Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sepiolite Modification with Zirconium(IV) Propoxide
2.3. Characterization of the Samples
2.4. Adsorption Experiments
2.5. Desorption
3. Results and Discussion
3.1. Characterization of the Samples
3.2. Adsorption
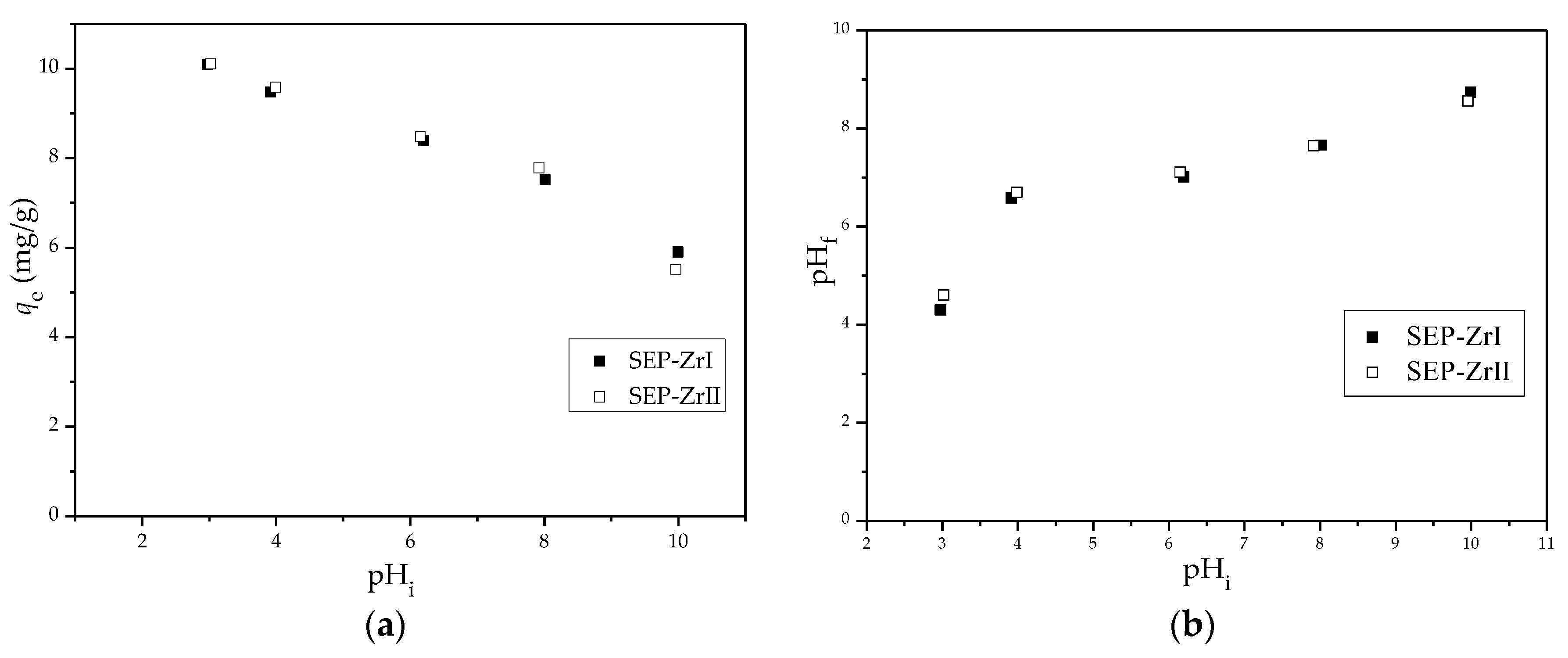
3.2.1. Effect of Solution pH
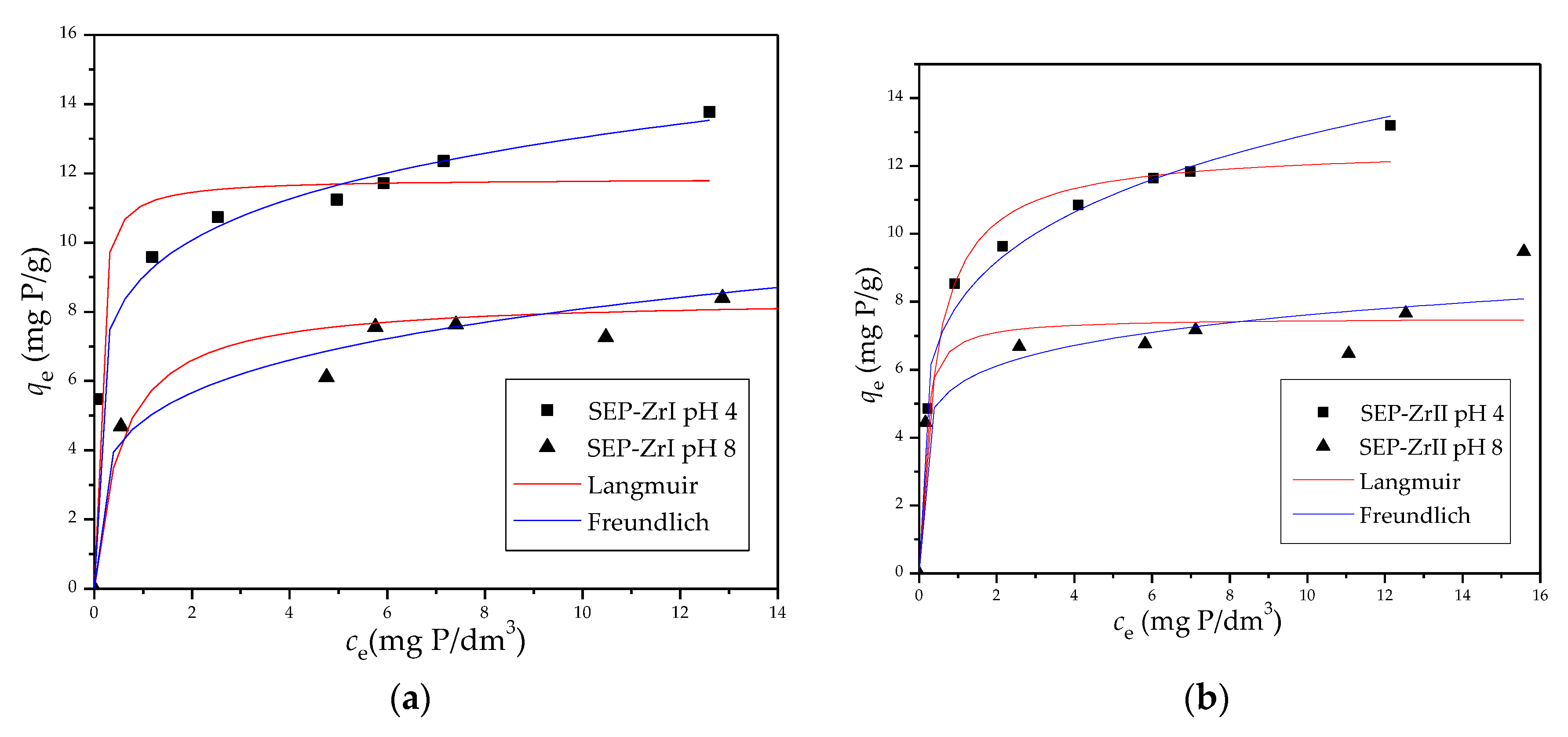
3.2.2. Adsorption Isotherms
| Adsorbent | Capacity | pH | Reference |
|---|---|---|---|
| Zirconia-functionalized graphite oxide | 16.45 mg PO43-/g | 6 | 27 |
| ZrO2/Fe3O4 composite | 59.9 mg PO43-/g | 4 | 46 |
| Amorphous-ZrO2 | 99.01 mg PO43-/g | 6.2 | 11 |
| Zirconium-modified bentonite | 8.90 mg PO43-/g | 7 | 17 |
| Zirconium (IV) loaded cross-linked chitosan particles | 71.68 mg PO43-/g | 3 | 21 |
| La-Zr modified magnetite | 49.1 mg PO43-/g | 2 | 47 |
| Magnetic zirconium-based metal–organic frameworks | 12.82 mg P/g | 6.5 | 1 |
| Zirconium-modified zeolite | 10.2 mg P/g | 7 | 14 |
| Zirconium(IV) loaded lignocellulosic butanol residue | 8.75 mg P/g | 6 | 41 |
| Sep-Zr I | 13.5 mg P/g (41.4 mg PO43-/g) | 4 | This study |
| Sep-Zr I | 9.8 mg P/g (30.0 mg PO43-/g) | 8 | This study |
| Sep-Zr II | 13.2 mg P/g (40.45 mg PO43-/g) | 4 | This study |
| Sep-Zr II | 9.4 mg P/g (28.8 mg PO43-/g) | 8 | This study |
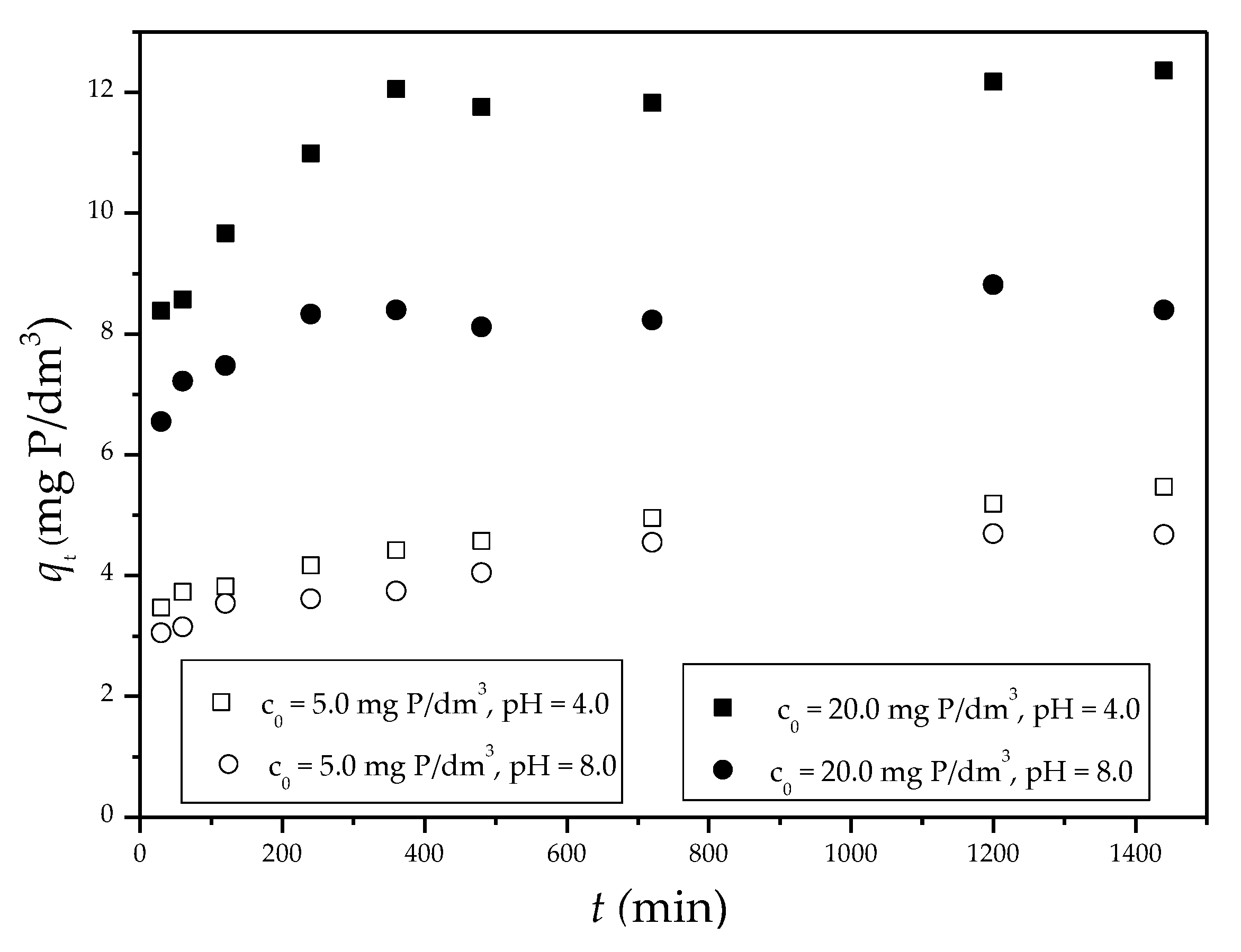
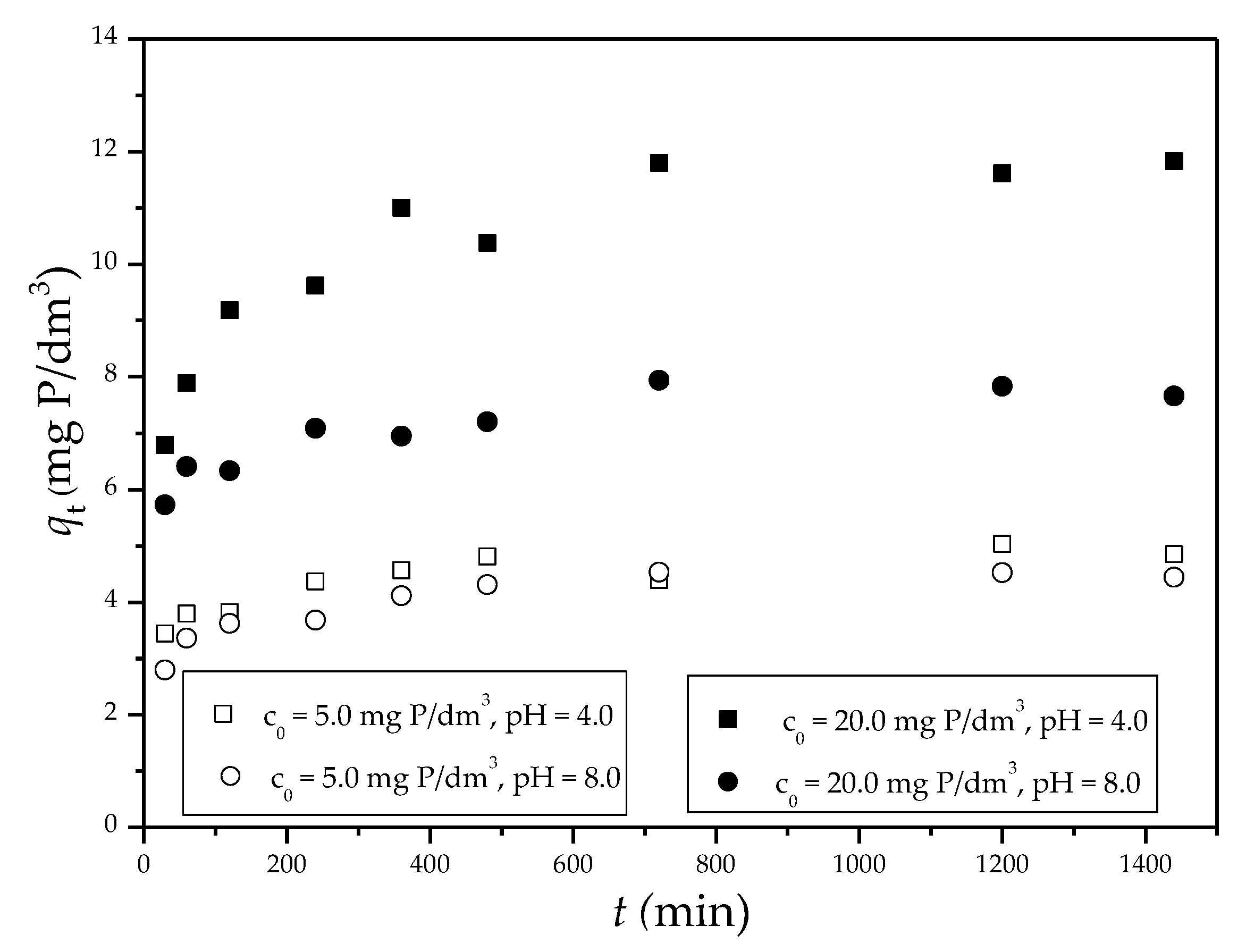
3.2.3. Phosphate Adsorption Kinetics
3.2.4. ATR-FTIR Study
3.2.5. XPS Analysis
3.2.6. Desorption
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Zheng, S.; Yang, L. Magnetic zirconium-based metal–organic frameworks for selective phosphate adsorption from water, J. Colloid Interf. Sci. 2019, 552, 134–141. [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)2 treated natural clinoptilolite, Chem. Eng. J. 2017, 320, 510–522. [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism, J. Colloid Interf. Sci. 2017, 493, 17–23. [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash, J. Colloid Interf. Sci. 2018, 513, 72–81. [CrossRef]
- Han, C.; Lalley, J.; Iyanna, N.; Nadagouda, M.N. Removal of phosphate using calcium and magnesium-modified iron based adsorbents, Mater. Chem. Phys. 2017, 198, 115-124. [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated Magnetite, J. Colloid Interf. Sci. 2018, 530, 704–713. [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites, Water Res. 2017, 126, 179-188.
- Xu, Y.; Hong, H.; Yang, F.; Zhang, L.; Xu, J.; Dou, L.; Hao, Y.; Qian, G.; Zhou, J. Removal behaviors and mechanisms of orthophosphate and pyrophosphate by calcined dolomite with ferric chloride assistance, Chemosphere 2019, 235, 1015-1021. [CrossRef]
- Ya, W.; Zhao, H.D.L.; Liud, Q.; Tao, Q.; Zhua, Y.; Yanga, J.; Zhang, Y.M. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents, Chem. Eng. J. 2014, 236, 191-201.
- Liua, R.; Chia, L.; Wanga, X.; Suia, Y.; Wang, Y.; Arandiyan, H. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water, J. Environ. Chem. Eng. 2018, 6, 5269–5286.
- Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles, Water Res. 2013, 47, 5018-5026. [CrossRef]
- Liu, H.; Sun, X.; Yin, C.; Hu, C. Removal of phosphate by mesoporous ZrO2, J. Hazard. Mater. 2008, 151, 616-622. [CrossRef]
- Tao, Y.; Liu, S.; Dong, S.; Wang, C.; Qu, T.; Li, S.; Lib, L.; Ma, Z. An in situ grown amorphous ZrO2 layer on zeolite for enhanced phosphate adsorption, RSC Adv. 2022, 12, 16751–16762. [CrossRef]
- Yanga, M.; Lina, J.; Zhana, Y.; Zhang, H. Adsorption of phosphate from water on lake sediments amended with zirconium-modified zeolites in batch mode, Ecol. Eng. 2014, 71, 223–233.
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhou, J.L.; Wang, J.; Liang, H.; Li, G. Phosphorus elimination from aqueous solution using ‘zirconium loaded okara’ as a biosorbent. Bioresource Technol. 2014, 170, 30–37.
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Pham, T.Q.; Li, F.M.; Nguyen, T.V.; Bui, X.T. Adsorption of phosphate from aqueous solutions and sewage using zirconium loaded okara (ZLO): fixed-bed column study, Sci. Total Environ. 2015, 523, 40–49. [CrossRef]
- Lin, J.; Jiang, B.; Zhan, Y. Effect of pre-treatment of bentonite with sodium and calcium ions on phosphate adsorption onto zirconium-modified bentonite, J. Environ. Manage. 2018, 217, 183-195. [CrossRef]
- Tang, Y.; Zong, E.; Wan, H.; Xu, Z.; Zheng, S.; Zhu, D. Zirconia functionalized SBA-15 as effective adsorbent for phosphate removal, Micropor. Mesopor. Mat. 2012, 155, 192–200. [CrossRef]
- Padungthon, S.; German, M.; Wiriyathamcharoen, S.; SenGupta, A.K. Polymeric anion exchanger supported hydrated Zr(IV) oxide nanoparticles: A reusable hybrid sorbent for selective trace arsenic removal, React. Funct.Polym. 2015, 93, 84-94. [CrossRef]
- Chena, L.; Zhaoa, X.; Pana, B.; Zhang, W.; Huaa, M.; Lv, L.; Zhanga, W. Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability, J. Hazard Mater. 2015, 284, 35–42.
- Liu, Q.; Hu, P.; Wang, J.; Zhang, L.; Huang, R. Phosphate adsorption from aqueous solutions by Zirconium (IV) loaded cross-linked chitosan particles, J. Taiwan. Inst. Chem. E. 2015, 000, 1–9. [CrossRef]
- Marjanović, V.; Lazarević, S.; Janković-Častvan, I.; Jokić, B.; Janaćković, Đ.; Petrović, R., Adsorption of chromium (VI) from aqueous solutions onto amine- functionalized natural and acid-activated sepiolites, Appl. Clay. Sci. 2013, 80-81, 202-210.
- Zhang, Z.; Liu, T.; Wu, D. Facile synthesis of hydrous zirconia-impregnated chitosan beads as a filter medium for efficient removal of phosphate from water, Cellulose 2022, 29, 8749–8768. [CrossRef]
- Zhan, Y.; Zhang, H.; Lin, J.; Zhang, Z.; Gao, J. Role of zeolite's exchangeable cations in phosphate adsorption onto zirconium-modified zeolite, J. Mol. Liq. 2017, 243, 624–637.
- Shao, Y.; Li, J.; Fang, X.; Yang, Z.; Qu, Y.; Yang, M.; Tan, W.; Li, G.; Wang, H. Chemical modification of bamboo activated carbon surface and its adsorption property of simultaneous removal of phosphate and nitrate, Chemosphere 2022, 287, 132118. [CrossRef]
- Shana, S.; Tanga, H.; Zhaoa, Y.; Wanga, W.; Cuia, F. Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal, Chem. Eng. J. 2019, 359, 779–789.
- Zong, E.; Wei, D.; Wan, H.; Zheng, S.; Xu, Z.; Zhu, D. Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide, Chem. Eng. J. 2013, 221, 193-203. [CrossRef]
- Marjanović, V.; Lazarević, S.; Janković-Častvan, I.; Potkonjak, B.; Janaćković, Đ.; Petrović, R. Chromium (VI) removal from aqueous solutions using mercaptosilane functionalized sepiolites, Chem. Eng. J. 2011, 166, 198-206.
- Lazarević, S.; Janković-Častvan, I.; Jovanović, D.; Milonjić, S.; Janaćković, Đ.; Petrović, R. Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural andacid-activated sepiolites, Appl. Clay. Sci. 2007, 37, 47–57.
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids, Academic Press, 1975.
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms, J. Am. Chem. Soc. 1951, 73, 373-380. [CrossRef]
- Lippens, B.C.; De, Boer J.H. Studies on pore systems in catalysts V. The t method, J. Catal., 1965, 4, 319-323.
- Waseem, M.; Mustafa, S.; Naeem, A.; Koper, G.J.M.; Shah, K.H. Cd2+sorption characteristics of iron coated silica, Desalinatio, 2011, 277, 221-226. [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc. 1918, 40, 1361–1403. [CrossRef]
- Freundlich, H. Concerning adsorption in solutions, Zeitschriftfür Physikalische Chemie,1906, 57, 385–470.
- Lagergren, S. About the theory of so-called adsorption of soluble substances, K. Sven.Vetenskapsakad.Handl.1989 , 24, 1–39.
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes, Process Biochem. 1999, 34, 451–465. [CrossRef]
- Weber, Jr. W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution, J. Sanit. Eng. Div. ASCE 1963, 89, 31–59.
- Stankovic, J.B.; Milonjic, S.K.; Zec, S.P. The influence of chemical and thermal treatment on the point of zero charge of hydrous zirconium oxide. J. Serb. Chem.Soc. 2013, 78, 987–995. [CrossRef]
- Song, L.; Li, J.; Zhou, H.; Lin, Y.; Ding, H.; Huang, Y.; Zhang, P.; Lai, X.; Liu, G.; Fan, Y. Zirconia nano-powders with controllable polymorphs synthesized by a wet chemical method and their phosphate adsorption characteristics & mechanism, Ceram Int. 2022, 48, 6591–6599. [CrossRef]
- Zong, E.; Liu, X.; Jiang, J.; Fu, S.; Chu, F. Preparation and characterization of zirconia-loaded lingo cellulosic butanol residue as a biosorbent for phosphate removal from aqueous solution, Appl. Surf. Sci. 2016, 387, 419–430.
- Lin, J.; Zhang, Z.; Zhan, Y. Effect of humic acid preloading on phosphate adsorption onto zirconium-modified zeolite, Environ. Sci. Pollut. Res. 2017, 24, 12195–12211. [CrossRef]
- Kosmulski, M. The Significance of the Points of Zero Charge of Zirconium (Hydr)Oxide Reported in the Literature, J. Dispersion Sci. Technol. 2002, 23, 529-538. [CrossRef]
- Pettersson, A.; Marino, G.; Pursiheimo, A.; Rosenholm, J.B. Electrosteric Stabilization of Al2O3, ZrO2, and 3-Y-ZrO2 Suspensions: Effect of Dissociation and Type of Polyelectrolyte, J. Colloid Interf. Sci. 2000, 228, 73-81.
- Lin, J.; He, S.; Wang, X.; Zhang, H.; Zhana, Y. Removal of phosphate from aqueous solution by a novel Mg(OH)2/ZrO2 composite: Adsorption behavior and mechanism, Colloids and Surfaces 2019 , 561, 301–314.
- Lee, W.H.; Kim, J.O. Mechanisms and novel performance of ZrO2/Fe3O4 composite for.
- phosphate recovery from wastewater, Chem. Eng. J. 2023, 453, 139817.
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery, Chem. Eng. J. 2021, 413, 127530. [CrossRef]
- Zhou, K.; Wua, B.; Su, L.; Xin, W.; Chai, X. Enhanced phosphate removal using nanostructured hydrated ferric zirconium binary oxide confined in a polymeric anion exchanger, Chem. Eng. J. 2018, 345, 640-647. [CrossRef]
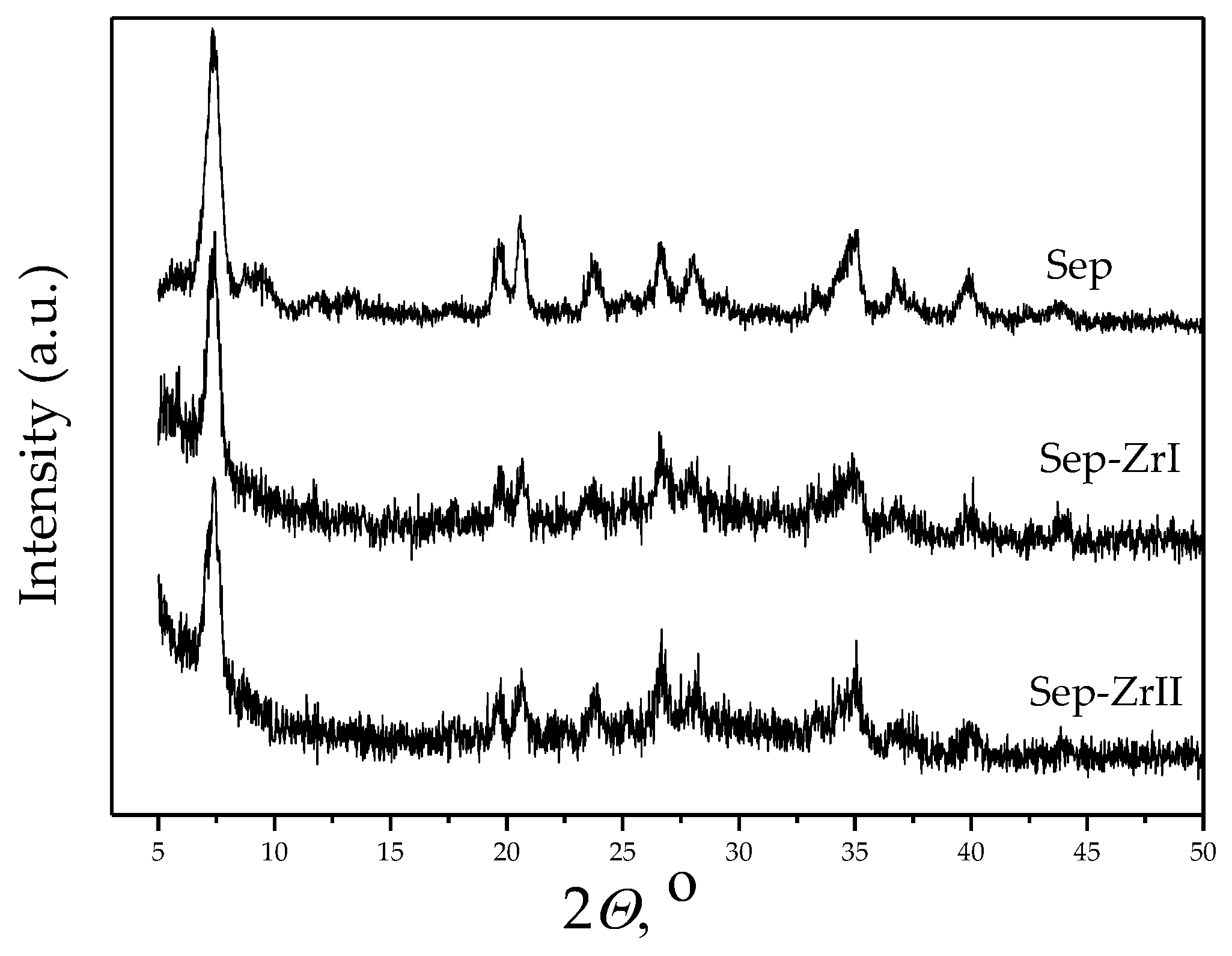
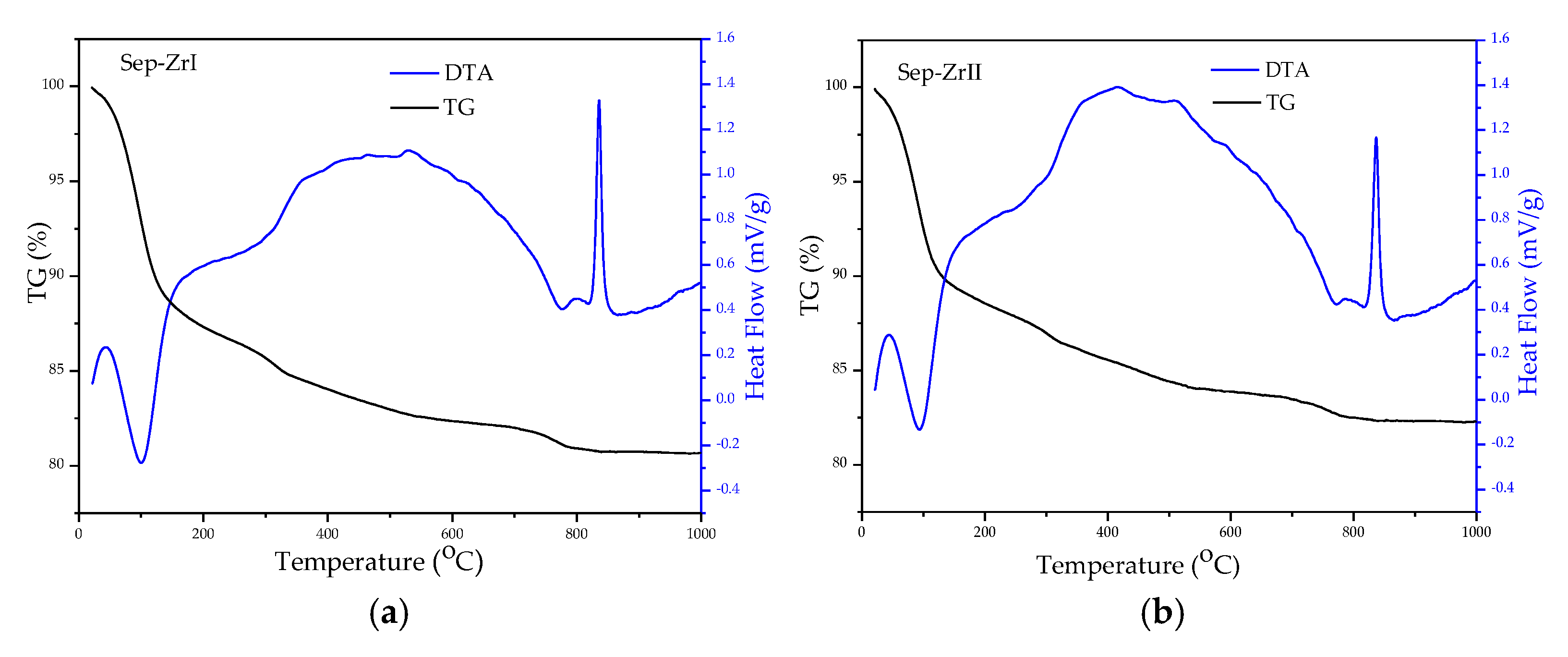
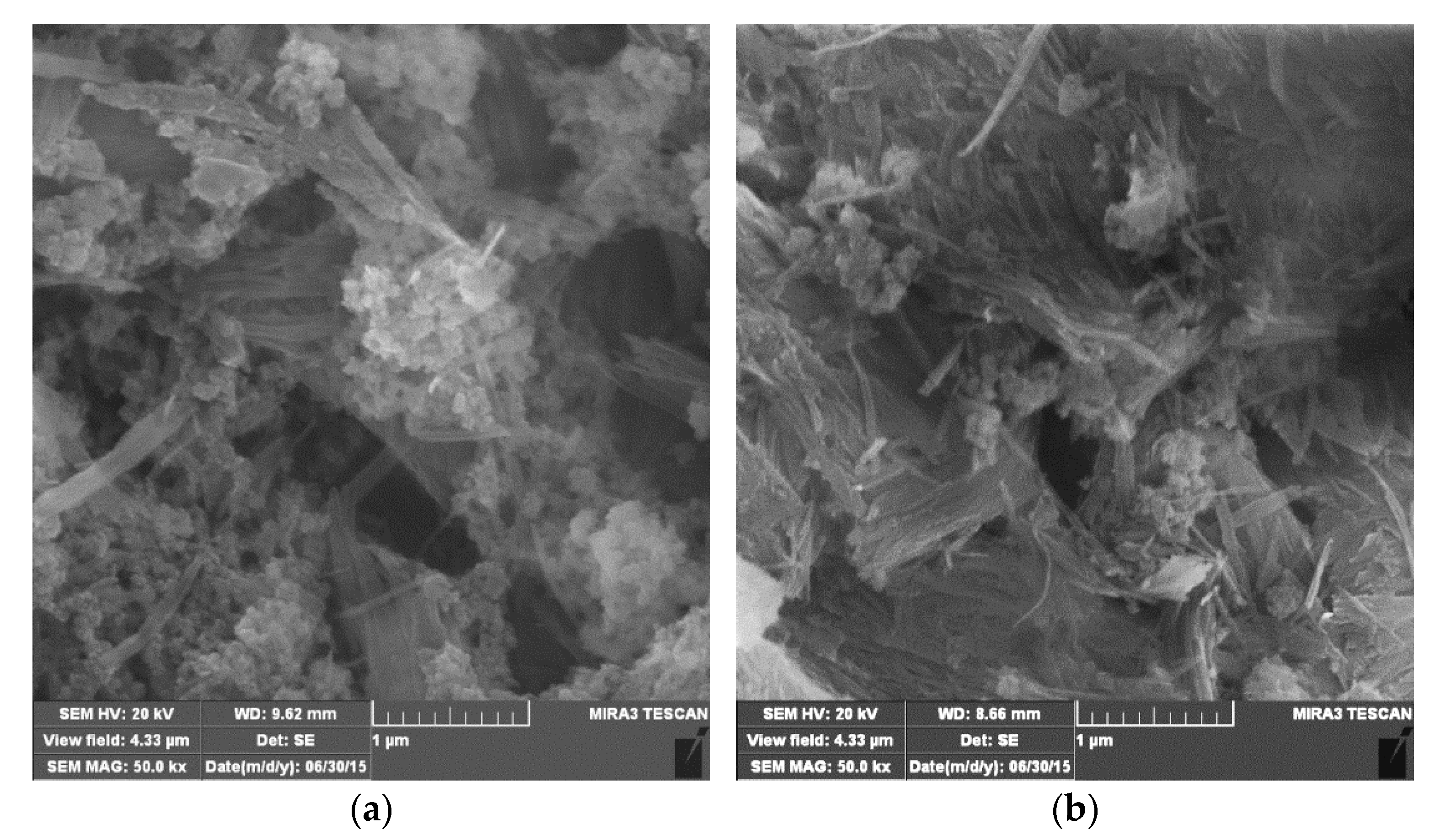
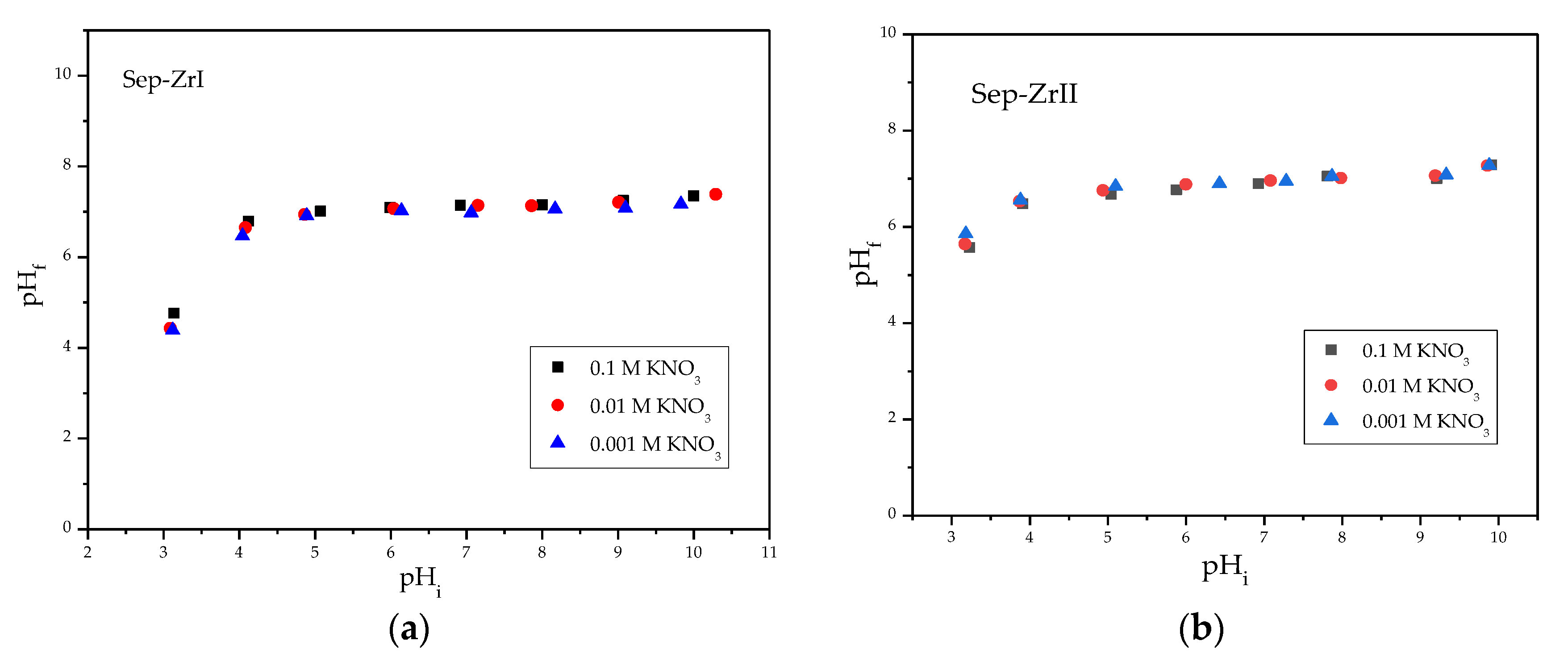
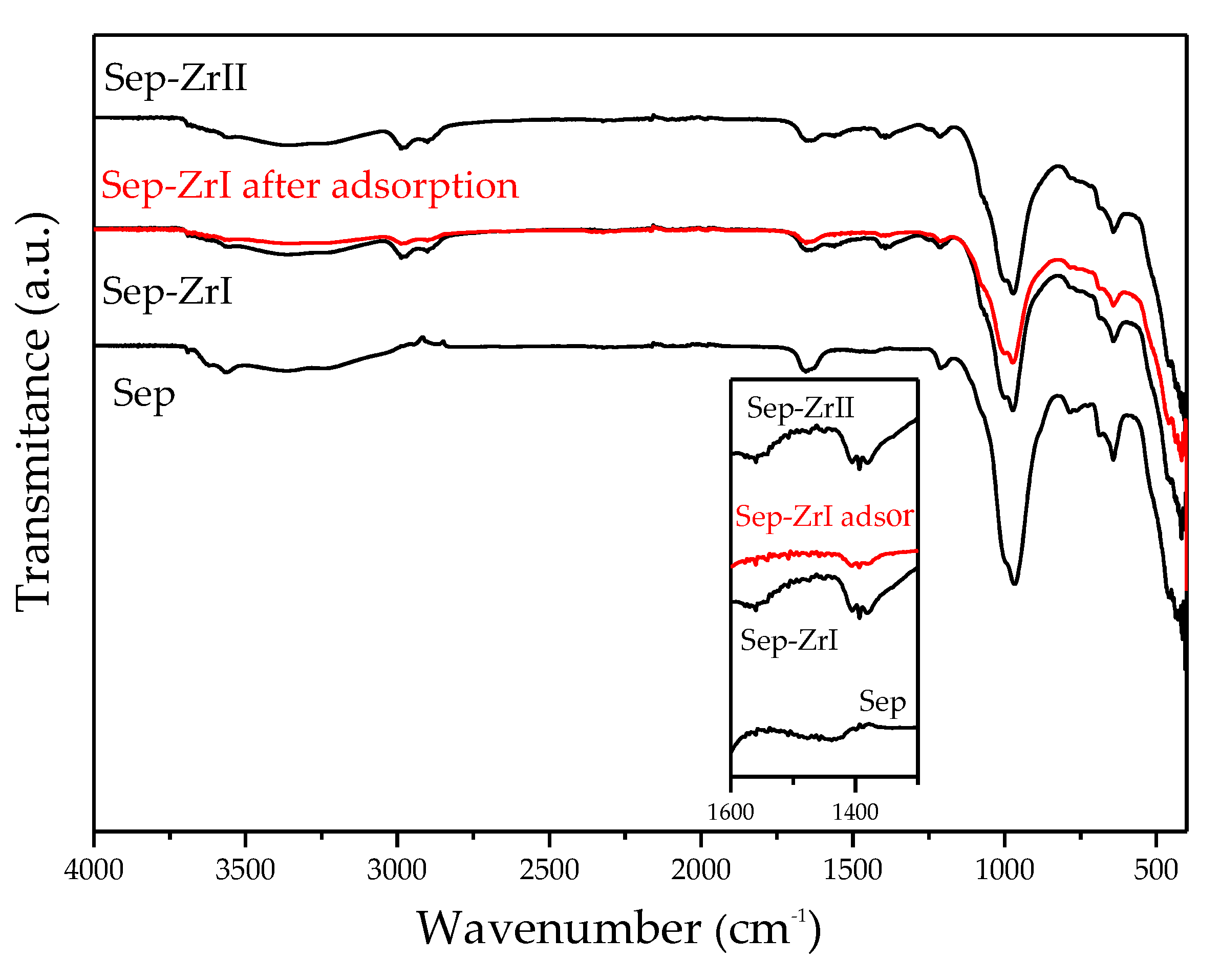
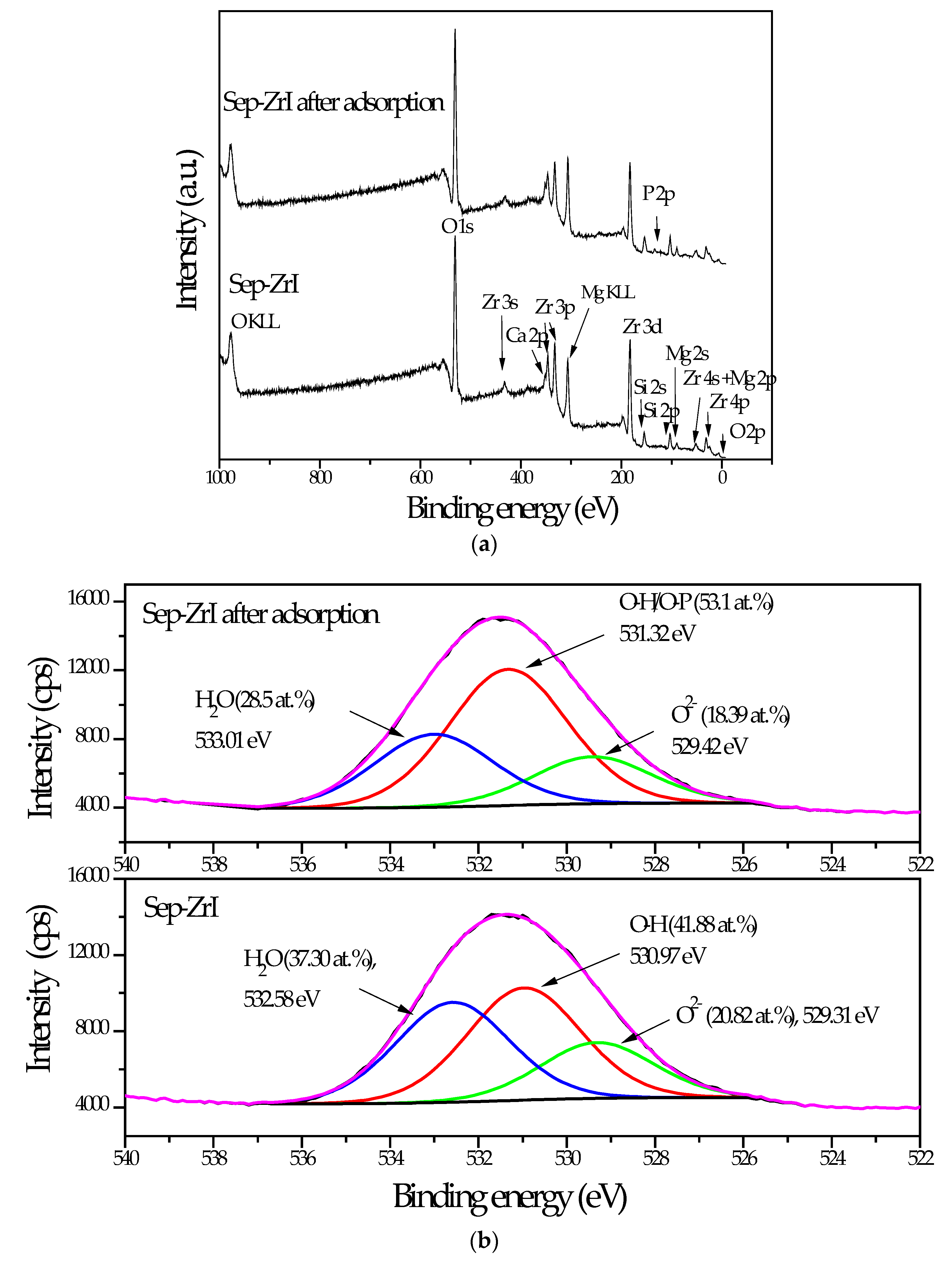
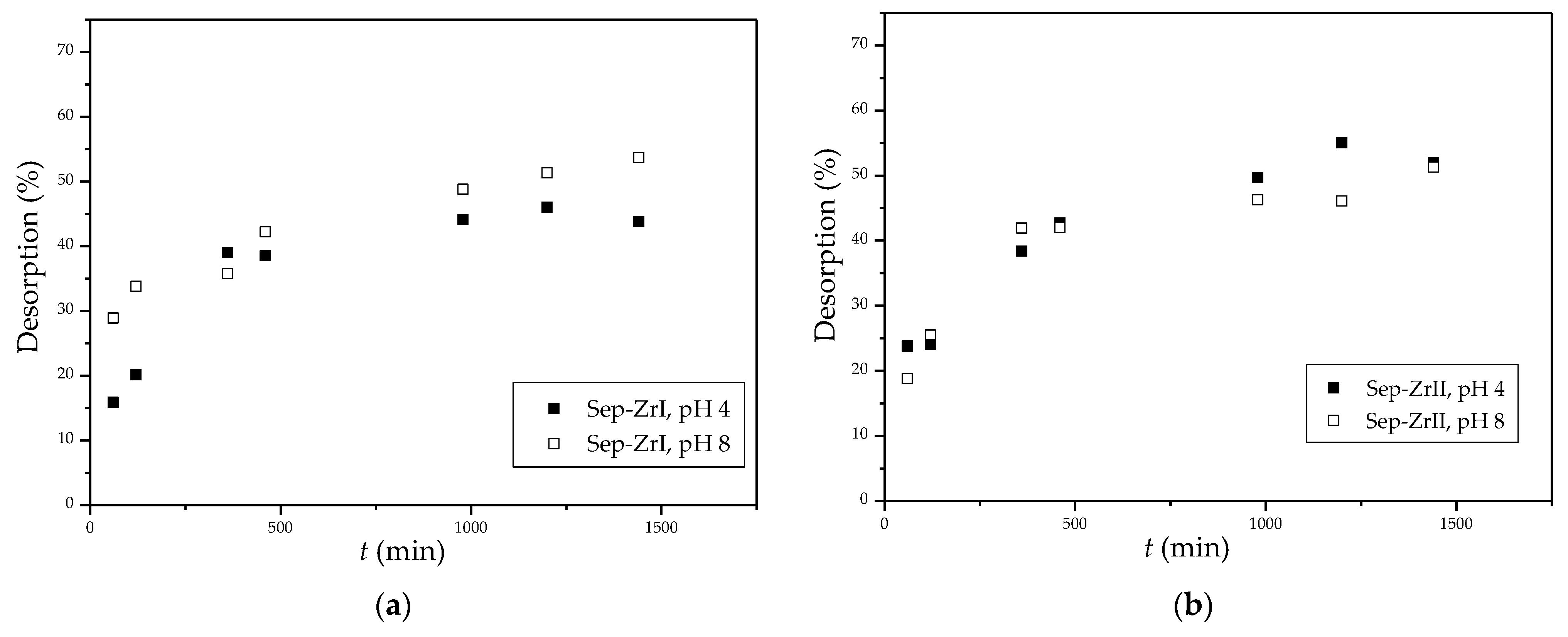
| Sample | O | Mg | Si | Fe | Zr |
|---|---|---|---|---|---|
| Sep-ZrI | 56.26±0.99 | 8.84±0.37 | 17.42±0.93 | 1.05±0.15 | 16.42±0.30 |
| Sep-ZrII | 57.87±3.53 | 7.38±0.72 | 14.45±1.19 | 0.96±0.16 | 19.35±2.39 |
| Sample | SBET, m2/g | Vtotal, m3/g | Vmeso, cm3/g | Vmicro, cm3/g | Dmean, nm | Dmax, nm |
|---|---|---|---|---|---|---|
| Sep | 311.4 | 0.351 | 0.265 | 0.126 | 6.63 | 4.00 |
| Sep–ZrI | 337.3 | 0.340 | 0.236 | 0.135 | 6.48 | 4.00 |
| Sep–ZrII | 352.2 | 0.398 | 0.306 | 0.135 | 6.47 | 4.00 |
| Sample | pHi | ||||||
|---|---|---|---|---|---|---|---|
|
qm mg/g |
KL dm3/mg |
R2 | 1/n |
Kf (mg/g)(dm3/mg)1/n |
R2 | ||
| Sep-ZrI | 4.0 | 11.85 | 14.45 | 0.935 | 0.160 | 9.01 | 0.996 |
| 8.0 | 8.47 | 1.81 | 0.905 | 0.221 | 4.85 | 0.957 | |
| Sep-ZrII | 4.0 | 12.55 | 2.32 | 0.982 | 0.212 | 7.93 | 0.988 |
| 8.0 | 7.52 | 8.41 | 0.897 | 0.137 | 5.56 | 0.932 | |
| Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adsorbent/pH |
Pseudo – first | Pseudo – second | Intraparticle | ||||||
| k1 (1/min) |
qe (mg/g) |
R2 | k2 (g/mg⋅min) |
qe (mg/g) |
R2 |
ki (mg/g⋅min1/2) |
C (mg/g) |
R2 | |
| Sep-ZrI c0 = 5.0 mg P/dm3, pH 4 |
0.0017 | 1.97 | 0.992 | 0.0030 | 5.52 | 0.996 | 0.0598 | 3.22 | 0.988 |
| Sep-ZrI c0 = 20.0 mg P/dm3, pH 4 |
0.0026 | 2.80 | 0.752 | 0.0029 | 12.51 | 0.999 | 0.2842 | 6.60 | 0.989 |
| Sep-ZrI c0 = 5.0 mg P/dm3, pH 8 |
0.0029 | 1.95 | 0.962 | 0.0036 | 4.85 | 1.00 | 0.0648 | 2.68 | 0.950 |
| Sep-ZrI c0 = 20.0 mg P/dm3, pH 8 |
0.0009 | 1.20 | 0.447 | 0.0036 | 8.55 | 0.988 | 0.1368 | 5.99 | 0.939 |
| Sep-ZrII c0 = 5.0 mg P/dm3, pH 4 |
0.0014 | 1.10 | 0.651 | 0.0067 | 4.97 | 0.997 | 0.0807 | 3.05 | 0.975 |
| Sep-ZrII c0 = 20.0 mg P/dm3, pH 4 |
0.0034 | 3.90 | 0.621 | 0.0020 | 12.09 | 0.999 | 0.2858 | 5.54 | 0.952 |
| Sep-ZrII c0 = 5.0 mg P/dm3, pH 8 |
0.0031 | 1.09 | 0.630 | 0.0071 | 4.58 | 1.00 | 0.0743 | 2.63 | 0.930 |
| Sep-ZrII c0 = 20.0 mg P/dm3, pH 8 |
0.0017 | 1.75 | 0.842 | 0.0054 | 7.87 | 0.999 | 0.0880 | 5.46 | 0.910 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





