Submitted:
13 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
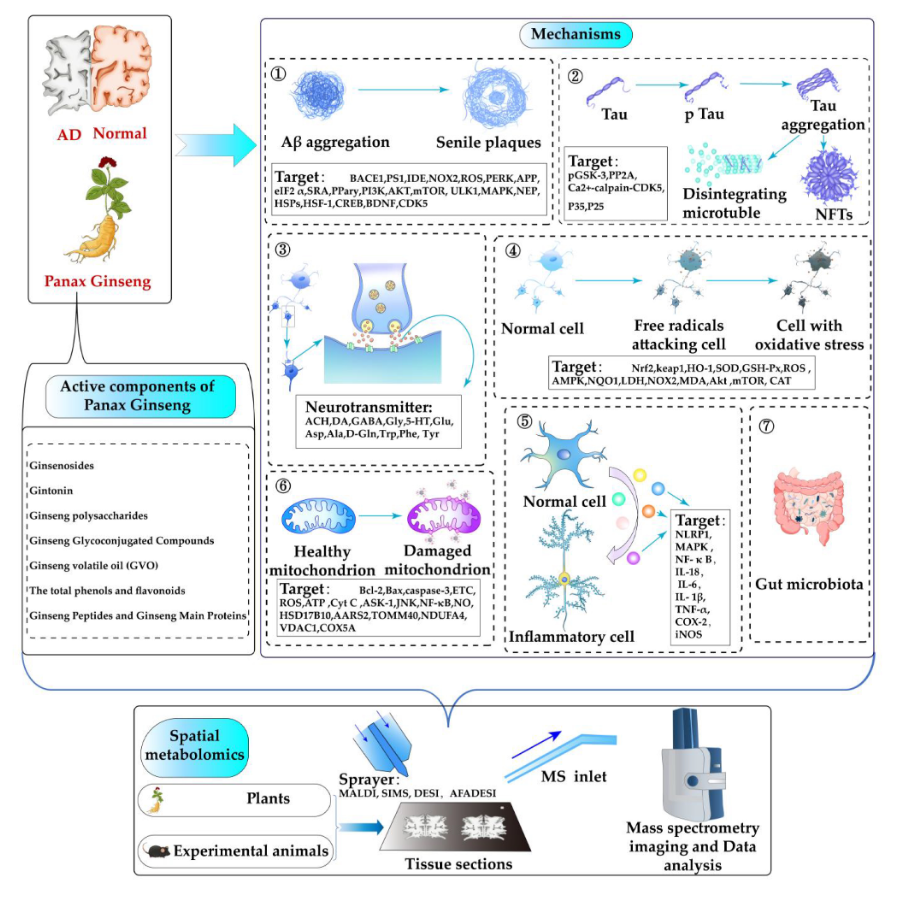
Keywords:
1. Introduction
2. Active Ingredients of P. ginseng
2.1. Ginsenosides
2.2. Gintonin
2.3. Ginseng Polysaccharides
2.4. Ginseng Peptides and Ginseng Main Proteins
2.5. Ginseng Glycoconjugated Compounds
2.6. Other Compounds
3. Mechanism of P.ginseng in the Treatment of Alzheimer's Disease
3.1. Interference Aβ Generation and Aggregation
3.2. Inhibition of the Hyperphosphorylation of Tau
3.3. Regulation of Neurotransmitter Levels
3.4. Antioxidant Stress and Anti-Inflammation
3.5. Prevention of Mitochondrial Damage
3.6. Regulation of Gut Microbiota
4. Application of Spatial Metabolomics in Alzheimer's Disease Research
5. Conclusions
Author Contributions
Funding
References
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in Saponin Diversity of Panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef]
- Villain, N.; Dubois, B. Alzheimer’s disease including focal presentations. Semin. Neurol. 2019, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Tang, W.; Li, B. Mass spectrometry imaging and its potential in food microbiology. Int. J. Food Microbiol. 2022, 371, 109675. [Google Scholar] [CrossRef] [PubMed]
- He, M.J.; Pu, W.; Wang, X.; Zhang, W.; Tang, D.; Dai, Y. Comparing DESI-MSI and MALDI-MSI Mediated Spatial Metabolomics and Their Applications in Cancer Studies. Front. Oncol. 2022, 12, 891018. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Chhillar, H.; Kim, Y.-J.; Jo, I.H.; Kim, S.T.; Gupta, R. Phytochemistry of ginsenosides: Recent advancements and emerging roles. Crit. Rev. Food Sci. Nutr. 2023, 63, 613–640. [Google Scholar] [CrossRef]
- Yao, W.; Guan, Y. Ginsenosides in cancer: A focus on the regulation of cell metabolism. Biomed. Pharmacother. Biomedecine Pharmacother. 2022, 156, 113756. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Li, W.; Li, J.; Wang, C.; Zhang, K. Insights into the antitumor mechanism of ginsenosides Rg3. Mol. Biol. Rep. 2021, 48, 2639–2652. [Google Scholar] [CrossRef]
- Im, D.-S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Zhang, Z.; Ou, W.; Xu, F.; Luo, L.; Liu, Y.; Chen, W.; Chen, J. Antiviral Effect of Ginsenosides rk1 against Influenza a Virus Infection by Targeting the Hemagglutinin 1-Mediated Virus Attachment. Int. J. Mol. Sci. 2023, 24, 4967. [Google Scholar] [CrossRef]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.F.B.; Guissoni Campos, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an Anti-Diabetic Agent and Its Underlying Mechanism Analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. Biomedecine Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Zhu, G.-X.; Zuo, J.-L.; Xu, L.; Li, S.-Q. Ginsenosides in vascular remodeling: Cellular and molecular mechanisms of their therapeutic action. Pharmacol. Res. 2021, 169, 105647. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Wu, A.; Cao, Y.; Dai, X.; Liang, Y.; Li, X. Ginsenosides in central nervous system diseases: Pharmacological actions, mechanisms, and therapeutics. Phytother. Res. PTR 2022, 36, 1523–1544. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A Potential Neuroprotective Agent. BioMed Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, W.; Yue, J.; Xu, S.; Ma, W.; Zhao, C.; Wang, X.; Wang, D. HSF-1 mediated combined ginsenosides ameliorating Alzheimer’s disease like symptoms in Caernorhabditis elegans. Nutr. Neurosci. 2022, 25, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Gantait, S.; Mitra, M.; Chen, J.-T. Biotechnological Interventions for Ginsenosides Production. Biomolecules 2020, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Huy, N.Q.; Tung, N.H. Microorganisms for Ginsenosides Biosynthesis: Recent Progress, Challenges, and Perspectives. Mol. Basel Switz. 2023, 28, 1437. [Google Scholar] [CrossRef] [PubMed]
- H, L.; H, J.; L, X.; Y, D.; J, X.; Y, Z. Effects of Different Extraction Methods in Pharmacopoeia on the Content and Structure Transformation of Ginsenosides. Mol. Basel Switz. 2022, 27. [Google Scholar] [CrossRef]
- Cao, L.; Wu, H.; Zhang, H.; Zhao, Q.; Yin, X.; Zheng, D.; Li, C.; Kim, M.-J.; Kim, P.; Xue, Z.; et al. Highly efficient production of diverse rare ginsenosides using combinatorial biotechnology. Biotechnol. Bioeng. 2020, 117, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-N.; Wang, R.-F.; Zhao, S.-J.; Wang, Z.-T. Advance in glycosyltransferases, the important bioparts for production of diversified ginsenosides. Chin. J. Nat. Med. 2020, 18, 643–658. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, R.; Nam, S.M.; Kim, D.-G.; Cho, I.-H.; Kim, H.-C.; Cho, Y.; Rhim, H.; Nah, S.-Y. Ginseng gintonin, aging societies, and geriatric brain diseases. Integr. Med. Res. 2021, 10, 100450. [Google Scholar] [CrossRef]
- Kim, D.-G.; Jang, M.; Choi, S.-H.; Kim, H.-J.; Jhun, H.; Kim, H.-C.; Rhim, H.; Cho, I.-H.; Nah, S.-Y. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int. J. Biol. Macromol. 2018, 114, 1325–1337. [Google Scholar] [CrossRef]
- Tao, R.; Lu, K.; Zong, G.; Xia, Y.; Han, H.; Zhao, Y.; Wei, Z.; Lu, Y. Ginseng polysaccharides: Potential antitumor agents. J. Ginseng Res. 2023, 47, 9–22. [Google Scholar] [CrossRef]
- Jiao, L.; Li, J.; Liu, F.; Wang, J.; Jiang, P.; Li, B.; Li, H.; Chen, C.; Wu, W. Characterisation, Chain Conformation and Antifatigue Effect of Steamed Ginseng Polysaccharides With Different Molecular Weight. Front. Pharmacol. 2021, 12, 712836. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.-G.; Liang, Q.-C.; Wu, Y.-Y.; Liu, J.-Q.; Liu, J.-W. Red ginseng polysaccharide exhibits anticancer activity through GPX4 downregulation-induced ferroptosis. Pharm. Biol. 2022, 60, 909–914. [Google Scholar] [CrossRef]
- Li, S.; Huo, X.; Qi, Y.; Ren, D.; Li, Z.; Qu, D.; Sun, Y. The Protective Effects of Ginseng Polysaccharides and Their Effective Subfraction against Dextran Sodium Sulfate-Induced Colitis. Foods Basel Switz. 2022, 11, 890. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, C.W.; Lee, S.J.; Park, H.-R.; Kim, S.H.; Son, S.-U.; Park, J.; Shin, K.-S. Anti-Cancer Effects of Panax ginseng Berry Polysaccharides via Activation of Immune-Related Cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Wang, D.; Shao, S.; Zhang, Y.; Zhao, D.; Wang, M. Insight Into Polysaccharides From Panax ginseng C. A. Meyer in Improving Intestinal Inflammation: Modulating Intestinal Microbiota and Autophagy. Front. Immunol. 2021, 12, 683911. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; He, M.; Zheng, W.; Qi, D.; Zhang, Y.; Han, C.-C. Ginseng polysaccharides: A potential neuroprotective agent. J. Ginseng Res. 2021, 45, 211–217. [Google Scholar] [CrossRef]
- Guo, M.; Shao, S.; Wang, D.; Zhao, D.; Wang, M. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, C.; Gao, X.; Wang, F.; Li, X.; Kassim, R.M.; Tai, G.; Zhou, Y. Neuroprotective effects of ginseng pectin through the activation of ERK/MAPK and Akt survival signaling pathways. Mol. Med. Rep. 2012, 5, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Bai, Y.; Liu, D.; Zhao, N.; Gao, H.; Zhang, X. Quinetides: diverse posttranslational modified peptides of ribonuclease-like storage protein from Panax quinquefolius as markers for differentiating ginseng species. J. Ginseng Res. 2020, 44, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Cheng, M.; Lv, W.; Wu, Y.; Liu, D.; Zhang, X. Peptides as Potential Biomarkers for Authentication of Mountain-Cultivated Ginseng and Cultivated Ginseng of Different Ages Using UPLC-HRMS. J. Agric. Food Chem. 2020, 68, 2263–2275. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, D.; Wang, Y.; Chen, Y.; Jiang, R.; Yu, P.; Qiu, Z. Study on the Structure of Ginseng Glycopeptides with Anti-Inflammatory and Analgesic Activity. Mol. Basel Switz. 2018, 23, 1325. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, S.B.; Jo, Y.H.; Ahn, J.H.; Turk, A.; Kim, D.E.; Chang, B.Y.; Kim, S.Y.; Jeong, C.-S.; Hwang, B.Y.; et al. Curcubinoyl flavonoids from wild ginseng adventitious root cultures. Sci. Rep. 2021, 11, 12212. [Google Scholar] [CrossRef]
- Yao, F.; Xue, Q.; Li, K.; Cao, X.; Sun, L.; Liu, Y. Phenolic Compounds and Ginsenosides in Ginseng Shoots and Their Antioxidant and Anti-Inflammatory Capacities in LPS-Induced RAW264.7 Mouse Macrophages. Int. J. Mol. Sci. 2019, 20, 2951. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiao, P.; Ouyang, Z.; Li, D.; Zheng, J.; Wang, G.; Wang, F. Ginseng volatile oil prolongs the lifespan and healthspan of Caenorhabditis elegans. Biogerontology 2022, 23, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Han, J.; Du, Z.; Lim, M.H. Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer’s Disease. Acc. Chem. Res. 2021, 54, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.; Gupta, M.; Ahmad, M.; Shah, A.M.; Ahsan, A.U.; Qazi, P.H.; Malik, F.; Singh, G.; Sharma, P.R.; Kaddoumi, A.; et al. Alborixin clears amyloid-β by inducing autophagy through PTEN-mediated inhibition of the AKT pathway. Autophagy 2019, 15, 1810–1828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lin, J.; Zhang, H.; Liu, Y.; Kan, M.; Xiu, Z.; Chen, X.; Lan, X.; Li, X.; Shi, X.; et al. Ginsenoside Compound K Regulates Amyloid β via the Nrf2/Keap1 Signaling Pathway in Mice with Scopolamine Hydrobromide-Induced Memory Impairments. J. Mol. Neurosci. MN 2019, 67, 62–71. [Google Scholar] [CrossRef]
- Quan, Q.; Li, X.; Feng, J.; Hou, J.; Li, M.; Zhang, B. Ginsenoside Rg1 reduces β-amyloid levels by inhibiting CDK5-induced PPARγ phosphorylation in a neuron model of Alzheimer’s disease. Mol. Med. Rep. 2020, 22, 3277–3288. [Google Scholar] [CrossRef]
- Karpagam, V.; Sathishkumar, N.; Sathiyamoorthy, S.; Rasappan, P.; Shila, S.; Kim, Y.-J.; Yang, D.-C. Identification of BACE1 inhibitors from Panax ginseng saponins-An Insilco approach. Comput. Biol. Med. 2013, 43, 1037–1044. [Google Scholar] [CrossRef]
- Yun, Y.-J.; Park, B.-H.; Hou, J.; Oh, J.-P.; Han, J.-H.; Kim, S.-C. Ginsenoside F1 Protects the Brain against Amyloid Beta-Induced Toxicity by Regulating IDE and NEP. Life 2022, 12, 58. [Google Scholar] [CrossRef]
- Yang, L.; Hao, J.; Zhang, J.; Xia, W.; Dong, X.; Hu, X.; Kong, F.; Cui, X. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J. Pharm. Pharmacol. 2009, 61, 375–380. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665–675. [Google Scholar] [CrossRef]
- Mi, L.; Fan, M.; Liu, T.; Wu, D.; Wang, Y.; Li, F.; Cai, Y.; Qiu, Z.; Liu, D.; Cao, L. Ginsenoside Rd protects transgenic Caenorhabditis elegans from β-amyloid toxicity by activating oxidative resistant. Front. Pharmacol. 2022, 13, 1074397. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Su, P.; Zhang, S.; Guo, L.; Zhang, H.; Liang, Y.; Qin, C.; Zhang, W. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016, 793, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.J.; Roy, A.; Jung, H.J.; Ali, M.Y.; Min, B.-S.; Park, C.H.; Yokozawa, T.; Fan, T.-P.; Choi, J.S.; Jung, H.A. BACE1 molecular docking and anti-Alzheimer’s disease activities of ginsenosides. J. Ethnopharmacol. 2016, 190, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Chen, Q.; Liu, Y.; Wei, X.; Wu, M.; Zhang, K.; Liu, Y.; Wei, W. Inhibition of mGluR5/PI3K-AKT Pathway Alleviates Alzheimer’s Disease-Like Pathology Through the Activation of Autophagy in 5XFAD Mice. J. Alzheimers Dis. 2023, 91, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lv, J.; Lu, J.; Fan, L.; Huang, X.; Hu, L.; Wang, J.; Shen, X. Protopanaxadiol derivative DDPU improves behavior and cognitive deficit in AD mice involving regulation of both ER stress and autophagy. Neuropharmacology 2018, 130, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.W.; Jang, S.K.; Jo, B.R.; Kim, H.S.; Park, J.Y.; Park, H.Y.; Yoo, Y.-M.; Joo, S.S. A therapeutic intervention for Alzheimer’s disease using ginsenoside Rg3: its role in M2 microglial activation and non-amyloidogenesis. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, W.; Yue, J.; Xu, S.; Ma, W.; Zhao, C.; Wang, X.; Wang, D. HSF-1 mediated combined ginsenosides ameliorating Alzheimer’s disease like symptoms in Caernorhabditis elegans. Nutr. Neurosci. 2022, 25, 2136–2148. [Google Scholar] [CrossRef]
- Han, J.; Oh, J.-P.; Yoo, M.; Cui, C.-H.; Jeon, B.-M.; Kim, S.-C.; Han, J.-H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol. Brain 2019, 12, 77. [Google Scholar] [CrossRef]
- Liu, M.; Bai, X.; Yu, S.; Zhao, W.; Qiao, J.; Liu, Y.; Zhao, D.; Wang, J.; Wang, S. Ginsenoside Re Inhibits ROS/ASK-1 Dependent Mitochondrial Apoptosis Pathway and Activation of Nrf2-Antioxidant Response in Beta-Amyloid-Challenged SH-SY5Y Cells. Molecules 2019, 24, 2687. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Li, K.-X.; Xue, W. Pharmacokinetics and acetylcholine releasing effects of ginsenoside Rg1 in hippocampus of beta-amyloid model rats. J. Asian Nat. Prod. Res. 2019, 21, 772–781. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, X.; Xu, Q.; Wei, G.; Zhang, L.; Fan, Y.; Wen, L.; Liu, Y.; Zhang, T.; Zhang, L.; et al. Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 2022, 282, 114598. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Bakota, L.; Brandt, R. Microtubule-modulating Agents in the Fight Against Neurodegeneration: Will it ever Work? Curr. Neuropharmacol. 2022, 20, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Skuntz, S.; Pant, H.C. Deregulated Cdk5 activity is involved in inducing Alzheimer’s disease. Arch. Med. Res. 2012, 43, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Di, J.; Liu, W.; Liu, H.; Lai, H.; Lü, Y. Involvement of GSK3 and PP2A in ginsenoside Rb1’s attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013, 241, 228–234. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141. [Google Scholar] [CrossRef]
- Chen, X.; Huang, T.; Zhang, J.; Song, J.; Chen, L.; Zhu, Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1’s attenuation of beta-amyloid peptide25-35-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008, 1200, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pi, Z.; Song, F.; Liu, Z. Ginsenosides attenuate d-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 194, 188–195. [Google Scholar] [CrossRef]
- She, L.; Xiong, L.; Li, L.; Zhang, J.; Sun, J.; Wu, H.; Ren, J.; Wang, W.; Zhao, X.; Liang, G. Ginsenoside Rk3 ameliorates Aβ-induced neurotoxicity in APP/PS1 model mice via AMPK signaling pathway. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 158, 114192. [Google Scholar] [CrossRef]
- Kandimalla, R.; Reddy, P.H. Therapeutics of Neurotransmitters in Alzheimer’s Disease. J. Alzheimers Dis. JAD 2017, 57, 1049–1069. [Google Scholar] [CrossRef]
- Kamecki, F.; Knez, D.; Carvalho, D.; Marcucci, C.; Rademacher, M.; Higgs, J.; Žakelj, S.; Marcos, A.; de Tezanos Pinto, F.; Abin-Carriquiry, J.A.; et al. Multitarget 2’-hydroxychalcones as potential drugs for the treatment of neurodegenerative disorders and their comorbidities. Neuropharmacology 2021, 201, 108837. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Xie, J.; Tan, Y.; Zhang, M.; Zhao, Y.; Liu, X. Neurotransmitter-stimulated neuron-derived sEVs have opposite effects on amyloid β-induced neuronal damage. J. Nanobiotechnology 2021, 19, 324. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.F.; Adewale, Q.; Baumeister, T.R.; Carbonell, F.; Zilles, K.; Palomero-Gallagher, N.; Iturria-Medina, Y. Personalized brain models identify neurotransmitter receptor changes in Alzheimer’s disease. Brain 2021, 145, 1785–1804. [Google Scholar] [CrossRef]
- Lv, J.; Lu, C.; Jiang, N.; Wang, H.; Huang, H.; Chen, Y.; Li, Y.; Liu, X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 2021, 35, 337–345. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, X.; Jin, P.; Li, N.; Zhang, Q.; Yan, W.; Zhang, H.; Sun, J. Screening and determination for potential acetylcholinesterase inhibitory constituents from ginseng stem–leaf saponins using ultrafiltration (UF)-LC-ESI-MS2. Phytochem. Anal. 2019, 30, 26–33. [Google Scholar] [CrossRef]
- Lu, C.; Lv, J.; Dong, L.; Jiang, N.; Wang, Y.; Wang, Q.; Li, Y.; Chen, S.; Fan, B.; Wang, F.; et al. Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phytother. Res. 2018, 32, 1056–1063. [Google Scholar] [CrossRef]
- Benishin, C.G.; Lee, R.; Wang, L.C.; Liu, H.J. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology 1991, 42, 223–229. [Google Scholar] [CrossRef]
- Shi, J.; Xue, W.; Zhao, W.; Li, K. Pharmacokinetics and dopamine/acetylcholine releasing effects of ginsenoside Re in hippocampus and mPFC of freely moving rats. Acta Pharmacol. Sin. 2013, 34, 214–220. [Google Scholar] [CrossRef]
- Kim, J.; Shim, J.; Lee, S.; Cho, W.-H.; Hong, E.; Lee, J.H.; Han, J.-S.; Lee, H.J.; Lee, K.W. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC Complement. Altern. Med. 2016, 16, 66. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorganic Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 Prevents Cognitive Impairment by Improving Mitochondrial Dysfunction in the Rat Model of Alzheimer’s Disease. J. Agric. Food Chem. 2019, 67, 10048–10058. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Pi, Z.; Liu, S.; Zheng, Z.; Liu, Z.; Song, F. Mass spectrometry-based urinary metabolomics for exploring the treatment effects of Radix ginseng-Schisandra chinensis herb pair on Alzheimer’s disease in rats. J. Sep. Sci. 2021, 44, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- ZHAO, Y.; CHEN, J.; XIAO, H.; TIAN, Y.; LI, H.; TIAN, J.; YANG, J. Study on the Inhibition of Oxidative Stress Injury in H2O2-induced N2a Cells by Ginsenoside Rg1 via Activation of Nrf2/HO-1 Signaling Pathway. Res. Pract. Chin. Med. 2022, 36, 26–31. [Google Scholar] [CrossRef]
- Chen, L.; Yao, H.; Chen, X.; Wang, Z.; Xiang, Y.; Xia, J.; Liu, Y.; Wang, Y. Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by d-Galactose. Neurochem. Res. 2018, 43, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Yu, S.; Zhao, C.; Xu, B.; Liu, R.; Xu, L.; Guo, Y. Neuroprotective Effect of Ginseng Fibrous Root Enzymatic Hydrolysate against Oxidative Stress. Molecules 2022, 27, 7824. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Zhang, B.; Yin, Y.; Zhang, J.; Huang, D.; Huang, R.; Li, W.; Li, W. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int. J. Mol. Med. 2017, 40, 1134–1142. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J.; Kim, S.-H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol. Ther. 2013, 21, 381–390. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Q.; Fan, R.; Wang, J.; Li, Y. Anti-Inflammation Effect of Small Molecule Oligopeptides Prepared from Panax ginseng C. A. Meyer in Rats. Mol. Basel Switz. 2019, 24, 858. [Google Scholar] [CrossRef]
- Piaceri, I.; Rinnoci, V.; Bagnoli, S.; Failli, Y.; Sorbi, S. Mitochondria and Alzheimer’s disease. J. Neurol. Sci. 2012, 322, 31–34. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A.; Reddy, P.H. Defective Mitophagy in Alzheimer’s Disease. Ageing Res. Rev. 2020, 64, 101191. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, L.; Cheng, Y.; Ji, X.; Chi, T.; Liu, P.; Zou, L. PINK1 overexpression prevents forskolin-induced tau hyperphosphorylation and oxidative stress in a rat model of Alzheimer’s disease. Acta Pharmacol. Sin. 2022, 43, 1916–1927. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.; Zhao, Q.; Zhang, X.; Lou, Y. Ginsenoside Rg1 exerts a protective effect against Aβ25-35-induced toxicity in primary cultured rat cortical neurons through the NF-κB/NO pathway. Int. J. Mol. Med. 2016, 37, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Song, M.-Y.; Yim, S.-V.; Lee, S.-E.; Park, K.-S. Global analysis of ginsenoside Rg1 protective effects in β-amyloid-treated neuronal cells. J. Ginseng Res. 2017, 41, 566. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Guo, Y.; Du, X.; Qin, C. Gut microbiota regulate cognitive deficits and amyloid deposition in a model of Alzheimer’s disease. J. Neurochem. 2020, 155, 448–461. [Google Scholar] [CrossRef]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.-H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef]
- Chen, H.; Shen, J.; Li, H.; Zheng, X.; Kang, D.; Xu, Y.; Chen, C.; Guo, H.; Xie, L.; Wang, G.; et al. Ginsenoside Rb1 exerts neuroprotective effects through regulation of Lactobacillus helveticus abundance and GABAA receptor expression. J. Ginseng Res. 2020, 44, 86–95. [Google Scholar] [CrossRef]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.-P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Watling, M.; Imbimbo, B.P. Time to test antibacterial therapy in Alzheimer’s disease. Brain 2019, 142, 2905–2929. [Google Scholar] [CrossRef]
- de J.R. De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Garcez, M.L.; Jacobs, K.R.; Guillemin, G.J. Microbiota Alterations in Alzheimer’s Disease: Involvement of the Kynurenine Pathway and Inflammation. Neurotox. Res. 2019, 36, 424–436. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic Supplementation in Patients with Alzheimer’s Dementia - An Explorative Intervention Study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Zeng, Y.; Guo, Y.; Wu, C.; Zhao, H.; Zheng, H.; Jiao, J. Improving Alzheimer’s disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol. Lett. 2020, 367, fnaa011. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, M.; Guo, W.; Zhang, Y.; Sui, X.; Lin, J.; Liu, X.; Li, H.; Li, J.; Yang, Q.; et al. Microbiome-Metabolomics Reveals Endogenous Alterations of Energy Metabolism by the Dushen Tang to Attenuate D-Galactose-Induced Memory Impairment in Rats. BioMed Res. Int. 2021, 2021, 6649085. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Solas, M. Dysbiosis and Alzheimer’s Disease: Cause or Treatment Opportunity? Cell. Mol. Neurobiol. 2022, 42, 377–387. [Google Scholar] [CrossRef]
- Schwamborn, K.; Kriegsmann, M.; Weichert, W. MALDI imaging mass spectrometry — From bench to bedside. Biochim. Biophys. Acta BBA - Proteins Proteomics 2017, 1865, 776–783. [Google Scholar] [CrossRef]
- Nishidate, M.; Hayashi, M.; Aikawa, H.; Tanaka, K.; Nakada, N.; Miura, S.; Ryu, S.; Higashi, T.; Ikarashi, Y.; Fujiwara, Y.; et al. Applications of MALDI mass spectrometry imaging for pharmacokinetic studies during drug development. Drug Metab. Pharmacokinet. 2019, 34, 209–216. [Google Scholar] [CrossRef]
- Connolly, L.; Jamzad, A.; Kaufmann, M.; Farquharson, C.E.; Ren, K.; Rudan, J.F.; Fichtinger, G.; Mousavi, P. Combined Mass Spectrometry and Histopathology Imaging for Perioperative Tissue Assessment in Cancer Surgery. J. Imaging 2021, 7, 203. [Google Scholar] [CrossRef]
- Drake, R.R.; West, C.A.; Mehta, A.S.; Angel, P.M. MALDI Mass Spectrometry Imaging of N-Linked Glycans in Tissues. In Glycobiophysics; Advances in Experimental Medicine and Biology; Yamaguchi, Y., Kato, K., Eds.; Springer: Singapore, 2018; pp. 59–76. ISBN 9789811321580. [Google Scholar]
- Sun, C.; Li, T.; Song, X.; Huang, L.; Zang, Q.; Xu, J.; Bi, N.; Jiao, G.; Hao, Y.; Chen, Y.; et al. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 52–57. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, W.; Huo, M.; He, B.; Liu, Y.; Tian, L.; Li, W.; Zhou, Z.; Wang, B.; Xia, J.; et al. Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharm. Sin. B 2021, 11, 3665–3677. [Google Scholar] [CrossRef] [PubMed]
- Shariatgorji, M.; Nilsson, A.; Fridjonsdottir, E.; Vallianatou, T.; Källback, P.; Katan, L.; Sävmarker, J.; Mantas, I.; Zhang, X.; Bezard, E.; et al. Comprehensive mapping of neurotransmitter networks by MALDI–MS imaging. Nat. Methods 2019, 16, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, Z.; Fu, W.; Tian, L.; Li, W.; Zhou, Z.; Chen, Y.; Wei, J.; Abliz, Z. Spatially Resolved Metabolomics Based on Air-Flow-Assisted Desorption Electrospray Ionization–Mass Spectrometry Imaging Reveals Region-Specific Metabolic Alterations in Diabetic Encephalopathy. J. Proteome Res. 2021, 20, 3567–3579. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Gao, S.; Ga, M.; Zhang, J.; Luo, Z.; Chen, Y.; Zhang, R.; He, J.; Abliz, Z. Mapping Metabolic Networks in the Brain by Ambient Mass Spectrometry Imaging and Metabolomics. Anal. Chem. 2021, 93, 6746–6754. [Google Scholar] [CrossRef] [PubMed]
- Michno, W.; Stringer, K.M.; Enzlein, T.; Passarelli, M.K.; Escrig, S.; Vitanova, K.; Wood, J.; Blennow, K.; Zetterberg, H.; Meibom, A.; et al. Following spatial Aβ aggregation dynamics in evolving Alzheimer’s disease pathology by imaging stable isotope labeling kinetics. Sci. Adv. 2021, 7, eabg4855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, C.; Tan, W. Brain Lipid Dynamics in Amyloid Precursor Protein/Presenilin 1 Mouse Model of Early Alzheimer’s Disease by Desorption Electrospray Ionization and Matrix Assisted Laser Desorption Ionization–Mass Spectrometry Imaging Techniques. J. Proteome Res. 2021, 20, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Z.; Li, H.; An, Y.; Qu, H.; Yao, C.; Zhang, J.; Li, J.; Zhang, G.; Shi, Y.; et al. Exploration of tissue distribution of ginsenoside Rg1 by LC-MS/MS and nanospray desorption electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2021, 198, 113999. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, R.; Wang, Y.; Chen, C.; Liu, S. Investigation on property differences of ginseng and American ginseng by spatial metabolomics of neurochemicals with desorption electrospray ionization mass spectrometry imaging. J. Ethnopharmacol. 2023, 303, 116006. [Google Scholar] [CrossRef]

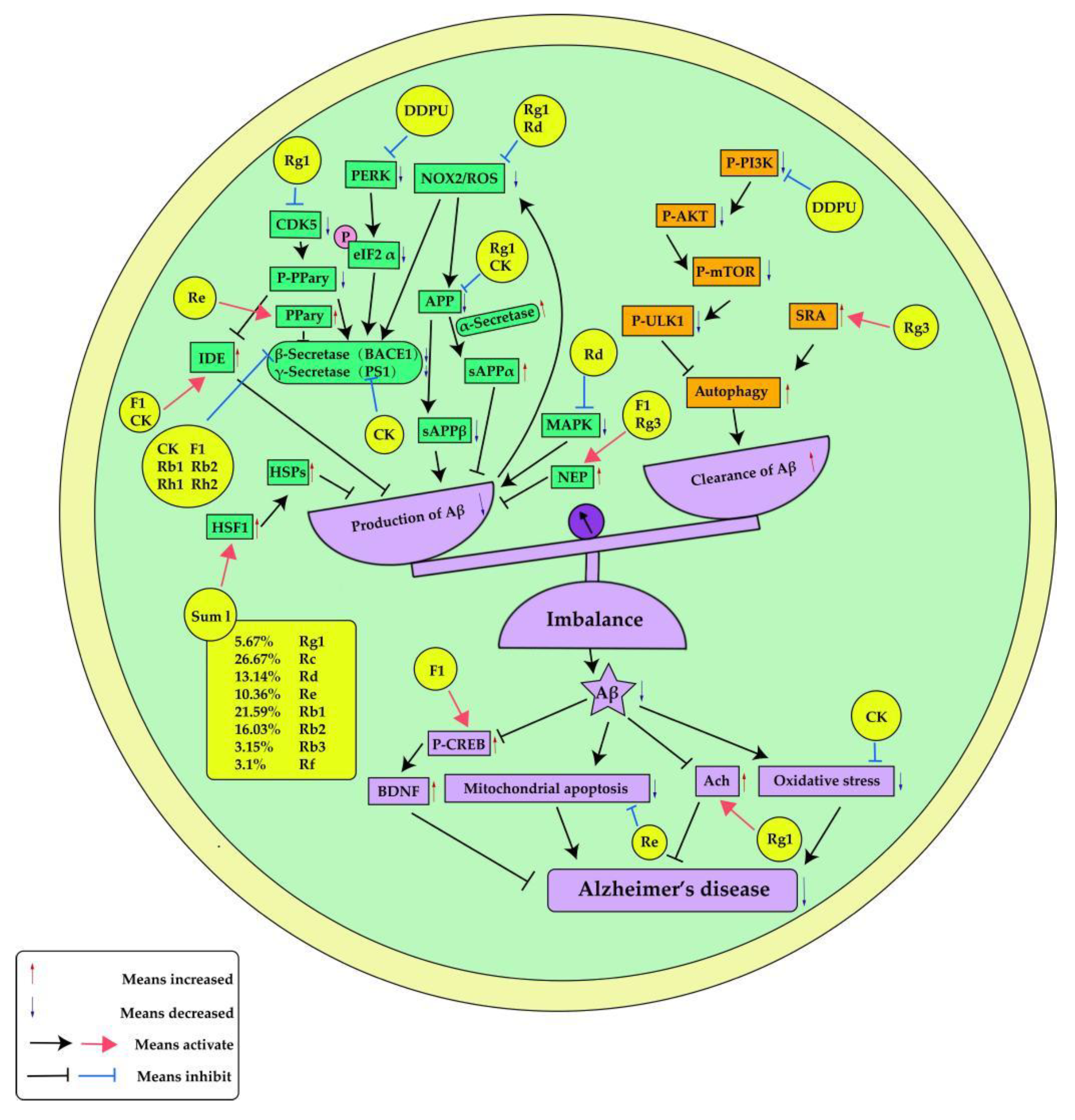
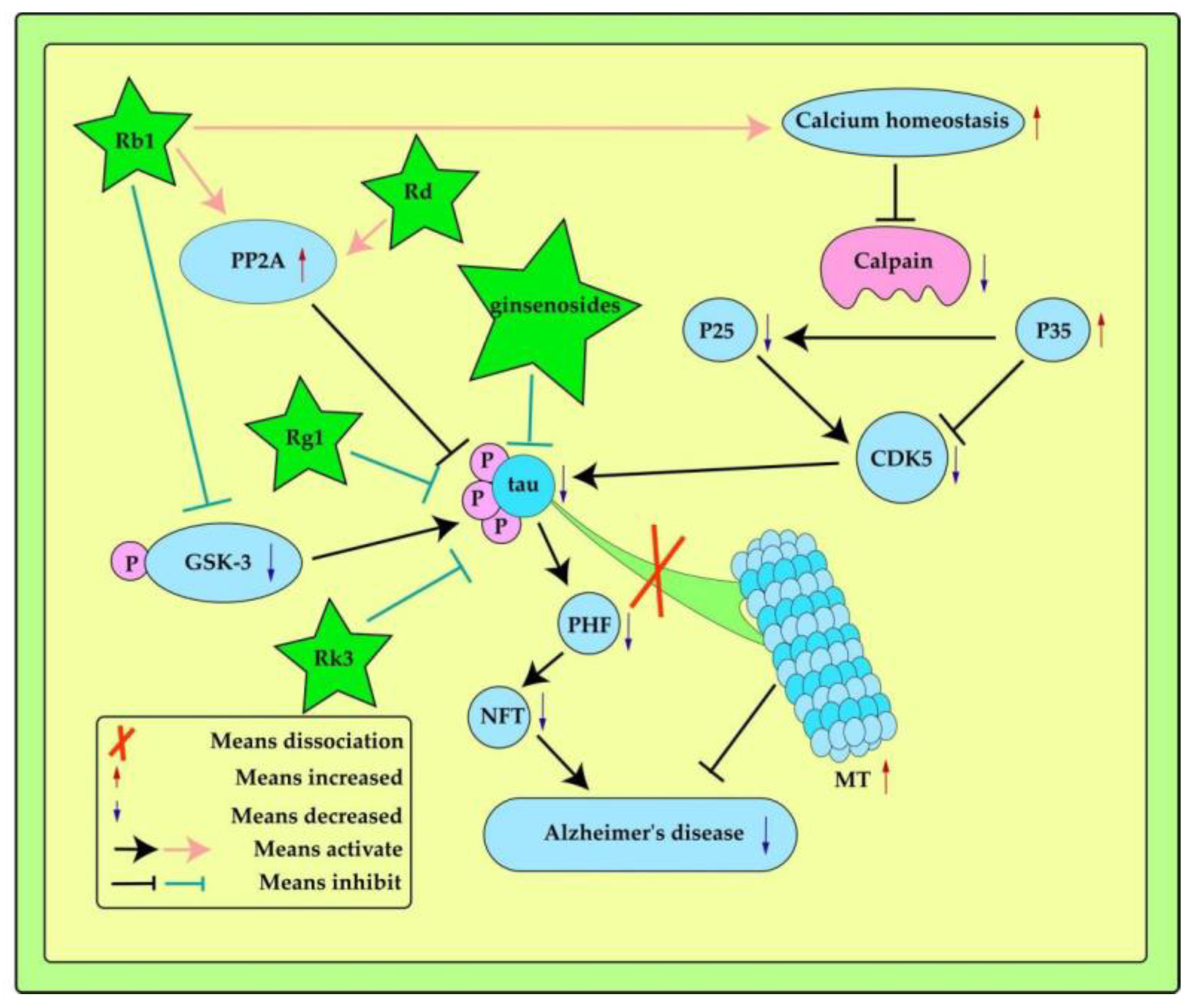
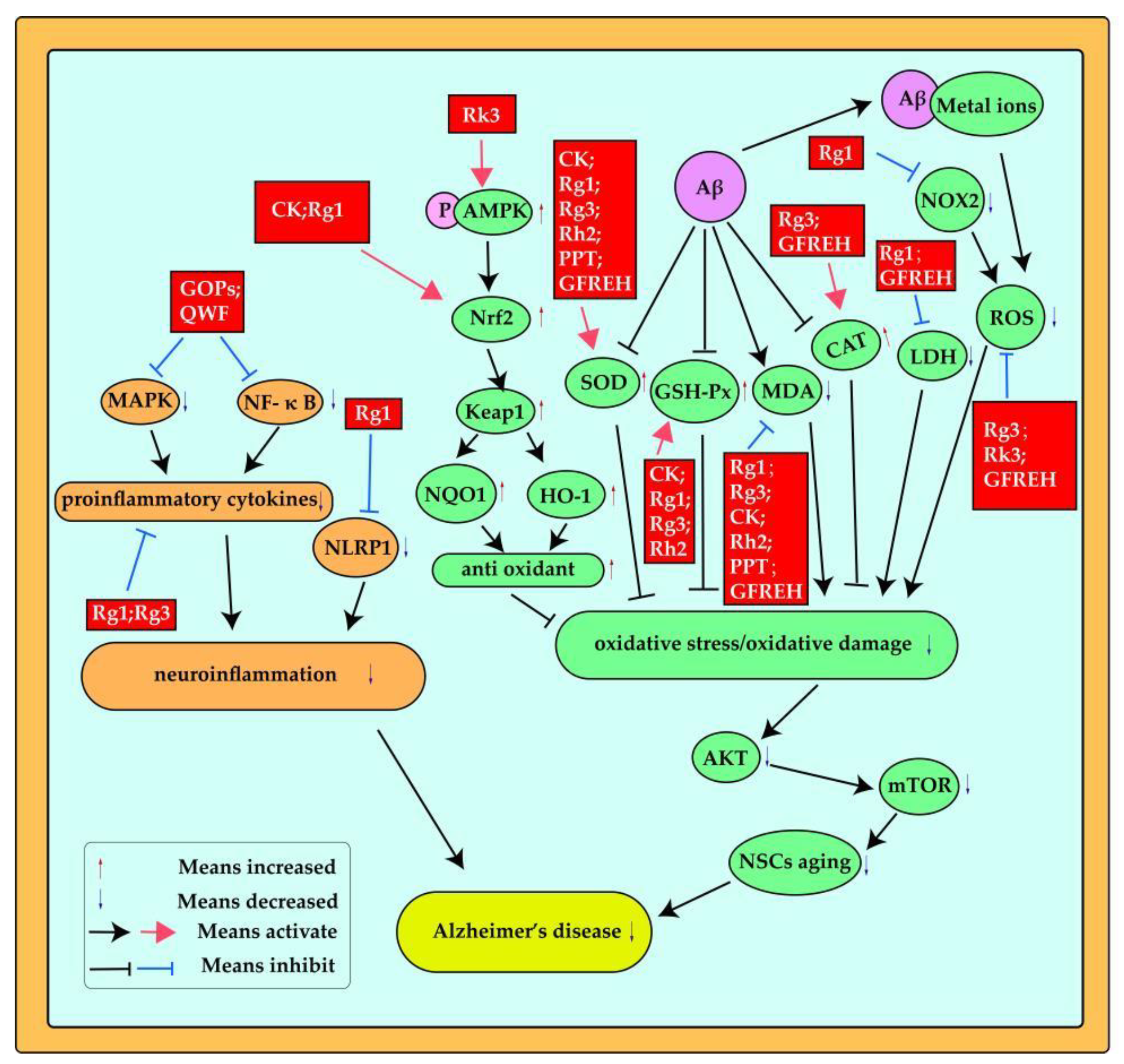
| Ginseng ingredients | Experimental model | Effects | Ref. |
|---|---|---|---|
| Ginsenoside compound K(CK) | ICR mice | BACE1,PS1,IDE | [45] |
| Ginsenoside Rg1 | Sprague Dawley rats | APP,CDK5,PPary,IDE,BACE1 | [46] |
| Ginsenoside CK, F1, Rh1 and Rh2 | Molecular docking | BACE1 | [47] |
| Ginsenoside F1 | Mouse neuroblastoma neuro-2a (N2a) cells and Human neuroblastoma SH-SY5Y cells | IDE,NEP | [48] |
| Ginsenoside Rg3 | SK-N-SH cells | NEP | [49] |
| Ginsenoside Rg1 | Wild type (WT) and APP/PS1 AD mice | NOX2,ROS,APP,BACE | [50] |
| Ginsenoside Rd | C. elegans | ROS,MAPK | [51] |
| Ginsenoside Re | N2a/APP695 cells | BACE1,PPary | [52] |
| Ginsenoside Rb1,Rb2 | Molecular docking | BACE1 | [53] |
| DDPU | APP/PS1 transgenic mice,SH-SY5Y cells, HEK293-APP swe cells, CHO-APP cells, primary neurons or primary astrocytes | BACE1,PERK,eIF2 α,PI3K, AKT, mTOR, ULK1, | [55] |
| Ginsenoside Rg3 | Neuro-2a (N2a) murine neuroblastoma and HMO6 humanmicroglial cells | SRA | [56] |
| combined ginsenosides(SumI) | AD worms | HSPs,HSF-1 | [57] |
| Ginsenoside F1 | APPswe/PSEN1dE9 double-transgenic AD mice with a B6 × C3 background and B6 × C3 wild type mice | CREB,BDNF | [58] |
| Ginseng ingredients | Experimental model | Effects | Ref. |
|---|---|---|---|
| Ginsenoside Rb1 | ICR mice | pGSK-3,PP2A | [65] |
| Ginsenoside Rd | SD rats | PP2A | [66] |
| Ginsenoside Rb1 | SD rats | Ca2+-calpain-CDK5,P35,P25 | [67] |
| Ginseng ingredients or Herb pairs | Experimental model | Effects | Ref. |
|---|---|---|---|
| Ginsenoside Rh2 | Male ICR mice | Ach | [74] |
| Ginsenoside Rg1 | Adult male Sprague–Dawley rats | Ach | [60] |
| GSL | Cell | Ach | [75] |
| PPT | Male Institute of Cancer Research mice | Ach | [76] |
| Ginsenoside Re,Rg3 | In vitro enzyme assays | ACHE,BCHE | [53] |
| Ginsenoside Rb1 | Male Sprague-Dawley rats | Ach | [77] |
| Ginsenoside Re | Adults male Sprague Dawley rats | DA,Ach | [78] |
| Ginsenoside Rg3 | Male C57BL/6 mice | Ach | [79] |
| Ginsenosides | Male Wistar rats | GABA,Ach,DA,Gly,5-HT,Glu,Asp | [68] |
| Ginsenoside Rg3 | Male Wistar rats | Ala,Asp,Glu,D-Gln,D-Glu,Trp | [81] |
| Ginseng Schisandra chinensis | Sprague-Dawley male rats | Phe, Tyr,Trp | [82] |
| Ginseng ingredients and Classic Chinese formulation | Experimental model | Effects | Ref. |
|---|---|---|---|
| Ginsenoside compound K(CK) | ICR mice | Nrf2, keap1, HO-1,SOD, GSH-Px, MDA | [45] |
| Ginsenoside Rk3 | APP/PS1 mice,PC12 Cells | ROS, AMPK, Nrf2,HO -1, NQO1, Keap1 | [69] |
| Ginsenoside Rg1 | mouse neuroblastoma N2a cells | Nrf2, HO-1, SOD, MDA, LDH | [85] |
| Ginsenoside Rg1 | Male APP/PS1 mice | ROS, NOX2 | [50] |
| Ginsenoside Rg1 | C57BL/6 mice,mice NSCs | SOD, GSH-Px, MDA, ROS, Akt, mTOR | [86] |
| Ginsenoside Rg3 | Male Wistar rats | SOD, CAT, GSH-Px, MDA, ROS | [81] |
| Ginsenoside Rh2 | Male ICR mice | SOD, GSH, MDA | [74] |
| PPT | Male Institute of Cancer Research mice | SOD, MDA | [76] |
| GFREH | C. elegans | ROS, SOD, CAT, MDA, LDH | [87] |
| Ginsenoside Rg1 | Male ICR mice | NLRP1,proinflammatory cytokines( IL-1β ,IL-18) | [88] |
| Ginsenoside Rg3 | Male Sprague-Dawley (SD) rats | proinflammatory cytokines(TNF-α, IL- 1β, and COX-2) | [89] |
| Ginsenoside Rg3 | Neuro-2a (N2a) murine neuroblastoma and HMO6 humanmicroglial cells | proinflammatory cytokines(iNOS, IL-6, and TNF-α) | [56] |
| Ginseng oligopeptides (GOPs) | Male Sprague-Dawley (SD) rats | MAPK , NF- κ Bproinflammatory cytokines(TNF-α IL-β) | [90] |
| Qisheng Wan formula (QWF) | Adult Sprague Dawley rats | NF-κB, IL-6, TNF-α | [61] |
| Ginseng ingredients | Experimental model | Effects | Ref. |
|---|---|---|---|
| Ginsenoside compound K (CK) | ICR mice | Bcl-2, Bax, caspase-3 | [45] |
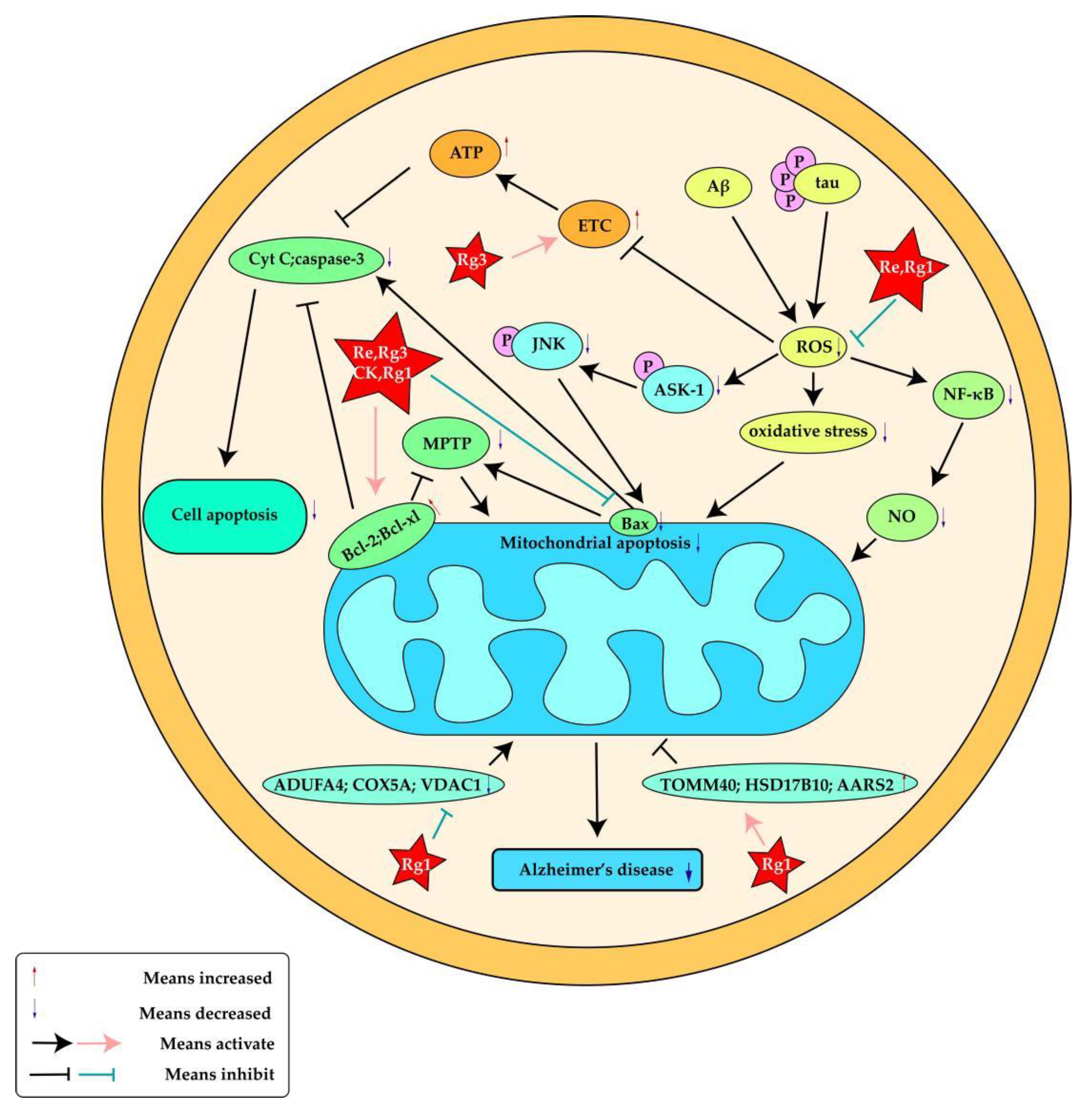
| Ginsenoside Rg3 | Male Wistar rats | ETC, ROS, ATP ,Cyt C, Bax, Bcl-2 | [81] |
| Ginsenoside Re | SH-SY5Y human neuroblastoma cells | Bax, ROS, ASK-1, JNK | [59] |
| Ginsenoside Rg1 | Primary cultured cortical neurons were preparedfrom embryonic day (D17-18) Sprague Dawley (SD) rat fetuses | NF-κB, NO, ROS, Bcl-2, Bax | [94] |
| Ginsenoside Rg1 | SH-SY5Y cells | HSD17B10, AARS2, TOMM40,NDUFA4, VDAC1, COX5A | [95] |
| Ginseng ingredients and Classic Chinese formulation and Herb pairs | Experimental model | Effects | Ref. |
|---|---|---|---|
| Ginsenoside Rb1 | Sprague–Dawley rats | GABAA (α2, β2, and γ2) , GABAB (1b and 2) ,Bif.L, Bif.D, Lac.B, Lac.H, Lac.R | [100] |
| Ginseng Schisandra chinensis | Sprague-Dawley male rats | regulating intestinal microbiota disorderPhe, Tyr,Trp | [82] |
| Qisheng Wan formula (QWF) | Adult Sprague Dawley rats | Reduces the richness and diversity of gut microbiota | [61] |
| Ginsenoside Rg1 | Male conventional tree shrews | Alter the composition and abundance of gut microbiota | [109] |
| DushenTang (DST) | Adult male Sprague-Dawley rats | Correct the disturbance of the gut microbiota | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




