Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. History of medical gloves
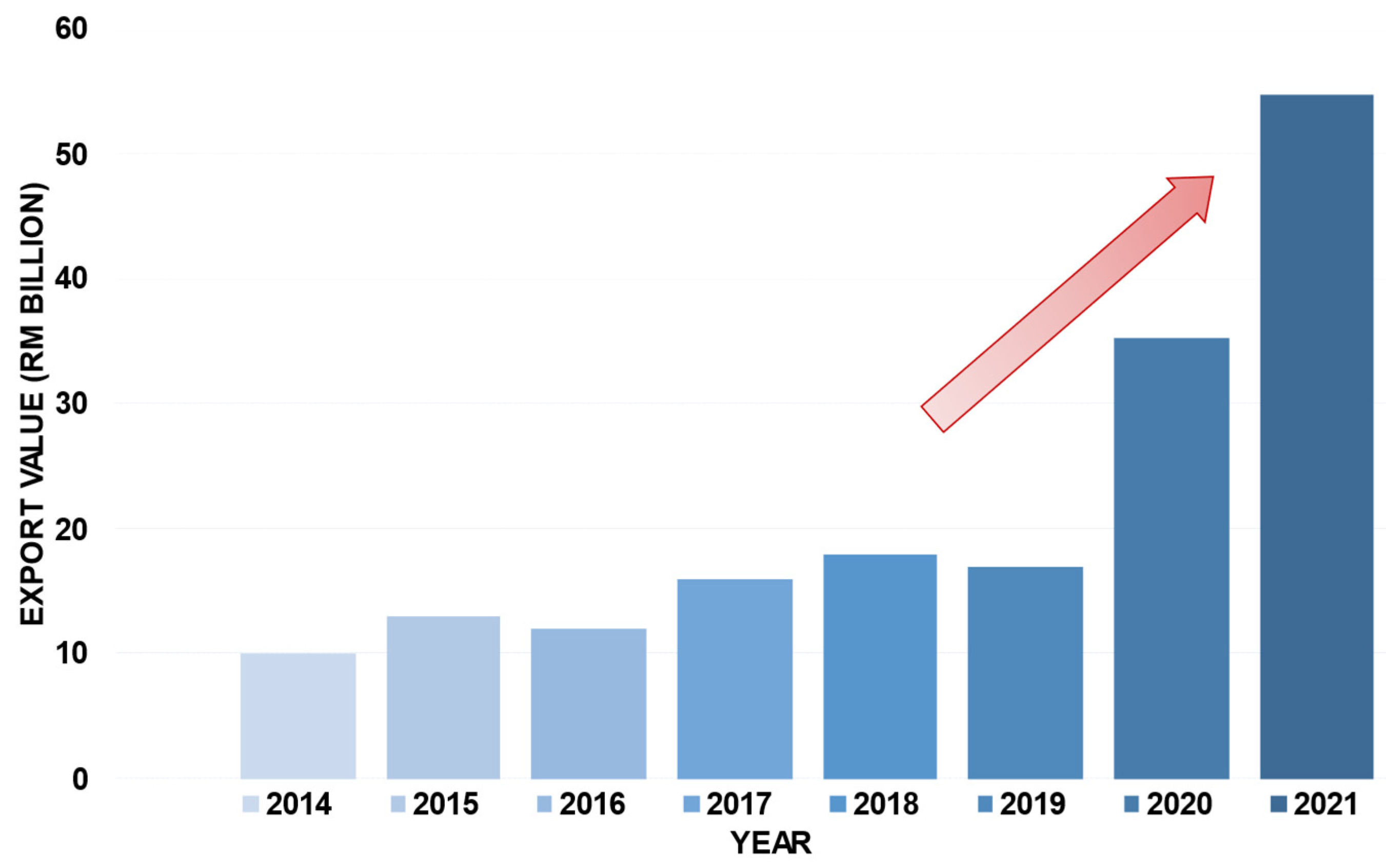
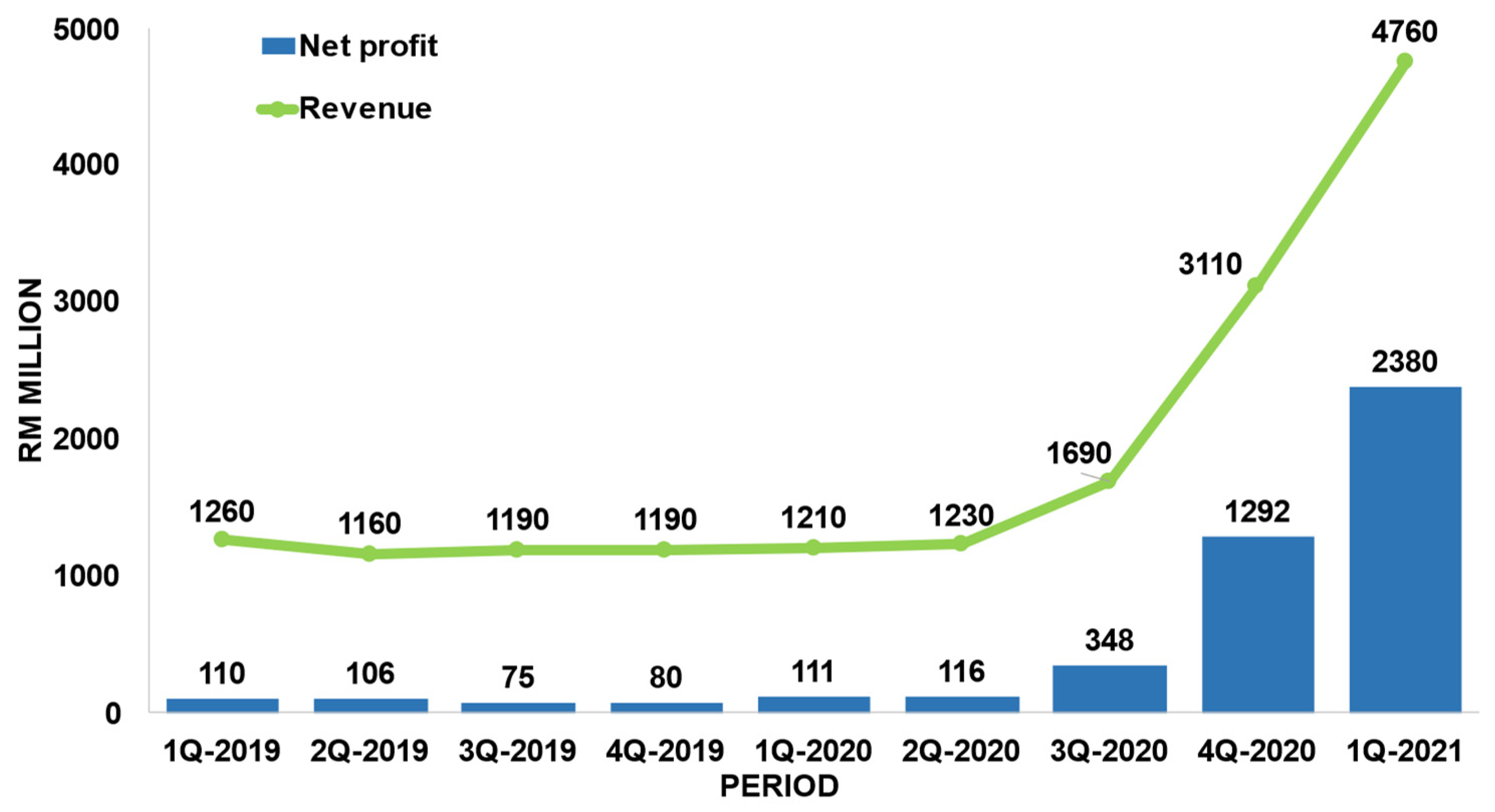
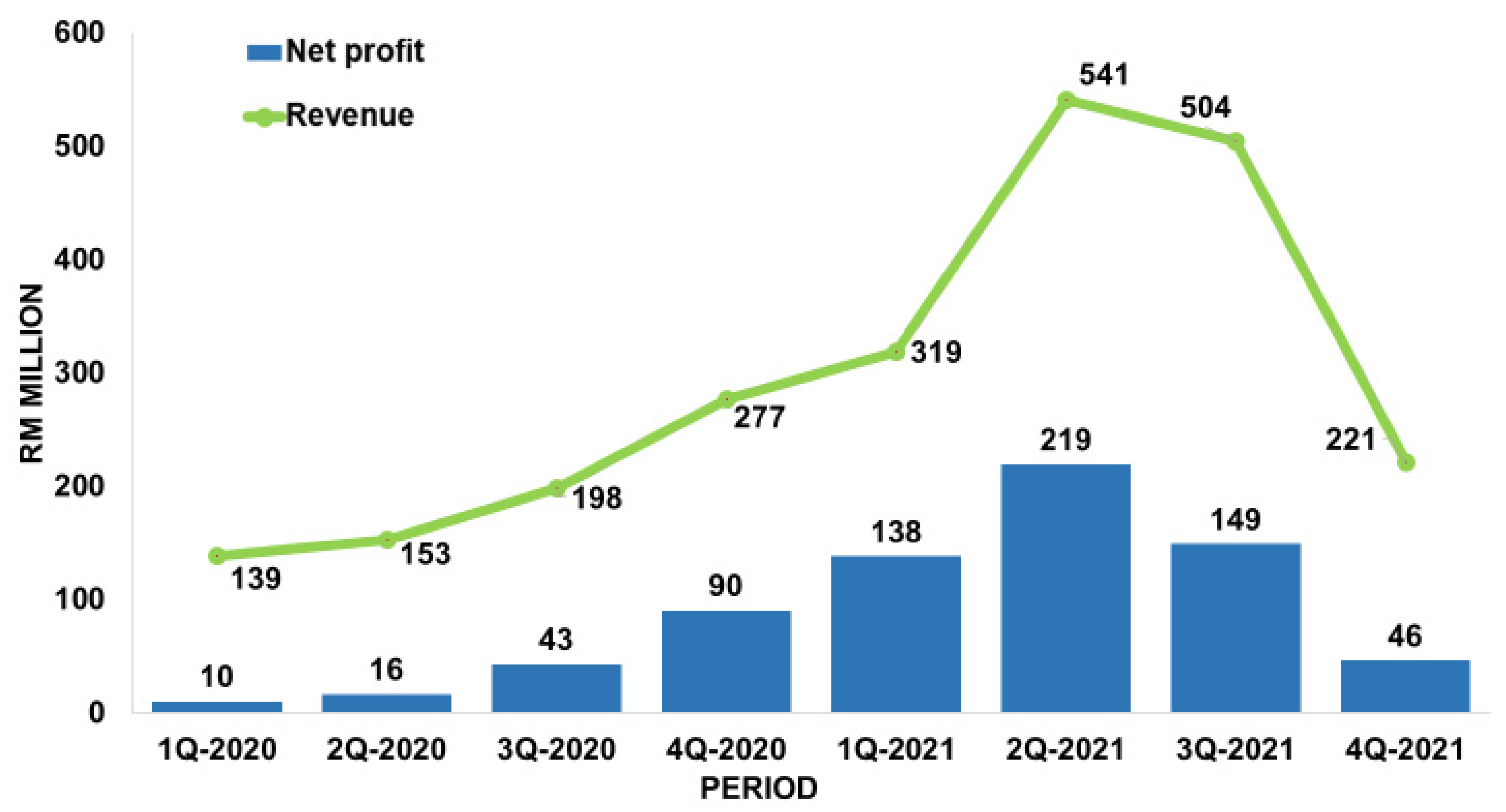
1.2. Market of medical gloves
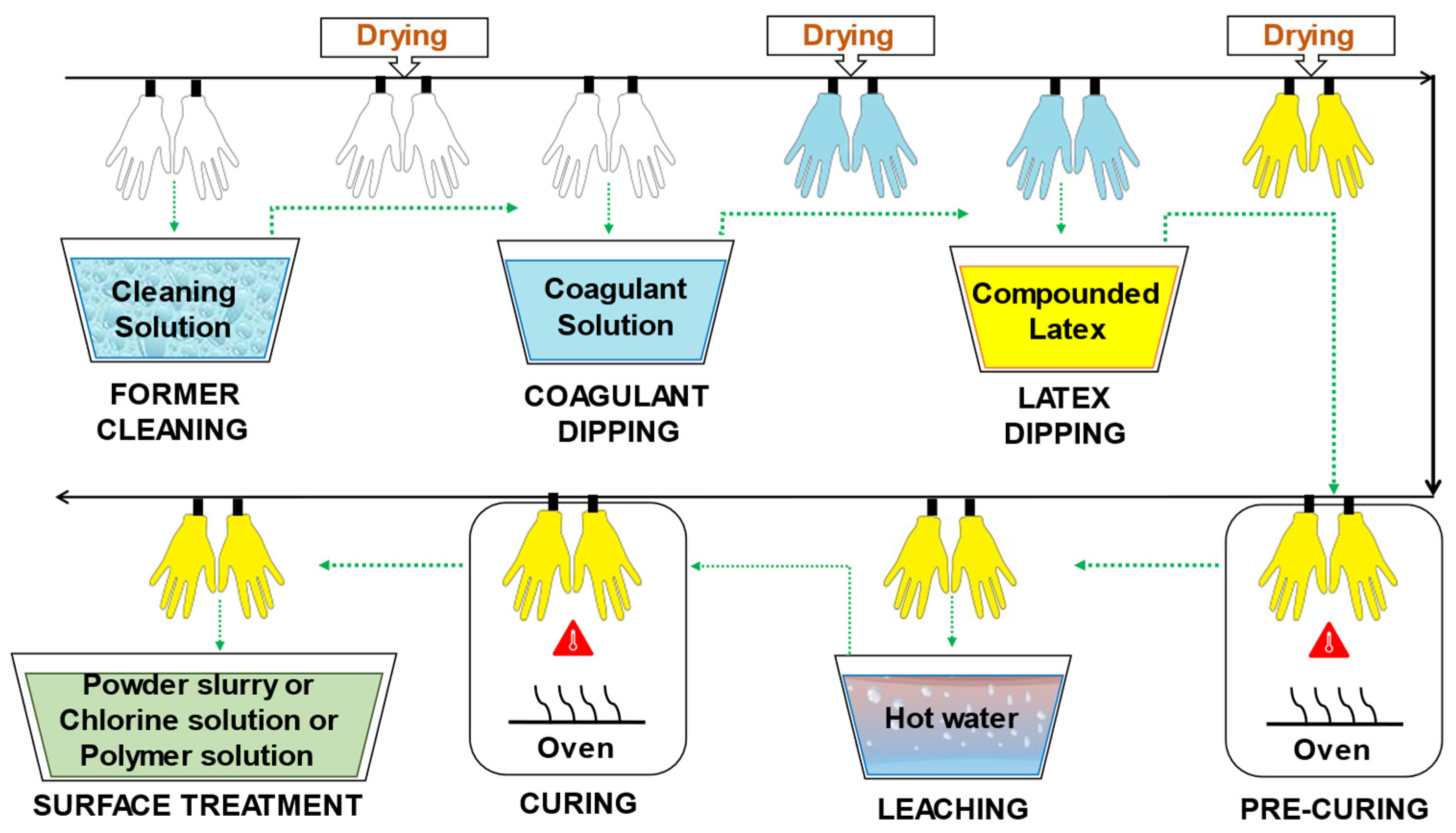
1.3. Production process of medical gloves
1.4. Environmental concerns related to medical gloves
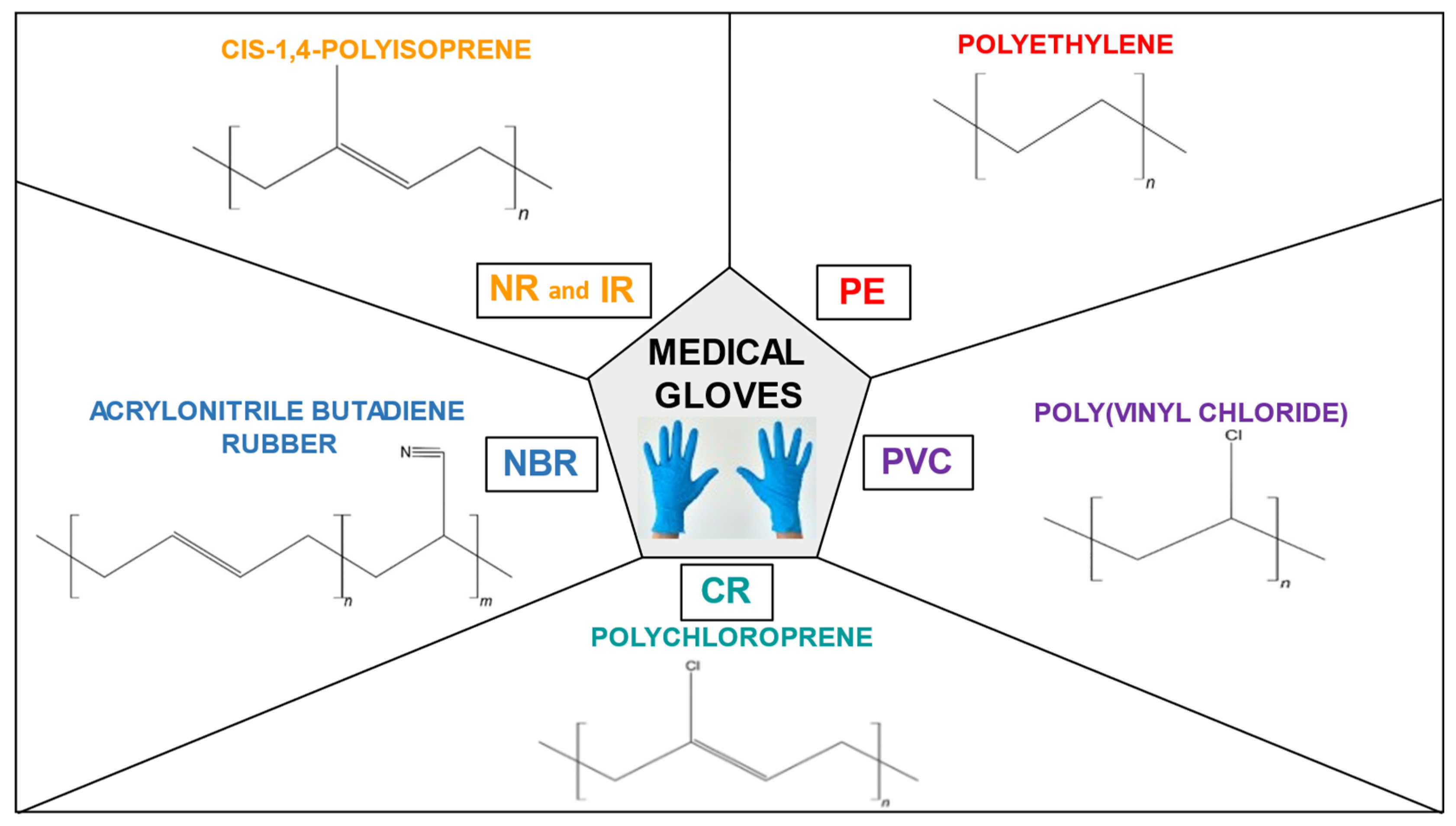
2. Types of medical gloves
2.1. Natural rubber (NR)
2.2. Polyisoprene (IR)
2.3. Acrylonitrile butadiene rubber (NBR)
2.4. Polychloroprene (CR)
2.5. Polyethylene (PE)
2.6. Poly(vinyl chloride) (PVC)
3. Mechanical properties of medical gloves
4. Prototypes of medical gloves with smart materials
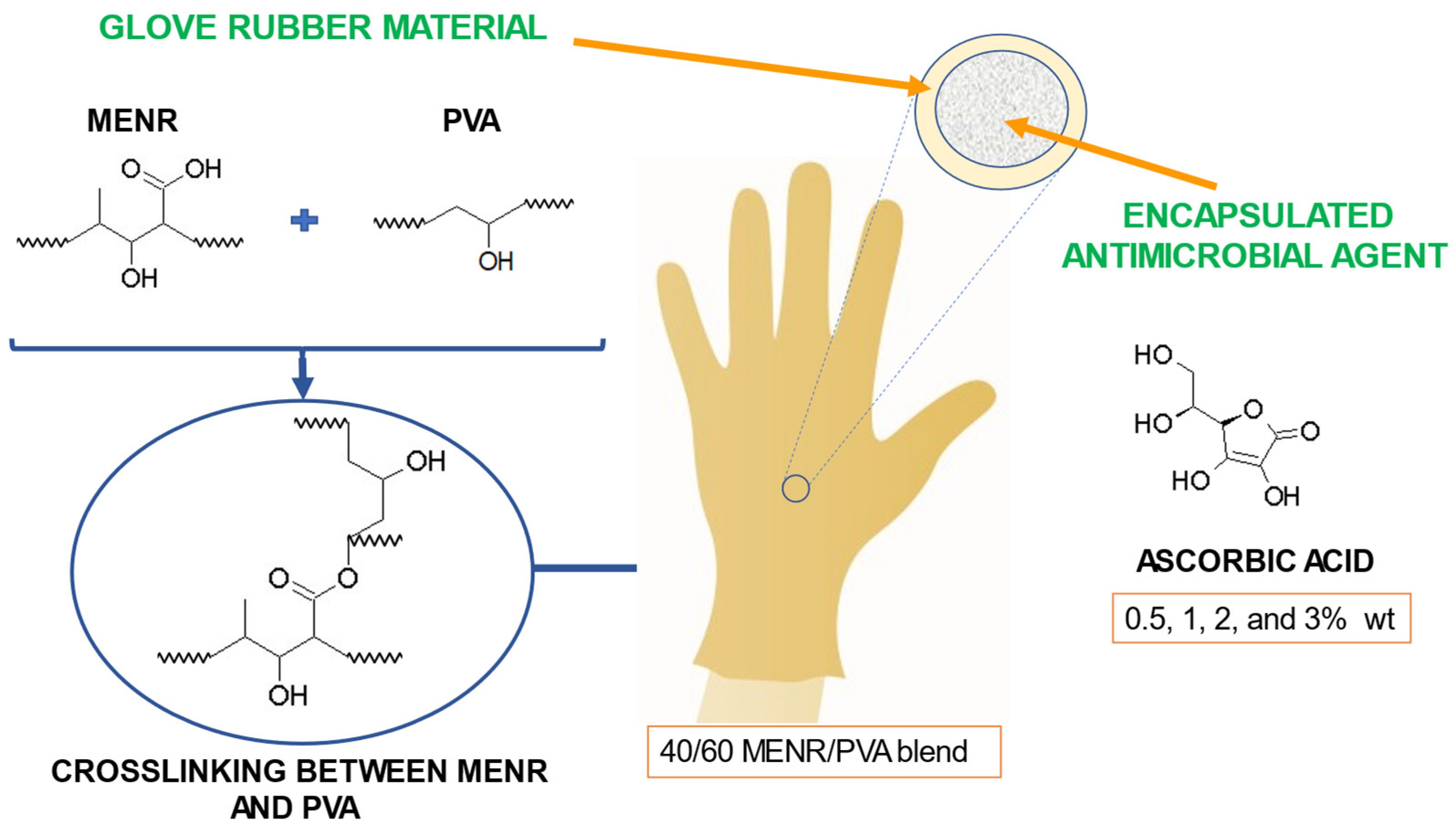
4.1. Biodegradable Green Glove Containing Ascorbic Acid from Maleate Epoxidized Natural Rubber/Poly(vinyl alcohol) Blend
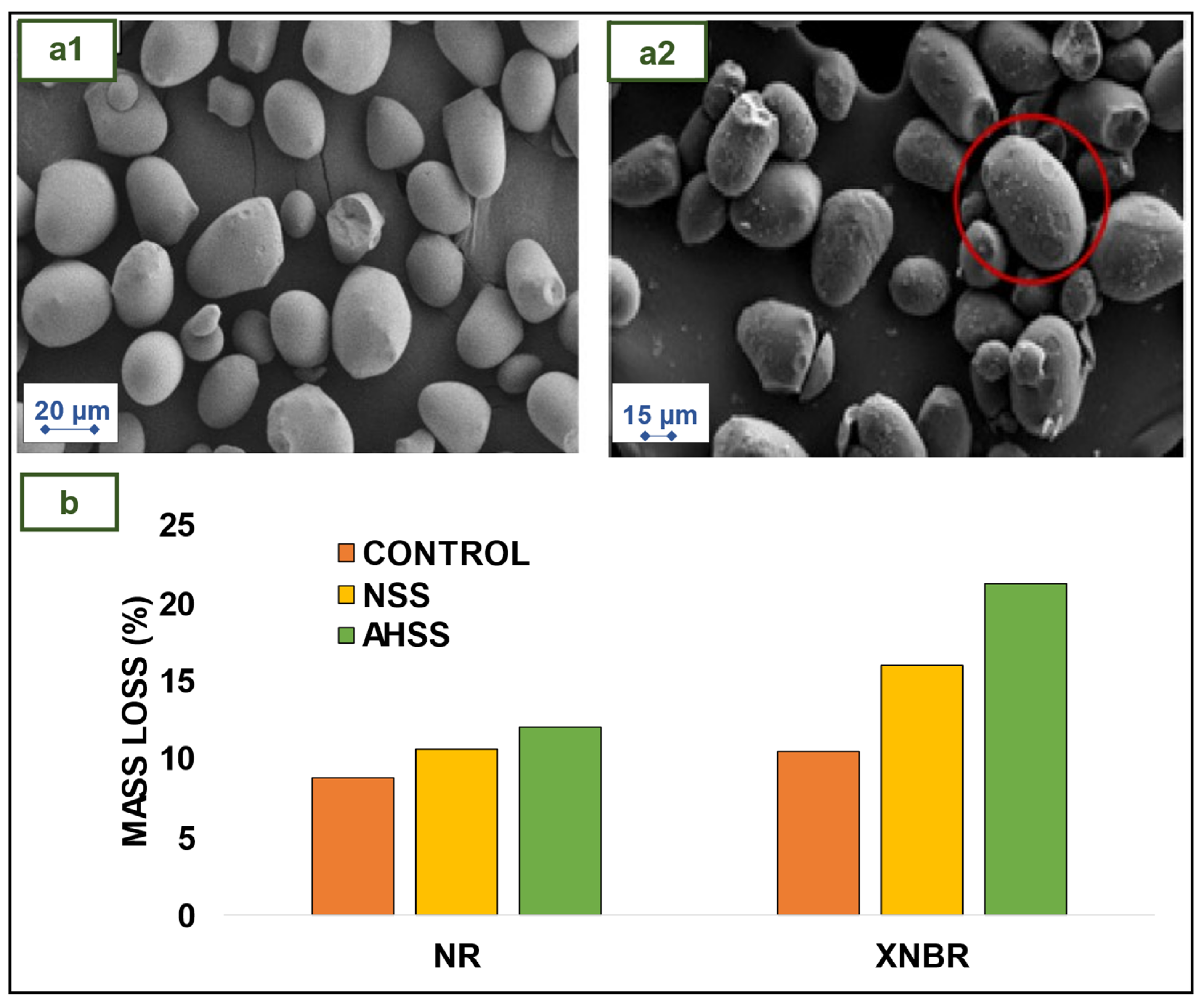
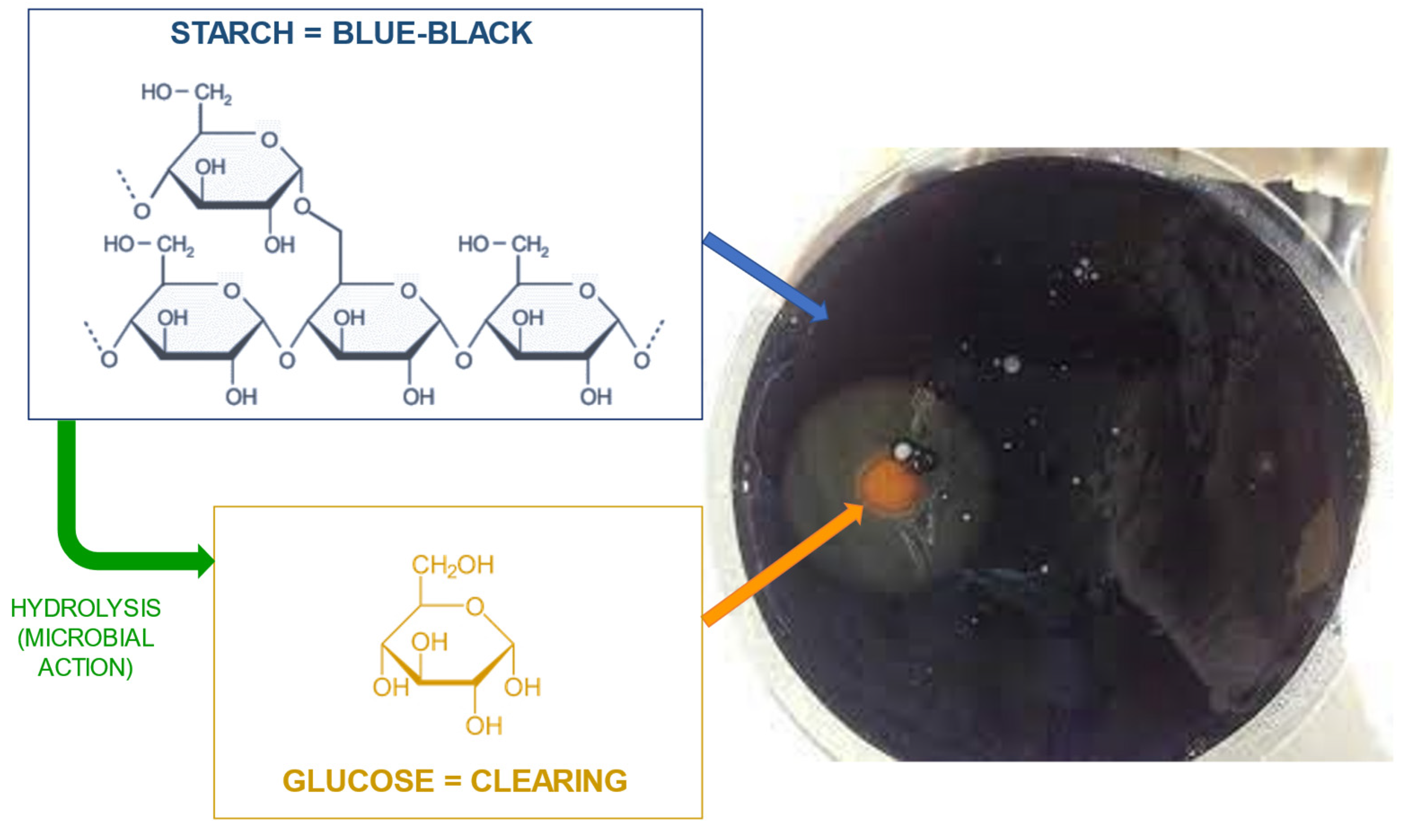
4.2. NR films/gloves and carboxylated-NBR (XNBR) films containing sago starch as bio-filler
4.3. Mangosteen peel as antimicrobial agent in NR gloves
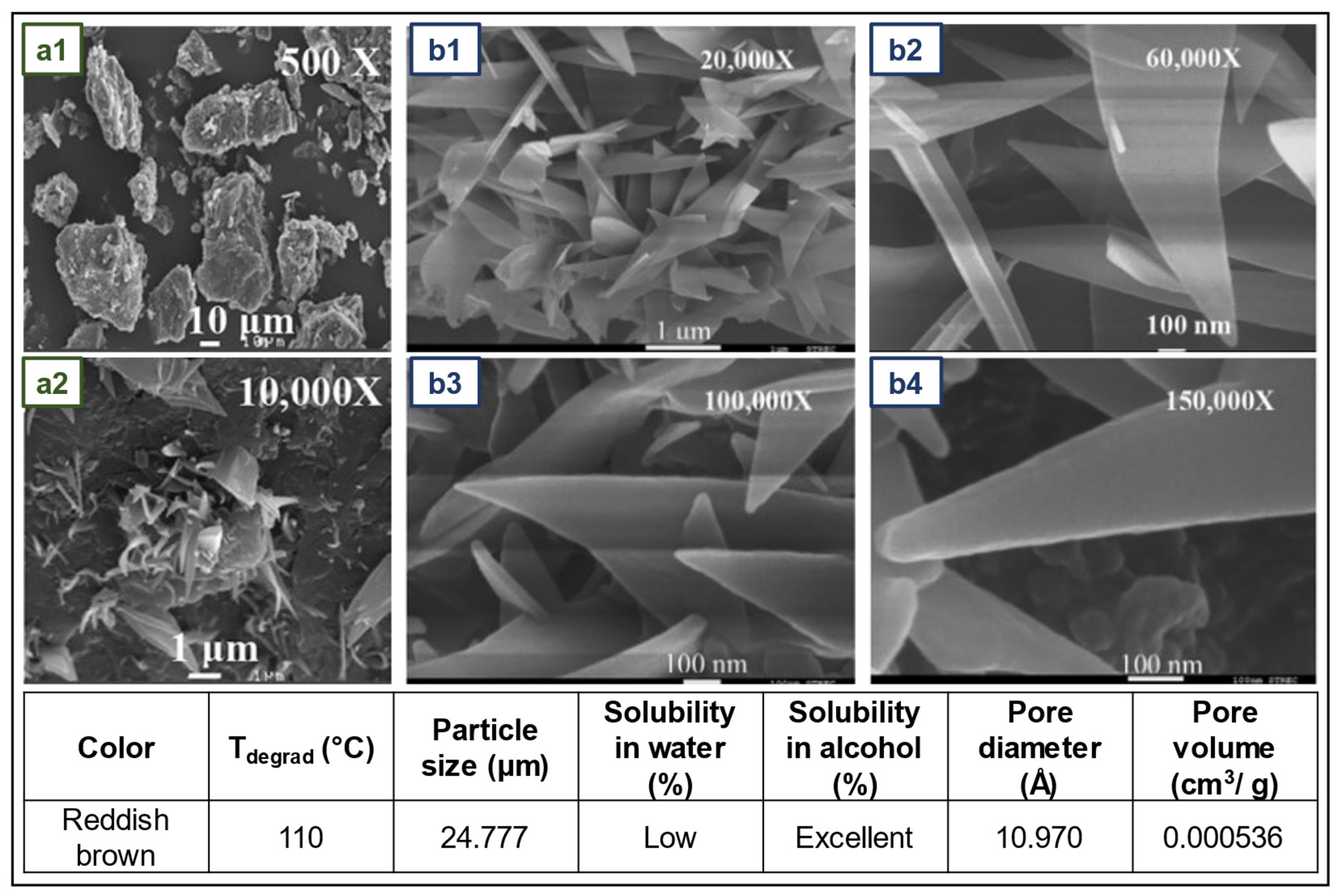
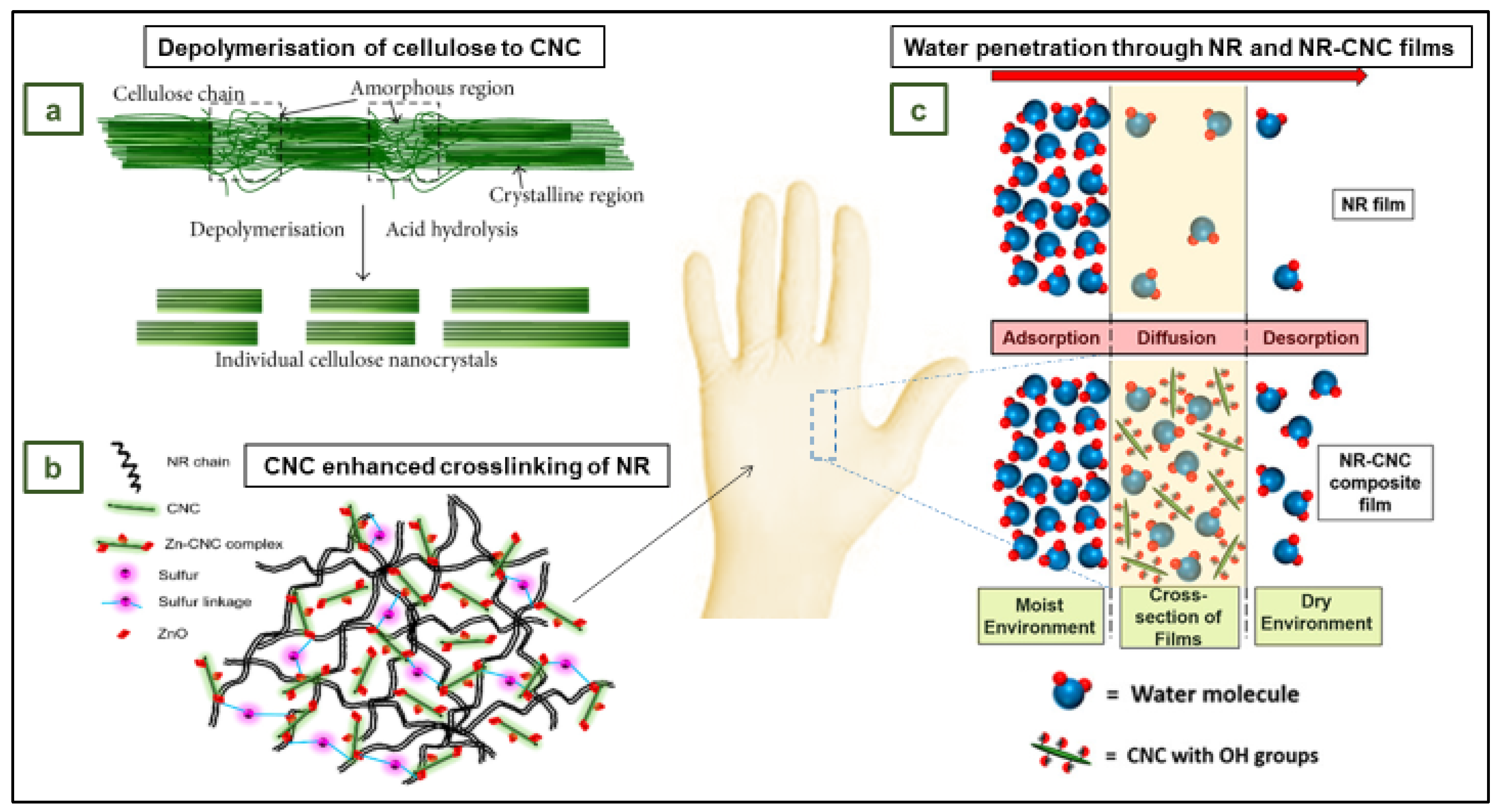
4.4. NR films with cellulose nanocrystals as reinforcing and crosslinking agent for application in gloves
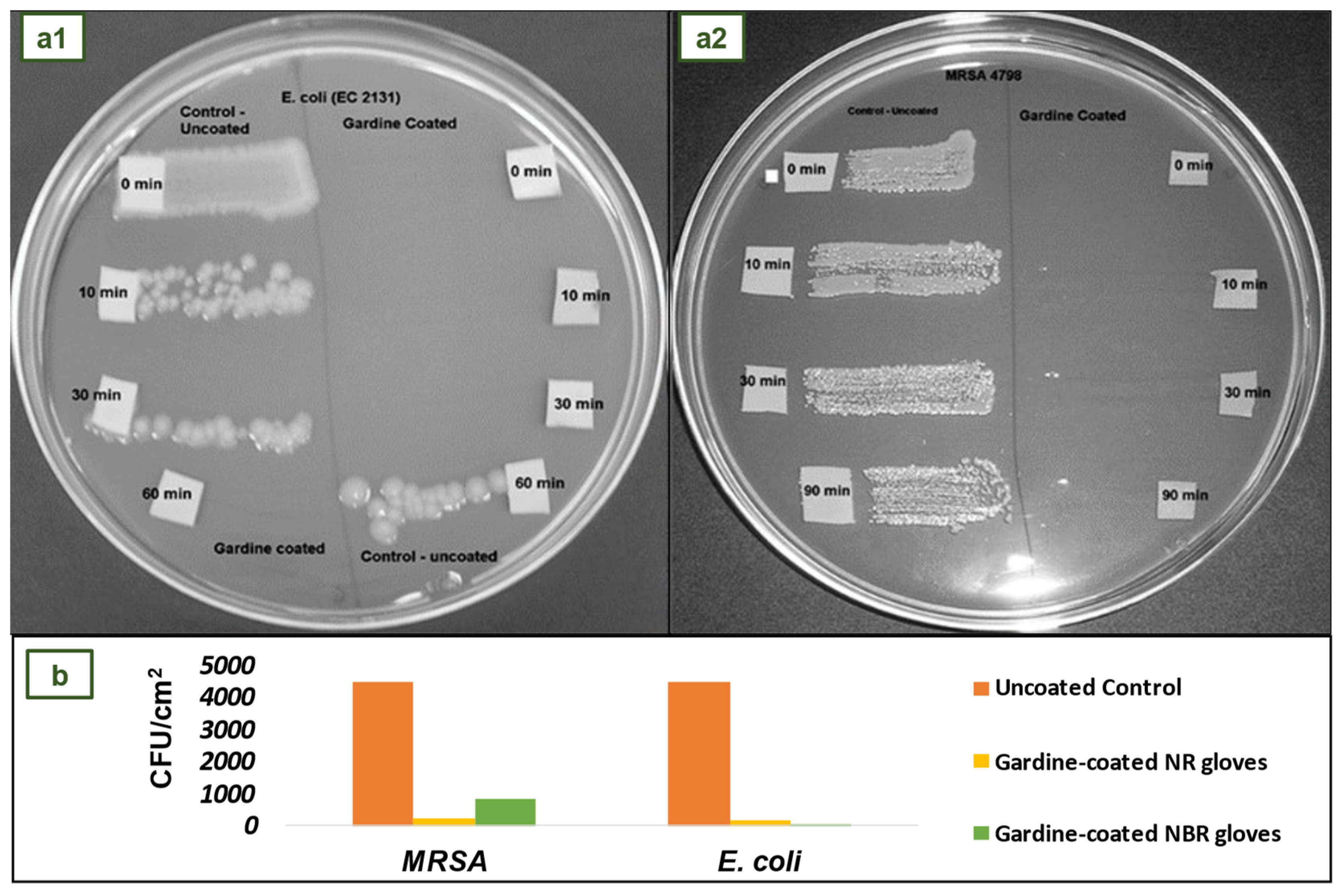
4.5. NR and NBR gloves coated with Gardine solution
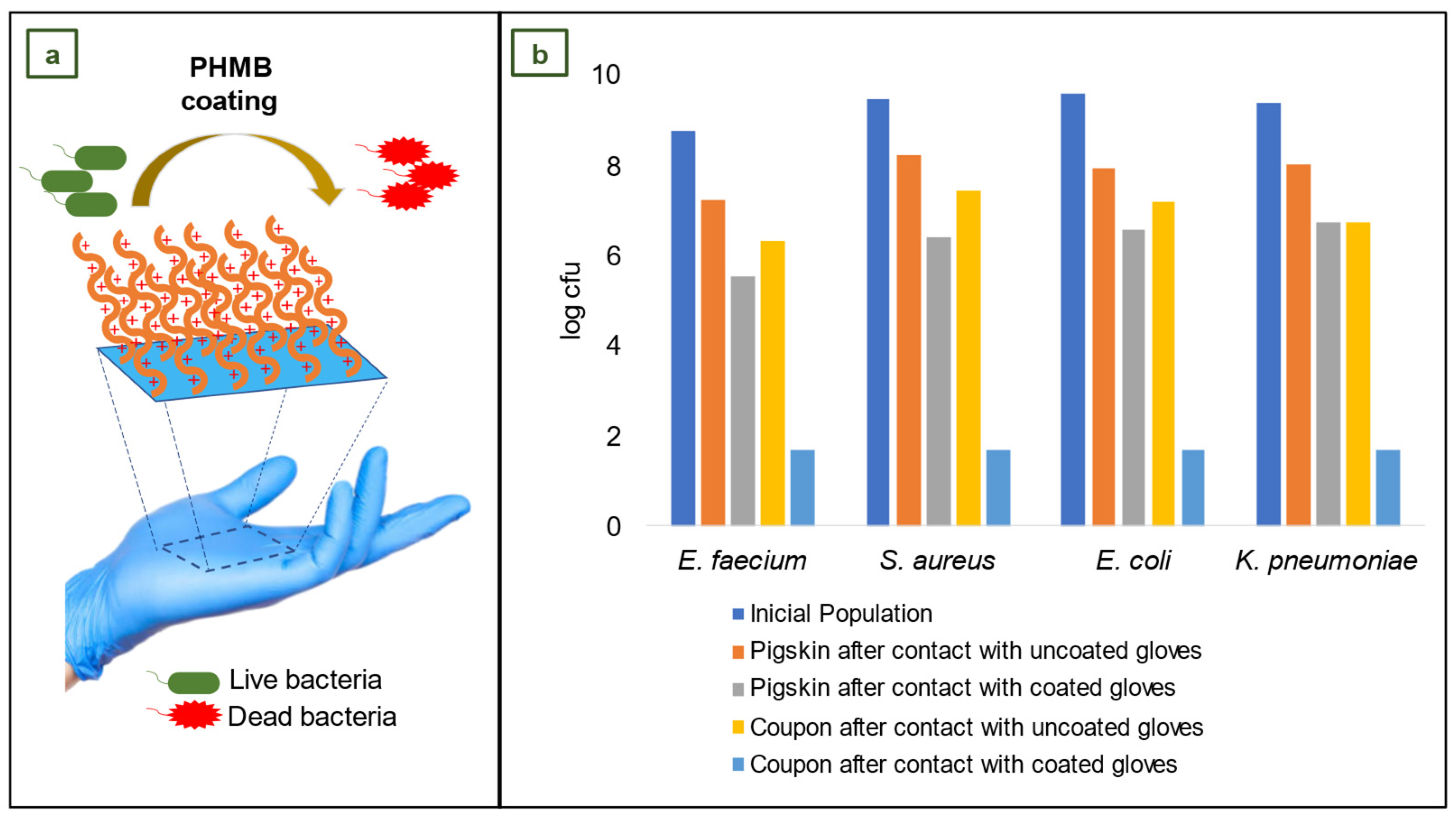
4.6. NBR gloves coated with Poly(hexamethylene biguanide) hydrochloride
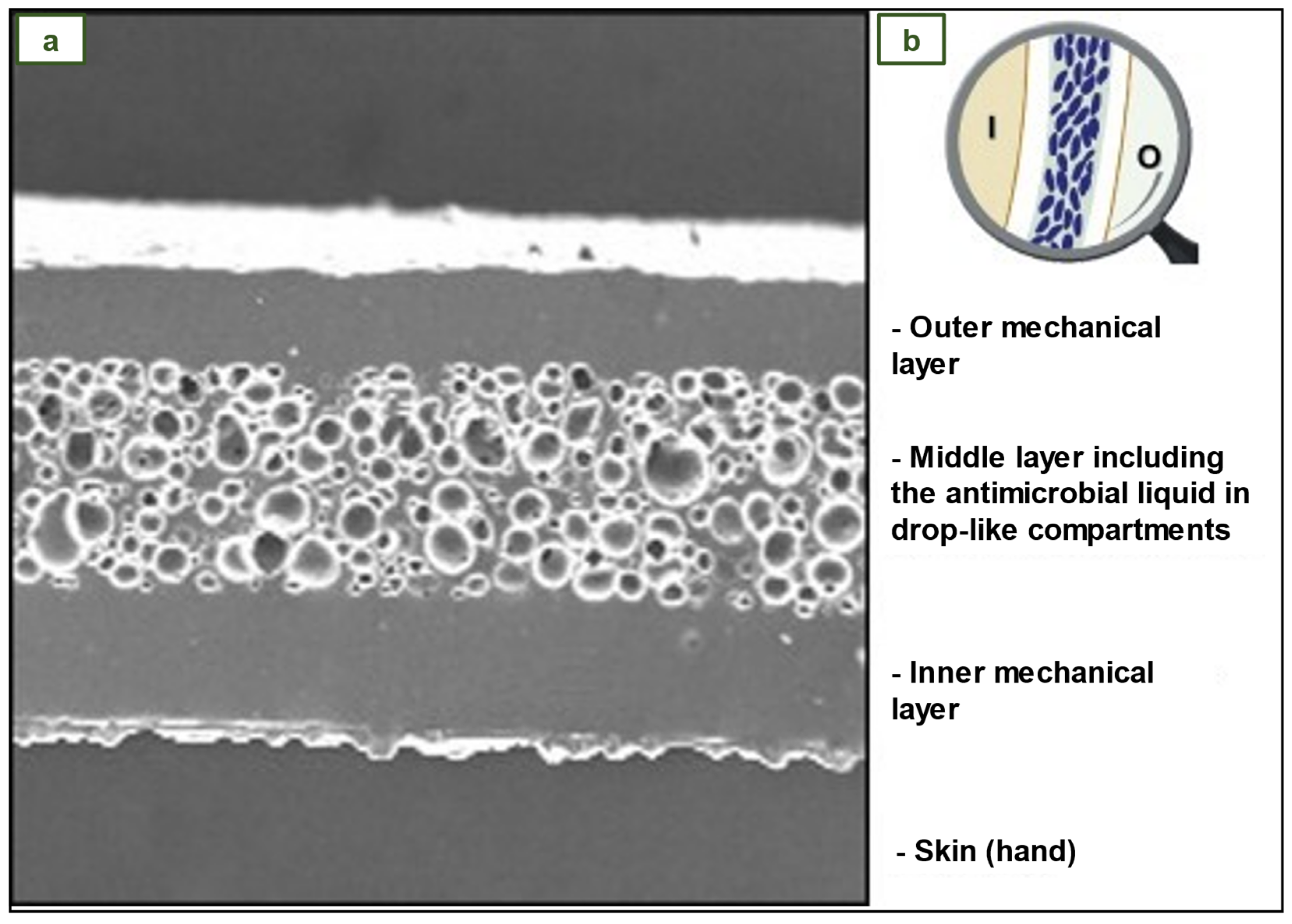
4.7. NR antimicrobial three-layer glove
4.8. NBR antimicrobial gloves coated by electrospun trimethylated chitosan (TMCh)-loaded (PVA) fibers
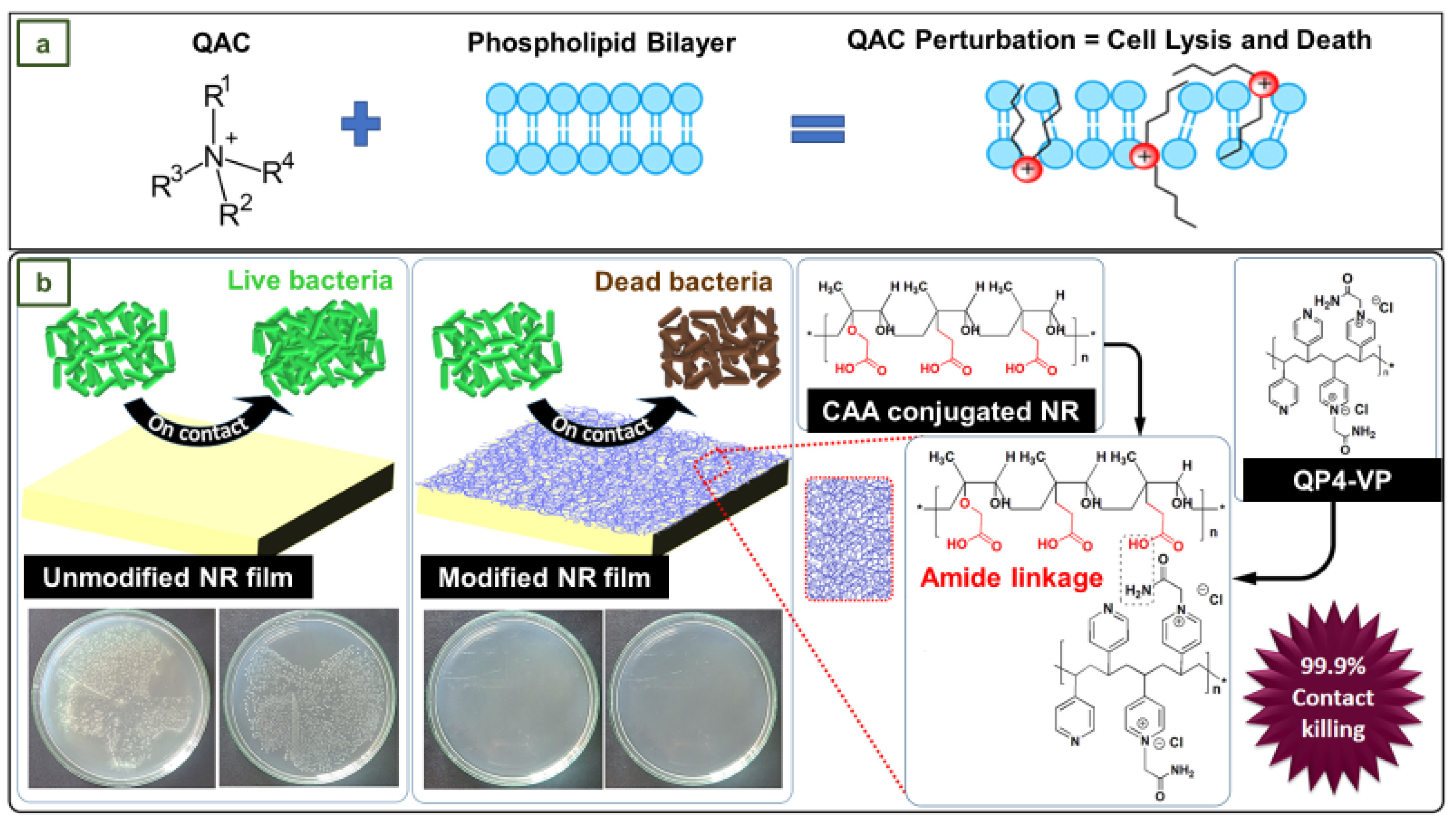
4.9. Antibacterial NR films with surface-anchored QP4-VP for application in medical gloves
4.10. NR, NBR and PE medical gloves with blood-repellent, antibacterial, and wound healing properties, modified by spraying process
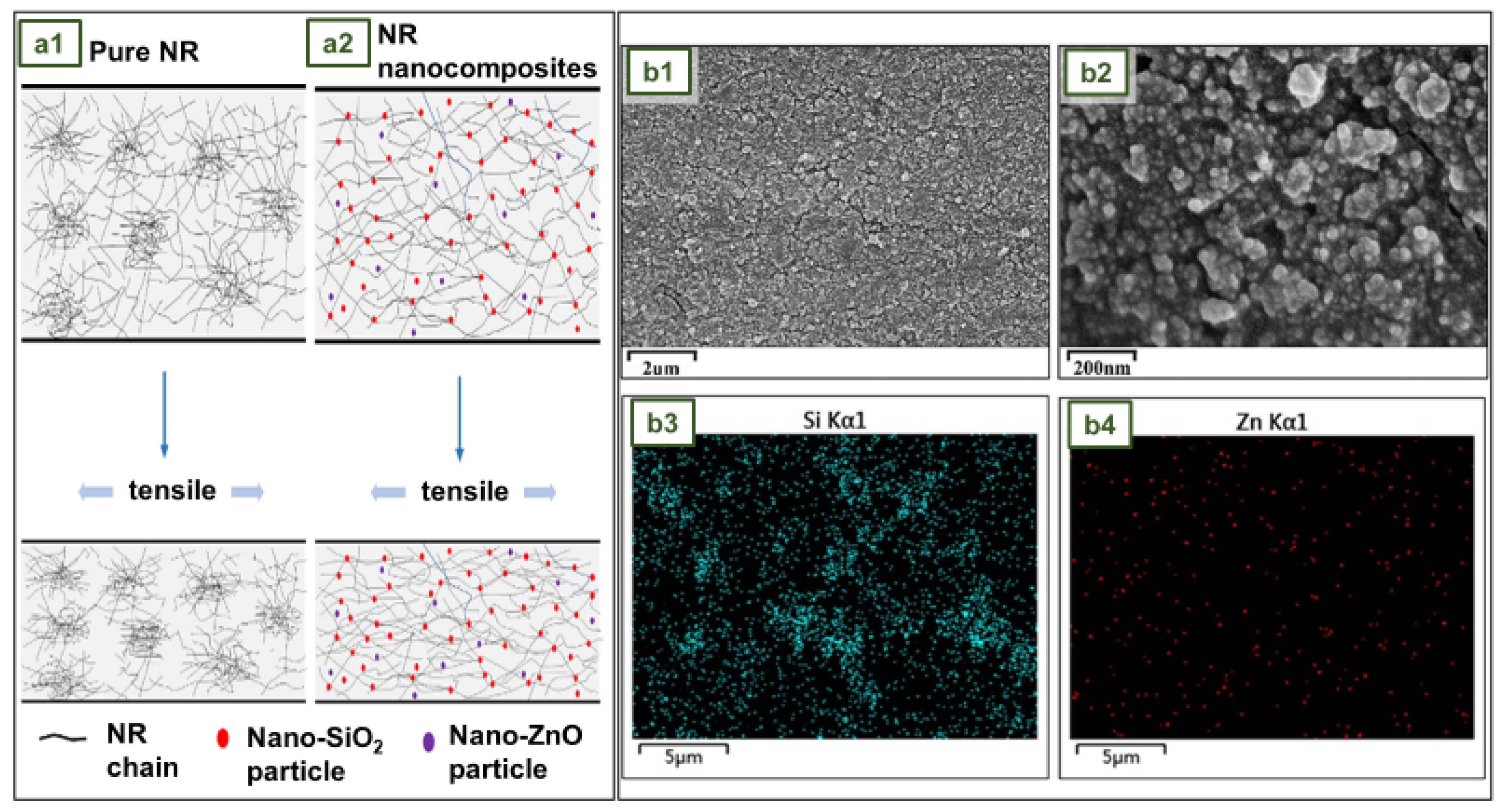
4.11. NR gloves with SiO2 and ZnO hybrid nanofillers
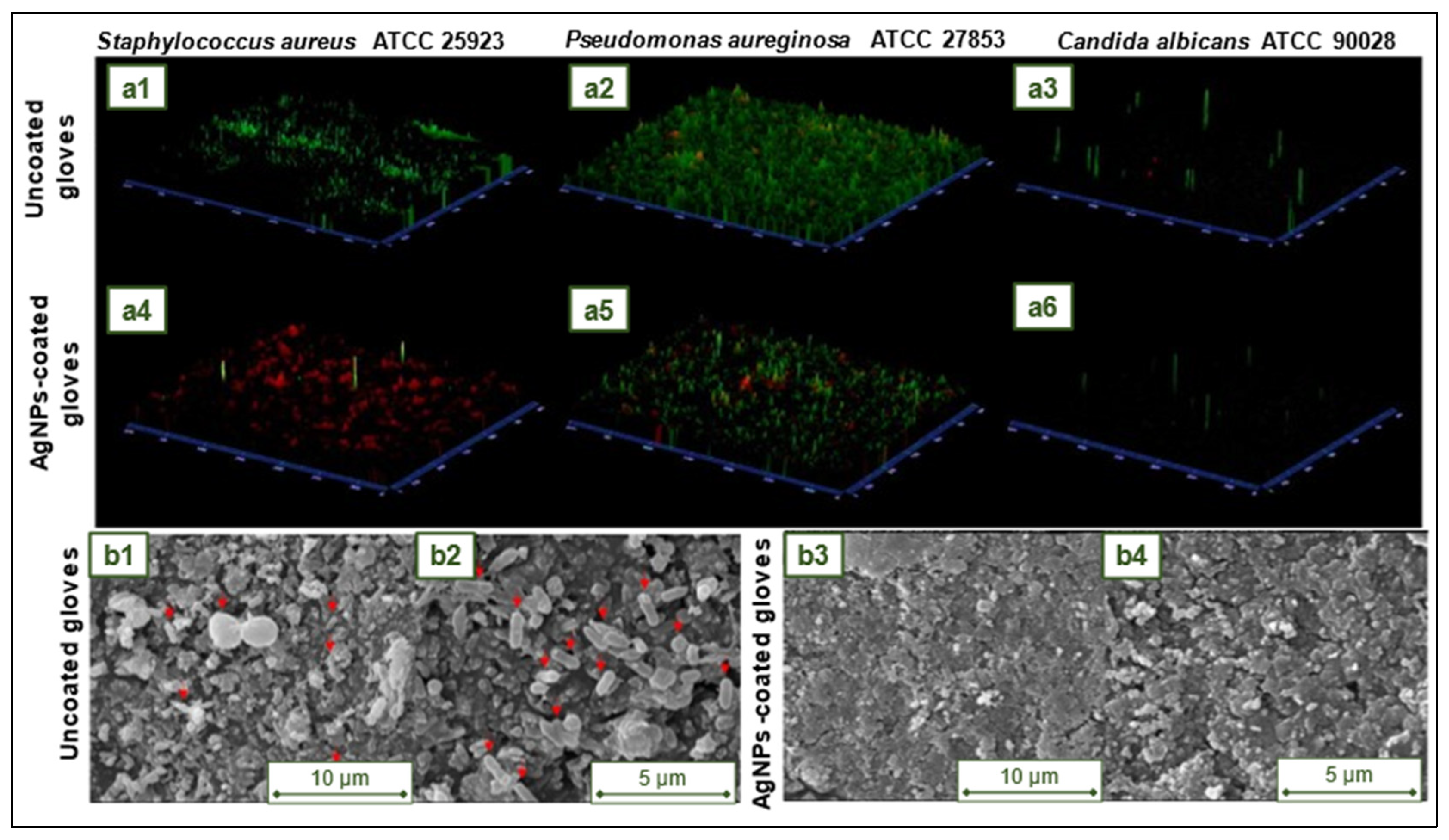
4.12. NR antimicrobial gloves impregnated with biosynthesized silver nanoparticles
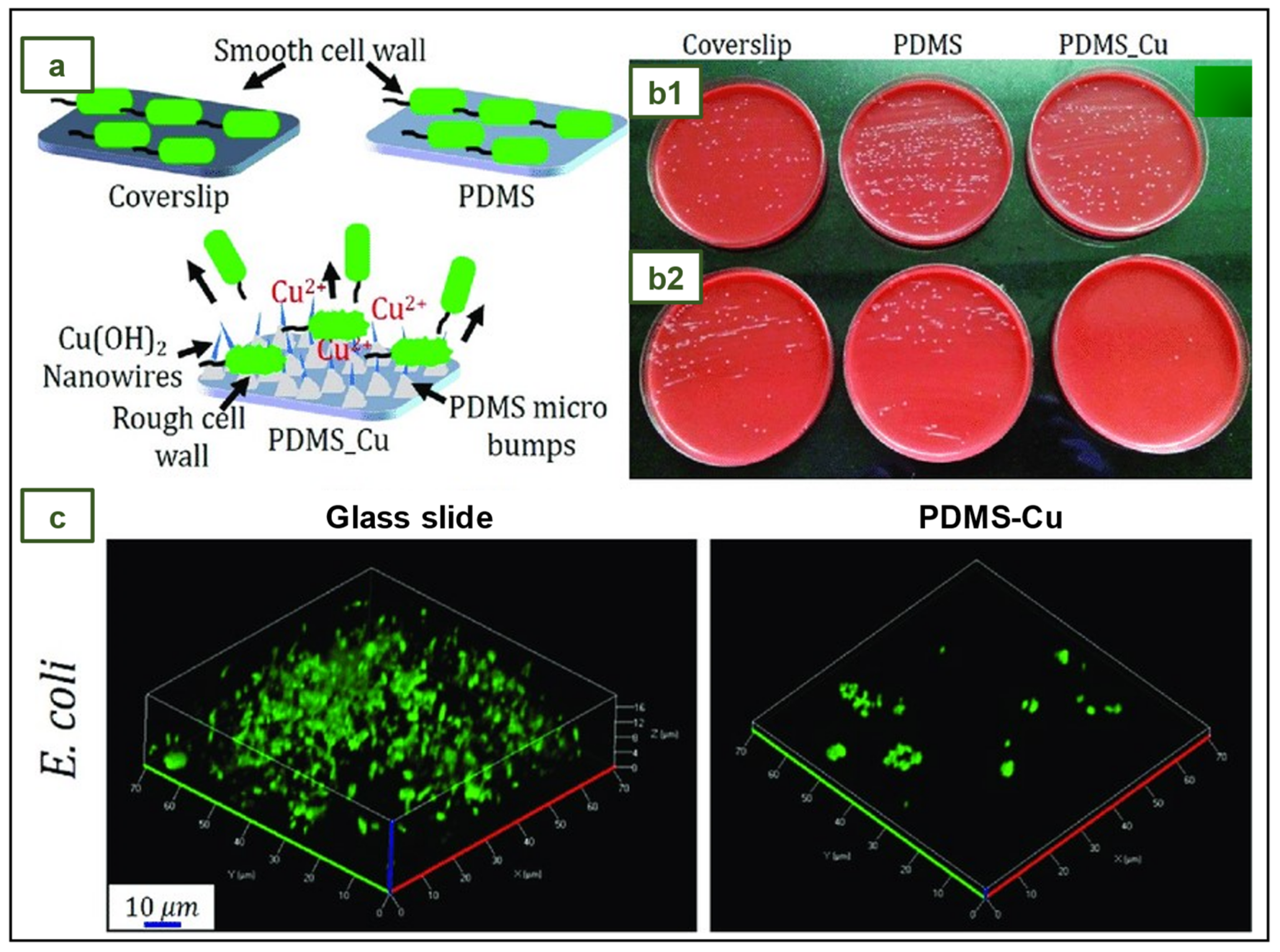
4.13. NR antimicrobial gloves with poly(dimethylsiloxane)- copper coating
5. Conclusions
Author Contributions
Funding
Acknowledges
Conflicts of Interest
References
- Mazón, L.; Orriols, R.M. Sanitary Gloves Management. Adequate Protection-Effectiveness and Environmental Responsibility. Revista de la Asociación Española de Especialistas en Medicina del Trabajo 2018, 27, 175–181. [Google Scholar]
- Delves, P.J.; Martin, S.J.; Burton, D.R.; Roitt, I.M. Roitt’s Essential Immunology; Thirteen edition.; John Wiley and Sons, 2017; ISBN 9781118415771.
- Ford, C.; Park, L.J. How to Apply and Remove Gloves. British Journal of Nursing 2019, 28, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Babadi, A.A.; Bagheri, S.; Hamid, S.B.A. Progress on Antimicrobial Surgical Gloves: A Review. Rubber Chemistry and Technology 2016, 89, 117–125. [Google Scholar] [CrossRef]
- Ellis, H. Surgical Gloves. J Perioper Pract 2010, 20, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H. Evolution of the Surgical Glove. J Am Coll Surg 2008, 207, 948–950. [Google Scholar] [CrossRef]
- Carraher, C.E. Introduction to Polymer Chemistry; CRC Press Taylor & Francis Group, Ed.; Fourth.; 2017; ISBN 978-1-4987-3761-6.
- Ikeda, Y. Understanding Network Control by Vulcanization for Sulfur Cross-Linked Natural Rubber (NR). Chemistry, Manufacture and Applications of Natural Rubber. [CrossRef]
- Boyd, D.A. Sulfur and Its Role In Modern Materials Science. Angewandte Chemie 2016, 128, 15486–15502. [Google Scholar] [CrossRef]
- Hill, D.M. The Science and Technology of Latex Dipping; First edition.; Smithers Rapra, 2018; ISBN 9781909030053.
- von Manteuffel, W.Z. Rubber Gloves in the Practice of Surgery. Centralblatt for Surgery 1897, 24, 553–556. [Google Scholar]
- Palma, S. How the World’s Largest Maker of Rubber Gloves Is Coping with Covid | Financial Times. Available online: https://www.ft.com/content/359047c2-89fb-11ea-a109-483c62d17528 (accessed on 10 October 2022).
- Kim Man, M.M. Glove Industry Spikes during Covid-19 Pandemic: A Case Study of Comfort Gloves Berhad (CGB). International Business Research 2021, 14, 105. [Google Scholar] [CrossRef]
- Ugalmugle, S.; Swain, R. Medical Gloves Market Size By Product, By Form, By Application, By Usage, By Sterility, By Distribution Channel, By End-Use, COVID19 Impact Analysis, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2021 – 2027. Available online: https://www.gminsights.com/industry-analysis/medical-gloves-market (accessed on 4 July 2022).
- Grand View Research Disposable Gloves Market Size Report, 2022-2030. Available online: https://www.grandviewresearch.com/industry-analysis/disposable-gloves-market (accessed on 9 March 2023).
- Eurostat EU Trade since 2015 of COVID-19 Medical Supplies by Categories. Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 8 June 2022).
- Mok, M.K.M. Glove Industry Spikes during Covid-19 Pandemic: A Case Study of Comfort Gloves Berhad (CGB). International Business Research 2021, 14, 105–114. [Google Scholar] [CrossRef]
- Tey, C. MARGMA Expects Global Glove Demand to Resume Growth next Year, after 19% Drop | The Edge Markets. Available online: https://www.theedgemarkets.com/article/margma-expects-global-glove-demand-resume-growth-next-year-after-19-drop (accessed on 7 October 2022).
- MarketWatch Disposable Gloves Market 2022 Recent Developments, Size, Share, Growth Strategies, Segment by Type, Region and Future Forecast 2028 - MarketWatch. Available online: https://www.marketwatch.com/press-release/disposable-gloves-market-2022-recent-developments-size-share-growth-strategies-segment-by-type-region-and-future-forecast-2028-2022-08-01 (accessed on 7 October 2022).
- Lim, J. Top Glove Makes the Highest Quarterly Profit among Malaysia’s Top 10 Companies | The Edge Markets. Available online: https://www.theedgemarkets.com/article/top-glove-makes-highest-quarterly-profit-among-malaysias-top-10-companies (accessed on 10 October 2022).
- Wong, E.L. Top Glove Posts Record Net Profit of RM348m in 3Q, Declares 10 Sen Dividend | The Edge Markets. Available online: https://www.theedgemarkets.com/article/top-glove-posts-record-3q-net-profit-rm348m-declares-10-sen-dividend (accessed on 10 October 2022).
- Syafiqah, S. Comfort Gloves Quarterly Net Profit Declines on Two Fronts | The Edge Markets. Available online: https://www.theedgemarkets.com/article/comfort-gloves-quarterly-net-profit-declines-two-fronts (accessed on 10 October 2022).
- Kuala Lumpur Stock Exchange (KLSE) COMFORT (2127): Quarterly Results for Last 10 Financial Years | I3investor. Available online: https://klse.i3investor.com/web/stock/financial-quarter/2127 (accessed on 10 October 2022).
- Sajeev, S.; Chandra, G. Disposable Gloves Market Size, Share, Trends & Industry Analysis 2023. Available online: https://www.alliedmarketresearch.com/disposable-gloves-market (accessed on 19 May 2020).
- Maximize Market Research Nitrile Gloves Market: Global Industry Analysis and Forecast (2022-2029). Available online: https://www.maximizemarketresearch.com/market-report/nitrile-gloves-market/126531/ (accessed on 7 October 2022).
- Akabane, T. Production Method & Market Trend of Rubber Gloves. International Polymer Science and Technology 2016, 43, 369–373. [Google Scholar] [CrossRef]
- Crépy, M.-N.; Hoerner, P. Gloves: Types, Materials, and Manufacturing. In Protective Gloves for Occupational Use; CRC Press: Boca Raton, 2022; pp. 17–44. ISBN 9781003126874. [Google Scholar]
- Yip, E.; Cacioli, P. The Manufacture of Gloves from Natural Rubber Latex. Journal of Allergy and Clinical Immunology 2002, 110, S3–S14. [Google Scholar] [CrossRef]
- Tyres, I.; Compounds, R.; Monomer, E.; Reinforcement, E. Rubber Technologist ’ s Handbook, Volume 1; 2001; Vol. 2; ISBN 1859572626.
- Mellström, G.A.; Boman, A. Gloves: Types, Materials, and Manufacturing. In Protective Gloves for Occupational Use; Wahlberg, J.E., Boman, A., Estlander, T., Maibach, H.I., Eds.; CRC Press, 2005; pp. 15–28 ISBN 9780367393854.
- Simpson, R.B. Rubber Basics; Rapra Technology Limited, 2002; ISBN 185957307X.
- Srinivasan, S. Powdered Gloves: Time to Bid Adieu. J Postgrad Med 2018, 64, 68. [Google Scholar] [CrossRef] [PubMed]
- Preece, D.; Hong Ng, T.; Tong, H.K.; Lewis, R.; Carré, M.J. The Effects of Chlorination, Thickness, and Moisture on Glove Donning Efficiency. Ergonomics 2021, 64, 1205–1216. [Google Scholar] [CrossRef]
- Daud, S.; You, Y.S.; Azura, A.R. The Effect of Acid Hydrolyzed Sago Starch on Mechanical Properties of Natural Rubber and Carboxylated Nitrile Butadiene Rubber Latex. Mater Today Proc 2019, 17, 1047–1055. [Google Scholar] [CrossRef]
- Rahman, M.F.A.; Rusli, A.; Adzami, N.S.; Azura, A.R. Studies on the Influence of Mixed Culture from Buried Soil Sample for Biodegradation of Sago Starch Filled Natural Rubber Latex Gloves. IOP Conf Ser Mater Sci Eng 2019, 548. [Google Scholar] [CrossRef]
- Misman, M.A.; Azura, A.R. Overview on the Potential of Biodegradable Natural Rubber Latex Gloves for Commercialization. Adv Mat Res 2013, 844, 486–489. [Google Scholar] [CrossRef]
- Jawjit, W.; Pavasant, P.; Kroeze, C. Evaluating Environmental Performance of Concentrated Latex Production in Thailand. J Clean Prod 2015, 98, 84–91. [Google Scholar] [CrossRef]
- Rattanapan, C.; Suksaroj, T.; Ounsaneha, W. Development of Eco-Efficiency Indicators for Rubber Glove Product by Material Flow Analysis. Procedia Soc Behav Sci 2012, 40, 99–106. [Google Scholar] [CrossRef]
- Yew, G.Y.; Tham, T.C.; Show, P.L.; Ho, Y.C.; Ong, S.K.; Law, C.L.; Song, C.; Chang, J.S. Unlocking the Secret of Bio-Additive Components in Rubber Compounding in Processing Quality Nitrile Glove. Applied Biochemistry and Biotechnology 2020 191:1 2020, 191, 1–28. [Google Scholar] [CrossRef]
- Kloepfer, A.; Jekel, M.; Reemtsma, T. Occurrence, Sources, and Fate of Benzothiazoles in Municipal Wastewater Treatment Plants. Environ Sci Technol 2005, 39, 3792–3798. [Google Scholar] [CrossRef]
- Lenko, D.; Schlögl, S.; Temel, A.; Schaller, R.; Holzner, A.; Kern, W. Dual Crosslinking of Carboxylated Nitrile Butadiene Rubber Latex Employing the Thiol-Ene Photoreaction. J Appl Polym Sci 2013, 129, 2735–2743. [Google Scholar] [CrossRef]
- Yew, G.Y.; Tham, T.C.; Law, C.L.; Chu, D.T.; Ogino, C.; Show, P.L. Emerging Crosslinking Techniques for Glove Manufacturers with Improved Nitrile Glove Properties and Reduced Allergic Risks. Mater Today Commun 2019, 19, 39–50. [Google Scholar] [CrossRef]
- Nik Yahya, N.Z.; Zulkepli, N.N.; Ismail, H.; Ting, S.S.; Abdullah, M.M.A.B.; Kamarudin, H.; Hamzah, R. Properties of Natural Rubber/Styrene Butadiene Rubber/Recycled Nitrile Glove (NR/SBR/RNBRg) Blends: The Effects of Recycled Nitrile Glove (RNBRg) Particle Sizes. Key Eng Mater 2016, 673, 151–160. [Google Scholar] [CrossRef]
- Linos, A.; Reichelt, R.; Keller, U.; Steinbüchel, A. A Gram-Negative Bacterium, Identified as Pseudomonas Aeruginosa AL98, Is a Potent Degrader of Natural Rubber and Synthetic Cis-1,4-Polyisoprene. FEMS Microbiol Lett 2000, 182, 155–161. [Google Scholar] [CrossRef]
- Rose, K.; Steinbüchel, A. Biodegradation of Natural Rubber and Related Compounds: Recent Insights into a Hardly Understood Catabolic Capability of Microorganisms. Appl Environ Microbiol 2005, 71, 2803–2812. [Google Scholar] [CrossRef]
- Chengalroyen, M.D.; Dabbs, E.R. The Biodegradation of Latex Rubber: A Minireview. J Polym Environ 2013, 21, 874–880. [Google Scholar] [CrossRef]
- Arenskötter, M.; Baumeister, D.; Berekaa, M.; Pötter, G.; Kroppenstedt, R.M.; Linos, A.; Steinbüchel, A. Taxonomic Characterization of Two Rubber Degrading Bacteria Belonging to the Species Gordonia Polyisoprenivorans and Analysis of Hyper Variable Regions of 16S RDNA Sequences. FEMS Microbiol Lett 2001, 205, 277–282. [Google Scholar] [CrossRef]
- Preece, D.; Lewis, R.; Carré, M.J. A Critical Review of the Assessment of Medical Gloves. Tribology - Materials, Surfaces and Interfaces 2021, 15, 10–19. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative Sources of Natural Rubber. Appl Microbiol Biotechnol 2000, 53, 355–365. [Google Scholar] [CrossRef]
- Cacioli, P. Introduction to Latex and the Rubber Industry. Rev Fr Allergol Immunol Clin 1997, 37, 1173–1176. [Google Scholar] [CrossRef]
- Das, D.; Nag, S.; Naskar, H.; Acharya, S.; Bakchi, S.; Ali, S.S.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R. Personal Protective Equipment for COVID-19: A Comprehensive Review. In Healthcare Informatics for Fighting COVID-19 and Future Epidemics. EAI/Springer Innovations in Communication and Computing; Garg, L., Chakraborty, C., Mahmoudi, S., Sohmen, V.S., Eds.; Springer Cham, 2022; pp. 141–154 ISBN 978-3-030-72751-2.
- Hänninen, A.R.; Mikkola, J.H.; Kalkkinen, N.; Turjanmaa, K.; Ylitalo, L.; Reunala, T.; Palosuo, T. Increased Allergen Production in Turnip (Brassica Rapa) by Treatments Activating Defense Mechanisms. Journal of Allergy and Clinical Immunology 1999, 104, 194–201. [Google Scholar] [CrossRef]
- Mercurio, J. Creating a Latex-Safe Perioperative Environment. OR Nurse 2011, 5, 18–25. [Google Scholar] [CrossRef]
- Charous, B.L.; Tarlo, S.M.; Charous, M.A.; Kelly, K. Natural Rubber Latex Allergy in the Occupational Setting. Methods 2002, 27, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, K.; Kelly, K.J. Latex Allergy Risks Live On. Journal of Allergy and Clinical Immunology: In Practice 2018, 6, 1877–1878. [Google Scholar] [CrossRef] [PubMed]
- McMillan, F.M. The Chain Straighteners; First.; The MacMillan Press Ltd., 1979; ISBN 978-1-349-04432-0.
- Zetune, K.; Dombrowski, R.; Day, J.; Wagner, N.J. Puncture And/Or Cut Resistant Glove Having Maximized Dexterity, Tactility, And Comfort 2013.
- Cuillo, P.A.; Hewitt, N. The Rubber Formulary; Noyes Publications/William Andrew Publishing, LLC, 1999; ISBN 0−8155−1434−4.
- Konrad, E.; Tschunkur, E. “`Rubber like Masses from Butadiene Hydrocarbons and Polymerizable Nitrils”’ U.S. Patent US1973000 1934, 0–2.
- SmartPractice Polychloroprene Gloves vs Nitrile Gloves: What You Need to Know » SmartPractice Blog. Available online: https://blog.smartpractice.com/polychloroprene-gloves-vs-nitrile-gloves-what-you-need-to-know/ (accessed on 13 March 2023).
- Wypych, G. Handbook of Polymers; Second.; 2016; ISBN 9781895198928.
- Sugiura, K.; Sugiura, M.; Shiraki, R.; Hayakawa, R.; Shamoto, M.; Sasaki, K.; Itoh, A. Contact Urticaria Due to Polyethylene Gloves. Contact Dermatitis 2002, 46, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Carroll, W.F.; Johnson, R.W.; Moore, S.S.; Paradis, R.A. Poly(Vinyl Chloride). In Applied Plastics Engineering Handbook: Processing, Materials, and Applications: Second Edition; William Andrew Publishing, 2017; pp. 73–89 ISBN 9780323390408.
- Tapia Fuentes, J.; Cruz Salas, A.; Álvarez Zeferino, C.; Martínez Salvador, C.; Pérez Aragón, B.; Vázquez-Morillas, A. Bioplastics in Personal Protective Equipment. Biodegradable Materials and Their Applications 2022, 173–210. [Google Scholar] [CrossRef]
- Gnaneswaran, V.; Mudhunuri, B.; Bishu, R.R. A Study of Latex and Vinyl Gloves: Performance versus Allergy Protection Properties. Int J Ind Ergon 2008, 38, 171–181. [Google Scholar] [CrossRef]
- Wallemacq, P.E.; Capron, A.; Vanbinst, R.; Boeckmans, E.; Gillard, J.; Favier, B. Permeability of 13 Different Gloves to 13 Cytotoxic Agents under Controlled Dynamic Conditions. American Journal of Health-System Pharmacy 2006, 63, 547–556. [Google Scholar] [CrossRef]
- Landeck, L.; Gonzalez, E.; Koch, O.M. Handling Chemotherapy Drugs - Do Medical Gloves Really Protect? Int J Cancer 2015, 137, 1800–1805. [Google Scholar] [CrossRef]
- ASTM International D3578 − 19 Standard Specification for Rubber Examination Gloves. 2019, 1–5. [CrossRef]
- ASTM International D6319 - 19 Standard Specification for Nitrile Examination Gloves for Medical Application. 2019, 1–4. [CrossRef]
- ASTM International D6977 - 19 Standard Specification for Polychloroprene Examination Gloves for Medical Application. 2019, 1–4. [CrossRef]
- European Committee for Standardization UNE - EN 455 - 2 Medical Gloves for Single Use - Part 2: Requirements and Testing for Physical Properties. 2015, 1–13.
- Kossan Rubber Industries Bhd Rubber Disposable Gloves. Available online: https://kossan.com.my/products/gloves/healthcare.html (accessed on 18 October 2022).
- Jayathilaka, I.; Ariyadasa, T.U.; Egodage, S.M. Powdered Corn Grain and Cornflour on Properties of Natural Rubber Latex Vulcanizates: Effect of Filler Loading. MERCon 2018 - 4th International Multidisciplinary Moratuwa Engineering Research Conference 2018, 235–240. [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin Microbiol Rev 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R. Electrospun Polymer Nanofibers with Antimicrobial Activities. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Muñoz-Bonilla, A., Cerrada, M.L., Fernández-García, M., Eds.; Royal Society of Chemistry, 2013; pp. 208–223.
- Riyajan, S.A.; Chaiponban, S.; Tanbumrung, K. Investigation of the Preparation and Physical Properties of a Novel Semi-Interpenetrating Polymer Network Based on Epoxised NR and PVA Using Maleic Acid as the Crosslinking Agent. Chemical Engineering Journal 2009, 153, 199–205. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wakisaka, N.; Maeda, O.; Yamamoto, T. Vitamin C Inhibits the Growth of a Bacterial Risk Factor for Gastric Carcinoma: Helicobacter Pylori. Cancer 2000, 80, 1897–1903. [Google Scholar] [CrossRef]
- Sánchez-Najera, R.I.; Nakagoshi-Cepeda, S.; Martínez-Sanmiguel, J.J.; Hernandez-Delgadillo, R.; Cabral-Romero, C. Ascorbic Acid On Oral Microbial Growth and Biofilm Formation. The Pharma Innovation Journal 2013, 2, 103–109. [Google Scholar]
- Vilchèze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R. Mycobacterium Tuberculosis Is Extraordinarily Sensitive to Killing by a Vitamin C-Induced Fenton Reaction. Nat Commun 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Thomas, N.; Mandal, A.; Mullick, A.; Chandra, D.; Mukherjee, S.; Sett, S.; Kumar Mitra, A. In Vitro Analysis of Antibacterial Activity of Vitamin C alone and in Combination with Antibiotics on Gram Positive Rod isolated from Soil of a Dumping Site of Kolkata. Int J Pharm Biol Sci 2013, 3, 101–110. [Google Scholar]
- El-Gebaly, E.; Essam, T.; Hashem, S.; El-Baky, R.A. Effect of Levofloxacin and Vitamin C on Bacterial Adherence and Preformed Biofilm on Urethral Catheter Surfaces. J Microb Biochem Technol 2012, 4, 131–136. [Google Scholar] [CrossRef]
- Verghese, R.; Mathew, S.; David, A. Antimicrobial Activity of Vitamin C Demonstrated on Uropathogenic Escherichia Coli and Klebsiella Pneumoniae. Journal of Current Research in Scientific Medicine 2017, 3, 88. [Google Scholar] [CrossRef]
- Riyajan, S. ad Biodegradable Green Glove Containing Ascorbic Acid from Maleated Epoxidized Natural Rubber/Poly(Vinyl Alcohol) Blend: Preparation and Physical Properties. J Polym Environ 2022, 30, 1141–1150. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int J Polym Sci 2015, 2015. [Google Scholar] [CrossRef]
- Thomas, S.K.; Parameswaranpillai, J.; Krishnasamy, S.; Begum, P.M.S.; Nandi, D.; Siengchin, S.; George, J.J.; Hameed, N.; Salim, Nisa. V.; Sienkiewicz, N. A Comprehensive Review on Cellulose, Chitin, and Starch as Fillers in Natural Rubber Biocomposites. Carbohydrate Polymer Technologies and Applications 2021, 2, 1–17. [Google Scholar] [CrossRef]
- Bode, H.B.; Kerkhoff, K.; Jendrossek, D. Bacterial Degradation of Natural and Synthetic Rubber. Biomacromolecules 2001, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Afiq, M.M.; Azura, A.R. Effect of Sago Starch Loadings on Soil Decomposition of Natural Rubber Latex (NRL) Composite Films Mechanical Properties. Int Biodeterior Biodegradation 2013, 85, 139–149. [Google Scholar] [CrossRef]
- Taghvaei-Ganjali, S.; Motiee, F.; Shakeri, E.; Abbasian, A. Effect of Amylose/Amylopectin Ratio on Physico-Mechanical Properties of Rubber Compounds Filled by Starch. Journal of Applied Chemical Researches 2010, 4, 53–60. [Google Scholar]
- Wei, B.; Xu, X.; Jin, Z.; Tian, Y. Surface Chemical Compositions and Dispersity of Starch Nanocrystals Formed by Sulfuric and Hydrochloric Acid Hydrolysis. PLoS One 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Abiola, C.; Oyetayo, V.O. Isolation and Biochemical Characterization of Microorganisms Associated with the Fermentation of Kersting’s Groundnut (Macrotyloma Geocarpum). Res J Microbiol 2016, 11, 47–55. [Google Scholar] [CrossRef]
- Riscoe, M.; Kelly, J.X.; Winter, R. Xanthones as Antimalarial Agents: Discovery, Mode of Action, and Optimization. Curr Med Chem 2005, 12, 2539–2549. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal Properties of Mangosteen (Garcinia Mangostana). Food and Chemical Toxicology 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Moopayak, W.; Tangboriboon, N. Mangosteen Peel and Seed as Antimicrobial and Drug Delivery in Rubber Products. J Appl Polym Sci 2020, 137, 1–14. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, D.; Fu, X.; Chen, Y. Green Method to Reinforce Natural Rubber with Tunicate Cellulose Nanocrystals via One-Pot Reaction. Cellulose 2018, 25, 4551–4563. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain Chem Eng 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Lee, H. V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Scientific World Journal 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in Advanced Functional Material Applications of Nanocellulose. Processes 2019, Vol. 7, Page 10 2018, 7, 10. [Google Scholar] [CrossRef]
- Parambath Kanoth, B.; Claudino, M.; Johansson, M.; Berglund, L.A.; Zhou, Q. Biocomposites from Natural Rubber: Synergistic Effects of Functionalized Cellulose Nanocrystals as Both Reinforcing and Cross-Linking Agents via Free-Radical Thiol-Ene Chemistry. ACS Appl Mater Interfaces 2015, 7, 16303–16310. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, R.; Ogunsona, E.O.; Hojabr, S.; Berry, R.; Mekonnen, T.H. Synergistic Cross-Linking and Reinforcing Enhancement of Rubber Latex with Cellulose Nanocrystals for Glove Applications. ACS Appl Polym Mater 2020, 2, 887–898. [Google Scholar] [CrossRef]
- Thakore, S. Nanosized Cellulose Derivatives as Green Reinforcing Agents at Higher Loadings in Natural Rubber. J Appl Polym Sci 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Reitzel, R.A.; Dvorak, T.L.; Hachem, R.Y.; Fang, X.; Jiang, Y.; Raad, I. Efficacy of Novel Antimicrobial Gloves Impregnated with Antiseptic Dyes in Preventing the Adherence of Multidrug-Resistant Nosocomial Pathogens. Am J Infect Control 2009, 37, 294–300. [Google Scholar] [CrossRef]
- Koburger, T.; Hübner, N.O.; Braun, M.; Siebert, J.; Kramer, A. Standardized Comparison of Antiseptic Efficacy of Triclosan, PVP-Iodine, Octenidine Dihydrochloride, Polyhexanide and Chlorhexidine Digluconate. Journal of Antimicrobial Chemotherapy 2010, 65, 1712–1719. [Google Scholar] [CrossRef]
- Peng, J.; Liu, P.; Peng, W.; Sun, J.; Dong, X.; Ma, Z.; Gan, D.; Liu, P.; Shen, J. Poly(Hexamethylene Biguanide) (PHMB) as High-Efficiency Antibacterial Coating for Titanium Substrates. J Hazard Mater 2021, 411, 1–13. [Google Scholar] [CrossRef]
- Sowlati-Hashjin, S.; Karttunen, M.; Carbone, P. Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. Journal of Physical Chemistry B 2020, 124, 4487–4497. [Google Scholar] [CrossRef]
- Leitgeb, J.; Schuster, R.; Eng, A.H.; Yee, B.N.; Teh, Y.P.; Dosch, V.; Assadian, O. In-Vitro Experimental Evaluation of Skin-to-Surface Recovery of Four Bacterial Species by Antibacterial and Non-Antibacterial Medical Examination Gloves. Antimicrob Resist Infect Control 2013, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Kramer, A.; Arnold, A.; Ladwig, A.; Seabrook, G.R.; Edmiston, C.E. Evaluation of an Innovative Antimicrobial Surgical Glove Technology to Reduce the Risk of Microbial Passage Following Intraoperative Perforation. Am J Infect Control 2011, 39, 98–103. [Google Scholar] [CrossRef]
- Vongsetskul, T.; Wongsomboon, P.; Sunintaboon, P.; Tantimavanich, S.; Tangboriboonrat, P. Antimicrobial Nitrile Gloves Coated by Electrospun Trimethylated Chitosan-Loaded Polyvinyl Alcohol Ultrafine Fibers. Polymer Bulletin 2015, 72, 2285–2296. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl Environ Microbiol 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int J Food Microbiol 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Schrank, C.L.; Minbiole, K.P.C.; Wuest, W.M. Are Quaternary Ammonium Compounds, the Workhorse Disinfectants, Effective against Severe Acute Respiratory Syndrome-Coronavirus-2? ACS Infect Dis 2020, 6, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Arakkal, A.; Rathinam, P.; Sirajunnisa, P.; Gopinathan, H.; Vengellur, A.; Bhat, S.G.; Sailaja, G.S. Antibacterial Natural Rubber Latex Films with Surface-Anchored Quaternary Poly(4-Vinylpyridine) Polyelectrolyte. React Funct Polym 2022, 172, 1–10. [Google Scholar] [CrossRef]
- Zhuo, Y.; Cheng, X.; Fang, H.; Zhang, Y.; Wang, B.; Jia, S.; Li, W.; Yang, X.; Zhang, Y.; Wang, X. Medical Gloves Modified by a One-Minute Spraying Process with Blood-Repellent, Antibacterial and Wound-Healing Abilities. Biomater Sci 2022, 10, 939–946. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Z.; Kong, L.X.; Huang, M.F.; Li, P.W. Natural Rubber Nanocomposite Reinforced with Nano Silica. Polym Eng Sci 2008, 48, 1674–1677. [Google Scholar] [CrossRef]
- Mou, W.; Li, J.; Fu, X.; Huang, C.; Chen, L.; Liu, Y. SiO2 and ZnO Hybrid Nanofillers Modified Natural Rubber Latex: Excellent Mechanical and Antibacterial Properties. Polym Eng Sci 2022. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO Nanofluids: Green Synthesis, Characterization, and Antibacterial Activity. Mater Chem Phys 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Leung, Y.H.; Chan, C.M.N.; Ng, A.M.C.; Chan, H.T.; Chiang, M.W.L.; Djurišić, A.B.; Ng, Y.H.; Jim, W.Y.; Guo, M.Y.; Leung, F.C.C.; et al. Antibacterial Activity of ZnO Nanoparticles with a Modified Surface under Ambient Illumination. Nanotechnology 2012, 23, 475703. [Google Scholar] [CrossRef]
- Turner, R.J. Is Silver the Ultimate Antimicrobial Bullet? Antibiotics 2018, 7, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Salayová, A. Green-Synthesized Silver Nanoparticles and Their Potential for Antibacterial Applications. Bacterial Pathogenesis and Antibacterial Control 2018, 73–94. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A. De; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 1–27. [Google Scholar] [CrossRef]
- Paosen, S.; Lethongkam, S.; Wunnoo, S.; Lehman, N.; Kalkornsurapranee, E.; Septama, A.W.; Voravuthikunchai, S.P. Prevention of Nosocomial Transmission and Biofilm Formation on Novel Biocompatible Antimicrobial Gloves Impregnated with Biosynthesized Silver Nanoparticles Synthesized Using Eucalyptus Citriodora Leaf Extract. Biotechnol J 2021, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Airey, P.; Verran, J. Potential Use of Copper as a Hygienic Surface; Problems Associated with Cumulative Soiling and Cleaning. Journal of Hospital Infection 2007, 67, 271–277. [Google Scholar] [CrossRef]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S.J. Role of Copper in Reducing Hospital Environment. Journal of Hospital Infection 2010, 74, 72–77. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential Use of Copper Surfaces to Reduce Survival of Epidemic Meticillin-Resistant Staphylococcus Aureus in the Healthcare Environment. Journal of Hospital Infection 2006, 63, 289–297. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin Microbiol Rev 2019, 32, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Kumar, A.; Chowdhury, A.R.; Karmakar, K.; Purighalla, S.; Sambandamurthy, V.; Chakravortty, D.; Sen, P. A Nanowire-Based Flexible Antibacterial Surface Reduces the Viability of Drug-Resistant Nosocomial Pathogens. ACS Appl Nano Mater 2018, 1, 2678–2688. [Google Scholar] [CrossRef]


| Function | Description |
|---|---|
| pH increasing | Generally, KOH is added to latex to raise its pH to 10-11. |
| Surfactants | Suspensions of chemicals in water can be made more stable with the help of ionic and non-ionic additives. |
| Rosin resins | Some synthetic latexes, such as CR and IR, are formulated with colophonium resins, which effectively perform the functions of particle stability and film forming. |
| Material | Advantages | Disadvantages |
|---|---|---|
| NR | Good resistance to alkali and acids. Comfortable, good fitting and feeling to hands. High elasticity and ability to adapt to shapes. High tear strength. Waterproof. |
Permeable to several solvents. Poor resistance to chemicals. Possible allergies due to residual protein. |
| IR | Absence of allergy associated to proteins in NR gloves. Good elasticity and break resistance. |
It is costly. |
| NBR | Good alternative for people that have allergic to NR gloves. Resistance to various chemicals, especially oils, fuels, weak acids, caustics, and some organic solvents. Eligible to handle most food materials. Good resistance to mechanical stress. |
It has limited sensitivity, which may limit how comfortable the hands conform to, and work with, the gloves. Poor resistance to alcohols, amines, ketones, ester, ethers, concentrated acids, halogenated hydrocarbons, aromatic hydrocarbons. |
| CR | Resistant to temperature and harsh chemicals. Mechanical and flammability resistance are superior to NBR gloves. CR gloves fit and feel like NR gloves. Appropriate for NR-allergic people. |
It is costly. |
| PE | Can be used for food material. Inexpensive option. |
Poor resistance and barrier protection. |
| PVC | It is cost effective, since PVC is inexpensive. Good for those suffering from skin and chemical allergy as it is skin-friendly. |
Due to plasticiser, not adequate to handle fatty food since there is possibility of migration of the plasticiser into the food. Less stretch, comfort, and elongation than NR. Poor resistance to chemical degradation. High permeability to chemotherapy drugs. |
| Property of examination gloves | ASTM D3578 – 19 (NR) [68] | ASTM D6319 – 19 (NBR) [69] | ASTM D6977-19 (CR) [70] | |||||
|---|---|---|---|---|---|---|---|---|
| Before Aging | After Aging | Before Aging | After Aging | Before Aging | After Aging | |||
| Type I | Type II | Type I | Type II | |||||
| Minimum Tensile Strength (MPa) | 18 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Maximum Stress at 500 % elongation (MPa) | 5.5 | 2.8 | - | - | - | - | - | - |
|
Minimum Ultimate Elongation (%) |
650 | 650 | 500 | 500 | 500 | 400 | 500 | 400 |
| Property | Latex examination glove PS60Y | Nitrile examination glove CS30 | ||
|---|---|---|---|---|
| Unaged | Aged | Unaged | Aged | |
| Tensile Strength (MPa) | 20 – 24 | 16 – 20 | 28 – 32 | 29 – 33 |
|
Ultimate Elongation (%) |
700 – 740 | 600 – 640 | 500 – 540 | 460 - 500 |
| Force at break (N) | 7.0 - 7.5 | 7.0 - 7.5 | 6.0 - 6.3 | 6.0 - 6.3 |
| Microorganisms tested | Uo (cfu/cm2) | Ut (cfu/cm2) | At (cfu/cm2) | R | Antibacterial rate (%) |
|---|---|---|---|---|---|
| E. coli | 2,1 x 104 | 2,9 x 105 | <0,6 | >5,3 | >99,9 |
| S. aureus | 2,1 x 104 | 2,3 x 105 | <0,6 | >5,2 | >99,9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





