Submitted:
01 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
Assessment of SNV and non-SNV energy change distribution in experimental databases and types of amino acid changes
Assessment of leading algorithms performance
3. Discussion
4. Methods
Databases of experimentally measured changes of ΔGfolding and ΔGbinding.
Databases of binding free energy changes of protein-protein interactions (main source SKEMPI database)
Databases of binding free energy changes of protein-DNA interactions
Computational methods for predicting ΔGfolding or ΔGbinding
Methods for predicting folding free energy change caused by mutation.
- SAAFEC-SEQ [34]: SAAFEC-SEQ is a gradient-boosting decision tree machine learning method that uses physicochemical properties, sequence features, and evolutionary information features to predict changes in folding free energy caused by amino acid mutation. The method utilizes amino acid sequence as the input for making predictions.
- I-mutant 2.0 [30]: I-mutant 2.0 is a support vector machine (SVM) based method for prediction of folding free energy as an effect of mutation. The method is implemented as both sequence and structure based.
- mCSM [26]: mCSM is a web-based predictor that uses a graph-based approach to predict the impact of missense mutations on protein stability. The predictive models in mCSM are trained with the atomic distance patterns of different amino acid residues.
- MAESTRO [54]: MAESTRO is a structure-based method that utilizes a multi-agent machine learning system for predicting the impact of mutation on folding free energy.
- PoPMuSic [60]: PoPMuSiC is a web server that predicts the thermodynamic stability changes caused by single site mutations in proteins, using a linear combination of statistical potentials whose coefficients depend on the solvent accessibility of the mutated residue.
- SDM [61] : Site Directed Mutator (SDM) uses statistical potential energy function to calculate the stability score which uses amino-acid substitution frequencies within homologous protein families. The metric is analogous to the free energy difference between wild-type and mutant protein. The method is 3D structure based and is available as a webserver.
- DUET [56]: DUET is a 3D structure-based method that uses mCSM and SDM for the consensus prediction. The results from these methods are combined and optimized using Support Vector Machines (SVM) to make the final prediction. The method is available as a webserver.
Methods for predicting binding free energy changes of protein-protein interactions caused by mutation.
- SAAMBE-SEQ [42]: It is a sequence-based machine-learning technique that can predict how a single mutation will affect the binding energy of protein-protein complexes. In contrast to other methods already in use, SAAMBE-SEQ does not require a 3D protein-protein complex structure as input. Note that it uses features that require the length of interacting partners to be longer than 20 amino acids and thus it is not expected to perform well on protein-peptide binding cases.
- SAAMBE-3D [36]: SAAMBE-3D is a machine learning-based method that takes a PDB file as its input and can estimate the effect of a single amino acid modification on protein-protein binding. This tool enables the investigation of two types of inquiries: (1) forecasting alterations in binding free energy resulting from a mutation, and (2) predicting whether a mutation causes a disturbance in protein-protein interactions.
- mCSM-PPI2 [40]: mCSM-PPI2 is a computational technique that uses machine learning to forecast the impact of missense mutations on protein-protein binding affinity. It employs an enhanced graph-based signature strategy to model changes in the network of non-covalent interactions between residues using graph kernels, complex network metrics, evolutionary data, and energetic terms. This approach is available for free at https://biosig.lab.uq.edu.au/mcsm_ppi2/
- MutaBind2 [41]: MutaBind2 is a tool that assesses the influence of individual-site and multi-site mutations on protein-protein binding affinities in soluble complexes. This method utilizes statistical potentials, molecular mechanics, force fields, and the structure of the protein-protein complex.
- BeAtMuSiC [39]: BeAtMuSiC is a method based on a set of statistical potentials derived from known protein structures, in addition, it accounts for the effect of the mutation on the strength of the interactions at the interface as well as the overall stability of the complex. This method is available as an online web server free of charge at http://babylone.3bio.ulb.ac.be/beatmusic/index.php
Methods for predicting binding free energy changes of protein-DNA interactions caused by mutation.
- SAMPDI-3D [47]: SAMPDI-3D uses a gradient-boosting decision tree machine learning method to predict the change in the protein-DNA binding free energy brought on by mutations in the binding protein or the bases of the corresponding DNA. It takes the structure of the complex i.e., a PDB file as an input.
- mCSM-NA [44]: The mCSM-NA method is based on graph-based structural signatures to predict the DDG caused by mutations in proteins bound to DNA/RNA.
- PREMPDI [45]: PremPDI is a physics-based method that relies on the 3D structure of the protein-nucleic acid complex for making predictions. The method is based on molecular mechanics force fields and fast side-chain optimization algorithms.
SNV vs non-SNV cases
Free energy changes
Assessment of Predictions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kent, S.; Marshall, G.R.; Wlodawer, A. Determining the 3D Structure of HIV-1 Protease. Science (1979) 2000, 288, 1590. [CrossRef]
- Naddaf, M. How Do We Smell? First 3D Structure of Human Odour Receptor Clues. Nature 2023. [CrossRef]
- Pacheco-Fiallos, B.; Vorländer, M.K.; Riabov-Bassat, D.; Fin, L.; O’Reilly, F.J.; Ayala, F.I.; Schellhaas, U.; Rappsilber, J.; Plaschka, C. MRNA Recognition and Packaging by the Human-Export Complex. Nature 2023, 616, 828–835. [CrossRef]
- Pradhan, B.; Kanno, T.; Umeda Igarashi Miki and Loke, M.S.; Baaske, M.D.; Wong, J.S.K.; Jeppsson, K.; Björkegren, C.; Kim, E. The Smc5/6 Complex Is a DNA Loop-Extruding Motor. Nature 2023, 616, 843–848. [CrossRef]
- Bell, D.R.; Cheng, S.Y.; Salazar, H.; Ren, P. Capturing RNA Folding Free Energy with Coarse-Grained Dynamics Simulations. Sci. Rep. 2017, 7, 45812. [CrossRef]
- Hazel, A.J.; Walters, E.T.; Rowley Christopher N and Gumbart, J.C. Folding Free Energy Landscapes of -Sheets with Non-polarizable and Polarizable CHARMM Force Fields. J. Chem. Phys. 2018, 149, 72317. [CrossRef]
- Krivov, S. V Protein Folding Free Energy Landscape along the Committor - the Optimal Folding Coordinate. J. Chem. Theory Comput. 2018, 14, 3418–3427. [CrossRef]
- Nawrocki, G.; Leontyev, I.; Sakipov, S.; Darkhovskiy, M.; Kurnikov, I.; Pereyaslavets Leonid and Kamath, G.; Voronina, E.; Butin, O.; Illarionov, A.; Olevanov, M.; et al. Protein-Ligand Binding Free-Energy Calculations with ARROW─A Purely First-Principles Parameterized Polarizable Field. J. Chem. Theory Comput. 2022, 18, 7751–7763. [CrossRef]
- Fu, H.; Chipot, C.; Shao, X.; Cai, W. Achieving Accurate Standard Protein-Protein Binding Free Energy through the Geometrical Route and Ergodic Sampling. J. Chem. Inf. Model. 2023, 63, 2512–2519. [CrossRef]
- Molani, F.; Webb, S.; Cho, A.E. Combining QM/MM Calculations with Classical Mining Minima to Predict Protein-Ligand Binding Free Energy. J. Chem. Inf. Model. 2023, 63, 2728–2734. [CrossRef]
- Proniewicz, E.; Burnat, G.; Domin, H.; Małuch, I.; Makowska, M.; Prahl, A. Application of Alanine Scanning to Determination of Amino Acids for Peptide Adsorption at the Solid/Solution and Binding to the Receptor: Surface-Enhanced/Infrared Spectroscopy versus Bioactivity Assays. J. Med. Chem. 2021, 64, 8410–8422. [CrossRef]
- Tanaka, T.; Asano, T.; Sano, M.; Takei, J.; Hosono, H.; Nanamiya, R.; Tateyama, N.; Kaneko, M.K.; Kato, Y. Epitope Mapping of the Anti-California Sea Lion Podoplanin Antibody PMab-269 Using Alanine-Scanning and ELISA. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 196–200. [CrossRef]
- Isoda, Y.; Tanaka, T.; Suzuki, H.; Asano, T.; Kitamura, K.; Kudo, Y.; Ejima, R.; Ozawa, K.; Yoshikawa, T.; Kaneko, M.K.; et al. Epitope Mapping of the Novel Anti-Human CCR9 Monoclonal (CMab-11) by 2 Alanine Scanning. Monoclon. Antib. Immunodiagn. Immunother. 2023, 42, 73–76. [CrossRef]
- Gromiha, M.M.; An, J.; Kono, H.; Oobatake, M.; Uedaira H and Sarai, A. ProTherm: Thermodynamic Database for Proteins and Mutants. Nucleic Acids Res. 1999, 27, 286–288. [CrossRef]
- Nikam, R.; Kulandaisamy, A.; Harini, K.; Sharma Divya and Gromiha, M.M. ProThermDB: Thermodynamic Database for Proteins and Mutants after 15 Years. Nucleic Acids Res. 2021, 49, D420–D424. [CrossRef]
- Prabakaran, P.; An, J.; Gromiha, M.M.; Selvaraj, S.; Uedaira, H.; Kono, H.; Sarai, A. Thermodynamic Database for Protein-Nucleic Acid Interactions(ProNIT). Bioinformatics 2001, 17, 1027–1034. [CrossRef]
- Harini, K.; Srivastava, A.; Kulandaisamy Arulsamy and Gromiha, M.M. ProNAB: Database for Binding Affinities of Protein-Nucleic Acid and Their Mutants. Nucleic Acids Res. 2022, 50, D1528–D1534. [CrossRef]
- Moal, I.H.; Fernández-Recio, J. SKEMPI: A Structural Kinetic and Energetic Database of Mutant Interactions and Its Use in Empirical Models. Bioinformatics 2012, 28, 2600–2607. [CrossRef]
- Jankauskaite, J.; Jiménez-Garc\’\ia, B.; Dapkunas, J.; Fernández-Recio, J.; Moal, I.H. SKEMPI 2.0: An Updated Benchmark of Changes in Protein-Protein Energy, Kinetics and Thermodynamics upon Mutation. Bioinformatics 2019, 35, 462–469. [CrossRef]
- Gromiha, M.M.; Huang, L.-T. Machine Learning Algorithms for Predicting Protein Folding rates and Stability of Mutant Proteins: Comparison with Statistical. Curr. Protein Pept. Sci. 2011, 12, 490–502. [CrossRef]
- Fang, J. A Critical Review of Five Machine Learning-Based Algorithms for predicting Protein Stability Changes upon Mutation. Brief. Bioinform. 2020, 21, 1285–1292. [CrossRef]
- Pucci, F.; Schwersensky, M.; Rooman, M. Artificial Intelligence Challenges for Predicting the Impact of mutations on Protein Stability. Curr. Opin. Struct. Biol. 2022, 72, 161–168. [CrossRef]
- Sora, V.; Laspiur, A.O.; Degn Kristine and Arnaudi, M.; Utichi, M.; Beltrame, L.; De Menezes, D.; Orlandi, M.; Stoltze, U.K.; Rigina, O.; Sackett, P.W.; et al. RosettaDDGPrediction for High-Throughput Mutational Scans: From to Binding. Protein Sci. 2023, 32, e4527. [CrossRef]
- Guerois, R.; Nielsen, J.E.; Serrano, L. Predicting Changes in the Stability of Proteins and Protein: A Study of More than 1000 Mutations. J. Mol. Biol. 2002, 320, 369–387. [CrossRef]
- Gilis, D.; Rooman, M. PoPMuSiC, an Algorithm for Predicting Protein Mutant Stability: Application to Prion Proteins. Protein Eng. 2000, 13, 849–856. [CrossRef]
- Pires, D.E. V; Ascher, D.B.; Blundell, T.L. MCSM: Predicting the Effects of Mutations in Proteins Using-Based Signatures. Bioinformatics 2014, 30, 335–342. [CrossRef]
- Quan, L.; Lv, Q.; Zhang, Y. STRUM: Structure-Based Prediction of Protein Stability changes upon Single-Point Mutation. Bioinformatics 2016, 32, 2936–2946. [CrossRef]
- Pandurangan, A.P.; Ochoa-Montaño, B.; Ascher, D.B.; Blundell, T.L. SDM: A Server for Predicting Effects of Mutations on Protein. Nucleic Acids Res. 2017, 45, W229–W235. [CrossRef]
- Getov, I.; Petukh, M.; Alexov, E. SAAFEC: Predicting the Effect of Single Point Mutations on Protein Folding Free Energy Using a Knowledge-Modified/PBSA Approach. Int. J. Mol. Sci. 2016, 17, 512. [CrossRef]
- Capriotti, E.; Fariselli, P.; Casadio, R. I-Mutant2.0: Predicting Stability Changes upon Mutation from the Protein Sequence or Structure. Nucleic Acids Res. 2005, 33, W306–10. [CrossRef]
- Folkman, L.; Stantic, B.; Sattar, A.; Zhou, Y. EASE-MM: Sequence-Based Prediction of Mutation-Induced Changes with Feature-Based Multiple Models. J. Mol. Biol. 2016, 428, 1394–1405. [CrossRef]
- Savojardo, C.; Fariselli, P.; Martelli, P.L.; Casadio, R. INPS-MD: A Web Server to Predict Stability of Protein variants from Sequence and Structure. Bioinformatics 2016, 32, 2542–2544. [CrossRef]
- Lv, X.; Chen, J.; Lu, Y.; Chen, Z.; Xiao, N.; Yang, Y. Accurately Predicting Mutation-Caused Stability Changes from Protein Sequences Using Extreme Gradient Boosting. J. Chem. Inf. Model. 2020, 60, 2388–2395. [CrossRef]
- Li, G.; Panday, S.K.; Alexov, E. SAAFEC-SEQ: A Sequence-Based Method for Predicting the Effect of Single Point Mutations on Protein Thermodynamic Stability. Int. J. Mol. Sci. 2021, 22. [CrossRef]
- Petukh, M.; Dai, L.; Alexov, E. SAAMBE: Webserver to Predict the Charge of Binding Free Energy by Amino Acids Mutations. Int. J. Mol. Sci. 2016, 17, 547. [CrossRef]
- Pahari, S.; Li, G.; Murthy, A.K.; Liang, S.; Fragoza, R.; Yu, H.; Alexov, E. SAAMBE-3D: Predicting Effect of Mutations on Protein-Protein. Int. J. Mol. Sci. 2020, 21. [CrossRef]
- Xiong, P.; Zhang, C.; Zheng, W.; Zhang, Y. BindProfX: Assessing Mutation-Induced Binding Affinity Change by Protein Interface Profiles with Pseudo-Counts. J. Mol. Biol. 2017, 429, 426–434. [CrossRef]
- Geng, C.; Vangone, A.; Folkers, G.E.; Xue, L.C.; Bonvin, A.M.J.J. ISEE: Interface Structure, Evolution, and Energy-Based Machine Predictor of Binding Affinity Changes upon Mutations. Proteins 2019, 87, 110–119. [CrossRef]
- Dehouck, Y.; Kwasigroch, J.M.; Rooman, M.; Gilis, D. BeAtMuSiC: Prediction of Changes in Protein-Protein Binding on Mutations. Nucleic Acids Res. 2013, 41, W333–9. [CrossRef]
- Rodrigues, C.H.M.; Myung, Y.; Pires Douglas E V and Ascher, D.B. MCSM-PPI2: Predicting the Effects of Mutations on protein-Protein Interactions. Nucleic Acids Res. 2019, 47, W338–W344. [CrossRef]
- Zhang, N.; Chen, Y.; Lu, H.; Zhao, F.; Alvarez, R.V.; Goncearenco, A.; Panchenko, A.R.; Li, M. MutaBind2: Predicting the Impacts of Single and Multiple on Protein-Protein Interactions. iScience 2020, 23, 100939. [CrossRef]
- Li, G.; Pahari, S.; Murthy, A.K.; Liang, S.; Fragoza, R.; Yu, H.; Alexov, E. SAAMBE-SEQ: A Sequence-Based Method for Predicting Mutation on Protein-Protein Binding Affinity. Bioinformatics 2021, 37, 992–999. [CrossRef]
- Jemimah, S.; Sekijima, M.; Gromiha, M.M. ProAffiMuSeq: Sequence-Based Method to Predict the Binding Free Change of Protein-Protein Complexes upon Mutation Using Classification. Bioinformatics 2020, 36, 1725–1730. [CrossRef]
- Pires, D.E. V; Ascher, D.B. MCSM-NA: Predicting the Effects of Mutations on Protein-Nucleic Interactions. Nucleic Acids Res. 2017, 45, W241–W246. [CrossRef]
- Zhang, N.; Chen, Y.; Zhao, F.; Yang, Q.; Simonetti, F.L.; Li, M. PremPDI Estimates and Interprets the Effects of Missense on Protein-DNA Interactions. PLoS Comput. Biol. 2018, 14, e1006615. [CrossRef]
- Peng, Y.; Sun, L.; Jia, Z.; Li, L.; Alexov, E. Predicting Protein-DNA Binding Free Energy Change upon Missense Using Modified MM/PBSA Approach: SAMPDI Webserver. Bioinformatics 2018, 34, 779–786. [CrossRef]
- Li, G.; Panday, S.K.; Peng, Y.; Alexov, E. SAMPDI-3D: Predicting the Effects of Protein and DNA on Protein-DNA Interactions. Bioinformatics 2021, 37, 3760–3765. [CrossRef]
- Benevenuta, S.; Birolo, G.; Sanavia, T.; Capriotti, E.; Fariselli, P. Challenges in Predicting Stabilizing Variations: An Exploration. Front Mol Biosci 2022, 9, 1075570. [CrossRef]
- Usmanova, D.R.; Bogatyreva, N.S.; Ariño Bernad, J.; Eremina, A.A.; Gorshkova, A.A.; Kanevskiy, G.M.; Lonishin, L.R.; Meister, A. V; Yakupova, A.G.; Kondrashov, F.A.; et al. Self-Consistency Test Reveals Systematic Bias in Programs for prediction Change of Stability upon Mutation. Bioinformatics 2018, 34, 3653–3658. [CrossRef]
- Iqbal, S.; Li, F.; Akutsu, T.; Ascher, D.B.; Webb, G.I.; Song, J. Assessing the Performance of Computational Predictors for estimating Protein Stability Changes upon Missense Mutations. Brief. Bioinform. 2021, 22. [CrossRef]
- Fang, J. The Role of Data Imbalance Bias in the Prediction of Protein Change upon Mutation. PLoS One 2023, 18, e0283727. [CrossRef]
- Brock, K.; Talley, K.; Coley, K.; Kundrotas, P.; Alexov, E. Optimization of Electrostatic Interactions in Protein-Protein. Biophys. J. 2007, 93, 3340–3352. [CrossRef]
- Fariselli, P.; Martelli, P.L.; Savojardo, C.; Casadio, R. INPS: Predicting the Impact of Non-Synonymous Variations on protein Stability from Sequence. Bioinformatics 2015, 31, 2816–2821. [CrossRef]
- Laimer, J.; Hofer, H.; Fritz, M.; Wegenkittl, S.; Lackner, P. MAESTRO–Multi Agent Stability Prediction upon Point Mutations. BMC Bioinformatics 2015, 16, 116. [CrossRef]
- Dehouck, Y.; Grosfils, A.; Folch, B.; Gilis, D.; Bogaerts, P.; Rooman, M. Fast and Accurate Predictions of Protein Stability Changes upon mutations Using Statistical Potentials and Neural Networks: PoPMuSiC-2.0. Bioinformatics 2009, 25, 2537–2543. [CrossRef]
- Pires, D.E. V; Ascher, D.B.; Blundell, T.L. DUET: A Server for Predicting Effects of Mutations on Protein Using an Integrated Computational Approach. Nucleic Acids Res. 2014, 42, W314–9. [CrossRef]
- Zhang, X.; Mei, L.; Gao, Y.; Hao, G.; Song, B. Web Tools Support Predicting Protein–Nucleic Acid Complexes Stability with Affinity Changes. WIREs RNA 2023. [CrossRef]
- Pandey, P.; Ghimire, S.; Wu, B.; Alexov, E. On the Linkage of Thermodynamics and Pathogenicity. Curr Opin Struct Biol 2023, 80, 102572. [CrossRef]
- Kumar, M.D.S. ProTherm and ProNIT: Thermodynamic Databases for Proteins and Protein-Nucleic Acid Interactions. Nucleic Acids Res 2006, 34, D204–D206. [CrossRef]
- Dehouck, Y.; Kwasigroch, J.M.; Gilis, D.; Rooman, M. PoPMuSiC 2.1: A Web Server for the Estimation of Protein Changes upon Mutation and Sequence Optimality. BMC Bioinformatics 2011, 12, 1–12. [CrossRef]
- Worth, C.L.; Preissner, R.; Blundell, T.L. SDM–a Server for Predicting Effects of Mutations on Protein and Malfunction. Nucleic Acids Res. 2011, 39, W215–22. [CrossRef]
- Peng, Y.; Alexov, E. Investigating the Linkage between Disease-Causing Amino Acid and Their Effect on Protein Stability and Binding. Proteins 2016, 84, 232–239. [CrossRef]
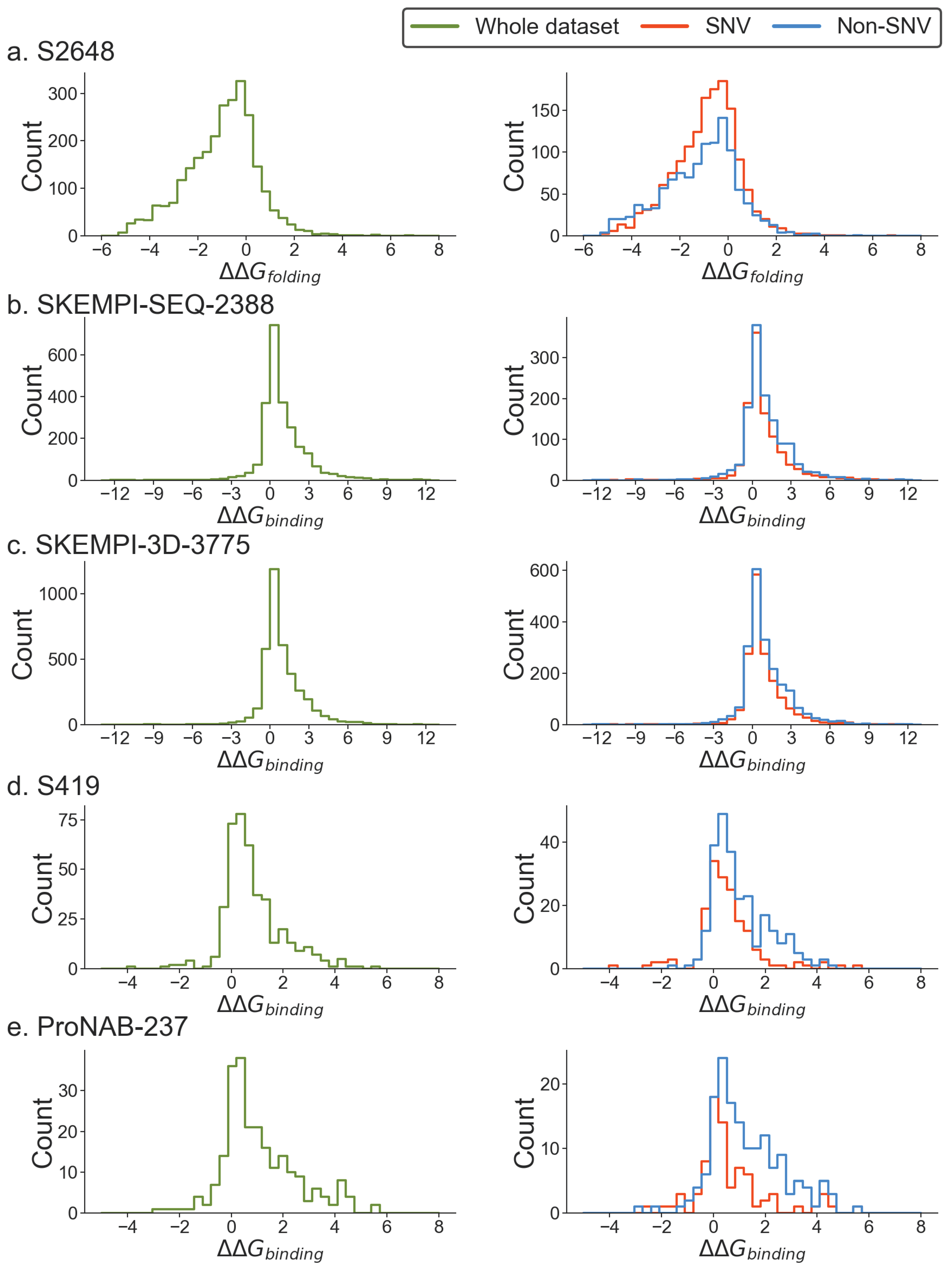
| Datasets | Cut-off | |||
| > 2 kcal/mol | > 1 kcal/mol | |||
| No. of stabilizing mutations | No. of destabilizing mutations | No. of stabilizing mutations | No. of destabilizing mutations | |
| S2648 | 42 | 621 | 152 | 1192 |
| SKEMPI-SEQ-2388 | 56 | 470 | 117 | 903 |
| SKEMPI-3D-3775 | 67 | 742 | 159 | 1398 |
| S419 | 4 | 64 | 10 | 137 |
| ProNAB-237 | 3 | 53 | 9 | 100 |
| Methods | S2648 | ||||||||
| Whole Dataset | SNV | Non-SNV | |||||||
| PCC | MSE | Slope | PCC | MSE | Slope | PCC | MSE | Slope | |
| SAAFEC-SEQa | 0.91 | 0.45 | 0.66 | 0.90 | 0.44 | 0.64 | 0.92 | 0.46 | 0.69 |
| I-mutant 2.0a | 0.55 | 1.68 | 0.45 | 0.52 | 1.50 | 0.38 | 0.57 | 1.92 | 0.52 |
| I-mutant 2.0 | 0.60 | 1.51 | 0.51 | 0.56 | 1.40 | 0.44 | 0.63 | 1.66 | 0.57 |
| INPSa | 0.57 | 1.56 | 0.39 | 0.52 | 1.50 | 0.33 | 0.60 | 1.65 | 0.43 |
| INPS-3D | 0.64 | 1.30 | 0.41 | 0.59 | 1.27 | 0.35 | 0.68 | 1.33 | 0.46 |
| mCSM | 0.69 | 1.15 | 0.42 | 0.62 | 1.19 | 0.34 | 0.74 | 1.11 | 0.50 |
| MAESTRO | 0.66 | 1.29 | 0.52 | 0.58 | 1.31 | 0.43 | 0.72 | 1.26 | 0.61 |
| PoPMuSiC | 0.62 | 1.34 | 0.41 | 0.55 | 1.33 | 0.33 | 0.67 | 1.36 | 0.48 |
| SDM | 0.46 | 2.34 | 0.43 | 0.40 | 2.14 | 0.36 | 0.50 | 2.59 | 0.50 |
| DUET | 0.68 | 1.17 | 0.50 | 0.62 | 1.19 | 0.41 | 0.74 | 1.14 | 0.59 |
| Methods | SKEMPI-SEQ-2388 | ||||||||
| Whole Dataset | SNV | Non-SNV | |||||||
| PCC | MSE | Slope | PCC | MSE | Slope | PCC | MSE | Slope | |
| SAAMBE-SEQ | 0.88 | 0.82 | 0.72 | 0.86 | 0.86 | 0.69 | 0.89 | 0.78 | 0.73 |
| SKEMPI-3D-3775 | |||||||||
| Whole Dataset | SNV | Non-SNV | |||||||
| PCC | MSE | Slope | PCC | MSE | Slope | PCC | MSE | Slope | |
| SAAMBE 3D | 0.90 | 0.66 | 0.64 | 0.90 | 0.64 | 0.63 | 0.91 | 0.68 | 0.65 |
| MutaBind2 | 0.90 | 0.62 | 0.70 | 0.90 | 0.58 | 0.69 | 0.90 | 0.64 | 0.70 |
| mCSM-PPI2 | 0.91 | 0.65 | 0.65 | 0.88 | 0.75 | 0.60 | 0.93 | 0.57 | 0.68 |
| BeAtMuSiC | 0.35 | 2.73 | 0.18 | 0.31 | 2.53 | 0.13 | 0.37 | 2.90 | 0.21 |
| Methods | S419 | ||||||||
| Whole Dataset | SNV | Non-SNV | |||||||
| PCC | MSE | Slope | PCC | MSE | Slope | PCC | MSE | Slope | |
| SAMPDI-3D | 0.83 | 0.46 | 0.53 | 0.84 | 0.48 | 0.52 | 0.81 | 0.45 | 0.52 |
| mCSM-NA | 0.37 | 1.56 | 0.33 | 0.42 | 1.45 | 0.36 | 0.28 | 1.63 | 0.25 |
| PremPDI | 0.44 | 1.53 | 0.45 | 0.40 | 1.31 | 0.30 | 0.41 | 1.67 | 0.47 |
| ProNAB-237 | |||||||||
| Whole Dataset | SNV | Non-SNV | |||||||
| PCC | MSE | Slope | PCC | MSE | Slope | PCC | MSE | Slope | |
| SAMPDI-3D | 0.58 | 1.39 | 0.30 | 0.50 | 1.59 | 0.21 | 0.59 | 1.29 | 0.31 |
| mCSM-NA | 0.43 | 2.08 | 0.35 | 0.28 | 3.21 | 0.28 | 0.52 | 1.51 | 0.38 |
| PremPDI | 0.52 | 1.76 | 0.42 | 0.45 | 2.20 | 0.38 | 0.51 | 1.54 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





