Submitted:
03 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
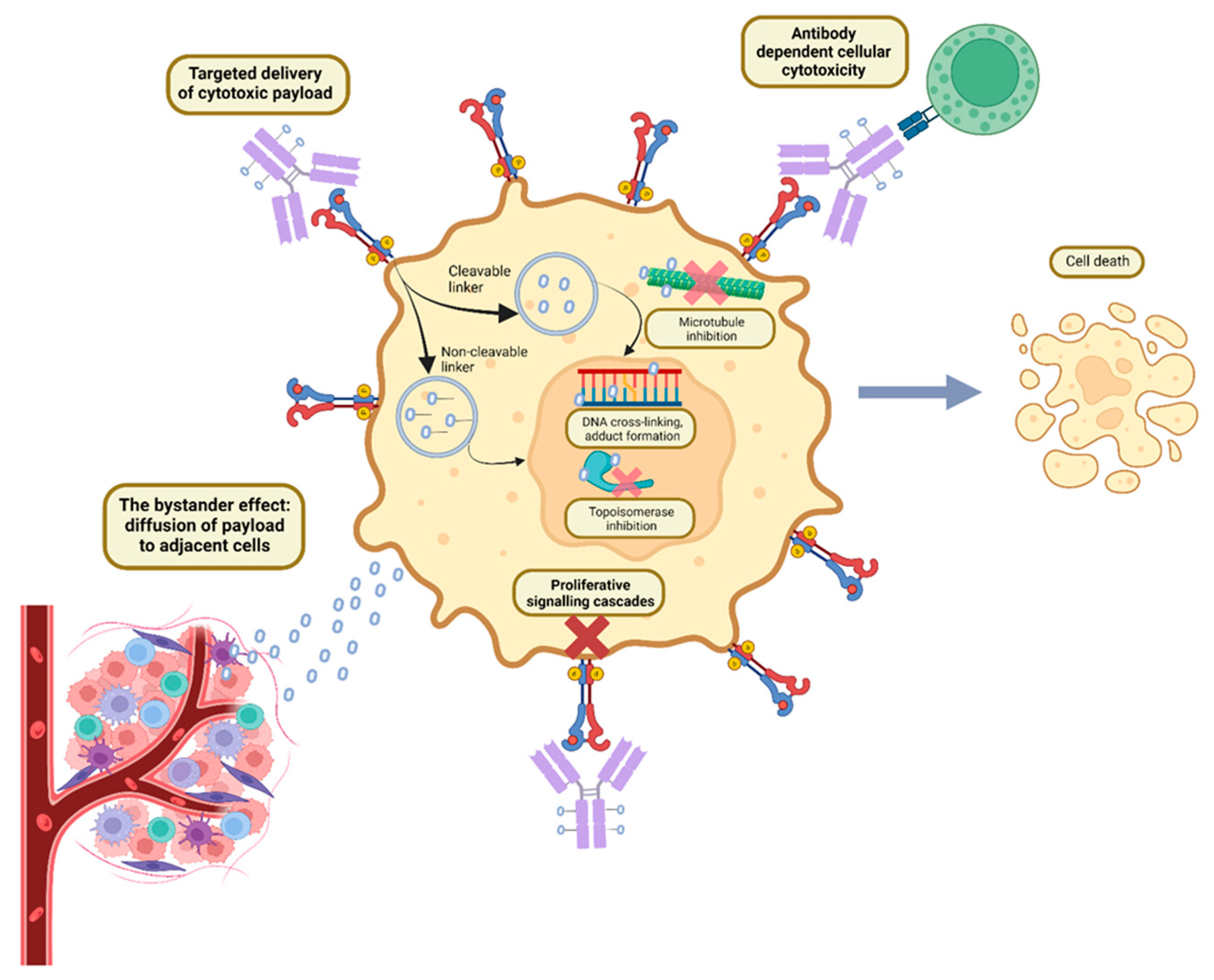
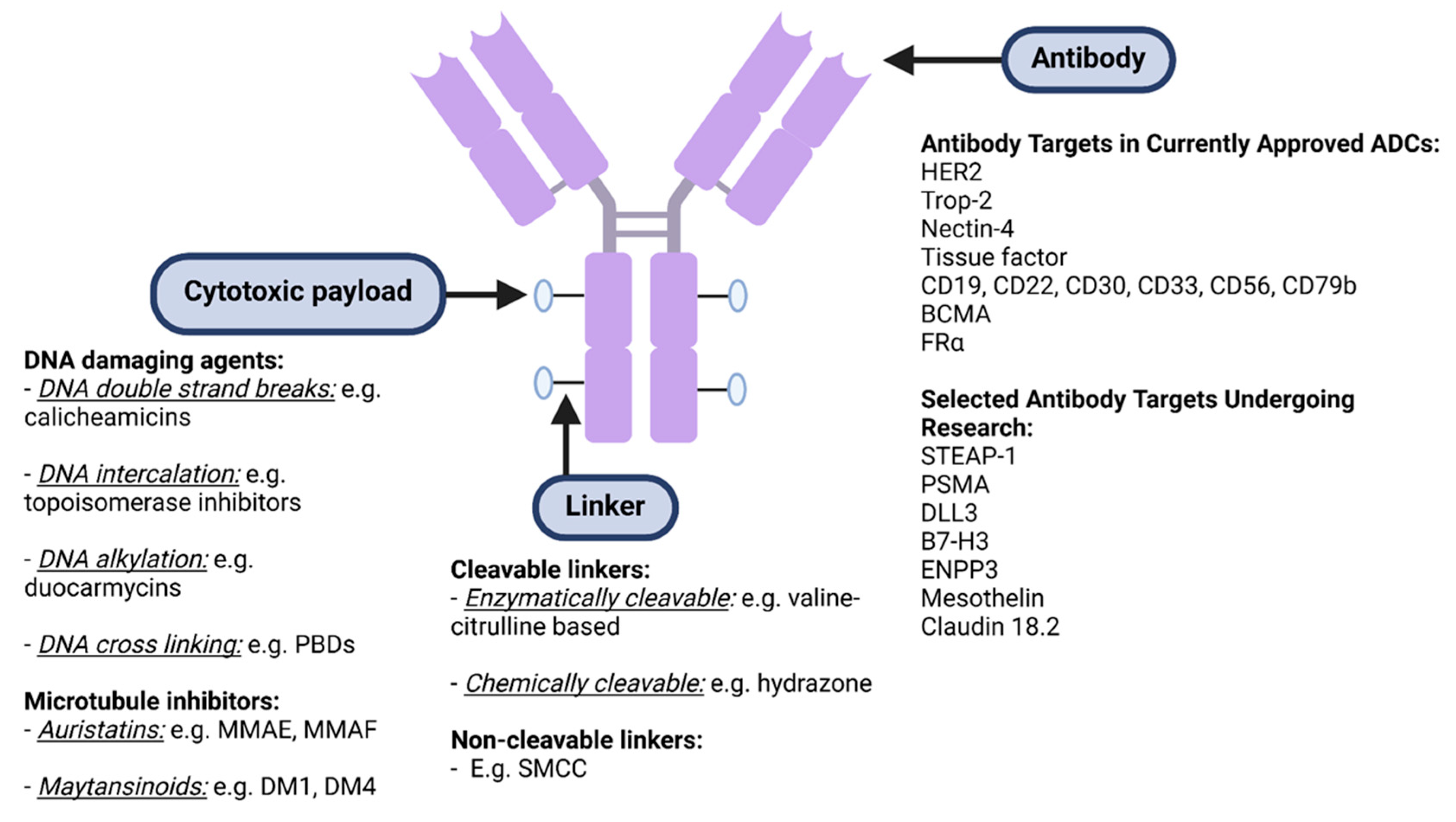
ADC Structure, Pharmacokinetics and Mechanism of Action:
Pharmacokinetics and Pharmacodynamics of ADCs:
Bystander Effect:
Seminal Phase II/III Trials of Antibody Drug Conjugates in Cancer
| Drug | FDA approval | Pivotal trial(s) | Population | Number of patients | Antibody Target, Linker and Payload | Results with Intervention vs Comparator |
|---|---|---|---|---|---|---|
|
Trastuzumab emtansine (T-DM1) |
2013 | EMILIA [52] (phase III) |
Advanced HER2+ breast cancer with PD after trastuzumab + taxane. | T-DM1: 495 Capecitabine + lapatinib: 496 |
Ab target: HER2 Linker: SMCC (non-cleavable) Payload: DM1 |
ORR 43.6% vs 30.8%, mPFS 9.6 vs 6.4 mths, mOS 30.9 vs 25.1 mths. |
| 2019 | KATHERINE [53] (phase III) |
Early stage HER2+ breast cancer with residual disease after NACT. | T-DM1: 743 Trastuzumab: 743 |
3 yr iDFS 88.3% vs 77.0%. | ||
|
Trastuzumab deruxtecan (T-DXd) |
2022 | DESTINY-Breast03 [54] (phase III) |
Advanced HER2+ breast cancer with PD after trastuzumab + taxane. | T-DXd: 261 T-DM1: 263 |
Ab target: HER2 Linker: GGFG tetrapeptide (cleavable) Payload: Deruxtecan |
ORR 79.7% vs 34.2%, mPFS not reached vs 6.8 mths with T-DM1, mOS both not reached. |
| 2022 | DESTINY-Breast02 [55] (phase III) |
Advanced HER2+ breast cancer with PD after T-DM1. | T-DXd: 406 TPC: 202 |
ORR 70% vs 29%, mPFS 17.8 vs 6.9 mths, mOS 39.2 vs 26.5 mths. |
||
| 2022 | DESTINY-Breast04 [56] (phase III) |
Advanced HER2 low breast cancer with PD after 1-2 lines of chemotherapy. | T-DXd: 373 TPC: 184 |
ORR 52.3% vs 16.3%, mPFS 9.9 vs 5.1 mths, mOS 23.4 vs 16.8 mths. |
||
| 2021 | DESTINY-Gastric01 [57] (phase II) |
Advanced HER2+ gastric/GOJ cancers after ≥2 lines of therapy. | T-DXd: 125 TPC: 62 |
ORR 51% vs 14%, mPFS 5.6 vs 3.5 mths, mOS 12.5 vs 8.4 mths. |
||
| 2022 | DESTINY-Lung01 [58] (phase II) | Advanced HER2+ NSCLC refractory to standard therapy. | T-DXd: 91 (single arm) | ORR 55%, mPFS 8.2 mths, mOS 17.8 mths. |
||
|
Sacituzumab govitecan (SG) |
2023 | TROPiCS-02 [59] (phase III) | Advanced HR+ breast cancer, HER2- or low with PD after ET and ≥2 systemic therapies. | SG: 272 TPC: 271 |
Ab target: Trop-2 Linker: CL2A (cleavable) Payload: SN-38 |
ORR 21% vs 14%, mPFS 5.5 vs 4.0 mths, mOS 13.9 vs 12.3 mths. |
| 2020 | ASCENT [60] (phase III) |
Advanced TNBC with PD after ≥2 lines of chemotherapy. | SG: 235 TPC: 233 |
ORR 35% vs 5%, mPFS 5.6 vs 1.7 mths, mOS 12.1 vs 6.7 mths. |
||
| 2021 | TROPHY [61] (phase II) |
Advanced urothelial cancer with PD after platinum and immunotherapy. | SG: 113 (single arm) |
ORR 27%, mPFS 5.4 mths, mOS 10.9 mths. |
||
| 2020 | IMMU-132-01 [62] (phase I/II) | Advanced TNBC after ≥2 lines of chemotherapy. | SG: 108 (single arm) |
ORR 33.3%, mPFS 5.5 mths, mOS 13.0 mths. |
||
|
Enfortumab vedotin (EV) |
2019 | EV-201 [63,64] (phase II) | Advanced urothelial carcinoma. Cohort 1: PD after platinum + immunotherapy. Cohort 2: PD after immunotherapy, no prior platinum. |
Cohort 1: 125 Cohort 2: 89 (single arm) |
Ab target: Nectin-4 Linker: mc-VC-PABC (cleavable) Payload: MMAE |
Cohort 1: ORR 44%, mPFS 5.8 mths, mOS 11.7 mths Cohort 2: ORR 52%, mPFS 5∙8 mths, mOS 14.7 mths. |
| 2019 | EV-301 [65] (phase III) | Advanced urothelial carcinoma with PD after platinum and immunotherapy. | EV: 301 TPC: 307 |
ORR 40.6% vs 17.9%, mPFS 5.6 vs 3.7 mths, mOS 12.9 vs 9.0 mths. |
||
|
Disitamab vedotin* (DV) |
2021 | [66] (phase II) |
Advanced HER2+ urothelial carcinoma with PD after ≥1 prior therapy. | DV: 43 (single arm) |
Ab target: HER2 Linker: mc-VC-PABC (cleavable) Payload: MMAE |
ORR 51.2%, mPFS 6.9 mths, mOS 13.9 mths. |
|
Tisotumab vedotin (TV) |
2021 | InnovaTV 204 [67] (phase II) | Recurrent/advanced cervical cancer with PD after ≤2 lines of chemotherapy. | TV: 102 (single arm) |
Ab target: tissue factor Linker: mc-VC-PABC (cleavable) Payload: MMAE |
ORR 24%, mPFS 4.2 mths, mOS 12.1 mths. |
| Mirvetuximab soravtansine (MIRV) | 2022 | SORAYA [68] (phase II) |
FRα high platinum- resistant ovarian cancer with ≤3 prior systemic therapies, including bevacizumab. | MIRV: 106 (single arm) |
Ab target: FRα Linker: disulfide hydrophilic sulfo-SPDB (cleavable) Payload: DM4 |
ORR 32.4%, mPFS 4.3 mths, mOS 13.8 mths. |
| Drug | FDA approval | Pivotal trial(s) | Population | Number of patients | Antibody Target, Linker and Payload | Results with Intervention vs Comparator |
|---|---|---|---|---|---|---|
| Gemtuzumab ozogamicin (GO) | 2017 | ALFA-0701 [69–71] (phase III) | Newly diagnosed, CD33+ AML, age 50-70. | GO + standard therapy: 140 SOC: 140 |
Ab target: CD33 Linker: hydrazone (cleavable) Payload: calicheamicin |
2 year EFS 40·8% vs 17.1%, RFS 50.3% vs 22.7%. |
| 2017 | AAML0531 [72] (phase III) |
Newly diagnosed AML age 0-29 years. | GO + standard therapy: 511 SOC: 511 |
3 yr EFS 53.1% vs 46.9%, 3 yr OS 69.4% vs 65.4%. | ||
| 2017 | AML-19 [73] (phase III) | Newly diagnosed AML, >75 yrs or 61-75 yrs and unfit for intensive chemotherapy. | GO: 118 BSC: 119 |
mOS 4.9 vs 3.6 mths. | ||
| 2017 | MyloFrance-1 [74] (phase II) |
CD33+ AML in first relapse. | GO: 57 (single arm) |
ORR 33.3%, mOS 8.4 mths, mRFS 11.0 mths. | ||
|
Brentuximab vedotin (BV) |
2018 | ECHELON-2 [75] (phase III) |
Untreated CD30+ peripheral T cell lymphomas. | BV + CHP: 226 CHOP: 226 |
Ab target: CD30 Linker: mc-VC-PABC (cleavable) Payload: MMAE |
5 yr PFS 51.4% vs 43.0%, 5 yr OS 70.1% vs 61.0%. |
| 2018 | ECHELON-1 [76] (phase III) |
Untreated stage III-IV classical Hodgkin lymphoma. | BV + AVD: 664 ABVD: 670 |
5 yr PFS 82.2% vs 75.3%, OS immature. | ||
| 2017 | ALCANZA [77] (phase III) |
Relapsed primary cutaneous anaplastic large cell lymphoma or CD30+ mycosis fungoides. | BV: 64 TPC: 64 |
ORR 54.7% vs 12.5%, mPFS 16.7 vs 3.5 mths, 3 year OS 64.4% vs 61.9%. | ||
| Polatuzumab vedotin (PV) | 2019 | Study GO29365 [78] (phase Ib/II) | Relapsed or refractory DLBCL with ≥2 prior therapies. | 1. PV + BG: 20 2. PV + BR: 40 3. BR: 40 |
Ab target: CD79b Linker: mc-VC-PABC (cleavable) Payload: MMAE |
Phase I: PV + BG mOS 10.8 mths. Phase II: PV + BR vs BR mPFS 12.4 vs 4.7 mths. |
| Belantamab mafodotin (BM) | 2020 | DREAMM-2 [79] (phase II) |
Relapsed or refractory multiple myeloma with ≥4 prior therapies. |
Cohort 1 (BM 2.5 mg/kg): 97 Cohort 2 (BM 3.4 mg/kg): 99 |
Ab target: BCMA Linker: mc (non-cleavable) Payload: MMAF |
Cohort 1: ORR 31%, mPFS 2.9 mths. Cohort 2: ORR 34%, mPFS 4.9 mths. |
| Inotuzumab ozogamicin (InO) | 2017 | INO-VATE [80] (phase III) |
Relapsed or refractory B-cell precursor ALL. | InO: 164 TPC: 162 |
Ab target: CD22 Linker: hydrazone (cleavable) Payload: calicheamicin |
mOS: 7.7 vs 6.2 mths, 2 yr OS: 22.8% vs 10.0%. |
|
Moxetumomab pasudotox (MP) |
2018 | Study 1503 [81] (phase II) |
Relapsed or refractory hairy cell leukaemia. | MP: 80 (single arm) |
Ab target: CD22 Linker: hydrazone (cleavable) Payload: pasudotox |
Durable CR rate of 36%, median CR duration 62.8 mths, mPFS 41.5 mths. |
|
Loncastuximab tesirine (LT) |
2021 | LOTIS-2 [82] (phase II) | Relapsed or refractory DLBCL after ≥2 therapies. | LT: 145 (single arm) |
Ab target: CD19 Linker: valine-alanine (cleavable) Payload: PBD dimer |
ORR 48.3%, mPFS 4.9 mths, mOS 9.9 mths. |
Trials of ADCs in Solid Organ Malignancies
ADCs in Haematological Malignancies
Challenges in the Clinical Development of ADCs and Limitations of Current ADCs
Mechanisms of Resistance to ADCs
Future Directions
Developing Novel Antigenic Targets and Antibodies
Improving Linker Technology
Development of Improved Cytotoxic and Other Payloads
Immunotherapy and ADCs
References
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduction and Targeted Therapy 2022, 7, 93. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2023. MAbs 2023, 15, 2153410. [Google Scholar] [CrossRef]
- Theocharopoulos, C.; Lialios, P.P.; Samarkos, M.; Gogas, H.; Ziogas, D.C. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef]
- McKertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: dawn of a new era? npj Precision Oncology 2023, 7, 5. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody–drug conjugates (ADCs) for cancer therapy. Cancer Cell International 2022, 22, 255. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Groner, B.; Schumacher, U.; Superti-Furga, G.; Busslinger, M.; Kralovics, R.; Zielinski, C.; Penninger, J.M.; Kerjaschki, D.; Stingl, G.; et al. Paul Ehrlich (1854-1915) and His Contributions to the Foundation and Birth of Translational Medicine. J Innate Immun 2016, 8, 111–120. [Google Scholar] [CrossRef]
- Loadman, P. Anticancer Drug Development. British Journal of Cancer 2002, 86, 1665–1666. [Google Scholar] [CrossRef]
- Firer, M.A.; Luboshits, G. Antibody-Drug-Conjugate Therapy for Hematological Cancers: Matching Cell Biology with Clinical Benefit. Advanced Functional Materials 2021, 31, 2100032. [Google Scholar] [CrossRef]
- Perez, H.L.; Cardarelli, P.M.; Deshpande, S.; Gangwar, S.; Schroeder, G.M.; Vite, G.D.; Borzilleri, R.M. Antibody–drug conjugates: current status and future directions. Drug Discovery Today 2014, 19, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Jerjian, T.V.; Glode, A.E.; Thompson, L.A.; O'Bryant, C.L. Antibody-Drug Conjugates: A Clinical Pharmacy Perspective on an Emerging Cancer Therapy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2016, 36, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Antrás, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody–drug conjugates: in search of partners of choice. Trends in Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef]
- Mahmood, I. Clinical Pharmacology of Antibody-Drug Conjugates. Antibodies (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Stepan, L.P.; Trueblood, E.S.; Hale, K.; Babcook, J.; Borges, L.; Sutherland, C.L. Expression of Trop2 Cell Surface Glycoprotein in Normal and Tumor Tissues:Potential Implications as a Cancer Therapeutic Target. Journal of Histochemistry & Cytochemistry 2011, 59, 701–710. [Google Scholar] [CrossRef]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload diversification: a key step in the development of antibody–drug conjugates. Journal of Hematology & Oncology 2023, 16, 3. [Google Scholar] [CrossRef]
- Gébleux, R.; Casi, G. Antibody-drug conjugates: Current status and future perspectives. Pharmacol Ther 2016, 167, 48–59. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nature reviews Drug discovery 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001, 7, 1490–1496. [Google Scholar]
- Choi-Sledeski, Y.M.; Wermuth, C.G. Chapter 28 - Designing Prodrugs and Bioprecursors. In The Practice of Medicinal Chemistry (Fourth Edition), Wermuth, C.G., Aldous, D., Raboisson, P., Rognan, D., Eds.; Academic Press: San Diego, 2015; pp. 657–696. [Google Scholar]
- Agarwal, P.; Bertozzi, C.R. Site-specific antibody–drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate chemistry 2015, 26, 176–192. [Google Scholar] [CrossRef]
- Giugliano, F.; Corti, C.; Tarantino, P.; Michelini, F.; Curigliano, G. Bystander effect of antibody–drug conjugates: fact or fiction? Current Oncology Reports 2022, 24, 809–817. [Google Scholar] [CrossRef]
- Su, D.; Zhang, D. Linker Design Impacts Antibody-Drug Conjugate Pharmacokinetics and Efficacy via Modulating the Stability and Payload Release Efficiency. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.-F.; Ma, Y.; Xu, K.; Dragovich, P.S.; Pillow, T.H.; Liu, L.; Del Rosario, G.; He, J.; Pei, Z.; et al. Chemical Structure and Concentration of Intratumor Catabolites Determine Efficacy of Antibody Drug Conjugates. Drug Metabolism and Disposition 2016, 44, 1517. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.V.; Kaur, S.; Saad, O.M. Conjugation Site Influences Antibody-Conjugated Drug PK Assays: Case Studies for Disulfide-Linked, Self-Immolating Next-Generation Antibody Drug Conjugates. Analytical Chemistry 2020, 92, 12168–12175. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Molecular Cancer Research 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kinneer, K.; Meekin, J.; Tiberghien, A.C.; Tai, Y.T.; Phipps, S.; Kiefer, C.M.; Rebelatto, M.C.; Dimasi, N.; Moriarty, A.; Papadopoulos, K.P.; et al. SLC46A3 as a Potential Predictive Biomarker for Antibody-Drug Conjugates Bearing Noncleavable Linked Maytansinoid and Pyrrolobenzodiazepine Warheads. Clin Cancer Res 2018, 24, 6570–6582. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein & Cell 2016, 9, 33–46. [Google Scholar] [CrossRef]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opinion on Therapeutic Targets 2013, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Doronina, S.O.; Bovee, T.D.; Meyer, D.W.; Miyamoto, J.B.; Anderson, M.E.; Morris-Tilden, C.A.; Senter, P.D. Novel Peptide Linkers for Highly Potent Antibody−Auristatin Conjugate. Bioconjugate Chemistry 2008, 19, 1960–1963. [Google Scholar] [CrossRef]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody–drug conjugates—A tutorial review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody-drug conjugates: Recent advances in linker chemistry. Acta Pharm Sin B 2021, 11, 3889–3907. [Google Scholar] [CrossRef] [PubMed]
- Brun, M.-P.; Gauzy-Lazo, L. Protocols for Lysine Conjugation. In Antibody-Drug Conjugates, Ducry, L., Ed.; Humana Press: Totowa, NJ, 2013; pp. 173–187. [Google Scholar]
- Matsuda, Y.; Mendelsohn, B.A. An overview of process development for antibody-drug conjugates produced by chemical conjugation technology. Expert Opinion on Biological Therapy 2021, 21, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Azar, I.; Alkassis, S.; Fukui, J.; Alsawah, F.; Fedak, K.; Al Hallak, M.N.; Sukari, A.; Nagasaka, M. Spotlight on Trastuzumab Deruxtecan (DS-8201,T-DXd) for HER2 Mutation Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl) 2021, 12, 103–114. [Google Scholar] [CrossRef]
- Nadkarni, D.V. Conjugations to Endogenous Cysteine Residues. In Antibody-Drug Conjugates: Methods and Protocols, Tumey, L.N., Ed.; Springer US: New York, NY, 2020; pp. 37–49. [Google Scholar]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nature biotechnology 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.V.; Cabel, L.; Bidard, F.C. Does sacituzumab-govitecan act as a conventional antibody drug conjugate (ADC), a prodrug of SN-38 or both? Ann Transl Med 2021, 9, 1113. [Google Scholar] [CrossRef]
- Singh, D.; Dheer, D.; Samykutty, A.; Shankar, R. Antibody drug conjugates in gastrointestinal cancer: From lab to clinical development. J Control Release 2021, 340, 1–34. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, P.; Li, Z.; Roy, P.; Sahajwalla, C.G. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J Clin Pharmacol 2013, 53, 314–325. [Google Scholar] [CrossRef]
- Su, D.; Kozak, K.R.; Sadowsky, J.; Yu, S.-F.; Fourie-O’Donohue, A.; Nelson, C.; Vandlen, R.; Ohri, R.; Liu, L.; Ng, C.; et al. Modulating Antibody–Drug Conjugate Payload Metabolism by Conjugation Site and Linker Modification. Bioconjugate Chemistry 2018, 29, 1155–1167. [Google Scholar] [CrossRef]
- Pillow, T.H.; Sadowsky, J.D.; Zhang, D.; Yu, S.F.; Del Rosario, G.; Xu, K.; He, J.; Bhakta, S.; Ohri, R.; Kozak, K.R.; et al. Decoupling stability and release in disulfide bonds with antibody-small molecule conjugates. Chem Sci 2017, 8, 366–370. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. Journal of Experimental & Clinical Cancer Research 2018, 37, 20. [Google Scholar] [CrossRef]
- Vezina, H.E.; Cotreau, M.; Han, T.H.; Gupta, M. Antibody–Drug Conjugates as Cancer Therapeutics: Past, Present, and Future. The Journal of Clinical Pharmacology 2017, 57, S11–S25. [Google Scholar] [CrossRef]
- Seligson, J.M.; Patron, A.M.; Berger, M.J.; Harvey, R.D.; Seligson, N.D. Sacituzumab Govitecan-hziy: An Antibody-Drug Conjugate for the Treatment of Refractory, Metastatic, Triple-Negative Breast Cancer. Annals of Pharmacotherapy 2020, 55, 921–931. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nature Reviews Clinical Oncology 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Dan, N.; Setua, S.; Kashyap, V.K.; Khan, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Antibody-drug conjugates for cancer therapy: chemistry to clinical implications. Pharmaceuticals 2018, 11, 32. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Li, W.; Jeanty, C.; Xiang, G.; Dong, Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharmaceutica Sinica B 2020, 10, 1589–1600. [Google Scholar] [CrossRef]
- Schönberger, S.; van Beekum, C.; Götz, B.; Nettersheim, D.; Schorle, H.; Schneider, D.T.; Casati, A.; Craveiro, R.B.; Calaminus, G.; Dilloo, D. Brentuximab vedotin exerts profound antiproliferative and pro-apoptotic efficacy in CD30-positive as well as cocultured CD30-negative germ cell tumour cell lines. J Cell Mol Med 2018, 22, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Fan, S.; Xiao, D.; Xie, F.; Li, W.; Zhong, W.; Zhou, X. Antibody-Drug Conjugate Using Ionized Cys-Linker-MMAE as the Potent Payload Shows Optimal Therapeutic Safety. Cancers 2020, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. New England Journal of Medicine 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. New England Journal of Medicine 2018, 380, 617–628. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. New England Journal of Medicine 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- André, F.; Hee Park, Y.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 2023. [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. New England Journal of Medicine 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. New England Journal of Medicine 2021, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. Journal of Clinical Oncology 2022, 40, LBA1001–LBA1001. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. Journal of Clinical Oncology 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Yu, E.Y.; Petrylak, D.P.; O'Donnell, P.H.; Lee, J.-L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial. The Lancet Oncology 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O'Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. New England Journal of Medicine 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.; Yao, X.; Luo, H.; Shi, B.; Liu, J.; He, Z.; Yu, G.; et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res 2021, 27, 43–51. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. The Lancet Oncology 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study. Journal of clinical oncology 2023, JCO2201900–JCO2201900. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Pautas, C.; Terré, C.; Renneville, A.; Gardin, C.; Suarez, F.; Caillot, D.; Berthon, C.; Rousselot, P.; Preudhomme, C.; et al. Final Analysis of the ALFA 0701 Study. Blood 2014, 124, 376. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. The Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Gamis, A.S.; Alonzo, T.A.; Meshinchi, S.; Sung, L.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Kahwash, S.B.; Heerema-McKenney, A.; Winter, L.; et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol 2014, 32, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Annino, L.; Venditti, A.; Voso, M.T.; Mazzone, C.; et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J Clin Oncol 2016, 34, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Taksin, A.L.; Legrand, O.; Raffoux, E.; de Revel, T.; Thomas, X.; Contentin, N.; Bouabdallah, R.; Pautas, C.; Turlure, P.; Reman, O.; et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007, 21, 66–71. [Google Scholar] [CrossRef]
- Horwitz, S.; O'Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 2022, 33, 288–298. [Google Scholar] [CrossRef]
- Straus, D.J.; Długosz-Danecka, M.; Connors, J.M.; Alekseev, S.; Illés, Á.; Picardi, M.; Lech-Maranda, E.; Feldman, T.; Smolewski, P.; Savage, K.J.; et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. The Lancet Haematology 2021, 8, e410–e421. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Scarisbrick, J.J.; Dummer, R.; Whittaker, S.; Duvic, M.; Kim, Y.H.; Quaglino, P.; Zinzani, P.L.; Bechter, O.; Eradat, H.; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician's choice in cutaneous T-cell lymphoma: final data. Blood Adv 2021, 5, 5098–5106. [Google Scholar] [CrossRef]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. The lancet oncology 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.M.; Jabbour, E.; Wang, T.; Liang White, J.; et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019, 125, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Robak, T.; le Coutre, P.D.; Gjertsen, B.T.; Troussard, X.; Roboz, G.J.; Karlin, L.; et al. Moxetumomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): long-term follow-up from the pivotal trial. J Hematol Oncol 2021, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology 2021, 22, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Chen, M.B.; Zhou, L.N.; Tang, M.; Liu, C.Y.; Lu, P.H. Impact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysis. Sci Rep 2016, 6, 33658. [Google Scholar] [CrossRef] [PubMed]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Raggi, D.; Miceli, R.; Sonpavde, G.; Giannatempo, P.; Mariani, L.; Galsky, M.D.; Bellmunt, J.; Necchi, A. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol 2016, 27, 49–61. [Google Scholar] [CrossRef]
- Wen, Y.; Ouyang, D.; Zou, Q.; Chen, Q.; Luo, N.; He, H.; Anwar, M.; Yi, W. A literature review of the promising future of TROP2 : a potential drug therapy target. Annals of Translational Medicine 2022, 10, 1403. [Google Scholar] [CrossRef]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Santaolalla, A.; Osborn, G.; Khiabany, A.; Grandits, M.; López-Abente, J.; Palhares, L.C.G.F.; Chan Wah Hak, C.; et al. Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes. British Journal of Cancer 2023, 128, 342–353. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Pereira, D.; Wimberger, P.; Oaknin, A.; Mirza, M.R.; Follana, P.; Bollag, D.; Ray-Coquard, I.; Weber, B.; et al. Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. Journal of clinical oncology 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Li, X.; Cao, D.; Zheng, X.; Wang, G.; Liu, M. Tissue factor as a new target for tumor therapy—killing two birds with one stone: a narrative review. Annals of Translational Medicine 2022, 10. [Google Scholar] [CrossRef]
- Chelariu-Raicu, A.; Mahner, S.; Moore, K.N.; Lorusso, D.; Coleman, R.L. Integrating antibody drug conjugates in the management of gynecologic cancers. Int J Gynecol Cancer 2023, 33, 420–429. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef]
- Brock, K.; Homer, V.; Soul, G.; Potter, C.; Chiuzan, C.; Lee, S. Is more better? An analysis of toxicity and response outcomes from dose-finding clinical trials in cancer. BMC Cancer 2021, 21, 777. [Google Scholar] [CrossRef]
- Liao, M.Z.; Lu, D.; Kågedal, M.; Miles, D.; Samineni, D.; Liu, S.N.; Li, C. Model-Informed Therapeutic Dose Optimization Strategies for Antibody-Drug Conjugates in Oncology: What Can We Learn From US Food and Drug Administration-Approved Antibody-Drug Conjugates? Clin Pharmacol Ther 2021, 110, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Investigational New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 low, ultra-low, and novel complementary biomarkers: Expanding the spectrum of HER2 positivity in breast cancer. Frontiers in Molecular Biosciences 2022, 9. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Wang, K.; Zhu, H. Treatment-related adverse events of antibody–drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer 2023, 129, 283–295. [Google Scholar] [CrossRef]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—an emerging class of cancer treatment. British journal of cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Erickson, H.K.; Widdison, W.C.; Mayo, M.F.; Whiteman, K.; Audette, C.; Wilhelm, S.D.; Singh, R. Tumor delivery and in vivo processing of disulfide-linked and thioether-linked antibody-maytansinoid conjugates. Bioconjug Chem 2010, 21, 84–92. [Google Scholar] [CrossRef]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Xu, Y.-y.; Shao, Z.-M.; Yu, K.-D. Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions. Cancer Communications 2023, 43, 297–337. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther 2015, 14, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R. Caveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1) altered trafficking as mechanism of ADC resistance. Molecular cancer therapeutics 2018, 17, 243–253. [Google Scholar] [CrossRef]
- Coates, J.T.; Sun, S.; Leshchiner, I.; Thimmiah, N.; Martin, E.E.; McLoughlin, D.; Danysh, B.P.; Slowik, K.; Jacobs, R.A.; Rhrissorrakrai, K. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer discovery 2021, 11, 2436. [Google Scholar] [CrossRef]
- Li, G.; Guo, J.; Shen, B.-Q.; Yadav, D.B.; Sliwkowski, M.X.; Crocker, L.M.; Lacap, J.A.; Phillips, G.D.L. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer CellsMechanisms of Resistance to Trastuzumab Emtansine (T-DM1). Molecular cancer therapeutics 2018, 17, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)–positive breast cancer after prior HER2-directed therapy. Journal of Clinical Oncology 2011, 29, 398–405. [Google Scholar] [CrossRef]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: a look into novel targets. Journal of Hematology & Oncology 2021, 14, 20. [Google Scholar] [CrossRef]
- Matsumura, Y. Barriers to antibody therapy in solid tumors, and their solutions. Cancer Science 2021, 112, 2939–2947. [Google Scholar] [CrossRef]
- Sardinha, M.; Palma Dos Reis, A.F.; Barreira, J.V.; Fontes Sousa, M.; Pacey, S.; Luz, R. Antibody-Drug Conjugates in Prostate Cancer: A Systematic Review. Cureus 2023, 15, e34490. [Google Scholar] [CrossRef]
- You, W.-K.; Schuetz, T.J.; Lee, S.H. Targeting the DLL/Notch Signaling Pathway in Cancer: Challenges and Advances in Clinical Development. Molecular Cancer Therapeutics 2023, 22, 3–11. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Kantoff, P.; Vogelzang, N.J.; Mega, A.; Fleming, M.T.; Stephenson, J.J.; Frank, R.; Shore, N.D.; Dreicer, R.; McClay, E.F.; et al. Phase 1 study of PSMA ADC, an antibody-drug conjugate targeting prostate-specific membrane antigen, in chemotherapy-refractory prostate cancer. The Prostate 2019, 79, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Moreaux, J.; Kassambara, A.; Hose, D.; Klein, B. STEAP1 is overexpressed in cancers: a promising therapeutic target. Biochem Biophys Res Commun 2012, 429, 148–155. [Google Scholar] [CrossRef]
- Danila, D.C.; Szmulewitz, R.Z.; Vaishampayan, U.; Higano, C.S.; Baron, A.D.; Gilbert, H.N.; Brunstein, F.; Milojic-Blair, M.; Wang, B.; Kabbarah, O.; et al. Phase I Study of DSTP3086S, an Antibody-Drug Conjugate Targeting Six-Transmembrane Epithelial Antigen of Prostate 1, in Metastatic Castration-Resistant Prostate Cancer. Journal of Clinical Oncology 2019, 37, 3518–3527. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Motzer, R.J.; Molina, A.M.; Choueiri, T.K.; Heath, E.I.; Redman, B.G.; Sangha, R.S.; Ernst, D.S.; Pili, R.; Kim, S.K.; et al. Phase I Trials of Anti-ENPP3 Antibody-Drug Conjugates in Advanced Refractory Renal Cell Carcinomas. Clin Cancer Res 2018, 24, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, W.; Zhao, Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int J Biol Sci 2020, 16, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Carneiro, B.A.; Dowlati, A.; Razak, A.R.A.; Chae, Y.K.; Villella, J.A.; Coppola, S.; Englert, S.; Phillips, A.C.; Souers, A.J.; et al. A first-in-human study of mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: Dose escalation results. Journal of Clinical Oncology 2021, 39, 3015–3015. [Google Scholar] [CrossRef]
- Chu, Q. Targeting Mesothelin in Solid Tumours: Anti-mesothelin Antibody and Drug Conjugates. Curr Oncol Rep 2023, 25, 309–323. [Google Scholar] [CrossRef]
- Hong, Y.; Nam, S.M.; Moon, A. Antibody-drug conjugates and bispecific antibodies targeting cancers: applications of click chemistry. Arch Pharm Res 2023. [CrossRef]
- Zong, H.-F.; Zhang, B.-H.; Zhu, J.-W. Generating a Bispecific Antibody Drug Conjugate Targeting PRLR and HER2 with Improving the Internalization. Pharmaceutical Fronts 2022, 04, e113–e120. [Google Scholar] [CrossRef]
- Fang, J.; Xiao, L.; Joo, K.-I.; Liu, Y.; Zhang, C.; Liu, S.; Conti, P.S.; Li, Z.; Wang, P. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. International Journal of Cancer 2016, 138, 1013–1023. [Google Scholar] [CrossRef]
- Ostermann, E.; Garin-Chesa, P.; Heider, K.H.; Kalat, M.; Lamche, H.; Puri, C.; Kerjaschki, D.; Rettig, W.J.; Adolf, G.R. Effective Immunoconjugate Therapy in Cancer Models Targeting a Serine Protease of Tumor Fibroblasts. Clinical Cancer Research 2008, 14, 4584–4592. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nature Communications 2021, 12, 3528. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: changing partners in the dance towards death. Cell Death & Differentiation 2018, 25, 65–80. [Google Scholar]
- Wang, L.; Doherty, G.A.; Judd, A.S.; Tao, Z.-F.; Hansen, T.M.; Frey, R.R.; Song, X.; Bruncko, M.; Kunzer, A.R.; Wang, X.; et al. Discovery of A-1331852, a First-in-Class, Potent, and Orally-Bioavailable BCL-XL Inhibitor. ACS Medicinal Chemistry Letters 2020, 11, 1829–1836. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Tupone, M.G.; Desideri, M.; Di Martile, M.; Gabellini, C.; Buglioni, S.; Pallocca, M.; Alessandrini, G.; D’Aguanno, S.; Del Bufalo, D. BCL-XL overexpression promotes tumor progression-associated properties. Cell Death & Disease 2017, 8, 3216. [Google Scholar] [CrossRef]
- Borges, V.F.; Ferrario, C.; Aucoin, N.; Falkson, C.; Khan, Q.; Krop, I.; Welch, S.; Conlin, A.; Chaves, J.; Bedard, P.L.; et al. Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncology 2018, 4, 1214–1220. [Google Scholar] [CrossRef]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.A.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int J Clin Oncol 2021, 26, 1812–1821. [Google Scholar] [CrossRef]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treatment Reviews 2022, 106, 102395. [Google Scholar] [CrossRef]
- Bauzon, M.; Drake, P.M.; Barfield, R.M.; Cornali, B.M.; Rupniewski, I.; Rabuka, D. Maytansine-bearing antibody-drug conjugates induce in vitro hallmarks of immunogenic cell death selectively in antigen-positive target cells. OncoImmunology 2019, 8, e1565859. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. The Lancet Oncology 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Janku, F.; Han, S.-W.; Doi, T.; Ajani, J.; Kuboki, Y.; Mahling, P.; Subramanian, K.; Pelletier, M.; Askoxylakis, V.; Siena, S. 378 A first in-human, multicenter, open-label, dose-finding phase 1 study of the immune stimulator antibody conjugate NJH395 in patients with nonbreast HER2+ advanced malignancies. Journal for ImmunoTherapy of Cancer 2020, 8, A230–A230. [Google Scholar] [CrossRef]
- Janku, F.; Han, S.-W.; Doi, T.; Amatu, A.; Ajani, J.A.; Kuboki, Y.; Cortez, A.; Cellitti, S.E.; Mahling, P.C.; Subramanian, K.; et al. Preclinical Characterization and Phase I Study of an Anti–HER2-TLR7 Immune-Stimulator Antibody Conjugate in Patients with HER2+ Malignancies. Cancer Immunology Research 2022, 10, 1441–1461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





