Submitted:
01 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
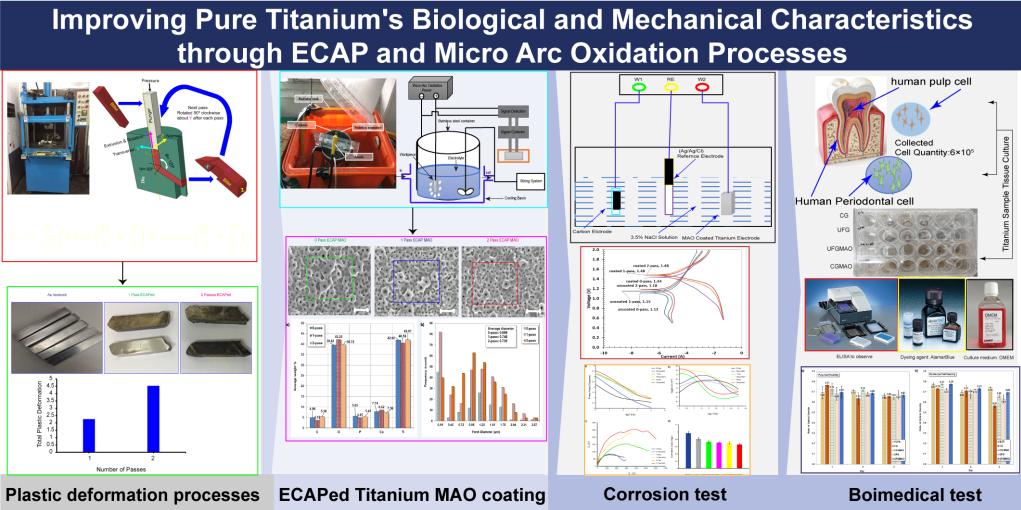
Keywords:
1. Introduction
2. Materials and Methods
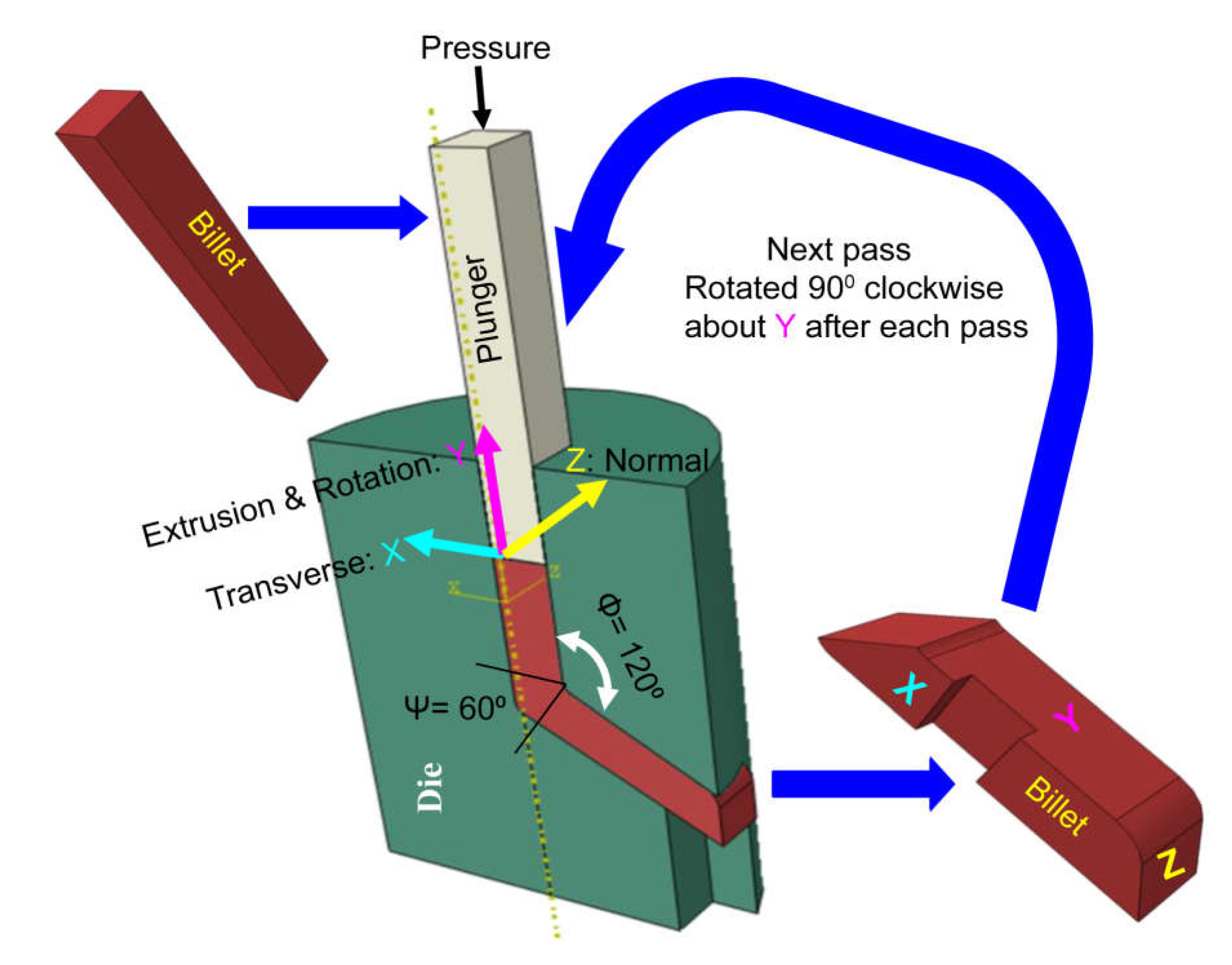
2.1. Equal Channel Angular Pressing (ECAP) Process
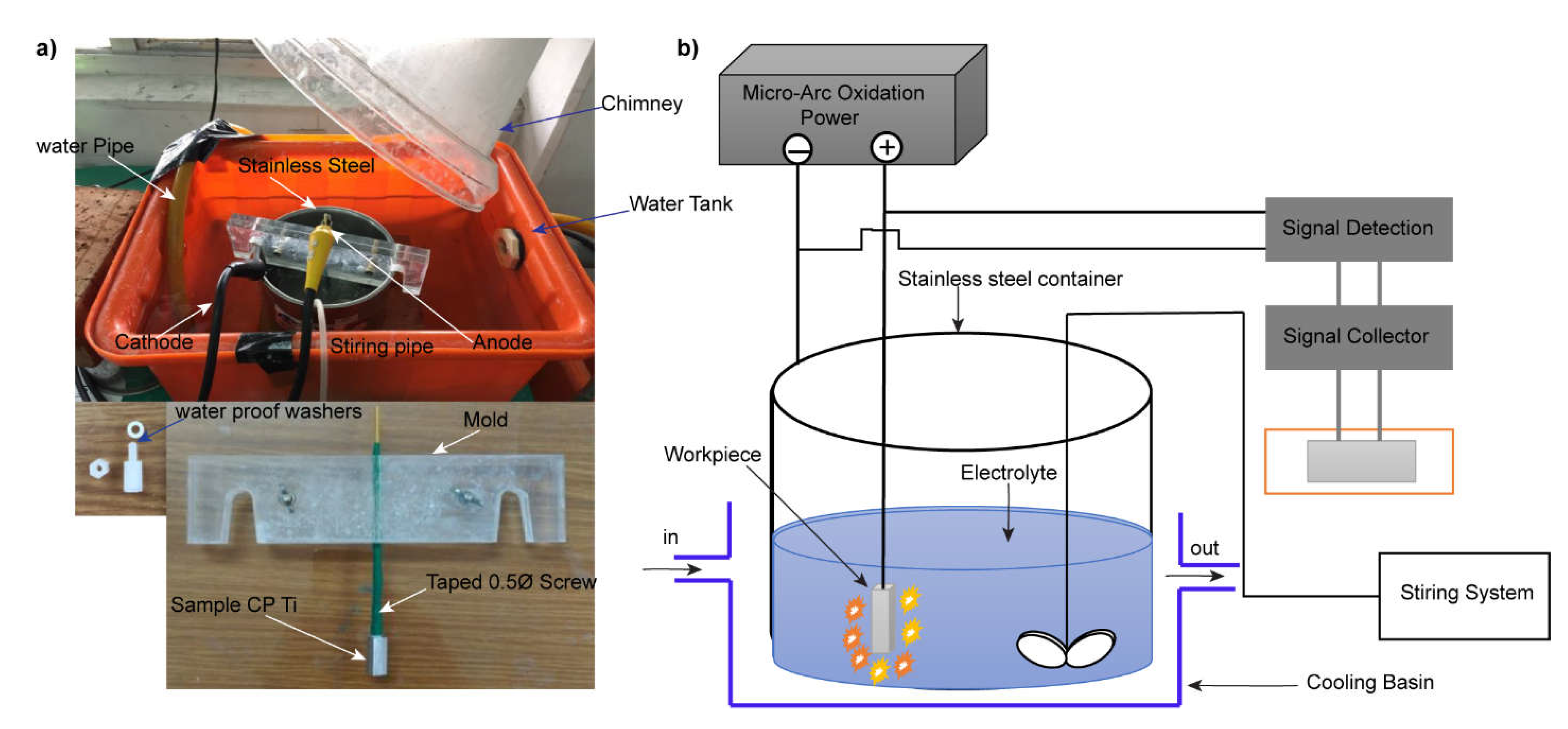
2.2. Micro Arc Oxidation (MAO) Coating
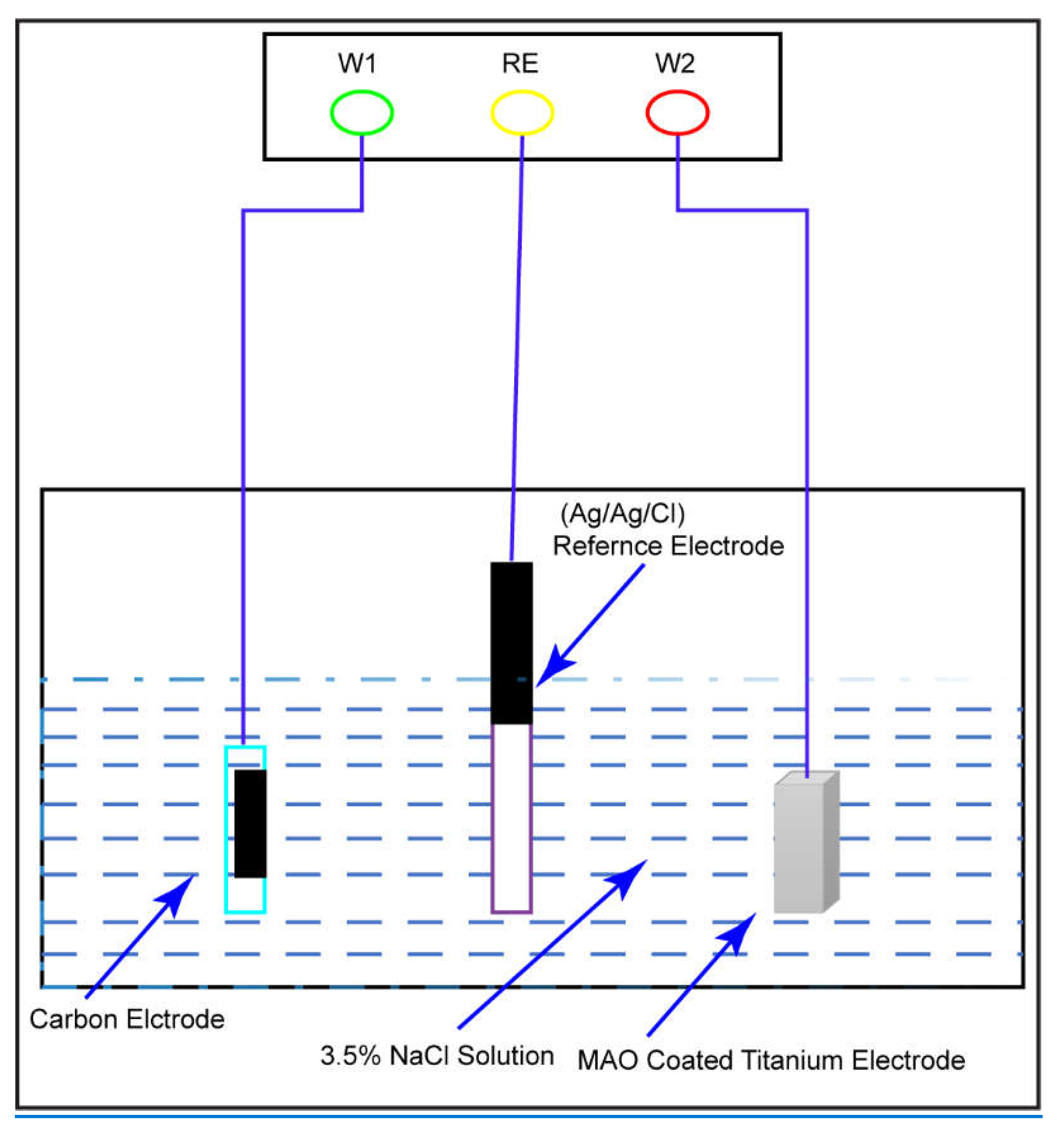
3.2. Electrochemical Test
2.3.1. Tafel extrapolation
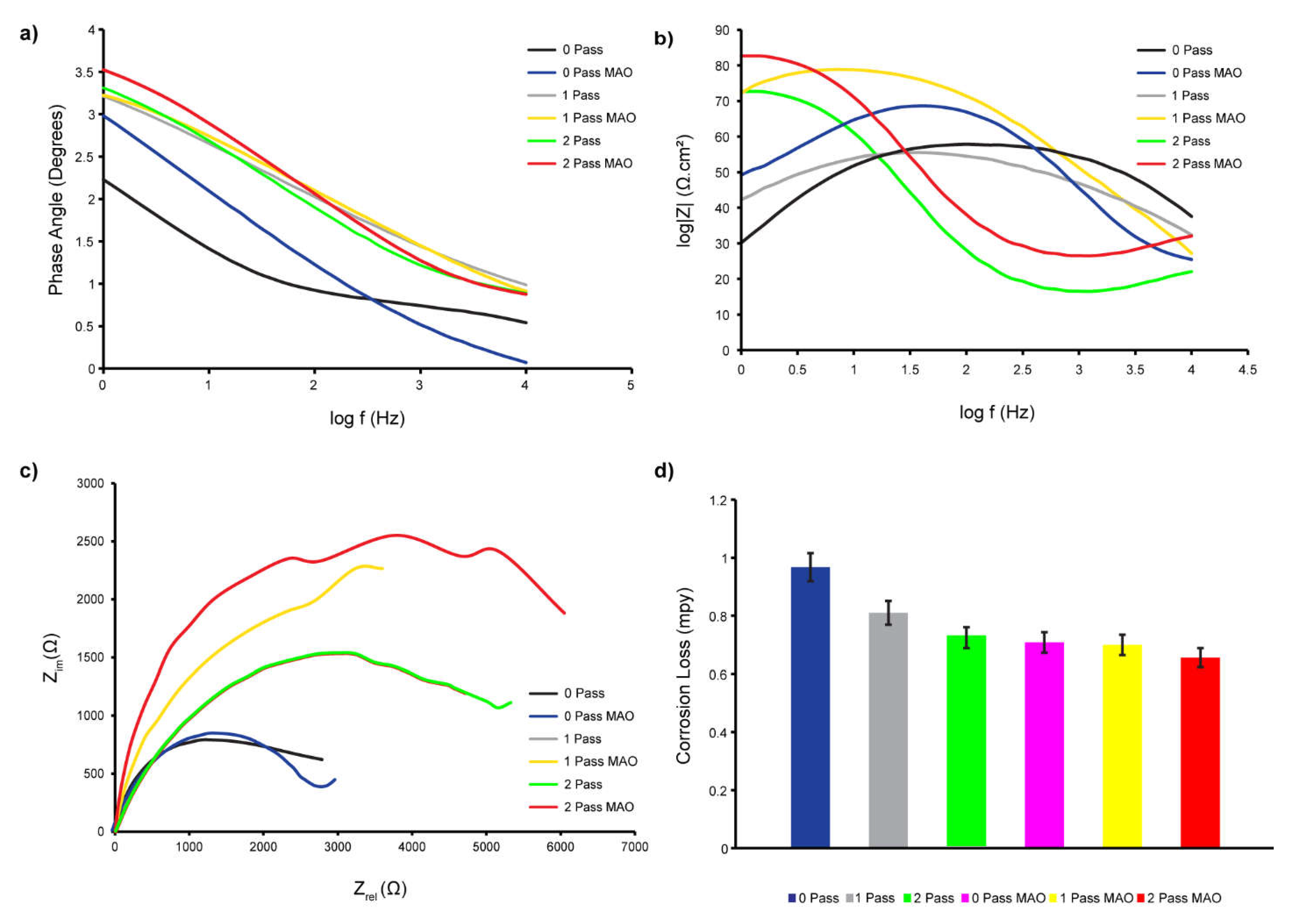
2.3.2. Electrochemical Impedance Spectroscopy
2.4. Material Characterization
2.4.1. X-ray diffraction (XRD)
2.4.2. Elemental Composition (EDS)
2.4.3. Surface Morphology
2.5. Biomedical Investigation
2.5.1. Pulp and Periodontal Cells on Modified Titanium
3. Results and Discussion
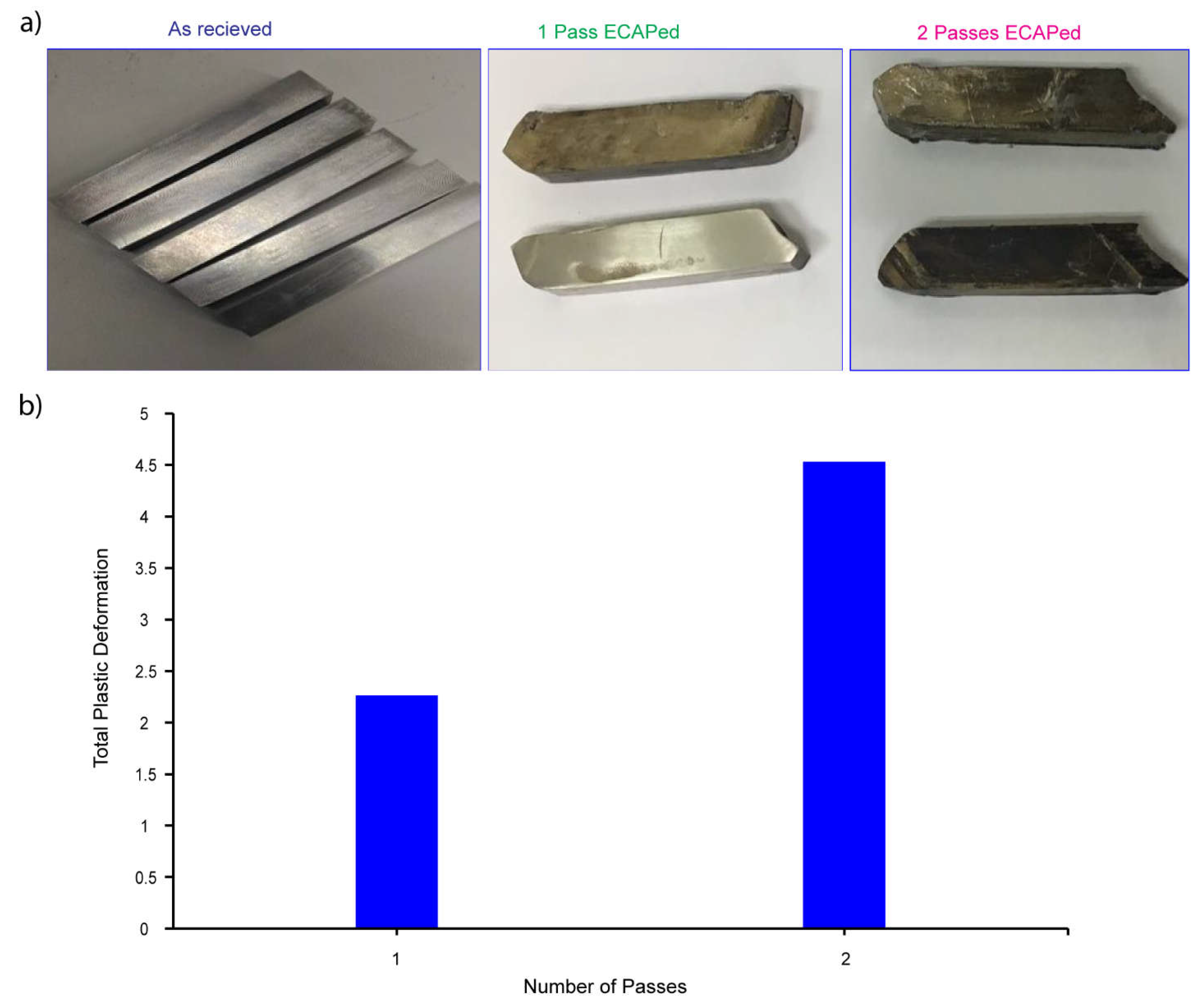
3.1. Sever Plastic Deformation
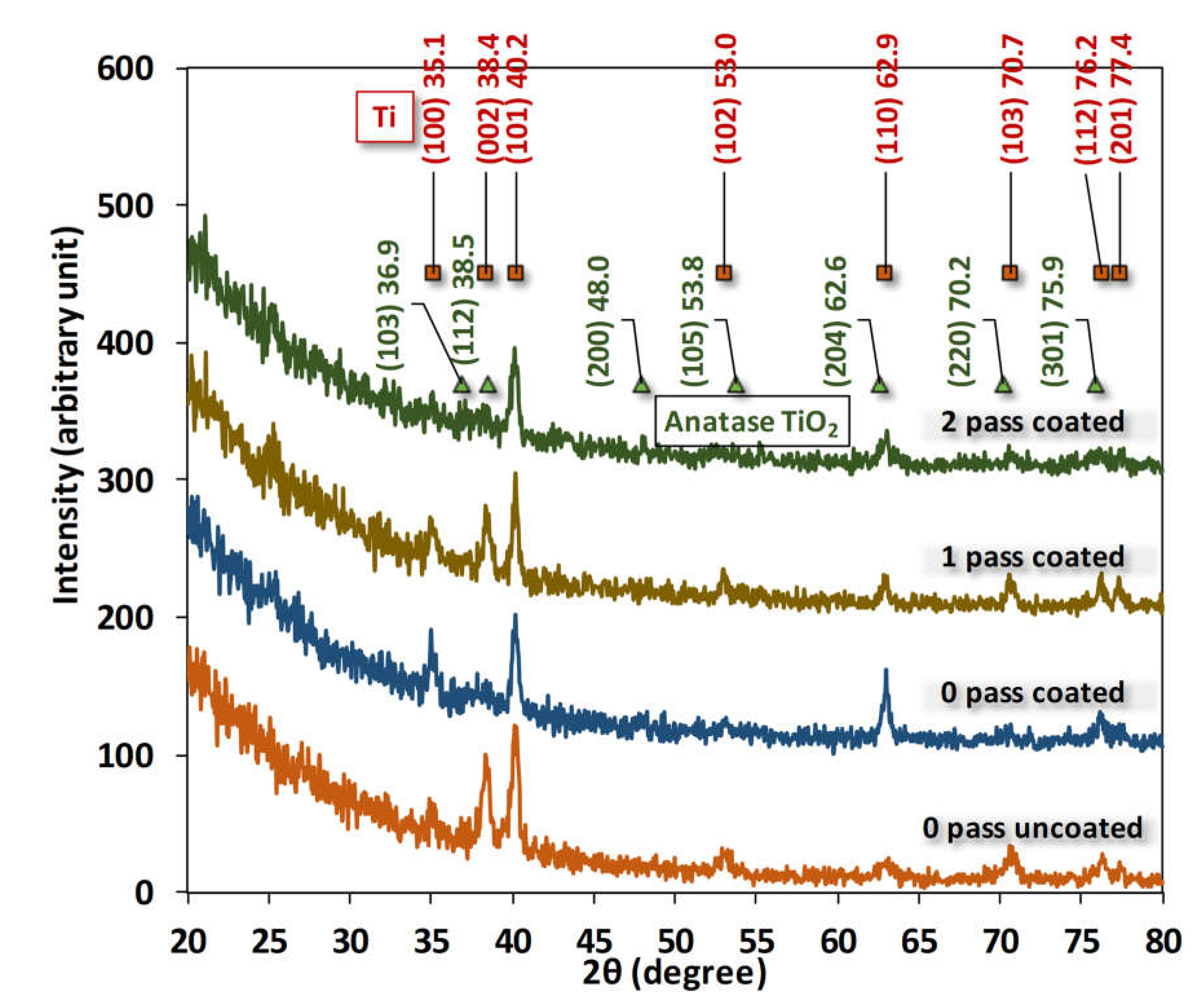
3.2. X-ray diffraction
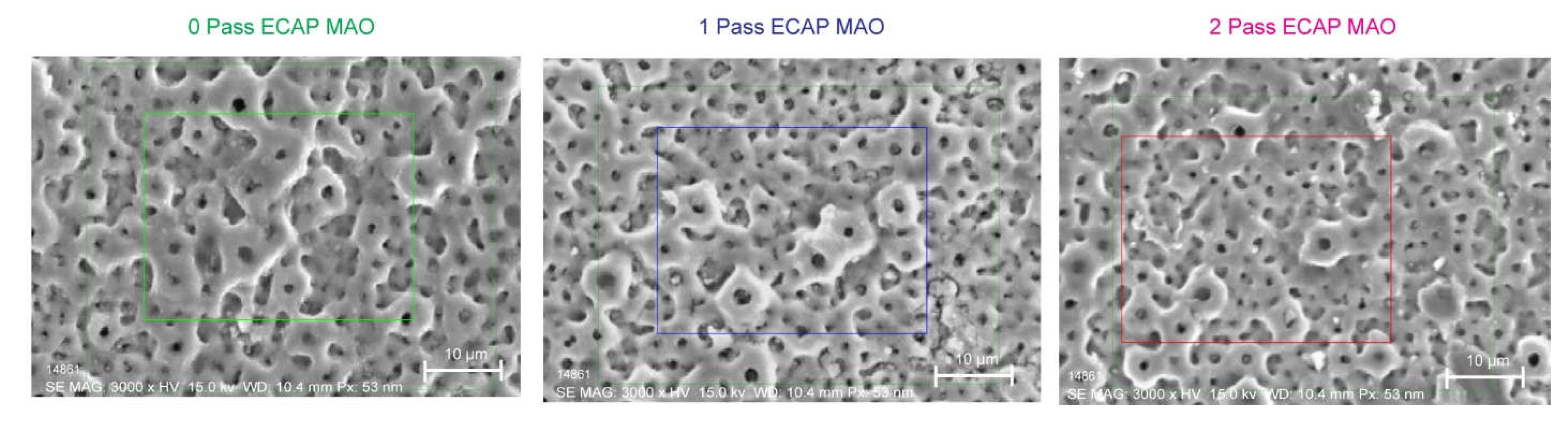
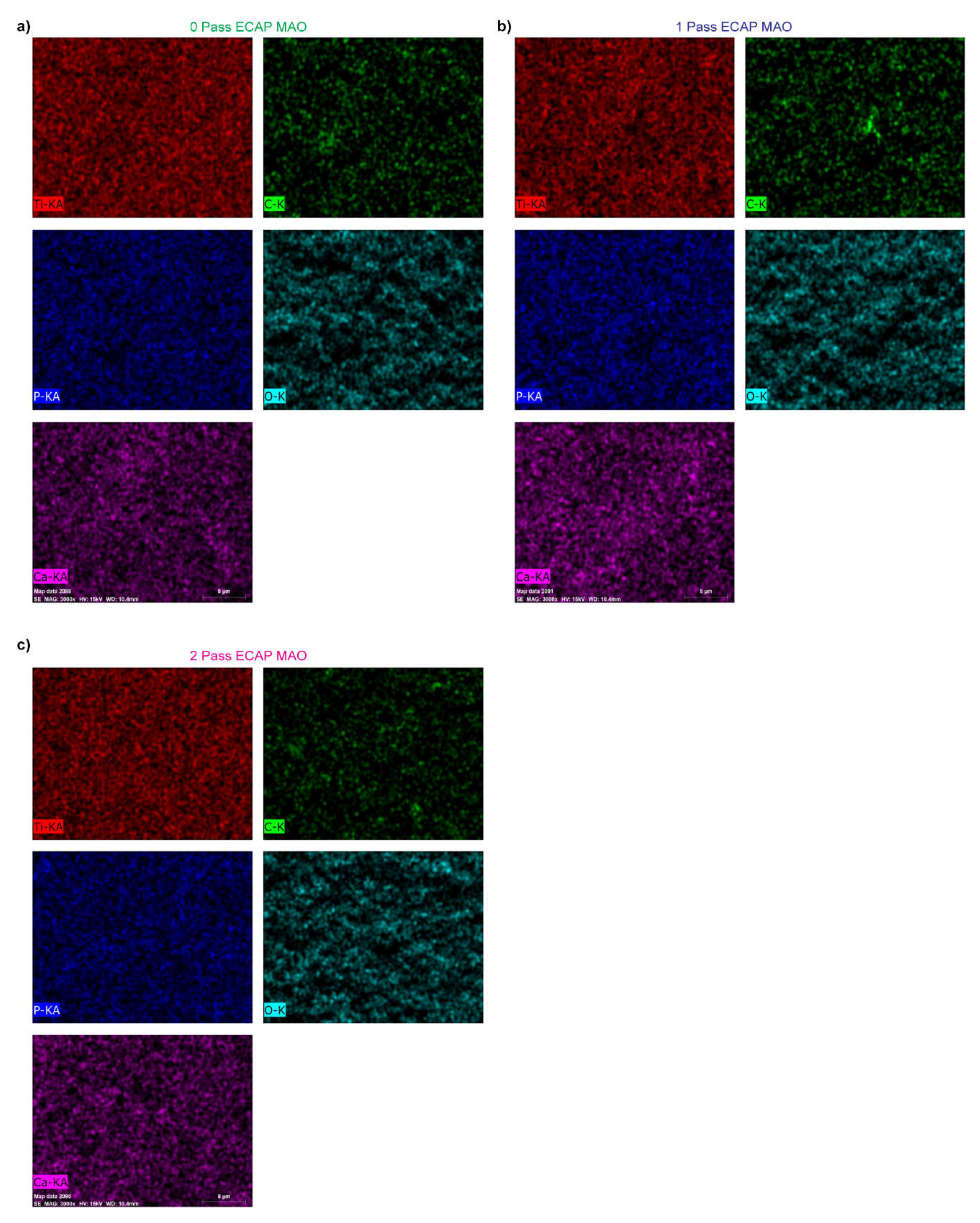
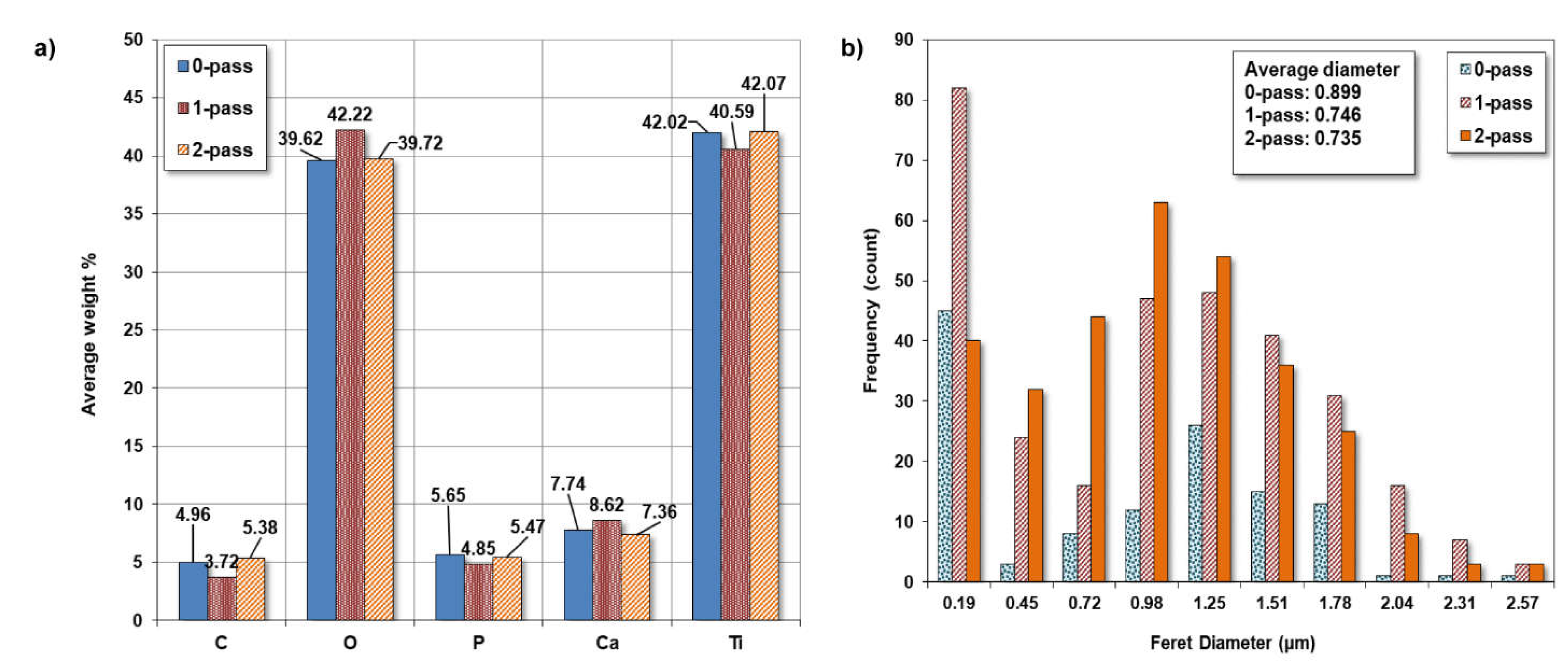
3.3. SEM and EDS
3.3.1. Pore Size.
3.4. Tafel Plot
3.4.1. Corrosion Loss
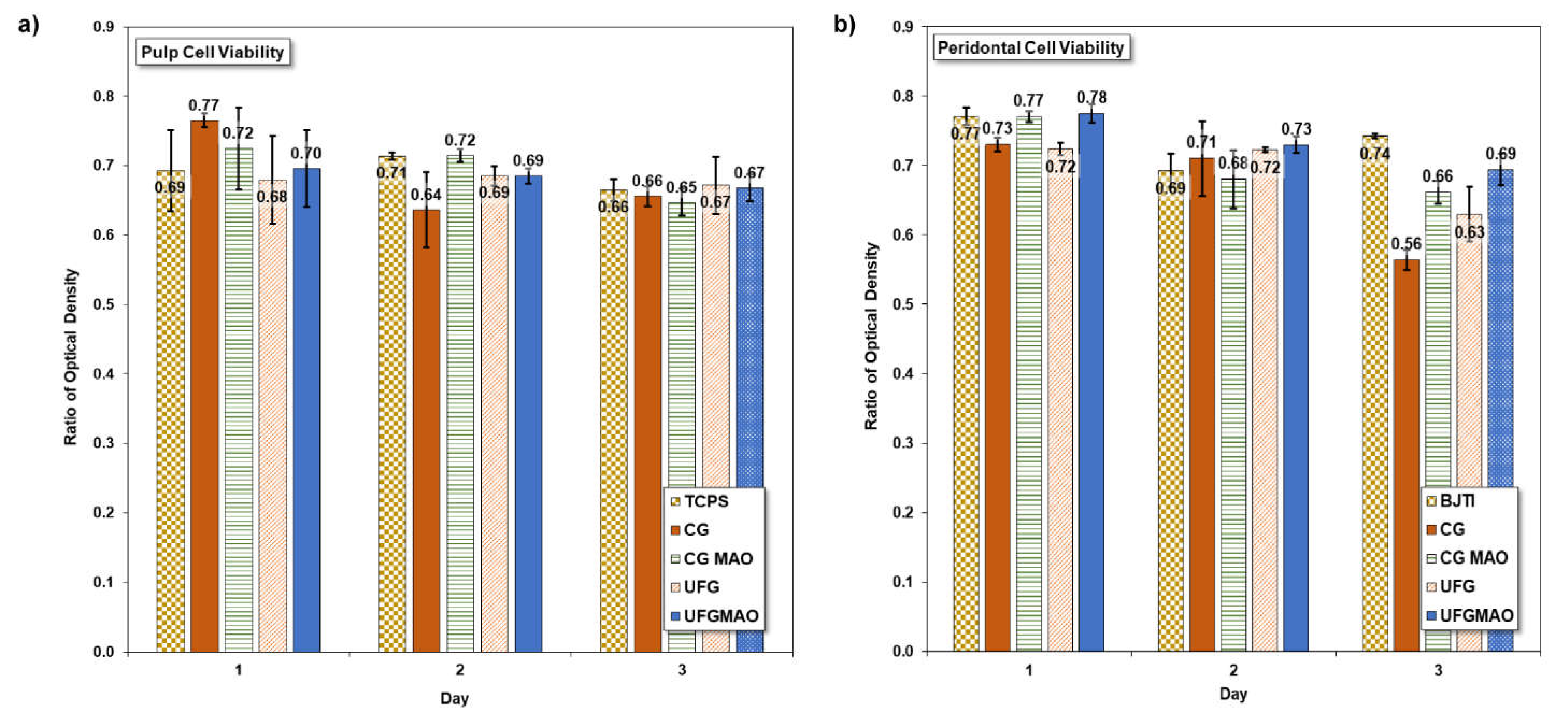
3.5. Biomedical Experiment
4. Conclusion
- The surface modification process using MAO and mineral solutions containing Ca and P can change the composition and shape of the surface of pure titanium.
- The corrosion resistance of commercially pure titanium can be enhanced by integrating ECAP and MAO surface modification.
- Human pulp and periodontal cell viability can be enhanced by modifying commercially pure titanium samples (CGMAO and UFGMAO) with micro arc oxidation (MAO) and ECAP technology; however, additional testing methods must be developed for improved biocompatibility in medical implants.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, K.M. , Tyagi, R.K., Singhal, A., Bhatia, D.: Effect of ECAP on the mechanical properties of titanium and its alloys for biomedical applications. Mater. Sci. Energy Technol. 2020, 3, 921–927. [Google Scholar] [CrossRef]
- Niinomi, M. : Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Elias, C.N. , Lima, J.H.C., Valiev, R., Meyers, M.A.: Biomedical applications of titanium and its alloys. Jom. 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Arora, H. : Titanium: The Ideal Dental Implant Material Choice BT - Surface Modification of Titanium Dental Implants. Presented at the ( 2023.
- Bordbar-Khiabani, A. , Gasik, M.: Electrochemical and biological characterization of Ti–Nb–Zr–Si alloy for orthopedic applications. Sci. Rep. 2023, 13, 2312. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A. , Azeem, M.A., Kumar, A.M., Saravanan, S., Ankah, N., Sorour, A.A.: Design and processing of near-β Ti–Nb–Ag alloy with low elastic modulus and enhanced corrosion resistance for orthopedic implants. J. Mater. Res. Technol. 2023, 24, 259–273. [Google Scholar] [CrossRef]
- Aslan Çakır, M. , Yetim, T., Yetim, A.F., Çelik, A.: Superamphiphobic TiO2 Film by Sol–Gel Dip Coating Method on Commercial Pure Titanium. J. Mater. Eng. Perform. ( 2023. [CrossRef]
- Pesode, P. , Barve, S., Wankhede, S. V, Jadhav, D.R., Pawar, S.K.: Titanium alloy selection for biomedical application using weighted sum model methodology. Mater. Today Proc. 2023, 72, 724–728. [Google Scholar] [CrossRef]
- Aslantas, K. , Demir, B., Guldibi, A.S., Niinomi, M., Dikici, B.: A comparative study on the machinability of β-type novel Ti29Nb13Ta4.6Zr (TNTZ) biomedical alloys under micro-milling operation. J. Manuf. Process. 2023, 92, 135–146. [Google Scholar] [CrossRef]
- Barboza, K., Carobolante, A., Rajan, S.S., Bortolini, C., Sabino, R.M., Nakazato, R.Z., Popat, K.C., Paula, A., Alves, R.: Studies of the New Ti-25Ta-25Nb-5Sn Alloy. 1–13 (2023).
- Shi, X. , Wang, X., Chen, B., Umeda, J., Bahador, A., Kondoh, K., Shen, J.: Precision control of oxygen content in CP-Ti for ultra-high strength through titanium oxide decomposition: An in-situ study. Mater. Des. 2023, 227, 111797. [Google Scholar] [CrossRef]
- Elshalakany, A.B., Abdel-Mottaleb, M.M., Salunkhe, S., Alqahtani, B.: 7 - Mechanical properties of titanium alloys additive manufacturing for biomedical applications. In: Salunkhe, S., Amancio-Filho, S.T., and Davim, J.P.B.T.-A. in M.A.M. (eds.) Woodhead Publishing Reviews: Mechanical Engineering Series. pp. 219–231. Woodhead Publishing (2023).
- Kati, J., Krivaˇ, S.: Titanium Implant Alloy Modified by Electrochemically Deposited Functional Bioactive Calcium Phosphate Coatings. (2023).
- Bandyopadhyay, A. , Ciliveri, S., Bose, S.: Metal additive manufacturing for load-bearing implants. J. Indian Inst. Sci. 2022, 102, 561–584. [Google Scholar] [CrossRef]
- Soro, N. , Brodie, E.G., Abdal-hay, A., Alali, A.Q., Kent, D., Dargusch, M.S.: Additive manufacturing of biomimetic Titanium-Tantalum lattices for biomedical implant applications. Mater. Des. 2022, 218, 110688. [Google Scholar] [CrossRef]
- Zhang, L. , Chen, L.: A review on biomedical titanium alloys: recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Fernandes, D.J. , Elias, C.N., Valiev, R.Z.: Properties and performance of ultrafine grained titanium for biomedical applications. Mater. Res. 2015, 18, 1163–1175. [Google Scholar] [CrossRef]
- Rack, H.J. , Qazi, J.I.: Titanium alloys for biomedical applications. Mater. Sci. Eng. C. 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Elias, C.N. , Meyers, M.A., Valiev, R.Z., Monteiro, S.N.: Ultrafine grained titanium for biomedical applications: An overview of performance. J. Mater. Res. Technol. 2013, 2, 340–350. [Google Scholar] [CrossRef]
- Quinn, J. , McFadden, R., Chan, C.-W., Carson, L.: Titanium for orthopedic applications: an overview of surface modification to improve biocompatibility and prevent bacterial biofilm formation. IScience. 2020, 23, 101745. [Google Scholar] [CrossRef]
- Mahajan, A. , Sidhu, S.S.: Surface modification of metallic biomaterials for enhanced functionality: a review. Mater. Technol. 2018, 33, 93–105. [Google Scholar] [CrossRef]
- Asri, R.I.M. , Harun, W.S.W., Samykano, M., Lah, N.A.C., Ghani, S.A.C., Tarlochan, F., Raza, M.R.: Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C. 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Liu, L., Ma, F., Kang, B., Liu, P., Qi, S., Li, W., Zhang, K., Chen, X.: Preparation and mechanical and biological performance of the Sr-containing microarc oxidation layer on titanium implants. Surf. Coatings Technol. 129530 (2023).
- Kumar, R., Agrawal, A.: Micro-hydroxyapatite reinforced Ti-based composite with tailored characteristics to minimize stress-shielding impact in bio-implant applications. J. Mech. Behav. Biomed. Mater. 105852 (2023). 2023.
- Spriano, S. , Yamaguchi, S., Baino, F., Ferraris, S.: A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mitra, I. , Bose, S., Dernell, W.S., Dasgupta, N., Eckstrand, C., Herrick, J., Yaszemski, M.J., Goodman, S.B., Bandyopadhyay, A.: 3D Printing in alloy design to improve biocompatibility in metallic implants. Mater. Today. 2021, 45, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Modina, I.M. , Dyakonov, G.S., Stotskiy, A.G., Yakovleva, T. V, Semenova, I.P.: Effect of the Texture of the Ultrafine-Grained Ti-6Al-4V Titanium Alloy on Impact Toughness. Materials (Basel). 2023, 16, 1318. [Google Scholar] [CrossRef] [PubMed]
- Nafikov, R.K. , Kulyasova, O.B., Khudododova, G.D., Enikeev, N.A.: Microstructural Assessment, Mechanical and Corrosion Properties of a Mg-Sr Alloy Processed by Combined Severe Plastic Deformation. Materials (Basel). 2023, 16, 2279. [Google Scholar] [CrossRef]
- Sadrkhah, M., Faraji, G., Khorasani, S., Mesbah, M.: Excellent Mechanical Properties, Wettability and Biological Response of Ultrafine-Grained Pure Ti Dental Implant Surface Modified by SLActive. J. Mater. Eng. Perform. 1–14 (2023).
- Huang, S. , Ali, A.N.: Experimental investigations of e ff ects of SiC contents and severe plastic deformation on the microstructure and mechanical properties of SiCp / AZ61 magnesium metal matrix composites. J. Mater. Process. Tech. 2019, 272, 28–39. [Google Scholar] [CrossRef]
- Singh, N., Agrawal, M.K., Saxena, K.K., Kumar, S., Prakash, C.: Advancement and influence of designing of ECAP on deformation and microstructure properties of the AA5083 under thermal effects. Int. J. Interact. Des. Manuf. 1–19 (2023).
- Medeiros, M.P. , Lopes, D.R., Kawasaki, M., Langdon, T.G., Figueiredo, R.B.: An Overview on the Effect of Severe Plastic Deformation on the Performance of Magnesium for Biomedical Applications. Materials (Basel). 2023, 16, 2401. [Google Scholar] [CrossRef]
- Kumar, H. , Devade, K., Singh, D.P., Giri, J.M., Kumar, M., Arun, V.: Severe plastic deformation: A state of art. Mater. Today Proc. ( 2023.
- Semenova, I.P. , Modina, Y.M., Stotskiy, A.G., Polyakov, A.V., Pesin, M.V.: Fatigue Properties of Ti Alloys with an Ultrafine Grained Structure: Challenges and Achievements. Metals (Basel). 2022, 12, 312. [Google Scholar] [CrossRef]
- Sotniczuk, A. , Kuczyńska-Zemła, D., Majchrowicz, K., Kijeńska-Gawrońska, E., Kruszewski, M., Nikiforow, K., Pisarek, M., Swieszkowski, W., Garbacz, H.: Tailoring mechanical and surface properties of UFG CP-Ti by the low-temperature annealing. Appl. Surf. Sci. 2023, 607, 155038. [Google Scholar] [CrossRef]
- Song, C. , Liu, L., Deng, Z., Lei, H., Yuan, F., Yang, Y., Li, Y., Yu, J.: Research progress on the design and performance of porous titanium alloy bone implants. J. Mater. Res. Technol. ( 2023.
- Shimizu, T. , Fujibayashi, S., Yamaguchi, S., Yamamoto, K., Otsuki, B., Takemoto, M., Tsukanaka, M., Kizuki, T., Matsushita, T., Kokubo, T.: Bioactivity of sol–gel-derived TiO2 coating on polyetheretherketone: In vitro and in vivo studies. Acta Biomater. 2016, 35, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M. , Fatimah, S., Nashrah, N., Gun, Y.: Progress in Materials Science Recent progress in surface modification of metals coated by plasma electrolytic oxidation : Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Zakaria, A., Todoh, M., Jusoff, K.: Bio-Functional Coating on Ti6Al4V Surface Produced. (2020). [CrossRef]
- Costa, A.I. , Gemini-Piperni, S., Alves, A.C., Costa, N.A., Checca, N.R., Leite, P.E., Rocha, L.A., Pinto, A.M.P., Toptan, F., Rossi, A.L.: TiO2 bioactive implant surfaces doped with specific amount of Sr modulate mineralization. Mater. Sci. Eng. C. 2021, 120, 111735. [Google Scholar] [CrossRef]
- Guo, T. , Scimeca, J.-C., Ivanovski, S., Verron, E., Gulati, K.: Enhanced Corrosion Resistance and Local Therapy from Nano-Engineered Titanium Dental Implants. Pharmaceutics. 2023, 15, 315. [Google Scholar] [CrossRef]
- Amirabad, A.A., Johari, M., Parichehr, R., Aghdam, R.M., Dehghanian, C., Allahkaram, S.R.: Improving corrosion, antibacterial and biocompatibility properties of MAO-coated AZ31 magnesium alloy by Cu (II)-chitosan/PVA nanofibers post-treatment. Ceram. Int. (2023).
- Makurat-Kasprolewicz, B., Ossowska, A.: Recent advances in electrochemically surface treated titanium and its alloys for biomedical applications: A review of anodic and plasma electrolytic oxidation methods. Mater. Today Commun. 105425 (2023).
- Matos, A.O. , Ricomini-Filho, A.P., Beline, T., Ogawa, E.S., Costa-Oliveira, B.E., de Almeida, A.B., Junior, F.H.N., Rangel, E.C., da Cruz, N.C., Sukotjo, C.: Three-species biofilm model onto plasma-treated titanium implant surface. Colloids Surfaces B Biointerfaces. 2017, 152, 354–366. [Google Scholar] [CrossRef]
- Wang, Y. , Yu, H., Chen, C., Zhao, Z.: Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Li, H. , Wang, P., Wen, C.: Recent Progress on Nanocrystalline Metallic Materials for Biomedical Applications. Nanomaterials. 2022, 12, 2111. [Google Scholar] [CrossRef] [PubMed]
- Huvelle, L.: Superplasticity of a 3D-printed aluminium alloy, (2023).
- Claudia, G.-M. , Ivan, G., Laia, O.-M., Emilio, J.-P., Maria-Pau, G., Maurizio, V., Luís, C.J., Marta, P.: Influence of ECAP process on mechanical, corrosion and bacterial properties of Zn-2Ag alloy for wound closure devices. Mater. Des. 2023, 228, 111817. [Google Scholar] [CrossRef]
- Rzepa, S. , Trojanová, Z., Džugan, J., Valiev, R.Z., Koukolíková, M., Melzer, D., Brázda, M.: Effect of ECAP processing on microstructure and mechanical behaviour of Ti-6Al-4V manufactured by directed energy deposition. Mater. Charact. 2023, 196, 112622. [Google Scholar] [CrossRef]
- Qadir, M. , Li, Y., Munir, K., Wen, C.: Calcium phosphate-based composite coating by micro-arc oxidation (MAO) for biomedical application: a review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 392–416. [Google Scholar] [CrossRef]
- Laino, L., La Noce, M., Fiorillo, L., Cervino, G., Nucci, L., Russo, D., Herford, A.S., Crimi, S., Bianchi, A., Biondi, A.: Dental pulp stem cells on implant surface: An in vitro study. Biomed Res. Int. 2021, (2021).
- Zhang, W. , Walboomers, X.F., van Kuppevelt, T.H., Daamen, W.F., Bian, Z., Jansen, J.A.: The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials. 2006, 27, 5658–5668. [Google Scholar] [CrossRef]
- Roy, M. , Corti, A., Dominici, S., Pompella, A., Cerea, M., Chelucci, E., Dorocka-Bobkowska, B., Daniele, S.: Biocompatibility of Subperiosteal Dental Implants: Effects of Differently Treated Titanium Surfaces on the Expression of ECM-Related Genes in Gingival Fibroblasts. J. Funct. Biomater. 2023, 14, 59. [Google Scholar]
- Atrens, A.: Understanding the Corrosion of Mg and Mg Alloys. Elsevier (2018).
- Sunday, O. , Fayomi, I., Akande, I.G., Popoola, A., Popoola, I., Molifi, H.: Potentiodynamic polarization studies of Cefadroxil and Dicloxacillin drugs on the corrosion susceptibility of aluminium AA6063 in 0. 5 M nitric acid. Integr. Med. Res. 2019, 8, 3088–3096. [Google Scholar] [CrossRef]
- Li, W. , Gao, J., Ma, Y., Zheng, K., Zhi, J., Xin, Y., Xie, S., Yu, S.: Undoped and diamond-doped MAO coatings prepared on Ti6Al4V: Microstructure, wear, corrosion, and biocompatibility properties. Surf. Coatings Technol. 2023, 458, 129340. [Google Scholar] [CrossRef]
- Aun, D.P. , Houmard, M., Mermoux, M., Latu-Romain, L., Joud, J.-C., Berthomé, G., Buono, V.T.L.: Development of a flexible nanocomposite TiO2 film as a protective coating for bioapplications of superelastic NiTi alloys. Appl. Surf. Sci. 2016, 375, 42–49. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A. , Ibrahim, H., Amerinatanzi, A., Hashemi, M., Moghaddam, N.S., Elahinia, M.: Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Wang, Y.-C. , Lin, S.-H., Chien, C.-S., Kung, J.-C., Shih, C.-J.: In vitro bioactivity and antibacterial effects of a silver-containing mesoporous bioactive glass film on the surface of titanium implants. Int. J. Mol. Sci. 2022, 23, 9291. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J., Saidin, S., Lo, A.Z.K., Koshy, P., Abdullah, H.Z., Idris, M.I., Lee, T.C.: In Vitro Surface Efficacy of CaP-based Anodised Titanium for Bone Implants. Surfaces and Interfaces. 102872 (2023).
- Zhang, X. , Zhang, T., Lv, Y., Zhang, Y., Lu, X., Xiao, J., Ma, C., Li, Z., Dong, Z.: Enhanced uniformity, corrosion resistance and biological performance of Cu-incorporated TiO2 coating produced by ultrasound-auxiliary micro-arc oxidation. Appl. Surf. Sci. 2021, 569, 150932. [Google Scholar] [CrossRef]
- Lin, Z. , Wang, T., Yu, X., Sun, X., Yang, H.: Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Du, Q. , Wei, D., Wang, S., Cheng, S., Wang, Y., Li, B., Jia, D., Zhou, Y.: TEM analysis and in vitro and in vivo biological performance of the hydroxyapatite crystals rapidly formed on the modified microarc oxidation coating using microwave hydrothermal technique. Chem. Eng. J. 2019, 373, 1091–1110. [Google Scholar]
- Wang, Y.-H. , Liao, C.-C., Chen, Y.-C., Ou, S.-F., Chiu, C.-Y.: The feasibility of eco-friendly electrical discharge machining for surface modification of Ti: a comparison study in surface properties, bioactivity, and cytocompatibility. Mater. Sci. Eng. C. 2020, 108, 110192. [Google Scholar] [CrossRef]
- Grebņevs, V. , Leśniak-Ziółkowska, K., Wala, M., Dulski, M., Altundal, Ş., Dutovs, A., Avotiņa, L., Erts, D., Viter, R., Vīksna, A.: Modification of physicochemical properties and bioactivity of oxide coatings formed on Ti substrates via plasma electrolytic oxidation in crystalline and amorphous calcium phosphate particle suspensions. Appl. Surf. Sci. 2022, 598, 153793. [Google Scholar]
- Chang, C.-L. , Huang, C.-H., Lin, C.-Y., Yang, F.-C., Tang, J.-F.: Mechanical properties of amorphous and crystalline CrN/CrAlSiN multilayer coating fabricated using HPPMS. Surfaces and Interfaces. 2022, 31, 102064. [Google Scholar] [CrossRef]
- Bignoli, F. , Rashid, S., Rossi, E., Jaddi, S., Djemia, P., Terraneo, G., Bassi, A.L., Idrissi, H., Pardoen, T., Sebastiani, M.: Effect of annealing on mechanical properties and thermal stability of ZrCu/O nanocomposite amorphous films synthetized by pulsed laser deposition. Mater. Des. 2022, 221, 110972. [Google Scholar]
- Buabthong, P. , Ifkovits, Z.P., Kempler, P.A., Chen, Y., Nunez, P.D., Brunschwig, B.S., Papadantonakis, K.M., Lewis, N.S.: Failure modes of protection layers produced by atomic layer deposition of amorphous TiO 2 on GaAs anodes. Energy Environ. Sci. 2020, 13, 4269–4279. [Google Scholar]
- Feng, Z., Hu, H., Wang, J., Dong, H., Zhang, X., Ma, J., Wang, J., Liu, D., Li, J., Zhang, X.: Revealing the evolution mechanisms of microstructure, texture and mechanical behavior in Zr702 alloy plates fabricated by accumulative roll bonding. Mater. Sci. Eng. A. 144609 (2023).
- Chen, W.J. , Xu, J., Liu, D.T., Shan, D. Bin, Guo, B., Langdon, T.G.: Thermal stability of ultrafine-grained pure titanium processed by high-pressure torsion. In: Materials Science Forum. pp. 338–344. 2021. [Google Scholar]
- Majchrowicz, K. , Sotniczuk, A., Malicka, J., Choińska, E., Garbacz, H.: Thermal Stability and Mechanical Behavior of Ultrafine-Grained Titanium with Different Impurity Content. Materials (Basel). 2023, 16, 1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. , Huang, S., Li, X., Zhao, D., Cao, Y., Liu, B., Huang, Q.: Oxide ceramic coatings with amorphous/nano-crystalline dual-structures prepared by micro-arc oxidation on Ti–Nb–Zr medium entropy alloy surfaces for biomedical applications. Ceram. Int. 2023, 49, 18114–18124. [Google Scholar] [CrossRef]
- Miyamoto, H.: Revealing What Enhance the Corrosion Resistance beside Grain Size in Ultrafine Grained Materials by Severe Plastic Deformation: Stainless Steels Case. Mater. Trans. MT-MF2022034 (2023).
- Li, G., Ma, F., Liu, P., Qi, S., Li, W., Zhang, K., Chen, X.: Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 169773 (2023).
- Pandoleon, P. , Bakopoulou, A., Papadopoulou, L., Koidis, P.: Evaluation of the biological behaviour of various dental implant abutment materials on attachment and viability of human gingival fibroblasts. Dent. Mater. 2019, 35, 1053–1063. [Google Scholar] [CrossRef]
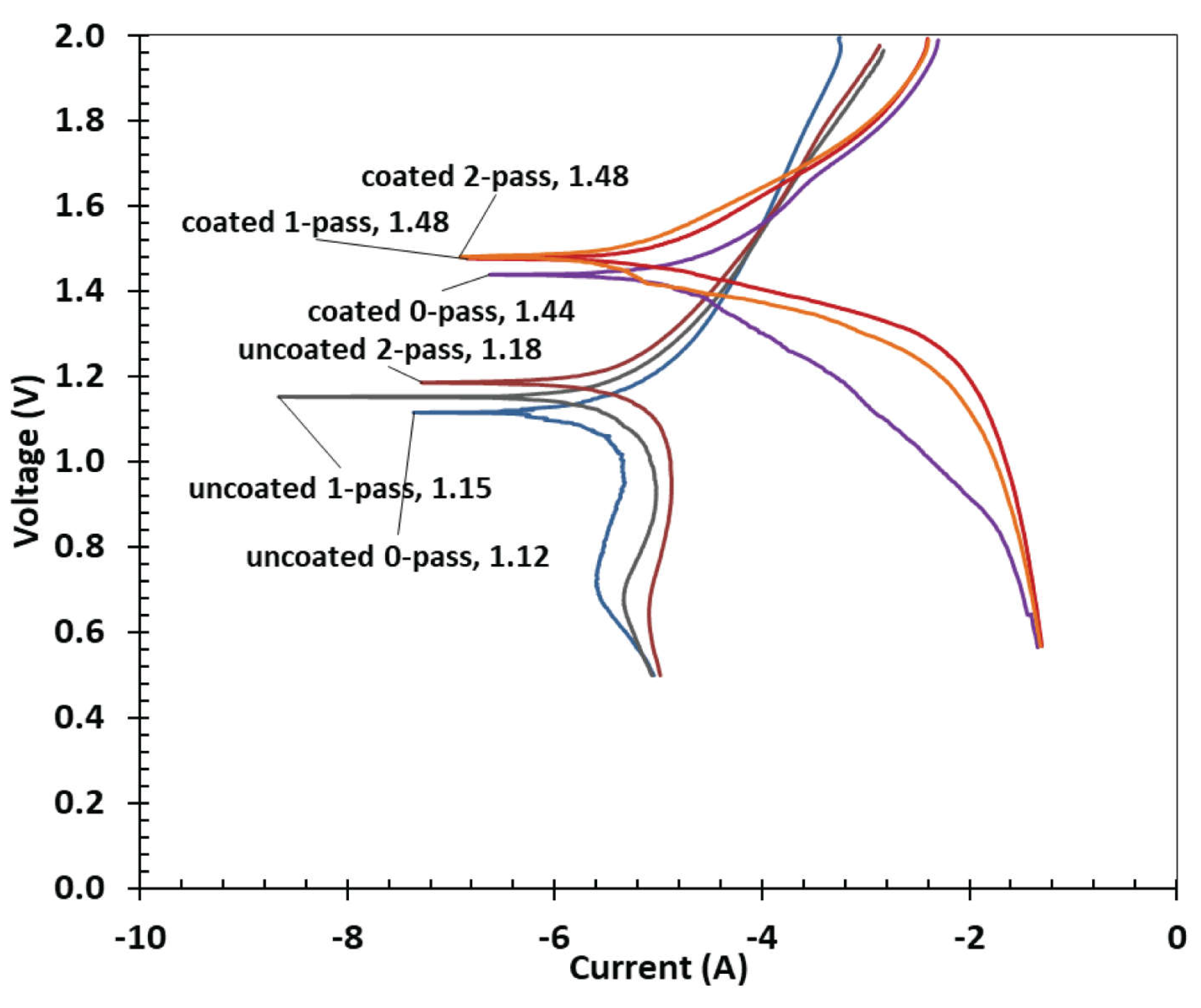
| Ecorr | Ba | Bc | Rp | icorr | CR | |
|---|---|---|---|---|---|---|
| Samples | (V) | (V) | (V) | (Ohms.cm2) | (µA/cm2) | mpy |
| CG | 1.21 | 0.01 | 2.18 | 0.01 | 0.70 | 0.97 |
| UFG1 | 1.49 | 0.04 | 0.07 | 0.08 | 0.59 | 0.81 |
| UFG2 | 1.16 | 0.00 | 0.25 | 0.00 | 0.53 | 0.72 |
| CGMAO | 1.52 | 0.04 | 0.11 | 0.05 | 0.51 | 0.71 |
| UFG1AMO | 1.11 | 0.02 | 0.31 | 0.02 | 0.51 | 0.70 |
| UFG2MAO | 1.47 | 0.20 | 0.16 | -0.83 | 0.48 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





