Submitted:
31 May 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Challenges in TB drug discovery
3. Emerging Mtb drug targets
3.1. Cell Wall
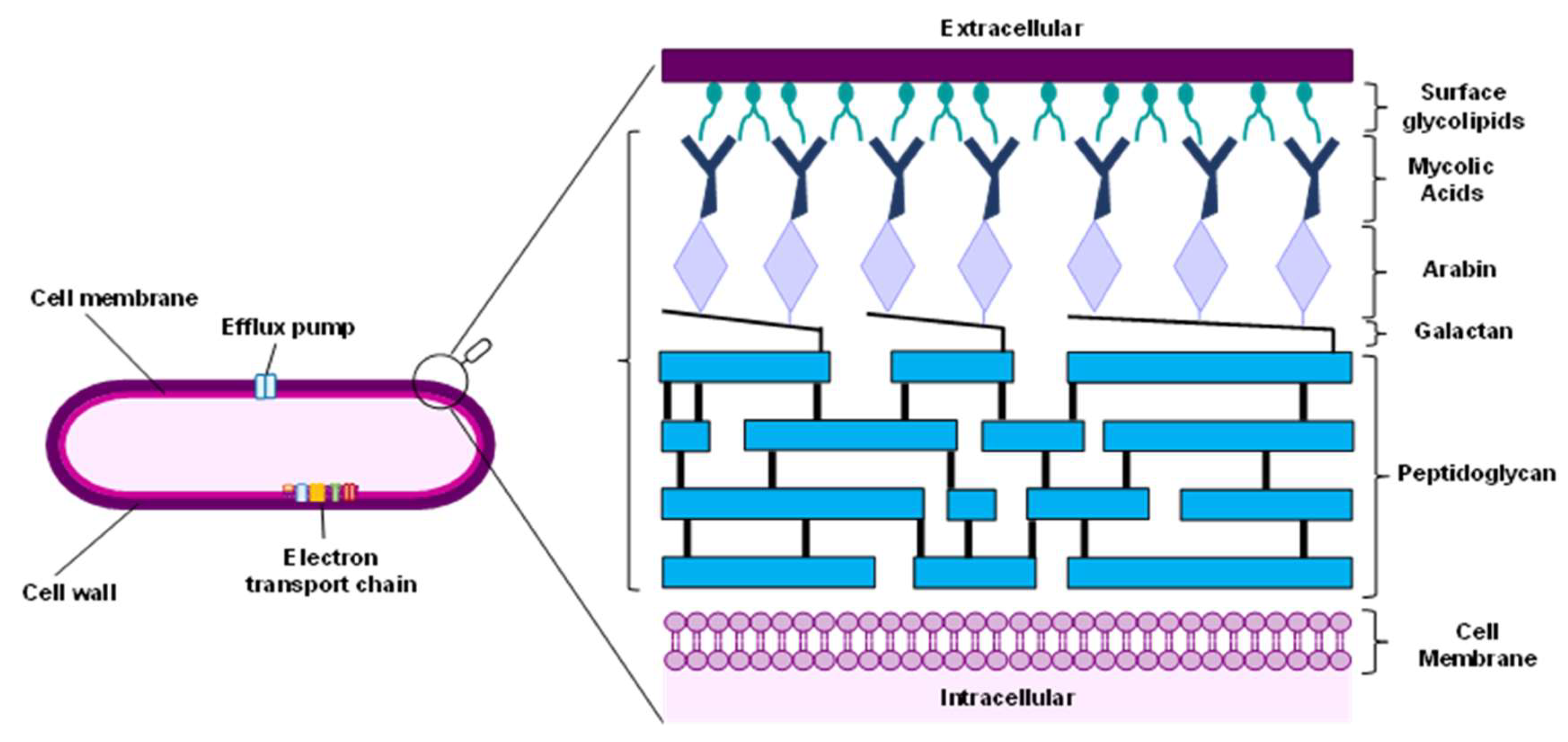
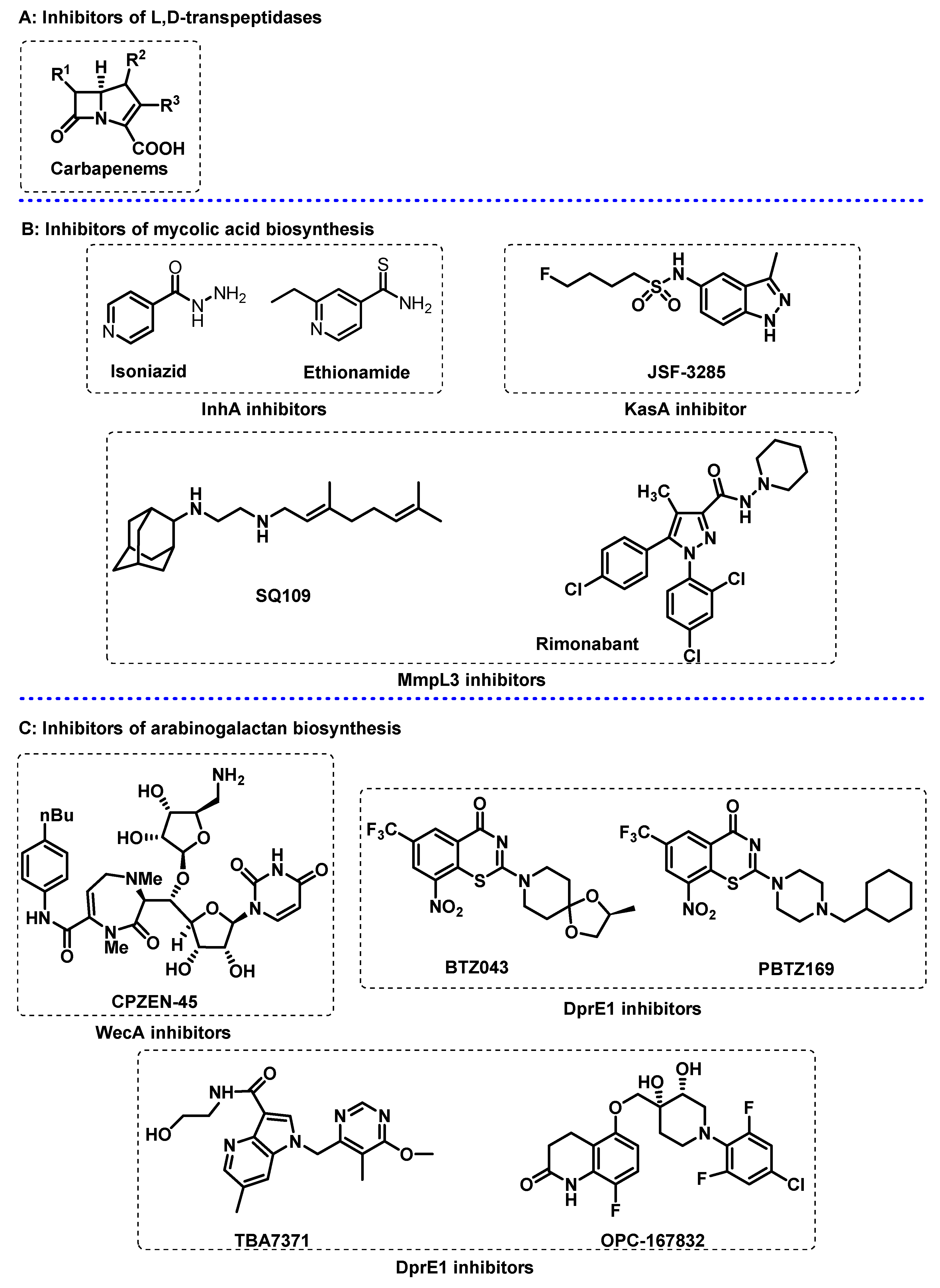
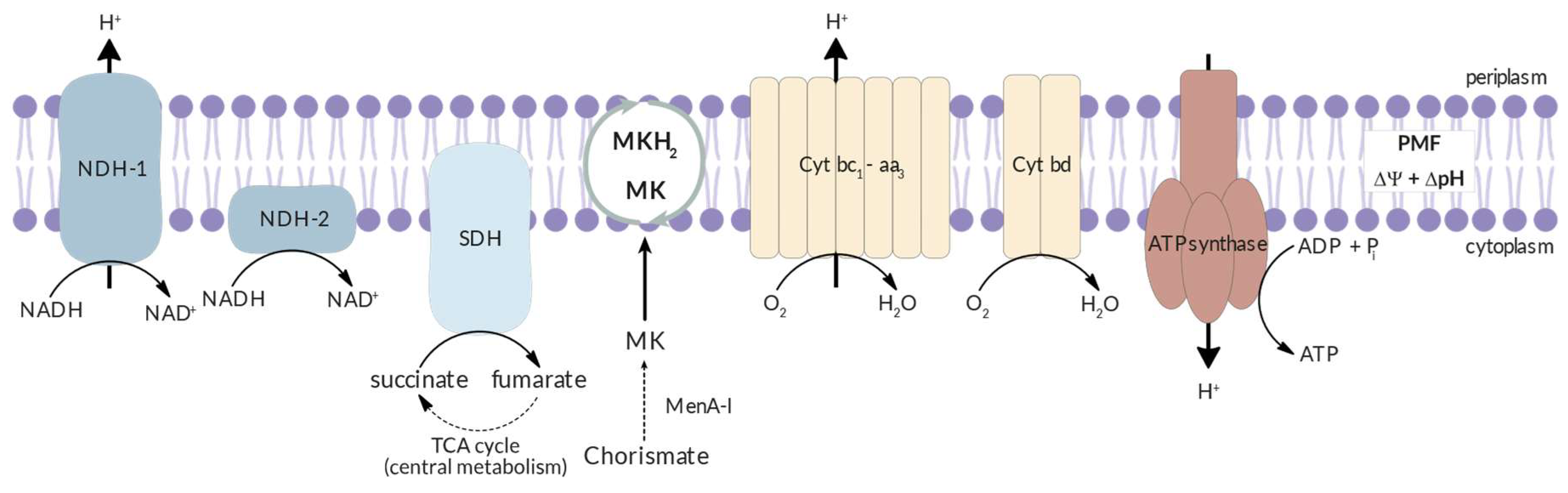
3.2. Energy Metabolism
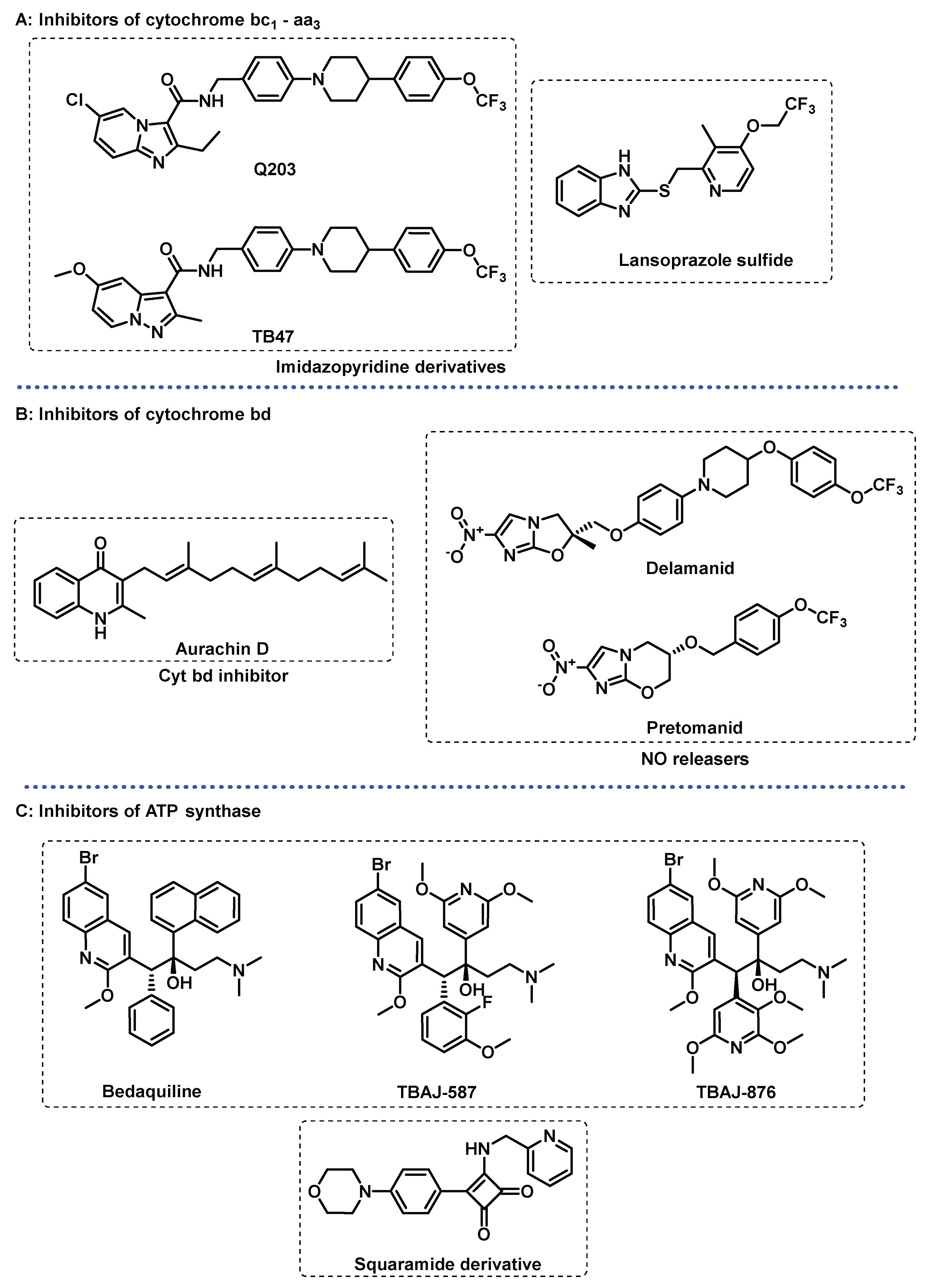
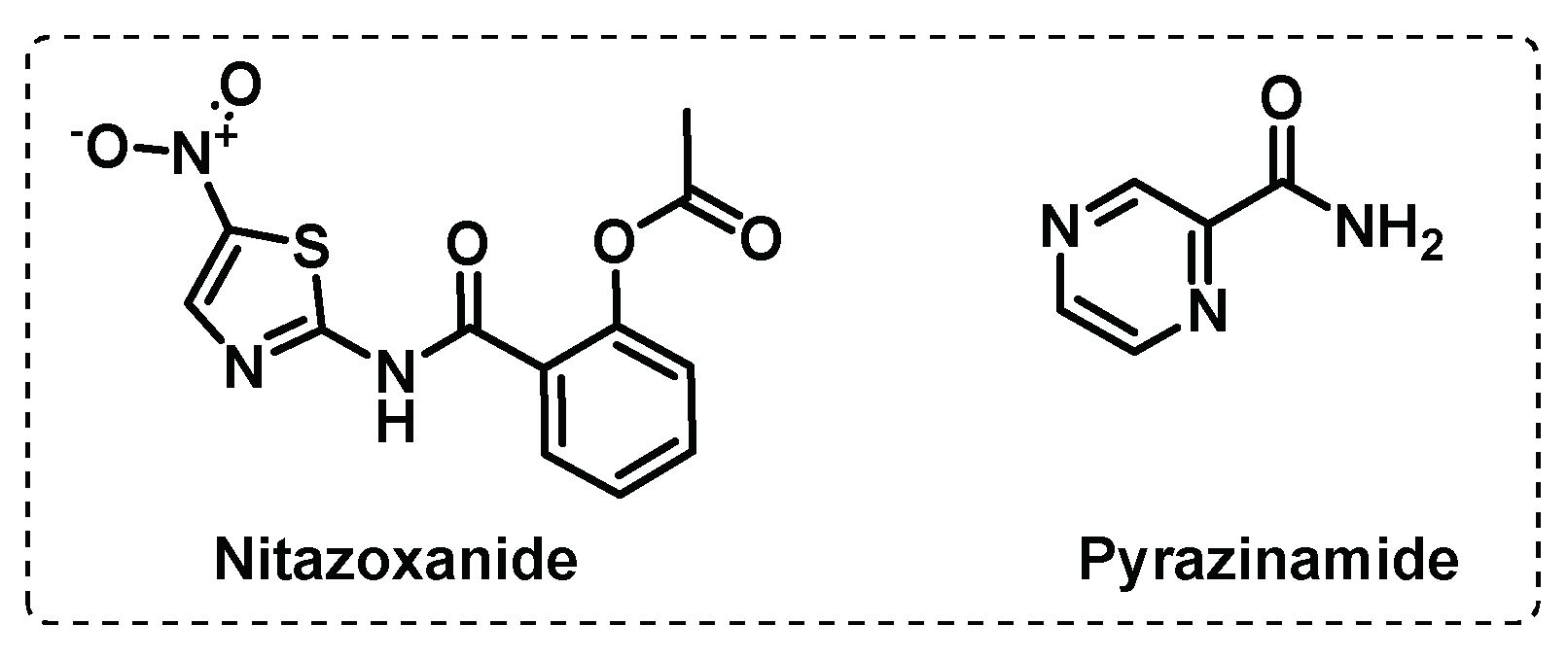
3.3. Other Targets
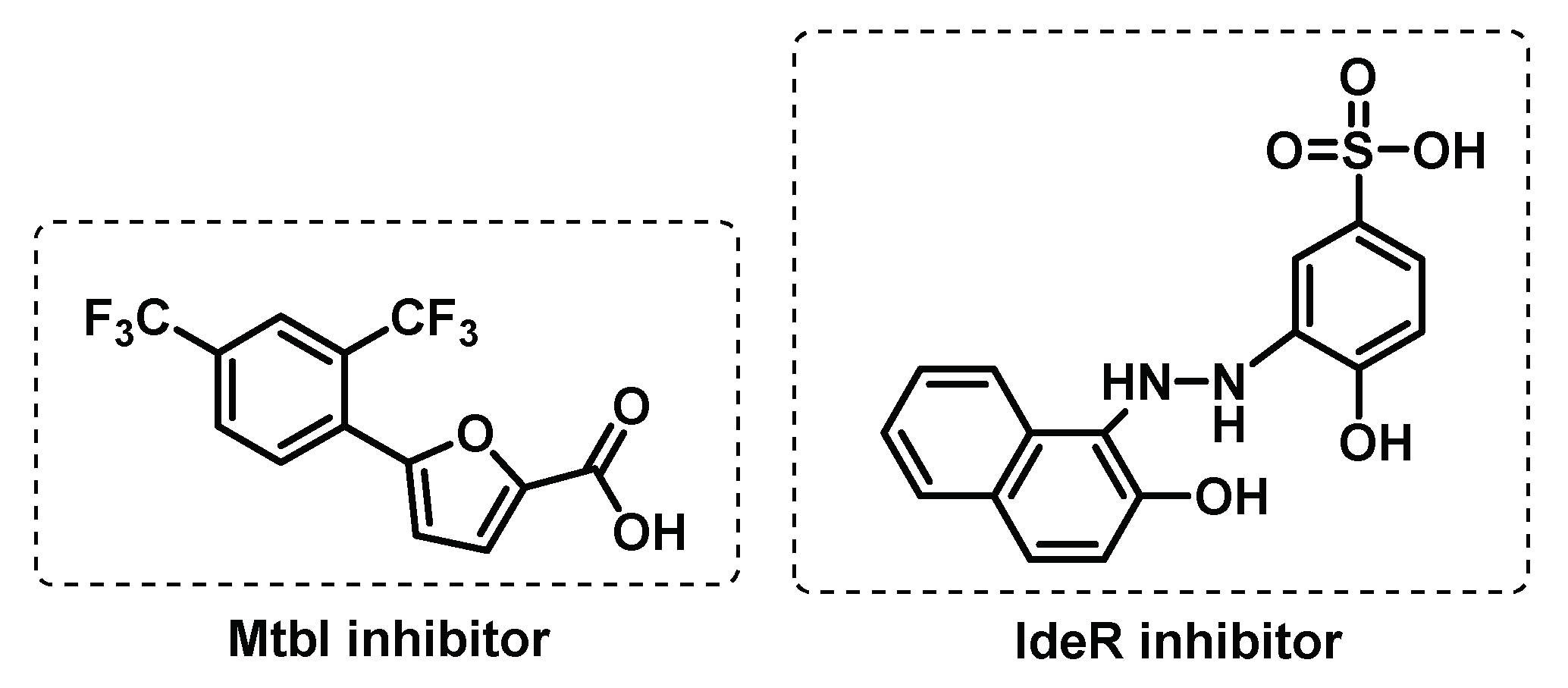
3.3.1. Iron uptake
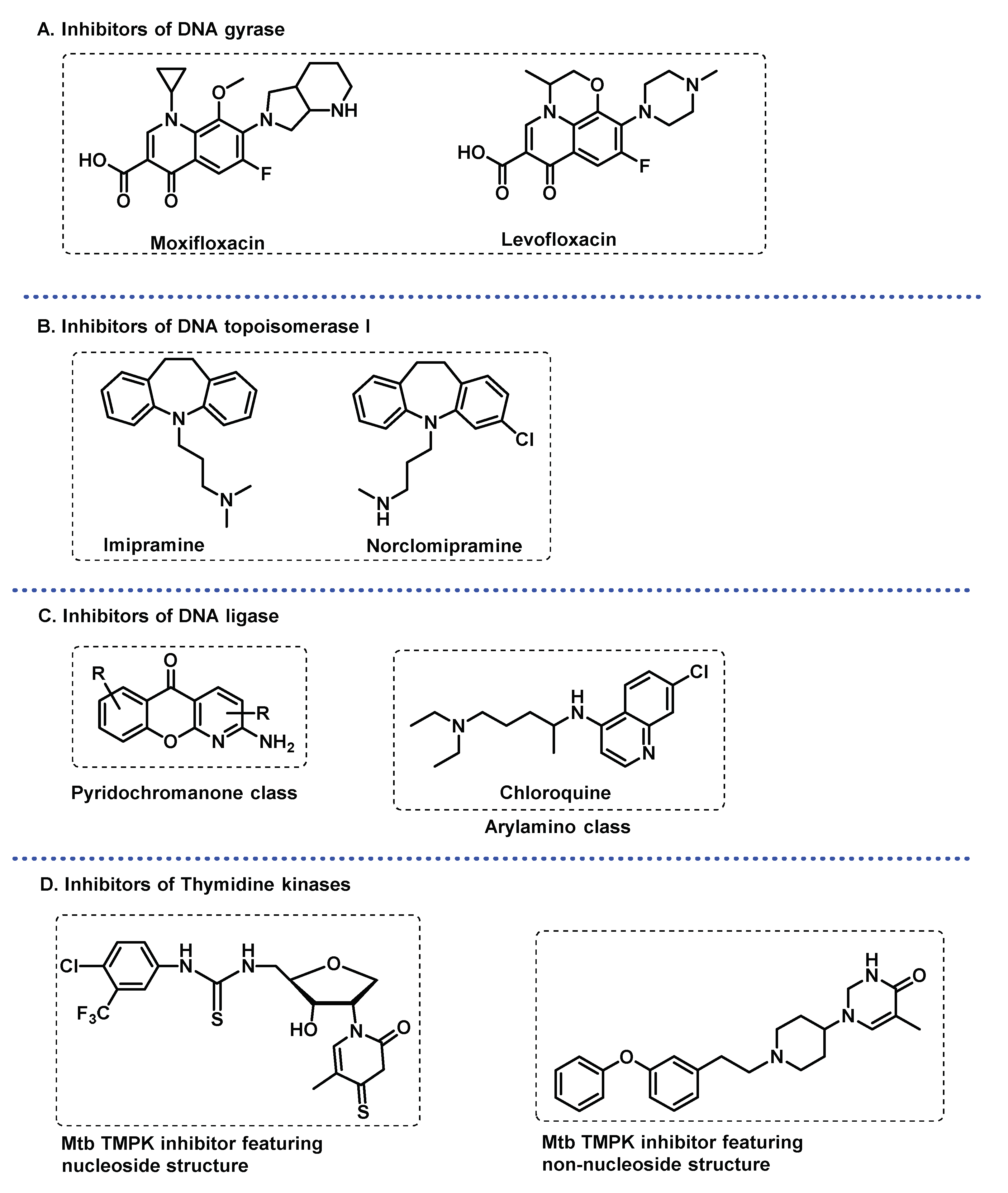
3.3.2. DNA related enzymes
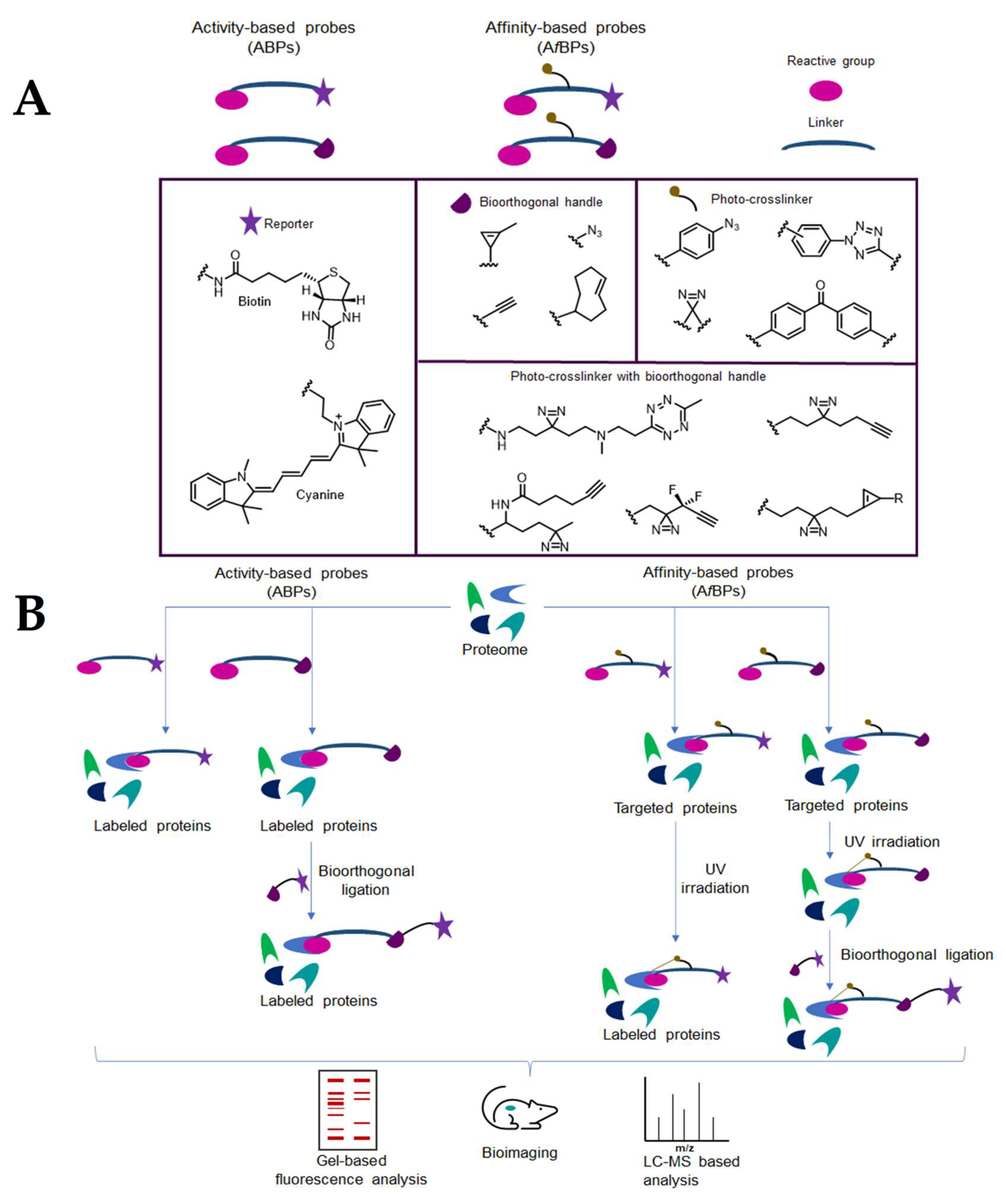
4. Chemical Probes for Target Identification in Mycobacteria
4.1. Activity-based protein profiling (ABPP)
4.1.1. Cytosolic serine hydrolases
4.1.2. Membrane serine and cysteine hydrolases
4.1.3. Other membrane targets
4.1.4. ATP-binding enzymes
4.2. Affinity-based probes (AfBP)
5. Conclusions
Funding
Conflicts of Interest
References
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nature Reviews Microbiology 2018, 16, 202-213. [CrossRef]
- Mashabela Gabriel, T.; de Wet Timothy, J.; Warner Digby, F. Mycobacterium tuberculosis Metabolism. Microbiology Spectrum 2019, 7, 1-26. [CrossRef]
- Woodman, M.; Haeusler, I.L.; Grandjean, L. Tuberculosis Genetic Epidemiology: A Latin American Perspective. Genes 2019, 10. [CrossRef]
- Kiazyk, S.; Ball, T.B. Latent tuberculosis infection: An overview. Can Commun Dis Rep 2017, 43, 62-66. [CrossRef]
- Organization, W.H. Global tuberculosis report 2022; Geneva, 2022.
- Campaniço, A.; Moreira, R.; Lopes, F. Drug discovery in tuberculosis. New drug targets and antimycobacterial agents. European Journal of Medicinal Chemistry 2018, 150, 525-545. [CrossRef]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: progress and advances in development of new drugs, treatment regimens, and host-directed therapies. The Lancet Infectious Diseases 2018, 18, 183-198. [CrossRef]
- Reid, M.J.A.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L.; et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. The Lancet 2019, 393, 1331-1384. [CrossRef]
- Nuermberger Eric, L. Preclinical Efficacy Testing of New Drug Candidates. Microbiology Spectrum 2017, 5, 1-22. [CrossRef]
- Şenol, G. Recent and New Strategies for Extensively Drug-resistant Tuberculosis. Mediterranean Journal of Infection, Microbes and Antimicrobials 2018, 7. [CrossRef]
- Gupta, V.K.; Kumar, M.M.; Singh, D.; Bisht, D.; Sharma, S. Drug targets in dormant Mycobacterium tuberculosis: can the conquest against tuberculosis become a reality? Infectious Diseases 2018, 50, 81-94. [CrossRef]
- Reddy, D.S.; Sinha, A.; Kumar, A.; Saini, V.K. Drug re-engineering and repurposing: A significant and rapid approach to tuberculosis drug discovery. Archiv der Pharmazie 2022, 355, 1-26. [CrossRef]
- Macalino, S.J.Y.; Billones, J.B.; Organo, V.G.; Carrillo, M.C.O. In Silico Strategies in Tuberculosis Drug Discovery. Molecules 2020, 25. [CrossRef]
- Huszár, S.; Chibale, K.; Singh, V. The quest for the holy grail: new antitubercular chemical entities, targets and strategies. Drug Discovery Today 2020, 25, 772-780. [CrossRef]
- Velayati, A.A.; Farnia, P.; Hoffner, S. Drug-resistant Mycobacterium tuberculosis: Epidemiology and role of morphological alterations. Journal of Global Antimicrobial Resistance 2018, 12, 192-196. [CrossRef]
- Sarathy, J.; Dartois, V.; Dick, T.; Gengenbacher, M. Reduced Drug Uptake in Phenotypically Resistant Nutrient-Starved Nonreplicating Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy 2013, 57, 1648-1653. [CrossRef]
- Kumar, A.; Chettiar, S.; Parish, T. Current challenges in drug discovery for tuberculosis. Expert Opinion on Drug Discovery 2017, 12, 1-4. [CrossRef]
- da Silva, P.E.A.; Machado, D.; Ramos, D.; Couto, I.; Von Groll, A.; Viveiros, M. Efflux Pumps in Mycobacteria: Antimicrobial Resistance, Physiological Functions, and Role in Pathogenicity. In Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications, Li, X.-Z., Elkins, C.A., Zgurskaya, H.I., Eds.; Springer International Publishing: Cham, 2016; pp. 527-559.
- Eoh, H.; Wang, Z.; Layre, E.; Rath, P.; Morris, R.; Branch Moody, D.; Rhee, K.Y. Metabolic anticipation in Mycobacterium tuberculosis. Nature Microbiology 2017, 2, 1-7. [CrossRef]
- Sharma, S.; Sharma, D.; Kalia, N.P. Editorial: Approaches to Address Resistance, Drug Discovery, and Vaccine Development in Mycobacterium tuberculosis: Challenges and Opportunities. Frontiers in Microbiology 2022, 13, 1-3. [CrossRef]
- Tomasi, F.G.; Rubin, E.J. Failing upwards: Genetics-based strategies to improve antibiotic discovery and efficacy in Mycobacterium tuberculosis. Front Cell Infect Microbiol 2022, 12, 1-8. [CrossRef]
- Mugumbate, G.; Mendes, V.; Blaszczyk, M.; Sabbah, M.; Papadatos, G.; Lelievre, J.; Ballell, L.; Barros, D.; Abell, C.; Blundell, T.L.; et al. Target Identification of Mycobacterium tuberculosis Phenotypic Hits Using a Concerted Chemogenomic, Biophysical, and Structural Approach. Frontiers in Pharmacology 2017, 8, 1-13. [CrossRef]
- Lechartier, B.; Rybniker, J.; Zumla, A.; Cole, S.T. Tuberculosis drug discovery in the post-post-genomic era. EMBO Molecular Medicine 2014, 6, 158-168. [CrossRef]
- Borsari, C.; Ferrari, S.; Venturelli, A.; Costi, M.P. Target-based approaches for the discovery of new antimycobacterial drugs. Drug Discovery Today 2017, 22, 576-584. [CrossRef]
- Cho Sang, H.; Warit, S.; Wan, B.; Hwang Chang, H.; Pauli Guido, F.; Franzblau Scott, G. Low-Oxygen-Recovery Assay for High-Throughput Screening of Compounds against Nonreplicating Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy 2007, 51, 1380-1385. [CrossRef]
- Wayne, L.G.; Hayes, L.G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infection and Immunity 1996, 64, 2062-2069. [CrossRef]
- Zhang, M.; Sala, C.; Hartkoorn Ruben, C.; Dhar, N.; Mendoza-Losana, A.; Cole Stewart, T. Streptomycin-Starved Mycobacterium tuberculosis 18b, a Drug Discovery Tool for Latent Tuberculosis. Antimicrobial Agents and Chemotherapy 2012, 56, 5782-5789. [CrossRef]
- Darby, C.M.; Ingólfsson, H.I.; Jiang, X.; Shen, C.; Sun, M.; Zhao, N.; Burns, K.; Liu, G.; Ehrt, S.; Warren, J.D.; et al. Whole Cell Screen for Inhibitors of pH Homeostasis in Mycobacterium tuberculosis. PLOS ONE 2013, 8, 1-12. [CrossRef]
- Gold, B.; Warrier, T.; Nathan, C. A Multistress Model for High Throughput Screening (HTS) Against Nonreplicating Mycobacterium tuberculosis (M. tuberculosis). In Mycobacteria Protocols, Parish, T., Kumar, A., Eds.; Springer US: New York, NY, 2021; pp. 611-635.
- Aguilar-Ayala, D.A.; Cnockaert, M.; Vandamme, P.; Palomino, J.C.; Martin, A.; Gonzalez-Y-Merchand, J. Antimicrobial activity against Mycobacterium tuberculosis under in vitro lipid-rich dormancy conditions. Journal of Medical Microbiology 2018, 67, 282-285. [CrossRef]
- Wang, F.; Sambandan, D.; Halder, R.; Wang, J.; Batt, S.M.; Weinrick, B.; Ahmad, I.; Yang, P.; Zhang, Y.; Kim, J.; et al. Identification of a small molecule with activity against drug-resistant and persistent tuberculosis. Proceedings of the National Academy of Sciences 2013, 110, E2510-E2517. [CrossRef]
- Egorova, A.; Salina, E.G.; Makarov, V. Targeting Non-Replicating Mycobacterium tuberculosis and Latent Infection: Alternatives and Perspectives (Mini-Review). International Journal of Molecular Sciences 2021, 22, 1-15. [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nature Reviews Disease Primers 2016, 2, 1-23. [CrossRef]
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nature Reviews Microbiology 2022, 20, 685-701. [CrossRef]
- Perveen, S.; Sharma, R. Screening approaches and therapeutic targets: The two driving wheels of tuberculosis drug discovery. Biochemical Pharmacology 2022, 197, 114906. [CrossRef]
- Abrahams, K.A.; Besra, G.S. Mycobacterial drug discovery. RSC Medicinal Chemistry 2020, 11, 1354-1365. [CrossRef]
- Xu, X.; Dong, B.; Peng, L.; Gao, C.; He, Z.; Wang, C.; Zeng, J. Anti-tuberculosis drug development via targeting the cell envelope of Mycobacterium tuberculosis. Frontiers in Microbiology 2022, 13. [CrossRef]
- Shetye, G.S.; Franzblau, S.G.; Cho, S. New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Translational Research 2020, 220, 68-97. [CrossRef]
- Nataraj, V.; Varela, C.; Javid, A.; Singh, A.; Besra, G.S.; Bhatt, A. Mycolic acids: deciphering and targeting the Achilles’ heel of the tubercle bacillus. Molecular Microbiology 2015, 98, 7-16. [CrossRef]
- PaweŁczyk, J.; Kremer, L. The Molecular Genetics of Mycolic Acid Biosynthesis. Microbiology Spectrum 2014, 2, 1-20. [CrossRef]
- DeJesus Michael, A.; Gerrick Elias, R.; Xu, W.; Park Sae, W.; Long Jarukit, E.; Boutte Cara, C.; Rubin Eric, J.; Schnappinger, D.; Ehrt, S.; Fortune Sarah, M.; et al. Comprehensive Essentiality Analysis of the Mycobacterium tuberculosis Genome via Saturating Transposon Mutagenesis. mBio 2017, 8, e02133-02116. [CrossRef]
- Inoyama, D.; Awasthi, D.; Capodagli, G.C.; Tsotetsi, K.; Sukheja, P.; Zimmerman, M.; Li, S.-G.; Jadhav, R.; Russo, R.; Wang, X.; et al. A Preclinical Candidate Targeting Mycobacterium tuberculosis KasA. Cell Chemical Biology 2020, 27, 560-570. [CrossRef]
- Abrahams, K.A.; Chung, C.-w.; Ghidelli-Disse, S.; Rullas, J.; Rebollo-López, M.J.; Gurcha, S.S.; Cox, J.A.G.; Mendoza, A.; Jiménez-Navarro, E.; Martínez-Martínez, M.S.; et al. Identification of KasA as the cellular target of an anti-tubercular scaffold. Nature Communications 2016, 7, 12581. [CrossRef]
- Kumar, P.; Capodagli Glenn, C.; Awasthi, D.; Shrestha, R.; Maharaja, K.; Sukheja, P.; Li, S.-G.; Inoyama, D.; Zimmerman, M.; Ho Liang Hsin, P.; et al. Synergistic Lethality of a Binary Inhibitor of Mycobacterium tuberculosis KasA. mBio 2018, 9, e02101-02117. [CrossRef]
- Ramesh, R.; Shingare, R.D.; Kumar, V.; Anand, A.; B, S.; Veeraraghavan, S.; Viswanadha, S.; Ummanni, R.; Gokhale, R.; Srinivasa Reddy, D. Repurposing of a drug scaffold: Identification of novel sila analogues of rimonabant as potent antitubercular agents. European Journal of Medicinal Chemistry 2016, 122, 723-730. [CrossRef]
- Kwofie, S.K.; Hanson, G.; Sasu, H.; Enninful, K.S.; Mensah, F.A.; Nortey, R.T.; Yeboah, O.P.; Agoni, C.; Wilson, M.D. Molecular Modelling and Atomistic Insights into the Binding Mechanism of MmpL3 Mtb. Chemistry & Biodiversity 2022, 19, e202200160. [CrossRef]
- Abrahams, K.A.; Besra, G.S. Mycobacterial cell wall biosynthesis: a multifaceted antibiotic target. Parasitology 2018, 145, 116-133. [CrossRef]
- Zhang, L.; Zhao, Y.; Gao, Y.; Wu, L.; Gao, R.; Zhang, Q.; Wang, Y.; Wu, C.; Wu, F.; Gurcha, S.S.; et al. Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Science 2020, 368, 1211-1219. [CrossRef]
- Kastrinsky, D.B.; McBride, N.S.; Backus, K.M.; LeBlanc, J.J.; Barry, C.E. 1.04–Mycolic Acid/Cyclopropane Fatty Acid/Fatty Acid Biosynthesis and Health Relations. In Comprehensive Natural Products II, Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, 2010; pp. 65-145.
- Zhang, L.; Zhao, Y.; Gao, R.; Li, J.; Yang, X.; Gao, Y.; Zhao, W.; Gurcha, S.S.; Veerapen, N.; Batt, S.M.; et al. Cryo-EM snapshots of mycobacterial arabinosyltransferase complex EmbB2-AcpM2. Protein & Cell 2020, 11, 505-517. [CrossRef]
- Goude, R.; Amin, A.G.; Chatterjee, D.; Parish, T. The Arabinosyltransferase EmbC Is Inhibited by Ethambutol in Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy 2009, 53, 4138-4146. [CrossRef]
- Meniche, X.; Sousa-d, d.; apos; Auria, C.; Van-der-Rest, B.; Bhamidi, S.; Huc, E.; Huang, H.; De Paepe, D.; Tropis, M.; et al. Partial redundancy in the synthesis of the d-arabinose incorporated in the cell wall arabinan of Corynebacterineae. Microbiology 2008, 154, 2315-2326. [CrossRef]
- Xu, L.; Qian, L.; Kang, J.; Sha, S.; Xin, Y.; Lu, S.; Ma, Y. Down-regulation of N-acetylglucosamine-1-phosphate transferase (WecA) enhanced the sensitivity of Mycobacterium smegmatis against rifampin. Journal of Applied Microbiology 2016, 121, 966-972. [CrossRef]
- Young, E.F.; Durham, P.G.; Perkowski, E.F.; Malik, S.; Hickey, A.J.; Braunstein, M. Efficacy of inhaled CPZEN-45 in treating tuberculosis in the guinea pig. Tuberculosis 2022, 135, 102207. [CrossRef]
- Sammartino, J.C.; Morici, M.; Stelitano, G.; Degiacomi, G.; Riccardi, G.; Chiarelli, L.R. Functional investigation of the antitubercular drug target Decaprenylphosphoryl-β-D-ribofuranose-2-epimerase DprE1/DprE2 complex. Biochemical and Biophysical Research Communications 2022, 607, 49-53. [CrossRef]
- Chhabra, S.; Kumar, S.; Parkesh, R. Chemical Space Exploration of DprE1 Inhibitors Using Chemoinformatics and Artificial Intelligence. ACS Omega 2021, 6, 14430-14441. [CrossRef]
- Iqbal, I.K.; Bajeli, S.; Akela, A.K.; Kumar, A. Bioenergetics of Mycobacterium: An Emerging Landscape for Drug Discovery. Pathogens 2018, 7, 1-30. [CrossRef]
- Wani, M.A.; Dhaked, D.K. Targeting the cytochrome bc1 complex for drug development in M. tuberculosis: review. Molecular Diversity 2022, 26, 2949-2965. [CrossRef]
- Hasenoehrl, E.J.; Wiggins, T.J.; Berney, M. Bioenergetic Inhibitors: Antibiotic Efficacy and Mechanisms of Action in Mycobacterium tuberculosis. Front Cell Infect Microbiol 2021, 10, 611683-611683. [CrossRef]
- Bajeli, S.; Baid, N.; Kaur, M.; Pawar, G.P.; Chaudhari, V.D.; Kumar, A. Terminal Respiratory Oxidases: A Targetables Vulnerability of Mycobacterial Bioenergetics? Front Cell Infect Microbiol 2020, 10, 1-22. [CrossRef]
- Foo, C.S.; Pethe, K.; Lupien, A. Oxidative Phosphorylation—an Update on a New, Essential Target Space for Drug Discovery in Mycobacterium tuberculosis. Applied Sciences 2020, 10. [CrossRef]
- Borisov, V.B.; Forte, E. Bioenergetics and Reactive Nitrogen Species in Bacteria. International Journal of Molecular Sciences 2022, 23. [CrossRef]
- Wiseman, B.; Nitharwal, R.G.; Fedotovskaya, O.; Schäfer, J.; Guo, H.; Kuang, Q.; Benlekbir, S.; Sjöstrand, D.; Ädelroth, P.; Rubinstein, J.L.; et al. Structure of a functional obligate complex III2IV2 respiratory supercomplex from Mycobacterium smegmatis. Nature Structural & Molecular Biology 2018, 25, 1128-1136. [CrossRef]
- Harrison Gregory, A.; Mayer Bridwell Anne, E.; Singh, M.; Jayaraman, K.; Weiss Leslie, A.; Kinsella Rachel, L.; Aneke Janessa, S.; Flentie, K.; Schene Miranda, E.; Gaggioli, M.; et al. Identification of 4-Amino-Thieno [2,3-d]Pyrimidines as QcrB Inhibitors in Mycobacterium tuberculosis. mSphere 2019, 4, e00606-00619. [CrossRef]
- Lupien, A.; Foo, C.S.-Y.; Savina, S.; Vocat, A.; Piton, J.; Monakhova, N.; Benjak, A.; Lamprecht, D.A.; Steyn, A.J.C.; Pethe, K.; et al. New 2-Ethylthio-4-methylaminoquinazoline derivatives inhibiting two subunits of cytochrome bc1 in Mycobacterium tuberculosis. PLOS Pathogens 2020, 16, e1008270. [CrossRef]
- Moraski, G.C.; Deboosère, N.; Marshall, K.L.; Weaver, H.A.; Vandeputte, A.; Hastings, C.; Woolhiser, L.; Lenaerts, A.J.; Brodin, P.; Miller, M.J. Intracellular and in vivo evaluation of imidazo [2,1-b]thiazole-5-carboxamide anti-tuberculosis compounds. PLOS ONE 2020, 15, e0227224. [CrossRef]
- Roy, K.K.; Wani, M.A. Emerging opportunities of exploiting mycobacterial electron transport chain pathway for drug-resistant tuberculosis drug discovery. Expert Opinion on Drug Discovery 2020, 15, 231-241. [CrossRef]
- Thompson, A.M.; Denny, W.A. Chapter Four–Inhibitors of enzymes in the electron transport chain of Mycobacterium tuberculosis. In Annual Reports in Medicinal Chemistry, Chibale, K., Ed.; Academic Press: 2019; Volume 52, pp. 97-130.
- Lee, B.S.; Kalia, N.P.; Jin, X.E.F.; Hasenoehrl, E.J.; Berney, M.; Pethe, K. Inhibitors of energy metabolism interfere with antibiotic-induced death in mycobacteria. Journal of Biological Chemistry 2019, 294, 1936-1943. [CrossRef]
- Yu, W.; Chiwala, G.; Gao, Y.; Liu, Z.; Sapkota, S.; Lu, Z.; Guo, L.; Khan, S.A.; Zhong, N.; Zhang, T. TB47 and clofazimine form a highly synergistic sterilizing block in a second-line regimen for tuberculosis in mice. Biomedicine & Pharmacotherapy 2020, 131, 110782. [CrossRef]
- Yu, W.; Yusuf, B.; Wang, S.; Tian, X.; Hameed, H.M.A.; Lu, Z.; Chiwala, G.; Alam Md, S.; Cook Gregory, M.; Maslov Dmitry, A.; et al. Sterilizing Effects of Novel Regimens Containing TB47, Clofazimine, and Linezolid in a Murine Model of Tuberculosis. Antimicrobial Agents and Chemotherapy 2021, 65, e00706-00721. [CrossRef]
- Cai, Y.; Jaecklein, E.; Mackenzie, J.S.; Papavinasasundaram, K.; Olive, A.J.; Chen, X.; Steyn, A.J.C.; Sassetti, C.M. Host immunity increases Mycobacterium tuberculosis reliance on cytochrome bd oxidase. PLOS Pathogens 2021, 17, e1008911. [CrossRef]
- Harikishore, A.; Chong, S.S.M.; Ragunathan, P.; Bates, R.W.; Grüber, G. Targeting the menaquinol binding loop of mycobacterial cytochrome bd oxidase. Molecular Diversity 2021, 25, 517-524. [CrossRef]
- Friedrich, T.; Wohlwend, D.; Borisov, V.B. Recent Advances in Structural Studies of Cytochrome bd and Its Potential Application as a Drug Target. International Journal of Molecular Sciences 2022, 23. [CrossRef]
- Foo Caroline, S.; Lupien, A.; Kienle, M.; Vocat, A.; Benjak, A.; Sommer, R.; Lamprecht Dirk, A.; Steyn Adrie, J.C.; Pethe, K.; Piton, J.; et al. Arylvinylpiperazine Amides, a New Class of Potent Inhibitors Targeting QcrB of Mycobacterium tuberculosis. mBio 2018, 9, e01276-01218. [CrossRef]
- Moosa, A.; Lamprecht Dirk, A.; Arora, K.; Barry Clifton, E.; Boshoff Helena, I.M.; Ioerger Thomas, R.; Steyn Adrie, J.C.; Mizrahi, V.; Warner Digby, F. Susceptibility of Mycobacterium tuberculosis Cytochrome bd Oxidase Mutants to Compounds Targeting the Terminal Respiratory Oxidase, Cytochrome c. Antimicrobial Agents and Chemotherapy 2017, 61, e01338-01317. [CrossRef]
- Sarathy, J.P.; Gruber, G.; Dick, T. Re-Understanding the Mechanisms of Action of the Anti-Mycobacterial Drug Bedaquiline. Antibiotics 2019, 8. [CrossRef]
- Gupta, S.; Fatima, Z.; Kumawat, S. Study of the bioenergetics to identify the novel pathways as a drug target against Mycobacterium tuberculosis using Petri net. Biosystems 2021, 209, 104509. [CrossRef]
- Jeon, A.B.; Ackart, D.F.; Li, W.; Jackson, M.; Melander, R.J.; Melander, C.; Abramovitch, R.B.; Chicco, A.J.; Basaraba, R.J.; Obregón-Henao, A. 2-aminoimidazoles collapse mycobacterial proton motive force and block the electron transport chain. Scientific Reports 2019, 9, 1513. [CrossRef]
- Odingo, J.; Bailey, M.A.; Files, M.; Early, J.V.; Alling, T.; Dennison, D.; Bowman, J.; Dalai, S.; Kumar, N.; Cramer, J.; et al. In Vitro Evaluation of Novel Nitazoxanide Derivatives against Mycobacterium tuberculosis. ACS Omega 2017, 2, 5873-5890. [CrossRef]
- Chao, A.; Sieminski, P.J.; Owens, C.P.; Goulding, C.W. Iron Acquisition in Mycobacterium tuberculosis. Chemical Reviews 2019, 119, 1193-1220. [CrossRef]
- Chiarelli, L.R.; Mori, M.; Barlocco, D.; Beretta, G.; Gelain, A.; Pini, E.; Porcino, M.; Mori, G.; Stelitano, G.; Costantino, L.; et al. Discovery and development of novel salicylate synthase (MbtI) furanic inhibitors as antitubercular agents. European Journal of Medicinal Chemistry 2018, 155, 754-763. [CrossRef]
- Chiarelli, L.R.; Mori, M.; Beretta, G.; Gelain, A.; Pini, E.; Sammartino, J.C.; Stelitano, G.; Barlocco, D.; Costantino, L.; Lapillo, M.; et al. New insight into structure-activity of furan-based salicylate synthase (MbtI) inhibitors as potential antitubercular agents. Journal of Enzyme Inhibition and Medicinal Chemistry 2019, 34, 823-828. [CrossRef]
- Rohilla, A.; Khare, G.; Tyagi, A.K. Virtual Screening, pharmacophore development and structure based similarity search to identify inhibitors against IdeR, a transcription factor of Mycobacterium tuberculosis. Scientific Reports 2017, 7, 4653. [CrossRef]
- Yang, L.; Hu, X.; Chai, X.; Ye, Q.; Pang, J.; Li, D.; Hou, T. Opportunities for overcoming tuberculosis: Emerging targets and their inhibitors. Drug Discovery Today 2022, 27, 326-336. [CrossRef]
- Khisimuzi, M.; Zhenkun, M. Mycobacterium tuberculosis DNA Gyrase as a Target for Drug Discovery. Infectious Disorders–Drug Targets 2007, 7, 159-168. [CrossRef]
- Khan, T.; Sankhe, K.; Suvarna, V.; Sherje, A.; Patel, K.; Dravyakar, B. DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomedicine & Pharmacotherapy 2018, 103, 923-938. [CrossRef]
- Godbole Adwait, A.; Ahmed, W.; Bhat Rajeshwari, S.; Bradley Erin, K.; Ekins, S.; Nagaraja, V. Targeting Mycobacterium tuberculosis Topoisomerase I by Small-Molecule Inhibitors. Antimicrobial Agents and Chemotherapy 2015, 59, 1549-1557. [CrossRef]
- Dwivedi, N.; Dube, D.; Pandey, J.; Singh, B.; Kukshal, V.; Ramachandran, R.; Tripathi, R.P. NAD+-Dependent DNA Ligase: A novel target waiting for the right inhibitor. Medicinal Research Reviews 2008, 28, 545-568. [CrossRef]
- Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. NAD+-dependent DNA Ligase (Rv3014c) from Mycobacterium tuberculosis: CRYSTAL STRUCTURE OF THE ADENYLATION DOMAIN AND IDENTIFICATION OF NOVEL INHIBITORS*. Journal of Biological Chemistry 2005, 280, 30273-30281. [CrossRef]
- Jian, Y.; Risseeuw, M.D.P.; Froeyen, M.; Song, L.; Cappoen, D.; Cos, P.; Munier-Lehmann, H.; van Calenbergh, S. 1-(Piperidin-3-yl)thymine amides as inhibitors of M. tuberculosis thymidylate kinase. Journal of Enzyme Inhibition and Medicinal Chemistry 2019, 34, 1730-1739. [CrossRef]
- Fang, H.; Peng, B.; Ong, S.Y.; Wu, Q.; Li, L.; Yao, S.Q. Recent advances in activity-based probes (ABPs) and affinity-based probes (AfBPs) for profiling of enzymes. Chemical Science 2021, 12, 8288-8310. [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365-370. [CrossRef]
- Vandal, O.H.; Pierini, L.M.; Schnappinger, D.; Nathan, C.F.; Ehrt, S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nature Medicine 2008, 14, 849-854. [CrossRef]
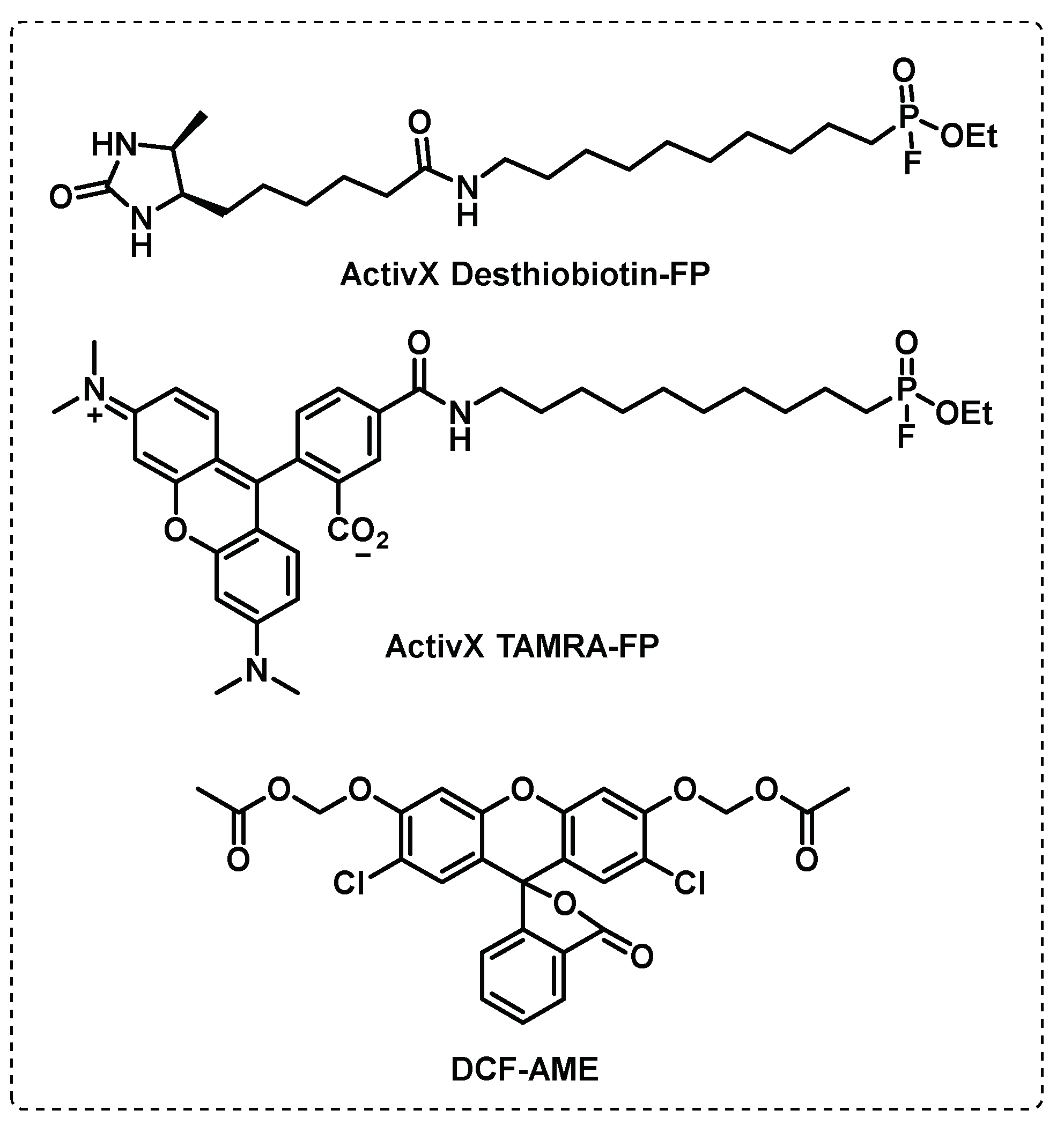
- Simon, G.M.; Cravatt, B.F. Activity-based Proteomics of Enzyme Superfamilies: Serine Hydrolases as a Case Study. Journal of Biological Chemistry 2010, 285, 11051-11055. [CrossRef]
- Ortega, C.; Anderson, Lindsey N.; Frando, A.; Sadler, Natalie C.; Brown, Robert W.; Smith, Richard D.; Wright, Aaron T.; Grundner, C. Systematic Survey of Serine Hydrolase Activity in Mycobacterium tuberculosis Defines Changes Associated with Persistence. Cell Chemical Biology 2016, 23, 290-298. [CrossRef]
- Bachovchin, D.A.; Ji, T.; Li, W.; Simon, G.M.; Blankman, J.L.; Adibekian, A.; Hoover, H.; Niessen, S.; Cravatt, B.F. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proceedings of the National Academy of Sciences 2010, 107, 20941-20946. [CrossRef]
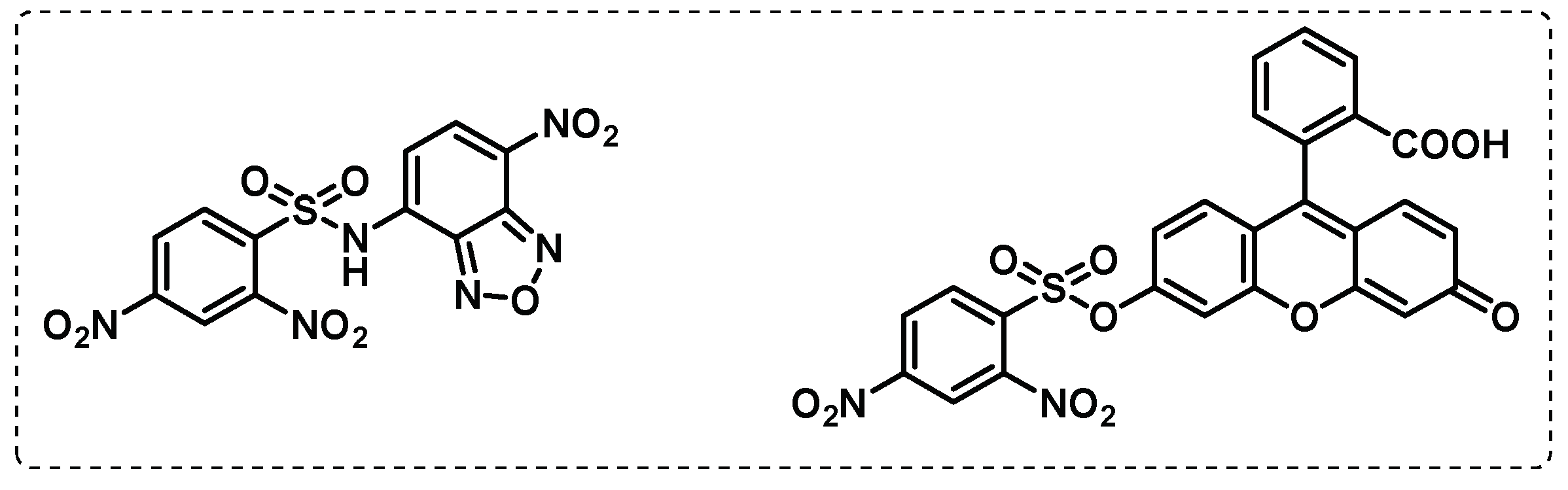
- Lentz, C.S.; Ordonez, A.A.; Kasperkiewicz, P.; La Greca, F.; O’Donoghue, A.J.; Schulze, C.J.; Powers, J.C.; Craik, C.S.; Drag, M.; Jain, S.K.; et al. Design of Selective Substrates and Activity-Based Probes for Hydrolase Important for Pathogenesis 1 (HIP1) from Mycobacterium tuberculosis. ACS Infectious Diseases 2016, 2, 807-815. [CrossRef]
- Babin, B.M.; Keller, L.J.; Pinto, Y.; Li, V.L.; Eneim, A.S.; Vance, S.E.; Terrell, S.M.; Bhatt, A.S.; Long, J.Z.; Bogyo, M. Identification of covalent inhibitors that disrupt M. tuberculosis growth by targeting multiple serine hydrolases involved in lipid metabolism. Cell Chemical Biology 2022, 29, 897-909.e897. [CrossRef]
- Li, M.; Patel, H.V.; Cognetta, A.B., III; Smith, T.C., II; Mallick, I.; Cavalier, J.-F.; Previti, M.L.; Canaan, S.; Aldridge, B.B.; Cravatt, B.F.; et al. Identification of cell wall synthesis inhibitors active against Mycobacterium tuberculosis by competitive activity-based protein profiling. Cell Chemical Biology 2022, 29, 883-896.e885. [CrossRef]
- Gun, M.A.; Bozdogan, B.; Coban, A.Y. Tuberculosis and beta-lactam antibiotics. Future Microbiology 2020, 15, 937-944. [CrossRef]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition. Frontiers in Microbiology 2022, 13. [CrossRef]
- Lopez Quezada, L.; Smith, R.; Lupoli, T.J.; Edoo, Z.; Li, X.; Gold, B.; Roberts, J.; Ling, Y.; Park, S.W.; Nguyen, Q.; et al. Activity-Based Protein Profiling Reveals That Cephalosporins Selectively Active on Non-replicating Mycobacterium tuberculosis Bind Multiple Protein Families and Spare Peptidoglycan Transpeptidases. Frontiers in Microbiology 2020, 11. [CrossRef]
- de Munnik, M.; Lohans, C.T.; Langley, G.W.; Bon, C.; Brem, J.; Schofield, C.J. A Fluorescence-Based Assay for Screening β-Lactams Targeting the Mycobacterium tuberculosis Transpeptidase LdtMt2. ChemBioChem 2020, 21, 368-372. [CrossRef]
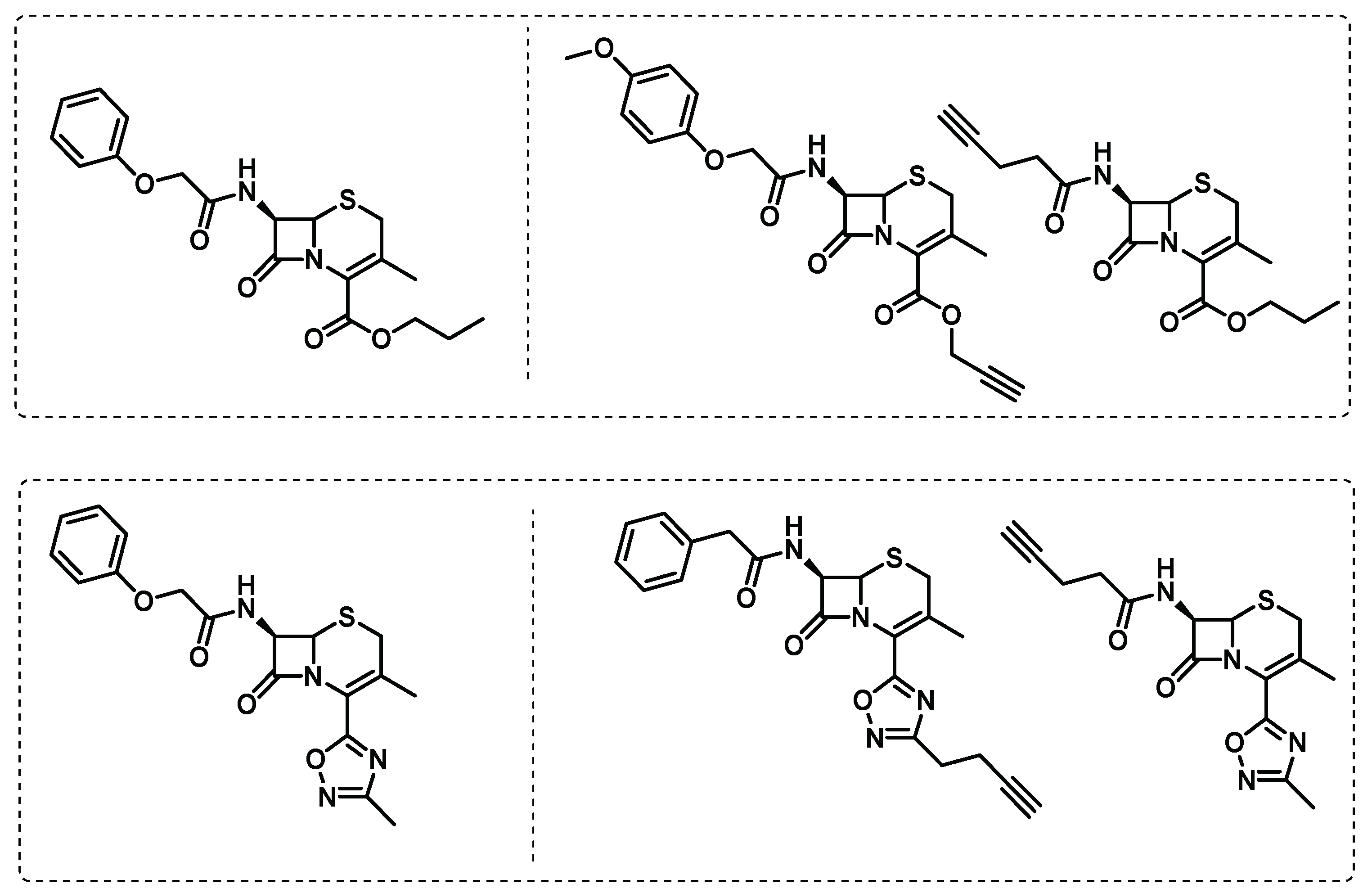
- Levine, S.R.; Beatty, K.E. Investigating β-Lactam Drug Targets in Mycobacterium tuberculosis Using Chemical Probes. ACS Infectious Diseases 2021, 7, 461-470. [CrossRef]
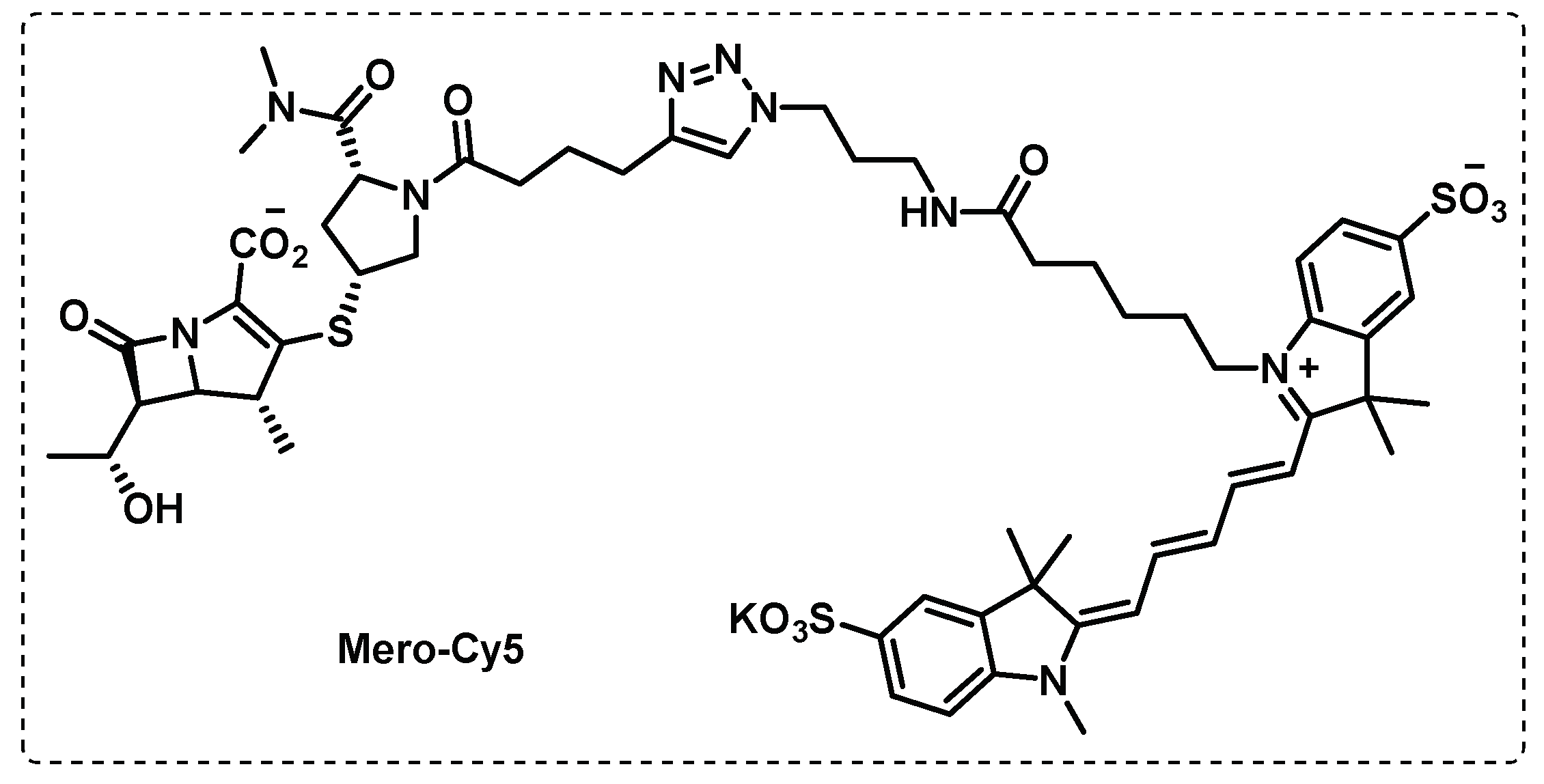
- Lehmann, J.; Cheng, T.-Y.; Aggarwal, A.; Park, A.S.; Zeiler, E.; Raju, R.M.; Akopian, T.; Kandror, O.; Sacchettini, J.C.; Moody, D.B.; et al. An Antibacterial β-Lactone Kills Mycobacterium tuberculosis by Disrupting Mycolic Acid Biosynthesis. Angewandte Chemie International Edition 2018, 57, 348-353. [CrossRef]
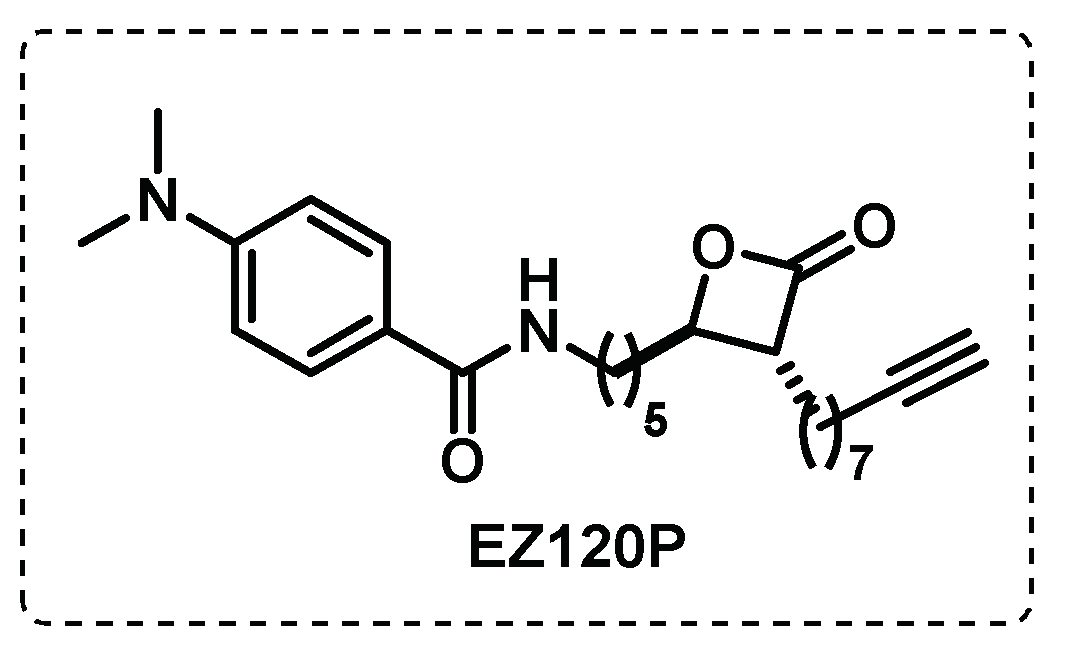
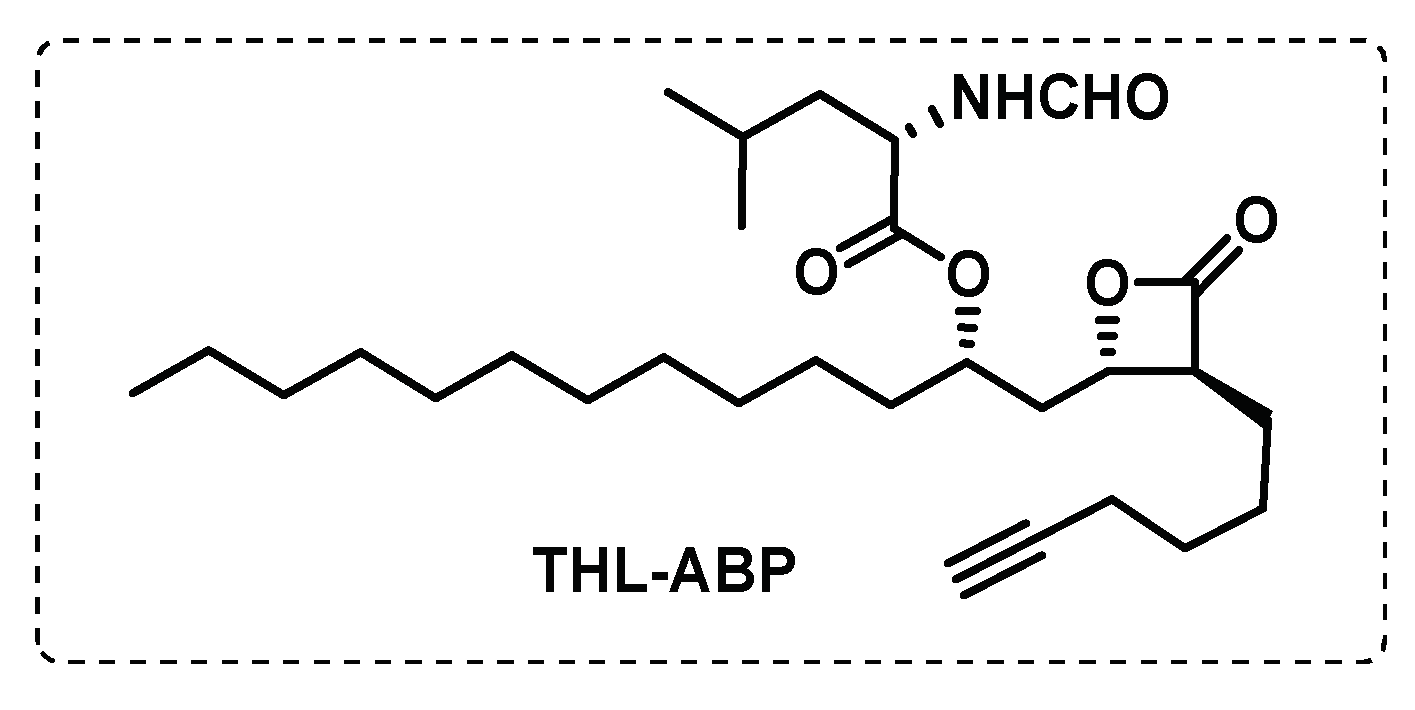
- Ravindran, M.S.; Rao, S.P.S.; Cheng, X.; Shukla, A.; Cazenave-Gassiot, A.; Yao, S.Q.; Wenk, M.R. Targeting Lipid Esterases in Mycobacteria Grown Under Different Physiological Conditions Using Activity-based Profiling with Tetrahydrolipstatin (THL). Molecular & Cellular Proteomics 2014, 13, 435-448. [CrossRef]
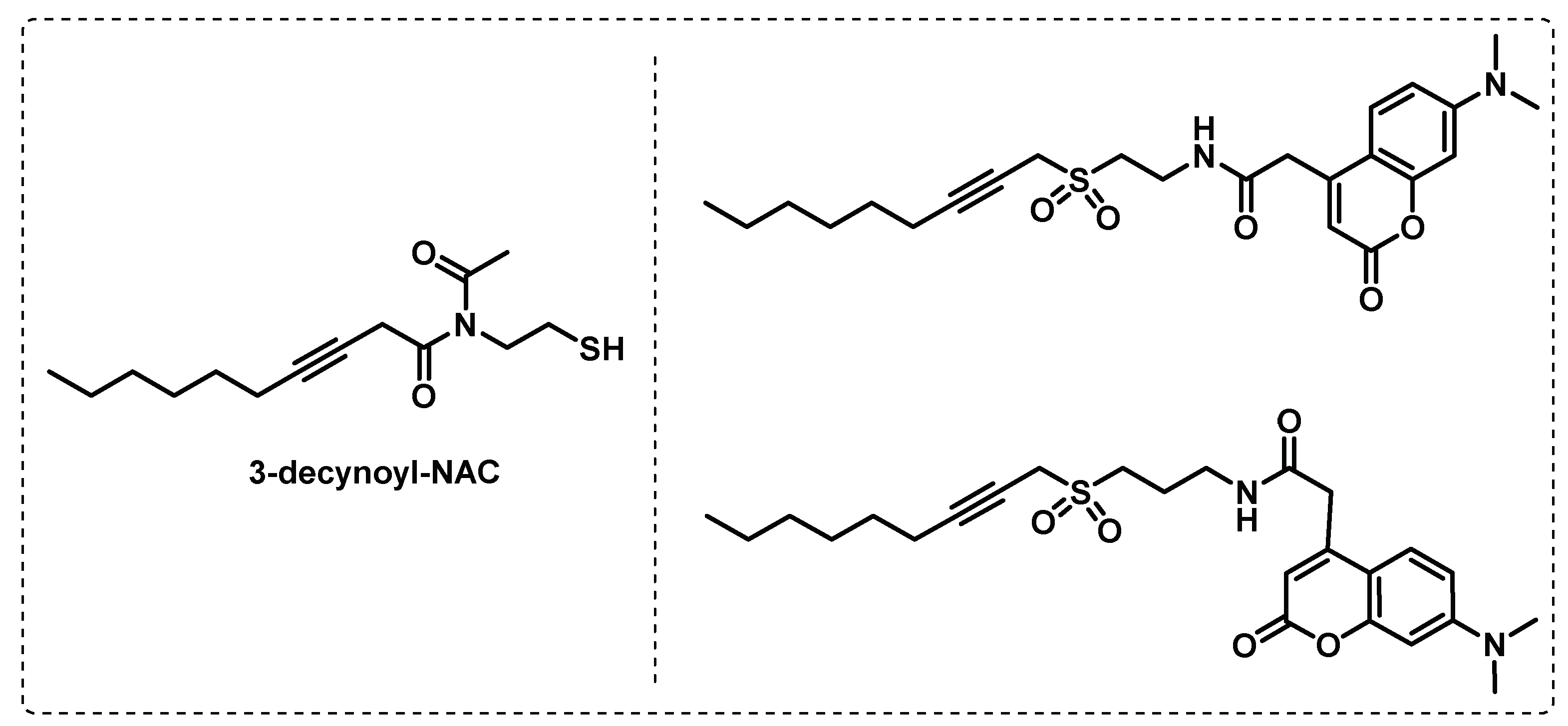
- Tallman, K.R.; Beatty, K.E. Far-red fluorogenic probes for esterase and lipase detection. Chembiochem : a European journal of chemical biology 2015, 16, 70-75. [CrossRef]
- Tallman, K.R.; Levine, S.R.; Beatty, K.E. Small-Molecule Probes Reveal Esterases with Persistent Activity in Dormant and Reactivating Mycobacterium tuberculosis. ACS Infectious Diseases 2016, 2, 936-944. [CrossRef]
- Tallman, K.R.; Levine, S.R.; Beatty, K.E. Profiling Esterases in Mycobacterium tuberculosis Using Far-Red Fluorogenic Substrates. ACS Chemical Biology 2016, 11, 1810-1815. [CrossRef]
- Touchette Megan, H.; Holsclaw Cynthia, M.; Previti Mary, L.; Solomon Viven, C.; Leary Julie, A.; Bertozzi Carolyn, R.; Seeliger Jessica, C. The rv1184c Locus Encodes Chp2, an Acyltransferase in Mycobacterium tuberculosis Polyacyltrehalose Lipid Biosynthesis. Journal of Bacteriology 2015, 197, 201-210. [CrossRef]
- Belardinelli, J.M.; Larrouy-Maumus, G.; Jones, V.; Sorio de Carvalho, L.P.; McNeil, M.R.; Jackson, M. Biosynthesis and Translocation of Unsulfated Acyltrehaloses in Mycobacterium tuberculosis. Journal of Biological Chemistry 2014, 289, 27952-27965. [CrossRef]
- Seeliger, J.C.; Holsclaw, C.M.; Schelle, M.W.; Botyanszki, Z.; Gilmore, S.A.; Tully, S.E.; Niederweis, M.; Cravatt, B.F.; Leary, J.A.; Bertozzi, C.R. Elucidation and Chemical Modulation of Sulfolipid-1 Biosynthesis in Mycobacterium tuberculosis*. Journal of Biological Chemistry 2012, 287, 7990-8000. [CrossRef]
- Ishikawa, F.; Tanabe, G.; Kakeya, H. Activity-Based Protein Profiling of Non-ribosomal Peptide Synthetases. In Activity-Based Protein Profiling, Cravatt, B.F., Hsu, K.-L., Weerapana, E., Eds.; Springer International Publishing: Cham, 2019; pp. 321-349.
- Li, W.; Deng, G.; Li, M.; Liu, X.; Wang, Y. Roles of Mucosal Immunity against Mycobacterium tuberculosis Infection. Tuberculosis Research and Treatment 2012, 2012, 791728. [CrossRef]
- Derrien, M.; van Passel, M.W.J.; van de Bovenkamp, J.H.B.; Schipper, R.; de Vos, W.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254-268. [CrossRef]
- Mougous, J.D.; Leavell, M.D.; Senaratne, R.H.; Leigh, C.D.; Williams, S.J.; Riley, L.W.; Leary, J.A.; Bertozzi, C.R. Discovery of sulfated metabolites in mycobacteria with a genetic and mass spectrometric approach. Proceedings of the National Academy of Sciences 2002, 99, 17037-17042. [CrossRef]
- Mougous, J.D.; Lee, D.H.; Hubbard, S.C.; Schelle, M.W.; Vocadlo, D.J.; Berger, J.M.; Bertozzi, C.R. Molecular Basis for G Protein Control of the Prokaryotic ATP Sulfurylase. Molecular Cell 2006, 21, 109-122. [CrossRef]
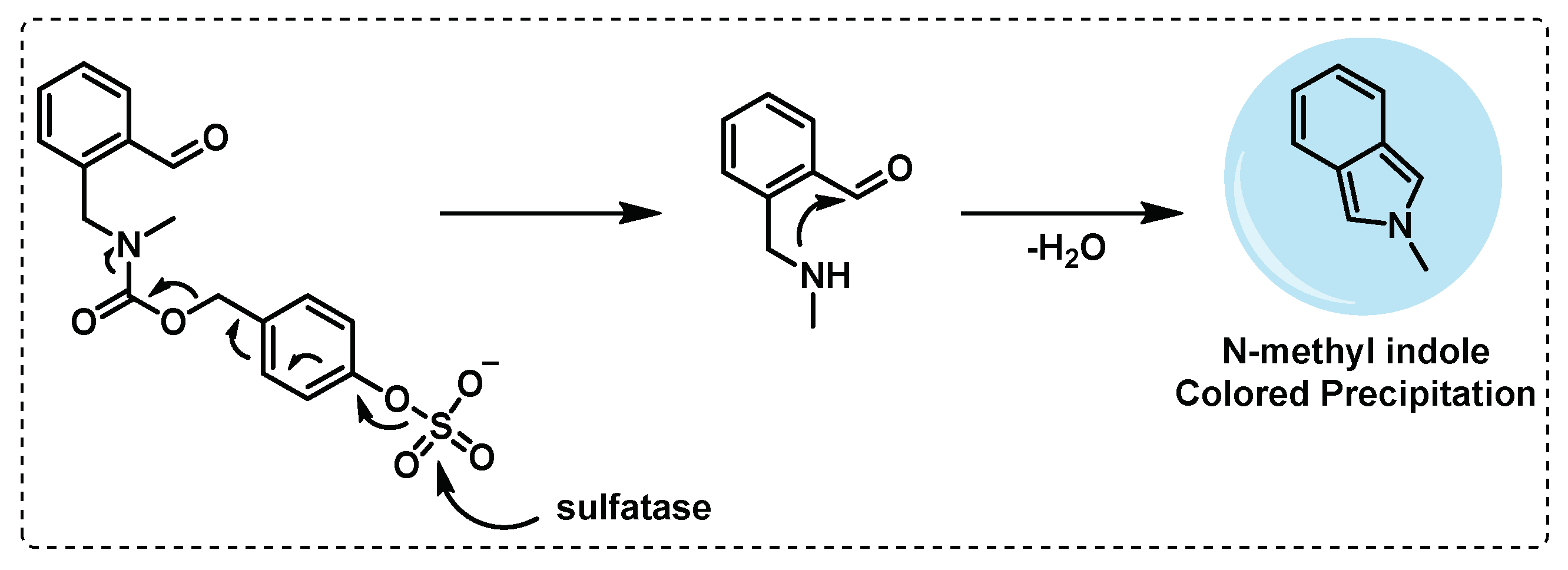
- Yoon, H.Y.; Kim, H.J.; Jang, S.; Hong, J.-I. Detection of bacterial sulfatase activity through liquid- and solid-phase colony-based assays. AMB Express 2017, 7, 150. [CrossRef]
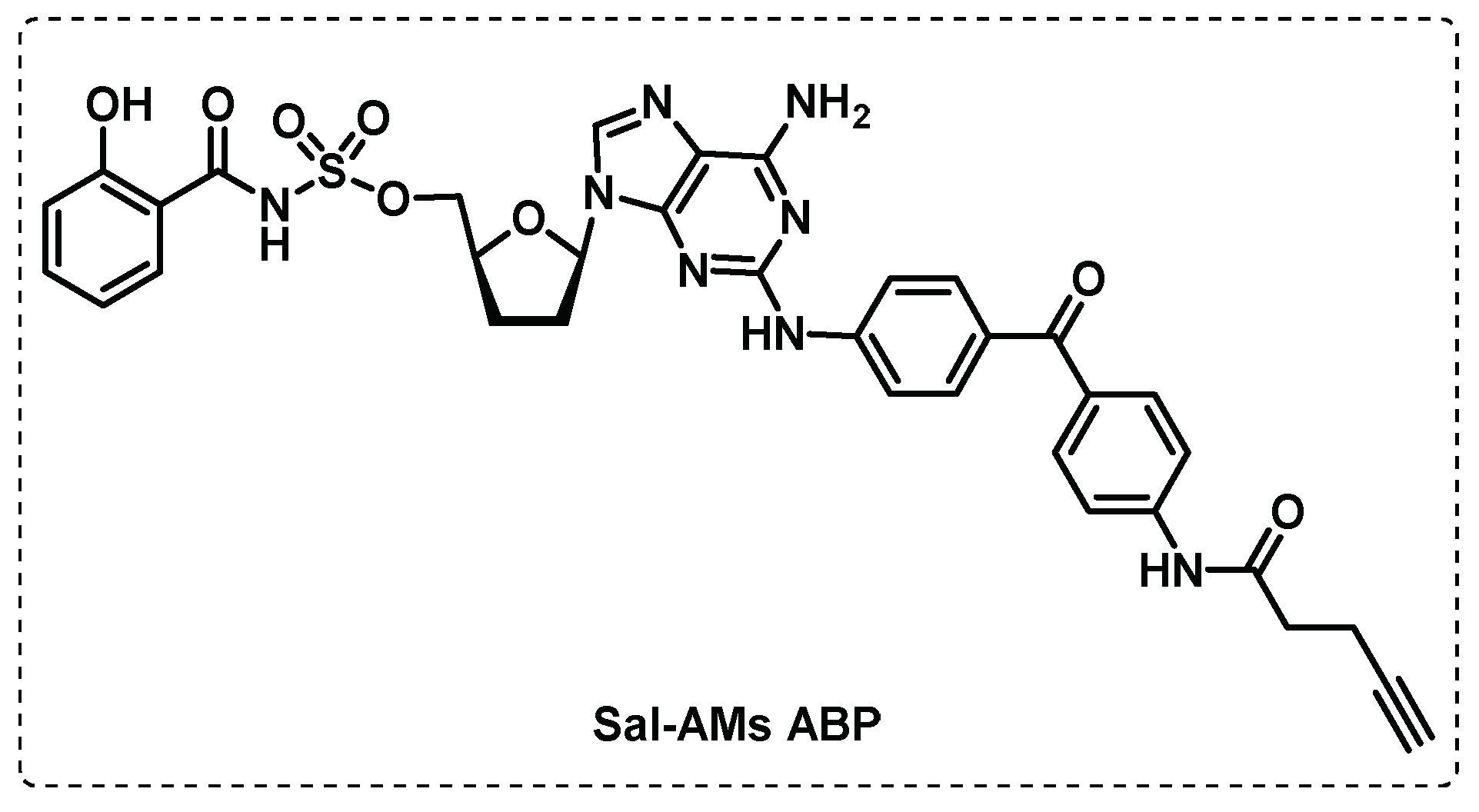
- Duckworth, B.P.; Wilson, D.J.; Nelson, K.M.; Boshoff, H.I.; Barry, C.E., III; Aldrich, C.C. Development of a Selective Activity-Based Probe for Adenylating Enzymes: Profiling MbtA Involved in Siderophore Biosynthesis from Mycobacterium tuberculosis. ACS Chemical Biology 2012, 7, 1653-1658. [CrossRef]
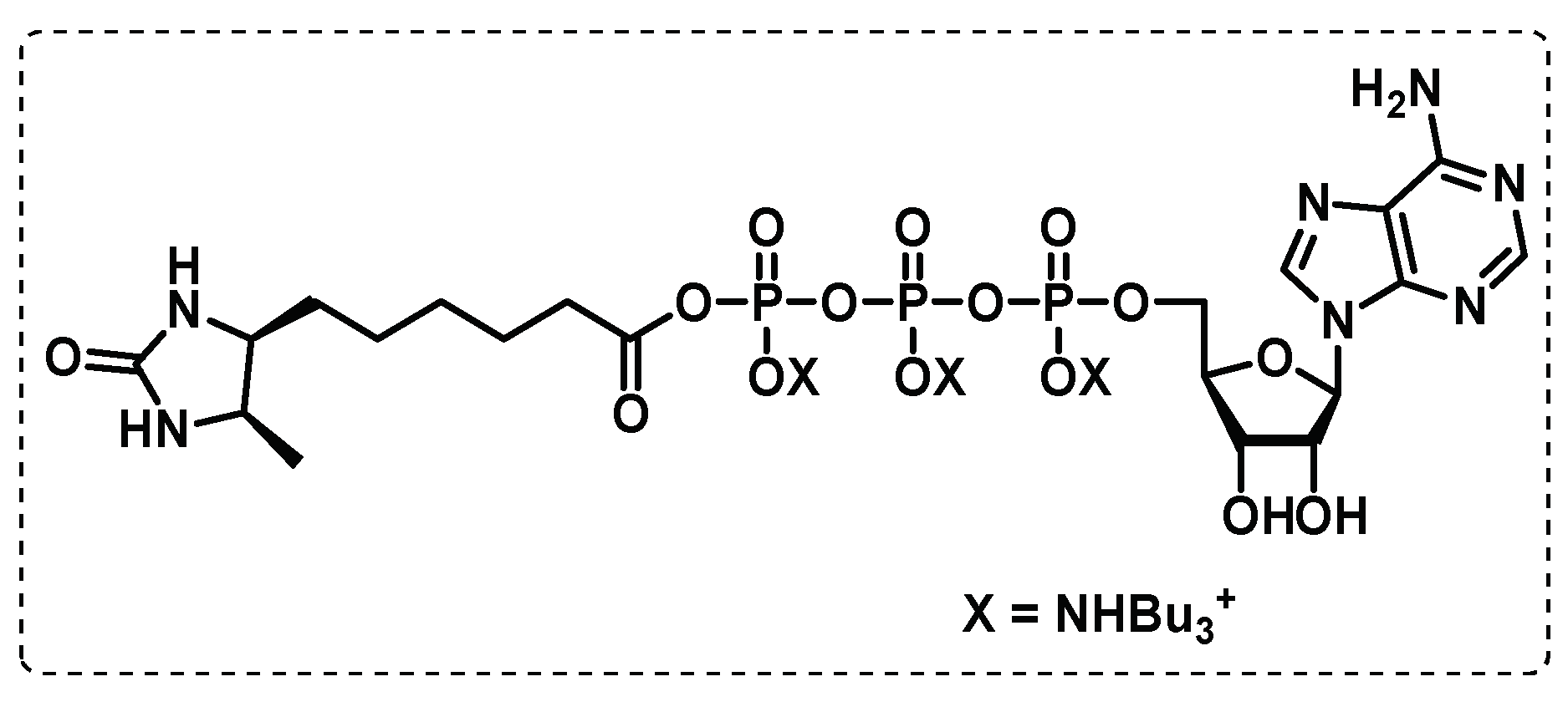
- Wolfe, L.M.; Veeraraghavan, U.; Idicula-Thomas, S.; Schürer, S.; Wennerberg, K.; Reynolds, R.; Besra, G.S.; Dobos, K.M. A chemical proteomics approach to profiling the ATP-binding proteome of Mycobacterium tuberculosis. Mol Cell Proteomics 2013, 12, 1644-1660. [CrossRef]
- Batt, S.M.; Minnikin, D.E.; Besra, G.S. The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host’s immune system. Biochemical Journal 2020, 477, 1983-2006. [CrossRef]
- Vilchèze, C. Mycobacterial Cell Wall: A Source of Successful Targets for Old and New Drugs. Applied Sciences 2020, 10. [CrossRef]
- Grzegorzewicz, A.E.; Eynard, N.; Quémard, A.; North, E.J.; Margolis, A.; Lindenberger, J.J.; Jones, V.; Korduláková, J.; Brennan, P.J.; Lee, R.E.; et al. Covalent Modification of the Mycobacterium tuberculosis FAS-II Dehydratase by Isoxyl and Thiacetazone. ACS Infectious Diseases 2015, 1, 91-97. [CrossRef]
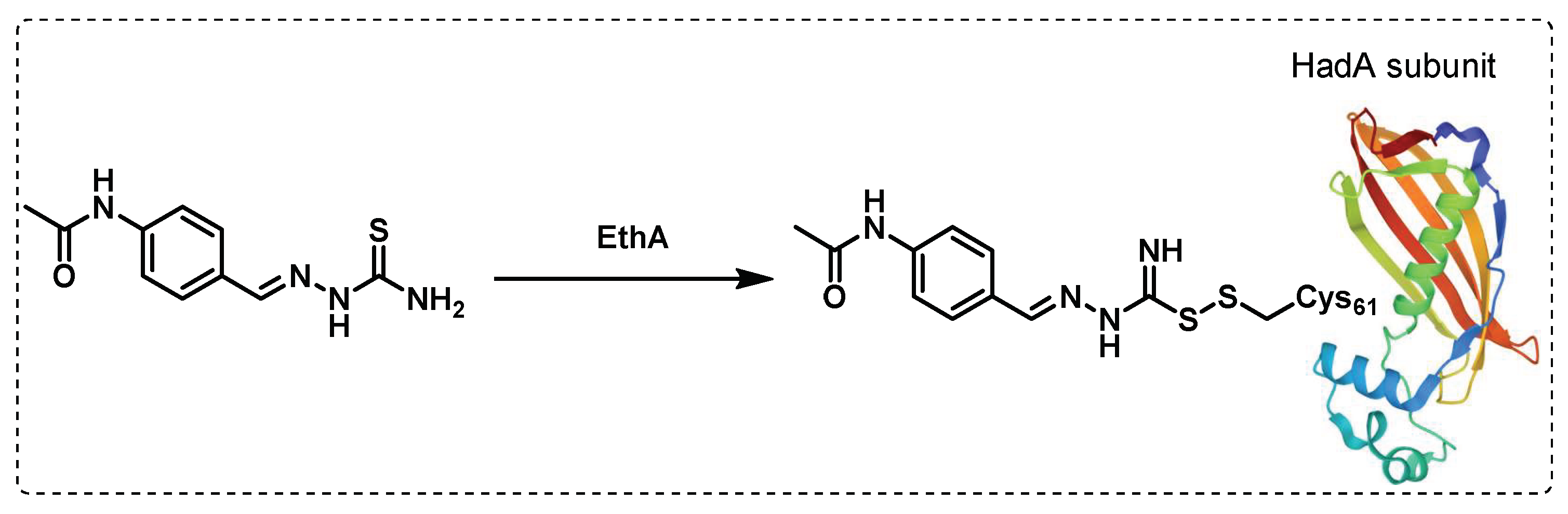
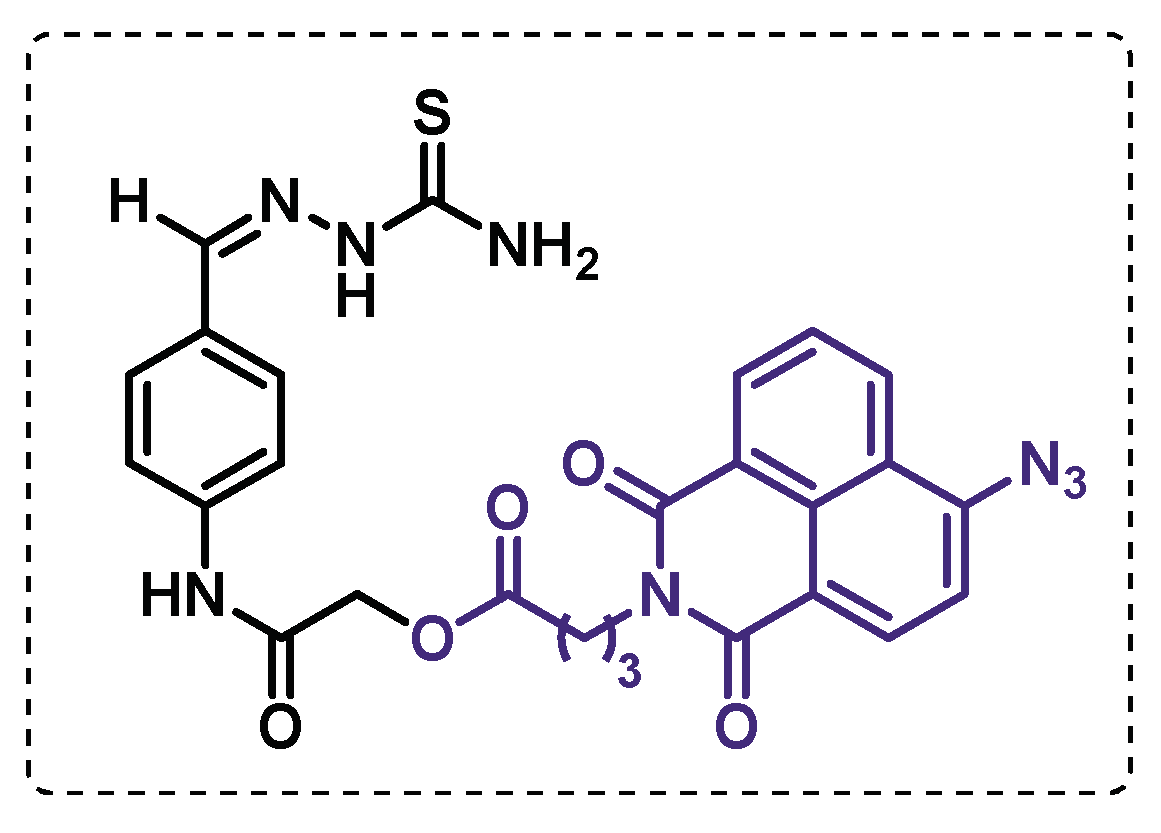
- Singh, B.K.; Singha, M.; Basak, S.; Biswas, R.; Das, A.K.; Basak, A. Fluorescently labelled thioacetazone for detecting the interaction with Mycobacterium dehydratases HadAB and HadBC. Organic & Biomolecular Chemistry 2022, 20, 1444-1452. [CrossRef]
- Rahlwes, K.C.; Dias, B.R.S.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [CrossRef]
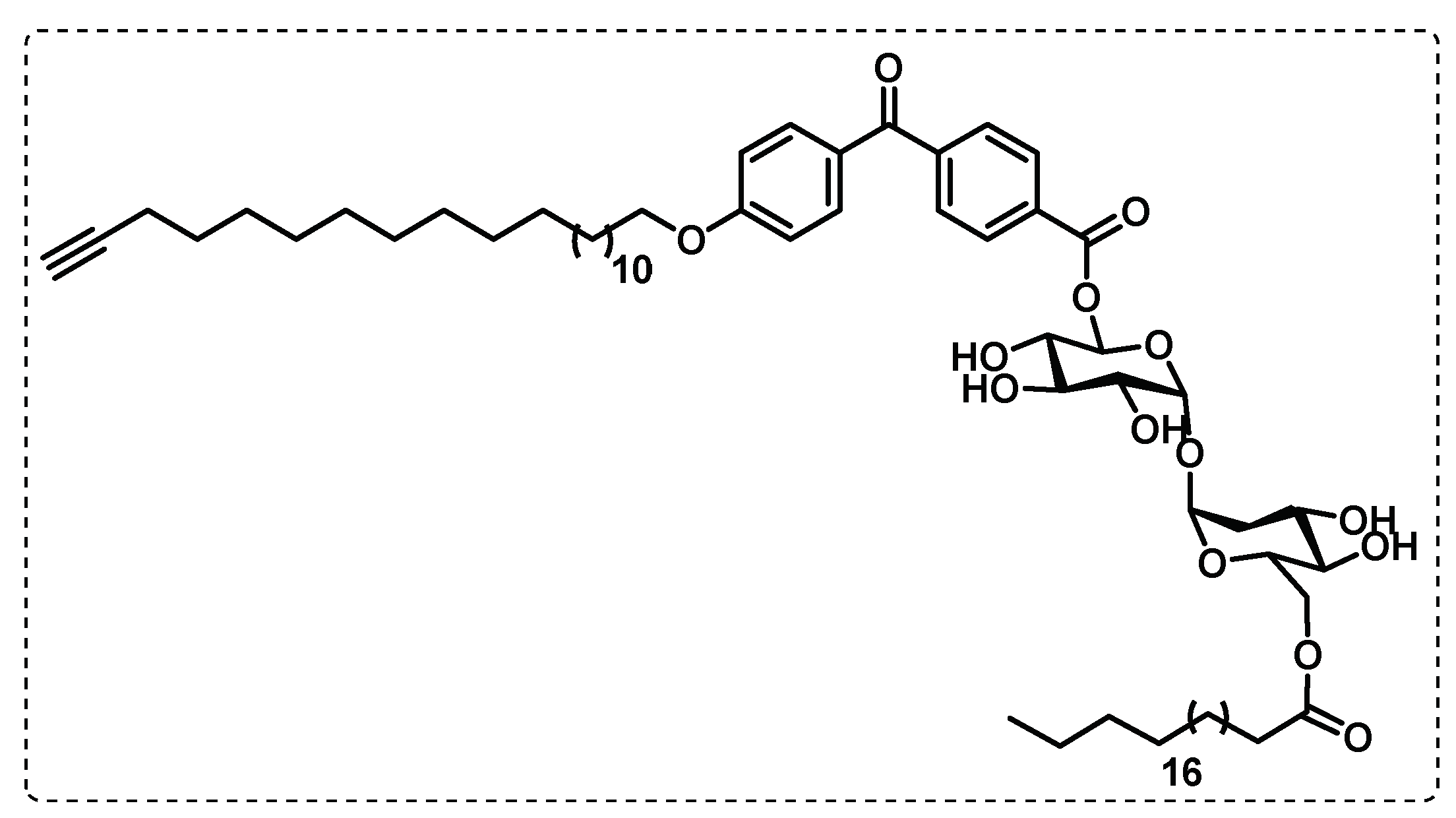
- Khan, A.A.; Kamena, F.; Timmer, M.S.M.; Stocker, B.L. Development of a benzophenone and alkyne functionalised trehalose probe to study trehalose dimycolate binding proteins. Organic & Biomolecular Chemistry 2013, 11, 881-885. [CrossRef]
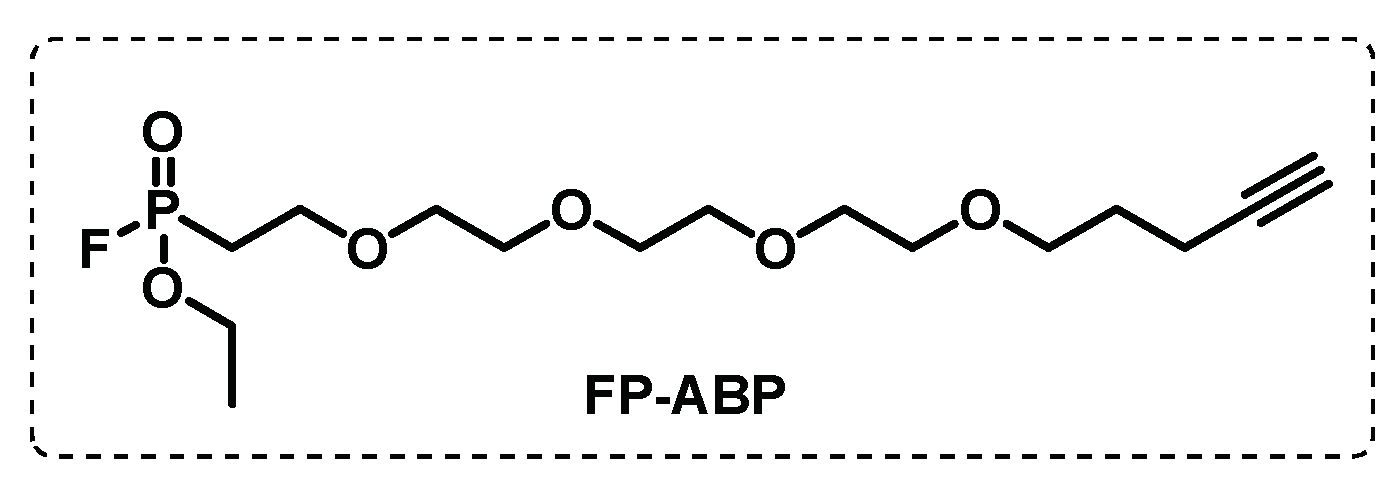
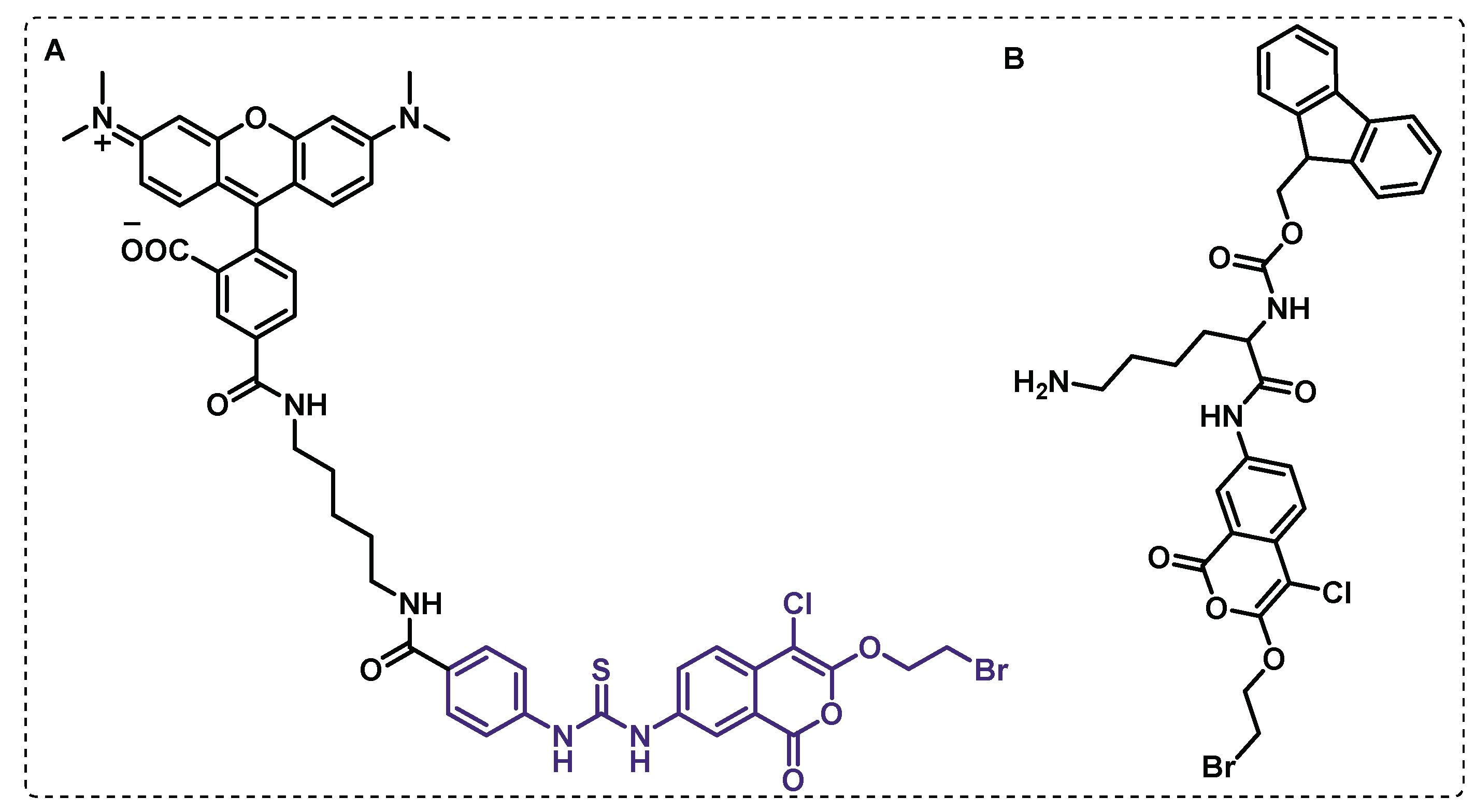
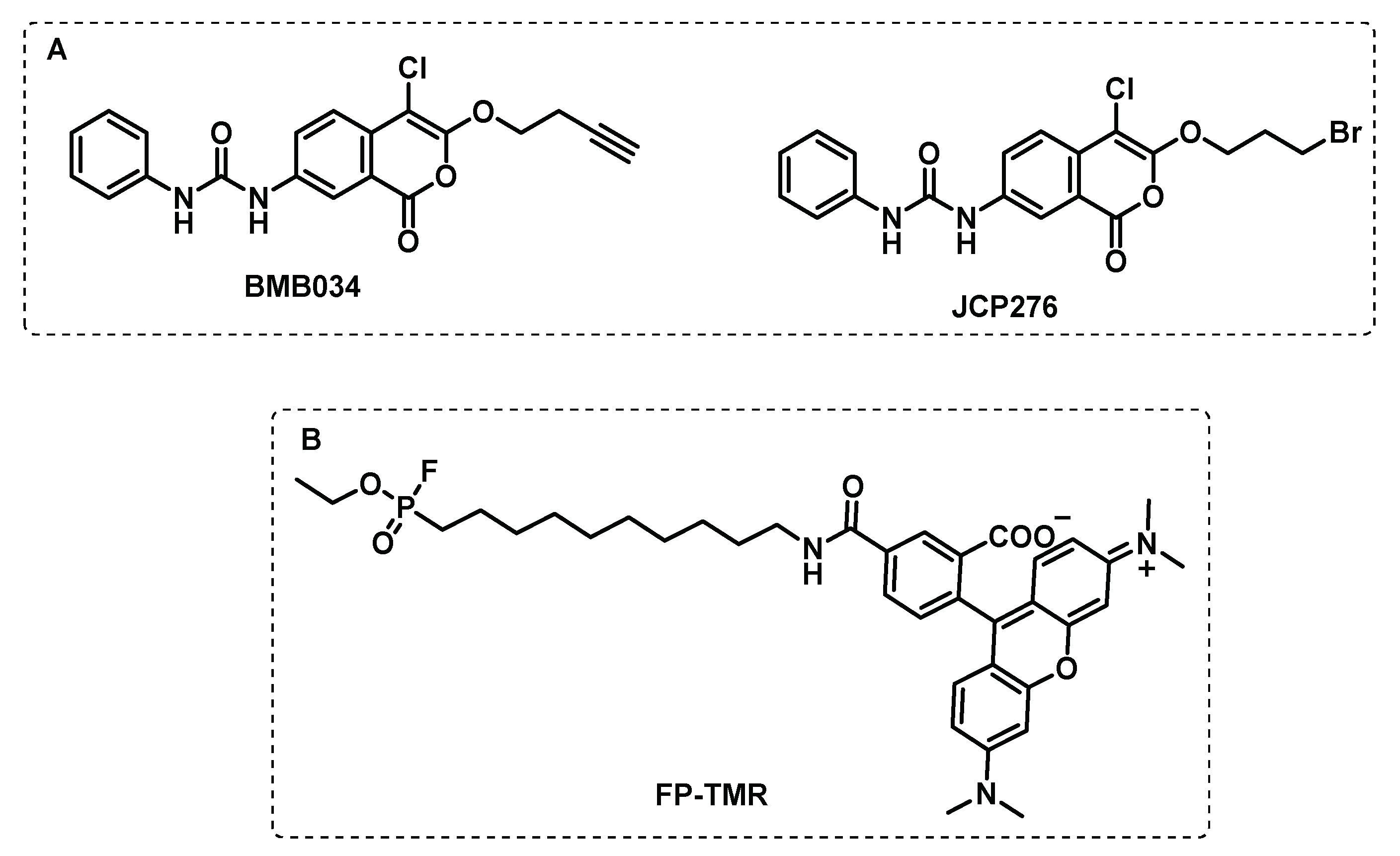
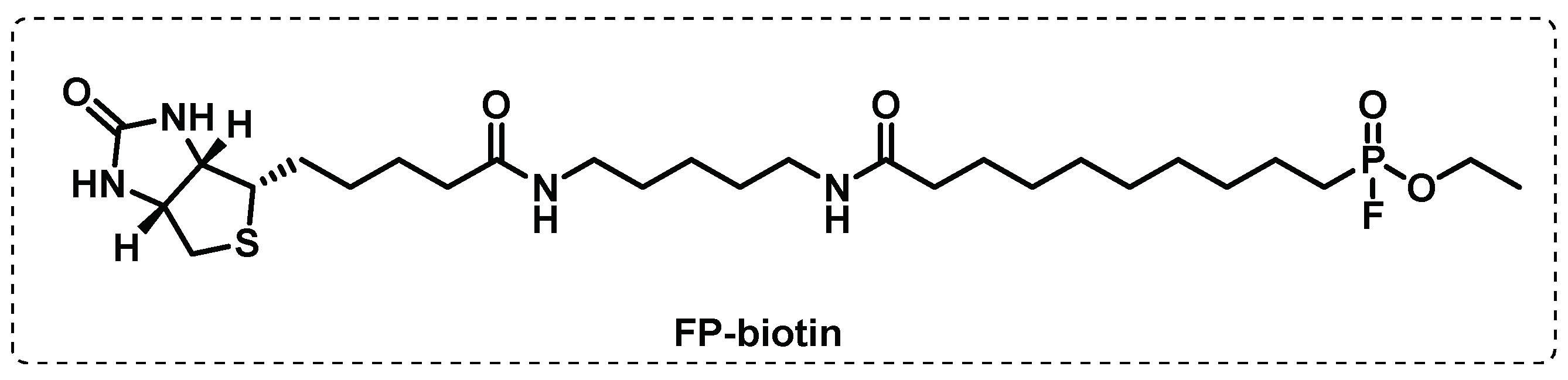
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




