Submitted:
05 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
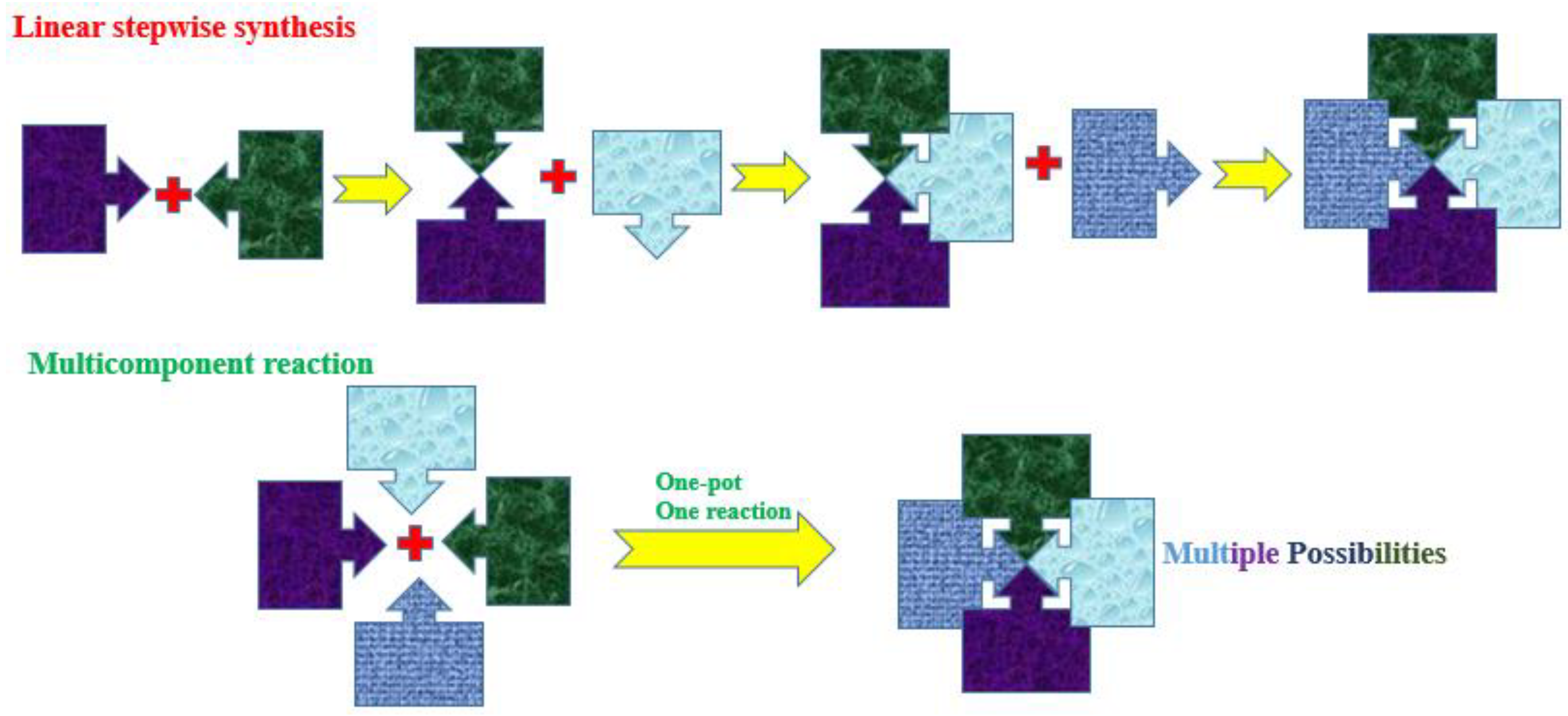
1. Introduction
2. Multicomponent Reactions in Synthesis and Applications
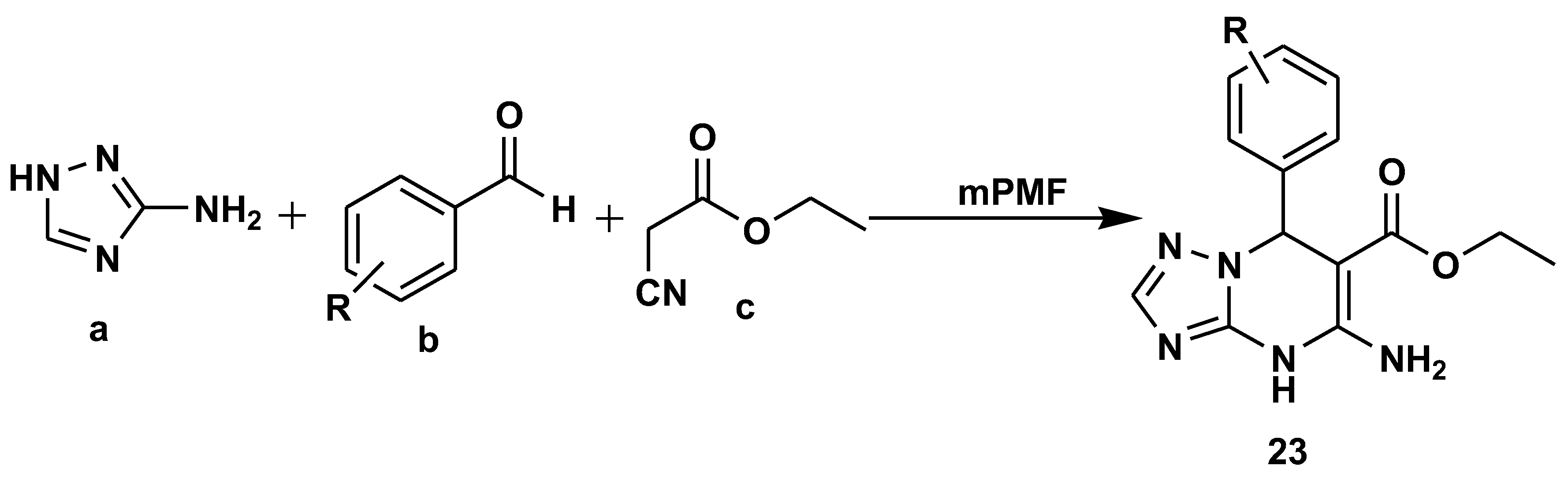
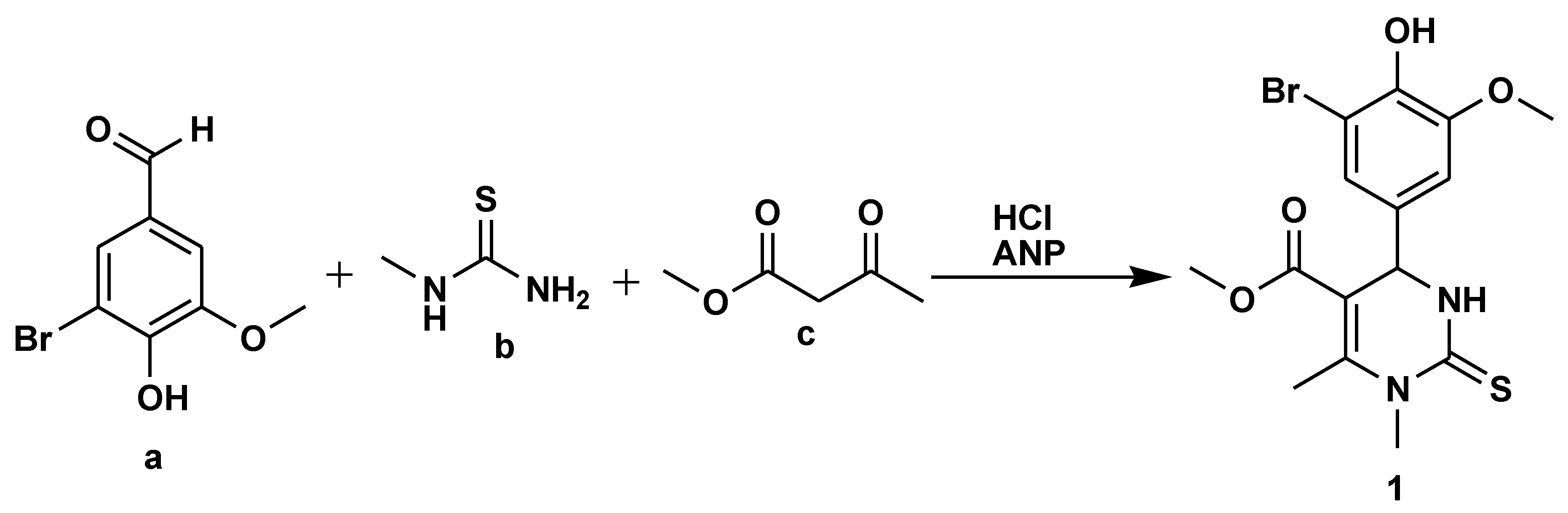
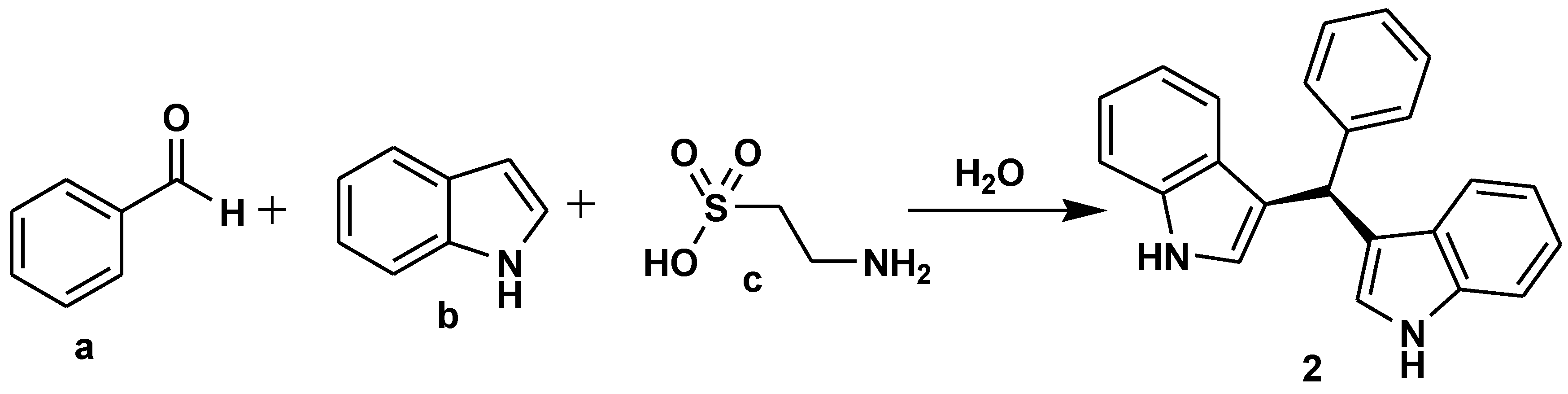
2.1. Medicinal Chemistry
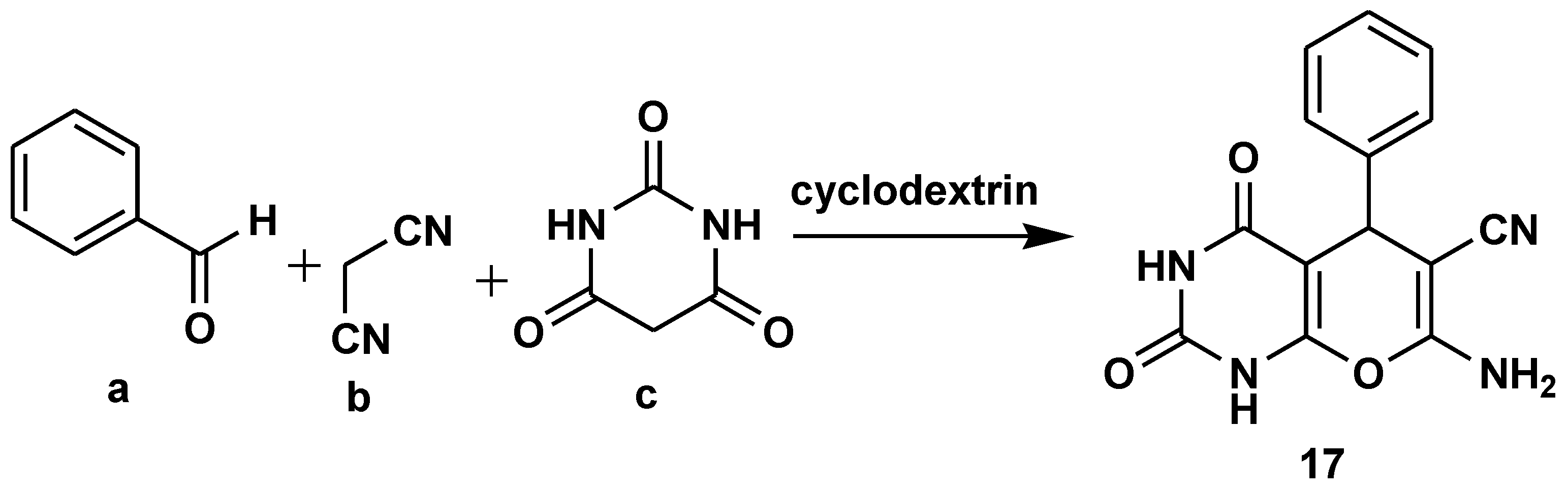
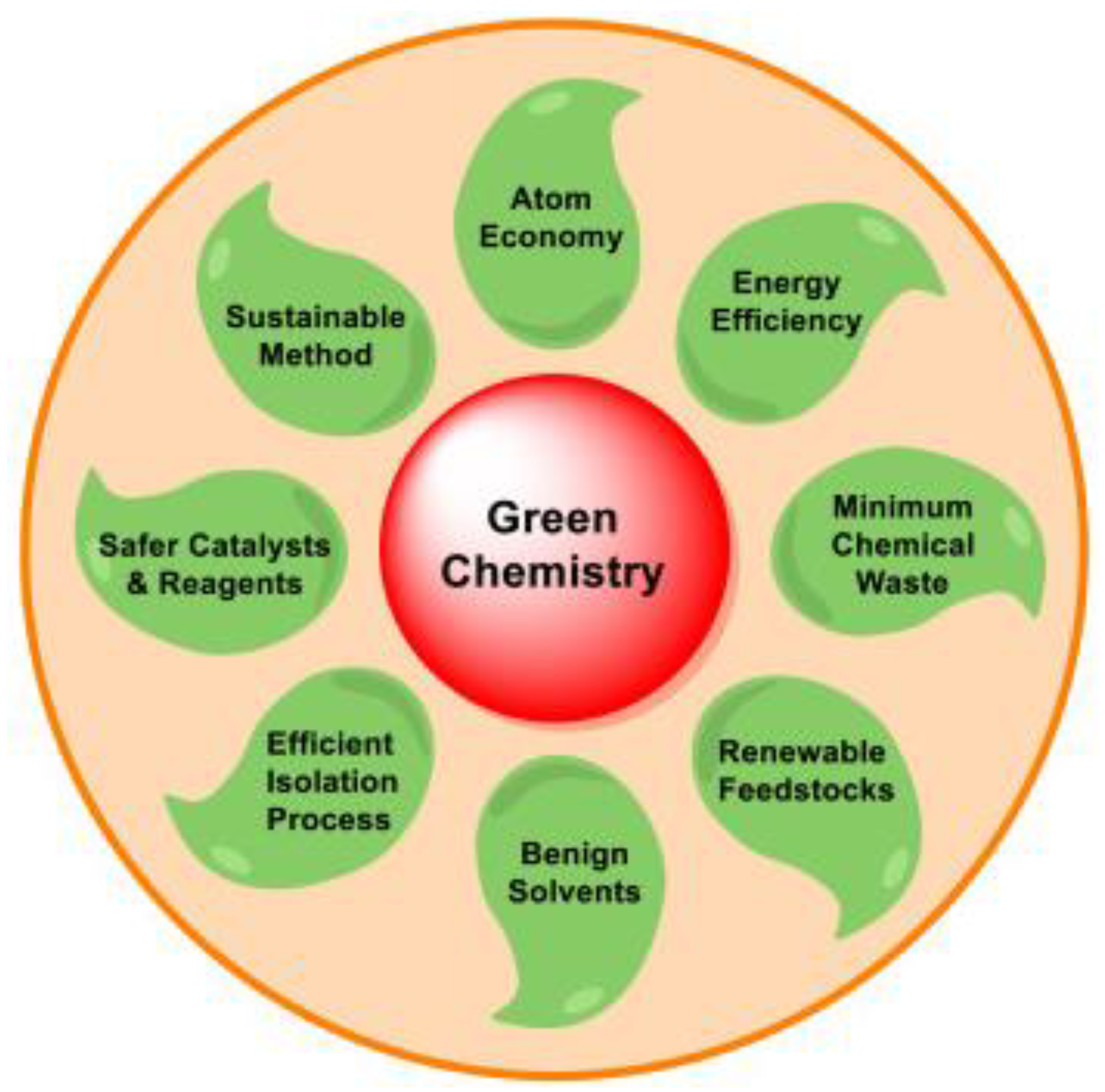
2.2. Green Chemistry
2.3. Polymerisations
2.4. Solid-Phase Synthesis
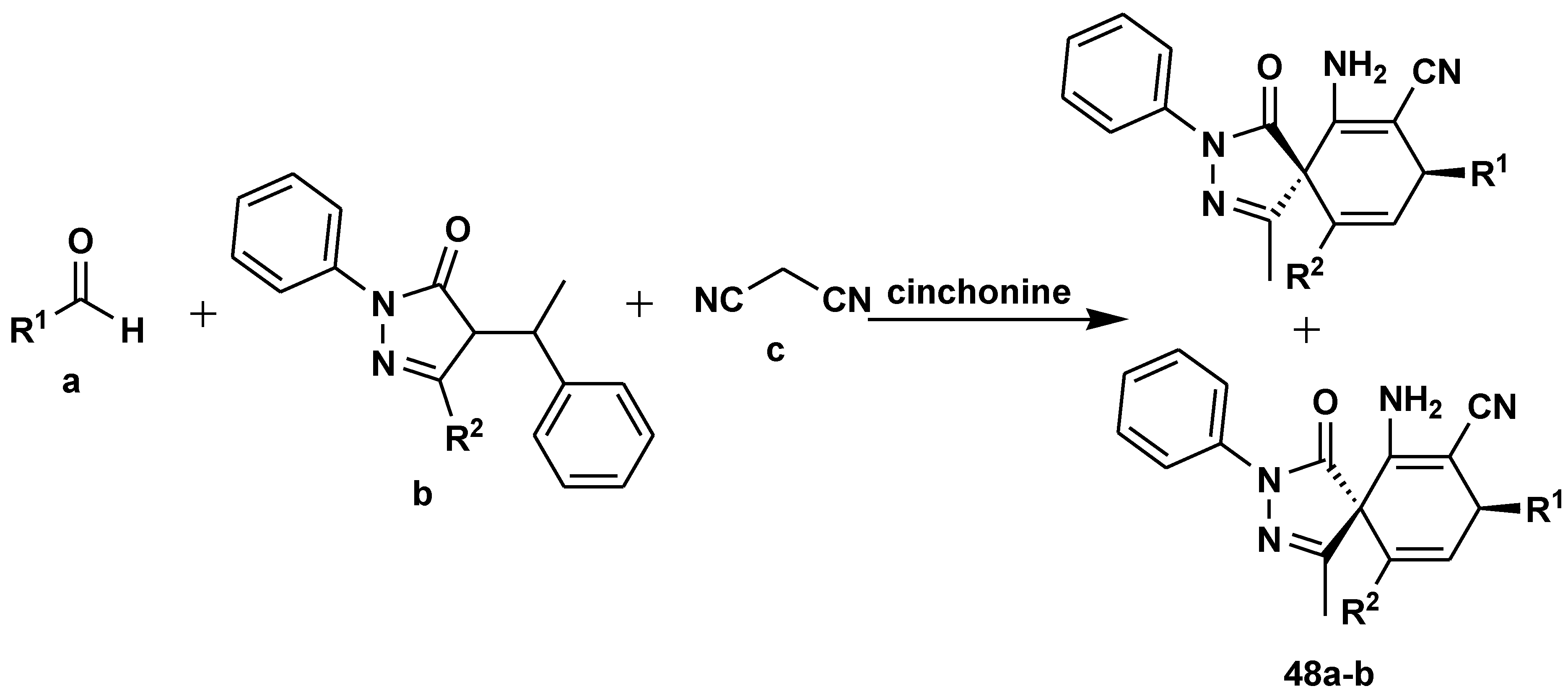
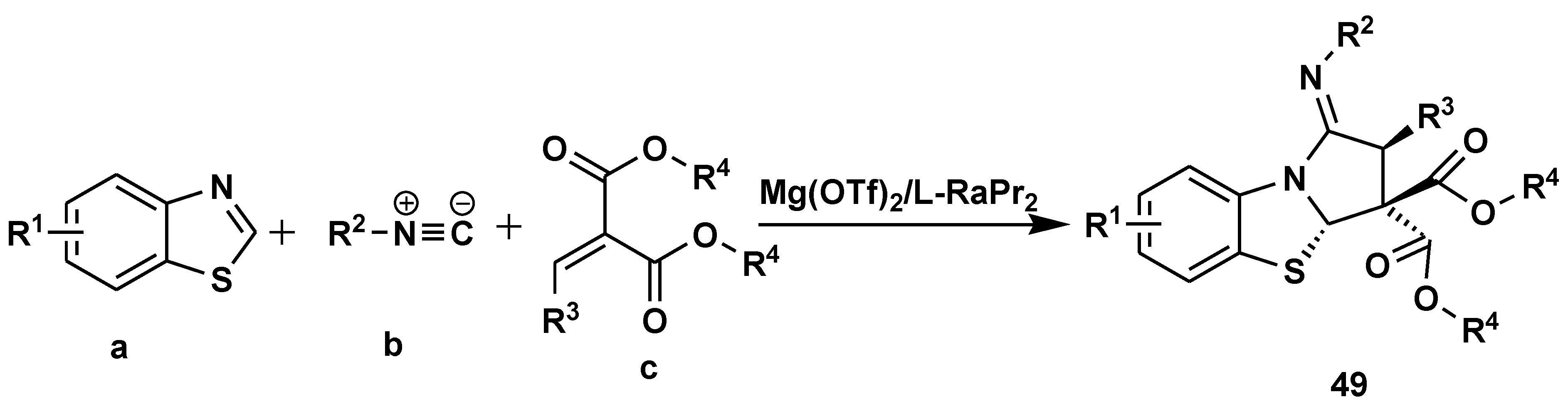
2.5. Asymmetric Catalysis
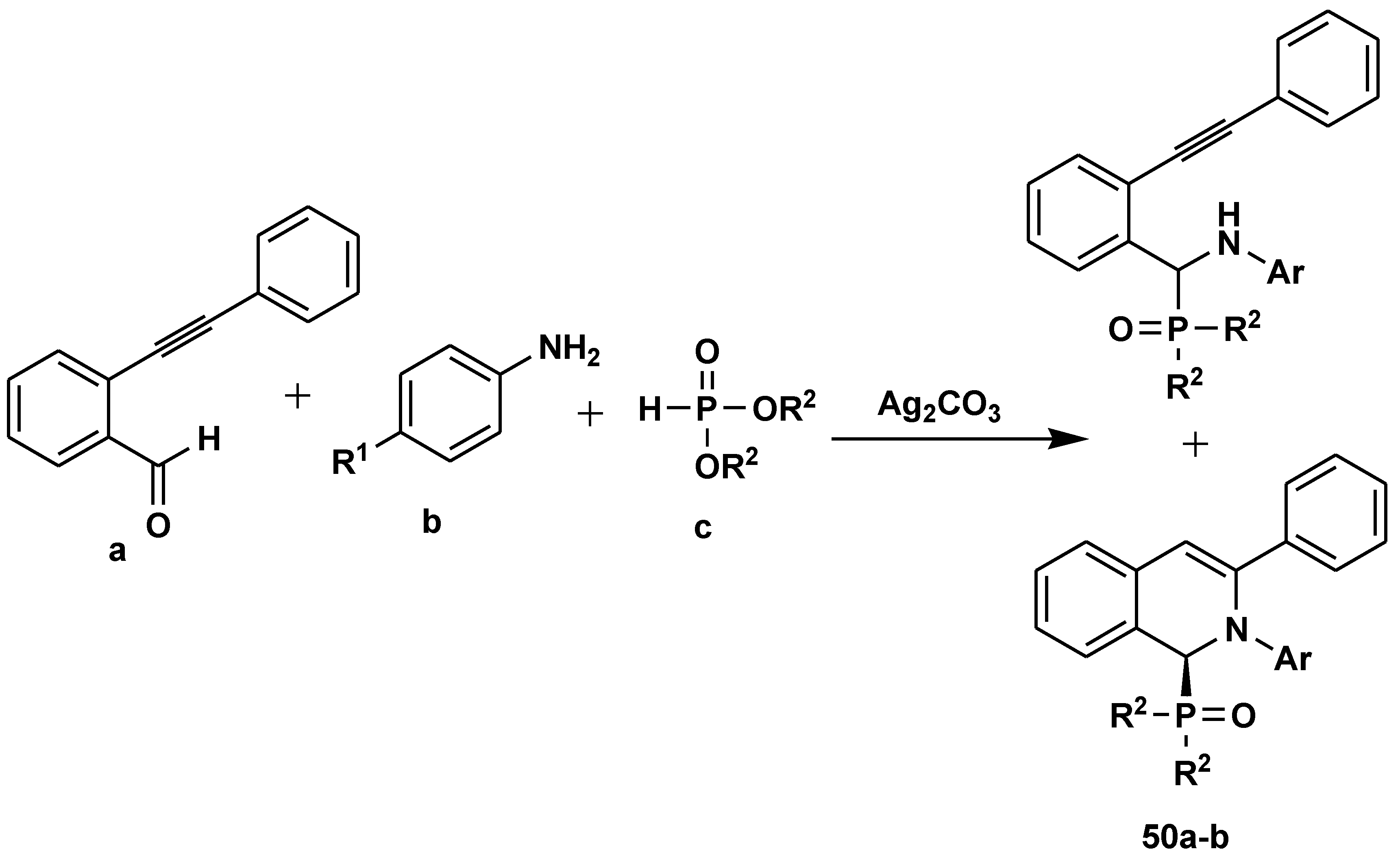
2.6. C-H Functionalisation
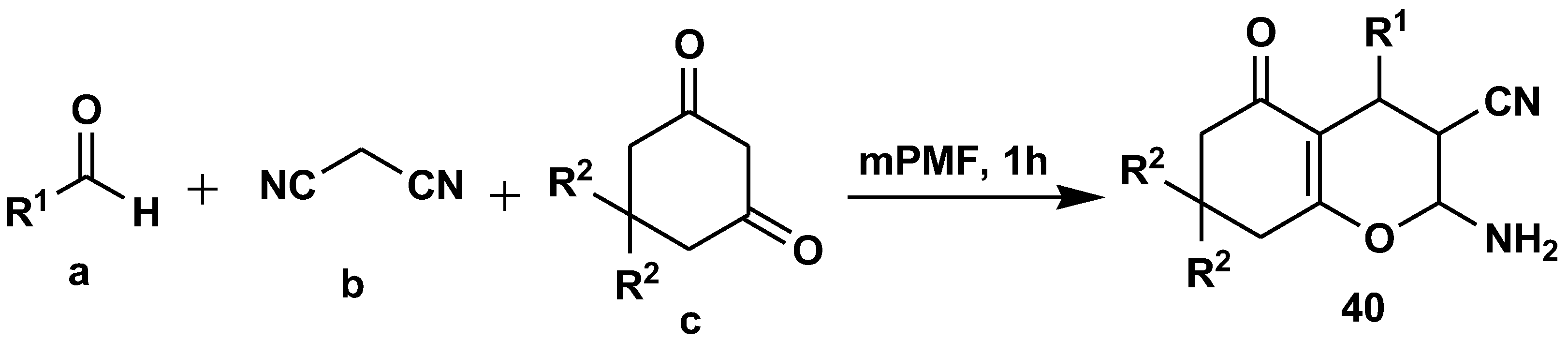
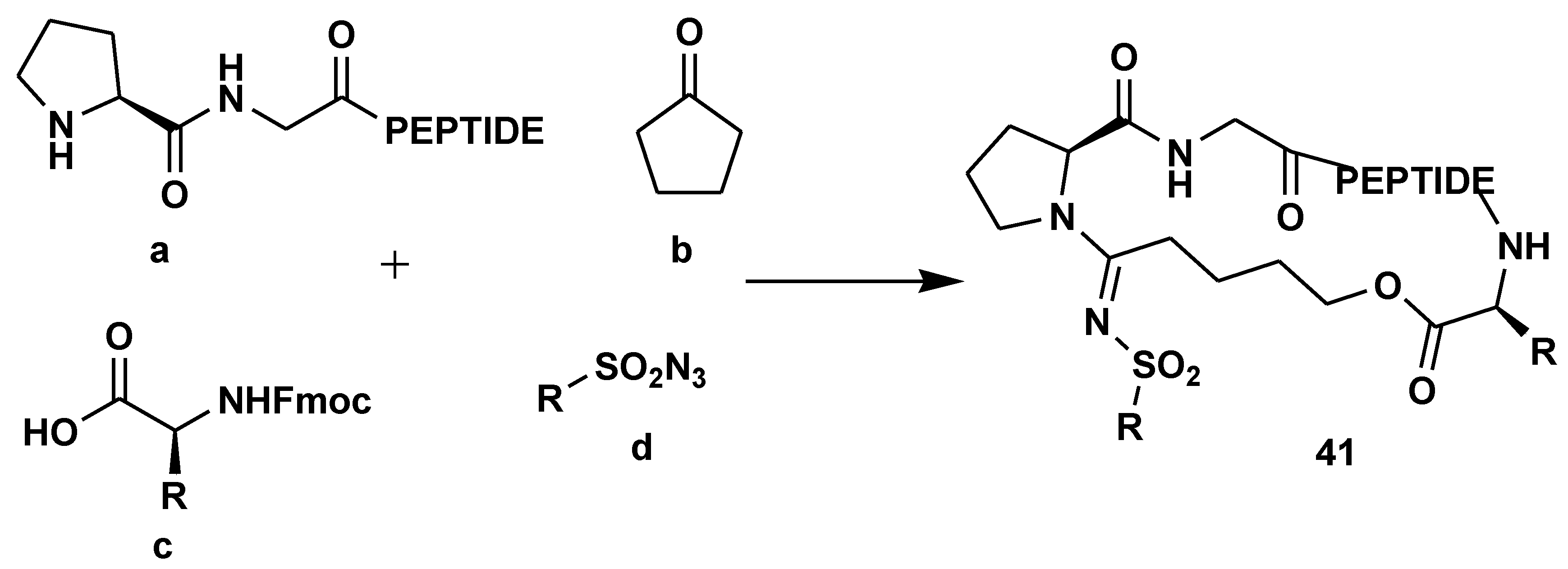
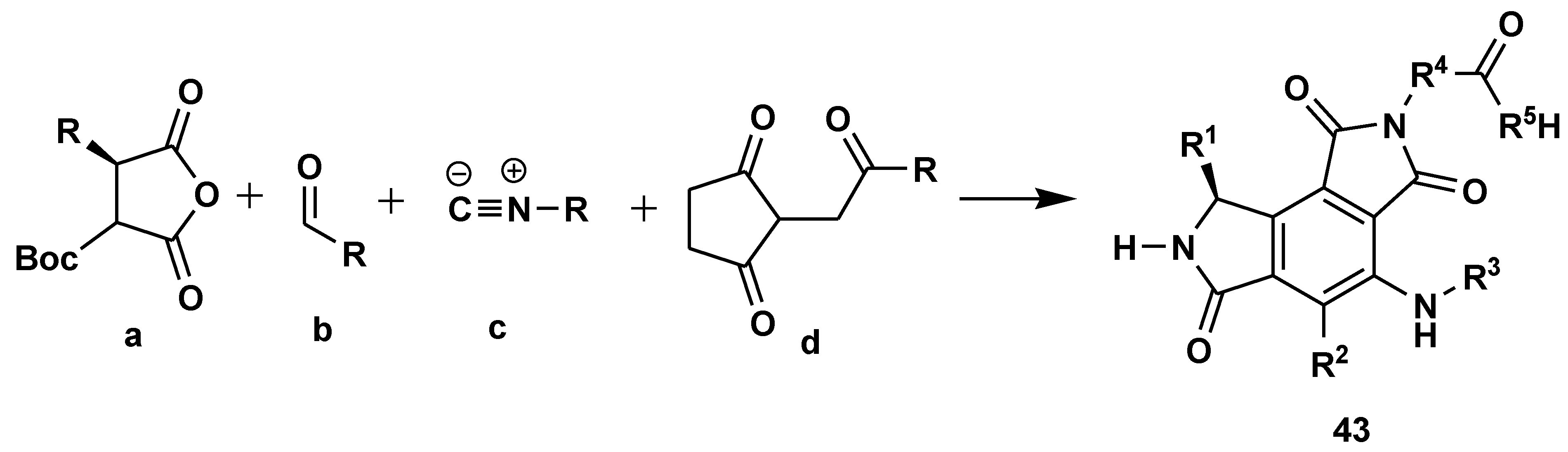
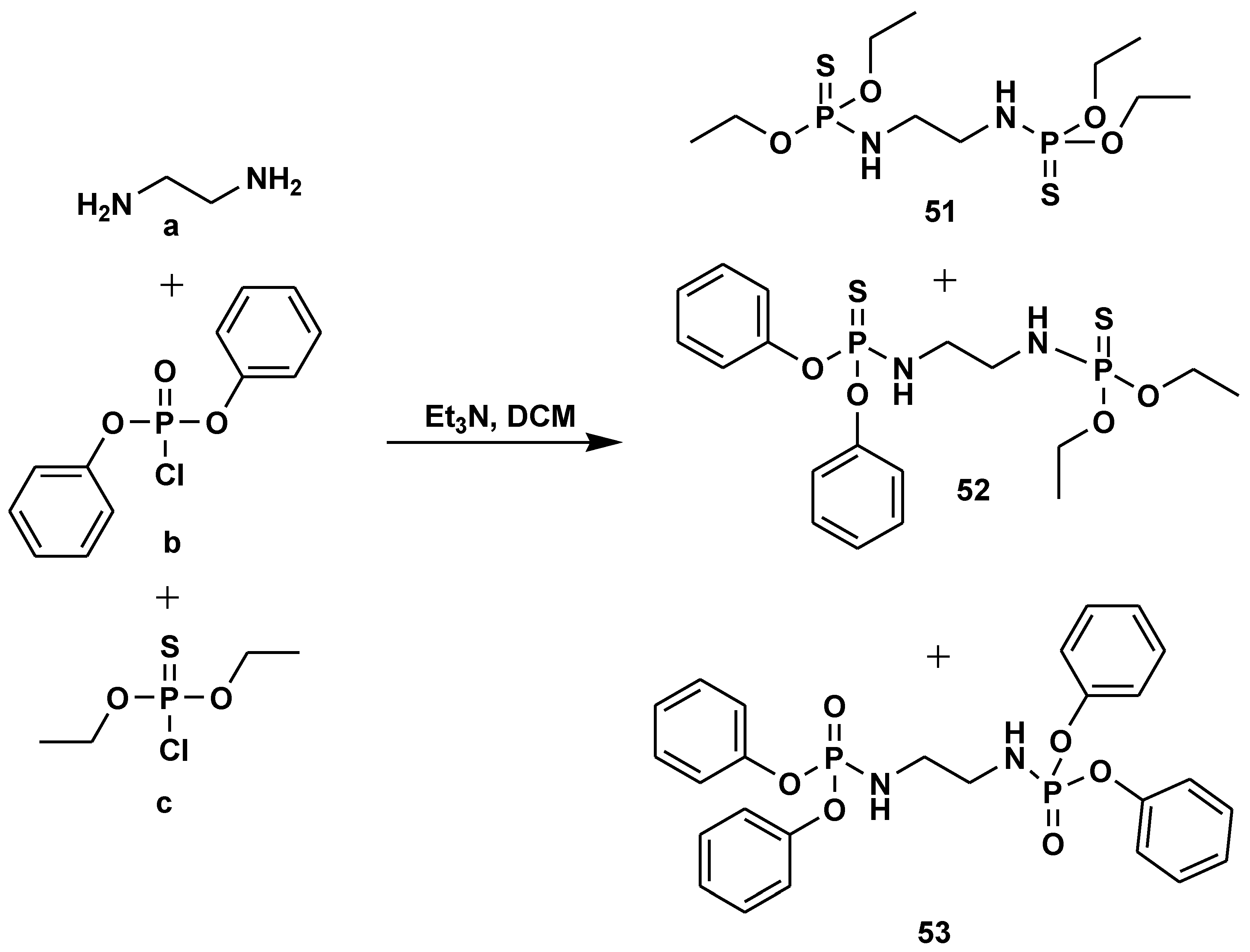
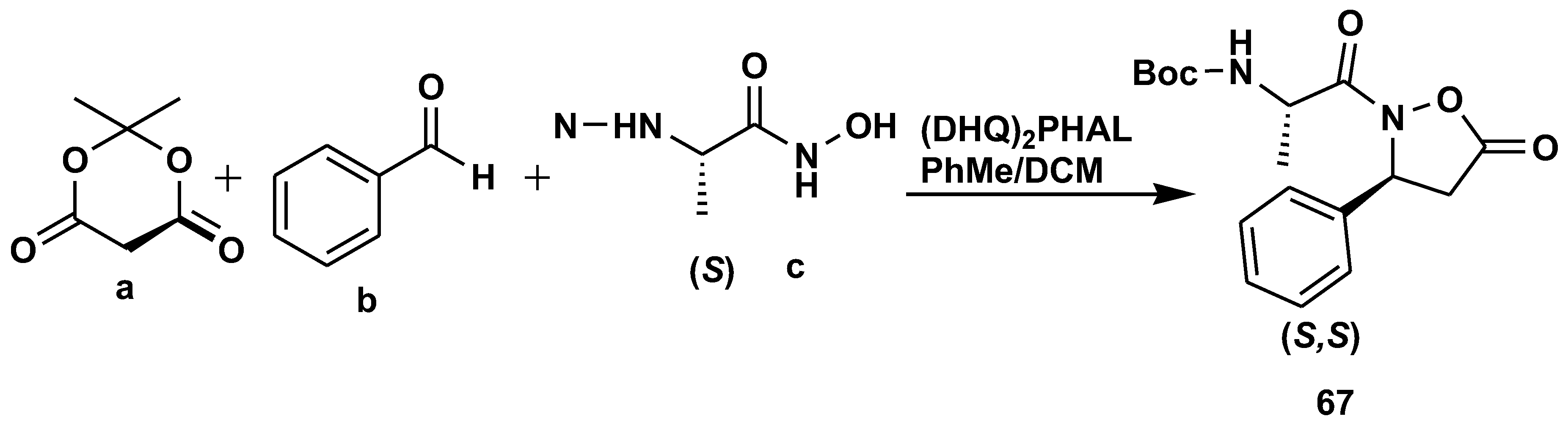
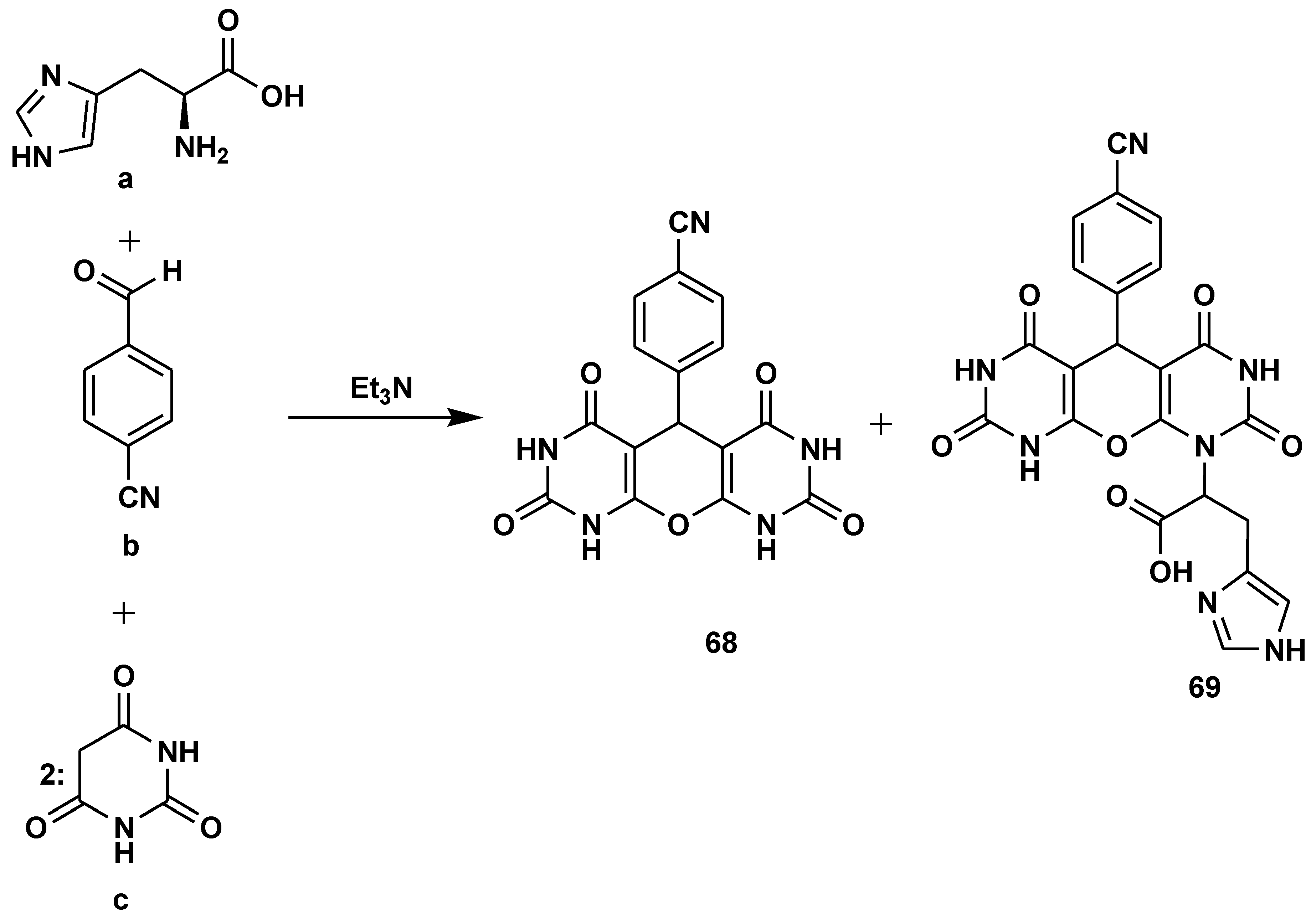
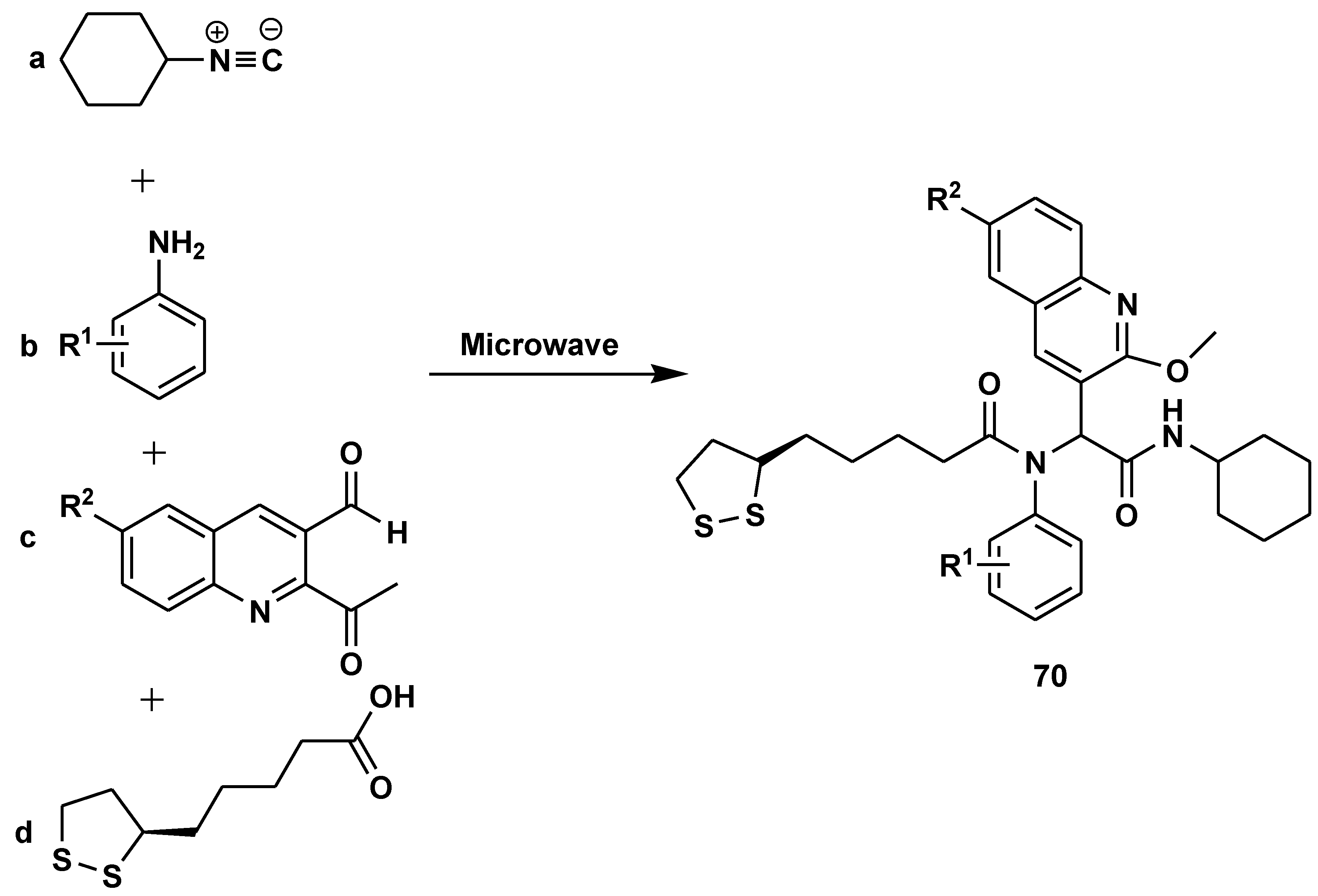
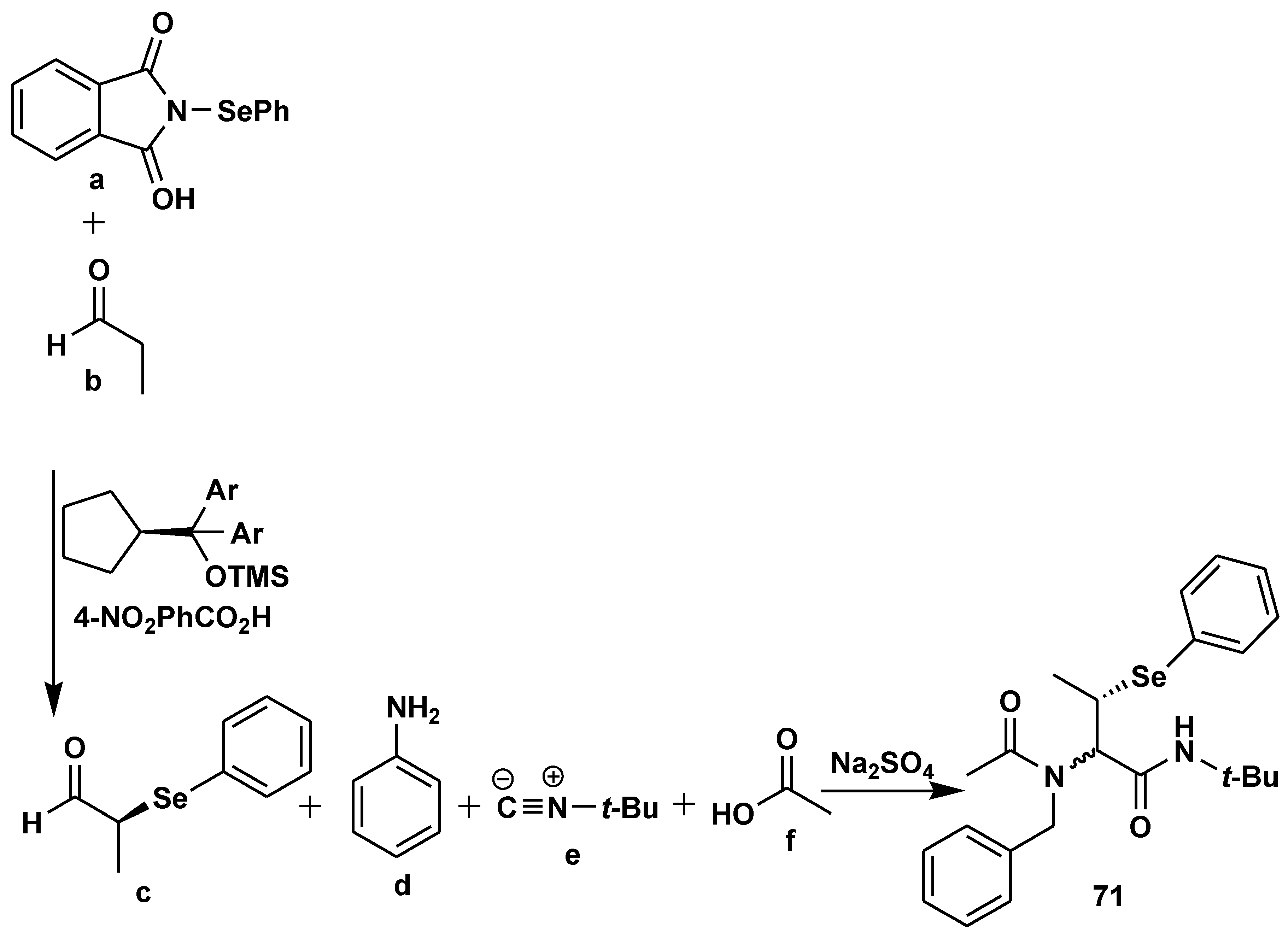
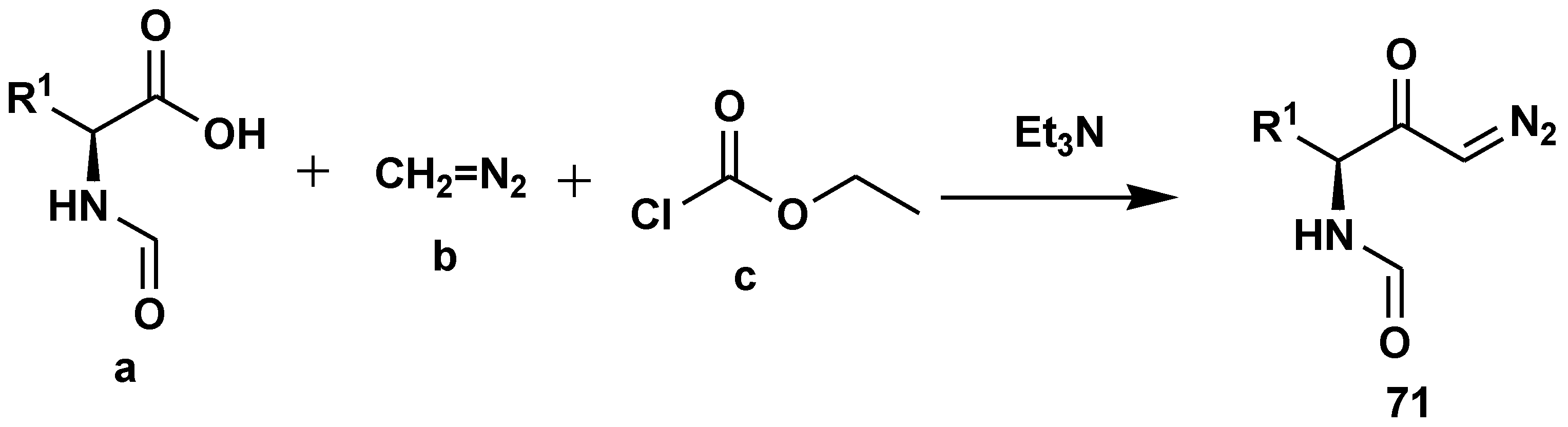
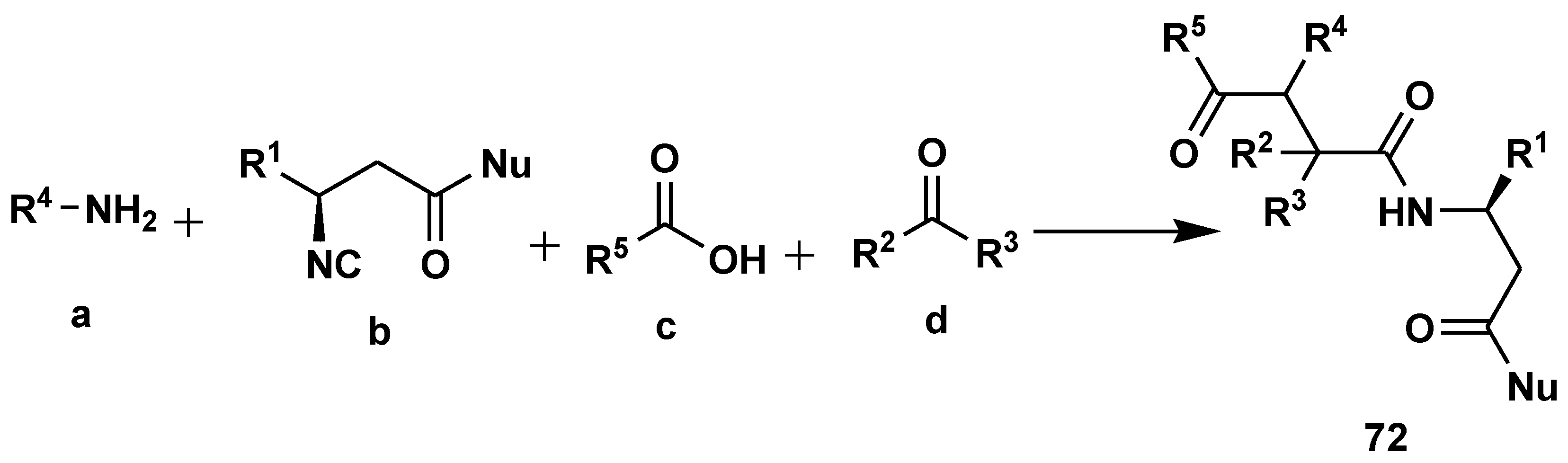
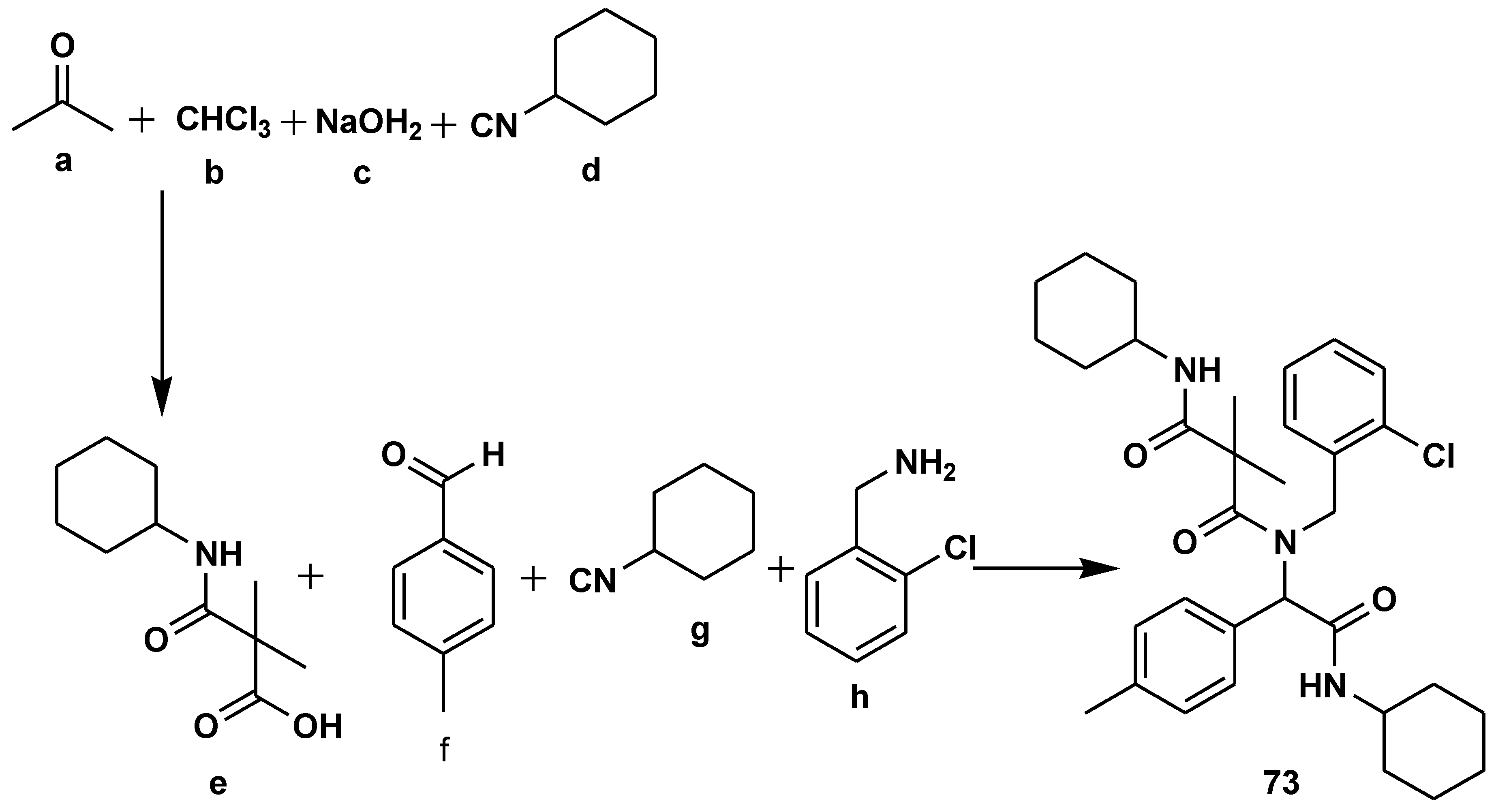
2.7. Peptide Synthesis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rocha, R.O.; Rodrigues, M.O.; Neto, B.A.D. Review on the Ugi Multicomponent Reaction Mechanism and the Use of Fluorescent Derivatives as Functional Chromophores. ACS Omega 2020, 5, 972–979. [Google Scholar] [CrossRef]
- Zhong, Y. Arylformylacetonitriles in Multicomponent Reactions Leading to Heterocycles. Eur. J. Org. Chem. 2022, 2022. [Google Scholar] [CrossRef]
- Yazdani, H.; Hooshmand, S.E.; Stenzel, M.H. Fusion of Cellulose and Multicomponent Reactions: Benign by Design. ACS Sustain. Chem. Eng. 2022, 10, 4359–4373. [Google Scholar] [CrossRef]
- Mohlala, R.L.; Coyanis, E.M.; Fish, M.Q.; Fernandes, M.A.; Bode, M.L. Synthesis of 6-Membered-Ring Fused Thiazine-Dicarboxylates and Thiazole-Pyrimidines via One-Pot Three-Component Reactions. Molecules 2021, 26, 5493. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, S.; Ma, Y.; Wen, R.; Cen, L.; Gong, P.; Wang, J. A Simple and Efficient Synthesis of Highly Substituted Indeno [1,2-b]pyrrole and Acenaphtho [1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions. Molecules 2018, 23, 3031. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem., Int. Ed., 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Li, Y. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo [1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. [Google Scholar] [CrossRef] [PubMed]
- Paprocki, D.; Madej, A.; Koszelewski, D.; Brodzka, A.; Ostaszewski, R. Multicomponent Reactions Accelerated by Aqueous Micelles. Front. Chem. 2018, 6, 502. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L. Synthesis of Novel 1,4-Naphthoquinones Possessing Indole Scaffolds Using In(OTf)3 in Solvent-Free Conditions. Molecules 2018, 23, 1954. [Google Scholar] [CrossRef]
- Shahedi, M.; Habibi, Z.; Yousefi, M.; Brask, J.; Mohammadi, M. Improvement of biodiesel production from palm oil by co-immobilization of Thermomyces lanuginosa lipase and Candida antarctica lipase B: Optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 170, 490–502. [Google Scholar] [CrossRef]
- Paprocki, D.; Madej, A.; Koszelewski, D.; Brodzka, A.; Ostaszewski, R. Multicomponent Reactions Accelerated by Aqueous Micelles. Front. Chem. 2018, 6, 502. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, S.; Ma, Y.; Wen, R.; Cen, L.; Gong, P.; Wang, J. A Simple and Efficient Synthesis of Highly Substituted Indeno [1,2-b]pyrrole and Acenaphtho [1,2-b]pyrrole Derivatives by Tandem Three-Component Reactions. Molecules 2018, 23, 3031. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Gioiello, A.; Piccinno, A.; Lozza, A.M.; Cerra, B. The Medicinal Chemistry in the Era of Machines and Automation: Recent Advances in Continuous Flow Technology. J. Med. Chem. 2020, 63, 6624–6647. [Google Scholar] [CrossRef]
- Milovi’c, E.; Jankovi´c, N.; Petronijevi´c, J.; Joksimovi´c, N.; Kosani´c, M.; Stanojkovic´, T. , Matic´, I. ; Grozdanic´, N.; Klisuric´, O.; Stefanovic, S. Synthesis, Characterization, and Biological Evaluation of Tetrahydropyrimidines: Dual-Activity and Mechanism of Action Pharmaceutics, 2022, 14, 2254, 1–12. [Google Scholar]
- Chavan, K.A.; Shukla, M.; Chauhan, A.N.S.; Maji, S.; Mali, G.; Bhattacharyya, S.; Erande, R.D. Effective Synthesis and Biological Evaluation of Natural and Designed Bis(indolyl)methanes via Taurine-Catalyzed Green Approach. ACS Omega 2022, 7, 10438–10446. [Google Scholar] [CrossRef]
- Mali, G.; Shaikh, B.A.; Garg, S.; Kumar, A.; Bhattacharyya, S.; Erande, R.D.; Chate, A.V. Design, Synthesis, and Biological Evaluation of Densely Substituted Dihydropyrano [2,3-c]pyrazoles via a Taurine-Catalyzed Green Multicomponent Approach. ACS Omega, 2021, 6, 30734–30742. [Google Scholar] [CrossRef]
- Popovics-Tóth, N.; Turpanova, M.; Németh, K.; Hackler, L.; Puskás, L.G.; Bálint, E. Synthesis of arylphosphinoyl-functionalized dihydroisoquinolines by Reissert-type reaction and their biological evaluation. Tetrahedron 2022, 111, 132720. [Google Scholar] [CrossRef]
- Venkatesh, R.; Shankar, G.; Narayanan, A.C.; Modi, G.; Sabiah, S.; Kandasamy, J. Multicomponent Synthesis of S-Benzyl Dithiocarbamates from para-Quinone Methides and Their Biological Evaluation for the Treatment of Alzheimer’s Disease. J. Org. Chem. 2022, 87, 6730–6741. [Google Scholar] [CrossRef]
- Butera, R.; Ważyńska, M.; Magiera-Mularz, K.; Plewka, J.; Musielak, B.; Surmiak, E.; Sala, D.; Kitel, R.; De Bruyn, M.; Nijman, H.W.; Elsinga, P.H.; Holak, T.A.; Dömling, A. Design, Synthesis, and Biological Evaluation of Imidazopyridines as PD-1/PD-L1 Antagonists. ACS Med. Chem. Lett. 2021, 12, 768–773. [Google Scholar] [CrossRef]
- Vahedi, M.M.; Asghari, S.; Tajbakhsh, M.; Mohseni, M.; Khalilpour, A. One-pot three-component synthesis of novel pyrano [3,2-e]pyrazolo [1,5-a]pyrimidines and investigation of their biological activities. J. Mol. Struct. 2023, 1284. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Gong, Q.-T.; Wang, Y.; Yu, Y.; Liu, Y.-H.; Wang, N.; Yu, X.-Q. Biocatalytic tandem multicomponent reactions for one-pot synthesis of 2-Amino-4H-Pyran library and in vitro biological evaluation. Mol. Catal. 2020, 491, 110983. [Google Scholar] [CrossRef]
- Martinez-Amezaga, M.; Giordano, R.A.; Gori, D.N.P.; Squizatto, C.P.; Giolito, M.V.; Scharovsky, O.G.; Rozados, V.R.; Rico, M.J.; Mata, E.G.; Delpiccolo, C.M.L. Synthesis of propargylamines via the A3 multicomponent reaction and their biological evaluation as potential anticancer agents. Org. Biomol. Chem. 2020, 18, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Ayoup, M.S.; Wahby, Y.; Abdel-Hamid, H.; Ramadan, E.S.; Teleb, M.; Abu-Serie, M.M.; Noby, A. Design, synthesis and biological evaluation of novel α-acyloxy carboxamides via Passerini reaction as caspase 3/7 activators. European Journal of Medicinal Chemistry, 168, 2019, 340-356.
- Pradeep, M.A.; Kumar, N.R.; Swaroop, D.K.; Reddy, N.S.; Sirisha, K.; Kumar, C.G.; Babu, N.J.; Ganapathi, T.; Narsaiah, B. Design and Synthesis of Novel Pyrimidine/Hexahydroquinazoline-Fused Pyrazolo [3,4-b]Pyridine Derivatives, Their Biological Evaluation and Docking Studies. ChemistrySelect. 2019, 4, 138–144. [Google Scholar] [CrossRef]
- Vasava, M.S.; Bhoi, M.N.; Rathwa, S.K.; Shetty, S.S.; Patel, R.D.; Rajani, D.P.; Rajani, S.D.; Patel, A.; Pandya, H.A.; Patel, H.D. Novel 1,4-dihydropyrano [2,3-c]pyrazole derivatives: Synthesis, characterization, biological evaluation and in silico study. Journal of Molecular Structure, 2019, 1181, 383–402. [Google Scholar] [CrossRef]
- Bhagat, S.; Supriy, M.; Pathak, S.; Sriram, D.; Chakraborti, A.K. α-Sulfonamidophosphonates as new anti-mycobacterial chemotypes: Design, development of synthetic methodology, and biological evaluation. Bioorganic Chemistry. 2019, 82, 246–252. [Google Scholar] [CrossRef]
- Karypidou, K.; Ribone, S.R.; Quevedo, M.A.; Persoons, L.; Pannecouque, C.; Helsen, C.; Claessens, F.; Dehaen, W. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorganic Med. Chem. Lett. 2018, 28, 3472–3476. [Google Scholar] [CrossRef]
- Chate, A.V.; Dongre, R.M.; Khaire, M.K.; Bondle, G.M.; Sangshetti, J.N.; Damale, M. β-CD-catalyzed multicomponent domino reaction: synthesis, characterization, in silico molecular docking and biological evaluation of pyrano [2,3-d]-pyrimidinone derivatives. Res. Chem. Intermed. 2018, 44, 6119–6136. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice, Oxford University Press, 1998.
- Asfaw, N.; Chebude, Y.; Ejigu, A.; Hurisso, B.B.; Licence, P.; Smith, R.L.; Tang, S.L.Y.; Poliakoff, M. The 13 Principles of Green Chemistry and Engineering for a Greener Africa. Green Chem. 2011, 13, 1059–1060. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
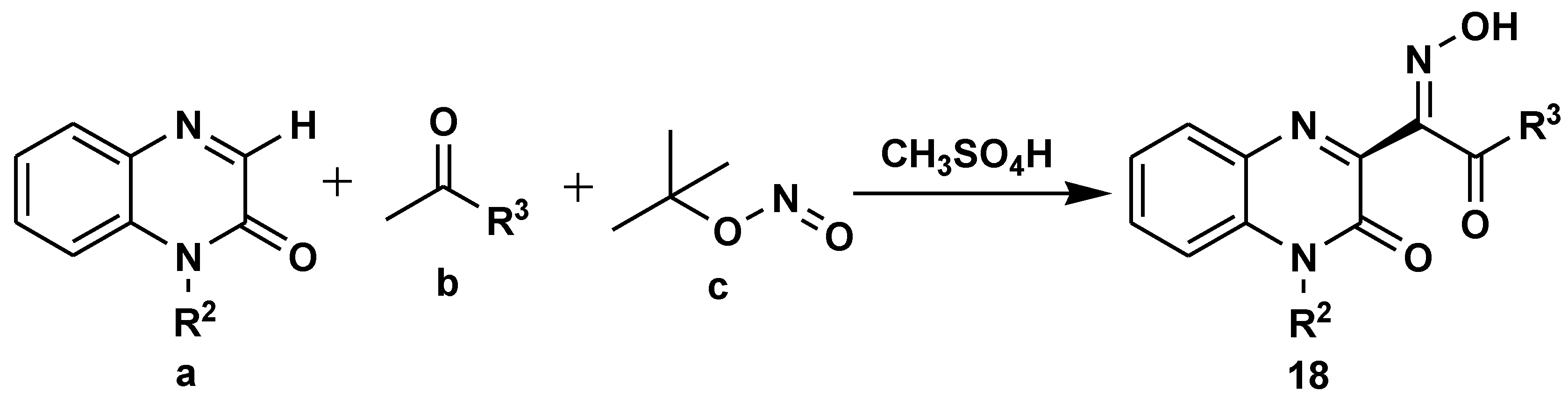
- Xu, J.; Yang, H.; He, L.; Huang, L.; Shen, J.; Li, W.; Zhang, P. Synthesis of (E)-Quinoxalinone Oximes through a Multicomponent Reaction under Mild Conditions. Org. Lett. 2020, 23, 195–201. [Google Scholar] [CrossRef]
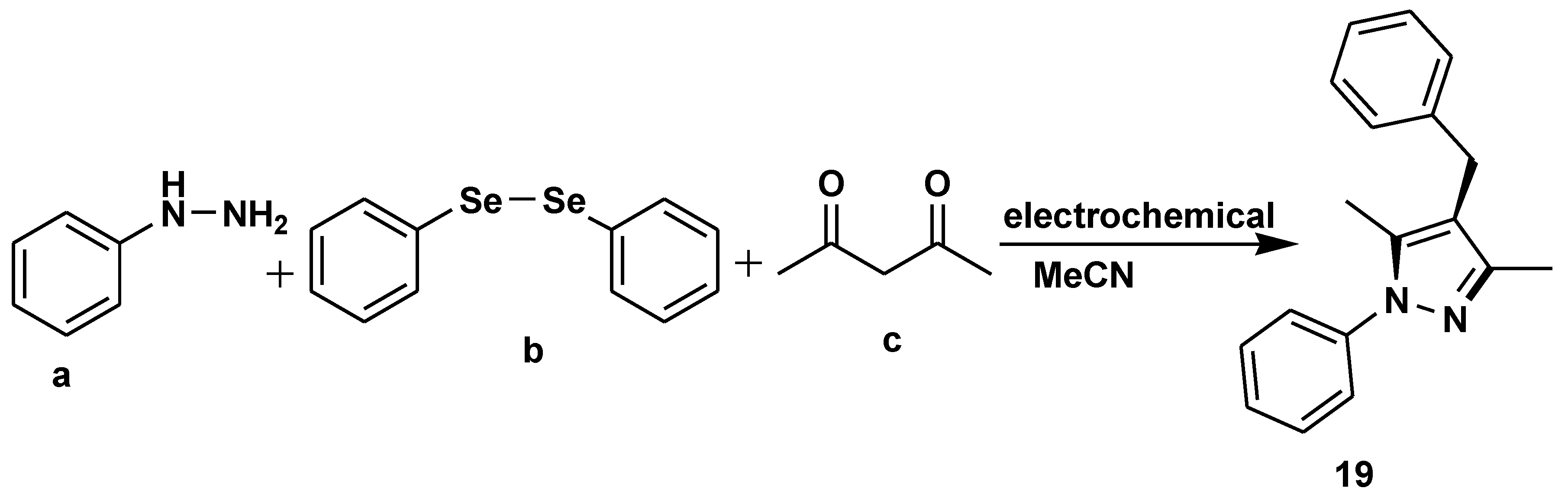
- Wu, Y.; Chen, J.-Y.; Ning, J.; Jiang, X.; Deng, J.; Deng, Y.; Xu, R.; He, W.-M. Electrochemical multicomponent synthesis of 4-selanylpyrazoles under catalyst- and chemical-oxidant-free conditions. Green Chem. 2021, 23, 3950–3954. [Google Scholar] [CrossRef]
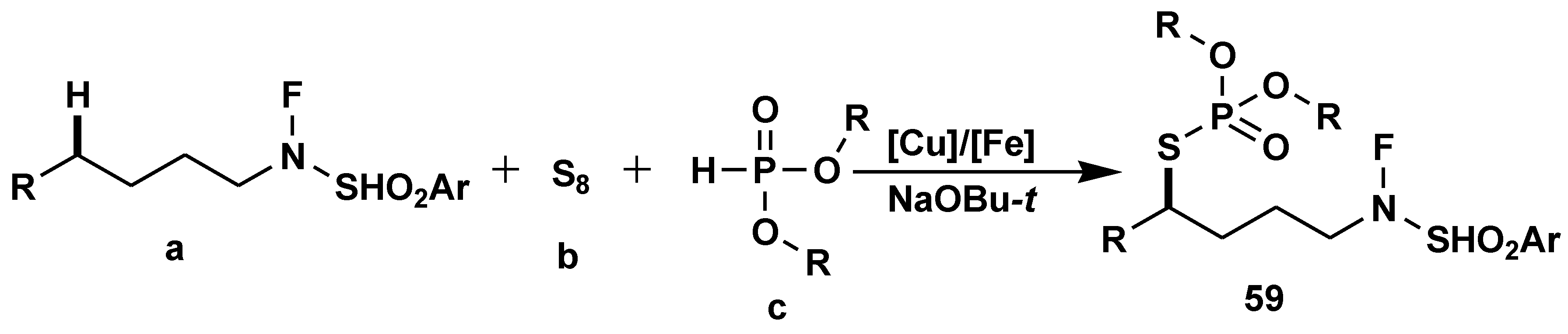
- Qu, C.; Liu, R.; Wang, Z.; Lv, Y.; Yue, H.; Wei, W. Visible-light-driven multicomponent reactions to access S-alkyl phosphorothioates using elemental sulfur as the sulfur source. Green Chem. 2022, 24, 4915–4920. [Google Scholar] [CrossRef]
- Das, S.; Paul, S.; Mitra, B.; Pariyar, G.C.; Ghosh, P. PEG-200: A versatile green solvent assisted catalyst-free one-pot three-component synthesis of functionalised N-amino-3-cyano-2-pyridone. Results Chem. 2023, 5. [Google Scholar] [CrossRef]
- Khaligh, N.G.; Mihankhah, T. Green and Solid-Phase Synthesis of New Dihydro-[1,2,4]Triazolo [1,5-a]Pyrimidine Scaffolds by Using Poly-Melamine-Formaldehyde as a Nitrogen-Rich Porous Organocatalyst. POLYCYCLIC AROMATIC COMPOUNDS, 2022, 42, 942–950. [Google Scholar] [CrossRef]
- Li, K.; Lv, Y.; Lu, Z.; Yun, X.; Yan, S. An environmentally benign multi-component reaction: Highly regioselective synthesis of functionalized 2-(diarylphosphoryl)-1,2-dihydro-pyridine derivatives. Green Synth. Catal. 2021, 3, 59–68. [Google Scholar] [CrossRef]
- Paul, S.; Das, S.; Mitra, B.; Pariyar, G.C.; Ghosh, P. β-Cyclodextrin: a green supramolecular catalyst assisted eco-friendly one-pot three-component synthesis of biologically active substituted pyrrolidine-2-one. RSC Adv. 2023, 13, 5457–5466. [Google Scholar] [CrossRef]
- Kerru, N.; Maddila, S.; Jonnalagadda, S.B. A Facile and Catalyst-Free Microwave-Promoted Multicomponent Reaction for the Synthesis of Functionalised 1,4-Dihydropyridines With Superb Selectivity and Yields. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Awad, J.M.; Xie, G.; Qiu, W.; Zhang, W. One-pot synthesis of tetrahydro-pyrrolobenzodiazepines and tetrahydro-pyrrolobenzodiazepinones through sequential 1,3-dipolar cycloaddition/N-alkylation (N-acylation)/Staudinger/aza-Wittig reactions. Green Chem. 2019, 21, 4489–4494. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Abdelhamid, A.A.; Khalaf, M.M.; Gouda, M.; Elkanzi, N.A.A.; El-Shamy, H.; Ali, A.M. Green Synthesis of Novel Pyridines via One-Pot Multicomponent Reaction and Their Anti-Inflammatory Evaluation. ACS Omega 2023, 8, 11326–11334. [Google Scholar] [CrossRef]
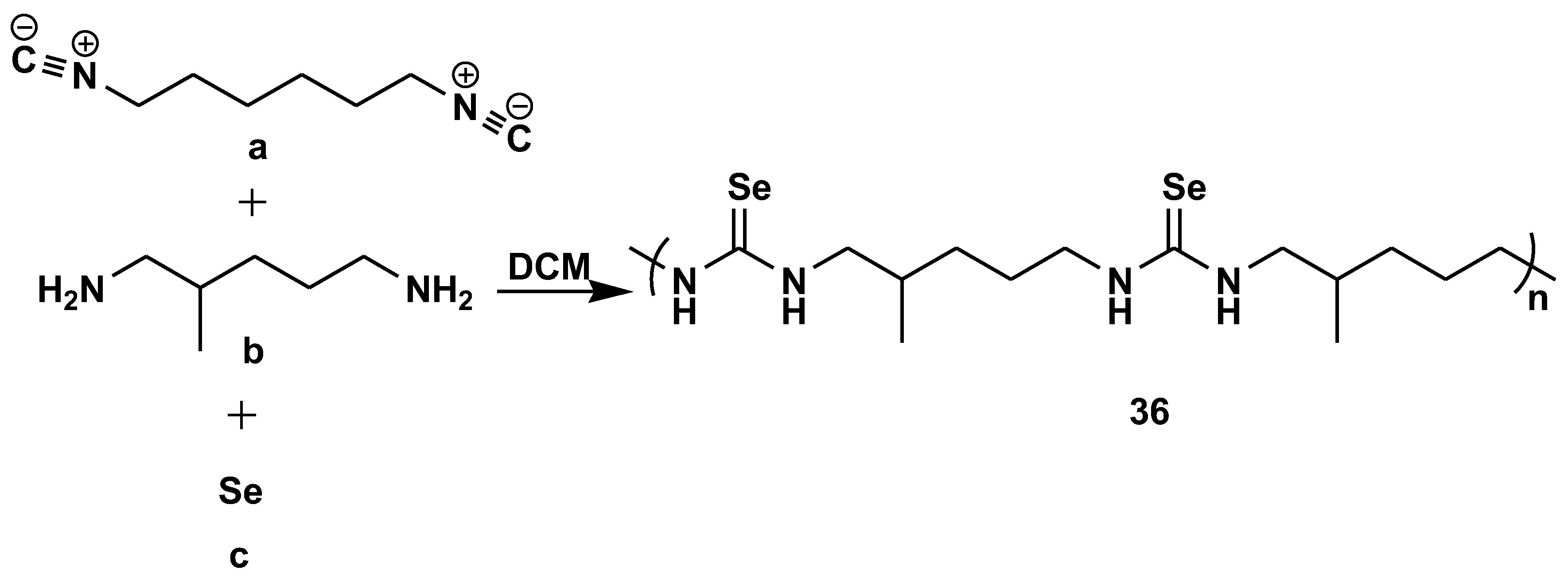
- Tian, T.; Hu, R.; Tang, B.Z. Room Temperature One-Step Conversion from Elemental Sulfur to Functional Polythioureas through Catalyst-Free Multicomponent Polymerizations. J. Am. Chem. Soc. 2018, 140, 6156–6163. [Google Scholar] [CrossRef]
- Velencoso, M. M.; Battig, A.; Markwart, J. C.; Schartel, B.; Wurm, F. R. Molekulare Brandbekämpfung—wie moderne Phosphorchemie zur Lösung der Flammschutzaufgabe beitragen kann. Angew. Chem.,Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
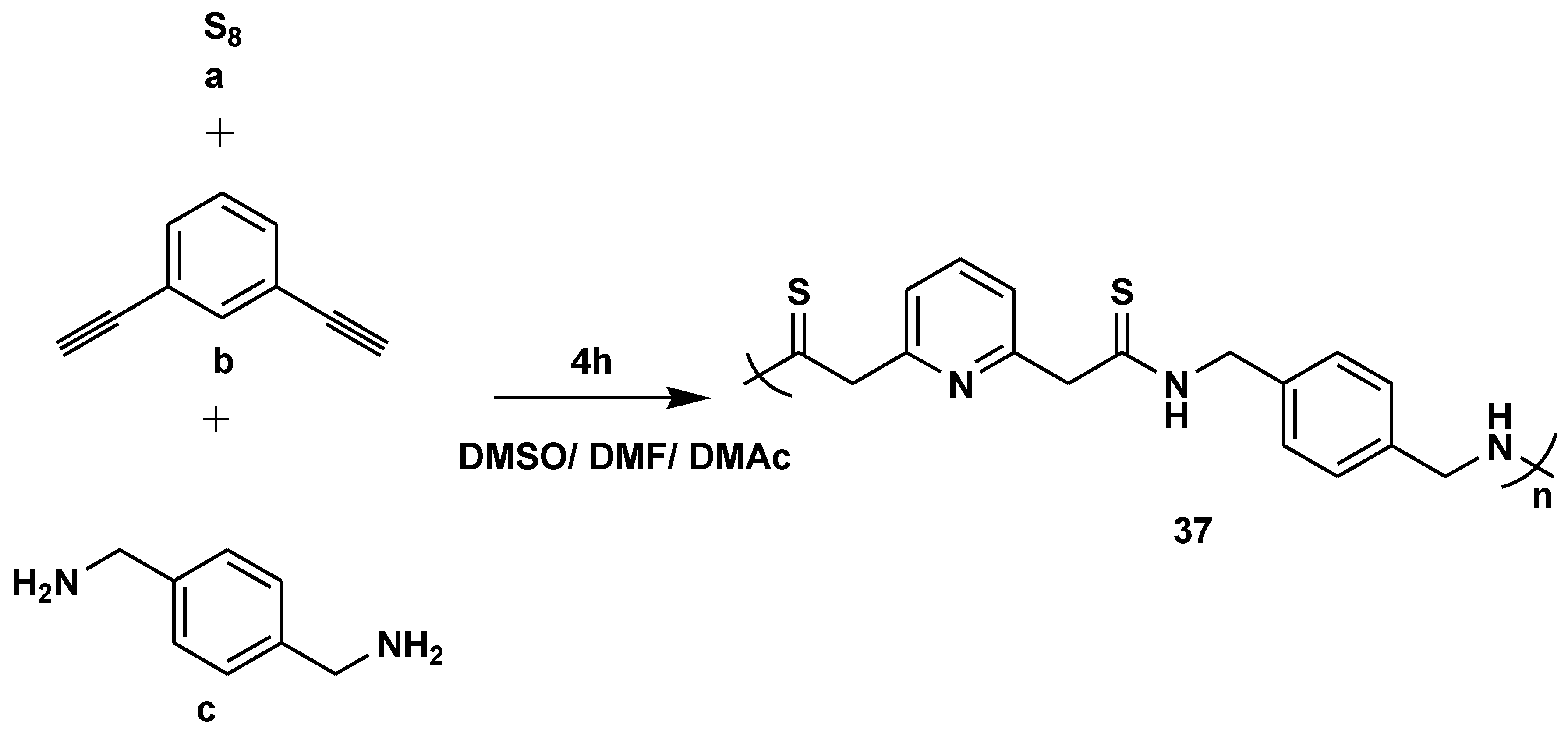
- Wang, T.; Zhang, N.; Bai, W.; Bao, Y. Synthesis of Stable Thiazole-Linked Covalent Organic Frameworks via a Multicomponent Reaction. One-Step Multicomponent Polymerizations for the Synthesis of Multifunctional AIE Polymers Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Han, T.; Niu, N.; Li, H.; Wang, D.; Tang, B.Z. Facile Multicomponent Polymerizations toward Multifunctional Heterochain Polymers with α,β-Unsaturated Amidines. Macromolecules, 2021, 54, 9906–9918. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, T.; Liao, M. , Hu; R. , Tang, Z.B. Multicomponent Polymerizations of Alkynes, Sulfonyl Azides, and 2-Hydroxybenzonitrile/2-Aminobenzonitrile toward Multifunctional Iminocoumarin/Quinoline-Containing Poly(N-sulfonylimine)s. ACS Macro Lett. 2019, 8, 2, 101–106. [Google Scholar]
- Tian, T.; Hu, R.; Tang, B.Z. Room Temperature One-Step Conversion from Elemental Sulfur to Functional Polythioureas through Catalyst-Free Multicomponent Polymerizations. J. Am. Chem. Soc. 2018, 140, 6156–6163. [Google Scholar] [CrossRef]
- Su, X.; Gao, Q.; Wang, D.; Han, T.; Tang, B.Z. One-Step Multicomponent Polymerizations for the Synthesis of Multifunctional AIE Polymers. Macromol. Rapid Commun. 2020, 42, e2000471. [Google Scholar] [CrossRef] [PubMed]
- Tuten, B.T.; Bloesser, F.R.; Marshall, D.L.; Michalek, L.; Schmitt, C.W.; Blanksby, S.J.; Barner-Kowollik, C. Polyselenoureas via Multicomponent Polymerizations Using Elemental Selenium as Monomer. ACS Macro Lett. 2018, 7, 898–903. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Hu, R.; Tang, B.Z. Room temperature synthesis of polythioamides from multicomponent polymerization of sulfur, pyridine-activated alkyne, and amines. Chem. Commun. 2022, 58, 1994–1997. [Google Scholar] [CrossRef]
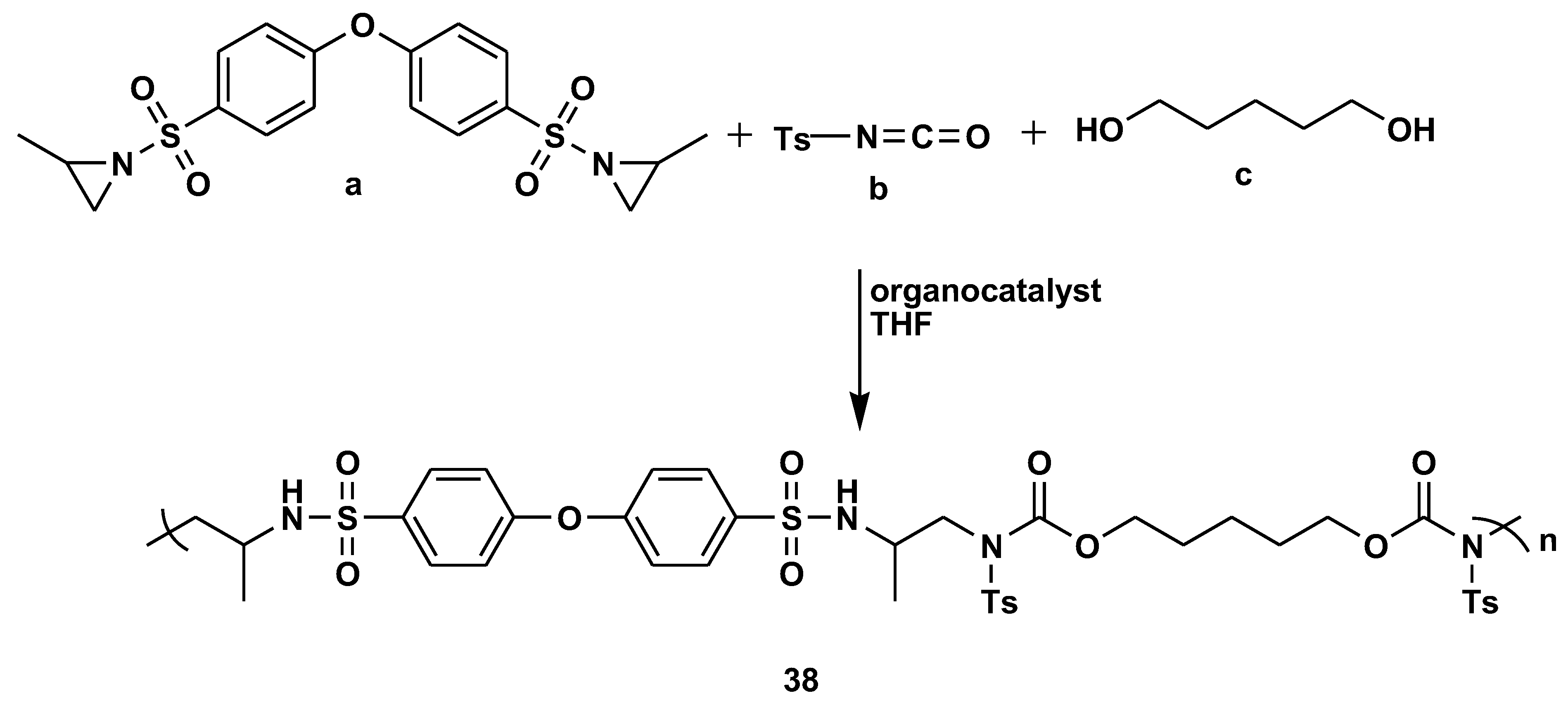
- Chen, Q.; Ye, J.; Zhu, L.; Luo, J.; Cao, X.; Zhang, Z. Organocatalytic multicomponent polymerization of bis(aziridine)s, diols, and tosyl isocyanate toward poly(sulfonamide urethane)s. Eur. Polym. J. 2022, 180. [Google Scholar] [CrossRef]
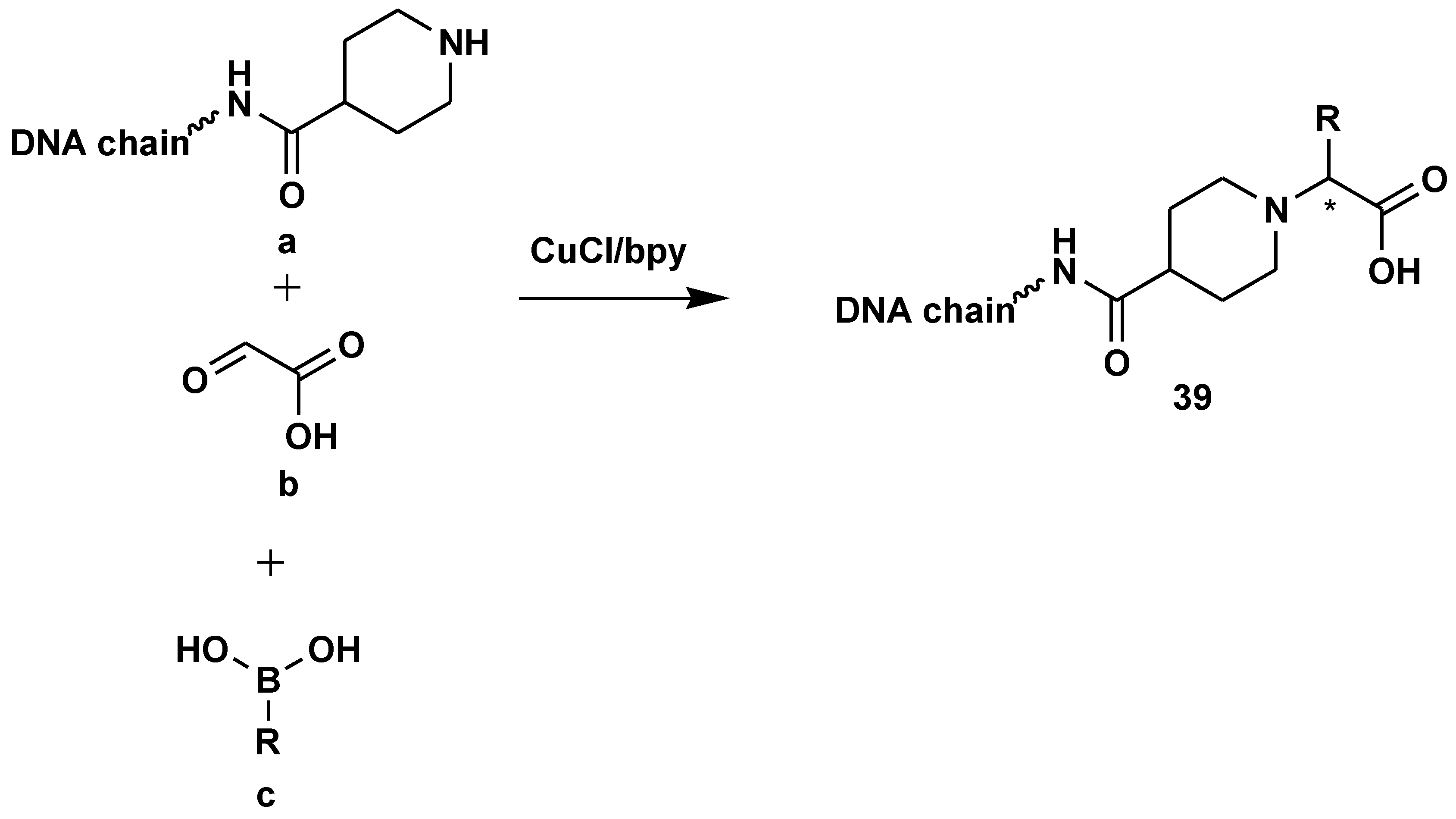
- Potowski, M.; Esken, R.; Brunschweiger, A. Translation of the copper/bipyridine-promoted Petasis reaction to solid phase-coupled DNA for encoded library synthesis. Bioorganic Med. Chem. 2020, 28, 115441. [Google Scholar] [CrossRef]
- Zaharani, L. ; Khaligh, N, G.; Mihankhah, T.; ·Johan, M.R. Application of nitrogen-rich porous organic polymer for the solid-phase synthesis of 2-amino-4H-benzo[b]pyran scaffolds using ball milling process. Molecular Diversity, 2021, 25:323-332.
- Bucci, R.; Dapiaggi, F.; Macut, H.; Pieraccini, S.; Sironi, M.; Gelmi, M.L.; Erba, E.; Pellegrino, S. On-resin multicomponent 1,3-dipolar cycloaddition of cyclopentanone–proline enamines and sulfonylazides as an efficient tool for the synthesis of amidino depsipeptide mimics. Amino Acids, 2020, 52, 15–24. [Google Scholar] [CrossRef]
- Méndez, Y.; De Armas, G.; Pérez, I.; Rojas, T.; Valdés-Tresanco, M.E.; Izquierdo, M.; del Rivero, M.A.; Álvarez-Ginarte, Y.M.; Valiente, P.A.; Soto, C.; et al. Discovery of potent and selective inhibitors of the Escherichia coli M1-aminopeptidase via multicomponent solid-phase synthesis of tetrazole-peptidomimetics. Eur. J. Med. Chem. 2018, 163, 481–499. [Google Scholar] [CrossRef]
- Massarano, T.; Mazir, A.; Lavi, R.; Byk, G. Solid-Phase Multicomponent Synthesis of 3-Substituted Isoindolinones Generates New Cell-Penetrating Probes as Drug Carriers. ChemMedChem 2020, 15, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Alvim, H.G.O.; Pinheiro, D.L.J.; Carvalho-Silva, V.H.; Fioramonte, M.; Gozzo, F.C.; da Silva, W.A.; Amarante, G.W.; Neto, B.A.D. Combined Role of the Asymmetric Counteranion-Directed Catalysis (ACDC) and Ionic Liquid Effect for the Enantioselective Biginelli Multicomponent Reaction. J. Org. Chem. 2018, 83, 12143–12153. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Yang, J.; Lin, L.; Feng, X.; Liu, X. Asymmetric Three-Component Reaction for the Synthesis of Tetrasubstituted Allenoates via Allenoate-Copper Intermediates. Chem 2018, 4, 1658–1672. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, L.; Yang, J.; Liu, X.; Feng, X. Asymmetric Synthesis of Tetrahydroindolizines by Bimetallic Relay Catalyzed Cycloaddition of Pyridinium Ylides. Angew. Chem. Int. Ed. 2018, 57, 12323–12327. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.-K.; Zhu, J.; Zhou, L.; Wang, L.; Wang, S.R.; Tang, Y. Synergetic Tandem Enantiomeric Enrichment in Catalytic Asymmetric Multi-Component Reactions (AMCRs): Highly Enantioselective Construction of Tetracyclic Indolines with Four Continuous Stereocenters. ACS Catal. 2018, 8, 4991–4995. [Google Scholar] [CrossRef]
- Ji, Y-L. ; Li, H-P.; Ai, Y-Y.; Li, G.; He, X-H.; Huang, W.; Huang, R-Z.; Han, B. Enantio- A nd diastereoselective synthesis of spiropyrazolones: Via an organocatalytic [1 + 2 + 3] multicomponent reaction. Org. Biomol. Chem., 2019, 17, 9217–9225.
- Xiong, Q.; Li, G.; Dong, S.; Liu, X.; Feng, X. Enantioselective Synthesis of Hydrothiazole Derivatives via an Isocyanide-Based Multicomponent Reaction. Org. Lett. 2019, 21, 8771–8775. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Huang, J.; Liao, N.; Liu, Y.; Guo, Q.; Peng, Y. Catalytic Asymmetric Three-Component Reaction of 2-Alkynylbenzaldehydes, Amines, and Dimethylphosphonate. Org. Lett. 2020, 22, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Medina, A.; Pérez-Hernández, N.; Castrejón-Flores, J.L.; Romero-García, S.; Prado-García, H.; Bañuelos-Hernández, A.; Franco-Pérez, M. Obtaining symmetric and asymmetric bisphosphoramidates and bisphosphoramidothioates by a single step multicomponent reaction. Phosphorus, Sulfur and Silicon and the Related Elements, 2021, 196, 634–642. [Google Scholar] [CrossRef]
- Borpatra, P.J.; Rastogi, G.K.; Saikia, B.; Deb, M.L.; Baruah, P.K. Multi-Component Reaction of 6-Aminouracils, Aldehydes and Secondary Amines: Conversion of the Products into Pyrimido [4,5-d]pyrimidines through C-H Amination/Cyclization. ChemistrySelect 2019, 4, 3381–3386. [Google Scholar] [CrossRef]
- Wang, X.-G.; Li, Y.; Liu, H.-C.; Zhang, B.-S.; Gou, X.-Y.; Wang, Q.; Ma, J.-W. ; Liang, Y.-M. Three-Component Ruthenium-Catalyzed Direct Meta-Selective C-H Activation of Arenes: A New Approach to the Alkylarylation of Alkenes. J. Am. Chem. Soc. 2019, 141, 13914–13922. [Google Scholar]
- Herraiz, A.G.; Cramer, N. Cobalt(III)-Catalyzed Diastereo- and Enantioselective Three-Component C–H Functionalization. ACS Catal. 2021, 11, 11938–11944. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, P.; Luo, C.; Zhuo, S.; Zhang, Y.; Tang, G.; Zhao, Y. Copper-Catalyzed Remote C(sp3)–H Phosphorothiolation of Sulfonamides and Carboxamides in a Multicomponent Reaction. Org. Lett. 2020, 22, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Dwari, S.; Jana, C.K. Metal Free C–H Functionalization Enabled Diastereoselective Multicomponent Reaction of N-Heterocycles to Fused Heteropolycycles. J. Org. Chem. 2018, 83, 8874–8887. [Google Scholar] [CrossRef] [PubMed]
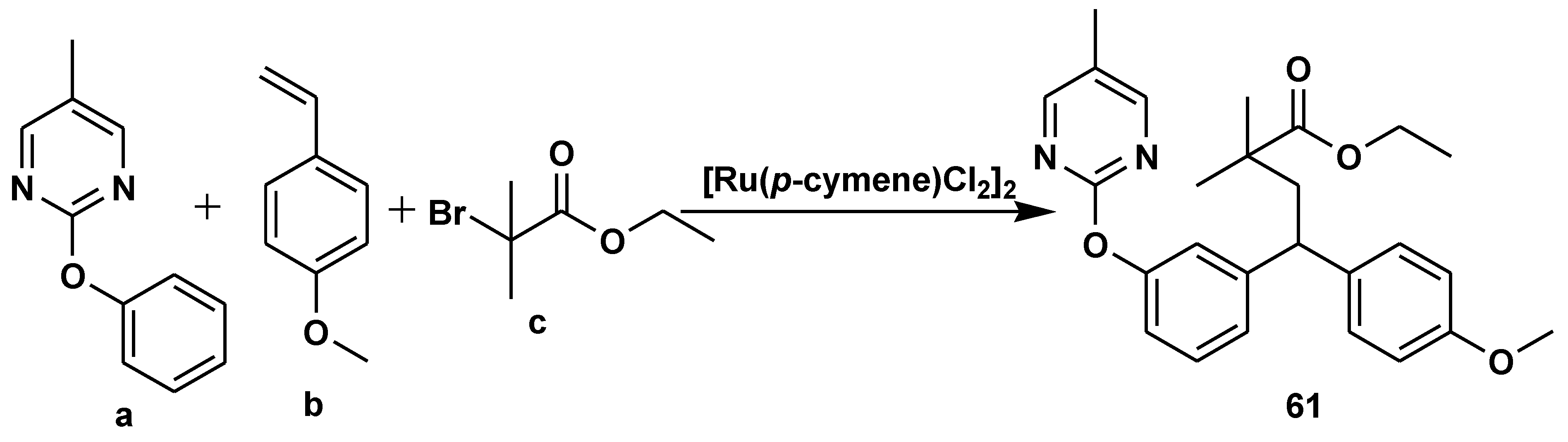
- Luan, Y.-Y.; Gou, X.-Y.; Shi, W.-Y.; Liu, H.-C.; Chen, X.; Liang, Y.-M. Three-Component Ruthenium-Catalyzed meta-C–H Alkylation of Phenol Derivatives. Org. Lett. 2022, 24, 1136–1140. [Google Scholar] [CrossRef]
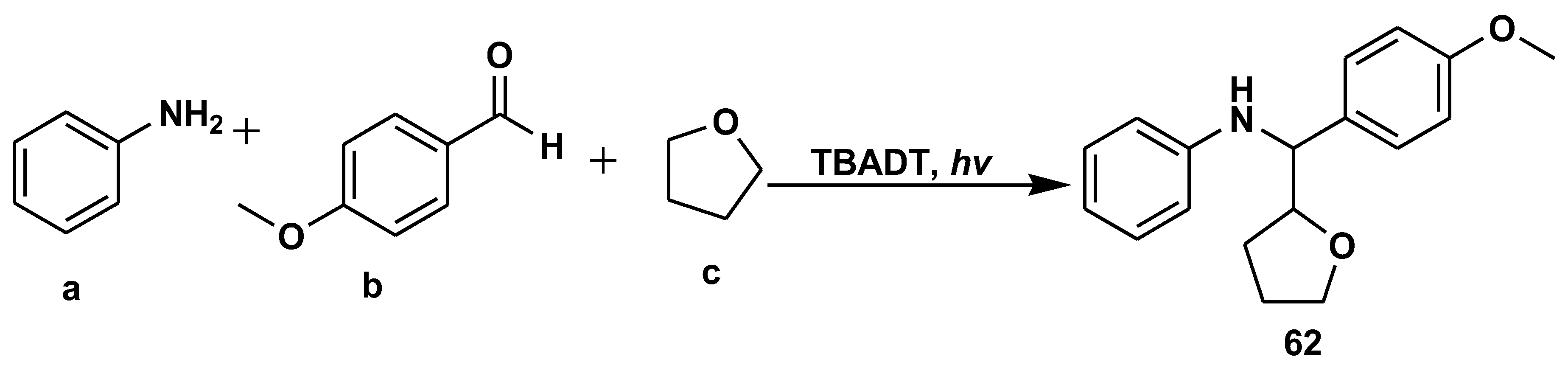
- Pillitteri, S.; Ranjan, P.; Voskressensky, L.G.; Van der Eycken, E.V.; Sharma, U.K. Alkylation of in situ generated imines via photoactivation of strong aliphatic C-H bonds. Mol. Catal. 2021, 514, 111841. [Google Scholar] [CrossRef]
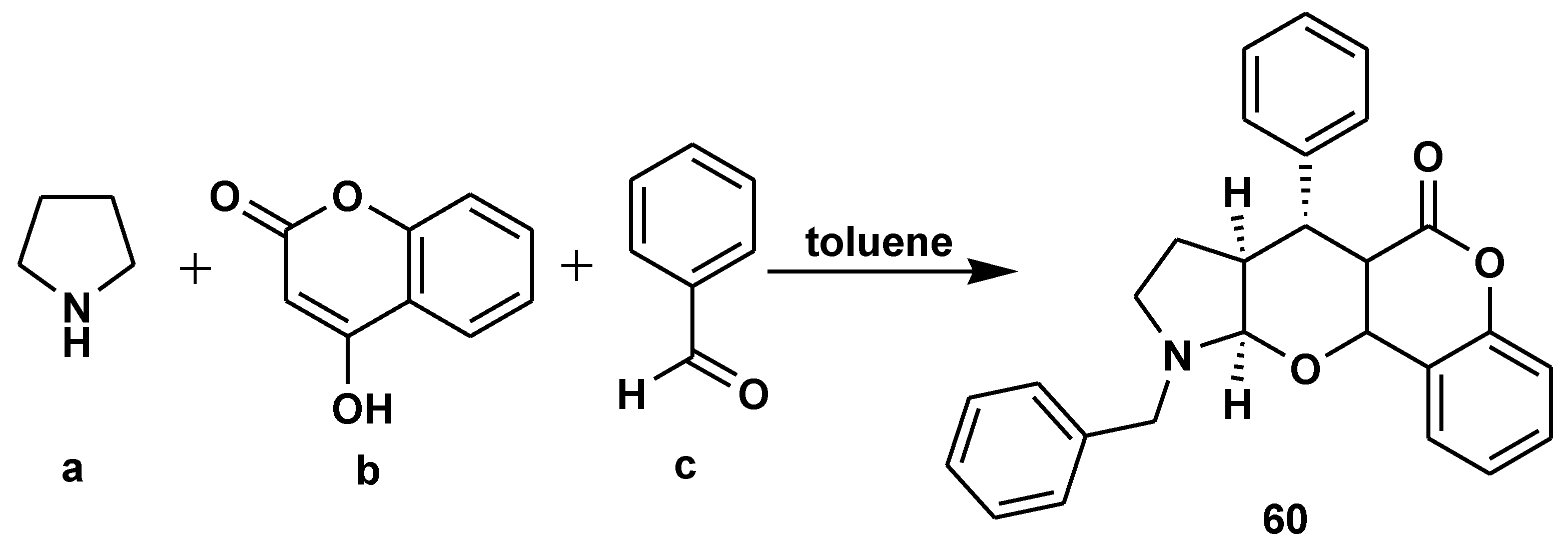
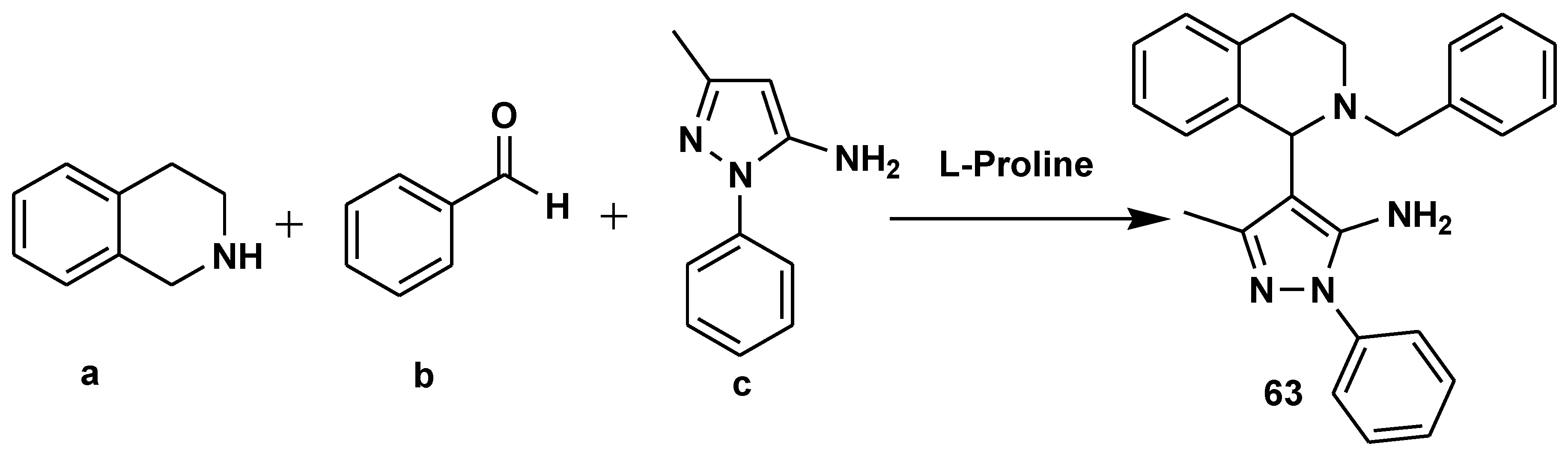
- Rahman, I.; Deka, B.; Thakuria, R.; Deb, M.L.; Baruah, P.K. l-Proline-catalyzed regioselective C1 arylation of tetrahydroisoquinolines through a multicomponent reaction under solvent-free conditions. Org. Biomol. Chem. 2020, 18, 6514–6518. [Google Scholar] [CrossRef]
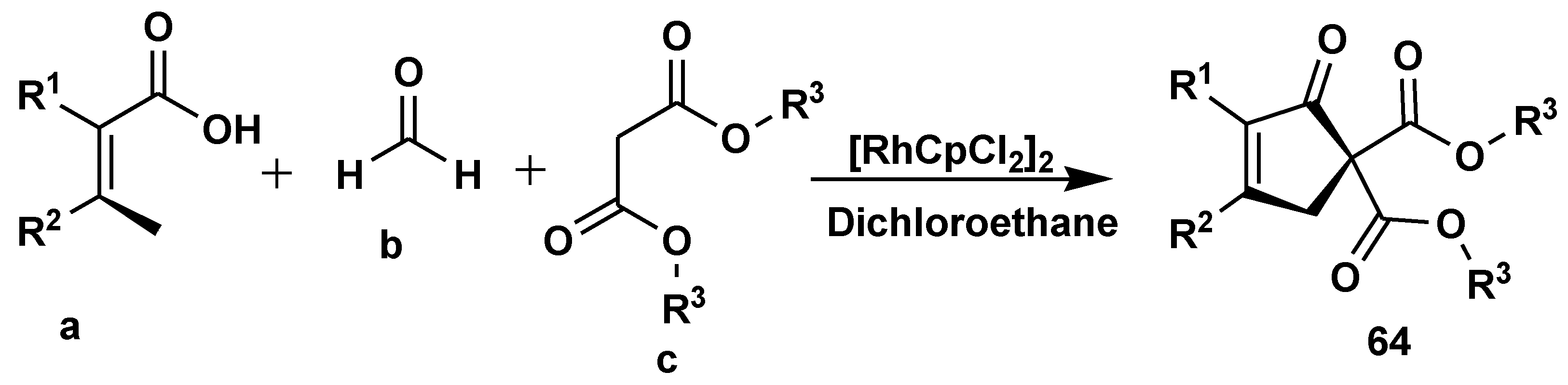
- Yu, S.; Hong, C.; Liu, Z.; Zhang, Y. Synthesis of Cyclopentenones through Rhodium-Catalyzed C–H Annulation of Acrylic Acids with Formaldehyde and Malonates. Org. Lett. 2021, 23, 5054–5059. [Google Scholar] [CrossRef]
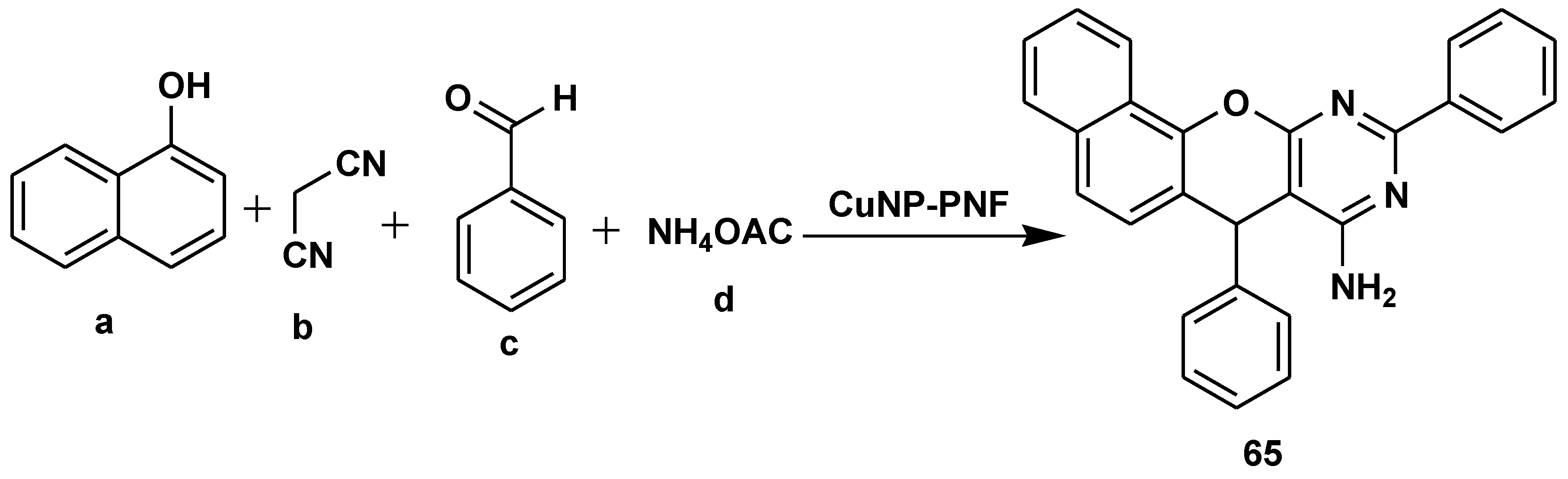
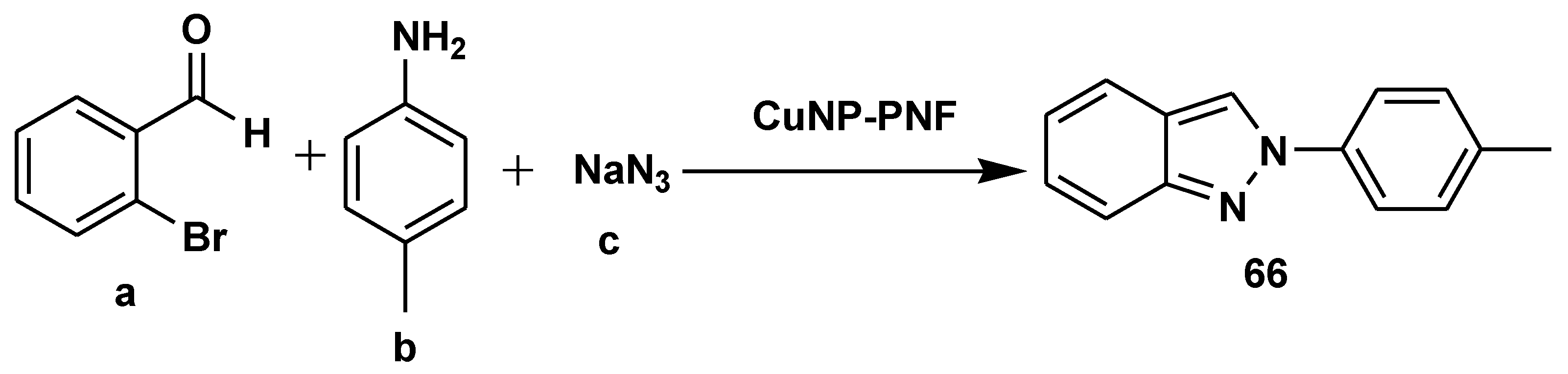
- Taherinia, Z.; Ghorbani-Choghamarani, A.; Hajjami, M. Decorated Peptide Nanofibers with Cu Nanoparticles: An Efficient Catalyst for the Multicomponent Synthesis of Chromeno [2, 3-d] pyrimidin-8-amines, Quinazolines and 2H- Indazoles. ChemistrySelect 2019, 4, 2753–2760. [Google Scholar] [CrossRef]
- Martzel, T.; Annibaletto, J.; Millet, P.; Pair, E.; Sanselme, M.; Oudeyer, S.; Levacher, V. ; Briere, J-F. Organocatalytic Multicomponent Synthesis of α/β-Dipeptide Derivatives. Chem. Eur. J. 2020, 26, 8541–8545. [Google Scholar] [PubMed]
- Nourisefat, M.; Panahi, F.; Khalafi-Nezhad, A. Amino acids and peptides as reactants in multicomponent reactions: modification of peptides with heterocycle backbones through combinatorial chemistry. Mol. Divers. 2018, 23, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, M.; Gengan, R.M.; Ranjan, B.; Muthusamy, R. Synthesis, molecular docking, antimicrobial, antioxidant and toxicity assessment of quinoline peptides. J. Photochem. Photobiol. B: Biol. 2018, 178, 287–295. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, A.F.; Ali, A.; Galetto, F.Z.; Braga, A.L.; Delgado, J.A.C.; Paixão, M.W. One-pot organocatalytic/multicomponent approach for the preparation of novel enantioenriched non-natural selenium-based peptoids and peptide–peptoid conjugates. Mol. Divers. 2019, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marigo, M.; Wabnitz, T.C.; Fielenbach, D.; Jørgensen, K.A. Enantioselective Organocatalyzed α Sulfenylation of Aldehydes. Angew. Chem. Int. Ed. 2005, 44, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Tiecco, M.; Carlone, A.; Sternativo, S.; Marini, F.; Bartoli, G.; Melchiorre, P. Organocatalytic asymmetric α-selenenylation of aldehydes. Angew Chem Int Ed, 2007, 46, 6882–6885. [Google Scholar] [CrossRef] [PubMed]
- Zarezin, D.P.; Shmatova, O.I. ; Nenajdenko, V,G. Chiral β3-isocyanopropionates for multicomponent synthesis of peptides and depsipeptides containing a β-amino acid fragment. Org. Biomol. Chem., 2018, 16, 5987–5998. [Google Scholar]
- Farhid, H.; Nazeri, M.T.; Shaabani, A.; Armaghan, M.; Janiak, C. Isocyanide-based consecutive Bargellini/Ugi reactions: an efficient method for the synthesis of pseudo-peptides containing three amide bonds. Amino Acids 2020, 53, 1–10. [Google Scholar] [CrossRef]
- Strecker, D. , Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Annalen der Chemie und Pharmacie,. Ann. Chem. Pharm. 1850, 75, 27–45. [Google Scholar] [CrossRef]
- Hantzsch, A. Ueber die Synthese pyridinartiger Verbindungen aus Acetessigäther und Aldehydammoniak. Eur. J. Org. Chem. 1882, 215, 1–82. [Google Scholar] [CrossRef]
- Mohlala, R.L.; Coyanis, E.M.; Fernandes, M.A.; Bode, M.L. Catalyst-free synthesis of novel 1,5-benzodiazepines and 3,4-dihydroquinoxalines using isocyanide-based one-pot, three- and four-component reactions. RSC Adv. 2021, 11, 24466–24473. [Google Scholar] [CrossRef] [PubMed]
- Mohlala, R.L.; Coyanis, E.M.; Fernandes, M.A.; Bode, M.L. Synthesis of highly functionalised 5-membered ring fused pyrimidine derivatives using an isocyanide-based one-pot, three component reaction. Tetrahedron Lett. 2020, 61, 151796. [Google Scholar] [CrossRef]
- Ugi, I.; Steinbrückner, C. Chem. Ber., 1961, 94, 734–742. [Google Scholar]
- Lamberth, C, Jeanguenat, A, Cederbaum, F, De Mesmaeker, A, Zeller, M, Kempf H.-J., Zeun R, Bioorg. Med. Chem., 2008, 16, 1531–1545.
- Bossert, F.; Meyer, H.; Wehinger, E. 4-Aryldihydropyridines, a New Class of Highly Active Calcium Antagonists. Angew. Chem. Int. Ed. 1981, 20, 762–769. [Google Scholar] [CrossRef]
- Rosenblum, S.B. ; Huynh. T.; Afonso, A.; Davis, H.R.; Yumibe, N.; Clader, J.W.; Burnett, D.A. Discovery of 1-(4-Fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)- hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (SCH 58235): A Designed, Potent, Orally Active Inhibitor of Cholesterol Absorption. J. Med. Chem., 1998, 41, 973–980. [Google Scholar] [CrossRef]
- Vázquez-Romero, A.; Cárdenas, L.; Blasi, E.; Verdaguer, X.; Riera, A. Synthesis of Prostaglandin and Phytoprostane B1 Via Regioselective Intermolecular Pauson−Khand Reactions. Org. Lett. 2009, 11, 3104–3107. [Google Scholar] [CrossRef]
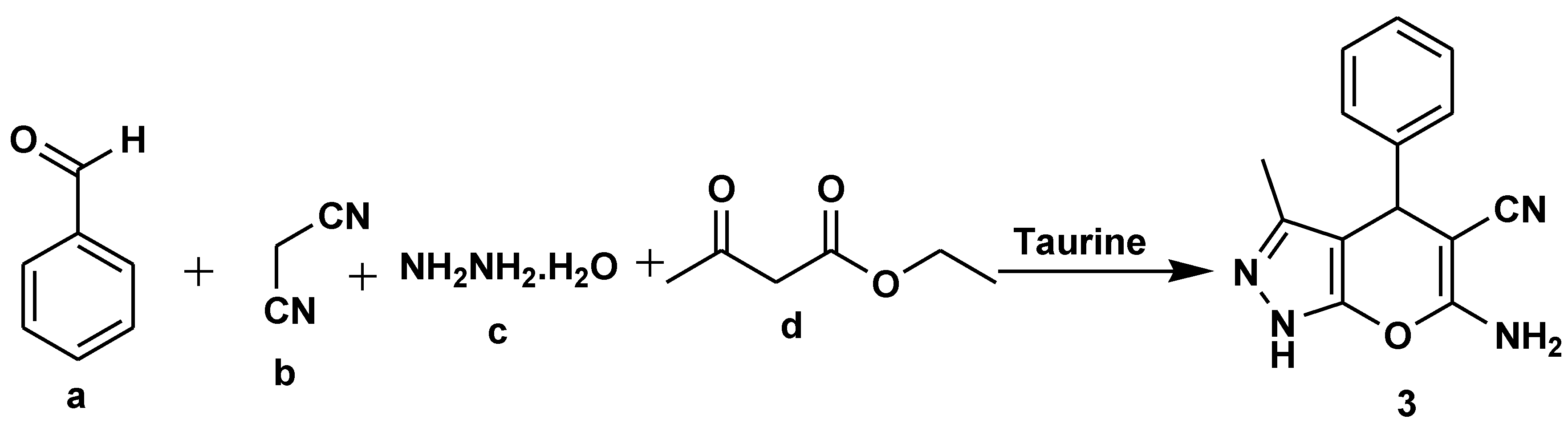
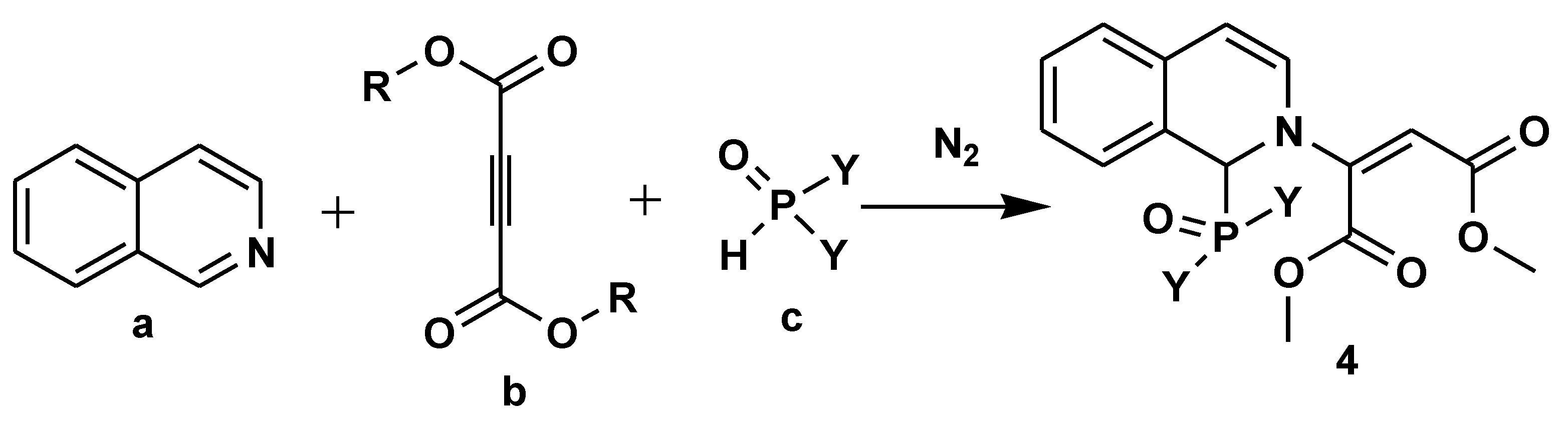
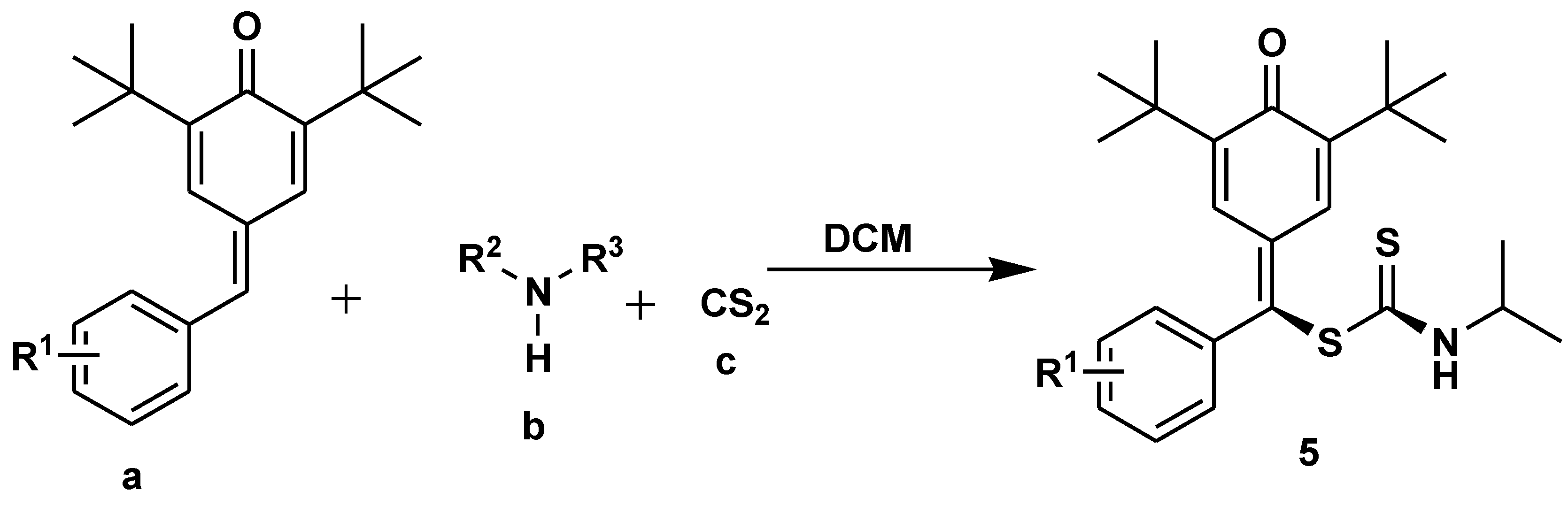
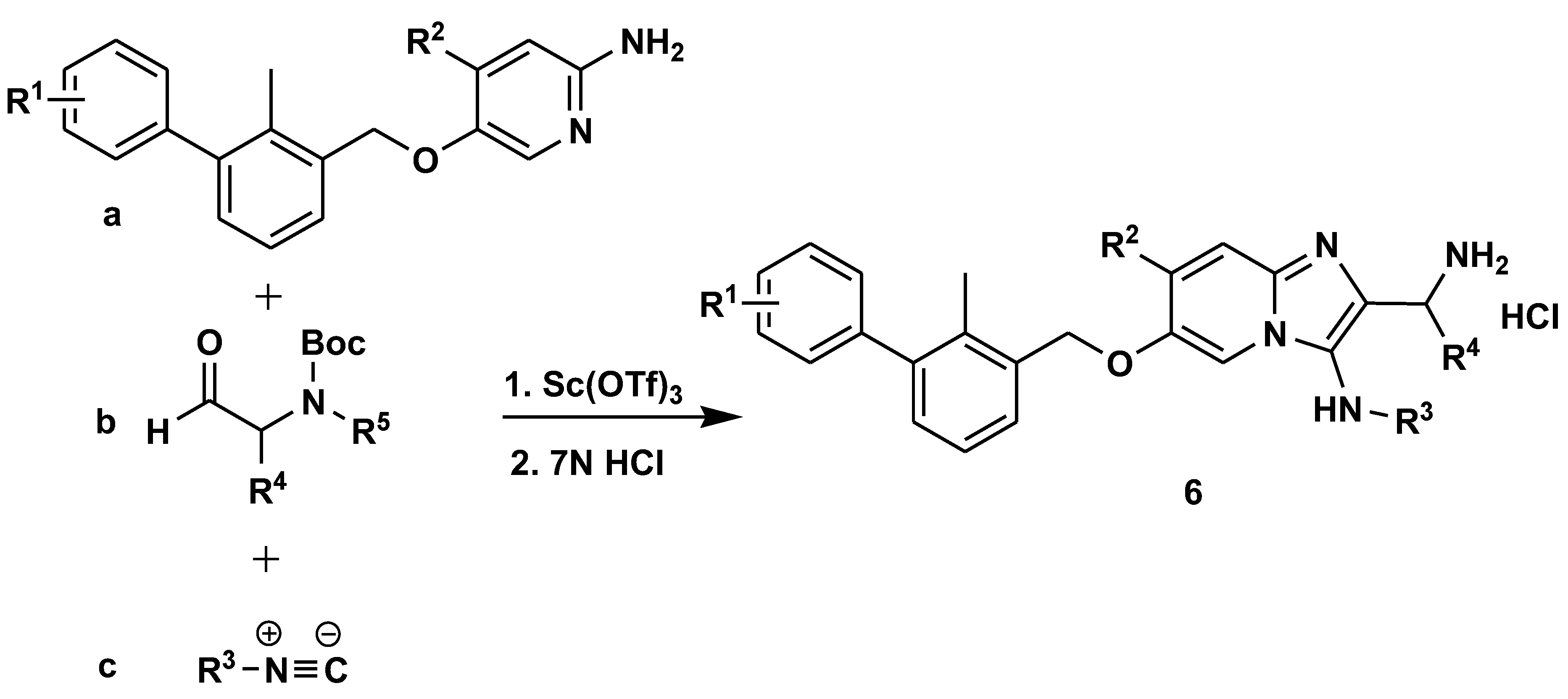
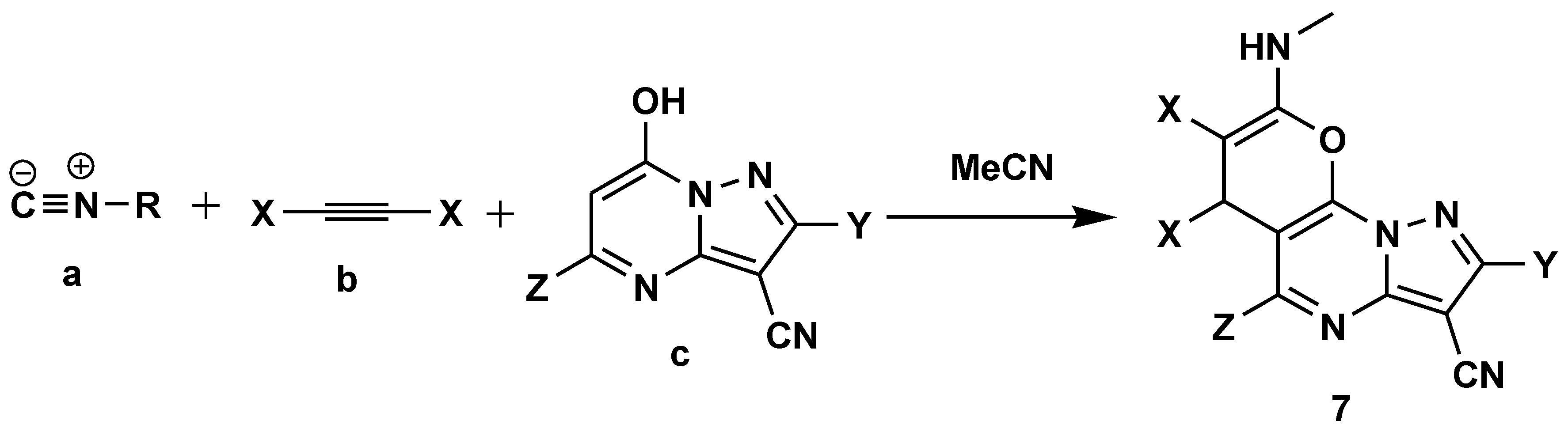
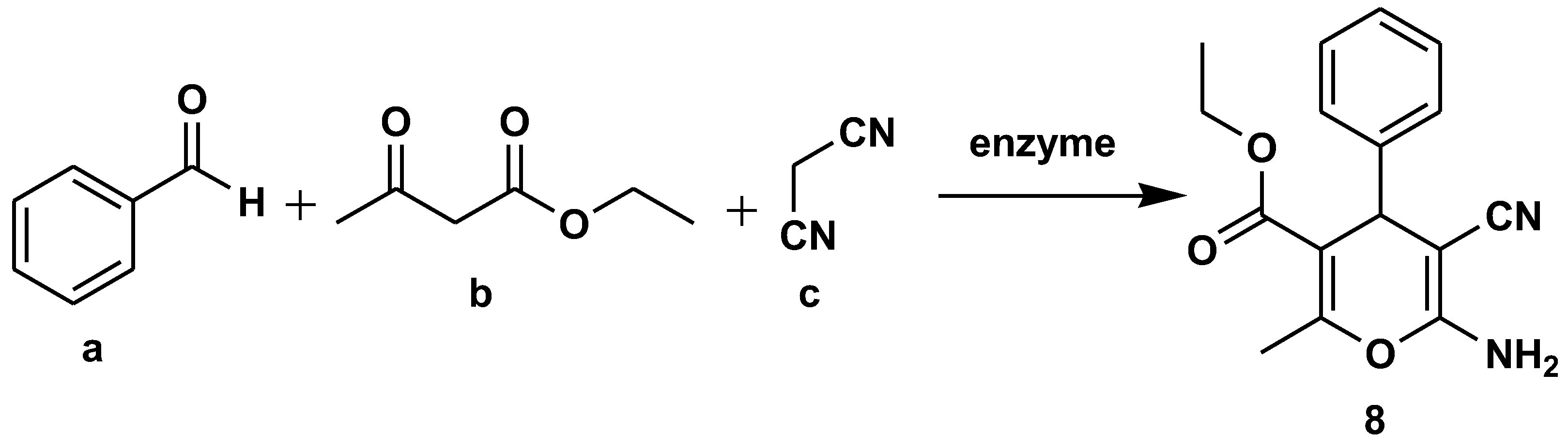
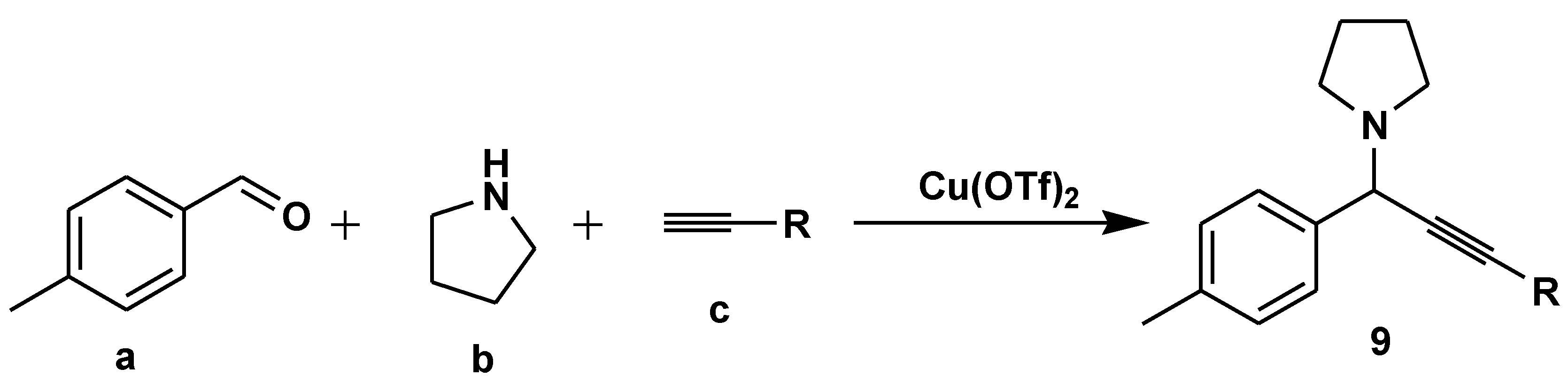
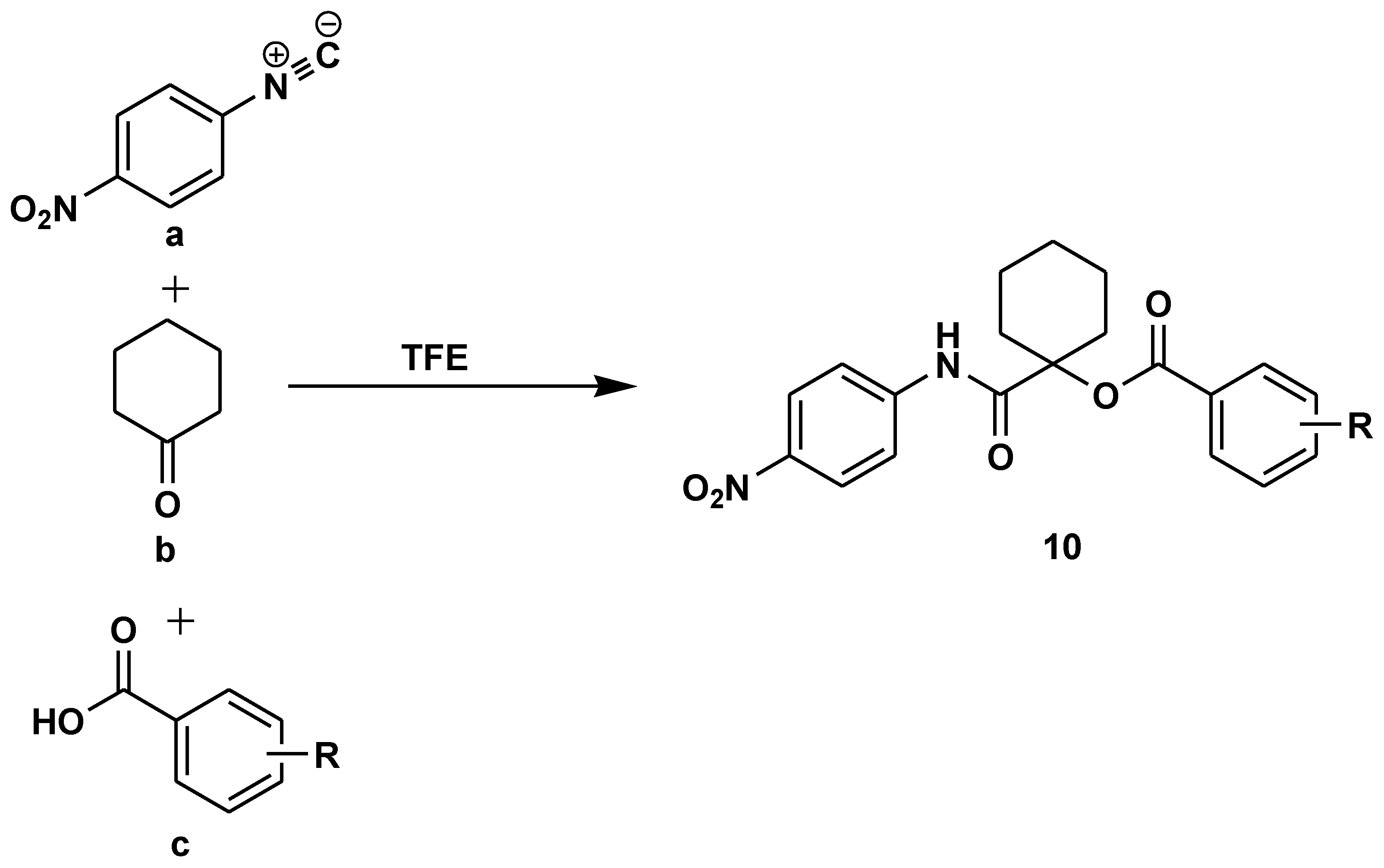
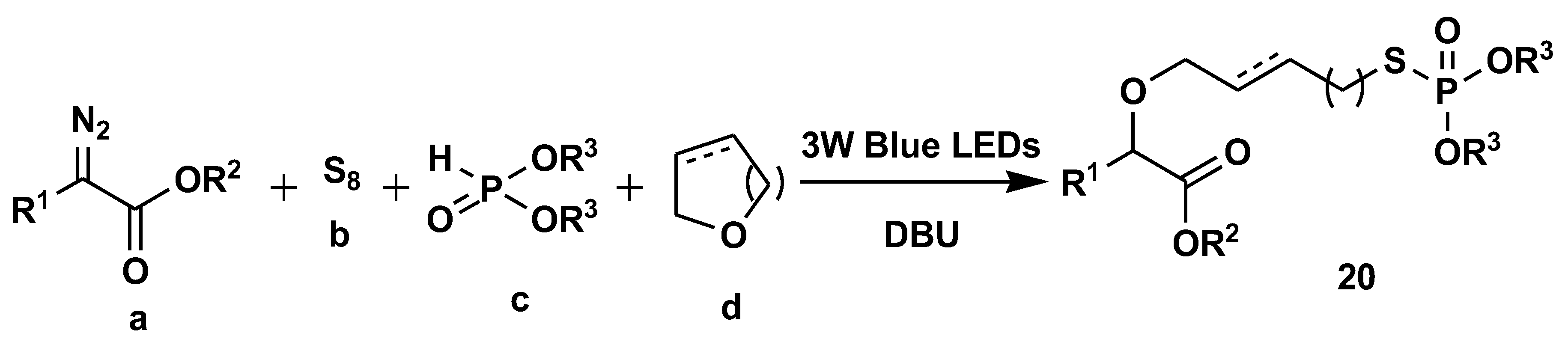
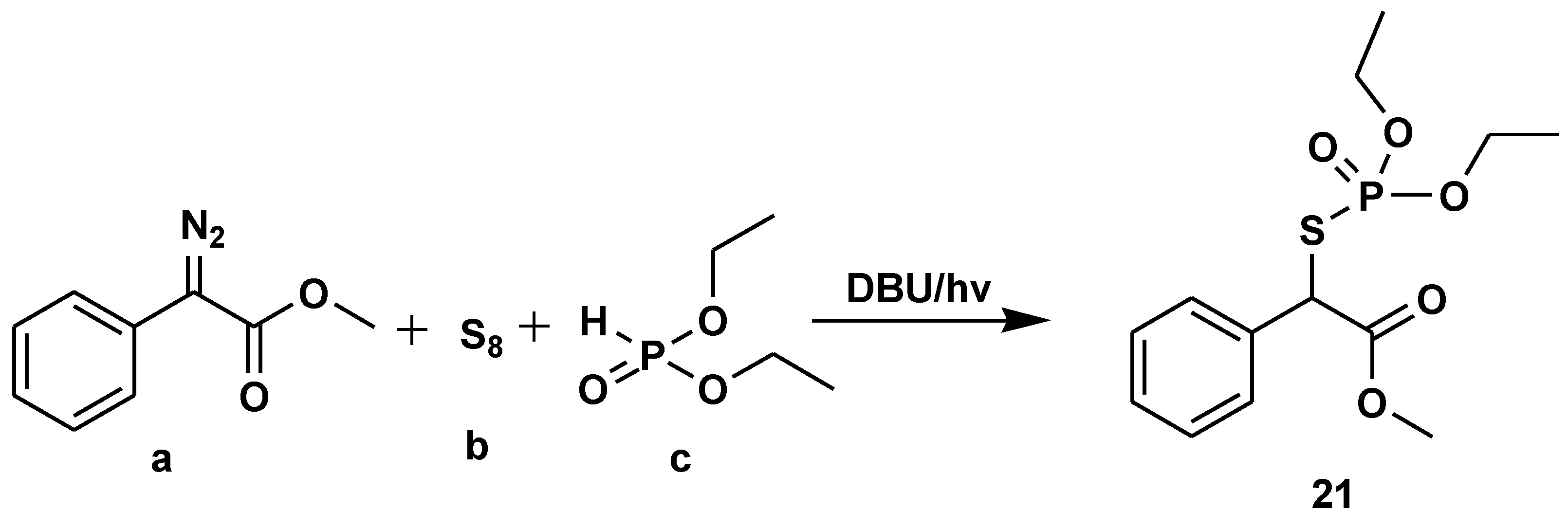
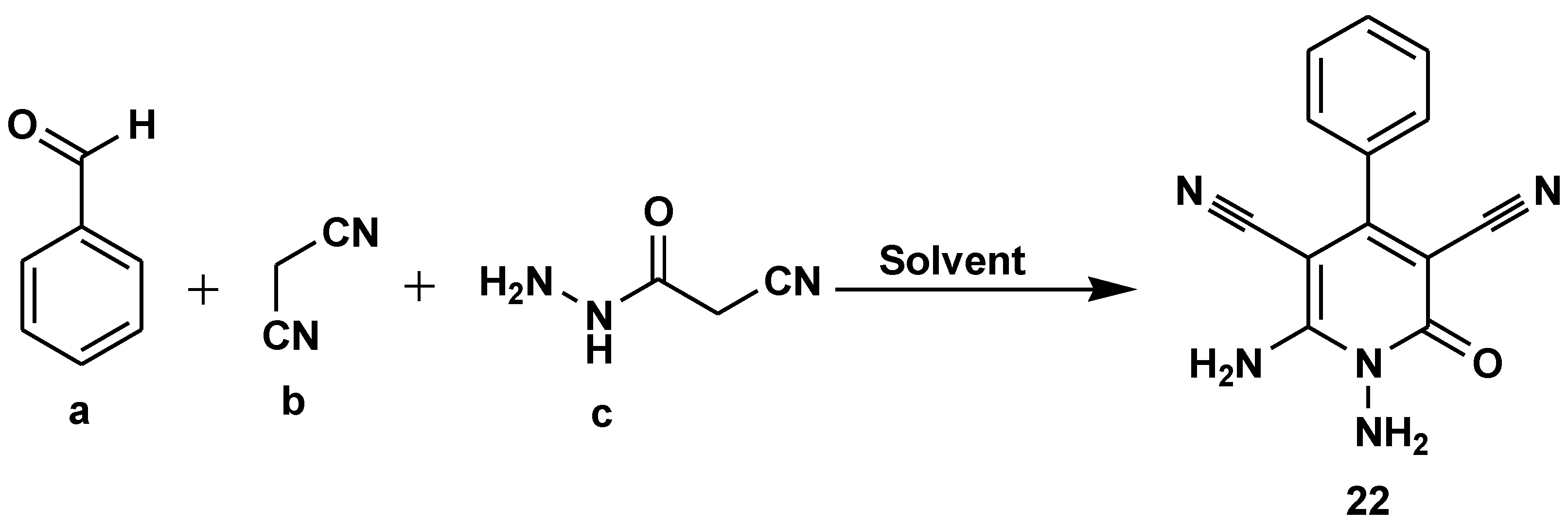
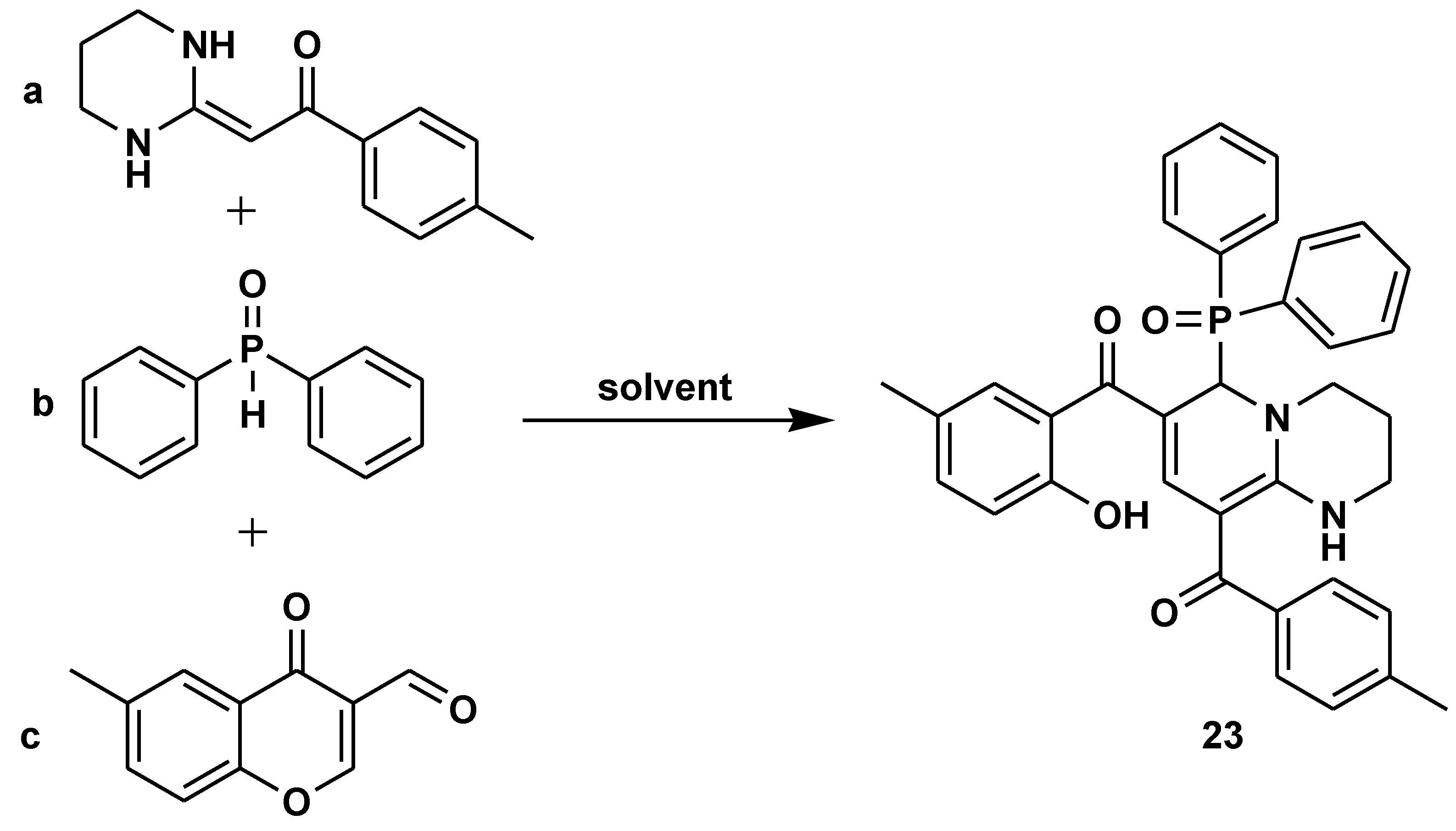
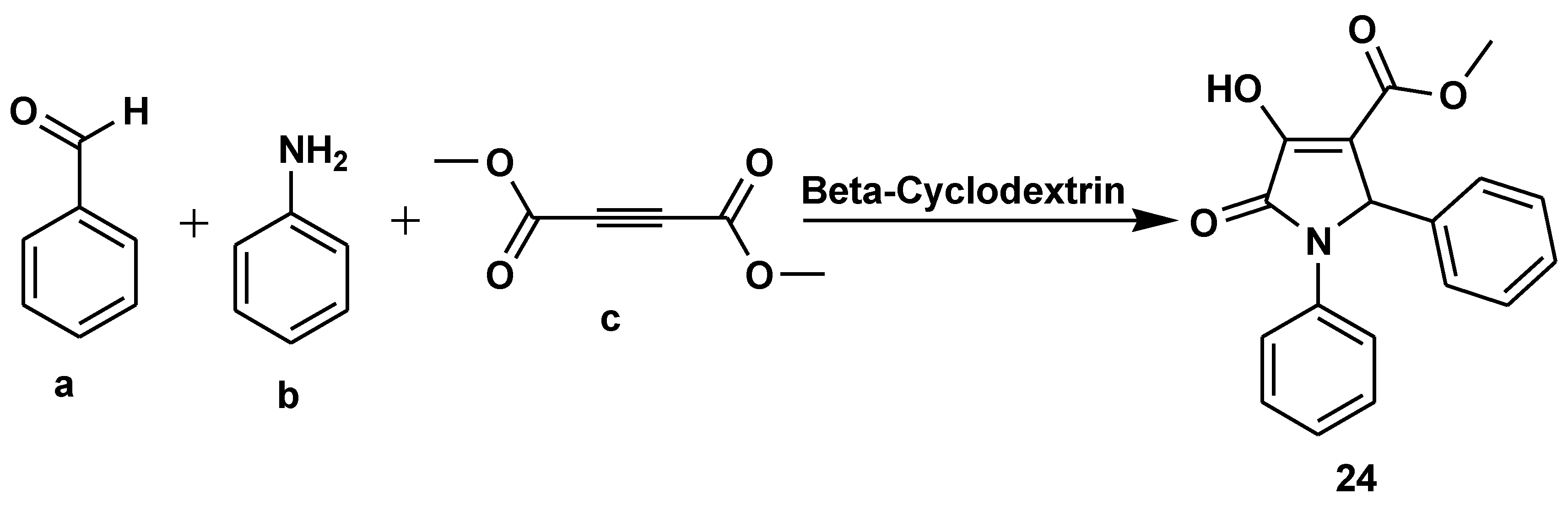
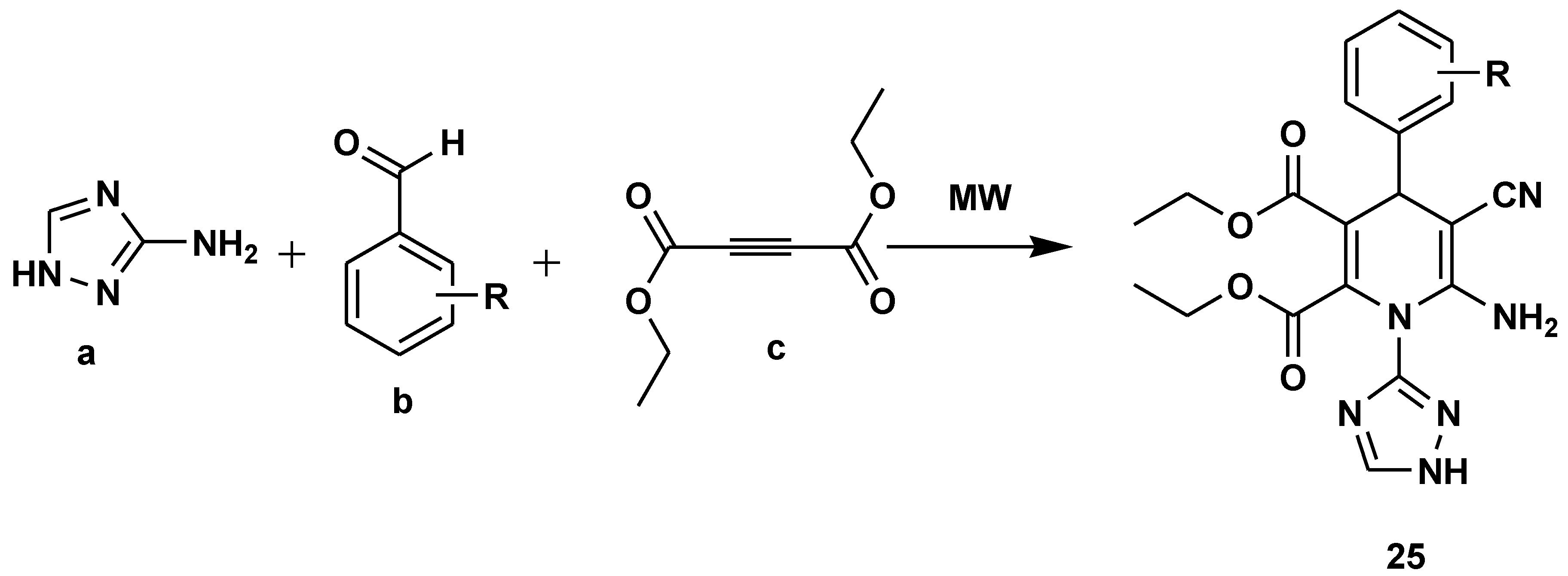
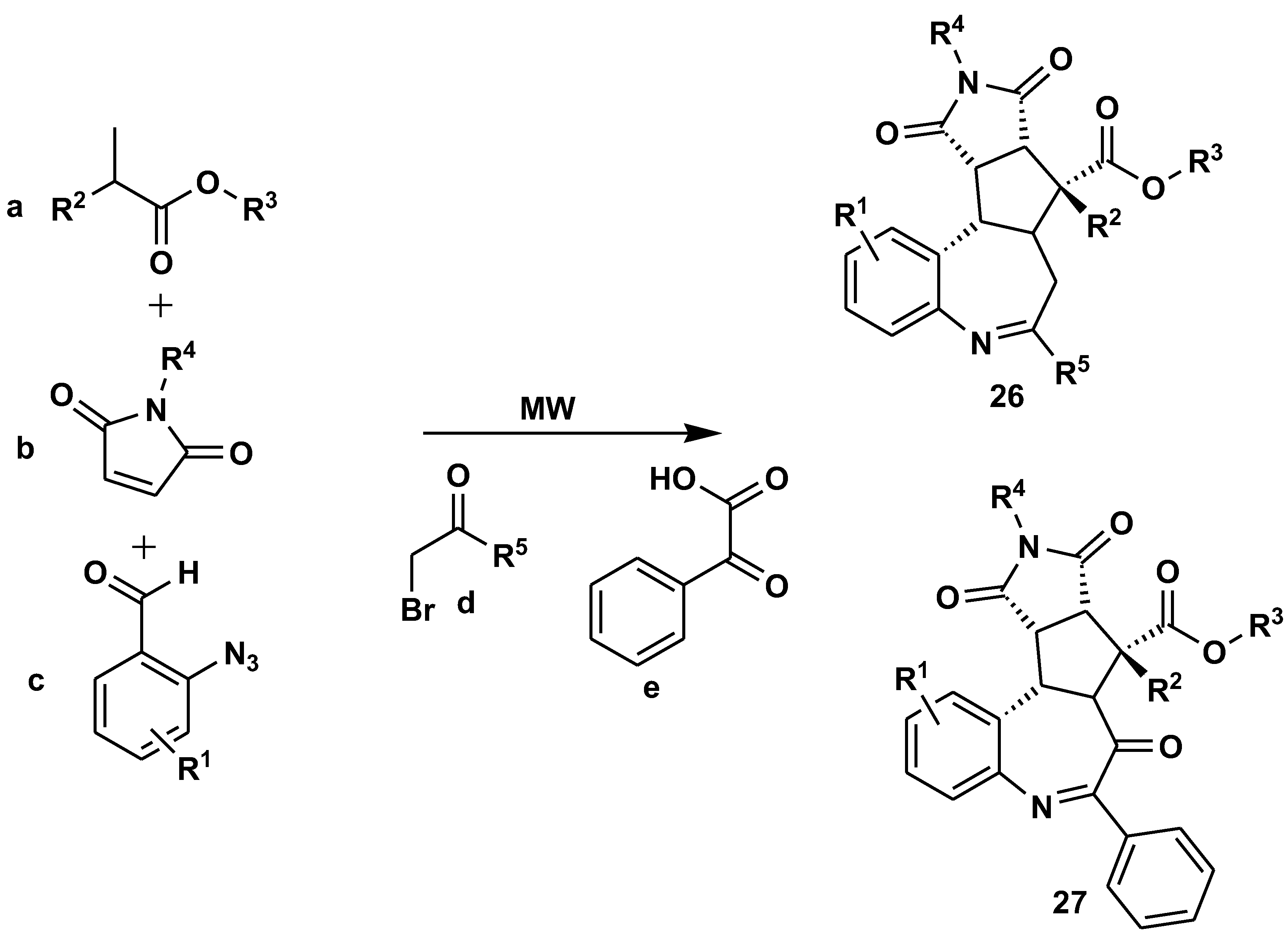
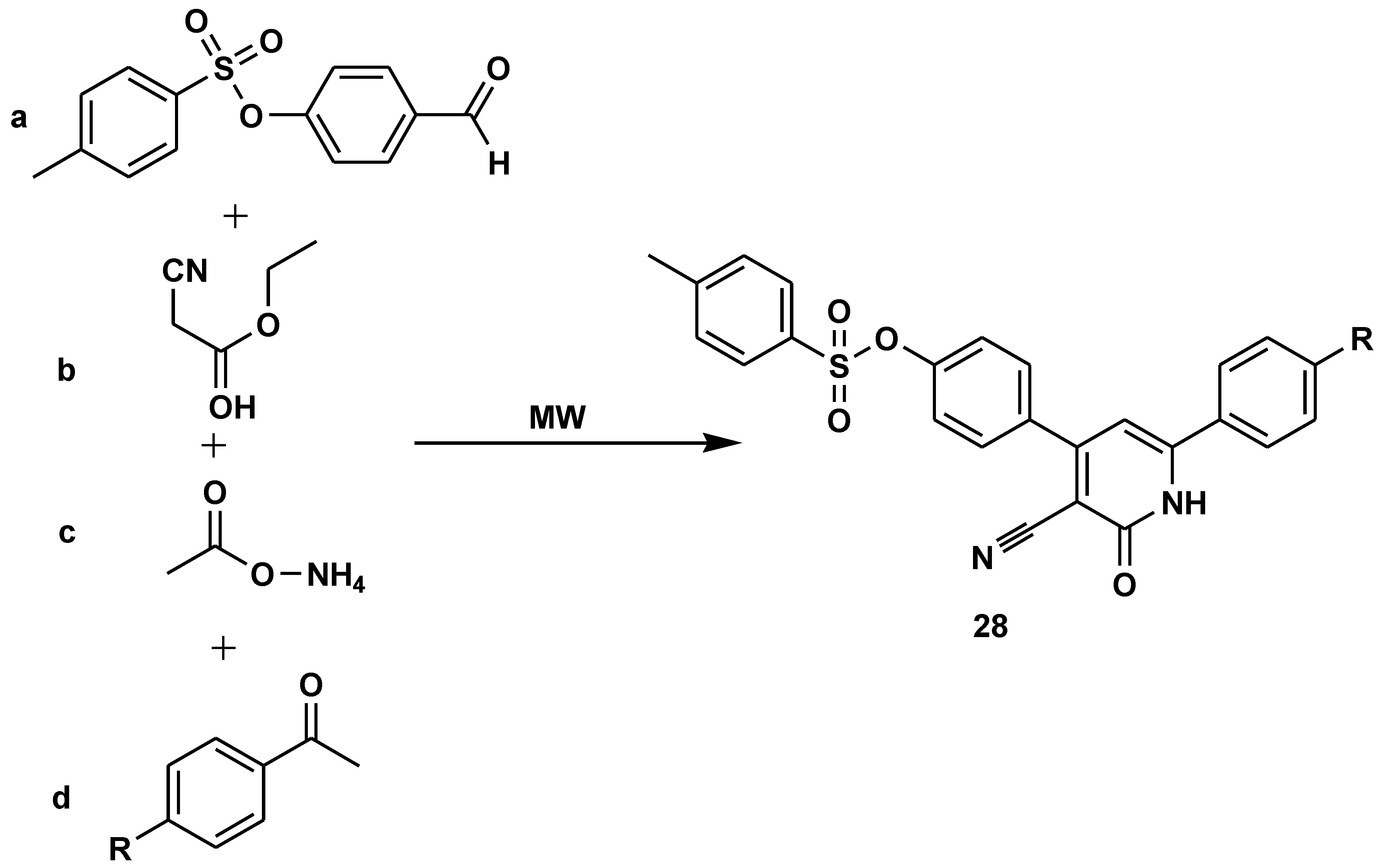
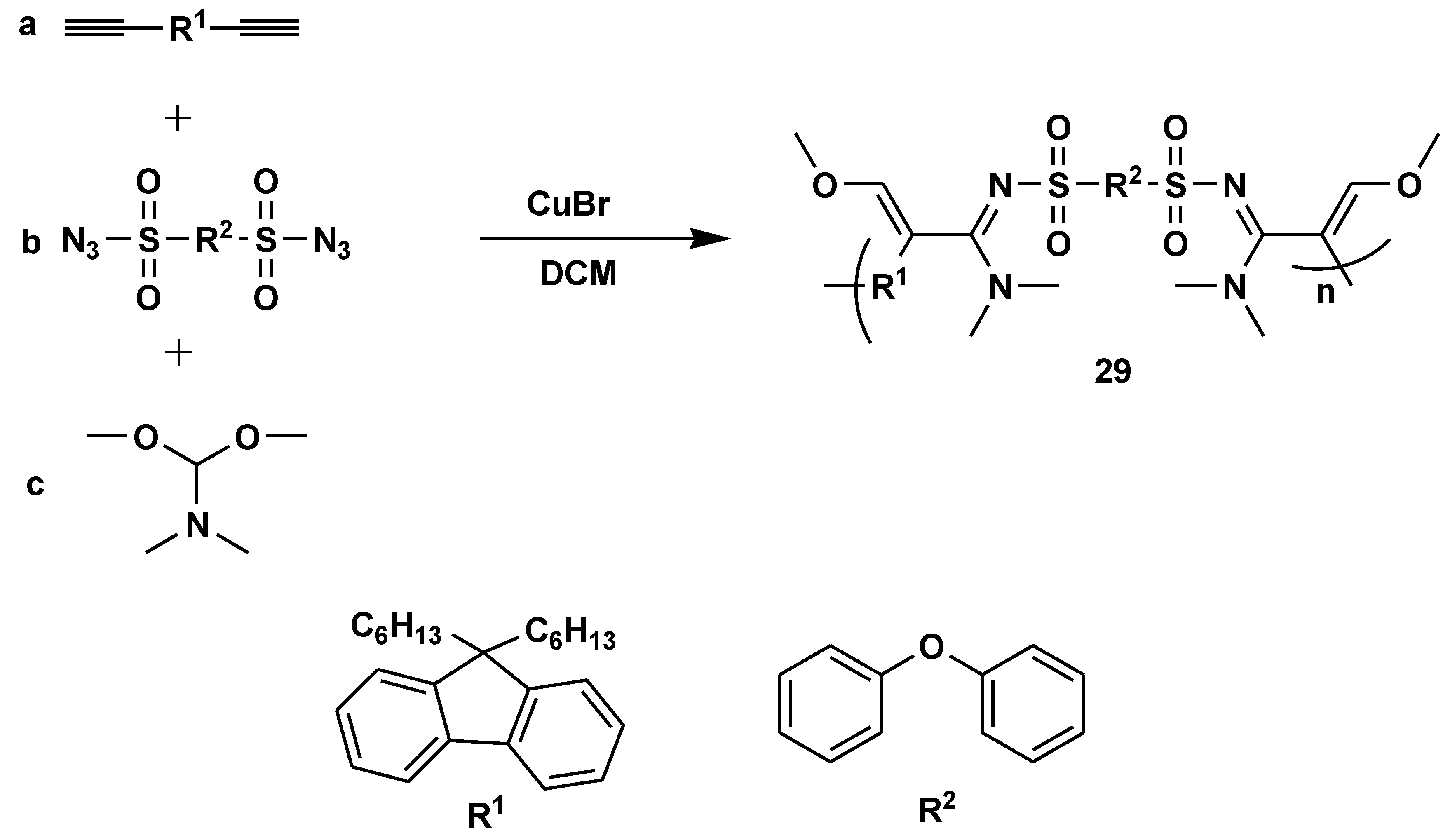
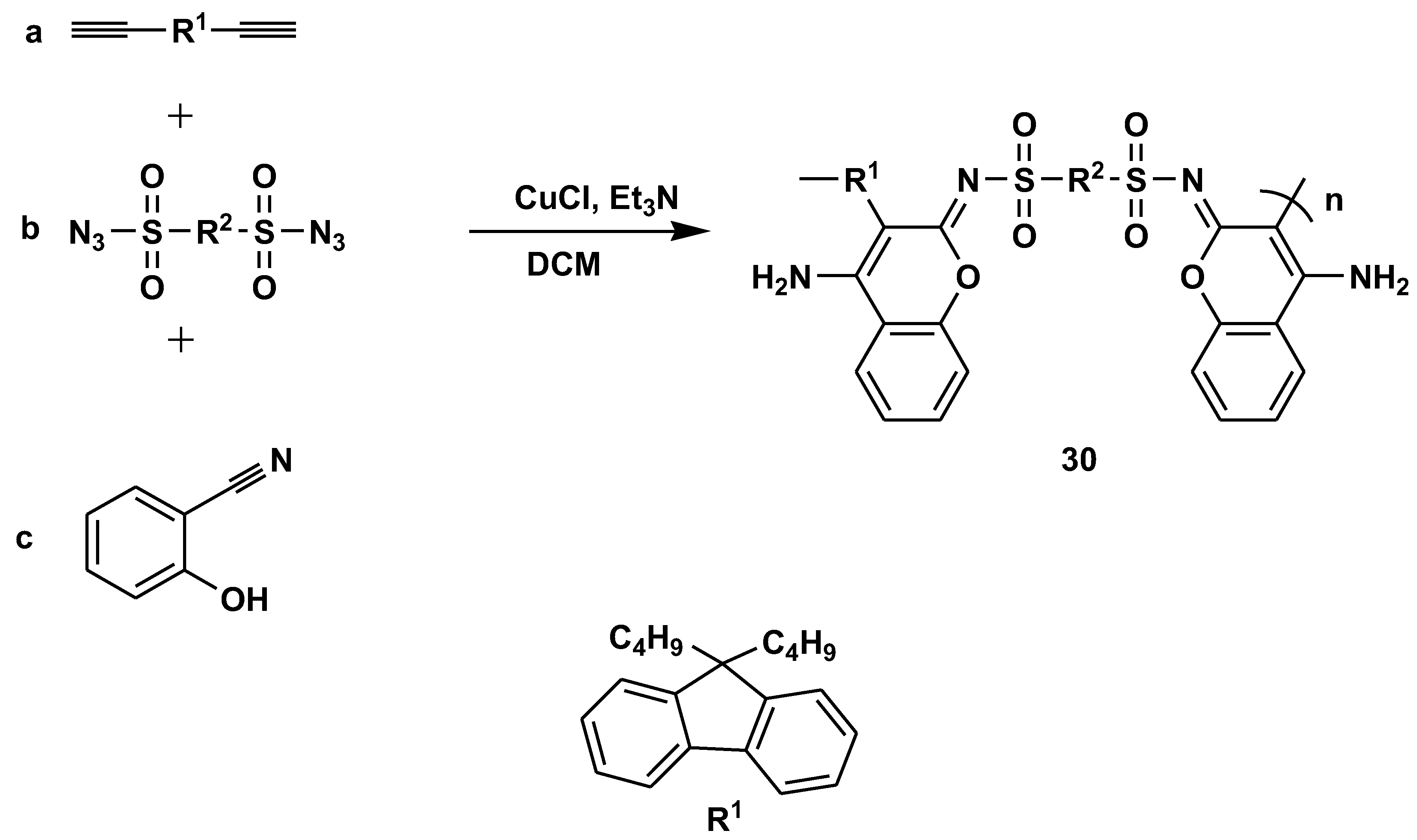
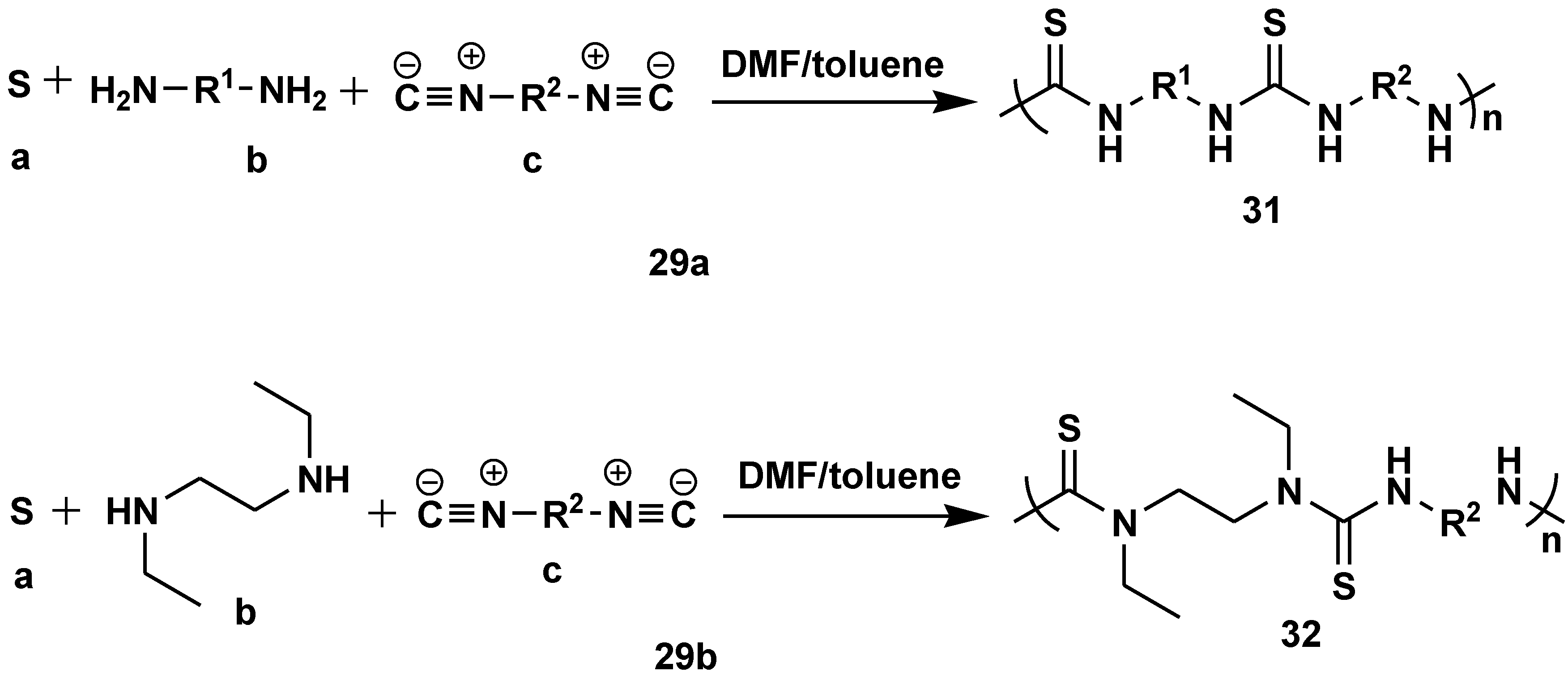
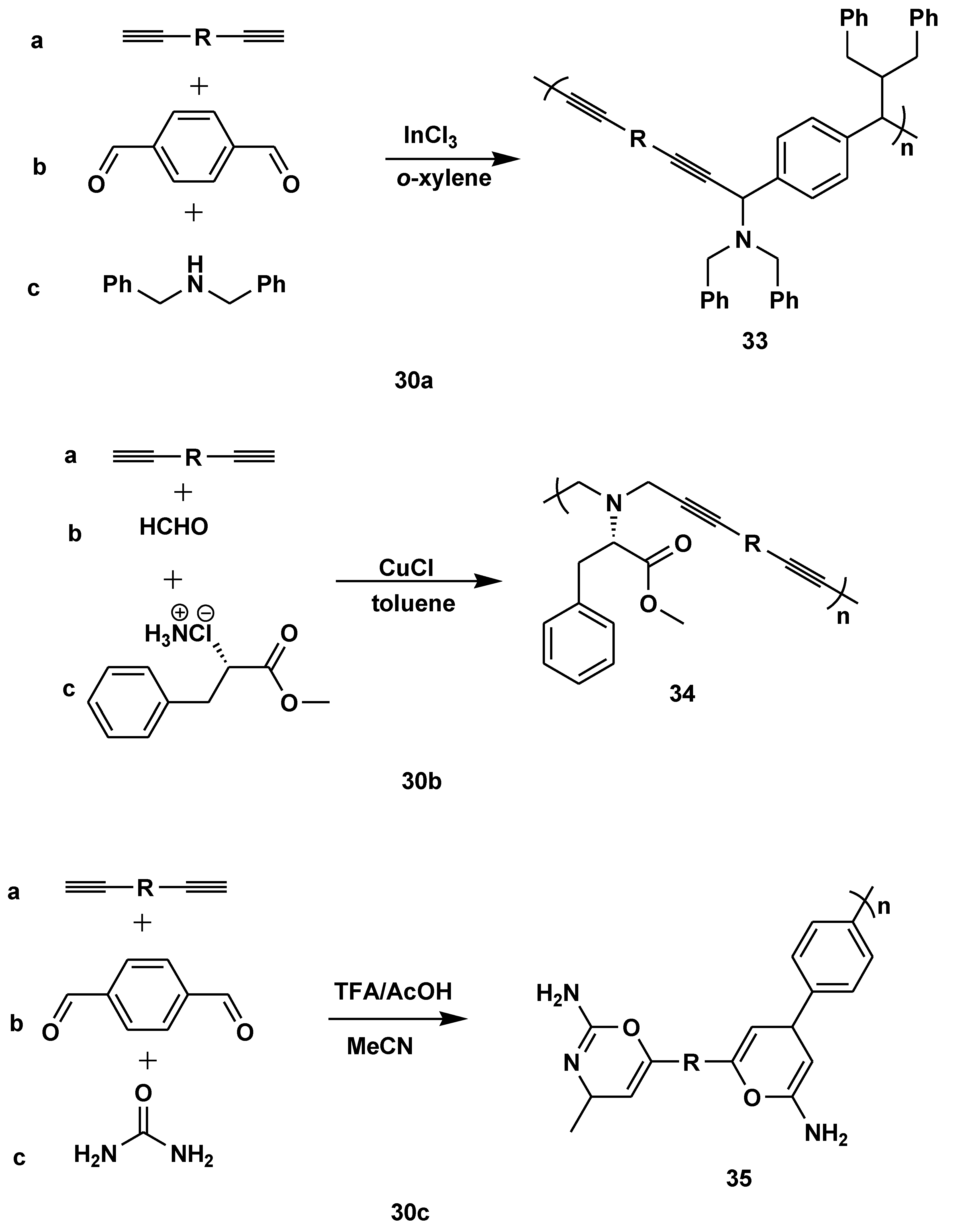
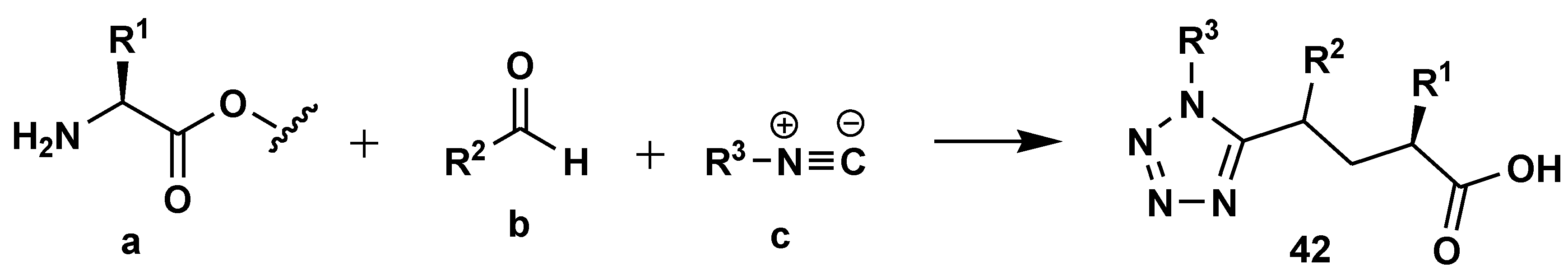
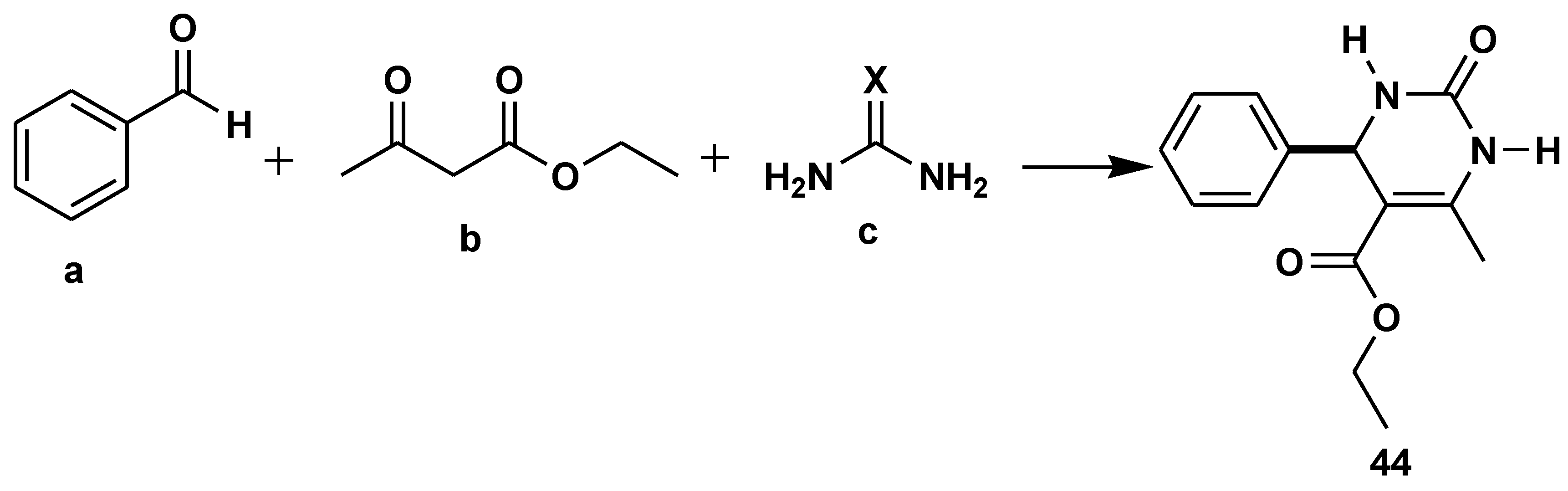
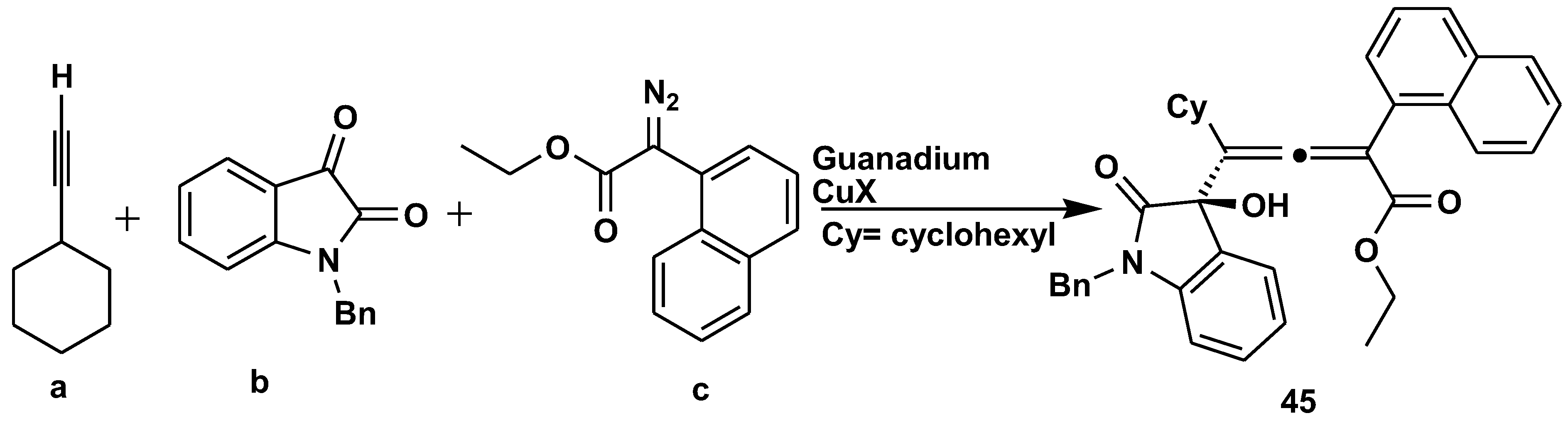
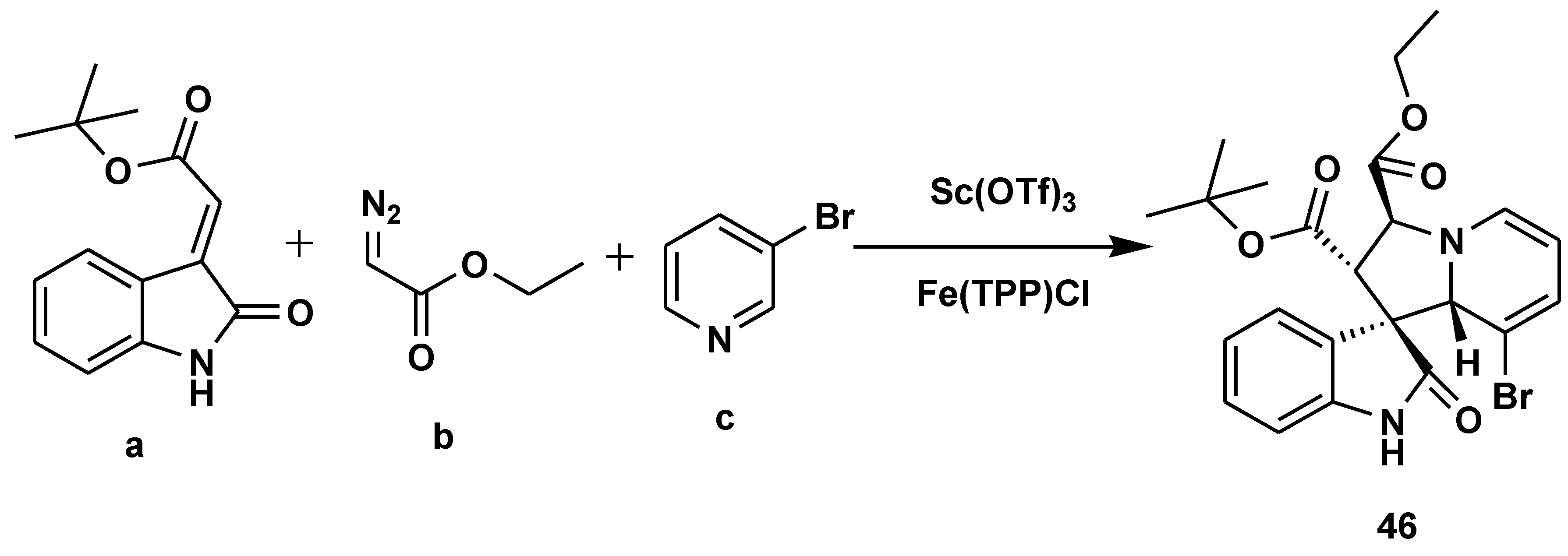
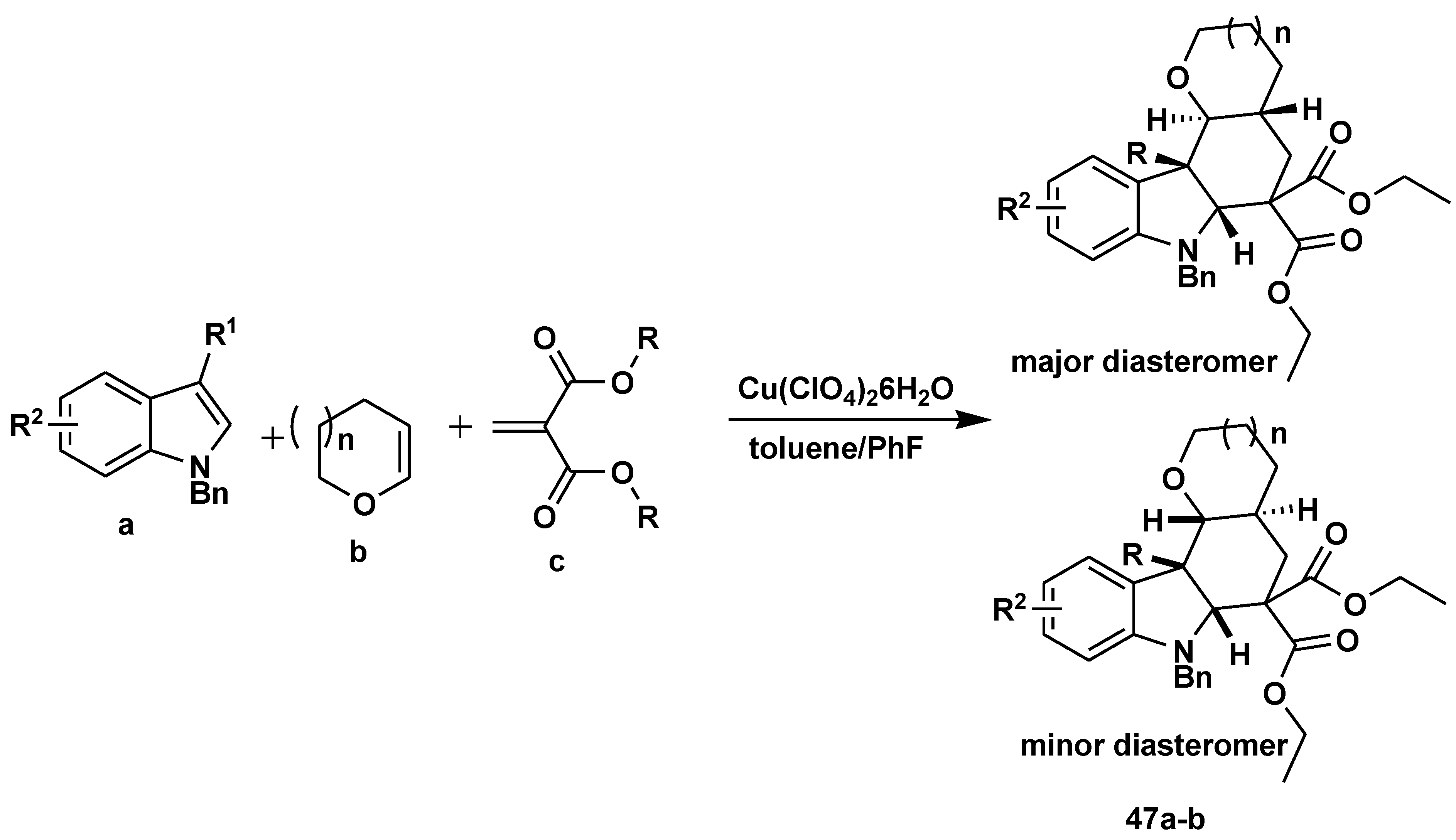
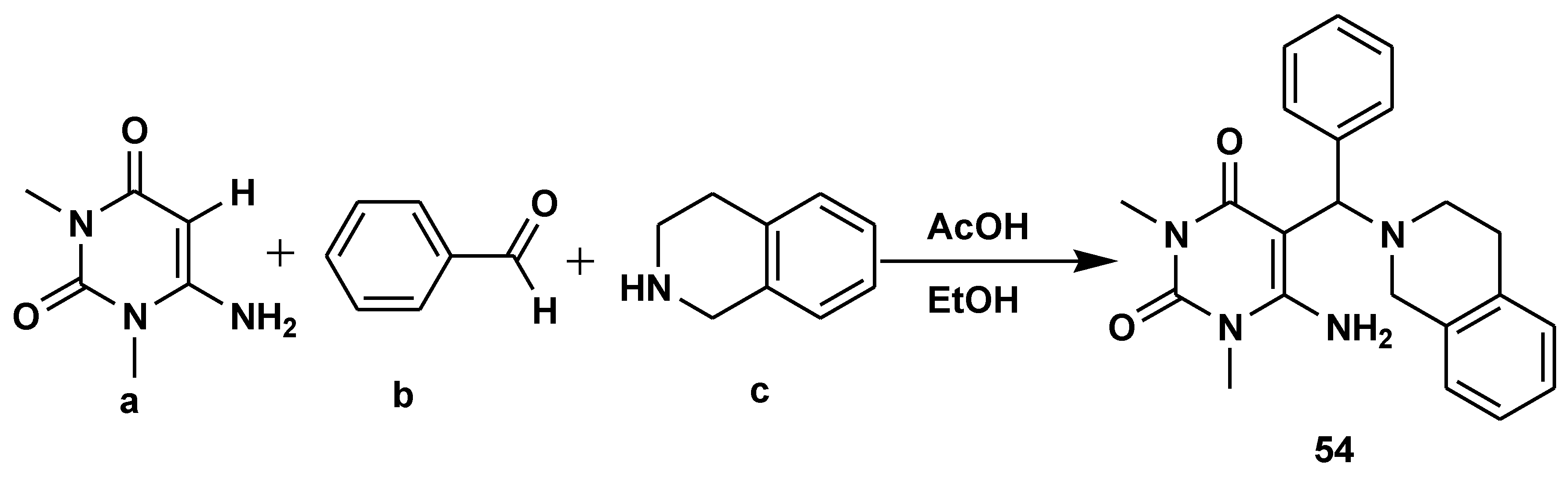
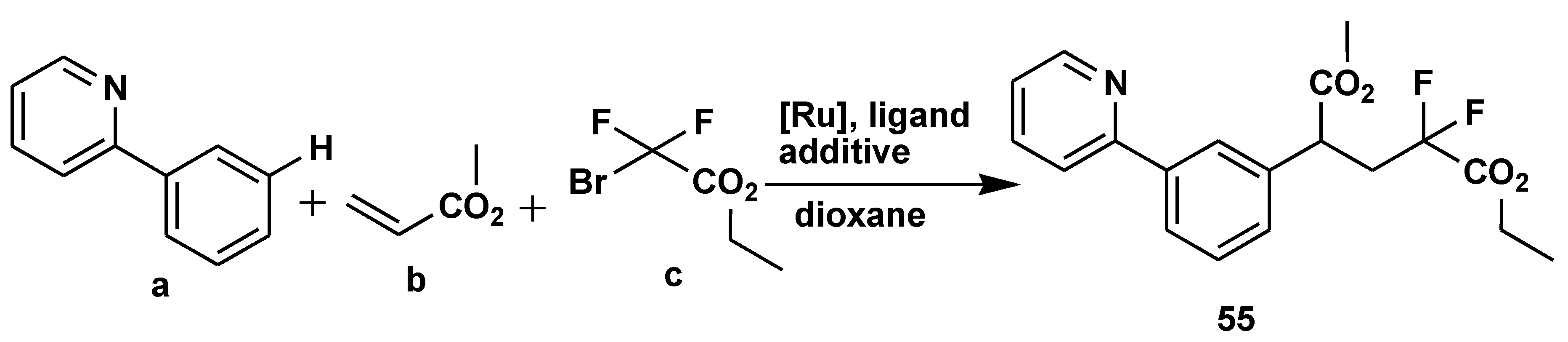
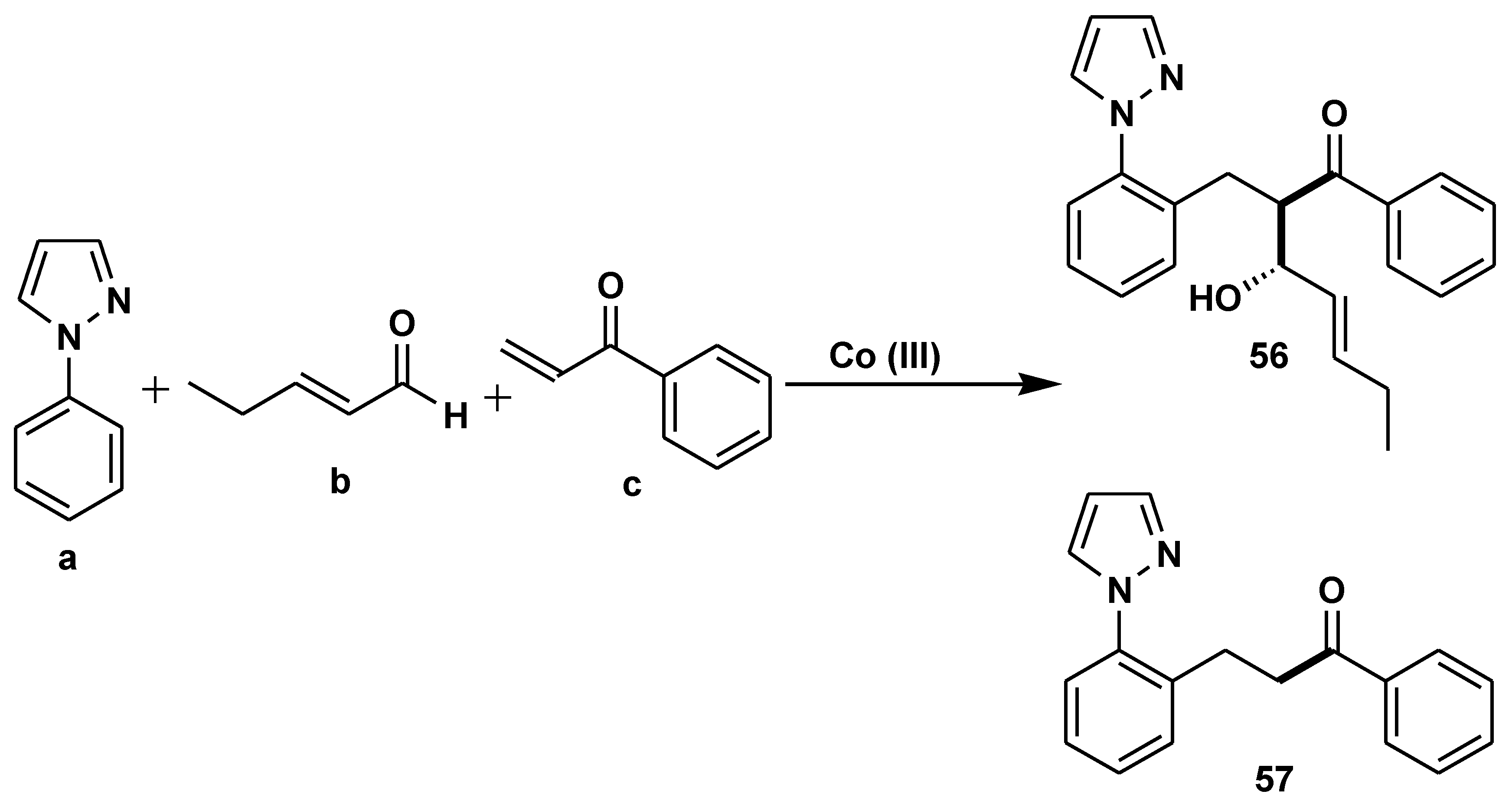
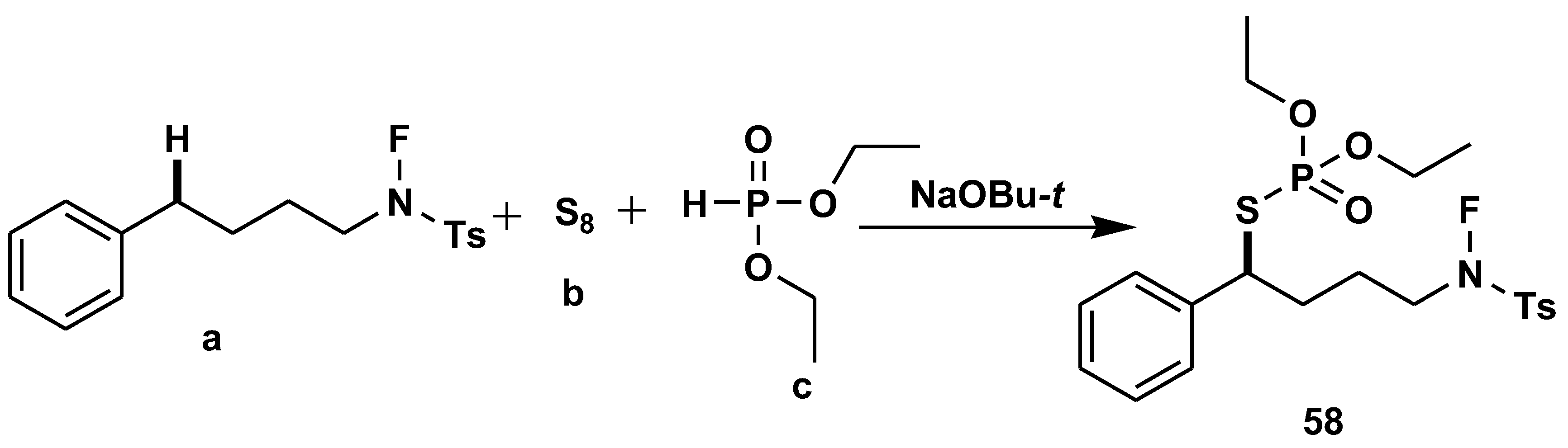
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).