Submitted:
12 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Study eligibility
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Sources
2.5. Study Selection
2.6. Data Extraction
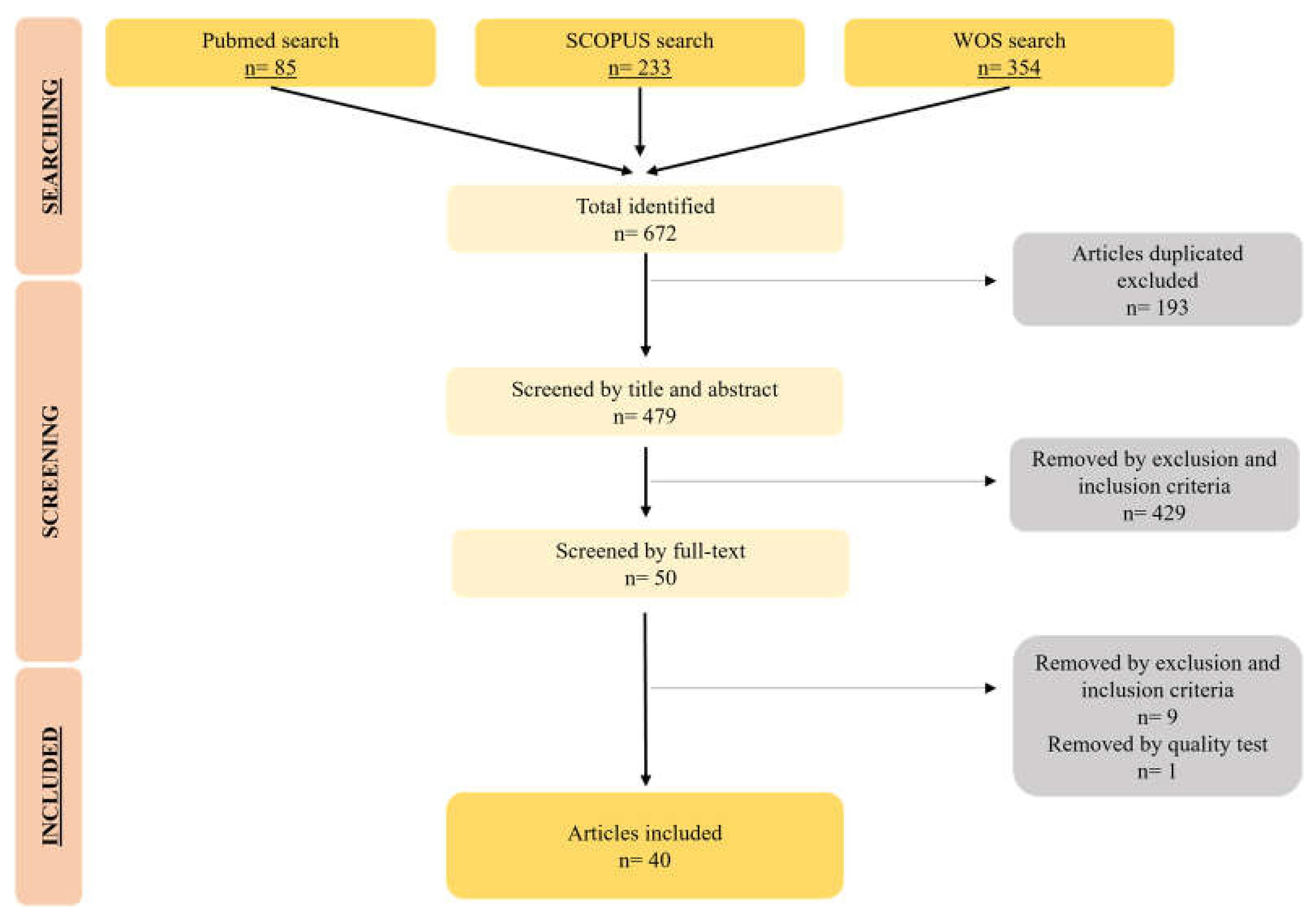
3. Results and Discussion
3.1. Study Description
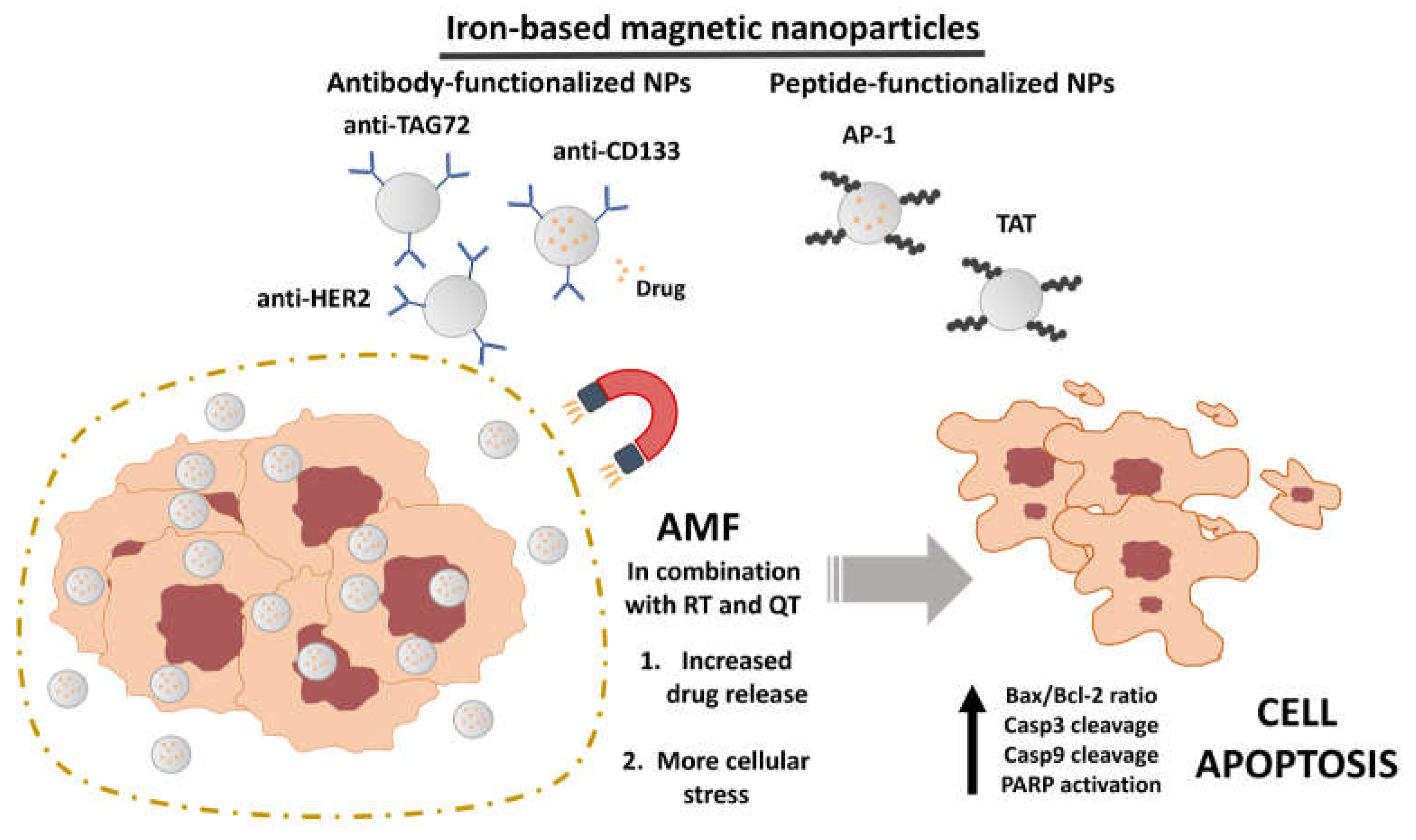
3.2. Characteristics of magnetic nanoformulations
3.3. Biocompatibility of hyperthermia assays
3.4. In vitro assays
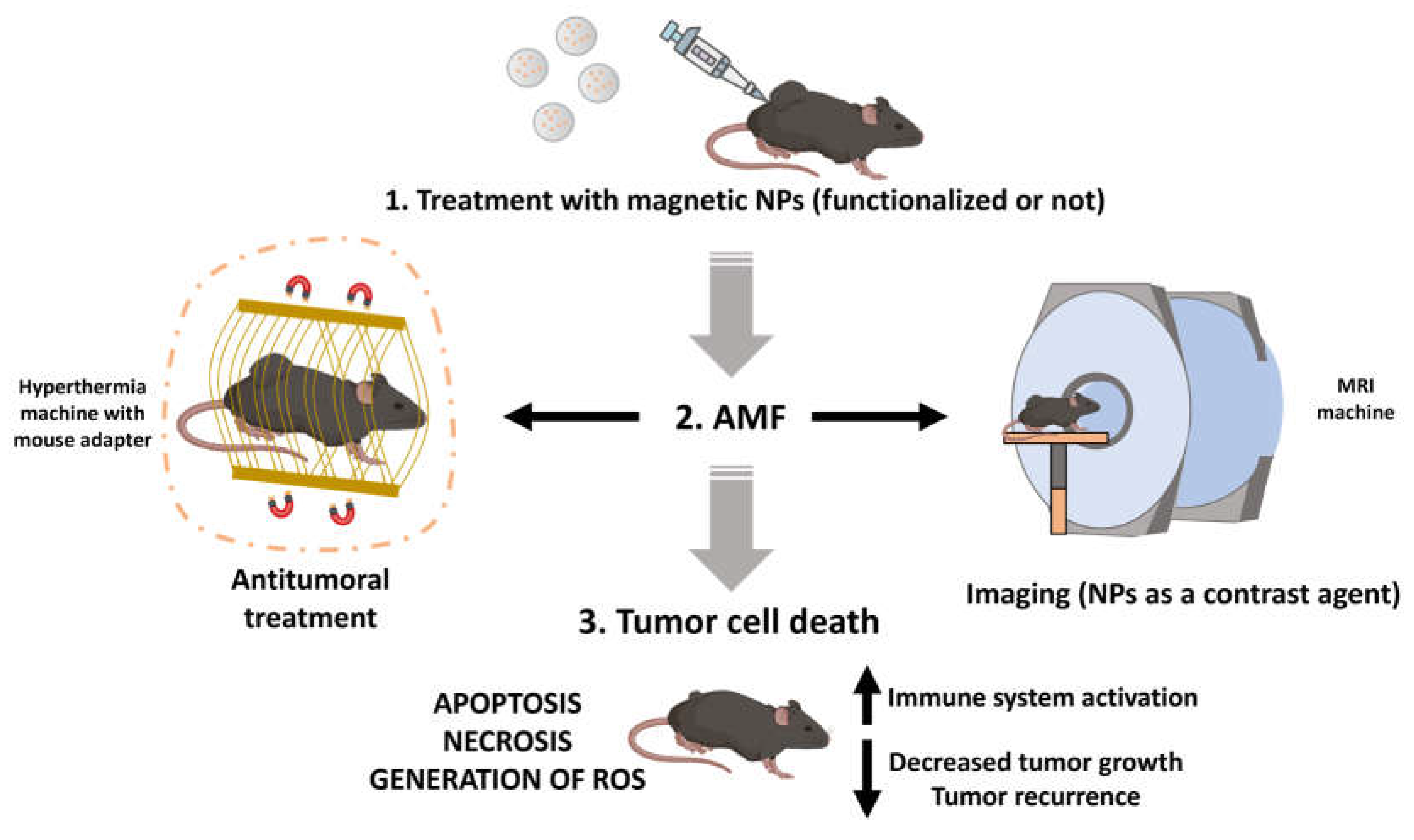
3.5. In vivo assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.L.; Yu, S.J. Esophageal Cancer: Risk Factors, Genetic Association, and Treatment. Asian J Surg 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric Cancer: A Comprehensive Review of Current and Future Treatment Strategies. Cancer and Metastasis Reviews 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent Progress in Multidisciplinary Treatment for Patients with Esophageal Cancer. Surg Today 2020, 50, 12–20. [Google Scholar] [CrossRef]
- Knowlton, C.A.; Mackay, M.K.; Speer, T.W.; Vera, R.B.; Arthur, D.W.; Wazer, D.E.; Lanciano, R.; Brashears, J.H.; Knowlton, C.A.; Mackay, M.K.; et al. Cancer Colon. In Encyclopedia of Radiation Oncology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp. 77–77. [Google Scholar]
- Shiga, T.; Hiraide, M. Cardiotoxicities of 5-Fluorouracil and Other Fluoropyrimidines. Curr Treat Options Oncol 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; AlAhmad, M.M.; Ali, B.R. Current Opinion on the Pharmacogenomics of Paclitaxel-Induced Toxicity. Expert Opin Drug Metab Toxicol 2021, 17, 785–801. [Google Scholar] [CrossRef]
- Garbayo, E.; Pascual-Gil, S.; Rodríguez-Nogales, C.; Saludas, L.; Estella-Hermoso de Mendoza, A.; Blanco-Prieto, M.J. Nanomedicine and Drug Delivery Systems in Cancer and Regenerative Medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2020, 12, e1637. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and Cancer Therapy: Perspectives for Application of Nanoparticles in the Treatment of Cancers. J Cell Physiol 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Kumar, D.N.; Shaik, R.A.; Eid, B.G.; Abdel-Naim, A.B.; Md, S.; Ahmad, A.; Agrawal, A.K. Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy. Int J Mol Sci 2022, 23, 10068. [Google Scholar] [CrossRef]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced Nanomedicine and Cancer: Challenges and Opportunities in Clinical Translation. Int J Pharm 2021, 599, 120438. [Google Scholar] [CrossRef]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal Irinotecan (Onivyde): Exemplifying the Benefits of Nanotherapeutic Drugs. Cancer Sci 2022, 113, 2224–2231. [Google Scholar] [CrossRef]
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.G.; Malatesta, M.; Calderan, L.; et al. Improving the Cellular Uptake of Biomimetic Magnetic Nanoparticles. Nanomaterials 2021, 11, 766. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv Healthc Mater 2020, 9, 1901058. [Google Scholar] [CrossRef]
- Li, Y.; Xin, J.; Sun, Y.; Han, T.; Zhang, H.; An, F. Magnetic Resonance Imaging-Guided and Targeted Theranostics of Colorectal Cancer. Cancer Biol Med 2020, 17, 307–327. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, Md.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic Nanoparticles for Hyperthermia in Cancer Treatment: An Emerging Tool. Environmental Science and Pollution Research 2020, 27, 19214–19225. [Google Scholar] [CrossRef] [PubMed]
- Acar, M.; Solak, K.; Yildiz, S.; Unver, Y.; Mavi, A. Comparative Heating Efficiency and Cytotoxicity of Magnetic Silica Nanoparticles for Magnetic Hyperthermia Treatment on Human Breast Cancer Cells. 3 Biotech 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Minaei, S.E.; Khoei, S.; Khoee, S.; Mahdavi, S.R. Sensitization of Glioblastoma Cancer Cells to Radiotherapy and Magnetic Hyperthermia by Targeted Temozolomide-Loaded Magnetite Tri-Block Copolymer Nanoparticles as a Nanotheranostic Agent. Life Sci 2022, 306, 120729. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.; Jagal, J.; Khurshid, H.; Al-Omari, I.A.; Haider, M.; Kamzin, A.S.; Obaidat, I.M.; Issa, B. Hyperthermia of Magnetically Soft-Soft Core-Shell Ferrite Nanoparticles. Int J Mol Sci 2022, 23, 14825. [Google Scholar] [CrossRef]
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.; Nucci, M.; Mamani, J.; Oliveira, F.; Marti, L.; Filgueiras, I.; Ferreira, J.; Real, C.; Faria, D.; Espinha, P.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int J Mol Sci 2020, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, R.; Hilger, I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-Step Guide on How to Design, Conduct, and Successfully Publish a Systematic Review and Meta-Analysis in Medical Research. Eur J Epidemiol 2020, 35, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Száva-Kováts, E. Unfounded Attribution of the “Half-Life” Index-Number of Literature Obsolescence to Burton and Kebler: A Literature Science Study. Journal of the American Society for Information Science and Technology 2002, 53, 1098–1105. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic Reviews in Nutrition: Standardized Methodology. British Journal of Nutrition 2012, 107, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Faruque, H. Al; Kee, H.; Kim, E.; Park, S. Exosome-Based Hybrid Nanostructures for Enhanced Tumor Targeting and Hyperthermia Therapy. Colloids Surf B Biointerfaces 2021, 205, 111915. [Google Scholar] [CrossRef]
- Lin, T.-C.; Lin, F.-H.; Lin, J.-C. In Vitro Characterization of Magnetic Electrospun IDA-Grafted Chitosan Nanofiber Composite for Hyperthermic Tumor Cell Treatment. J Biomater Sci Polym Ed 2013, 24, 1152–1163. [Google Scholar] [CrossRef]
- Park, J.; Jin, C.; Lee, S.; Kim, J.; Choi, H. Magnetically Actuated Degradable Microrobots for Actively Controlled Drug Release and Hyperthermia Therapy. Adv Healthc Mater 2019, 8, 1900213. [Google Scholar] [CrossRef]
- Li, C.; Ruan, J.; Yang, M.; Pan, F.; Gao, G.; Qu, S.; Shen, Y.L.; Dang, Y.J.; Wang, K.; Jin, W.L.; et al. Human Induced Pluripotent Stem Cells Labeled with Fluorescent Magnetic Nanoparticles for Targeted Imaging and Hyperthermia Therapy for Gastric Cancer. Cancer Biol Med 2015, 12, 163. [Google Scholar] [CrossRef]
- Yang, S.-J.; Tseng, S.-Y.; Wang, C.-H.; Young, T.-H.; Chen, K.-C.; Shieh, M.-J. Magnetic Nanomedicine for CD133-Expressing Cancer Therapy Using Locoregional Hyperthermia Combined with Chemotherapy. Nanomedicine 2020, 15, 2543–2561. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Matsumi, Y.; Aono, H.; Ohara, T.; Tazawa, H.; Shigeyasu, K.; Yano, S.; Takeda, S.; Komatsu, Y.; Hoffman, R.M.; et al. Immuno-Hyperthermia Effected by Antibody-Conjugated Nanoparticles Selectively Targets and Eradicates Individual Cancer Cells. Cell Cycle 2021, 20, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Mihailović, J.; Mirković, M.; Radović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; Mijović, M.; Vranješ-Đurić, S.; et al. Aminosilanized Flower-Structured Superparamagnetic Iron Oxide Nanoparticles Coupled to 131I-Labeled CC49 Antibody for Combined Radionuclide and Hyperthermia Therapy of Cancer. Int J Pharm 2020, 587, 119628. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Liu, T.Y.; Chan, T.Y.; Tsai, S.C.; Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Lu, R.H.; Jiang, J.K.; Yang, C.Y.; et al. Magnetically Triggered Nanovehicles for Controlled Drug Release as a Colorectal Cancer Therapy. Colloids Surf B Biointerfaces 2016, 140, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; He, Y.; Wu, C.; Zhang, M.; Gu, Z.; Zhang, J.; Liu, E.; Xu, Q.; Asrorov, A.M.; Huang, Y. Magnetism-Mediated Targeting Hyperthermia-Immunotherapy in “Cold” Tumor with CSF1R Inhibitor. Theranostics 2021, 11, 6860–6872. [Google Scholar] [CrossRef]
- Jahangiri, S.; Khoei, S.; Khoee, S.; Safa, M.; Shirvalilou, S.; Pirhajati Mahabadi, V. Potential Anti-Tumor Activity of 13.56 MHz Alternating Magnetic Hyperthermia and Chemotherapy on the Induction of Apoptosis in Human Colon Cancer Cell Lines HT29 and HCT116 by up-Regulation of Bax, Cleaved Caspase 3&9, and Cleaved PARP Proteins. Cancer Nanotechnol 2021, 12, 34. [Google Scholar] [CrossRef]
- Ha, P.T.; Le, T.T.H.; Ung, T.D.T.; Do, H.D.; Doan, B.T.; Mai, T.T.T.; Pham, H.N.; Hoang, T.M.N.; Phan, K.S.; Bui, T.Q. Properties and Bioeffects of Magneto–near Infrared Nanoparticles on Cancer Diagnosis and Treatment. New Journal of Chemistry 2020, 44, 17277–17288. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Lin, P.-Y.; Hsieh, S.-L.; Kirankumar, R.; Lin, H.-Y.; Li, J.-H.; Chen, Y.-T.; Wu, H.-M.; Hsieh, S. Utilizing Edible Agar as a Carrier for Dual Functional Doxorubicin-Fe3O4 Nanotherapy Drugs. Materials 2021, 14, 1824. [Google Scholar] [CrossRef]
- LIU, J.; LI, N.; LI, L.; LI, D.; LIU, K.; ZHAO, L.; TANG, J.; LI, L. Local Hyperthermia for Esophageal Cancer in a Rabbit Tumor Model: Magnetic Stent Hyperthermia versus Magnetic Fluid Hyperthermia. Oncol Lett 2013, 6, 1550–1558. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-Based Magnetic Nanofluids Used in Hyperthermia Applications. J Magn Magn Mater 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Fernández-Álvarez, F.; Caro, C.; García-García, G.; García-Martín, M.L.; Arias, J.L. Engineering of Stealth (Maghemite/PLGA)/Chitosan (Core/Shell)/Shell Nanocomposites with Potential Applications for Combined MRI and Hyperthermia against Cancer. J Mater Chem B 2021, 9, 4963–4980. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic Field-Inducible Drug-Eluting Nanoparticles for Image-Guided Thermo-Chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, S.; Ghin, L.; Conti, G.; Tambalo, S.; Lascialfari, A.; Orlando, T.; Benati, D.; Bernardi, P.; Betterle, N.; Bassi, R.; et al. Magnetic Nanoparticles from Magnetospirillum Gryphiswaldense Increase the Efficacy of Thermotherapy in a Model of Colon Carcinoma. PLoS One 2014, 9, e108959. [Google Scholar] [CrossRef]
- Muñoz de Escalona, M.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Arias, J.L. Magnetic Solid Lipid Nanoparticles in Hyperthermia against Colon Cancer. Int J Pharm 2016, 504, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-Engineering of 5-Fluorouracil-Loaded Magnetoliposomes for Combined Hyperthermia and Chemotherapy against Colon Cancer. European Journal of Pharmaceutics and Biopharmaceutics 2013, 85, 329–338. [Google Scholar] [CrossRef]
- Jabalera, Y.; Garcia-Pinel, B.; Ortiz, R.; Iglesias, G.; Cabeza, L.; Prados, J.; Jimenez-Lopez, C.; Melguizo, C. Oxaliplatin–Biomimetic Magnetic Nanoparticle Assemblies for Colon Cancer-Targeted Chemotherapy: An In Vitro Study. Pharmaceutics 2019, 11, 395. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S. V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-Controlled Magnetic Nanoparticles Hyperthermia Inhibits Primary Tumor Growth and Metastases Dissemination. Nanomedicine 2020, 25, 102171. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Castillo; Mendez, J. ; Rinaldi, C.; Soto, O.; Alvarez-Berrios, M.P. Hyperthermic Potentiation of Cisplatin by Magnetic Nanoparticle Heaters Is Correlated with an Increase in Cell Membrane Fluidity. Int J Nanomedicine 2013, 8, 1003–1013. [Google Scholar] [CrossRef]
- Mirzaghavami, P.S.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R.; Pirhajati Mahabadi, V. Radio-Sensitivity Enhancement in HT29 Cells through Magnetic Hyperthermia in Combination with Targeted Nano-Carrier of 5-Flourouracil. Materials Science and Engineering: C 2021, 124, 112043. [Google Scholar] [CrossRef]
- Pawlik, P.; Blasiak, B.; Depciuch, J.; Pruba, M.; Kitala, D.; Vorobyova, S.; Stec, M.; Bushinsky, M.; Konakov, A.; Baran, J.; et al. Application of Iron-Based Magnetic Nanoparticles Stabilized with Triethanolammonium Oleate for Theranostics. J Mater Sci 2022, 57, 4716–4737. [Google Scholar] [CrossRef]
- Fernandes, S.; Fernandez, T.; Metze, S.; Balakrishnan, P.B.; Mai, B.T.; Conteh, J.; De Mei, C.; Turdo, A.; Di Franco, S.; Stassi, G.; et al. Magnetic Nanoparticle-Based Hyperthermia Mediates Drug Delivery and Impairs the Tumorigenic Capacity of Quiescent Colorectal Cancer Stem Cells. ACS Appl Mater Interfaces 2021, 13, 15959–15972. [Google Scholar] [CrossRef] [PubMed]
- Beyk, J.; Tavakoli, H. Selective Radiofrequency Ablation of Tumor by Magnetically Targeting of Multifunctional Iron Oxide–Gold Nanohybrid. J Cancer Res Clin Oncol 2019, 145, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Guo, Y.; Yu, Z.; Hu, P.; Shi, J. Nanocatalytic Bacteria Disintegration Reverses Immunosuppression of Colorectal Cancer. Natl Sci Rev 2022, 9, nwac169. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, A.; Ansari, Md.M.; Vyawahare, A.; Jayamurugan, G.; Khan, R. Hyperbranched Polymer-Functionalized Magnetic Nanoparticle-Mediated Hyperthermia and Niclosamide Bimodal Therapy of Colorectal Cancer Cells. ACS Biomater Sci Eng 2020, 6, 1102–1111. [Google Scholar] [CrossRef]
- Shen, M.Y.; Liu, T.I.; Yu, T.W.; Kv, R.; Chiang, W.H.; Tsai, Y.C.; Chen, H.H.; Lin, S.C.; Chiu, H.C. Hierarchically Targetable Polysaccharide-Coated Solid Lipid Nanoparticles as an Oral Chemo/Thermotherapy Delivery System for Local Treatment of Colon Cancer. Biomaterials 2019, 197, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Oliva, J.; Cordova-Fraga, T.; Basurto-Islas, G.; Benal-Alvarado, J.J.; Mtz-Enriquez, A.I.; Quintana, M.; Gomez-Solis, C. High Heating Efficiency of Magnetite Nanoparticles Synthesized with Citric Acid: Application for Hyperthermia Treatment. J Electron Mater 2022, 51, 4425–4436. [Google Scholar] [CrossRef]
- Castellanos-Rubio, I.; Rodrigo, I.; Olazagoitia-Garmendia, A.; Arriortua, O.; Gil de Muro, I.; Garitaonandia, J.S.; Bilbao, J.R.; Fdez-Gubieda, M.L.; Plazaola, F.; Orue, I.; et al. Highly Reproducible Hyperthermia Response in Water, Agar, and Cellular Environment by Discretely PEGylated Magnetite Nanoparticles. ACS Appl Mater Interfaces 2020, 12, 27917–27929. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.; Wang, X.; Chen, B.; Zhang, H.; Yang, X.; Huang, Y.; Tang, J. Complex of TNF-α and Modified Fe 3 O 4 Nanoparticles Suppresses Tumor Growth by Magnetic Induction Hyperthermia. Cancer Biother Radiopharm 2017, 32, 379–386. [Google Scholar] [CrossRef]
- Matsumi, Y.; Kagawa, T.; Yano, S.; Tazawa, H.; Shigeyasu, K.; Takeda, S.; Ohara, T.; Aono, H.; Hoffman, R.M.; Fujiwara, T.; et al. Hyperthermia Generated by Magnetic Nanoparticles for Effective Treatment of Disseminated Peritoneal Cancer in an Orthotopic Nude-Mouse Model. Cell Cycle 2021, 20, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berríos, M.P.; Castillo, A.; Rinaldi, C.; Torres-Lugo, M. Magnetic Fluid Hyperthermia Enhances Cytotoxicity of Bortezomib in Sensitive and Resistant Cancer Cell Lines. Int J Nanomedicine 2014, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic Liposomes for Colorectal Cancer Cells Therapy by High-Frequency Magnetic Field Treatment. Nanoscale Res Lett 2014, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wydra, R.J.; Rychahou, P.G.; Evers, B.M.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. The Role of ROS Generation from Magnetic Nanoparticles in an Alternating Magnetic Field on Cytotoxicity. Acta Biomater 2015, 25, 284–290. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Martino, U.; Khan, R.; Bazzar, M.; Southern, P.; Tuncel, D.; Al-Jamal, K.T. Engineering Red-Emitting Multi-Functional Nanocapsules for Magnetic Tumour Targeting and Imaging. Biomater Sci 2020, 8, 2590–2599. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor Magnetic Hyperthermia Induced by RGD-Functionalized Fe 3 O 4 Nanoparticles, in an Experimental Model of Colorectal Liver Metastases. Beilstein Journal of Nanotechnology 2016, 7, 1532–1542. [Google Scholar] [CrossRef]
- Dabaghi, M.; Quaas, R.; Hilger, I. The Treatment of Heterotopic Human Colon Xenograft Tumors in Mice with 5-Fluorouracil Attached to Magnetic Nanoparticles in Combination with Magnetic Hyperthermia Is More Efficient than Either Therapy Alone. Cancers (Basel) 2020, 12, 2562. [Google Scholar] [CrossRef]
| Nanoformulation | Antitumor agent | AMF | In vitro assay | In vivo assay | Main results | Reference |
| Anti-131I-labeled CC49 SPIONs | - | 252 kHz, 15.9 kA/m | - | LS174T tumor-bearing mice | Decrease in tumor size | [35] |
| PMAO MNPs | - | 606 kHz, 14 kA/m | - | CC-531 tumor-bearing rats | Heterogeneous cytotoxicity results | [67] |
| (maghemite/PLGA)/CS NPs | - | 250 kHz, 4 kA/m | Cytotoxicity assay (T84) | Healthy mice | High cytotoxicity effect and good MRI results | [43] |
| Exosome-FA-MNP | DOXO | 310 kHz | Cytotoxicity assay (HT29) | HT29 tumor-bearing mice | High cytotoxicity effect and decrease in tumor size | [29] |
| PMAO-PEG MNPs | - | 650 kHz, 16.71 kA/m | Cytotoxicity assay (HCT116) | - | High cytotoxicity effect | [60] |
| Fluorescent MNP labeled iPS | - | 63 kHz, 7 kA/m | - | MGC803 tumor-bearing mice | Decrease in tumor size and good MRI results | [32] |
| Carboxydextran coated MNPs | - | 390 kHz, 28 kA/m | Cytotoxicity assay (HCT116) | Peritoneal-dissemination mice | High cytotoxicity effect and metastases decrease | [62] |
| MnFe2O4-Fe3O4 core-shell NPs | - | 384.5 kHz, 27.85 kA/m | Cytotoxicity assay (HT29) | - | High cytotoxicity effect | [22] |
| anti-HER2 carboxydextran and amphiphilic polimer SPIONs | - | 280 kHz, 31 kA/m | Cytotoxicity assay ( NUGC-4) | - | High cytotoxicity effect | [34] |
| APTES coated MNPs | - | 300 kHz | - | VX2 tumor-bearing rabbits | Decrease in tumor size | [41] |
| PLGA SPIONs | DOXO | 205 kHz, 2 kA/m | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | High cytotoxicity assay, drug release, decrease in tumor size and good MRI results | [45] |
| Liposome encapsulated citric acid-coated MNPs | DOXO | 300 kHz, 59.3 kA/m | Cytotoxicity assay (CT26) | - | High cytotoxicity effect and drug release | [64] |
| SPIO-APTES anti-CD133 MNPs | IRI | 1.3–1.8 kHz | Cytotoxicity (Caco-2, HCT116, DLD1) | HCT116 tumor-bearing mice | High cytotoxicity assay, decrease in tumor size and good MRI results | [33] |
| Iron oxide nanocubes | DOXO | 182 kHz | Patient-derived CSCs | Patient-derived CSCs tumor-bearing mice | High cytotoxicity assay, decrease in tumor size | [54] |
| Bacteria derived MNPs | - | 187 kHz, 23 kA/m | - | HT29 tumor-bearing mice | In vivo apoptotic and necrotic areas and good MRI results | [46] |
| Solid-lipid MNPs | - | 250 kHz, 4 kA/m | Cytotoxicity assay (HT29) | - | High cytotoxicity effect | [47] |
| MPVA-AP1 nanovehicles | DOXO | 50–100 kHz | Liberation assay | - | High drug liberation and drug release | [36] |
| TAT /CSF1R inhibitor functionalized magnetic liposomes | - | 288 kHz, 35 kA/m | - | CT26 tumor-bearing mice | Decrease in tumor size and increased magnetic targeting | [37] |
| Bacteria-derived MNPs | 5-FU | 250 kHz, 4 kA/m | Liberation assay | - | High drug release | [48] |
| Bacteria-derived MNPs | OXA | 197 kHz, 18 kA/m | Liberation assay | - | High drug release | [49] |
| Cobalt ferrite NPs | - | 261 kHz, 8–19.8 kA/m | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | High cytotoxicity effect and decrease in tumor size | [50] |
| Cs MNPs | 5-FU | 435 kHz, 15.4 kA/m | - | HT29 tumor-bearing mice | Decrease in tumor size | [25] |
| Agar encapsulated MNPs | DOXO | 400 kHz, 0.45 kA/m | Cytotoxicity assay (HT29) | - | High cytotoxicity effect | [40] |
| Acid citric and EDC/NHC functionalized MNPs | - | 87 kHz-340 kHz, 79.57 kA/m | Cytotoxicity assay (not specified) | - | High cytotoxicity effect | [59] |
| MNPs | - | 100 kHz, 4 kA/m | MRI assay | - | Good MRI results | [53] |
| MNPs loaded Cs nanofibers | - | 750–1150 kHz | Cytotoxicity assay (CT26) | - | High cytotoxicity effect | [30] |
| Alginate coated MPNPs and QDs | DOXO | 4-6.3 kA/m | - | CT26 tumor-bearing mice | Good MRI results | [39] |
| Iron oxide NPs/Au NPs core/shell nanohybrid | - | 13560 kHz | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | High cytotoxicity effect, decrease in tumor size, increased magnetic targeting and good MRI results | [55] |
| PEG-PBA-PEG coated SPIONs | 5-FU | 13560 kHz | Cytotoxicity assay (HT29, HCT116) | - | High cytotoxicity effect | [38] |
| APTS/PRO functionalized SPIONs loaded with TNF-alfa | - | 110 kHz, 8.75 kA/m | Cytotoxicity assay (SW480, HepG2) | - | High cytotoxicity effect | [61] |
| PLGA SPIONs | 930 kHz, 13 kA/m | - | CT26 tumor-bearing mice | Increased magnetic targeting | [66] | |
| Magnetic solid lipid NPs coated with FA and Dextran | DOXO | Not especified | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | High cytotoxicity effect, decrease in tumor size and metastases | [58] |
| MNPs | CDDP | 237 kHz, 20 kA/m | Cytotoxicity assay (Caco-2) | - | High cytotoxicity effect | [51] |
| Carboxydextran coated MNPs | Bortezomib | 233 kHz, 29.39 kA/m | Cytotoxicity assay (Caco-2) | - | High cytotoxicity effect | [63] |
| SPIONs loaded microrobots | 5-FU | 430 kHz, 45 kA/m | Cytotoxicity assay (HCT116) | - | High cytotoxicity effect | [31] |
| ZnCoFe2O4 and ZnMnFe2O4 NPs | - | 1.35 kA/m | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | High cytotoxicity effect, decrease in tumor size and better targeting | [56] |
| PEG-PCL-PEG/FA MNPs | 5-FU | 13560 kHz, 0.4 kA/m | Cytotoxicity assay (HT29) | - | High cytotoxicity effect | [52] |
| Monosaccharides coated MNPs | - | 292 kHz, 51.0 kA/m | Cytotoxicity assay (CT26) | - | High cytotoxicity effect | [65] |
| Cs MNPs | 5-FU | 435 kHz, 15.4 kA/m | - | HT29 tumor-bearing mice | Sensitizes cells for further therapies and DNA damage | [68] |
| Polymers functionalized MNPs | Niclosamide | 405 kHz | Cytotoxicity assay (HCT116) | - | High Cytotoxicity effect | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





