1. Introduction
The appearance of pathogens in crowded places can potentially lead to very serious consequences for any country in the world and for an individual. Well-known in the history of mankind, for example, the flu pandemics that occurred in 1918, 1957 1968, and Covid-19 clearly showed that the price of moral suffering, financial and material losses are very significant and often unacceptably high. Early and rapid diagnosis of cases epidemic infections with epidemic potential allows timely implementation and organization of number of measures to prevent the occurrence of an epidemic or even pandemic. Among them are quarantine, isolation, hospitalization, vaccination, treatment, etc. [
1,
2,
3].
Currently, the problem of rapid diagnosis pathogens is solved by various methods and technical means that perform, and often successfully, only individual tasks. Among them, methods in various variants can be distinguished: polymerase chain reaction (PCR), serological tests, enzyme immunoassay, etc.
PCR is based on detection of nucleic acid sites genetic material virus and determination their group membership by this criterion. The technique has high diagnostic value, is modern and high-quality way to confirm disease. It is considered the “gold standard” of diagnostics, and widely used in virology laboratory, inpatient and outpatient settings [
3].
Serological diagnostic methods – allow obtain data characteristics of pathogen and stage of clinical process. The analysis performed on basis of patient's blood serum with assumption of presence in body specific type antibodies and and/or antigen. This is retrospective laboratory study that uses known virus antigen as well. When disease occurs, specific antibodies, immune complexes AB+AG are formed in patient's body, which confirms method. Determination of presence antibodies in blood serum is carried out few days after onset of disease. Detection of immunoglobulin IgA against the SARS-CoV-2 virus is carried out from 2 days after infection, of immunoglobulin IgM on 7th day, of immunoglobulin IgG from about 3rd week. Method has high accuracy, sensitivity, reliability and allows us to obtain numerical estimates of titer AB, dynamics their development. It has particular value for prognosis of development pathology. Serology often used to diagnose erased forms of disease, obvious clinical signs or asymptomatic as well. In medicine and virology, the method is used to diagnose presence of pathogens in body: coronavirus, influenza A, B viruses, HIV infection, hepatitis, rubella and number of other pathogens [
3,
4,
5,
6].
Enzyme immunoassay (ELISA) is based on specific AB+AG reaction. Semi-quantitative analysis, the purpose of which is to determine presence of IgM and IgG antibodies in blood serum taken from a vein [
4,
5]. The principles of ELISA are based on modern knowledge in field of immunochemistry, analytical chemistry, physico-chemical laws AB+AG reaction, and molecular biology [
5,
6,
7]
A competitive alternative to traditional detection methods is rapidly developing direction of sensors based on field-effect transistors (SOI-FET). Ion-sensitive sensors were first proposed by Bergveld P. in 1970, 1972 [
8,
9]. Researchers are developing more advanced biosensors and methods for detecting biological particles based on fundamental laws of physics and chemistry, using an intuitive understanding of transfer analyzed substance as a result of its diffusion and convection, control kinetics of binding and chemical reactions in test sample. Various biosensors, their characteristics, sensitivity, of design elements are presented in literature [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. However, attention to problem of probability detection is insufficiently. Mistake detected of target pathogen by SOI-FET biosensor may be as result procedures of preparing its surface. During manufacture or storage of biosensor, background particles from the air atmosphere (oils, photoresistors, mechanical dust, etc.) can be adsorbed or chemically bound on its surface [
23]. They influence: properties of nanowires (NW), selective interaction of AB+AG, their effective eclectic charge, amplitude signal of detection. Mistake detected of target pathogen increases. The topology and the design of SOI-FET biosensor can be cause mistake of detection AB+AG complexes also.
Consider biosensor that uses field-effect transistor with two isolated gates [
14,
15,
16]. First gate is made in form of NW between source and drain of biosensor. Second one is located on reverse side of biosensor crystal. It is used to select initial current in source-drain circuit [
14]. Source of input signal is various ions chemical elements, molecules, viruses, proteins, bacteria, DNA, etc., which are present in tested suspension and partially adsorbed on surface of first gate – NW [
10,
11,
12,
13]. Adsorption of biological particles (proteins, viruses, DNA, etc.) on NW do to change in concentration of charge carriers in semiconductor and its conductivity in real time. Electric current is modulated in source-drain chain of SOI-FET as result of their absorption. If during adsorption semiconductor is depleted by electric charge carriers, and depletion region is comparable to size of sensor element, then one particle can potentially completely block its conductivity. Thus, potential sensitivity is achieved – one particle per sensitive element [
11]. Regulation of choice operating mode, optimization of response NW biosensors is considered in articles [
14,
15]. Sensitivity of biosensor is result of choice its operating mode, presence of receptor layers on surface of NW and design and technological parameters, etc. Among other factors, delivery of analyte to biosensor is indicated.
Aim of work is theoretical investigation of process detection AB+AG complexes by SOI-FET biosensor.
Whole detection process is divided into three main stages.
First stage. Formation of AB+AG complexes by introducing a specific serum (antibodies) into studied viral suspension, which is located on surface of biosensor. This stage of reaction occurs very quickly and has no visible changes. The interaction of antibodies with background particles suspended in solution can lead to cross-linking and the formation error of detection by SOI-FET biosensor also.
Second stage. Adsorption of complexes on surface NW causes current modulation in source–drain circuit. As a result, a target information signal is formed. It is defined as difference between value of a priori selected initial current biosensor set before introduction of a specific serum, and current after detection process is completed.
Third stage. Registration of electric current in source-drain sections of transistor using specialized devices.
Adsorption of AB+AG complexes from a liquid onto surface of NW is random event that depends on number of factors. Among them are radius of AB+AG complex, its diffusion coefficient, viscosity and temperature of suspension, etc. see, i.g, [
24,
25]. Listed parameters are selected a priori for practical reasons in order to achieve optimal detection of target complex. Development of express detection methods and systems based on biosensors is carried out in direction of increasing sensitivity, improving operational characteristics, simplicity of methodological procedures, minimizing material and time costs, use of middle-level personnel, as well as accessibility for wide range of users [
10,
11,
12,
13,
14,
15,
19,
20].
2. Materials and Methods
Most demanded characteristics of a biosensor are its high sensitivity, specificity and speed. Sensitivity is understood as minimum amount of a substance that can be detected with its help in test sample. Specificity refers to reliability of detection target substance (antibody or antigen), against background of others particles.
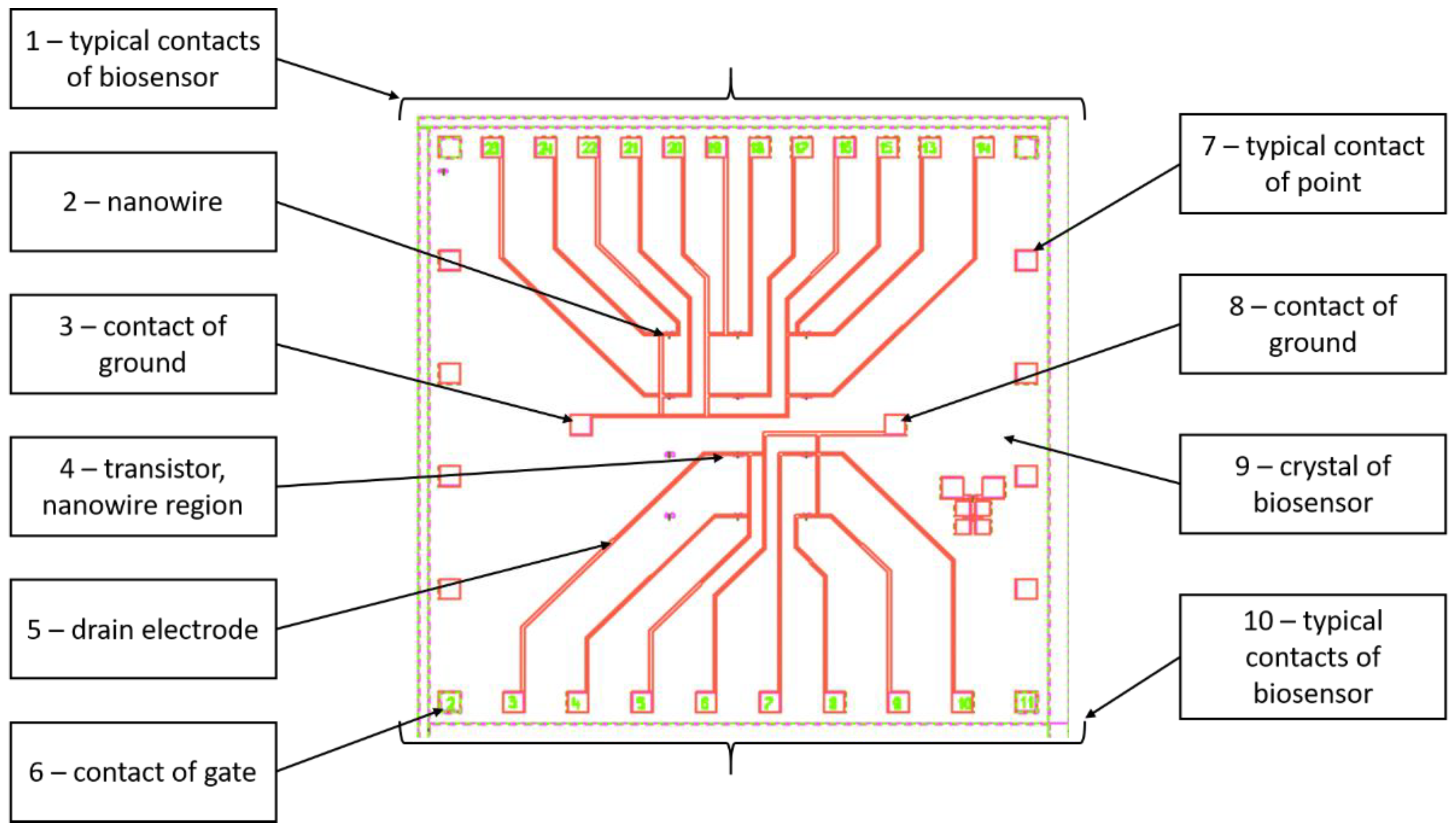
The topology of biosensor manufactured by Design center of Bio-microelectronic technology Vega shows at (
Figure 1). It contains following elements:
1, 10 - typical contacts of biosensor. Contacts are necessary to connect silicon crystal to contacts of case;
2 - NW (gate). NW is located between source and drain of each transistor;
3, 8 – contact of ground;
4 - transistor, NW region;
5 - drain electrode;
6 - contact of gate;
7 - typical contact of point;
9 - crystal of biosensor. All components of biosensor are located on surface of crystal.
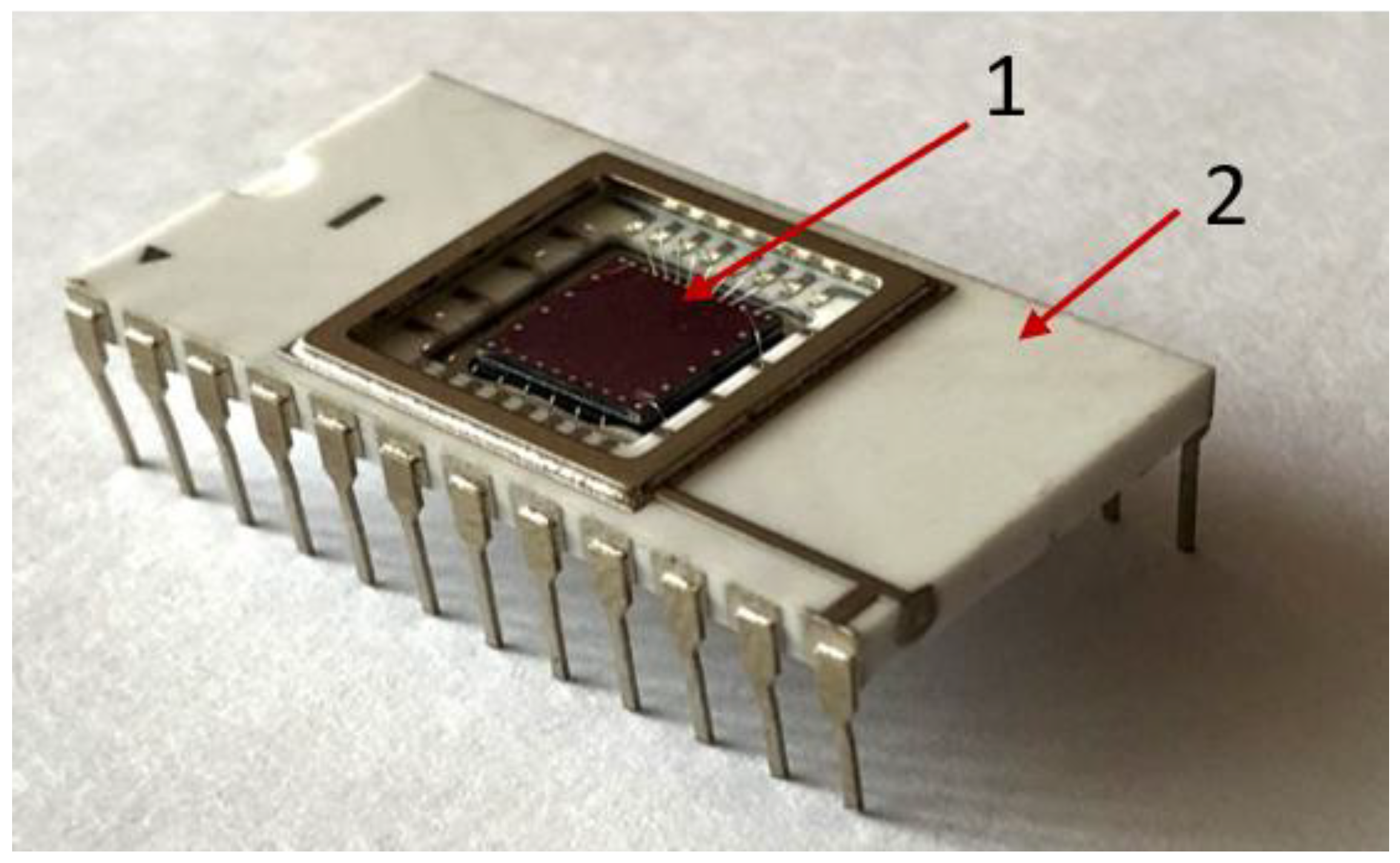
There are 10 independent biosensors located on surface of crystal. Overall dimensions of crystal are 6×6 mm. The back side of crystal is glued to body chip. Photo of biosensor case is shown at (
Figure 2).
Biosensor is connected to data acquisition and processing recorder, in which information signal is amplified in analog form, converted into digital and primary mathematical processing. Further signal processing is performed by a computer, which makes it possible to use its capabilities for statistical analysis of results obtained, data transmission via communication channels to a single information center, practically unlimited time for storing results, quick decision making, etc.
Operating principle of recorder is based on measuring value of current flowing in source-drain circuit of transistor. Registrar software performs following functions:
- -
plotting dependence electric current of transistor – time;
- -
calculation of average value current for given time interval of measurement. In suspension under study, all background particles, as well as antibodies and antigen, experience Brownian motion. Process of movement occurs spontaneously and proceeds constantly. Average value square of displacement Δ
2 particle during observation
t is found from expression of Einstein and Smolukhovsky, see i.g. [
24,
25]:
where:
R - universal gas constant 8.31 [
J/(mol
·K)];
T - absolute temperature [K];
η - viscosity of sample [Pa·s];
r - radius of suspended particles [m];
- Avogadro constant 6.02⋅1023 [mol⁻1];
t - observation time [s].
Numerical estimates show that colloidal particle, for example, complex of flu virus AB+AG with a radius
r = 5.00·10
-8 m during observation
t = 200
s in an aqueous medium with viscosity
η = 10
-3 Pa·s, at temperature of 300 K is able to overcome linear distance
l:
After substituting listed parameters in (1), we find:
Value of
l allows us to give reasonable estimate of measuring volume for one biosensor
v1:
During detection, both target AB+AG complexes and background particles, which are inevitably present in any test sample, are simultaneously adsorbed on surface of NW biosensor. Biosensor transforms adsorption of AB+AG complexes into a useful information signal in form of an electric current, and adsorption of background particles onto surface of NW into an electrical interference signal. As result, an additive (total) output signal
b is observed at output of recorder, which is described by equation:
where:
x - useful information signal of target complexes;
c - background particle interference signal;
A - logical parameter, takes values 0 or 1.
In general, solution to problem of express detection AB+AG is reduced to estimate value of b. Parameter A, takes one of two values, or A = 1 or A = 0.
In process of detection, following four logical events are likely according to the Latin square with two rows and two columns [
26,
27].
1. If target AB+AG complex is present in sample, and it is correctly detected against background of interference signal. Result – decision was made correctly, A = 1.
2. If target complex is present in sample, but it has not been detected. Result is an error of decision, A = 0.
3. If target complex is missing in sample, but it has been “detected". Result is an error of decision, A = 0.
4. If target complex is missing in sample, and it is not found. Result – decision was made correctly, A = 1.
Finally, detection process should end with one of two mutually exclusive solutions, or A = 1 or A = 0. Any other result of detection, any uncertainty in decision-making is not allowed. It is obvious that detection of a single AB+AG complex by biosensor will not be statistically reliable. Thus, expected potential sensitivity of biosensor at level of one particle becomes questionable. In order to obtain reliability, a series of independent samples is carried out, which will determine probability that AB+AG complexes will appear m times in measuring volume of biosensor, and parameter A will take value equal to one.
The Pawson distribution is closest description of probabilistic discrete m-time detection AB+AG complexes in measuring volume [28]. Following assumptions that are mandatory for it are fulfilled:
- -
measurements do not depend on each other; they can be considered as random processes both in time and (or) in space;
- -
probability of occurrence event A in a single unit dimension is small (0.05-0.1 and less) and constant;
- -
number of measurements - a sample of n measurements is quite large;
- -
dispersion index χ2 > 1:
where:
σ1 - standard deviation of distribution under study;
σ* - standard deviation of approximating distribution.
Pawson probability density for AB+AG is written as:
where:
λAB+AG =
n · p(1
) - average intensity of occurrence events in during observation;
m = 0, 1, 2 ÷ 25 – number of simultaneously expected events;
n - samples of process measurement;
p(1) - probability detection of target event in one count.
Pawson probability density for background particles is written similarly:
Likelihood ratio:
allows you to select most reliable hypothesis of occurrence event A.
It should be remembered that these probability densities generally differ in parameter λ, which is calculated separately for both AB+AG and background particles. An increase in ratio leads to an increase in reliability of detection target complex. Decision on presence of a useful information signal is made if likelihood ratio has a value greater than one.
Choice of hardware construction of recorder, its software, algorithm for analyzing and processing input data may be most optimal based on second and third conditions of four previously specified. Target AB+AG complex is absent in sample, but it was “detected” or target complex is present in sample, but it was not detected. For first condition, choice avoids possible significant material costs from organization of large-scale anti epidemic measures, for second condition, possible tragic events as a result of a single disease. In this variant, threshold level B-const should be chosen based on equality of their probabilities with some correction factor risk of missing or “false positive detection" complex. Considering equality of relations makes it possible to avoid analyzing a priori data on presence or absence of a signal already at stages of designing and building a schematic diagram recorder.
3. Numerical estimates
A biosensor with ten (
d) transistors is planned to detect AB+AG complexes. Detection is supposed to be carried out considering compliance with mandatory conditions of Poisson distribution, for
t = 300
s and
n = 200 samples of process measurement. Probability
p(1) of detecting single AB+AG complex by one biosensor is found from equation
where:
v1 - measuring volume for one SOI-FET biosensor for AB+AG particle [m
3];
Vs - total volume of suspension for investigation and detection on surface of crystal biosensor [m3];
S - number AB+AG complexes in volume of suspension for investigation and detection by biosensor.
Similarly, for ten biosensors [29]:
where:
d - number of biosensors on surface of crystal.
Number of complexes search from equation:
where:
C - number of complexes in total volume of suspension for investigation, in test tube.
After appropriate substitution, v1 = 4,23·10-13 m3, d = 10, C = 104 [number/ml], Vs = 10-2 [ml], S = 100 to equations (11) and (12), we find probability of detection p(1) = 8,38·10-2 for a single complex of AB+AG.
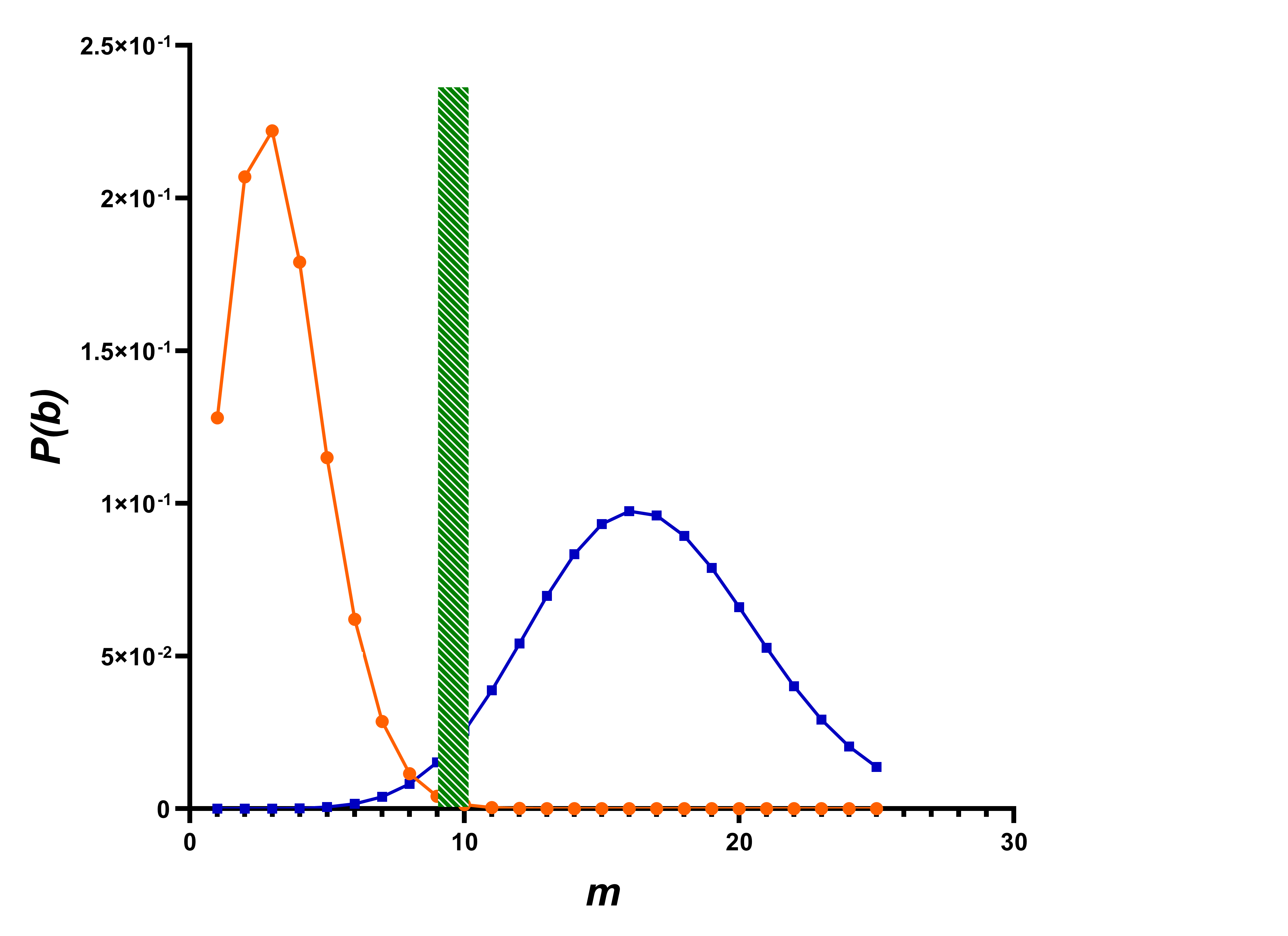
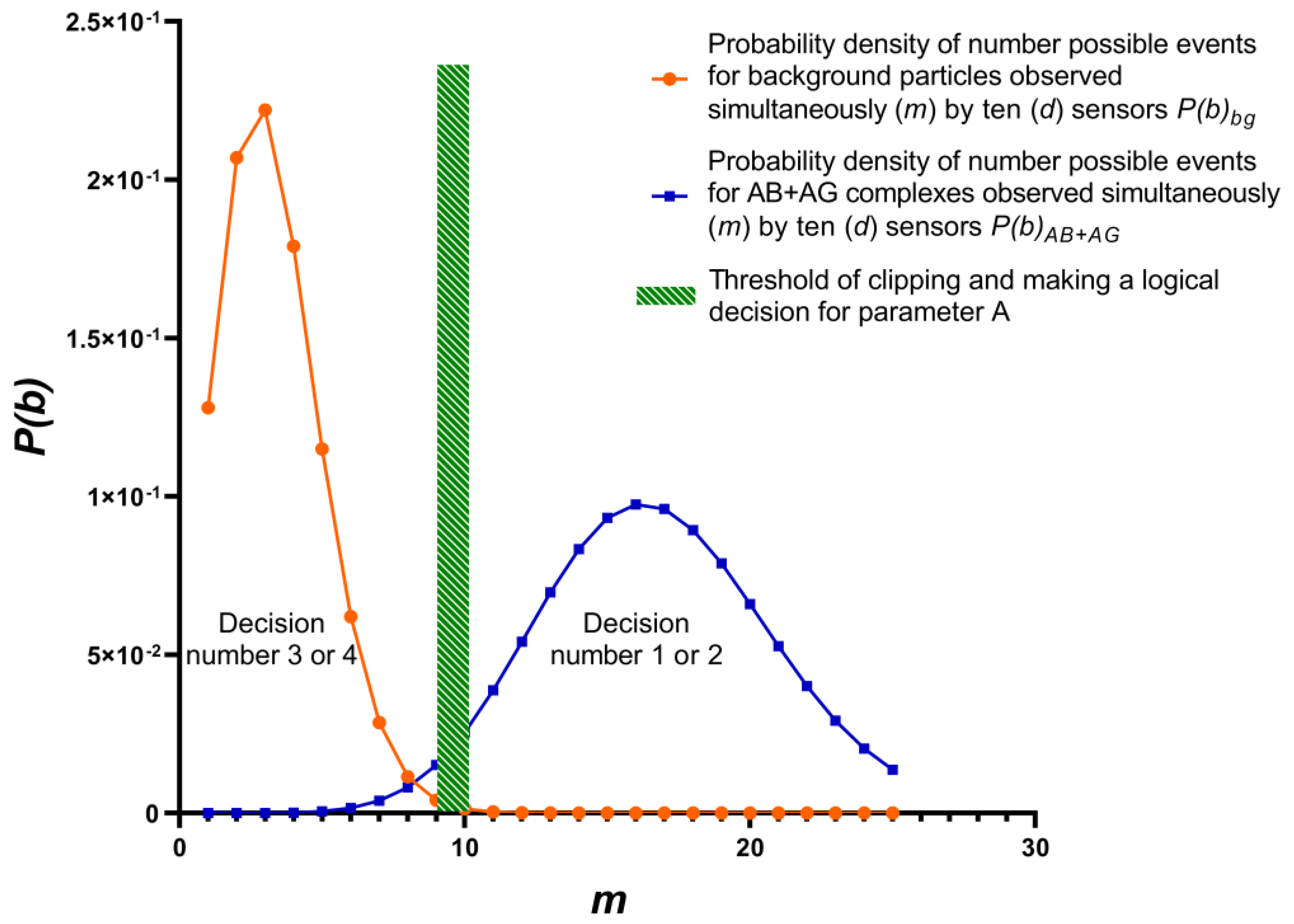
One of many possible variant’s probability density target complexes and background particles shows at (
Figure 3). Variant is determined by parameters included in equations (1-13):
1 - target complex is present in test sample, and it was correctly detected, A = 1, m = 10 - 25;
2 - target complex is present in sample, but it was not detected, A = 0; m = 10 - 25;
3 - target complex is absent in sample, but background particles are mistakenly detected as target complex, A = 0, m = 0 - 10;
4 - target complex is absent in sample, background particles present in sample and correctly detected as noise, A = 1, m = 0 - 10.
4. Discussion
Background particles are present in tested samples always. The source of particles are samples themselves, laboratory utensils, chemical reagents used, air of diagnostic laboratories, laboratory clothing, human breathing, surface of biosensor, etc. Numerous natural proteins are present in blood samples, which create a background signal (noise) as well also [23]. The complete removal of these particles is complex and expensive task that does not have simple solution. Thus, it is obvious that detection of viruses and antibodies in samples is always carried out in the presence of numerous and diverse background particles. They can have a negative impact on results of detection. Within framework of presented work, an attempt was made to investigate probability of detection influenza AB+AG complexes using biosensor and to establish factors affecting the reliability of detection. Studies have shown that use of biosensors imposes strict requirements on detection method, design of the biosensor, as well as qualifications of personnel. Currently, theory of experimental planning and methods of technical decision-making are being developed at junction of analytical, computational mathematics, statistics, optimization theory [26,27]. Planning gives an understanding of objectively existing limitations, gives confidence in reliability of results obtained or expected, allows you to trace links between various operational procedures of the experiment, etc.
The undeniable advantage of silicon-based biosensors on an insulator is their potential high sensitivity, high speed, low material and time costs, instrumental measurements that exclude subjective evaluation of results obtained, mathematically justified reliability of detection. A biosensor, like any other device, has its advantages and disadvantages, which must be treated carefully. For example, it should be emphasized that likelihood co-efficient is calculated for a sample already deposited on surface of biosensor. The likeli-hood coefficient of another sample is likely to be different from first one. This circumstance establishes methodical construction of experiment, first step of which is the calculation of probability densities for background particles and signal + background particles.
The development of rapid detection pathogens using sensor - SOI-FET is a step towards solving problem of diagnostics on new technological platform.
The use of sensor - SOI-FET will allow to obtain qualitatively new results and fundamental knowledge:
- -
understanding the sign of electric charge AB, AG, and AB+AG;
- -
adjusting sensitivity of biosensor threshold electronically;
- -
creation of automated, inexpensive stationary posts for rapid detection of pathogens in cities, airports, subways, stadiums and other real-time facilities;
- -
the use of modern digital technology for processing, storing, transmitting display results using a computer, via radio channels, the Internet;
- -
organization of production disposable biosensors by hundreds of thousands and even millions of pieces at a minimum price;
- -
use of modern digital technology for processing, storing, transmitting display results using a computer via a radio channel, the Internet;
- -
availability on market of ready-made computer programs for processing indication signal and statistical processing;
- -
ability to create biosensors for individual use.
- -
Investigation of probability density detection of AB+AG complexes by biosensor and considering account relationship of equations (1), (12), (13) allowed us to draw following conclusions:
- -
total measured volume of biosensors on surface crystal must coincide with volume of test sample on it;
- -
probability of detecting AB+AG complex using SOI-FET biosensors increases with an increase in number of biosensors on crystal;
- -
detection of single AB+AG complex, by biosensor cannot be considered reliable;
- -
ratio of probability density simultaneous detection AB+AG complexes and background particles at regular intervals in independent tests should be in range of 3-10 times, see (8-10);
- -
planning of experiment, making technical decisions and conclusions based on results of detection test sample, optimization of detection process are complex tasks and still remain within competence of a person.
5. Conclusion
This article presents the results of study possible errors, misses that arise due to the design features of sensors, methods of detection and sample preparation of suspension for research, characteristics of the pathogens themselves also. Statistical analysis revealed completely non-obvious factors that have a significant impact on the quality of sensors and their reliability.
Brownian motion, diffusion of complexes leads to their adsorption both on the FET gate and on the crystal surface. In the first case, the detection of the complex is possible, in the second case, detection is excluded. Continuous modification surface of crystal by specific antibodies to the pathogen will lead to adsorption of pathogen outside gate of FET, chemical binding of AB+AG and partial exclusion the pathogen from detection process. The sensitivity of detection pathogen by sensors on crystal is decreasing overall. The sensitivity of detection pathogen by sensors on crystal is decreasing overall. A number of FET on crystal surface must be such that the desired number of particles in the suspension must necessarily have the probability of being adsorbed onto the transistor gate.
Doctors, researchers associate the desired amount of pathogen with its infection dose for humans usually. It varies from single values to ten thousand for various pathogens [30]. It values determines the requirements for sensor sensitivity also.
These circumstances make us think about the necessary and sufficient amount of FET at the stage of select assigning the technique detection. Modern technologies make possible to create thousands and hundreds of thousands of transistors on the crystal surface, however, an excessive amount of FET leads to an increase of the price and time detection with a sequential survey of all FETs. An increase number of sensors reduces the detection time to units of seconds with parallel survey of all sensors on the other hand.
Continuous modification of crystal surface by specific antibodies to the target’s pathogen can will lead to adsorption it outside gate of FET and partial exclusion of its particles from detection process and, as consequence, an undesirable decrease in sensitivity of technique and the sensor as a whole. Parallel adsorption of background particles onto gate FET leads to detection errors. Sample preparation of studied suspension, elimination, reduction of the number of background particles should be carried out considering the diffusion coefficient for both background and the targets particles. These coefficients should be fundamentally different in value. So, a universal method of sample preparation, sensor topology, design solutions, registrar device and software for any pathogens is impractical. A line of specialized detection devices should be considered, which would be aimed at selective detection of bacteria, viruses, and proteins. We propose to use the particle diffusion coefficient (1) for sensors as a specialization criterion as well. This coefficient allows to estimate of effective area controlled by an individual FET, considering its size and the radius of the pathogen particle.
Summing up the work, we note. Pathogen detection is a complex interdisciplinary problem that has no unambiguous, universally recognized solution today. It affects to method detection and design of sensors in area: microelectronics of designing the topology of the sensor, radio engineering in development of schematic diagram of recorder, programming of detection algorithm, sensor survey, statistical analysis of results and their reliability, medicine in diagnosis of the disease, epidemiology in part of controlling the development of an epidemic or even a pandemic, molecular biology, virology, microbiology in the preparation of specific antibodies, viral, microbiological suspension in the detection procedure. Biosensors already has promising prospects for application in the field of pathogen detection and new big market potentially.
Author Contributions
Conceptualization, V.G. and A.S.; Data curation, A.G.; Formal analysis, V.G.; Methodology, M.K.; Project administration, A.G.; Resources, V.G., A.S., A.G. and V.Gr.; Supervision, M.K.; Validation, M.K.; Visualization, A.S.; Writing-original draft, V.G., A.S., and A.C.; Writing-review and editing, V.Gr., A.S. and M.K. All authors have read and agreed to published version of manuscript.
Funding
This investigation was supported State assignment of Rospotrebnadzor Russia. Investigation was carried out according to State task within framework of budget topic "Epidemiological monitoring of health status of population and study of molecular genetics and molecular biological mechanisms of development of common therapeutic diseases in Siberia to improve approaches to their diagnosis, prevention and treatment" (FWNR-2022-0024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Onischenko, G.G.; Netesov, S.V.; Agafonov, A.P.; Safatov, A.S.; Buryak, G.A.; Generalov, V.M.; Sergeyev, A.N.; Drozdov, I.G. Highly pathogenic avian influenza - a new pandemic threat and possibilities to resist it. Bulle-tin of the Russian Academy of Medical Sciences (In Russia). 2006, 12, 36–42. [Google Scholar]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 24 May 2023).
- Maia, R.; Carvalho, V.; Faria, B.; Miranda, I.; Catarino, S.; Teixeira, S.; Lima, R.; Minas, G.; Ribeiro, J. Diagnosis Methods for COVID-19: A Systematic Review. Micromachines 2022, 13, 1349. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clinical Chemistry 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Dolgov, V.V. Immunochemical analysis in laboratory medicine; Triad: Moscow, Russia, 2015 ; P. 418 (in Russia).
- Thomas, E.; Delabat, S.; Andrews, D.M. Diagnostic Testing for SARS-CoV-2 Infection. Curr Hepatology Rep. 2021, 20, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, R.; Reverberi, L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007, 5, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Development of an ion sensitive solid-state device for neurophysiological measurement. IEEE Trans Biomed Eng. 1970, 17, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Development, operation, and application of ion-sensitive field-effect transistor as a tool for electrophysiology. IEEE Trans. Biomed. Eng. 1972, 19, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Squires, T. M.; Messinger, R. J.; Manalis, S. R. Making it stick: convection, reaction and diffusion in surface-based sensors. Nat Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y.; Rajan, N.K.; Routenberg, D.A.; Modis, Y.; Reed, M.A. Quantification of the affinities and kinetics of protein interactions using silicon nanowire sensors. Nat Nanotechnol. 2012, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Zheng, G.; Lieber, C.M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.A.; Ivanov, Yu.D.; Pleshakova, T.O.; Kozlov, A.F.; Krohin, N.V.; Kaysheva, A.L.; Shumov, I.D.; Popov, V.P.; Naumova, O.V.; Fomin, B.I.; Nasimov, D.A. SOI-nanowire biosensor for detection of D-NFAT 1 protein. Biomed Khim. 2015, 61, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Naumova, O.V.; Fomin, B.I. Optimization of the response of nanowire biosensors. Optoelectronics, Instrumentation and Data Processing 2015, 52, 434–437. [Google Scholar] [CrossRef]
- Naumova, O.; Generalov, V.; Shcherbakov, D.; Zaitseva, E.; Zhivodkov, Yu.; Kozhukhov, A.; Latyshev, A.; Aseev, A.; Safatov, A.; Buryak, G.; Cheremiskina, A.; Merkuleva, Ju.; Rudometova, N. SOI-FET sensors with dielectrophoretic concentration of viruses and proteins. Biosensors 2022, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.A.; Pleshakova, T.O.; Kozlov, A.F.; Galiullin, R.A.; Popov, V.P.; Tikhonenko, F.V.; Glukhov, A.V.; Ziborov, V.S.; Shumov, I.D.; Petrov, O.F.; et al. Detection of influenza virus using a SOI-nanoribbon chip, based on an N-type field-effect transistor. Biosensors 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Kartalov, E.P.; Zhong, J.F.; Scherer, A.; Quake, S.R.; Taylor, C.R.; Anderson, W.F. High-throughput multi-antigen microfluidic fluorescence immunoassays. Biotechniques 2006, 40, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; Kim, S.-J.; Lee, J.-O; Kim, B.T.; Park, E.C.; Kim, S.I. Rapid detection of COVID-causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Hussain, C.M. Graphene-based field-effect transistor biosensors for the rapid detection and analysis of viruses: A perspective in view of COVID-19. Carbon Trends. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Hu, W.P.; Wu, Y.M.; Vu, C.A.; Chen, W.Y. Ultrasensitive detection of interleukin 6 by using silicon nanowire field-effect transistors. Sensors 2023, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Elfström, N.; Juhasz, R.; Sychugov, I.; Engfeldt, T.; Karlström, A.E.; Linnros, J. Surface charge sensitivity of silicon nanowires size dependence. Nano Lett. 2007, 7, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Nuzaihan, M.N. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Biosens Bioelectron. 2016, 83, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Cheremiskina A.A., Generalov V.M., Safatov A.S. and others. Preparation of the substrate surface of silicon nanowire field-effect transistors to create a biosensor // Technologies of living systems. 2021, 18, 62–70. (In Russia). [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Theoretical Physics, Hydrodynamics, Volume VI, 4rd ed.; Nauka: Moscow. Russian, 1988; pp. 330-332 (in Russia).
- Feynman, R.; Leighton, R.; Sands, M. Feynman lectures on physics. Volume 4. Kinetics. Warmth. Sound; Mir: Moscow, Russian, 1976; pp. 59-62 (in Russia).
- Ermakov, S.M.; Zhiglyavsky, A.A. Mathematical theory of optimal experiment. Studies. Manual; Science. Gl. ed. phys.-mat. lit.: Moscow, Russian, 1987; P. 320 (in Russia).
- Mushik, E.; Muller, P. Methods of technical decision-making: Trans. from German; Mir: Moscow, Russian, 1990; P. 208 (in Russia).
- Sachs, L. Statistische Auswertungsmethoden [Statistical evaluation methods]; Springer-Verlag: Berlin, Heidelberg, 1974; P. 548 (Russ. ed.: Sachs, L. Statisticheskoe otsenivanie; Statistika: Moscow, Russia, 1976; P. 598).
- Feller, W. Introduction to probability theory and its application. Vol. 1; Mir: Moscow, Russian, 1984; page. 33–35 (in Russia).
- E Raber, A Jin, K Noonan, R McGuire, R D Kirwell. Issues of disinfection of chemical and biological warfare agents: how clean is sufficient? International Journal of Environmental Research. 2001, 11, 128–148. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).