capsicum annuum L.) fruits containing diverse levels of capsaicinoids, Antioxidants 9 (2020) 878. [CrossRef]
- Z. Mao, Z. Liu, L. Chen, J. Yang, B. Zhao, Y.M. Jung, X. Wang, C. Zhao, Predictive value of the surface-enhanced resonance raman scattering-based MTT assay: A rapid and ultrasensitive method for cell viability in situ, Anal. Chem. 85 (2013) 7361-7368. [CrossRef]
- P.K. Smith, R.I. Krohn, G.T. Hermanson, A.K. Mallia, F.H. Gartner, M.D. Provenzano, E.K. Fujimoto, N.M. Goeke, B.J. Olson, D.C. Klenk, Measurement of protein using bicinchoninic acid, Anal Biochem. 150 (1985) 76-85. [CrossRef]
- H. Aebi, Catalase in vitro, Methods Enzym. 105 (1984) 121–126. [CrossRef]
- F.J. Corpas, J.B. Barroso, L.M. Sandalio, S. Distefano, J.M. Palma, J.A. Lupiáñez, L.A. del Río, A dehydrogenase-mediated recycling system of NADPH peroxisomes, Biochem. J. 330 (1998) 777–784. [CrossRef]
- F.J. Corpas, J.B. Barroso, L.M. Sandalio, J.M. Palma, J.A. Lupiáñez, L.A. del Río, Peroxisomal NADP-dependent isocitrate dehydrogenase. characterization and activity regulation during natural senescence, Plant Physiol. 121 (1999) 921-928. [CrossRef]
- C.M. Beauchamp, I. Fridovich, Superoxide dismutase. Improved assay and an assay applicable to acrylamide gels, Anal. Biochem. 44 (1971) 276–287. [CrossRef]
- C.L. Lin, H.J. Chen, W.C. Hou, Activity staining of glutathione peroxidase after electrophoresis on native and sodium dodecyl sulfate polyacrylamide gels, Electrophoresis, 23 (2002) 513-516.
- R. Roskoski Jr., ERK1/2 MAP kinases: structure, function, and regulation, Pharmacol. Res. 66 (2012)105-143. [CrossRef]
- Raskin, D.M. Ribnicky, S. Komarnytsky, N. Ilic, A. Poulev, N. Borisjuk, A. Brinker, D.A. Moreno, C. Ripoll, N. Yakoby, J.M. O’Neal, T. Cornwell, I. Pastor, B. Fridlender, Plants and human health in the twenty-first century, Trends Biotechnol. 20 (2020) 522-531. [CrossRef]
- E. Salmerón-Manzano, J.A. Garrido-Cardenas, F. Manzano-Agugliaro, Worldwide research trends on medicinal plants, Int. J. Environ. Res. Public Health 17 (2020) 3376. [CrossRef]
- S. Pattanayak, Plants in healthcare: past, present and future, Explor. Anim. Med. Res. 11 (2022) 140-144. [CrossRef]
- J.M. Palma, J. Pérez del Palacio, M. Rodríguez-Ruiz, S. González-Gordo, C. Díaz, María C. Ramos, B. Cautain, F. Vicente, F.J. Corpas, Pepper fruit as a nutraceutical food with antiproliferative activity against tumor cells potentiated by nitric oxide (NO), In: Nitric Oxide in Health and Disease: Therapeutic Applications in Cancer and Inflammatory Disorders (L. Morbidelli, B. Bonavida, J. Muntané, eds.) Academic Press (2023) 193-210.
- M. Materska, I. Perucka, Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L), J. Agric. Food Chem. 53 (2005) 1750-1756. [CrossRef]
- R. Roskoski Jr., Targeting ERK1/2 protein-serine/threonine kinases in human cancers, Pharmacol. Res. 142 (2019) 151-168. [CrossRef]
- L. Leon, J.F. Jeannin, A. Bettaieb, Post-translational modifications induced by nitric oxide (NO): Implication in cancer cells apoptosis, Nitric Oxide Biol. Chem. 19 (2008) 77-83. [CrossRef]
- N.T. Moldogazieva, S.V. Lutsenko, A.A. Terentiev, Reactive oxygen and nitrogen species-induced protein modifications: implication in carcinogenesis and anticancer therapy, Cancer Res. 78 (2018) 6040-6047. [CrossRef]
- P. Aguilar-Melero, G. Ferrin, J. Muntané, Effects of nitric oxide synthase-3 overexpres sion on post-translational modifications and cell survival in HepG2 cells, J. Proteomics 75 (2012), 740-755. [CrossRef]
- F.J. Corpas, S. González-Gordo, J.M. Palma. Nitric oxide: A radical molecule with potential biotechnological applications in fruit ripening. J Biotechnol. 20 (2020) 211-219. [CrossRef]
- Musaogullari, Y.C. Chai, Redox regulation by protein s-glutathionylation: from molecular mechanisms to implications in health and disease, Int. J. Mol. Sci. 21 (2020) 8113. [CrossRef]
- D. Pal, A. Rai, R. Checker, R.S. Patwardhan, B. Singh, D. Sharma, S.K. Sandur, Role of protein S-Glutathionylation in cancer progression and development of resistance to anti-cancer drugs, Arch. Biochem. Biophys. 704 (2021) 108890. [CrossRef]
- M. Skrzycki, M. Majewska, H. Czeczot, Superoxide dismutase mRNA and protein level in human colorectal cáncer, Cent. Eur. J. Biol. 5 (2010) 590-599. [CrossRef]
- M.H. Shah, G.S. Liu, E.W. Thompson, G.J. Dusting, H.M. Peshavariya, Differential effects of superoxide dismutase and superoxide dismutase/catalase mimetics on human breast cancer cells, Breast Cancer Res. Treat. 150 (2015) 523-534. [CrossRef]
- M. Liu, X. Sun, B. Chen, R. Dai, Z. Xi, H. Xu, Insights into manganese superoxide dismutase and human diseases. Int. J. Mol. Sci. 23 (2022) 24. [CrossRef]
- M.M. Ogle, R. Trevino Jr., J. Schell, M. Varmazyad, N. Horikoshi, D. Gius, Manganese superoxide dismutase acetylation and regulation of protein structure in breast cancer biology and therapy, Antioxidants 11 (2022) 4. [CrossRef]
- C. Di Ilio, P. Sacchetta, S. Angelucci, A. Zezza, R. Tenaglia, A. Aceto, Glutathione-peroxidase and glutathione-reductase activities in cancerous and non-cancerous human kidney tissues, Arch. Toxicol. 91 (1995) 19-23. [CrossRef]
- J. Gromadzinska, W. Wasowicz, M. Andrijewski, M. Skłodowska, O.Z. Quispe, P. Wołkanin, B. Ołborski, A. Pluzanska, Glutathione and glutathione metabolizing enzymes in tissues and blood of breast cancer patients, Neoplasma 44 (1997) 45-51.
- N. Saydam, A. Kirb, O. Demir, E. Hazan, O. Oto, O. Saydam, G. Guner, Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues, Cancer Lett. 119 (1997) 13-19. [CrossRef]
- R. Brigelius-Flohé, M. Maiorino, Glutathione peroxidases, Biochim. Biophys. Acta 1830 (2013) 3289-303. [CrossRef]
- J.M. Mates, J.A. Campos-Sandoval, J. de los Santos-Jiménez, J. Márquez, Glutaminases regulate glutathione and oxidative stress in cancer, Arch. Toxicol. 94 (2020) 2603-2623. [CrossRef]
- Y.J. Zhao, H. Wang, J.D. Zhou, Q.X. Shao, Glutathione peroxidase GPX1 and its dichotomous roles in cancer, Cancers 14 (2022) 2560. [CrossRef]
- J.M. Palma, R.M. Mateos, J. López-Jaramillo, M. Rodríguez-Ruiz, S. González-Gordo, A.M. Lechuga-Sancho, F.J. Corpas, Plant catalases as NO and H2S targets. Redox Biol. 34 (2020b) 101525. [CrossRef]
- C. Glorieux, N. Dejeans, B. Sid, R. Beck, P.B. Calderon, J. Verrax, Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy, Biochem. Pharmacol. 82 (2011) 1384-1390. [CrossRef]
- C. Glorieux, P.B. Calderon, Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach, Biol. Chem. 398 (2017) 1095-1108. [CrossRef]
- M. Galasso, S. Gambino, M.G. Romanelli, M. Donadelli, M.T. Scupoli, Browsing the oldest antioxidant enzyme: catalase and its multiple regulation in cancer, Free Rad. Biol. Med. 172 (2021) 264-272. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





