1. Introduction
A major challenge in the 21st Century is the protection of consumers against risks arising from consumption of contaminated food. Amongst the food contaminants most worrying for their impact on human health are mycotoxins, a chemically diverse group of toxic secondary fungal metabolites that can occur in a wide array of food commodities, resulting from fungal infection and proliferation in the field or during storage. Mycotoxins exhibit in vivo toxicity towards vertebrates after entering via a natural route (i.e., ingestion, inhalation, dermic contact) and may cause acute and/or chronic severe adverse effects in human health, even at the low levels they are usually present in food [
1]. Of the 400 mycotoxins known to date, only a very limited number is subject to legal guidance and regular monitoring, being aflatoxins, fumonisins, trichothecenes, zearalenone (ZEN) and ochratoxin А (OTA) the ones most often tested [
2,
3]. In some countries such as Algeria, however, the number of mycotoxin regulated is even more restrict, being limited to the aflatoxins [
4].
Nevertheless, some studies have reported mycotoxin occurrence in foods and feeds in Algeria with levels that in some cases were higher than the legal limit established in the European Union (EU) [
5,
6,
7,
8]. Madjoubi et al. [
5] study found that 21 maize samples, 7 wheat sample and 1 maize sample from Algerian markets presented levels of fumonisins (FB1+FB2), ZEN and deoxynivalenol (DON), respectively, above the maximum allowed level established by the EU [
2]. Riba et al. [
6] found several samples of wheat grains and wheat derived products exceeding OTA limits established by EU limit (3 μg/kg for raw cereals and 5 μg/kg for cereal products). In а previous study [
7], the same group had reported that 90% of nut samples analysed were contaminated with aflatoxins, with concentrations of AFB1 ranging from 0.2 to 20.52 μg/kg, although with only one sample exceeding the maximum limit allowed by Algerian and EU regulations (10 μg/kg).
Dietary exposure combining contaminant levels and consumption data is the process usually applied to estimate the human exposure to mycotoxins. However, in the last two decades a direct human biomonitoring of biological fluids such urine has been proposed as an alternative approach to assess health risk as it’s non-invasive and provides accurate exposure assessment, since it covers exposure from all possible sources [
9,
10]. This approach has been scarcely applied to African countries, in particular Algeria.
Quantification of mycotoxins in urine requires a previous extraction and clean-up procedure, followed by a chromatographic step for separation and quantification of the compounds, usually by means of liquid chromatography with tandem mass spectrometry (LC-MS/MS) [
11,
12,
13]. Protocols need to be appropriate to provide adequate sensitivity, that is acceptable limits of detection (LODs) and limits of quantification (LOQs), and be effective, fast and economic. An optimized sample pre-treatment guides to accurate and consistent results. Extraction protocols in urine are mostly based on LLE (liquid-liquid extraction), but recently these protocols have been optimized to allow lower volumes and provide a faster analysis. Some techniques that resulted from this optimization are SALLE (salting-out liquid-liquid extraction), QuEChERS (quick, easy, cheap, effective, rugged and safe) and DLLME (dispersive liquid-liquid micro extraction) [
14,
15,
16]. QuEChERS involves extraction, usually with acetonitrile, in the presence of great amounts of inorganic salts in order to provide the separation of the acetonitrile phase from the aqueous media. The following clean-up step is performed by dispersive solid-phase extraction through suitable sorbents [
17,
18]. Consequently, QuEChERS has the advantages of being cheap, time-efficient and simple to operate, while providing good recovery values, and recently it has been widely employed in mycotoxin biomonitoring analysis in urine, as reported by Martins et al. [
13] and Pallarés et al. [
10].
The current study aimed to develop a sensitive and accurate method for the analysis of 11 mycotoxins and metabolites [deoxynivalenol (DON), deoxynivalenol-3-glucoside (DON-3gluc), deepoxy-deoxynivalenol (DOM-1), T-2 toxin (T-2), HT-2 toxin (HT-2), OTA, ZEN, α-zearalenol (α-ZEL), aflatoxin-B1 (AFB1), aflatoxin-B2 (AFB2), fumonisin-B1 (FB1)], in urine using LC-MS/MS. These biomarkers were monitored in first-morning urine of Algerian workers from a plastic industry, in order to elucidate their exposure to mycotoxins. The workers of this company included adults of different social and economic areas, representing part of labored society of Algeria.
2. Materials and Methods
2.1. Reagents and standard solutions
The standards of AFB1, AFB2, T-2, HT-2, OTA, ZEN and α-ZEL were purchased from Sigma (West Chester, PA, USA) and Fluka (West Chester, PA, USA), all with purity higher than 97%. DON, DON-3-gluc, DOM-1, and FB1 were also purchased from Sigma, with purity higher than 92.5%.
The surrogate standard ochratoxin A-(phenyl-d5) (OTAd5) was purchased from Sigma at 10 mg/L, with purity higher than 95%. A working solution was prepared at 500 μg/L in MeOH. A 13C15-DON (deoxynivalenol-13C15) solution, used as an internal standard, was purchased from Fluka, at 25 μg/L in acetonitrile.
Two mixed solutions were prepared in solvent B, described below. A mixture of DON, DOM-1, DON-3-gluc and ZEN at 1 mg/L and a mixture of AFB1, AFB2, FB1, OTA, T-2, HT-2, and α-ZEL at 400 μg/L. The standards and solutions were always kept at -18 ºC when not in use.
Acetonitrile, formic acid and methanol were purchased from Merck (Darmstadt, German). Anhydrous magnesium sulfate was purchased from Sigma (West Chester, PA, USA), and treated at 500 ºC for 5 hours before use. Octadecylsilica (C18, particle size 55–105 mm) was purchased from Waters (Milford, MA, USA). Ultrapure water was obtained by purification with a “Seral” system (Ser-alPur, Pro 90 CN) for use in the mobile phase.
2.2. Sample collection
Including in a study of contaminants exposure, first-morning urine samples (collected from 08 am to 10 am) of 96 Algerian adults were obtained from a plastics industry, situated in the industrial zone of Didouche Mourad, which belongs to Hamma Bouziane. All samples correspond to male individuals, aged from twenty-eight to sixty years old, who worked eight hours a day. The urine samples collected were transported at -20 ºC from the place of collection to the place of storage.
The participants were not subjected to any dietary restrictions prior to sample collection, and their specific diets are unknown. However, it is worth mentioning that in the region where the study took place, it’s common for people to consume homemade bread, nuts and tea, foods that may contain high amounts of mycotoxins [
1,
19]. Additionally, the origin and storage conditions of these foods are unknown, although a significant portion of the food reserves are imported from China. All participants answered a questionnaire regarding their demographics (age, body mass index and place of residence), (Supplementary
Table 1). The average age of the workers in the study was 43 ± 8.03 years. The average body mass index was 24.72 ± 3.12, amidst a range of 16.01-34.60, with 5.2% being obese (BMI >30), 42.7% overweight (BMI 25-30), 50.0% normal (BMI 18.5-24.9) and 2.1% underweight (BMI <18).
This study’s authorization was formulated by the concerned authorities, and followed the standards of the Ethics Committee of the Scientific Committee of the Pharmacy Department of Constantine (Algeria) for Clinical Investigations (Ref CS/CE/01/2019).
2.3. LC-MS/MS conditions
Chromatographic analysis was performed as described by Caldeirão et al. [
20]. LC-MS/MS assays were performed using Waters Alliance 2695 HPLC system (Waters, Milford, MA, USA), that comes with a Quattro Micro triple quadrupole mass spectrometer (Waters, Manchester, UK). For separation was used a ACQUITY UPLC BEH C18 Column, 130 Å, 1.7 μm, 2.1 mm X 100 (Waters Manchester, UK), maintained at 30 ºC.
The mobile phase A was made up of 94% water, 5% methanol and 1% acetic acid [94:5:1 (v/v/v) and 5 mM ammonium acetate] and the mobile phase B was made up of 97% methanol, 2% water and 1% acetic acid [97:2:1 (v/v/v)]. A gradient elution was performed using these two mobile phases, starting at 95% mobile phase A with a linear decrease to 35% in 7 min. In the following 4 min the mobile phase A was decreased to 25% and at 11 min an isocratic gradient of 100% of mobile phase B started for 2 min. Initial column conditions were reached at 25 min and remained for 2 min until the next injection. The flow rate was set to 0.3 mL/min.
The MS/MS acquisition was done in positive-ion mode with multiple reaction monitoring (MRM). Argon 99.995% (Gasin, Portugal), with a pressure of 2.9 ×10-3 mbar in the collision cell, was used as the collision gas. Capillary voltages of 3 kV were used in the positive ionization mode. Nitrogen was the desolvation gas and cone gas, with flows of 350 and 60 L/h respectively. The desolvation temperature was set to 350 ºC and the source temperature to 150 ºC. Data was collected using MassLynx 4.1.
Precursor and product ions were selected according to different conjugations of cone voltages and collision energies, to obtain the most advantageous MRM transition for accurate mycotoxin identification. The optimized LC-MS/MS parameters for each mycotoxin analysed are listed in Supplementary
Table 2.
2.4. Extraction and cleanup
Mycotoxins were extracted using a QueChERS method previously developed [
21], with some modifications. Before analysis, the urine samples were thawed at room temperature, and 120 μL of OTAd5 (500 μg/L) was added. The extraction was performed with a 3 mL mixture of 99% acetonitrile and 1% formic acid (v/v). It was also added 1 g of MgSO4 and 0.25 g of sodium acetate. The resulting mixture was vortexed, and then agitated for 15 min (rotary shaker Multi RS- 60 Biosan) and centrifuged at 4000 g during 5 min. Then 2 mL of organic phase was transferred to a tube already containing 200 mg of C18, centrifuged, and 1.3 mL of the supernatant was aspirated and evaporated at 45 ºC, under a stream of nitrogen. The resulting residue was re-suspended with 500 μL of mobile phase B, along with 12.5 μL of 13C15-deoxynivalenol (13DON15) at 25 μg/L, and it was finally injected into the LC-MS/MS system.
2.5. Method validation
Matrix-matching calibration curves with six concentration levels were obtained from all mycotoxins. Based on previous works [
11,
14,
22], two distinct levels of concentration ranges were evaluated; from 6.75 to 225 μg/L for DON, DON-3-gluc, DOM-1 and ZEN, while for AFB1, AFB2, FB1, T-2, HT-2, OTA and α-ZEL the calibration ranged from 0.25 to 5.0 μg/L. Limit of quantification (LOQ) was set as the lowest level at the calibration curve quantified with acceptable accuracy and precision (relative standard deviation <20%), while limit of detection (LOD) was calculated as the concentration of analyte providing a signal-to-noise of 3. The intra-day precision of each mycotoxin was measured on the same day in five replicate experiments at two different levels of concentration. For DON, DON-3-gluc, DOM-1 and ZEN the first level at 50 μg/L and the second at 100 μg/L. For AFB1, AFB2, FB1, T-2, HT-2, OTA, and α- ZEL the first level at 2 μg/L and the second at 4 μg/L.
2.6. Statistical analysis
To determine whether the mycotoxin data followed a parametric or non-parametric distribution, a Kolmogorov-Smirnov test was performed. The results revealed a non-normal distribution, prompting the selection of a nonparametric Mann-Whitney U-test to compare mycotoxin concentration between rural and urban areas, given the sample size and distribution. Fisher’s exact test was applied to compare mycotoxin frequency between both groups. Statistical significance was determined by setting a significance level of 0.05. All statistical analyses were conducted using SPSS statistical package, version 27.0 (IBM Corporation, New York, USA).
3. Results
3.1. Cleanup optimization
Two different solid sorbents, EMR-lipid and C18, were assessed for dispersive cleanup step. Thus, 2 mL of extract obtained from QuEchERs extraction of 1.5 ml of spiked urine (25 μg/L) with 3 ml of acidified acetonitrile (1% formic acid) was treated with 200 mg of EMR-lipid or 200 mg of C18. After this, the extracts were centrifuged and 1 mL of supernatant was evaporated at 45 ºC, under a stream of nitrogen. Once evaporated, the residues were re-suspended with 500 μL of mobile phase B, so they could be injected into the LC-MS/MS system. The results obtained were compared in terms of analytical signal (See supplementary Figure S1).
Despite slightly better results were obtained with EMR, this sorbent is more expensive and requires an activation step, being less time-effective than C18. Consequently, without compromising the results, C18 was chosen as the sorbent.
3.2. Analytical validation
The performance results are listed in
Table 1.
Table 1.
Calibration range, correlation coefficient (r), LOQ, LOD, RSD (%) and Extraction Yield (%).
Table 1.
Calibration range, correlation coefficient (r), LOQ, LOD, RSD (%) and Extraction Yield (%).
| |
Calibration range (μg/L) |
|
|
|
Repeatability (RSD %) |
| Mycotoxins |
Correlation coefficient (r) |
LOQ (μg/L) |
LOD (μg/L) |
First level |
Second level |
| DON |
6.75-225 |
0.997 |
6.75 |
2.05 |
3.1* |
1.1* |
| DON-3-gluc |
6.75-225 |
0.981 |
6.75 |
2.05 |
1.1* |
0.2* |
| DOM-1 |
6.75-225 |
0.997 |
6.75 |
2.05 |
6.4* |
0.9* |
| ZEN |
6.75-225 |
0.997 |
6.75 |
2.05 |
7.5* |
26.8* |
| α-ZEL |
0.25-5 |
0.978 |
0.25 |
0.08 |
6.2** |
0.8** |
| OTA |
0.25-5 |
0.999 |
0.25 |
0.08 |
22.3** |
3.1** |
| T-2 |
0.25-5 |
0.997 |
0.25 |
0.08 |
1.9** |
2.8** |
| HT-2 |
0.25-5 |
0.907 |
0.25 |
0.08 |
17.9** |
14.1** |
| AFB1
|
0.25-5 |
0.997 |
0.25 |
0.08 |
43.0** |
33.3** |
| AFB2
|
0.25-5 |
0.995 |
0.25 |
0.08 |
6.0** |
6.8** |
| FB1
|
0.25-5 |
0.962 |
0.25 |
0.08 |
0.01** |
0.02** |
3.3. Sample results
3.3.1. Levels of mycotoxin and their metabolites
The frequency of positive samples is shown in
Table 2, along with the average, minimum, and maximum concentrations of each mycotoxin.
Table 2.
Frequency of positive samples, average, minimum and maximum concentrations.
Table 2.
Frequency of positive samples, average, minimum and maximum concentrations.
| Mycotoxin |
Positive samples (%) |
Average (µg/L) |
Min (µg/L) |
Max (µg/L) |
| DON |
0 |
ND |
ND |
ND |
| DON-3-gluc |
44 (45.8) |
13.28 |
6.8 |
37.80 |
| DOM-1 |
73 (76.0) |
47.97 |
6.9 |
189.1 |
| ZEN |
86 (89.6) |
28.87 |
7.6 |
126.8 |
| α-ZEL |
23 (24.9) |
0.43 |
0.3 |
1.0 |
| OTA |
83 (86.4) |
0.82 |
0.3 |
3.5 |
| T-2 |
89 (92.7) |
8.37 |
0.3 |
36.3 |
| HT-2 |
74 (77.1) |
2.05 |
0.3 |
11.0 |
| AFB1
|
18 (18.8) |
0.82 |
0.3 |
4.7 |
| AFB2
|
10 (10.4) |
1.17 |
0.3 |
5.8 |
| FB1
|
35 (36.5) |
12.99 |
0.5 |
96.2 |
Table 3.
Comparison of the results obtained with other studies that use mycotoxin biomarkers in urine.
Table 3.
Comparison of the results obtained with other studies that use mycotoxin biomarkers in urine.
| |
Mycotoxin |
Algeria |
Chile [24] |
Ivory Coast [34] |
Nigeria [30] |
Portugal [13,25] |
Rwanda [28] |
South Africa [32] |
Spain [29] |
| Mycotoxin Prevalence (%) |
DON |
0 |
55 |
21 |
0.8 |
30 |
19 |
87 |
23 |
| DON-3-gluc |
46 |
- |
- |
5 |
24 |
48 |
- |
- |
| DOM-1 |
76 |
- |
0 |
- |
32 |
24 |
- |
53 |
| ZEN |
91 |
1 |
37 |
0.8 |
57 |
30 |
100 |
40 |
| α-ZEL |
25 |
8 |
- |
- |
5 |
- |
92 |
43 |
| OTA |
86 |
1 |
- |
28 |
27 |
71 |
96 |
3 |
| T-2 |
93 |
- |
- |
- |
ND |
- |
- |
- |
| HT-2 |
77 |
- |
- |
- |
ND |
- |
- |
- |
| AFB1
|
19 |
8 |
- |
- |
2 |
8 |
- |
- |
| AFB2
|
10 |
- |
- |
- |
0 |
- |
- |
- |
| FB1
|
37 |
- |
27 |
13.3 |
- |
30 |
- |
- |
| Mycotoxin Average (µg/L) |
DON |
- |
60.70 |
10.00 |
2.00 |
0.38 |
18.80 |
4.94 |
9.07 |
| DON-3-gluc |
13.28 |
- |
- |
3.50 |
0.25 |
5.88 |
- |
- |
| DOM-1 |
47.97 |
- |
- |
- |
0.23 |
35.00 |
- |
20.28 |
| ZEN |
28.59 |
1.10 |
- |
0.30 |
1.30 |
1.58 |
0.20 |
6.70 |
| α-ZEL |
0.43 |
41.80 |
- |
- |
2.70 |
- |
0.25 |
27.44 |
| OTA |
0.82 |
1.30 |
0.42 |
0.20 |
0.01 |
0.03 |
0.02 |
11.73 |
| T-2 |
8.37 |
- |
- |
- |
ND |
- |
- |
- |
| HT-2 |
2.05 |
- |
- |
- |
ND |
- |
- |
- |
| AFB1
|
0.82 |
0.30 |
- |
- |
0.003 |
0.01 |
- |
- |
| AFB2
|
1.17 |
- |
- |
- |
< LOQ |
- |
- |
- |
| FB1
|
12.99 |
- |
15.30 |
4.60 |
0.24 |
0.01 |
- |
- |
3.2.3. Distribution of mycotoxin and their metabolites
In this study, it was possible to distinguish samples from rural areas from those of urban areas, as it’s displayed in
Table 4.
4. Discussion
4.1. Analytical validation
All analytes presented good linear responses, with r values above 0.962 for all mycotoxins, except HT-2 that had r = 0.907 (
Table 1).
The RSD (relative standard deviation) values obtained were satisfactory at both levels of concentration, being lower than 20% in most cases, with the exception of AFB1, that presented a higher RSD%.
The LOQ and LOD ranged, respectively, from 6.75 μg/L and 2.05 μg/L for DON, DON-3-gluc, DOM-1, and ZEN, and 0.25 μg/L and 0.08 μg/L for AFB1, AFB2, FB1, T-2, HT-2, OTA and α-ZEL. Our LOQ and LOD were within the range reported in the literature [
11,
23,
24], but slight higher than those reported by Martins et al. [
13], Martins et al. [
25] and Huybrechts et al. [
26].
4.2. Sample results
4.2.1. Levels of mycotoxin and their metabolites
In this study, DON was not found in any samples (
Table 2), having lower prevalence than previously reported in literature (
Table 3). In a study by Vidal et al. [
27] it was observed that DON is rapidly excreted, and therefore, to obtain a more representative analysis of DON exposure, at least 16h of urine collection is suggested. To further support this, a Portuguese study by Martins et al. [
13] analysed both first-morning-urine and 24h urine samples and found that DON occurrence was significantly higher in the 24h urine samples. Nevertheless, DON metabolites, DON-3-gluc and DOM-1 were found in this study, in 45.8% and 76.0% of samples, respectively, with average levels of 13.28 μg/L and 47.97 μg/L, ranging from 6.8 to 37.8 μg/L and 6.9 to 189.1 μg/L. These values are comparable to those found in Rwanda [
28] and Spain [
29], and higher than those found in Portugal [
13].
T-2 was found in 89 out of 96 samples (92.7%), with levels ranging from 0.3 to 36.3 μg/L (average of 8.37 μg/L). Its metabolite, HT-2, also presented a high prevalence (77.1%) and an average concentration of 2.05 μg/L, ranging from 0.3 to 11.0 μg/L. These compounds, in general, were not reported in urine biomonitoring studies [
13,
30,
31].
ZEN was found in 89.6% of the samples, with an average concentration of 28.87 μg/L, ranging from 7.6 to 126.8 μg/L. The average level in the present study was higher than previous studies in Rwanda (average of 1.58 μg/L) [
28], South Africa (0.20 μg/L) [
32], Portugal (1.30 μg/L) [
13], and Spain (6.70 μg/L) [
29]. Regarding α-ZEL metabolite, found in 24.9% of samples, the average was 0.43 μg/L (from 0.3 to 1.0 μg/L). This level was higher than those obtained in South Africa (0.25 μg/L) [
32], but lower than those obtained in Chile (41.80 μg/L) [
24] or in Portugal (2.70 μg/L) [
13].
OTA was detected in 86.4% of samples, with levels within 0.3 μg/L and 3.5 μg/L, and average of 0.82 μg/L, higher than those reported from Portugal [
13] and from all African countries indicated on
Table 3 [
28,
30,
32,
33,
34], but lower than those reported in Chile (1.30 μg/L) [
24].
Regarding aflatoxins, 18 samples (18.8%) were positive for AFB1, with an average concentration of 0.82 μg/L (from 0.3 to 4.7 μg/L), and 10 samples (10.4%) were positive for AFB2, with an average of 1.17 μg/L (from 0.3 to 5.8 μg/L). These results show higher prevalence and concentration for aflatoxins in Algeria, when comparing to other countries [
24,
25,
28].
FB1, present in 36.5% of samples, ranged from 0.5 to 96.2 μg/L, with an average of 12.99 μg/L, similar to the one reported in Ivory Coast (15.30 μg/L) [
34].
4.2.2. Co-occurrence
Food contamination by multiple mycotoxins is very common, as some fungal species can produce various types of mycotoxins simultaneously, and because food can also be contaminated by multiple fungal species. This is a serious issue for public health, since current legislation does not account for the hazards of multi-mycotoxin exposure and mycotoxins can have additive or synergistic effects, so their toxicity does not always correspond to individual toxicities summed together. Therefore, when evaluating mycotoxin exposure there is a necessity to consider co-occurrence of mycotoxins.
Several studies conducted in the Mediterranean region, where Algeria is located, revealed high prevalence of co-occurring mycotoxins in cereals. In Morrocco 51% of samples tested were found to be co-contaminated with two to six mycotoxins [
35]. Similarly, in Spain [
36] and in Italy [
37] 65% and 81% of samples under study, respectively, were contaminated with at least two mycotoxins. As to Algeria, a study by Mahdjoubi et al. [
5] found that 50% of samples were contaminated with two to nine mycotoxins. Given the potential health risks associated with the consumption of co-contaminated cereals, further research is needed to develop effective strategies to mitigate these risks, especially in this area.
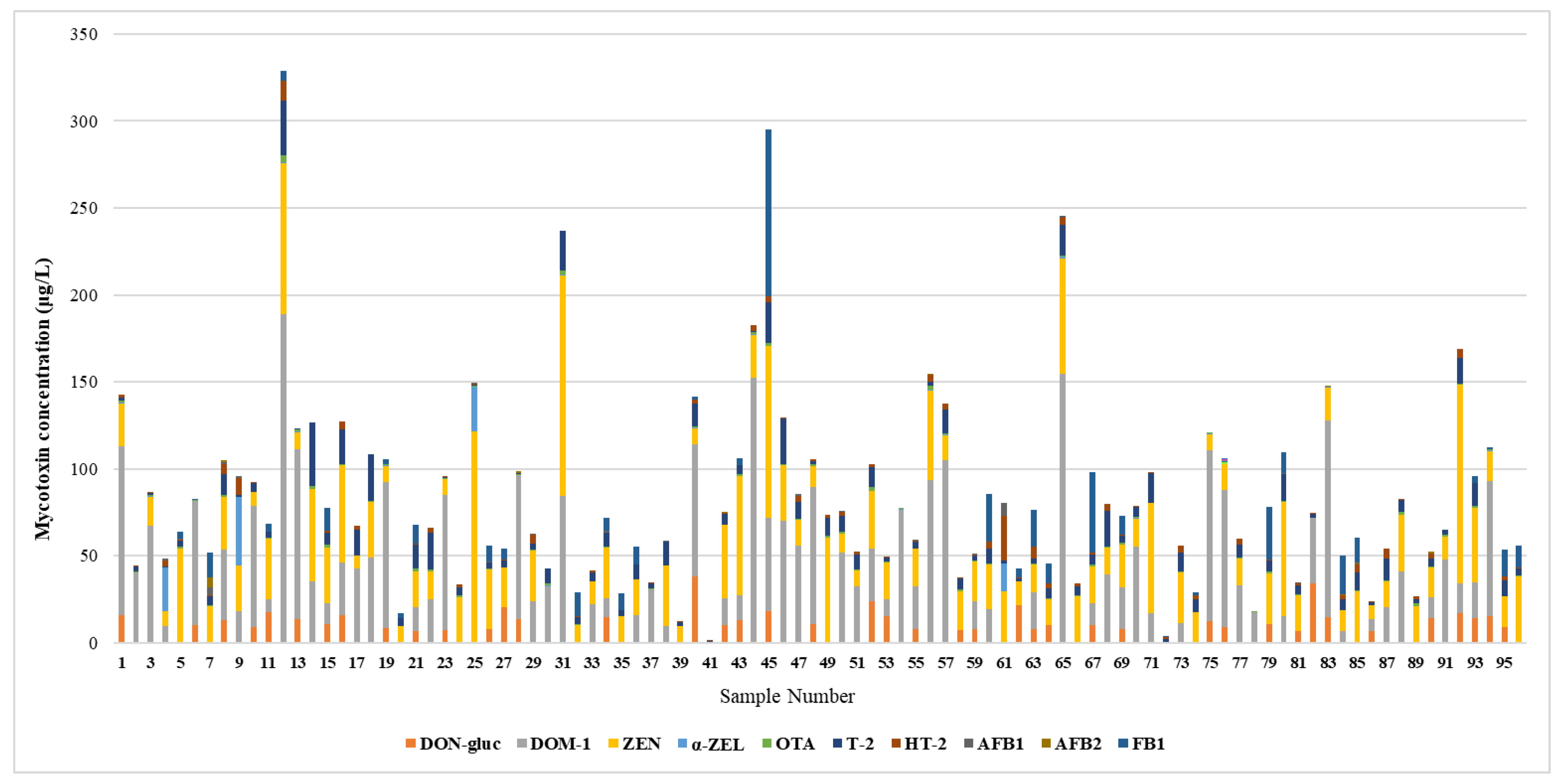
In this study, there is significant co-occurrence of mycotoxins, all samples including at least two mycotoxins. The average number of mycotoxins or metabolites in each sample was 5.6, with the maximum being 9 on sample nº 76 (DON-3-gluc + DOM-1 + ZEN + α-ZEL + T-2 + HT-2 + OTA + AFB1+ AFB2), as it’s demonstrated in
Figure 1.
4.2.3. Distribution of mycotoxin and their metabolites
In this study, it was possible to distinguish samples from rural areas from those of urban areas, as it’s displayed in
Table 4. There are 13 samples of subjects from rural areas and 82 from subjects from urban areas. There is one sample that is not specified in this regard (sample nº 48).
It was expected that people from rural areas would have a higher occurrence of some mycotoxins, especially DON, T-2 and ZEN, which are in grains, typically more consumed in these areas. In fact, DOM-1 had higher frequency and average concentration in samples from rural areas (p<0.05), all these samples testing positive, with an average of 88.58 μg/L, compared to 43.9% positive samples and average of 39.31 μg/L in people from urban areas. In contrast, T-2 had higher frequency and concentration in the urban group (p<0.05), with 98.8% positive samples and average of 9.01 μg/L, whilst the rural group had 69.2% positive samples and average of 1.37 μg/L. This may be due to several factors, including the longer times of grain storage in silos in urban agriculture, the higher levels of pollution that may contribute to higher incidence of fungal infections in crops, or also the fact that urban buildings are more likely to have high levels of mold [
38]. HT-2, a metabolite of T-2, also seemed to be more frequent in urban samples (81.7% in urban while 38.5% in rural, p<0.05) but average concentrations were similar between both groups (2.08 μg/L in urban and 2.06 μg/L in rural). ZEN also had similar average concentration in both groups (29.97 μg/L in urban and 20.32 μg/L in rural) and higher frequency in the urban group (93.9% positive samples, while only 61.5% in the rural group, p<0.05). As for α-ZEL, it was found more frequently in the rural group (76.9% in rural, 15.85% in urban, p<0.05), but average concentrations were similar in both (0.38 μg/L in rural and 0.48 μg/L in urban). OTA (100% with average of 0.92 μg/L in rural, and 84.1% with average of 0.82 μg/L in urban) and DON-3-gluc (53.8% with average of 11.71 μg/L in rural, and 43.9% with average of 13.66 μg/L in urban) had similar percentages of positive samples and average concentration in both groups (p>0.05). Regarding FB1, the differences in mycotoxin frequency and concentration between both groups weren’t statistically significant (40.2% positives with average of 13.67 μg/L in urban samples, while 23.1% positives with average of 1.37 μg/L in rural samples, p>0.05). AFB1 was only found in the urban group (15.4% of urban samples, with an average of 0.85 μg/L), however this doesn’t correlate to urban areas having a higher incidence of this mycotoxin, as there were significatively more samples from urban areas than from rural areas. AFB2 was present in two rural samples (15.4% with average of 0.85 μg/L) and 8 urban samples (9.8% with average of 1.25 μg/L).
The findings of this study showed that some mycotoxins, such as T-2 and ZEN, had higher incidence in samples from urban areas, thereby challenging the common belief that rural areas are more exposed to mycotoxins. However, it is worth noting that there are significant differences in the number of samples collected from each group, which makes it a challenge to draw definitive conclusions about which location is more susceptible to mycotoxin exposure, in this environment. Furthermore, it’s important to exercise caution when making generalizations about mycotoxin incidence in different locations, as it can vary greatly depending on various complex factors that may fluctuate in specific situations, so it’s essential to consider the unique environmental and socioeconomic circumstances of each area when examining mycotoxin exposure, rather than drawing generalizations based on whether the area is rural or not.
5. Conclusions
To the best of our knowledge, this was the first study to evaluate mycotoxin exposure in the Algerian population. The results are worrying, showing a high mycotoxin exposure, across workers coming from different socioeconomic backgrounds, emphasizing the need for awareness on this issue and preventive measures. In Algeria, where a significant amount of cereals are imported, and it is known that long shipping trips increase the possibility of fungal growth, there is an increased need for the control of mycotoxin-producing fungi and for monitorization of storage and harvesting conditions. It is also urgent to implement maximum allowed limits for mycotoxins in food, as currently, only legislation regarding aflatoxins in cattle feed, nuts and cereals exists. This study highlights the importance of addressing mycotoxin exposure in Algeria and serves as a call for action for Algerian authorities to implement measures to reduce exposure and protect public health.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Figure S1- Comparison of sorbents and extraction solvents in QuEChERS extraction; Table S1: Table S1- Sample characterization; Table S2- LC-MS/MS parameters for the mycotoxins analysed.
Author Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript. M.I.M: validation, Investigation, Writing – original draft. IR: resource, Writing - review & editing. J.O.F: Project administration, Writing - review & editing, funding acquisition. Sara C. Cunha: Project administration, Supervision, Writing - review & editing.
Funding
This research was funded by AgriFood XXI R&D&I project, operation No. NORTE-01-0145-FEDER-000041, co-financed by the European Regional Development Fund (ERDF) through NORTH 2020 (Northern Regional Operational Program 2014/2020) and from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects UIDP/50006/2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Université Salah Boubnider Constantine 3 (protocol code ReF CS/CE01/19 and 10 2019l).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.
Acknowledgments
Sara C. Cunha acknowledges FCT for the 2022.07841.CEECIND/CP1724/CT0014 contract
Conflicts of Interest
state The authors declare no conflict of interest.
References
- WHO (World Health Organization). Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 30 January 2022).
- European Comission. Comission Regulation (EC) No 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off. J. European Union 2006, L364, 5–24. [Google Scholar]
- U.S. DHHS. Chemical Contaminants, Metals, Natural Toxins & Pesticides > Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed; U.S. Food and Drug Administration: Washington, DC, USA. 2000. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-action-levels-poisonous-or-deleterious-substances-human-food-and-animal-feed (accessed on 4 February 2022).
- FAO, Food and Agriculture Organization of the United Nations Worldwide Regulations for Mycotoxins in Food and Feed in 2003. FAO Food and Nutrition Paper, 2004, Nº 81, Rome.
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-Mycotoxin Occurrence and Exposure Assessment Approach in Foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef]
- Riba, A.; Matmoura, A.; Mokrane Salim Mathieu, F.; Sabaou, N. Investigations on aflatoxigenic fungi and aflatoxins contamination in some nuts sampled in Algeria. Afr. J. Microbiol. Res. 2013, 7, 4974–4980. [Google Scholar] [CrossRef]
- Riba, A.; Zebiri, S.; Mokrane, S.; Sabaou, N. Occurrence of toxigenic fungi, aflatoxins and ochratoxin A in wheat and dried fruits commercialized in Algeria. International Congress of Mycotoxins and Cancer. 2016, 2016, 24–25. [Google Scholar]
- Tantaoui-Elaraki, A.; Riba, A.; Oueslati, S.; Zinedine, A. Toxigenic fungi and mycotoxin occurrence and prevention in food and feed in northern Africa – a review. World Mycotoxin J. 2018, 11, 385–400. [Google Scholar] [CrossRef]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, N.; Carballo, D.; Ferrer, E.; Rodríguez-Carrasco, Y.; Berrada, H. High-Throughput Determination of Major Mycotoxins with Human Health Concerns in Urine by LC-Q TOF MS and Its Application to an Exposure Study. Toxins 2022, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Ediage, E.N.; Di Mavungu, J.D.; Song, S.; Wu, A.; Van Peteghem, C.; De Saeger, S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assunção, R.; Alvito, P. Exposure assessment of Portuguese population to multiple mycotoxins: The human biomonitoring approach. Int. J. Hyg. Environ. Health 2019, 222, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ediage, E.N.; Wu, A.; De Saeger, S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. A. 2013, 1292, 111–120. [Google Scholar] [CrossRef]
- Dasí-Navarro, N.; Lozano, M.; Llop, S.; Esplugues, A.; Cimbalo, A.; Font, G.; Manyes, L.; Mañes, J.; Vila-Donat, P. Development and Validation of LC-Q-TOF-MS Methodology to Determine Mycotoxin Biomarkers in Human Urine. Toxins 2022, 14, 651. [Google Scholar] [CrossRef]
- Escrivá, L.; Manyes, L.; Font, G.; Berrada, H. Mycotoxin Analysis of Human Urine by LC-MS/MS: A Comparative Extraction Study. Toxins 2017, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; De Saeger, S.; Sun, C.; Wu, A. A QuEChERS-Based Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of Nine Zearalenone-Like Mycotoxins in Pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Rausch, A.K.; Brockmeyer, R.; Schwerdtle, T. Development and Validation of a QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Multi-Method for the Determination of 38 Native and Modified Mycotoxins in Cereals. J. Agric. Food Chem. 2020, 68, 4657–4669. [Google Scholar] [CrossRef] [PubMed]
- Sedova, I.; Kiseleva, M.; Tutelyan, V. Mycotoxins in Tea: Occurrence, Methods of Determination and Risk Evaluation. Toxins 2018, 10, 444. [Google Scholar] [CrossRef]
- Caldeirão, L.; Sousa, J.; Nunes, L.; Godoy, H.T.; Fernandes, J.O.; Cunha, S.C. Herbs and herbal infusions: Determination of natural contaminants (mycotoxins and trace elements) and evaluation of their exposure. Food Res. Int. 2021, 144, 110322. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Sá, S.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, X.; Li, J.; Niu, Y.; Shi, L.; Fang, Z.; Zhang, T.; Ding, H. Quantitative determination of carcinogenic mycotoxins in human and animal biological matrices and animal-derived foods using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1073, 191–200. [Google Scholar] [CrossRef]
- Warth, B.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Utilising an LC-MS/MS-based multi-biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 2040–2046. [Google Scholar] [CrossRef]
- Foerster, C.; Ríos-Gajardo, G.; Gómez, P.; Muñoz, K.; Cortés, S.; Maldonado, C.; Ferreccio, C. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins 2021, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Alvito, P.; Assunção, R. Burden of disease associated with dietary exposure to carcinogenic aflatoxins in Portugal using human biomonitoring approach. Food Res. Int. 2020, 134, 109210. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, B.; Martins, J.C.; Debongnie, P.; Uhlig, S.; Callebaut, A. Fast and sensitive LC-MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Arch. Toxicol. 2015, 89, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Walsh, J.P.; Renaud, J.B.; McMillan, A.; Rulisa, S.; Miller, J.D.; Reid, G.; Sumarah, M.W. Improved methods for biomarker analysis of the big five mycotoxins enables reliable exposure characterization in a population of childbearing age women in Rwanda. Food Chem. Toxicol. 2021, 147, 111854. [Google Scholar] [CrossRef]
- Carballo, D.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Assessment of Human Exposure to Deoxynivalenol, Ochratoxin A, Zearalenone and Their Metabolites Biomarker in Urine Samples Using LC-ESI-qTOF. Toxins 2021, 13, 530. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; Bandyopadhyay, R. Mycotoxin exposure in rural residents in northern Nigeria: a pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; Warth, B.; van der Westhuizen, L. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; Moundipa, P.F. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Lattanzio, V.M.; Ouattara, D.; Kouakou, B.; Visconti, A. Assessment of Mycotoxin Exposure in Côte d’ivoire (Ivory Coast) Through Multi-Biomarker Analysis and Possible Correlation with Food Consumption Patterns. Toxicol. Int. 2014, 21, 248–257. [Google Scholar] [CrossRef]
- Blesa, J.; Moltó, J.C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J. Mycotoxins: of molds and maladies. Environ. Health Perspect. 2000, 108, A20–A23. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).