Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. OA Etiopathogenesis
4. PRP Versus PRF
4.1. PRP
4.2. PRF
4.3. Hyaluronic Acid
4.4. The Role of Fibrin in Regeneration
4.4.1. Fibrinolytic Reactions
5. Conclusions
Conflicts of Interest
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. The Lancet 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clinics in Geriatric Medicine 2010. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Research 2017. [Google Scholar] [CrossRef] [PubMed]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; Santos, R.G. dos; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis – A Review. Journal of Clinical Orthopaedics and Trauma 2020. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. Journal of Pain Research 2018. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-Rich PRP for Knee Osteoarthritis: Current Concepts. Journal of Clinical Orthopaedics and Trauma 2019. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Annals of Long-Term Care 2010. [Google Scholar]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. Journal of Clinical Orthopaedics and Trauma 2020. [Google Scholar] [CrossRef]
- Santos Duarte Lana, J.F.; Furtado da Fonseca, L.; Mosaner, T.; Tieppo, C.E.; Marques Azzini, G.O.; Ribeiro, L.L.; Setti, T.; Purita, J. Bone Marrow Aspirate Clot: A Feasible Orthobiologic. Journal of Clinical Orthopaedics and Trauma 2020. [Google Scholar] [CrossRef]
- Dhillon, M.; Behera, P.; Patel, S.; Shetty, V. Orthobiologics and Platelet Rich Plasma. Indian Journal of Orthopaedics 2014. [Google Scholar] [CrossRef] [PubMed]
- Purita, J.; Duarte Lana, J.F.S.; Kolber, M.; Rodrigues, B.L.; Mosaner, T.; Santos, G.S.; Caliari-Oliveira, C.; Huber, S.C. Bone Marrow-Derived Products: A Classification Proposal - Bone Marrow Aspirate, Bone Marrow Aspirate Concentrate or Hybrid? World Journal of Stem Cells 2020. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, H.P.; Maheshwer, B.; Wong, S.E.; Chahla, J.; Cole, B.J.; Yanke, A.B. An Update on the Use of Orthobiologics: Use of Biologics for Osteoarthritis. Operative Techniques in Sports Medicine 2020. [Google Scholar] [CrossRef]
- Lana, J.F.; da Fonseca, L.F.; Azzini, G.; Santos, G.; Braga, M.; Cardoso Junior, A.M.; Murrell, W.D.; Gobbi, A.; Purita, J.; de Andrade, M.A.P. Bone Marrow Aspirate Matrix: A Convenient Ally in Regenerative Medicine. International Journal of Molecular Sciences 2021. [Google Scholar] [CrossRef]
- Godoi, T.T.F.; Rodrigues, B.L.; Huber, S.C.; Santana, M.H.A.; da Fonseca, L.F.; Santos, G.S.; Azzini, G.O.M.; Mosaner, T.; Paulus-Romero, C.; Lana, J.F.S.D. Platelet-Rich Plasma Gel Matrix (PRP-GM): Description of a New Technique. Bioengineering (Basel) 2022, 9, 817. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediators of Inflammation 2014. [Google Scholar] [CrossRef]
- Man, G.S.; Mologhianu, G. Osteoarthritis Pathogenesis - a Complex Process That Involves the Entire Joint. Journal of medicine and life 2014. [Google Scholar]
- Yuan, G.-H.; Tanaka, M.; Masuko-Hongo, K.; Shibakawa, A.; Kato, T.; Nishioka, K.; Nakamura, H. Characterization of Cells from Pannus-like Tissue over Articular Cartilage of Advanced Osteoarthritis. Osteoarthritis and Cartilage 2004, 12, 38–45. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nature Reviews Rheumatology 2010. [Google Scholar] [CrossRef]
- Lee, J.H.; Ort, T.; Ma, K.; Picha, K.; Carton, J.; Marsters, P.A.; Lohmander, L.S.; Baribaud, F.; Song, X.-Y.R.; Blake, S. Resistin Is Elevated Following Traumatic Joint Injury and Causes Matrix Degradation and Release of Inflammatory Cytokines from Articular Cartilage in Vitro. Osteoarthritis and Cartilage 2009, 17, 613–620. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential Distribution of Adipokines between Serum and Synovial Fluid in Patients with Osteoarthritis. Contribution of Joint Tissues to Their Articular Production. Osteoarthritis and Cartilage 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Hasegawa, M.; Segawa, T.; Maeda, M.; Yoshida, T.; Sudo, A. Thrombin-Cleaved Osteopontin Levels in Synovial Fluid Correlate with Disease Severity of Knee Osteoarthritis. J Rheumatol 2011, 38, 129–134. [Google Scholar] [CrossRef]
- Krasnokutsky, S.; Attur, M.; Palmer, G.; Samuels, J.; Abramson, S.B. Current Concepts in the Pathogenesis of Osteoarthritis. Osteoarthritis and Cartilage 2008, 16, S1–S3. [Google Scholar] [CrossRef]
- Ashraf, S.; Walsh, D.A. Angiogenesis in Osteoarthritis. Current Opinion in Rheumatology 2008, 20, 573–580. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage Homeostasis in Health and Rheumatic Diseases. Arthritis Res Ther 2009, 11, 224. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthritis. Instr Course Lect 2005, 54, 465–480. [Google Scholar]
- Heijink, A.; Gomoll, A.H.; Madry, H.; Drobnič, M.; Filardo, G.; Espregueira-Mendes, J.; Van Dijk, C.N. Biomechanical Considerations in the Pathogenesis of Osteoarthritis of the Knee. Knee Surg Sports Traumatol Arthrosc 2012, 20, 423–435. [Google Scholar] [CrossRef]
- Wang, M.; Peng, Z.; Vasilev, K.; Ketheesan, N. Investigation of Wear Particles Generated in Human Knee Joints Using Atomic Force Microscopy. Tribol Lett 2013, 51, 161–170. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthritis and Cartilage 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Parrish, W.R.; Roides, B. Musculoskeletal Regeneration. Musculoskeletal Regeneration 2017. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma: Evidence to Support Its Use. Journal of Oral and Maxillofacial Surgery 2004. [Google Scholar] [CrossRef]
- Rui, S.; Yuan, Y.; Du, C.; Song, P.; Chen, Y.; Wang, H.; Fan, Y.; Armstrong, D.G.; Deng, W.; Li, L. Comparison and Investigation of Exosomes Derived from Platelet-Rich Plasma Activated by Different Agonists. Cell Transplant 2021, 30, 9636897211017832. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The Regenerative Mechanisms of Platelet-Rich Plasma: A Review. Cytokine 2021. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of Platelet Concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for Topical and Infiltrative Use in Orthopedic and Sports Medicine: Current Consensus, Clinical Implications and Perspectives. Muscles, Ligaments and Tendons Journal 2014. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A Short Overview of Certain Bioactive Components. Open Medicine (Poland) 2016. [Google Scholar] [CrossRef]
- Maynard, D.M.; Heijnen, H.F.G.; Horne, M.K.; White, J.G.; Gahl, W.A. Proteomic Analysis of Platelet Alpha-Granules Using Mass Spectrometry. J Thromb Haemost 2007, 5, 1945–1955. [Google Scholar] [CrossRef]
- Parrish, W.R.; Roides, B.; Hwang, J.; Mafilios, M.; Story, B.; Bhattacharyya, S. Normal Platelet Function in Platelet Concentrates Requires Non-Platelet Cells: A Comparative in Vitro Evaluation of Leucocyte-Rich (Type 1a) and Leucocyte-Poor (Type 3b) Platelet Concentrates. BMJ Open Sport Exerc Med 2016, 2, e000071. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-Rich Plasma: A Milieu of Bioactive Factors. Arthroscopy - Journal of Arthroscopic and Related Surgery 2012. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-Rich Plasma: From Basic Science to Clinical Applications. American Journal of Sports Medicine 2009. [Google Scholar] [CrossRef]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-Articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy 2016, 32, 495–505. [Google Scholar] [CrossRef]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Sports Med 2021, 49, 249–260. [Google Scholar] [CrossRef]
- Hong, M.; Cheng, C.; Sun, X.; Yan, Y.; Zhang, Q.; Wang, W.; Guo, W. Efficacy and Safety of Intra-Articular Platelet-Rich Plasma in Osteoarthritis Knee: A Systematic Review and Meta-Analysis. Biomed Res Int 2021, 2021, 2191926. [Google Scholar] [CrossRef]
- Park, Y.-B.; Kim, J.-H.; Ha, C.-W.; Lee, D.-H. Clinical Efficacy of Platelet-Rich Plasma Injection and Its Association With Growth Factors in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized Double-Blind Controlled Clinical Trial As Compared With Hyaluronic Acid. Am J Sports Med 2021, 49, 487–496. [Google Scholar] [CrossRef]
- Nie, L.-Y.; Zhao, K.; Ruan, J.; Xue, J. Effectiveness of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Clinical Trials. Orthop J Sports Med 2021, 9, 2325967120973284. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hsu, C.-W.; Lin, G.-C.; Lin, H.-S.; Chou, Y.-J.; Liou, I.-H.; Sun, S.-F. Comparing Efficacy of a Single Intraarticular Injection of Platelet-Rich Plasma (PRP) Combined with Different Hyaluronans for Knee Osteoarthritis: A Randomized-Controlled Clinical Trial. BMC Musculoskelet Disord 2022, 23, 954. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Zhao, C.; Guo, S.; Liu, S.; Jia, W.; Tuan, R.S.; Zhang, C. Comparative Evaluation of MSCs from Bone Marrow and Adipose Tissue Seeded in PRP-Derived Scaffold for Cartilage Regeneration. Biomaterials 2012. [Google Scholar] [CrossRef]
- Van Buul, G.M.; Koevoet, W.L.M.; Kops, N.; Bos, P.K.; Verhaar, J.A.N.; Weinans, H.; Bernsen, M.R.; Van Osch, G.J.V.M. Platelet-Rich Plasma Releasate Inhibits Inflammatory Processes in Osteoarthritic Chondrocytes. American Journal of Sports Medicine 2011. [Google Scholar] [CrossRef]
- Giannopoulou, M.; Dai, C.; Tan, X.; Wen, X.; Michalopoulos, G.K.; Liu, Y. Hepatocyte Growth Factor Exerts Its Anti-Inflammatory Action by Disrupting Nuclear Factor-ΚB Signaling. American Journal of Pathology 2008. [Google Scholar] [CrossRef]
- Marathe, A.; Patel, S.J.; Song, B.; Sliepka, J.M.; Shybut, T.S.; Lee, B.H.; Jayaram, P. Double-Spin Leukocyte-Rich Platelet-Rich Plasma Is Predominantly Lymphocyte Rich With Notable Concentrations of Other White Blood Cell Subtypes. Arthrosc Sports Med Rehabil 2022, 4, e335–e341. [Google Scholar] [CrossRef]
- Kennedy, M.I.; Whitney, K.; Evans, T.; LaPrade, R.F. Platelet-Rich Plasma and Cartilage Repair. Current Reviews in Musculoskeletal Medicine 2018. [Google Scholar] [CrossRef]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet Rich Plasma (PRP) Induces Chondroprotection via Increasing Autophagy, Anti-Inflammatory Markers, and Decreasing Apoptosis in Human Osteoarthritic Cartilage. Experimental Cell Research 2017. [Google Scholar] [CrossRef]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy Maintains Stemness by Preventing Senescence. Nature 2016. [Google Scholar] [CrossRef]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R.A. Interleukin-10 Paradox: A Potent Immunoregulatory Cytokine That Has Been Difficult to Harness for Immunotherapy. Cytokine 2015. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. International Anesthesiology Clinics 2007. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in Fibrosis: Novel Roles and Mediators. Frontiers in Pharmacology 2014. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiological Reviews 2003. [Google Scholar] [CrossRef]
- Cavallo, C.; Filardo, G.; Mariani, E.; Kon, E.; Marcacci, M.; Pereira Ruiz, M.T.; Facchini, A.; Grigolo, B. Comparison of Platelet-Rich Plasma Formulations for Cartilage Healing: An in Vitro Study. Journal of Bone and Joint Surgery - Series A 2014. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of Platelets, the Coagulation and Fibrinolytic Systems to Cutaneous Wound Healing. Thrombosis Research 2019. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.I.; Hackeng, T.M.; Weber, C. Heterophilic Interactions of Platelet Factor 4 and RANTES Promote Monocyte Arrest on Endothelium. Blood 2005. [Google Scholar] [CrossRef]
- Xia, C.Q.; Kao, K.J. Effect of CXC Chemokine Platelet Factor 4 on Differentiation and Function of Monocyte-Derived Dendritic Cells. International Immunology 2003. [Google Scholar] [CrossRef]
- Scheuerer, B.; Ernst, M.; Dürrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.D.; Petersen, F. The CXC-Chemokine Platelet Factor 4 Promotes Monocyte Survival and Induces Monocyte Differentiation into Macrophages. Blood 2000. [Google Scholar] [CrossRef]
- Gratchev, A.; Kzhyshkowska, J.; Köthe, K.; Muller-Molinet, I.; Kannookadan, S.; Utikal, J.; Goerdt, S. Mφ1 and Mφ2 Can Be Re-Polarized by Th2 or Th1 Cytokines, Respectively, and Respond to Exogenous Danger Signals. Immunobiology 2006. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. American Journal of Pathology 2015. [Google Scholar] [CrossRef]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-Rich PRP versus Leukocyte-Poor PRP - The Role of Monocyte/Macrophage Function in the Healing Cascade. Journal of Clinical Orthopaedics and Trauma 2019. [Google Scholar] [CrossRef]
- Meszaros, A.J.; Reichner, J.S.; Albina, J.E. Macrophage-Induced Neutrophil Apoptosis. The Journal of Immunology 2000. [Google Scholar] [CrossRef]
- Saluja, H.; Dehane, V.; Mahindra, U. Platelet-Rich Fibrin: A Second Generation Platelet Concentrate and a New Friend of Oral and Maxillofacial Surgeons. Ann Maxillofac Surg 2011, 1, 53–57. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part I: Technological Concepts and Evolution. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 2006. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part IV: Clinical Effects on Tissue Healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Kállay-Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int J Mol Sci 2018, 19, 3433. [Google Scholar] [CrossRef]
- Sunitha Raja, V.; Munirathnam Naidu, E. Platelet-Rich Fibrin: Evolution of a Second-Generation Platelet Concentrate. Indian J Dent Res 2008, 19, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, F.; Ural, A.; Bekerecioglu, M. Platelet-Rich Fibrin: An Effective Chronic Wound Healing Accelerator. J Tissue Viability 2021, 30, 616–620. [Google Scholar] [CrossRef]
- Desai, C.B.; Mahindra, U.R.; Kini, Y.K.; Bakshi, M.K. Use of Platelet-Rich Fibrin over Skin Wounds: Modified Secondary Intention Healing. J Cutan Aesthet Surg 2013, 6, 35–37. [Google Scholar] [CrossRef]
- Cortese, A.; Pantaleo, G.; Borri, A.; Caggiano, M.; Amato, M. Platelet-Rich Fibrin (PRF) in Implant Dentistry in Combination with New Bone Regenerative Technique in Elderly Patients. International Journal of Surgery Case Reports 2016, 28, 52–56. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable Platelet Rich Fibrin (i-PRF): Opportunities in Regenerative Dentistry? Clin Oral Invest 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-Rich Fibrin: Basics of Biological Actions and Protocol Modifications. Open Med (Wars) 2021, 16, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Jeon, S.H.; Park, J.-Y.; Chung, J.-H.; Choung, Y.-H.; Choung, H.-W.; Kim, E.-S.; Choung, P.-H. Platelet-Rich Fibrin Is a Bioscaffold and Reservoir of Growth Factors for Tissue Regeneration. Tissue Eng Part A 2011, 17, 349–359. [Google Scholar] [CrossRef]
- Bielecki, T.; Ehrenfest, D.M.D.; Everts, P.A.; Wiczkowski, A. The Role of Leukocytes from L-PRP / L-PRF in Wound Healing and Immune Defense : New Perspectives. 2012, 1153–1162.
- Miron, R.J.; Bishara, M.; Choukroun, J. Basics of Platelet-Rich Fibrin Therapy. Dent Today 2017, 36, 74–76. [Google Scholar]
- Nicola, V.D. L-PRF in Osteoarthritis Treatment: Results of a Pilot Study. J Regen Biol Med 2020. [Google Scholar] [CrossRef]
- Kandel, L.; Agar, G.; Elkayam, O.; Sharipov, A.; Slevin, O.; Rivkin, G.; Dahan, M.; Aloush, V.; Pyeser, A.B.; Brin, Y.; et al. A Novel Approach for Knee Osteoarthritis Using High Molecular Weight Hyaluronic Acid Conjugated to Plasma Fibrinogen – Interim Findings of a Double-Blind Clinical Study. Heliyon 2020, 6, e04475. [Google Scholar] [CrossRef]
- Jang, J.D.; Moon, Y.S.; Kim, Y.S.; Choi, N.Y.; Mok, H.S.; Kim, Y.J.; Shetty, A.A.; Kim, S.J. Novel Repair Technique for Articular Cartilage Defect Using a Fibrin and Hyaluronic Acid Mixture. Tissue Engineering and Regenerative Medicine 2013. [Google Scholar] [CrossRef]
- Shoji, T.; Nakasa, T.; Yoshizuka, M.; Yamasaki, T.; Yasunaga, Y.; Adachi, N.; Ochi, M. Comparison of Fibrin Clots Derived from Peripheral Blood and Bone Marrow. Connective Tissue Research 2017. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.H.; Rai, B.; Tan, T.C.; Ramruttun, A.K.; Hui, J.H.; Nurcombe, V.; Teoh, S.H.; Cool, S.M. Autologous Bone Marrow Clot as an Alternative to Autograft for Bone Defect Healing. Bone and Joint Research 2019. [Google Scholar] [CrossRef] [PubMed]
- Cheeva-akrapan, V.; Turajane, T.; Cheeva-akrapan, V.; Turajane, T. The 36-Month Survival Analysis of Conservative Treatment Using Platelet-Rich Plasma Enhanced With Injectable Platelet-Rich Fibrin in Patients With Knee Osteoarthritis. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Işık, G. Application of Injectable Platelet-Rich Fibrin for the Treatment of Temporomandibular Joint Osteoarthritis: A Randomized Controlled Clinical Trial; clinicaltrials.gov, 2021.
- Manafikhi, M.; Ataya, J.; Heshmeh, O. Evaluation of the Efficacy of Platelet Rich Fibrin (I-PRF) Intra-Articular Injections in the Management of Internal Derangements of Temporomandibular Joints – a Controlled Preliminary Prospective Clinical Study. BMC Musculoskelet Disord 2022, 23, 454. [Google Scholar] [CrossRef]
- Lisignoli, G.; Cristino, S.; Piacentini, A.; Cavallo, C.; Caplan, A.I.; Facchini, A. Hyaluronan-Based Polymer Scaffold Modulates the Expression of Inflammatory and Degradative Factors in Mesenchymal Stem Cells: Involvement of Cd44 and Cd54. Journal of Cellular Physiology 2006. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Hackel, J.; Niazi, F.; Shaw, P.; Nicholls, M. Efficacy and Safety of Repeated Courses of Hyaluronic Acid Injections for Knee Osteoarthritis: A Systematic Review. Semin Arthritis Rheum 2018, 48, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mordin, M.; Parrish, W.; Masaquel, C.; Bisson, B.; Copley-Merriman, C. Intra-Articular Hyaluronic Acid for Osteoarthritis of the Knee in the United States: A Systematic Review of Economic Evaluations. Clin Med Insights Arthritis Musculoskelet Disord 2021, 14, 11795441211047284. [Google Scholar] [CrossRef]
- Bruyère, O.; Cooper, C.; Pelletier, J.P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; Hochberg, M.C.; Kanis, J.A.; Kvien, T.K.; Martel-Pelletier, J.; et al. An Algorithm Recommendation for the Management of Knee Osteoarthritis in Europe and Internationally: A Report from a Task Force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Seminars in Arthritis and Rheumatism 2014, 44, 253–263. [Google Scholar] [CrossRef]
- Brun, P.; Zavan, B.; Vindigni, V.; Schiavinato, A.; Pozzuoli, A.; Iacobellis, C.; Abatangelo, G. In Vitro Response of Osteoarthritic Chondrocytes and Fibroblast-like Synoviocytes to a 500-730 KDa Hyaluronan Amide Derivative. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2012, 100B, 2073–2081. [Google Scholar] [CrossRef]
- Kruel, A.V.S.; Ribeiro, L.L.; Gusmão, P.D.; Huber, S.C.; Lana, J.F.S.D. Orthobiologics in the Treatment of Hip Disorders. World J Stem Cells 2021, 13, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Rugolo, C.A.; Calatroni, A. Differential Effect of Molecular Mass Hyaluronan on Lipopolysaccharide-Induced Damage in Chondrocytes. Innate Immun 2010, 16, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; de la Motte, C.A. Hyaluronan Cross-Linking: A Protective Mechanism in Inflammation? Trends Immunol 2005, 26, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Rannou, F.; Reginster, J.Y. Efficacy and Safety of Hyaluronic Acid in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Seminars in Arthritis and Rheumatism 2016. [Google Scholar] [CrossRef] [PubMed]
- Julovi, S.M.; Yasuda, T.; Shimizu, M.; Hiramitsu, T.; Nakamura, T. Inhibition of Interleukin-1β-Stimulated Production of Matrix Metalloproteinases by Hyaluronan via CD44 in Human Articular Cartilage. Arthritis and Rheumatism 2004, 50, 516–525. [Google Scholar] [CrossRef]
- Kalaci, A.; Yilmaz, R.H.; Aslan, B.; Sög̈üt, S.; Yanat, A.N.; Uz, E. Effects of Hyaluronan on Nitric Oxide Levels and Superoxide Dismutase Activities in Synovial Fluid in Knee Osteoarthritis. Clinical Rheumatology 2007, 26, 1306–1311. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Surażyński, A.; Pałka, J.A. Protective Effect of Hyaluronic Acid on Interleukin-1-Induced Deregulation of Βeta 1 -Integrin and Insulin-like Growth Factor-I Receptor Signaling and Collagen Biosynthesis in Cultured Human Chondrocytes. Molecular and Cellular Biochemistry 2008, 308, 57–64. [Google Scholar] [CrossRef]
- Abate, M.; Pelotti, P.; De Amicis, D.; Di Iorio, A.; Galletti, S.; Salini, V. Viscosupplementation with Hyaluronic Acid in Hip Osteoarthritis (a Review). Upsala Journal of Medical Sciences 2008, 113, 261–278. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and Signaling. Biochim Biophys Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Panes, O.; Padilla, O.; Matus, V.; Sez, C.G.; Berkovits, A.; Pereira, J.; Mezzano, D. Clot Lysis Time in Platelet-Rich Plasma: Method Assessment, Comparison with Assays in Platelet-Free and Platelet-Poor Plasmas, and Response to Tranexamic Acid. Platelets 2012. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.D.; Harvey, J.A.; Kazmi, M.A.; Stout, A.J. Fibrinolysis and Angiogenesis in Wound Healing. The Journal of Pathology 1991. [Google Scholar] [CrossRef] [PubMed]
- Laurens, N.; Koolwijk, P.; de Maat, M.P. Fibrin Structure and Wound Healing. Journal of thrombosis and haemostasis : JTH 2006.
- Mullarky, I.K.; Szaba, F.M.; Berggren, K.N.; Parent, M.A.; Kummer, L.W.; Chen, W.; Johnson, L.L.; Smiley, S.T. Infection-Stimulated Fibrin Deposition Controls Hemorrhage and Limits Hepatic Bacterial Growth during Listeriosis. Infection and Immunity 2005. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The Impact of the Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors, and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Heissig, B.; Dhahri, D.; Eiamboonsert, S.; Salama, Y.; Shimazu, H.; Munakata, S.; Hattori, K. Role of Mesenchymal Stem Cell-Derived Fibrinolytic Factor in Tissue Regeneration and Cancer Progression. Cellular and Molecular Life Sciences 2015. [Google Scholar] [CrossRef] [PubMed]
- Sinclair And, R.D.; Ryan, T.J. PROTEOLYTIC ENZYMES IN WOUND HEALING: THE ROLE OF ENZYMATIC DEBRIDEMENT. Australasian Journal of Dermatology 1994. [Google Scholar] [CrossRef]
- Vallabhaneni, K.C.; Tkachuk, S.; Kiyan, Y.; Shushakova, N.; Haller, H.; Dumler, I.; Eden, G. Urokinase Receptor Mediates Mobilization, Migration, and Differentiation of Mesenchymal Stem Cells. Cardiovascular Research 2011. [Google Scholar] [CrossRef]
- Syrovets, T.; Lunov, O.; Simmet, T. Plasmin as a Proinflammatory Cell Activator. Journal of Leukocyte Biology 2012. [Google Scholar] [CrossRef]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting Innate Immunity Protein Kinase Signalling in Inflammation. Nature Reviews Drug Discovery 2009. [Google Scholar] [CrossRef]
- Rømer, J.; Bugge, T.; Pyke, C.; Lund, L.R.; Flick, M.J.; Degen, J.L.; Danø, K. Impaired Wound Healing in Mice with a Disrupted Plasminogen Gene. Fibrinolysis 1996. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Albiero, M.; De Kreutzenberg, S.V.; Boscaro, E.; Cappellari, R.; Marescotti, M.; Poncina, N.; Agostini, C.; Avogaro, A. Diabetes Impairs Stem Cell and Proangiogenic Cell Mobilization in Humans. Diabetes Care 2013. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, M.; Witte, M.; Becker, H.D. Models to Study Ischemia in Chronic Wounds. The International Journal of Lower Extremity Wounds 2002. [Google Scholar] [CrossRef] [PubMed]
- Basiouny, H.S.; Salama, N.M.; El Maadawi, Z.M.; Farag, E.A. Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat. International Journal of Stem Cells 2013. [Google Scholar] [CrossRef] [PubMed]
- Copland, I.B.; Lord-Dufour, S.; Cuerquis, J.; Coutu, D.L.; Annabi, B.; Wang, E.; Galipeau, J. Improved Autograft Survival of Mesenchymal Stromal Cells by Plasminogen Activator Inhibitor 1 Inhibition. Stem Cells 2009. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Kuo, I.H.; Chang, C.C.; Chu, C.Y.; Chen, H.Y.; Lin, B.R.; Sureshbabu, M.; Shih, H.J.; Kuo, M.L. Involvement of Hypoxia-Inducing Factor-1α-Dependent Plasminogen Activator Inhibitor-1 up-Regulation in Cyr61/CCN1-Induced Gastric Cancer Cell Invasion. Journal of Biological Chemistry 2008. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kawasaki, H.; Kerpedjieva, S.S.; Guan, J.; Ganju, R.K.; Sen, C.K. Differential Roles of Hypoxia Inducible Factor Subunits in Multipotential Stromal Cells under Hypoxic Condition. Journal of Cellular Biochemistry 2011. [Google Scholar] [CrossRef]
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional Expression of HGF and HGF Receptor/c-Met in Adult Human Mesenchymal Stem Cells Suggests a Role in Cell Mobilization, Tissue Repair, and Wound Healing. STEM CELLS 2004. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the Stem Cell Niche for Tissue Regeneration. Nature Biotechnology 2014. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, H.C.; Kon, V.; Fogo, A.B.; Lawrence, D.A.; Ma, J. Vitronectin-Binding PAI-1 Protects against the Development of Cardiac Fibrosis through Interaction with Fibroblasts. Laboratory Investigation 2014. [Google Scholar] [CrossRef]
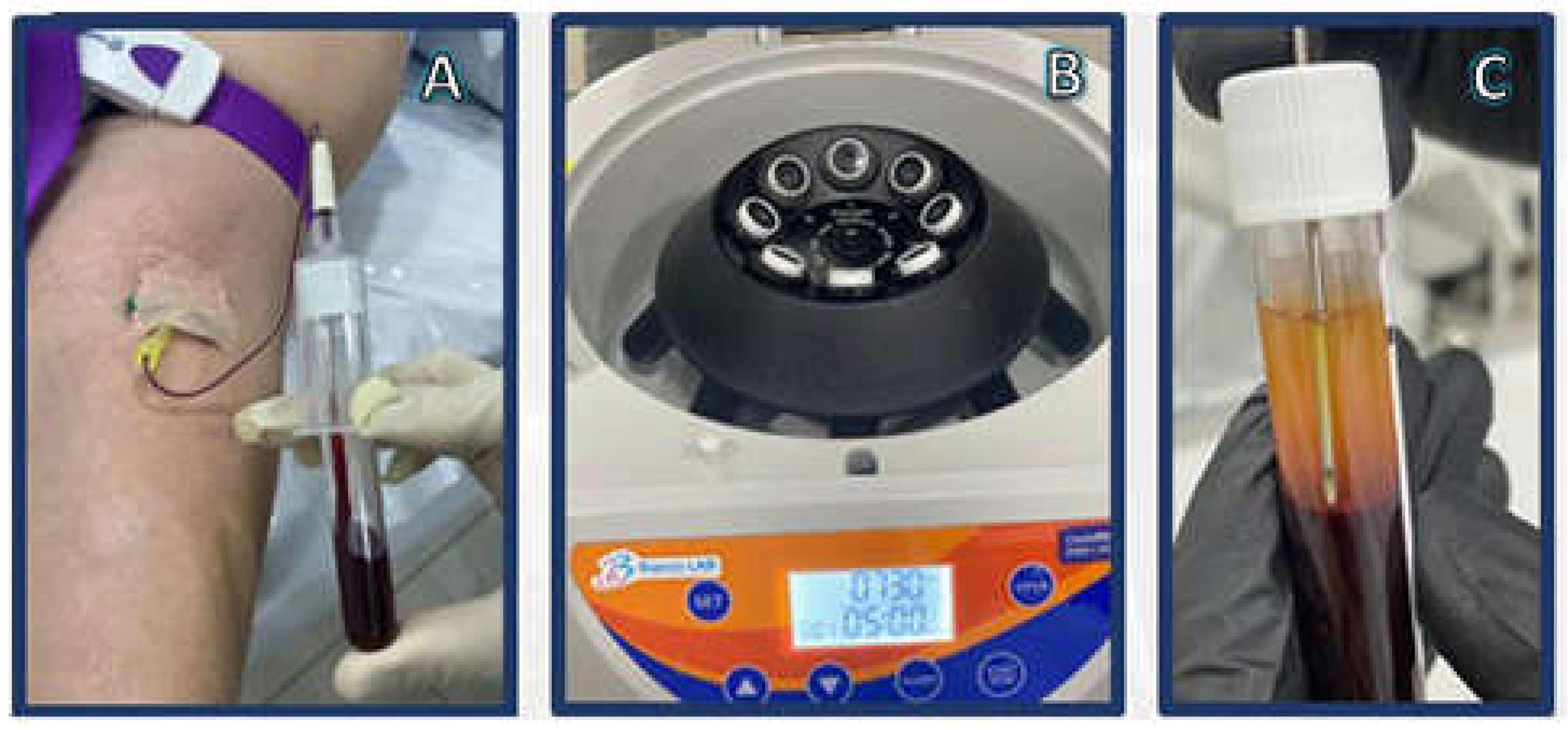
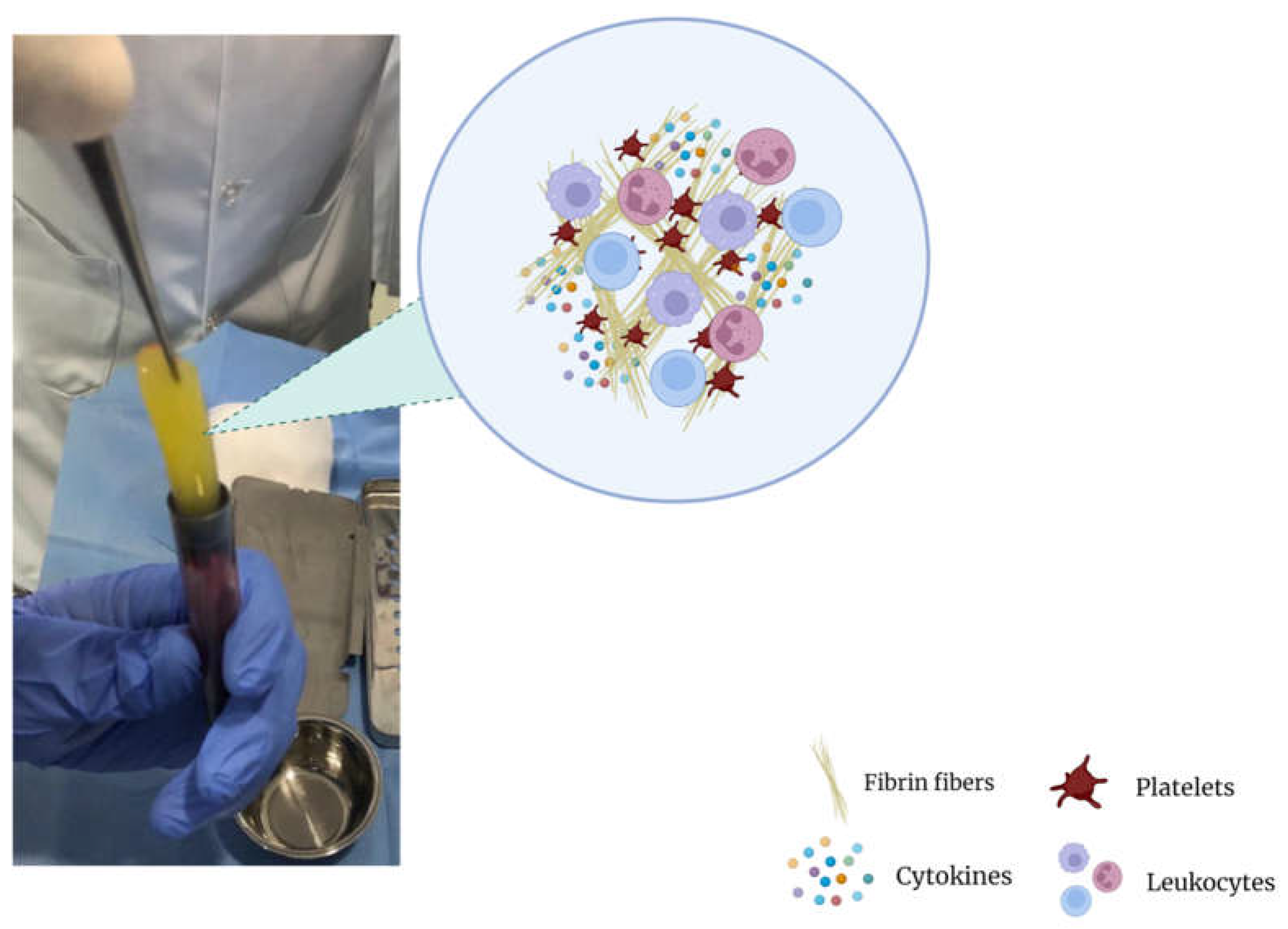
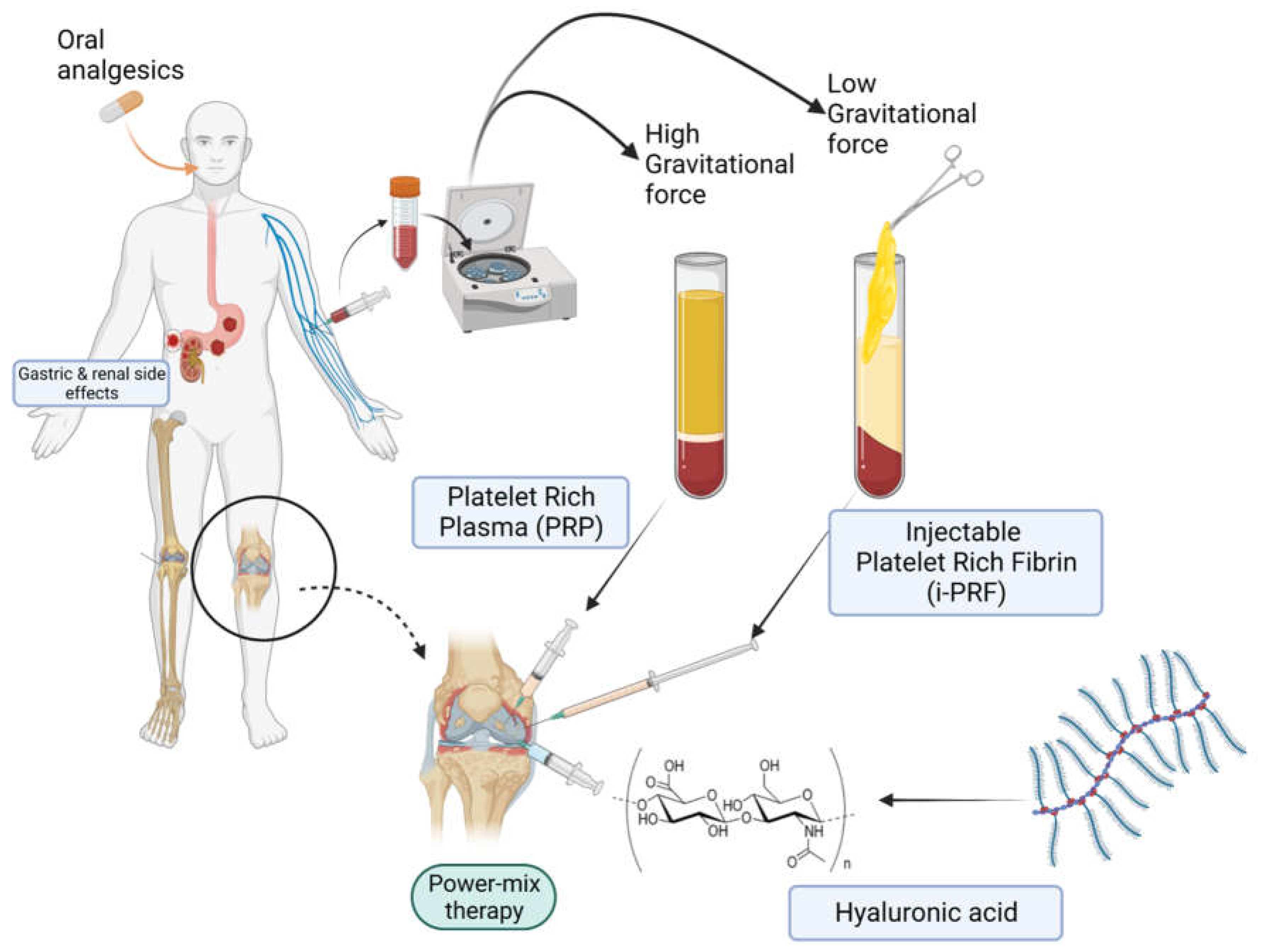
| Osteoarthritis Grade | Observation |
|---|---|
| Grade 0 (normal) | No radiological findings |
| Grade I (doubtful) | Possible signs of osteophytic lipping and narrowing of joint space |
| Grade II (mild) | Definite osteophytes and possible joint space narrowing |
| Grade III (moderate) | Definite joint space narrowing and multiple osteophytes |
| Grade IV (severe) | Large osteophytes, prominent demarcation of narrowed joint space, severe sclerosis and expressive deformity of bone contour |
| Name | Abbreviation | Biological Role |
|---|---|---|
| Fibroblast growth factor | FGF | Regulates cell proliferation, survival, migration, and differentiation. |
| Vascular endothelial growth factor | VEGF | Stimulates angiogenesis, macrophage and neutrophil chemotaxis, migration and mitosis of endothelial cells, and increases permeability of blood vessels. |
| Insulin-like growth factor | IGF | Regulates cell growth and differentiation, stimulates collagen synthesis and recruits cells from bone, endothelium, epithelium and other tissues. |
| Transforming growth factor-β | TGF-β | Boosts production of collagen type 1, stimulates angionesis and chemotaxis of immune cells, inhibits osteoclast formation and bone resorption |
| Hepatocyte growth factor | HGF | Secreted by mesenchymal cells, HGF stimulates mitogenesis, cell motility, and matrix invasion. |
| Platelet-derived growth factor | PDGF | Increases collagen expression, bone cell proliferation, chemotaxis and proliferation of fibroblasts, and macrophage activation. |
| Epidermal growth factor | EGF | Stimulates proliferation and differentiation of epithelial cells, promotes secretion of cytokines by mesenchymal and epithelial cells. |
| Insulin-like growth factor-1 | IGF-1 | Plays a key role in cell growth and healing via anabolic effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





