Submitted:
05 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
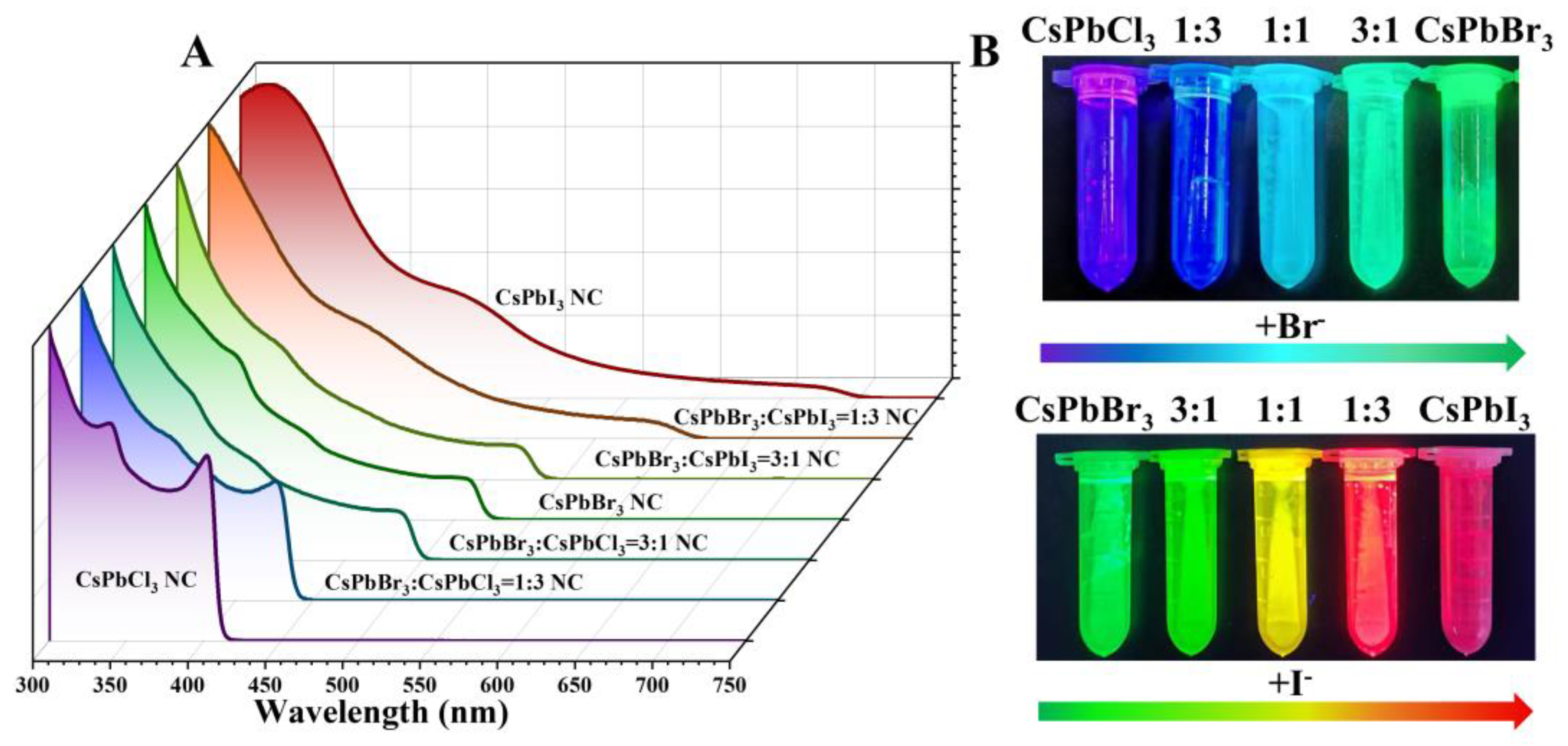
2. Results and Discussion
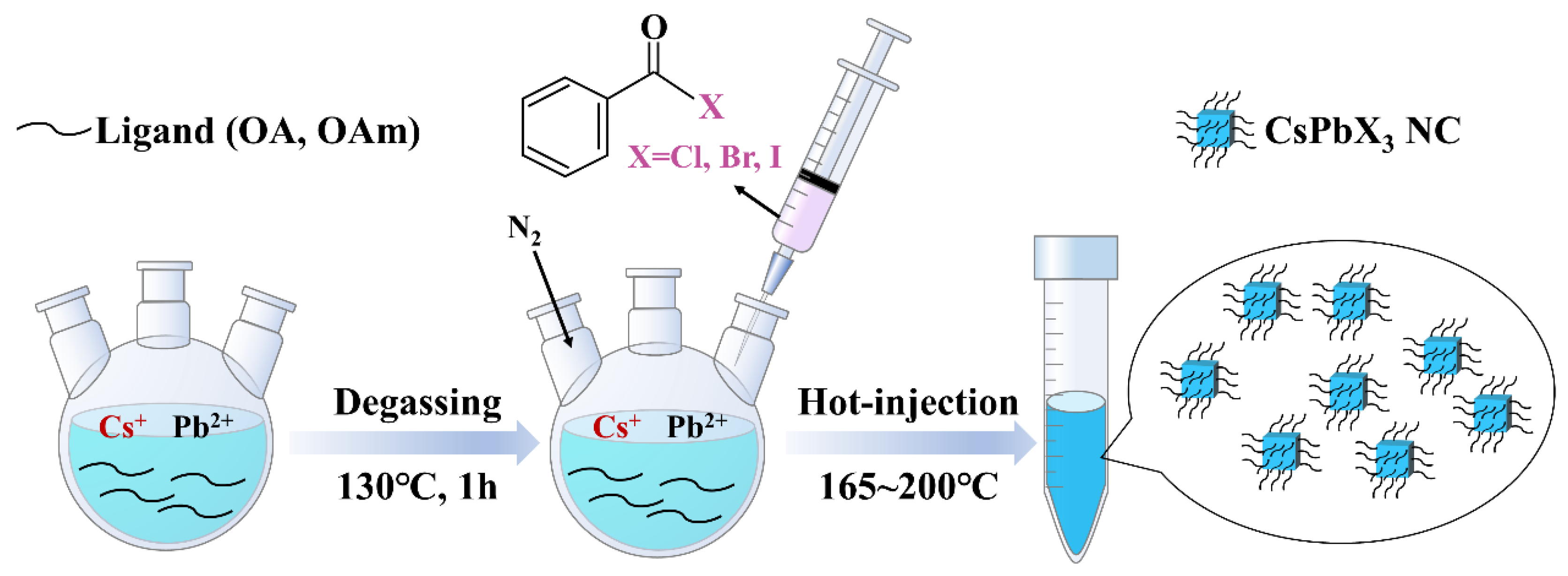
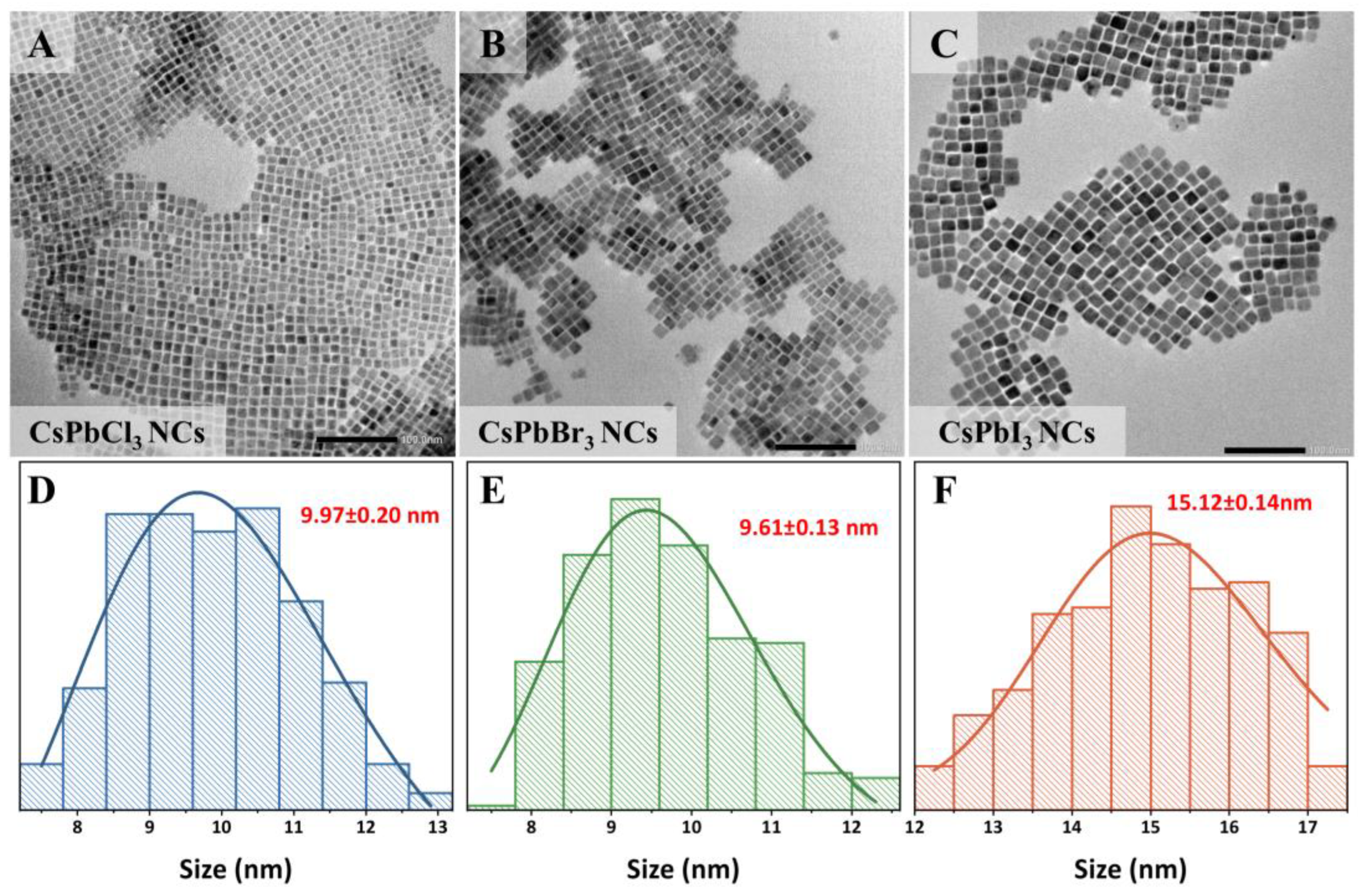
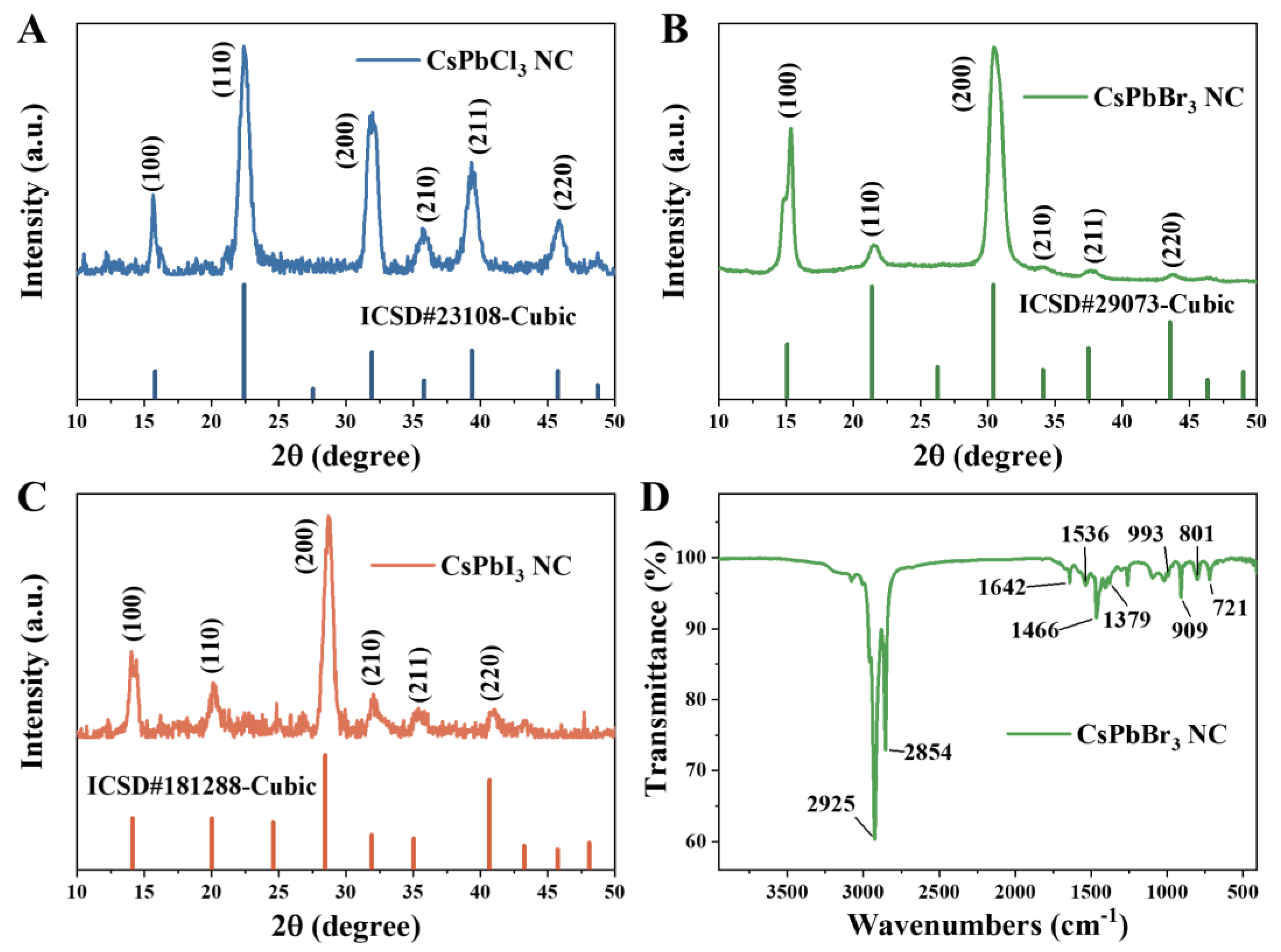
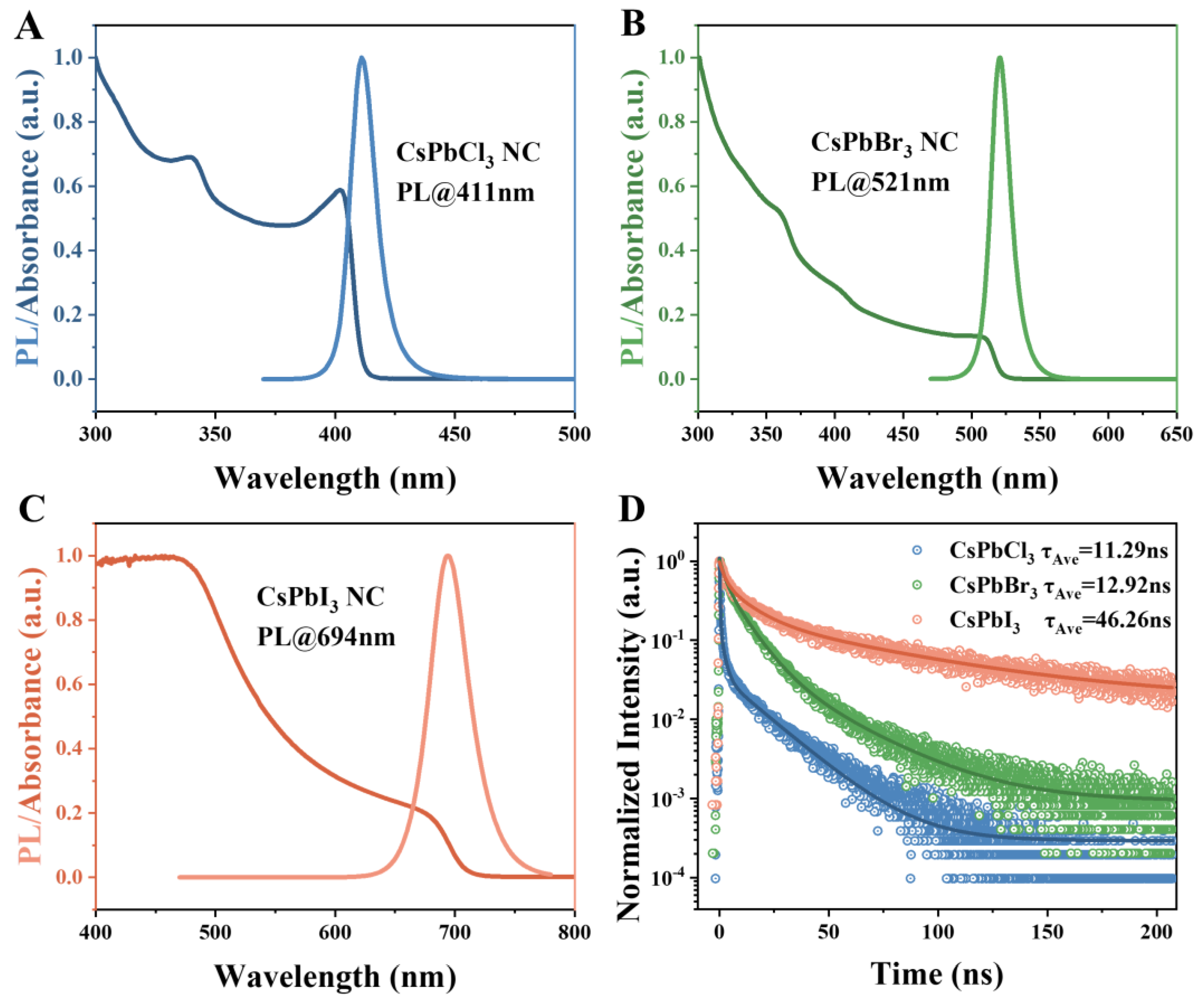
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis Scheme of All-Inorganic Perovskite Nanocrystals
3.2.1. Preparation of Benzoyl Iodine
3.2.2. Synthesis of CsPbX3 (X = Cl, Br, I) Nanocrystals (NCs)
3.2.3. Isolaiton of CsPbX3 (X = Cl, Br, I) Nanocrystals (NCs)
3.2.4. Anion Exchange Reactions
3.3. Materials Characterization
3.3.1. Transmission Electron Microscopy (TEM) Characterization
3.3.2. X-ray Diffraction (XRD) Characterization
3.3.3. Fourier Transform Infrared (FTIR) Spectroscopy Characterization
3.3.4. Fluorescence Spectrum Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhong, Y. Origins of p-Doping and Nonradiative Recombination in CsSnI3. Angew Chem Int Ed Engl 2022, 61, e202212002. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Mitzi, D.B. Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem Rev 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Ceratti, D.R.; Tenne, R.; Bartezzaghi, A.; Cremonesi, L.; Segev, L.; Kalchenko, V.; Oron, D.; Potenza, M.A.C.; Hodes, G.; Cahen, D. Self-Healing and Light-Soaking in MAPbI3 : The Effect of H2O. Adv Mater 2022, 34, e2110239. [Google Scholar] [CrossRef]
- Li, Y.; Ding, T.; Luo, X.; Tian, Y.; Lu, X.; Wu, K. Synthesis and Spectroscopy of Monodispersed, Quantum-Confined FAPbBr3 Perovskite Nanocrystals. Chem. Mater. 2019, 32, 549–556. [Google Scholar] [CrossRef]
- Seth, S.; Ahmed, T.; De, A.; Samanta, A. Tackling the Defects, Stability, and Photoluminescence of CsPbX3 Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 1610–1618. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, X.; Li, X.; Traore, B.; Spanopoulos, I.; Katan, C.; Even, J.; Kanatzidis, M.G.; Harel, E. Cation Engineering in Two-Dimensional Ruddlesden-Popper Lead Iodide Perovskites with Mixed Large A-Site Cations in the Cages. J. Am. Chem. Soc. 2020, 142, 4008–4021. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Guhrenz, C.; Benad, A.; Ziegler, C.; Haubold, D.; Gaponik, N.; Eychmüller, A. Solid-State Anion Exchange Reactions for Color Tuning of CsPbX3 Perovskite Nanocrystals. Chem. Mater. 2016, 28, 9033–9040. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D'Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Xu, W.; Yang, L.; Liu, Y.; Yao, D.; Zhang, D.; Zhang, H.; Yang, B. Engineering the Photoluminescence of CsPbX3 (X = Cl, Br, and I) Perovskite Nanocrystals Across the Full Visible Spectra with the Interval of 1 nm. ACS Appl Mater Interfaces 2019, 11, 14256–14265. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, C.; Xu, J.; Li, S.; Cui, M.; Fu, X.; Liu, Y.; Liu, Y.; Wan, H.; Wei, K.; et al. Synthesis-on-Substrate of Quantum Dot Solids. Nature 2022, 612, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Yettapu, G.R.; Talukdar, D.; Sarkar, S.; Swarnkar, A.; Nag, A.; Ghosh, P.; Mandal, P. Terahertz Conductivity within Colloidal CsPbBr3 Perovskite Nanocrystals: Remarkably High Carrier Mobilities and Large Diffusion Lengths. Nano Lett. 2016, 16, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, B.; Wang, W.; Liu, S.; Zheng, Y.; Chen, S.; Wang, K.; Sun, X.W. Plasmonic Perovskite Light-Emitting Diodes Based on the Ag-CsPbBr3 System. ACS Appl Mater Interfaces 2017, 9, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, W.; Olthof, S.; Liu, S.F. Defects in CsPbX3 Perovskite: From Understanding to Effective Manipulation for High-Performance Solar Cells. Small Methods 2021, 5, e2100725. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Cui, J.; Zhang, M.; Han, Y.; Yang, S.; Zhao, H.; Bian, H.; Yao, J.; Zhao, K.; Liu, Z.; et al. Precursor Engineering for Ambient-Compatible Antisolvent-Free Fabrication of High-Efficiency CsPbI2Br Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2000691. [Google Scholar] [CrossRef]
- Zhang, J.; Hodes, G.; Jin, Z.; Liu, S.F. All-Inorganic CsPbX3 Perovskite Solar Cells: Progress and Prospects. Angew. Chem. Int. Ed. 2019, 58, 15596–15618. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.H.; Kim, B.; Lee, S.H.; Lee, M.S.; Lee, J.S. All-Inorganic Cesium Lead Halide Perovskite Nanocrystals for Photodetector Applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef]
- He, Q.; Chen, G.; Wang, Y.; Liu, X.; Xu, D.; Xu, X.; Liu, Y.; Bao, J.; Wang, X. CsPbX3-ITO (X = Cl, Br, I) Nano-Heterojunctions: Voltage Tuned Positive to Negative Photoresponse. Small 2021, 17, e2101403. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, T.; Xu, T.; Ye, S.; Liu, R.; Sun, Z.; Tan, C.; Lv, X.; Yang, J.; et al. Unencapsulated CsPbClBr2 Film Photodetectors Grown by Thermal Vacuum Deposition Exhibit Exceptional Environmental Stability in High-Humidity Air. ACS Appl Energy Mater 2022, 5, 8709–8716. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Li, M.; Zeng, X.; Pi, M.; Liu, Z.; Liu, J.; Zhang, D.; Hu, Z.; Du, J. Low Threshold and Ultrastability of One-Step Air-Processed All-Inorganic CsPbX3 Thin Films toward Full-Color Visible Amplified Spontaneous Emission. ACS Appl Mater Interfaces 2022, 14, 26904–26912. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yu, X.; Ge, Y.; He, D.; Zhu, X.; Liu, H.; Guo, H.; Zhao, F.; Yu, S.; Qiu, J.; et al. Highly Stable and Controllable Lasing Actions from PVDF Encapsulated CsPbBr3 Perovskite Microcrystals. J. Mater. Chem. C 2022, 10, 16301–16308. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Nalla, V.; Zeng, H.; Sun, H. Solution-Processed Low Threshold Vertical Cavity Surface Emitting Lasers from All-Inorganic Perovskite Nanocrystals. Adv. Funct. Mater. 2017, 27, 1605088. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Deng, J.; Chu, Z.; Jiang, Q.; Meng, J.; Wang, P.; Zhang, L.; Yin, Z.; You, J. Efficient Green Light-Emitting Diodes Based on Quasi-Two-Dimensional Composition and Phase Engineered Perovskite with Surface Passivation. Nat Commun 2018, 9, 570. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite Light-Emitting Diodes Based on Spontaneously Formed Submicrometre-Scale Structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite Light-emitting Diodes with External Quantum Efficiency Exceeding 20 Percent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Gu, Z. All-Inorganic Perovskite Nanocrystal Materials: New Generation of Scintillators for High Quality X-ray Imaging. Sci Bull 2019, 64, 1205–1206. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, T.; Yang, Z.; Zhang, H.; Su, Y.; Chen, Z.; Chen, X.; Ma, Y.; Zhu, W.; Yu, X.; et al. Highly Resolved and Robust Dynamic X-Ray Imaging Using Perovskite Glass-Ceramic Scintillator with Reduced Light Scattering. Adv Sci 2021, 8, e2003728. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Yuan, R.; Chen, J.; Zhao, J.; Wang, S.; Liu, Y.; Li, Q.; Zhang, Z. A Novel Scintillation Screen for Achieving High-Energy Ray Detection with Fast and Full-Color Emission. J. Mater. Chem. C 2021, 9, 7905–7909. [Google Scholar] [CrossRef]
- Chan, K.K.; Giovanni, D.; He, H.; Sum, T.C.; Yong, K.-T. Water-Stable All-Inorganic Perovskite Nanocrystals with Nonlinear Optical Properties for Targeted Multiphoton Bioimaging. ACS Appl Nano Mater 2021, 4, 9022–9033. [Google Scholar] [CrossRef]
- Kar, M.R.; Kumar, S.; Acharya, T.K.; Goswami, C.; Bhaumik, S. Highly Water-Stable, Luminescent, and Monodisperse Polymer-Coated CsPbBr3 Nanocrystals for Imaging in Living Cells with Better Sensitivity. RSC Adv 2023, 13, 5946–5956. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Kumar, S.; Acharya, T.K.; Goswami, C.; Bhaumik, S. Highly Stable Multi-Encapsulated Red-Emitting Cesium Lead Halide Nanocrystals for Efficient Copper Ion Detection and Imaging in Live Cells. J. Alloys Compd. 2023, 947, 169453. [Google Scholar] [CrossRef]
- Xiao, M.; Hao, M.; Lyu, M.; Moore, E.G.; Zhang, C.; Luo, B.; Hou, J.; Lipton-Duffin, J.; Wang, L. Surface Ligands Stabilized Lead Halide Perovskite Quantum Dot Photocatalyst for Visible Light-Driven Hydrogen Generation. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Gong, W.; Guo, H.; Niu, X. Cobalt-doped CsPbBr3 perovskite quantum dots for photoelectrocatalytic hydrogen production via efficient charge transport. Colloids Surf. A 2023, 663. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Abdellah, M.; Sá, J. Hydrogen Evolution with CsPbBr3 Perovskite Nanocrystals under Visible Light in Solution. Mater Today Commun 2018, 16, 90–96. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J Am Chem Soc 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Gao, Z.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Inorganic Colloidal Perovskite Quantum Dots for Robust Solar CO2 Reduction. Chem 2017, 23, 9481–9485. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Ding, C.; Xu, J. Boosting charge separation and photocatalytic CO2 reduction of CsPbBr3 perovskite quantum dots by hybridizing with P3HT. Chem. Eng. J. 2021, 419. [Google Scholar] [CrossRef]
- Liu, H.; Bansal, S. Metal halide perovskite nanostructures and quantum dots for photocatalytic CO2 reduction: prospects and challenges. Mater. Today Energy 2023, 32. [Google Scholar] [CrossRef]
- Das, S.; Paul, T.; Maiti, S.; Chattopadhyay, K.K. Ambient Processed CsPbX3 Perovskite Cubes for Photocatalysis. Mater. Lett. 2020, 267, 127501. [Google Scholar] [CrossRef]
- Gao, G.; Xi, Q.; Zhou, H.; Zhao, Y.; Wu, C.; Wang, L.; Guo, P.; Xu, J. Novel inorganic perovskite quantum dots for photocatalysis. Nanoscale 2017, 9, 12032–12038. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Pak, Y.; Mitra, S.; Almalawi, D.; Alwadai, N.; Zhang, Y.; Roqan, I.S. Self-Patterned CsPbBr3 Nanocrystals for High-Performance Optoelectronics. ACS Appl Mater Interfaces 2019, 11, 5223–5231. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, C.; Peng, Z.; Xia, Y.; Zhang, J.; Luo, W.; Zhan, R.; Li, H.; Wang, Y.; Zhang, D. Self-Assembly and Photoactivation of Blue Luminescent CsPbBr3 Mesocrystals Synthesized at Ambient Temperature. J. Mater. Chem. C 2018, 6, 1701–1708. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Cui, X.; Wang, X.; Yang, D. Strategies for the Preparation of High-Performance Inorganic Mixed-Halide Perovskite Solar Cells. RSC Adv 2022, 12, 32925–32948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yasar, M.; Luo, Z.; Zhou, S.; Yu, Y.; Li, H.; Yang, R.; Wang, X.; Pan, A.; Gan, L.; et al. Temperature Difference Triggering Controlled Growth of All-Inorganic Perovskite Nanowire Arrays in Air. Small 2018, 14, e1803010. [Google Scholar] [CrossRef]
- Dong, S.; Hu, Z.Y.; Wei, P.; Han, J.; Wang, Z.; Liu, J.; Su, B.L.; Zhao, D.; Liu, Y. All-Inorganic Perovskite Single-Crystal Photoelectric Anisotropy. Adv Mater 2022, 34, e2204342. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-Mediated Synthesis of Shape-Controlled Cesium Lead Halide Perovskite Nanocrystals via Reprecipitation Process at Room Temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the Ligand-Mediated Synthesis of Colloidal CsPbBr3 Perovskite Nanocrystals: The Role of Organic Acid, Base, and Cesium Precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef]
- Chen, D.; Fang, G.; Chen, X.; Lei, L.; Zhong, J.; Mao, Q.; Zhou, S.; Li, J. Mn-Doped CsPbCl3 Perovskite Nanocrystals: Solvothermal Synthesis, Dual-Color Luminescence and Improved Stability. J. Mater. Chem. C 2018, 6, 8990–8998. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal Synthesis of High-Quality All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: From Nanocube to Ultrathin Nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.G.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X.; et al. Efficient Light-Emitting Diodes Based on in Situ Fabricated FAPbBr3 Nanocrystals: The Enhancing Role of the Ligand-Assisted Reprecipitation Process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Shamsi, J.; Akkerman, Q.A.; Imran, M.; Bertoni, G.; Brescia, R.; Manna, L. Low-Temperature Electron Beam-Induced Transformations of Cesium Lead Halide Perovskite Nanocrystals. ACS Omega 2017, 2, 5660–5665. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Wang, H.; Zhan, Q.; Liu, Q.; Xia, Z. Postsynthetic Surface Trap Removal of CsPbX3 (X = Cl, Br, or I) Quantum Dots via a ZnX2/Hexane Solution toward an Enhanced Luminescence Quantum Yield. Chem. Mater. 2018, 30, 8546–8554. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, D.; Kisielowski, C.; Dou, L.; Kornienko, N.; Bekenstein, Y.; Wong, A.B.; Alivisatos, A.P.; Yang, P. Atomic Resolution Imaging of Halide Perovskites. Nano Lett. 2016, 16, 7530–7535. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chou, H.-L.; Lin, J.-C.; Lee, Y.-C.; Pao, C.-W.; Chen, J.-L.; Chang, C.-C.; Chi, R.-Y.; Kuo, T.-R.; Lu, C.-W.; et al. Enhanced Luminescence and Stability of Cesium Lead Halide Perovskite CsPbX3 Nanocrystals by Cu2+-Assisted Anion Exchange Reactions. J Phy Chem C 2019, 123, 2353–2360. [Google Scholar] [CrossRef]
- Uddin, M.A.; Glover, J.D.; Park, S.M.; Pham, J.T.; Graham, K.R. Growth of Highly Stable and Luminescent CsPbX3 (X = Cl, Br, and I) Nanoplates via Ligand Mediated Anion Exchange of CsPbCl3 Nanocubes with AlX3. Chem. Mater. 2020, 32, 5217–5225. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



