1. Introduction
The
Alphavirus genus comprises over 31 species, most of which are zoonotic viruses transmitted by mosquitoes [
1]. Outbreaks caused by 30 of these species have had a significant impact on public health in the Americas, including diseases such as Chikungunya, Venezuelan equine encephalitis virus (VEEV), Eastern equine encephalitis virus (EEEV), and Western equine encephalitis virus (WEEV) [
2]. Alphaviruses can cause a range of diseases in humans, ranging from flu-like symptoms to arthritis, rashes, and potentially fatal encephalitis [
2].
Among the methods for diagnosing
Alphavirus, polymerase chain reaction (PCR) is a reliable and sensitive technique. Various PCR variants have been modified to reduce costs while maintaining accuracy [
3]. Universal PCR is one such variant that can detect virus families or genera by targeting multiple species in a single reaction [
4]. Although sequencing of the PCR product is necessary to determine the specific
Alphavirus species or strain [
5], this methodology allows for the discovery of new Alphaviruses and differentiation between various viral species affecting different hosts [
6]. Several universal PCR assays have been published for diagnosing the Alphavirus genus [
4,
6,
7,
8,
9]. However, given the variety of diagnostic options and potential universal primer candidates for
Alphavirus, it is crucial to properly compare and assess the conditions and limitations of the available sets of universal primers.
In Costa Rica, VEEV and EEEV are endemic [
10], while WEEV has not been detected [
11]. However, VEEV is more prevalent (35.9%) (confidence interval [CI]: 29.9-42.5) than EEEV (2.8%) (CI: 1.3-5.9) [
10]. VEE infection in humans can manifest as fever, headache, vomiting, and diarrhea, which can sometimes be misdiagnosed as dengue fever [
12]. Equines, on the other hand, may exhibit symptoms such as weight loss, ataxia, blindness, and even death [
13]. The aim of this study is to detect the presence of
Alphavirus in brain samples collected from 2012 to 2021, including 40 cattle and 30 horses with encephalitis that tested negative for rabies. To achieve this, four Universal RT-PCR methods were compared in order to determine the most sensitive and specific one for detecting
Alphavirus in these samples.
2. Materials and Methods
Control samples for this study were provided by the Gorgas Memorial Institute of Health. The TC83 VEEV IAB strain and the EEEV/Sinv chimeric virus [
14] were propagated in Vero E6 cell cultures. The monolayer of each virus was inoculated at a multiplicity of infection (MOI) of 0.1 in a 150 cm flask containing 15 mL of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2% fetal bovine serum (FBS). The flasks were incubated at 37°C for one hour, with rocking every 15 minutes to ensure even distribution of the virus. After harvesting, the titers of EEE/Sinv and TC83 were determined to be 1.4x10
8 plaque-forming units per milliliter (PFU/mL) and 7x10
9 PFU/mL, respectively.
Histological analysis was performed on various brain regions that were fixed in formalin, including the spinal cord, medulla oblongata, cerebellum, frontal colliculi, thalamus, hippocampus, occipital cortex, and basal ganglia. This analysis was conducted on a brain sample obtained from an equine with encephalitis, which was collected on October 22, 2015, and had tested positive for VEEV by IgM ELISA. No fixed tissue from these locations was preserved at -80°C for molecular analysis.
The control samples were stored at temperatures between -50°C and -80°C and were extracted using either the magnetic beads method (Applied Biosystems™ (Waltham, MA, USA), MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit) or the column method DNeasy Blood and Tissue Kit (Qiagen Germantown, MD USA).
To compare the sensitivity of different Universal RT-PCR methods, one RT-PCR and three nested and semi-nested RT-PCRs [
6,
7,
8,
9] were assessed. These PCR methods were evaluated for their ability to detect varying dilutions of Alphaviruses, ranging from 10
7 PFU/mL to 10
3 PFU/mL for the VEEV TC83 strain and from 10
6 PFU/mL to 10
2 PFU/mL for the EEEV/Sinv chimeric virus. The workflow for the comparison of the Universal RT-PCRs is depicted in
Figure 1.
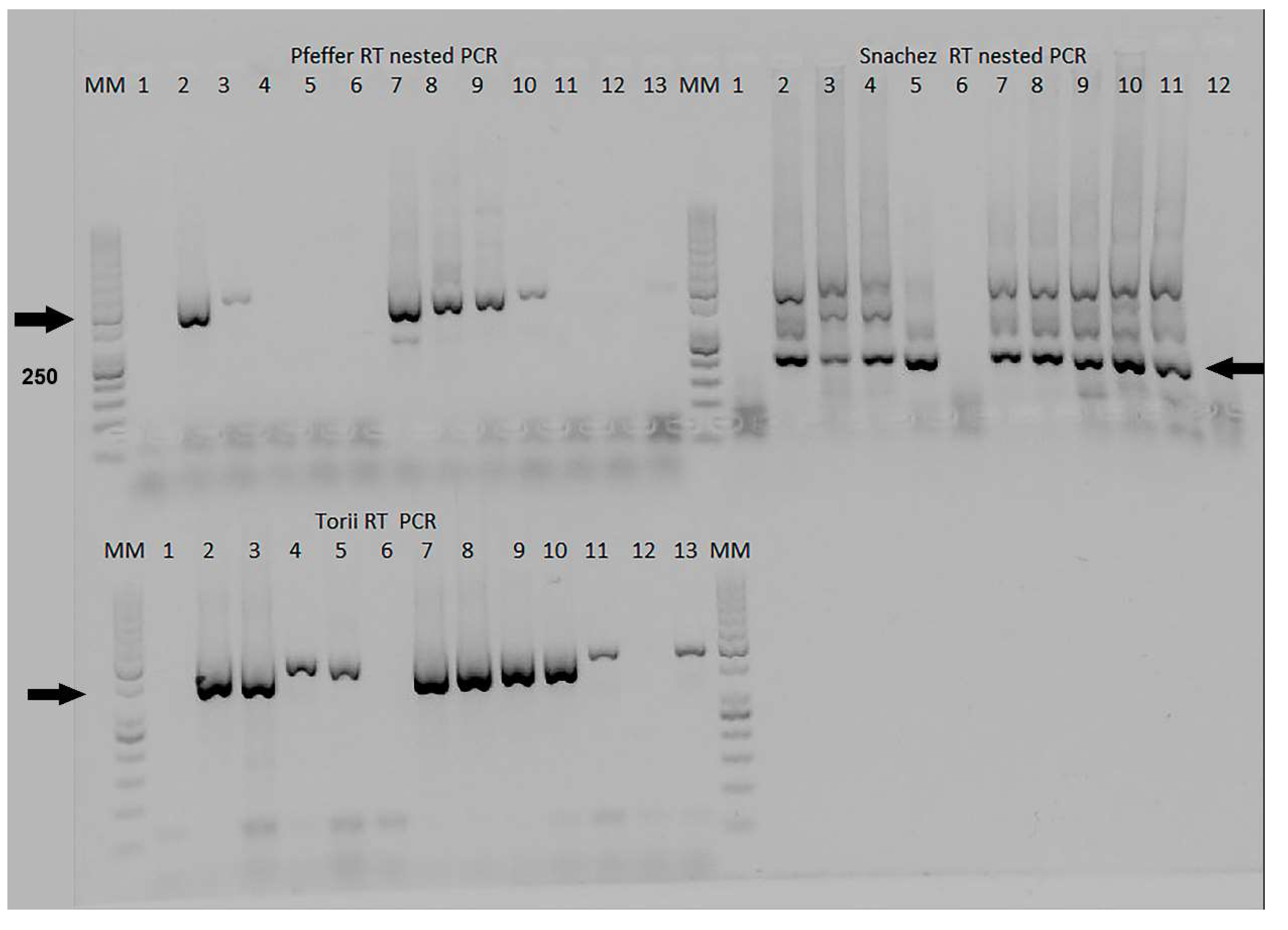
In the Phase I two-step nested RT-PCR, the reverse transcription step was the same for the Grywna, Pfeffer, and Sanchez protocols. The specific details of the reverse transcription master mix can be found in
Table S1 of the Supplementary Material. The PCR and nested PCR conditions, including the master mix reactions and thermocycling conditions, for the Grywna, Pfeffer, and Sanchez protocols are provided in
Table S2 of the Supplementary Material.
The Grywna protocols were performed using an Applied Biosystems Veriti Thermal Cycler, while other reactions without touchdown protocols were carried out in an Applied Biosystems 2720 Thermal Cycler, (Waltham, MA, USA). In the Grywna protocol, the amplicon of the second round of PCR targeted a region of the NSP4-polyprotein genes, resulting in a 210 bp amplicon. For the Pfeffer protocol, the amplicons were 434 bp and 310 bp for the first RT-PCR and the nested PCR, respectively, targeting the NSP1 gene. The Sanchez protocol produced amplicons of 481 bp and 195 bp for each round, respectively, targeting the NSP4 gene. In all RT-PCRs, water was added as necessary to complete the master mix volume reaction. Visualization of the amplicons was done using a 2% agarose gel stained with Gel-Red. A molecular weight ladder, specifically the 50 bp GeneRuler™ ladder, was used for size determination. The ladder contained bands ranging from 50 bp to 1000 bp, with the strongest bands corresponding to 250 bp and 500 bp.
In Phase II, the Pfeffer, Sanchez, and Torii protocols were compared in the one-step RT-PCR format. The details of these protocols can be found in
Table S3 RT-PCR step of the Pfeffer, Sanchez, and Torii protocols in the Supplementary Material. Specifically, the Torii RT-PCR protocol amplified a fragment of approximately 460 bp in the NS4 gene [
6].
In Phase III, the comparison focused on the volume of extraction, specifically using 1 µL and 5 µL volumes. The most sensitive RT-PCR protocol from Phase II was used for this comparison. The samples used in these assays included dilutions of VEEV TC83 (7x105 PFU/mL) and EEEV (1.2x105 PFU/mL), which were extracted with columns (30 months prior) and stored at -80ºC. Additionally, cell culture supernatants of VEEV (5x108 PFU/mL) and EEEV/Sinv (4.5x106 PFU/mL), were titrated on August 20, 2018 (40 months prior), and were used. These samples had been stored at -80ºC and were thawed for testing. Tenfold dilutions were prepared in DNAase and RNAse-free water, and 200 µL of each dilution was extracted using two commercial methods, columns and magnetic beads (Dneasy Blood and Tissue Kit (Qiagen Germantown, MD USA), magnetic beads method with Applied Biosystems™ MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit (Thermo Fisher Scientific, Waltham, MA USA), and Mag-MAX™-96 Total).
In one equine brain tested positive to VEEV for IgM ELISA, brain tissue samples ranging from 53 mg to 99 mg were collected from 8 identified locations. Pieces of tissue weighing between 20 mg to 25 mg were extracted from these samples using the Dneasy Blood and Tissue Kit (Qiagen Germantown, MD USA), and subsequently eluted in 200 µL of AE buffer. These extracted samples were then subjected to testing using the most suitable RT-PCR method determined in the study.
The brain tissue samples analyzed in the study were collected from a total of 70 animals with signs of encephalitis, including 30 equines and other samples from bovines. The samples were collected between 2012 and 2021 and were stored at temperatures between -20 and -80 ºC. The extraction of nucleic acids from the brain tissue samples was performed using either the magnetic beads method with the Applied Biosystems™ MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit, (Waltham, MA, USA) or the column method with the Dneasy Blood and Tissue Kit (Qiagen Germantown, MD USA).
In the case of amplified positive samples, they were further confirmed through sequencing using the Sanger protocol. Forward and reverse primer reactions were prepared separately, with each reaction containing a 20 µL mixture comprising 0.5x ready reaction premix, 0.5x sequencing buffer, 8 µM forward or reverse primers, and 10 µL of DNA (10-40 ng/µL). The PCR reactions underwent thermal cycling conditions, followed by purification using resin and SAMTM Solution. Depending on the band intensity observed, 10 to 20 µL of the supernatant was transferred to a 96-well plate. The sequencing step was performed using a SeqStudio genetic analyzer from Applied Biosystems™ (Waltham, MA, USA).
Ethics All samples used in this study were taken according to law 8495 “General Law of the National Animal Health Service”
4. Discussion
Three of the RT-PCR nested and semi-nested assays were validated against several species of the Alphavirus genus [
2,
3,
4], and the Torii RT-PCR was able to detect a new Alphavirus species in Zambia named Mwinilunga virus [
5]. The Grywna et al. protocol was validated using 10 alphaviruses and other RNA viruses. The Pfeffer et al. protocol, on the other hand, was validated using 30 viruses, which included strains from all six VEEV subtypes., while, the Sanchez et al. protocol was validated using 12 viruses. Our intention was not to validate these methods, but rather to verify and evaluate the performance of the assay under our specific laboratory conditions to ensure its accuracy and reliability. Factors such as the design of primers, the selection of target genes, and the conservation of the amplified regions can all impact the sensitivity and specificity of the assay. Additionally, variations in equipment, reaction volumes, and reagents used can also affect the assay's performance. This thorough evaluation helps to ensure that the assay is suitable for the intended purpose of detecting Alphavirus in the brain tissue of animals with encephalitis, providing accurate and reliable results.
It is interesting to note that three of the four Universal RT-PCR assays use the NSP4 gene as a target. In the case of the Grywna test, the limit of detection (LOD) corresponds to 10
4 PFU/ml, ranging from 5 to 100 RNA copies per reaction across all Alphavirus species [
9]. Meanwhile, Sanchez's LOD was 5X10
6 PFU/mL for EEEV in the first RT-PCR and 5X10
2 PFU/mL in the nested PCR, corresponding to 1 copy per reaction [
7]. No LOD information is available in the Torii manuscript [
6]. While the Pfeffer et al. method, targeted the NSP1 gene for its RT-PCR, the LOD was 1.2 X 10
3 PFU/mL of VEE strain for the first RT-PCR and 1.2 PFU/mL for the semi-nested PCR [
8]. The final reaction volume used in this validation was 12.5 µL, while in the Pfeffer protocol, it was 100 µL, 50 µL in Grywna and Sanchez protocols, and 15 µL in the Torii assay. Of the RT-PCRs only the Sanchez protocol shows several unspecific bands, however, the band corresponding to 195 bp band in the Chimera EEE/Sinv was confirmed as Sindbis virus by sequencing, Recombinant Sindbis virus (SINV)/VEEV and SINV/EEEV constructs have been developed to express the immunogenic structural proteins derived from VEEV or EEEV within the less virulent SINV backbone which include the NSPs. We use this chimeric virus because this approach allowed us to prepare control stocks of the chimeric virus while adhering to the appropriate biosafety guidelines and minimizing potential risks associated with handling VEEV or EEEV during their inoculation in Vero cells.
We observed that VEEV did not amplify in all of the brain locations tested. In studies of Alphavirus replication in mouse brains, histopathological evidence of EEEV damage was detected in the cortex, hippocampus, and thalamus [
16]. Conversely, VEEV viral replication in the brains of mice was multifocal and localized in association with brain capillaries [
17]. In humans, EEEV produces lesions in the basal ganglia, thalamus, and cerebral cortex, visible by computed tomography (CT) or magnetic resonance imaging (MRI) [
18]. In our study, we amplified VEEV RNA in the hippocampus, thalamus basal nuclei, and spinal cord of the equines. We were able to amplify pools of these locations from two positive equines, demonstrating the possibility of using these pools in VEEV diagnosis. Overall, these findings highlight the importance of selecting the correct sampling site in the brain for accurate diagnosis of viral infections. An interesting observation is the presence of nonspecific bands in the Torii RT-PCR when the brain tissue matrix and host DNA are included in the sample. This phenomenon can be attributed to the use of degenerate primers in the Torii et al. protocol. Degenerate primers contain nucleotide positions where multiple possibilities are allowed, increasing the chances of binding to nonspecific DNA sequences.
Despite the seropositive cases of VEEV in Costa Rica ranging from approximately 23% to 36% [
10,
11], only four out of the 30 equine brains evaluated in this study showed amplification for Alphavirus. Furthermore, only two samples could be sequenced and confirmed as VEEV subtype IE due to the low viral load observed in the other two samples. The challenges of detecting RNA are due to its susceptibility to degradation by multiple freeze and thaw cycles or RNase enzymes present in the tissue. To address this, the volume of RNA extraction was increased to 5 µl in the RT-PCR. Alphaviruses can be diagnosed not only in tissues but also in blood, although viremia is generally detected in blood for only 3 to 6 days after infection [
19]. A study used the detection of canine distemper virus (CDV) as an internal control to evaluate the extraction method, RNA degradation, and the presence of PCR inhibitors in bovine brain samples. CDV was detected in all the extracted samples when it was spiked directly into the extraction process but not in all the samples when CDV was spiked directly into the brain tissue sample before RNA extraction, possibly due to rapid CDV RNA degradation in the brain tissue matrix [
20]. The autophagy process, which is used by cells to maintain cellular health and homeostasis, may also contribute to the failure of viral RNA detection in tissue samples [
21].
We demonstrate that the Torii RT-PCR is well-suited for use in our laboratory conditions, with our reagents (master mix reaction volume, extraction methods), equipment (different thermocyclers, micropipettes), and workflow (one or two RT-PCR steps, different brain locations), it was also inexpensive, faster, sensitive, and specific in comparison with the nested RT-PCRs in the same conditions. It appears that the most suitable tissues for VEEV diagnosis in equines are the hippocampus, basal nuclei, and spinal cord. These specific tissue samples have shown positive amplification of VEEV RNA in our study, indicating their potential for accurate diagnosis of VEEV infection in equines. Finally, we were able to sequence two samples; according to the Basic Local Alignment Search Tool Blast [
6] (National Institute of Health, Bethesda, MD USA), both viruses belong to the VEEV subtype IE confirming the endemicity of this subtype at least in the northern provinces of Costa Rica. The presence of this virus highlights the importance of ongoing surveillance and understanding of these viruses to implement effective disease control measures under the one health protocol.