Submitted:
01 June 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fibrillar Type I Collagen
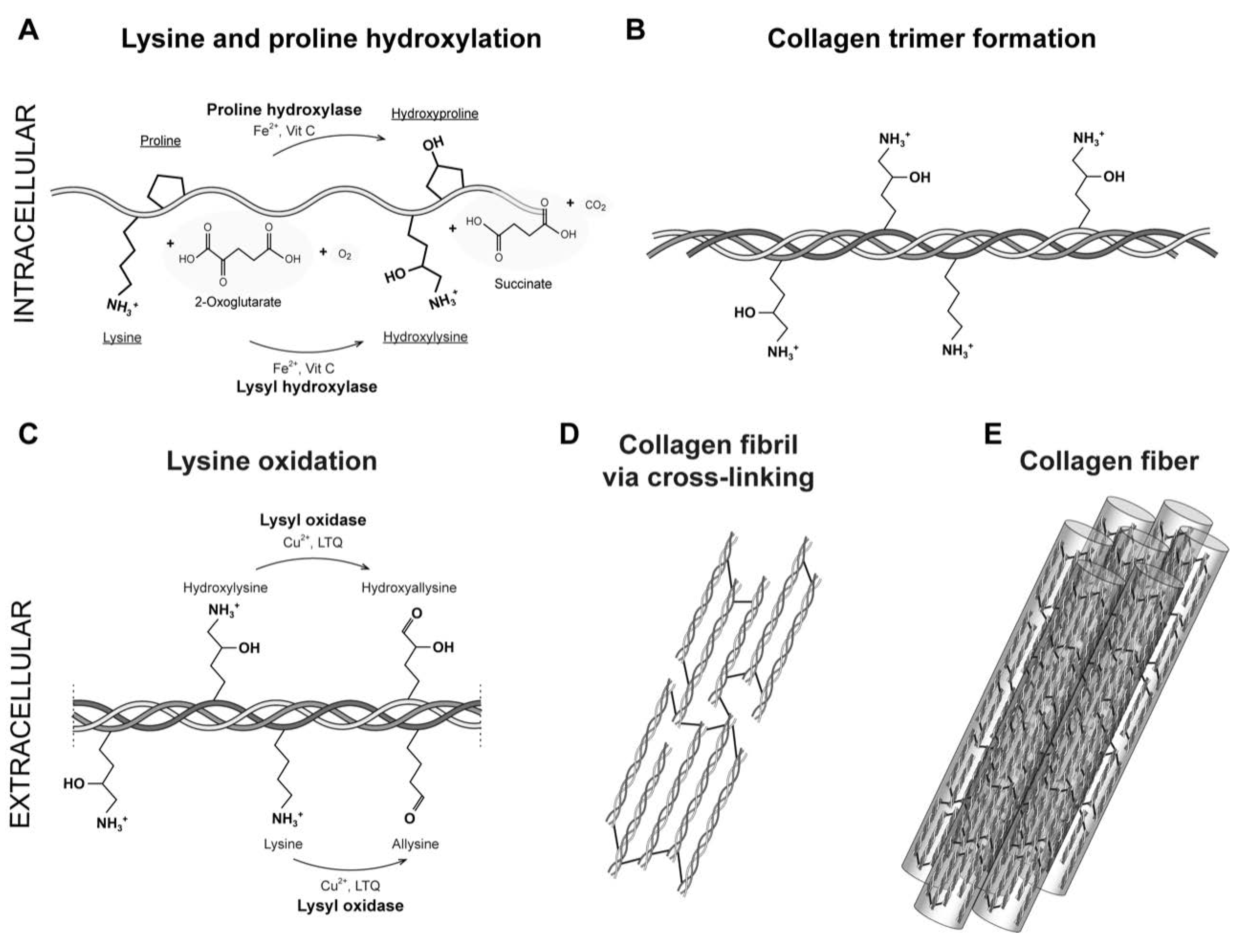
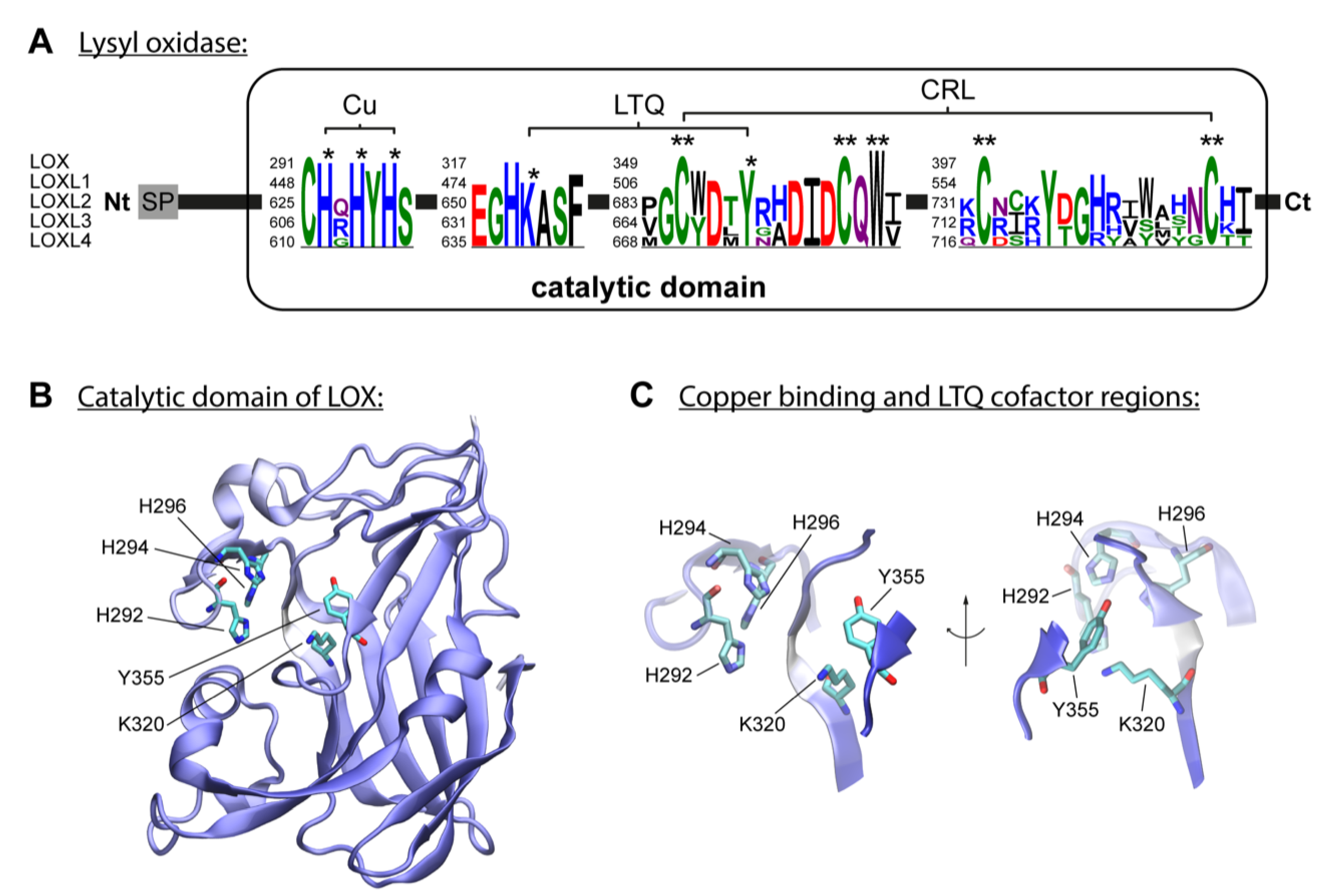
3. Enzymatic Collagen Cross-Linking Mediated by Lysyl Oxidases
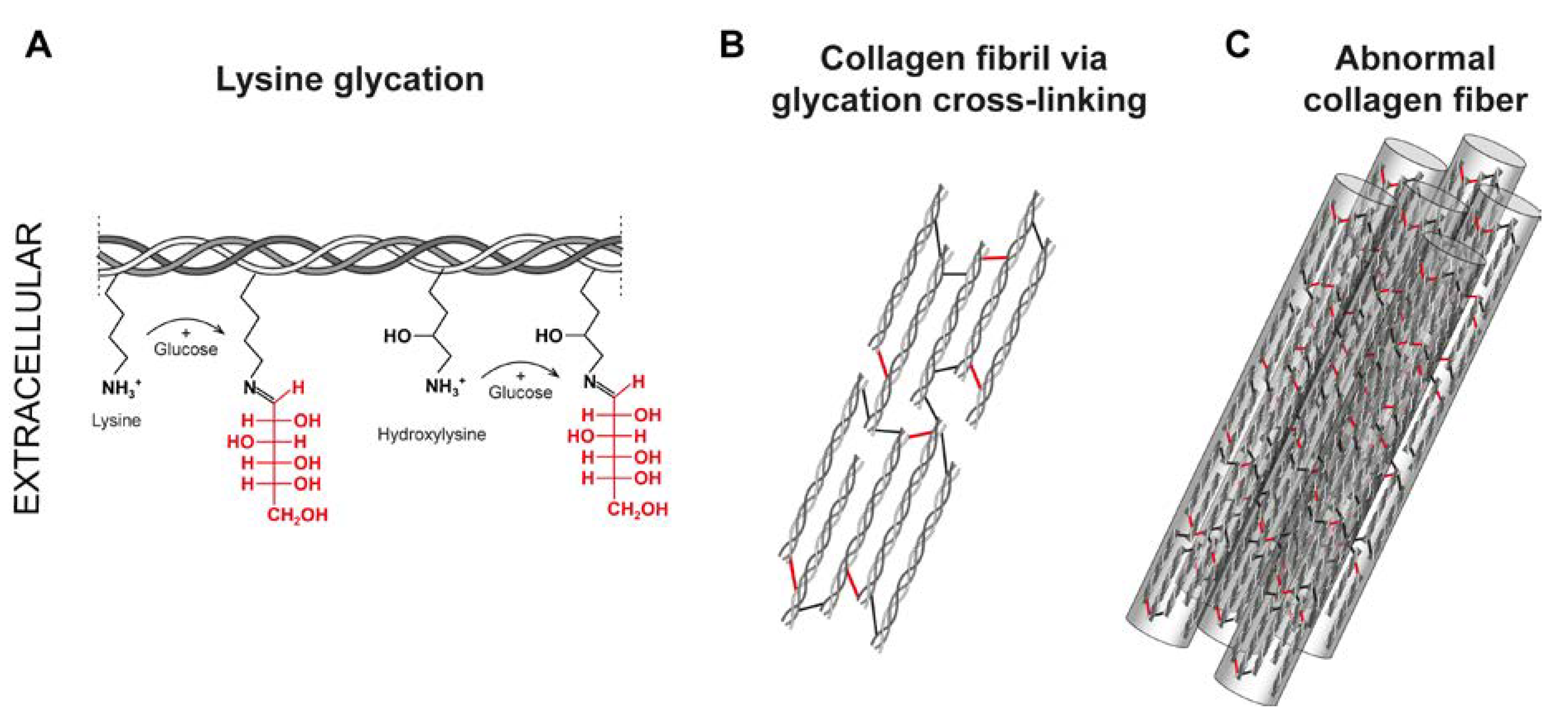
4. Non-Enzymatic Collagen Glycation
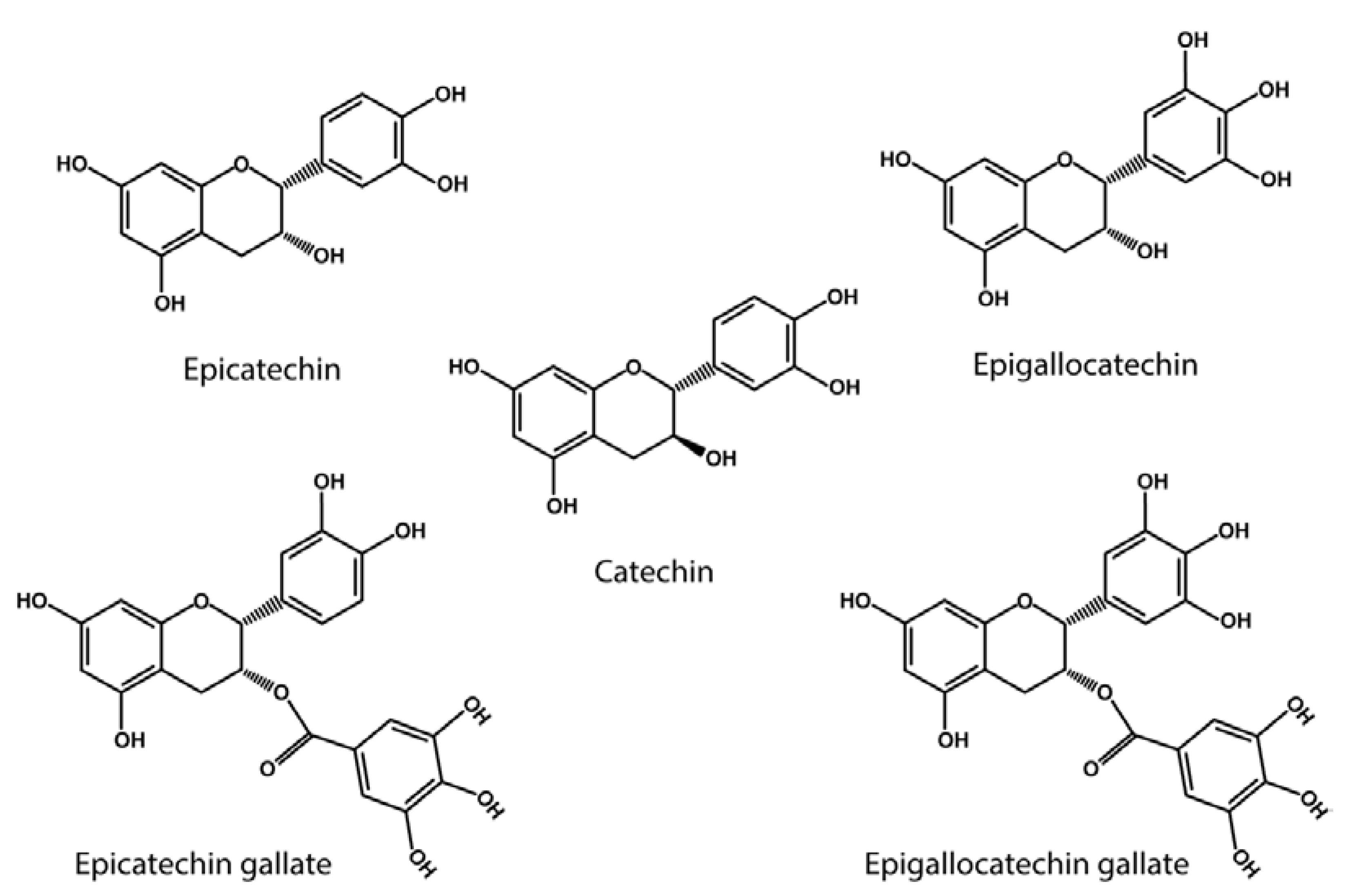
5. Lysyl Oxidase Activity of Polyphenols
6. Anti-Glycating Activity of Polyphenols
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Robins, S. Biochemistry and functional significance of collagen cross-linking. Biochem. Soc. Trans. 2007, 35, 849–852. [Google Scholar] [CrossRef]
- Heikkinen, J.; Risteli, M.; Wang, C.; Latvala, J.; Rossi, M.; Valtavaara, M.; Myllylä, R. Lysyl Hydroxylase 3 Is a Multifunctional Protein Possessing Collagen Glucosyltransferase Activity. J. Biol. Chem. 2000, 275, 36158–36163. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, M.; Deng, H.; Xu, Y. Heterogeneity in proline hydroxylation of fibrillar collagens observed by mass spectrometry. PLOS ONE 2021, 16, e0250544. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef]
- Lucero, H.A.; Kagan, H.M. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell. Mol. Life Sci. 2006, 63, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S., The collagen family. Cold Spring Harb Perspect Biol, 2011. 3(1): p. a004978.
- Siegel, R.C. Biosynthesis of Collagen Crosslinks: Increased Activity of Purified Lysyl Oxidase with Reconstituted Collagen Fibrils. Proc. Natl. Acad. Sci. 1974, 71, 4826–4830. [Google Scholar] [CrossRef]
- Trackman, P.C. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol. 2016, 52-54, 7–18. [Google Scholar] [CrossRef] [PubMed]
- van der Slot-Verhoeven, A.J., et al., The type of collagen cross-link determines the reversibility of experimental skin fibrosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2005. 1740(1): p. 60-67.
- Nagan, N.; Kagan, H. Modulation of lysyl oxidase activity toward peptidyl lysine by vicinal dicarboxylic amino acid residues. Implications for collagen cross-linking. J. Biol. Chem. 1994, 269, 22366–22371. [Google Scholar] [CrossRef]
- Finney, J., et al., Human copper-dependent amine oxidases. Archives of Biochemistry and Biophysics, 2014. 546(0): p. 19-32.
- Hudson, D.M.; Archer, M.; Rai, J.; Weis, M.; Fernandes, R.J.; Eyre, D.R. Age-related type I collagen modifications reveal tissue-defining differences between ligament and tendon. Matrix Biol. Plus 2021, 12, 100070. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.M.; Archer, M.; King, K.B.; Eyre, D.R. Glycation of type I collagen selectively targets the same helical domain lysine sites as lysyl oxidase–mediated cross-linking. J. Biol. Chem. 2018, 293, 15620–15627. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhang, J.-Q.; Li, L.; Guo, M.-M.; He, Y.-F.; Dong, Y.-M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef]
- Imran, M., et al., Health Benefits of Grapes Polyphenols. J. Environ. Agric. Sci, 2017. 10: p. 40-51.
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Shishido, S.; Miyano, R.; Nakashima, T.; Matsuo, H.; Iwatsuki, M.; Nakamura, K.; Kanno, T.; Egusa, H.; Niwano, Y. A novel pathway for the photooxidation of catechin in relation to its prooxidative activity. Sci. Rep. 2018, 8, 12888. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Itakura, M.; Kitazawa, R.; Lim, S.-Y.; Nagata, K.; Shibata, T.; Akagawa, M.; Uchida, K. Oxidative deamination of lysine residues by polyphenols generates an equilibrium of aldehyde and 2-piperidinol products. J. Biol. Chem. 2021, 297, 101035. [Google Scholar] [CrossRef]
- Akagawa, M.; Suyama, K. Amine oxidase-like activity of polyphenols. JBIC J. Biol. Inorg. Chem. 2001, 268, 1953–1963. [Google Scholar] [CrossRef]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Oxidative Deamination of Benzylamine and Lysine Residue in Bovine Serum Albumin by Green Tea, Black Tea, and Coffee. J. Agric. Food Chem. 2005, 53, 8019–8024. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L., Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutrition Reviews, 2009. 56(11): p. 317-333.
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Anazco, C.; Rojas, A.; Gonzalez, I.; Castro, M.A.; Robert, P.; Oyarzun-Ampuero, F. Dermal Collagen Stabilization by Polyphenols and Spray Drying as an Encapsulation Strategy. Curr. Top. Med. Chem. 2018, 18, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Hanauske-Abel, H.M., et al., Pyrroloquinoline quinone and molecules mimicking its functional domains Modulators of connective tissue formation? FEBS Letters, 1987. 214(2): p. 236-243.
- DiSilvestro, R.A.; Harris, E.D. Evaluation of (+)-catechin action on lysyl oxidase activity in aortic tissue. Biochem. Pharmacol. 1983, 32, 343–346. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2019, 129, 108843. [Google Scholar] [CrossRef]
- Odjakova, M., et al., Plant-derived agents with anti-glycation activity. Glycosylation, 2012. 10: p. 48186.
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef]
- Gordon, M.K. and R.A. Hahn, Collagens. Cell and Tissue Research, 2010. 339(1): p. 247-257.
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens – an ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Ramachandran, G. and G. Kartha, Structure of collagen. Nature, 1955. 176: p. 593-595.
- Zhang, X.; Xu, S.; Shen, L.; Li, G. Factors affecting thermal stability of collagen from the aspects of extraction, processing and modification. J. Leather Sci. Eng. 2020, 2, 1–29. [Google Scholar] [CrossRef]
- Reiser, K.; Amigable, M.; Last, J. Nonenzymatic glycation of type I collagen. The effects of aging on preferential glycation sites. J. Biol. Chem. 1992, 267, 24207–24216. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Redaelli, A.; Buehler, M.J.; Vesentini, S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: Candidate amino acids involved in Advanced Glycation End-products. Matrix Biol. 2014, 34, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Eekhoff, J.D.; Fang, F.; Lake, S.P. Multiscale mechanical effects of native collagen cross-linking in tendon. Connect. Tissue Res. 2018, 59, 410–422. [Google Scholar] [CrossRef]
- Sricholpech, M., et al., Lysyl hydroxylase 3-mediated glucosylation in type I collagen: molecular loci and biological significance. The Journal of biological chemistry, 2012. 287(27): p. 22998-23009.
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; A Perez-Romero, B.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; MacBarb, R.F.; Responte, D.J.; Hu, J.C.; Athanasiou, K.A. A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. FASEB J. 2013, 27, 2421–2430. [Google Scholar] [CrossRef]
- Knott, L.; Bailey, A. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone 1998, 22, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Reiser, K.; McCormick, R.J.; Rucker, R.B. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992, 6, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Fong, K.; He, Q.; Hayashi, K.; Kim, Y.; Fong, S.; Fogelgren, B.; Szauter, K.M.; Mink, M.; Csiszar, K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim. et Biophys. Acta (BBA) - Proteins Proteom. 2003, 1647, 220–224. [Google Scholar] [CrossRef]
- Trackman, P.; Kagan, H. Nonpeptidyl amine inhibitors are substrates of lysyl oxidase. J. Biol. Chem. 1979, 254, 7831–7836. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Liburkin-Dan, T.; Toledano, S.; Neufeld, G. Lysyl Oxidase Family Enzymes and Their Role in Tumor Progression. Int. J. Mol. Sci. 2022, 23, 6249. [Google Scholar] [CrossRef]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [CrossRef]
- Gacheru, S.; Trackman, P.; Shah, M.; O'Gara, C.; Spacciapoli, P.; Greenaway, F.; Kagan, H. Structural and catalytic properties of copper in lysyl oxidase. J. Biol. Chem. 1990, 265, 19022–19027. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Poe, A.; Yus, M.M.; Pak, L.; Nandakumar, K.; Santhanam, L. Lysyl oxidase-like 2 processing by factor Xa modulates its activity and substrate preference. Commun. Biol. 2023, 6, 1–12. [Google Scholar] [CrossRef]
- Añazco, C.; Lopez-Jimenez, A.J.; Rafi, M.; Vega-Montoto, L.; Zhang, M.Z.; Hudson, B.G.; Vanacore, R.M. Lysyl Oxidase-like-2 Cross-links Collagen IV of Glomerular Basement Membrane. J. Biol. Chem. 2016, 291, 25999–26012. [Google Scholar] [CrossRef] [PubMed]
- Bignon, M.; Pichol-Thievend, C.; Hardouin, J.; Malbouyres, M.; Bréchot, N.; Nasciutti, L.; Barret, A.; Teillon, J.; Guillon, E.; Etienne, E.; et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 2011, 118, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Busnadiego, O.; González-Santamaría, J.; Lagares, D.; Guinea-Viniegra, J.; Pichol-Thievend, C.; Muller, L.; Rodríguez-Pascual, F. LOXL4 Is Induced by Transforming Growth Factor β1 through Smad and JunB/Fra2 and Contributes to Vascular Matrix Remodeling. Mol. Cell. Biol. 2013, 33, 2388–2401. [Google Scholar] [CrossRef]
- Aronoff, M.R.; Hiebert, P.; Hentzen, N.B.; Werner, S.; Wennemers, H. Imaging and targeting LOX-mediated tissue remodeling with a reactive collagen peptide. Nat. Chem. Biol. 2021, 17, 865–871. [Google Scholar] [CrossRef]
- Moon, H.-J.; Finney, J.; Xu, L.; Moore, D.; Welch, D.R.; Mure, M. MCF-7 Cells Expressing Nuclear Associated Lysyl Oxidase-like 2 (LOXL2) Exhibit an Epithelial-to-Mesenchymal Transition (EMT) Phenotype and Are Highly Invasive in Vitro. J. Biol. Chem. 2013, 288, 30000–30008. [Google Scholar] [CrossRef]
- Añazco, C.; Delgado-López, F.; Araya, P.; González, I.; Morales, E.; Pérez-Castro, R.; Romero, J.; Rojas, A.; Tecer, D.; Sezgin, M.; et al. Lysyl oxidase isoforms in gastric cancer. Biomarkers Med. 2016, 10, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Añazco, C.; Cerro, S.; Pereira, N.; Rojas, C.; Torres. ; Vidal-Beltrán, I. Dysregulation of Lysyl Oxidases Expression in Diabetic Nephropathy and Renal Cell Carcinoma. Curr. Drug Targets 2021, 22, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.H., The glomerular basement membrane. Experimental Cell Research, 2012. 318(9): p. 973-978.
- Di Donato, A.; Ghiggeri, G.M.; Di Duca, M.; Jivotenko, E.; Acinni, R.; Campolo, J.; Ginevri, F.; Gusmano, R. Lysyl Oxidase Expression and Collagen Cross-Linking during Chronic Adriamycin Nephropathy. Nephron 1997, 76, 192–200. [Google Scholar] [CrossRef]
- Neusser, M.A.; Lindenmeyer, M.T.; Moll, A.G.; Segerer, S.; Edenhofer, I.; Sen, K.; Stiehl, D.P.; Kretzler, M.; Gröne, H.-J.; Schlöndorff, D.; et al. Human Nephrosclerosis Triggers a Hypoxia-Related Glomerulopathy. Am. J. Pathol. 2010, 176, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Li, Q.; Wong, W.R.; N’diaye, E.-N.; Caplazi, P.; Bender, H.; Huang, Z.; Arlantico, A.; Jeet, S.; Wong, A.; et al. LOXL4, but not LOXL2, is the critical determinant of pathological collagen cross-linking and fibrosis in the lung. Sci. Adv. 2023, 9, eadf0133. [Google Scholar] [CrossRef]
- Setargew, Y.F.; Wyllie, K.; Grant, R.D.; Chitty, J.L.; Cox, T.R. Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours. Cancers 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Grau-Bové, X.; Ruiz-Trillo, I.; Rodriguez-Pascual, F. Origin and evolution of lysyl oxidases. Sci. Rep. 2015, 5, 10568. [Google Scholar] [CrossRef]
- Bollinger, J.A.; Brown, D.E.; Dooley, D.M. The Formation of Lysine Tyrosylquinone (LTQ) Is a Self-Processing Reaction. Expression and Characterization of a Drosophila Lysyl Oxidase. Biochemistry 2005, 44, 11708–11714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Wu, J.; Wang, J.; Shi, Y.; Liu, M. Crystal structure of human lysyl oxidase-like 2 (hLOXL2) in a precursor state. Proc. Natl. Acad. Sci. 2018, 115, 3828–3833. [Google Scholar] [CrossRef]
- Shanbhag, V.; Jasmer-McDonald, K.; Zhu, S.; Martin, A.L.; Gudekar, N.; Khan, A.; Ladomersky, E.; Singh, K.; Weisman, G.A.; Petris, M.J. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 6836–6841. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Guéroult, M.; Belloy, N.; Dauchez, M.; Ricard-Blum, S. A Three-Dimensional Model of Human Lysyl Oxidase, a Cross-Linking Enzyme. ACS Omega 2019, 4, 8495–8505. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.A., K. Kuczera, and M. Mure, A 3D–Predicted Structure of the Amine Oxidase Domain of Lysyl Oxidase–Like 2. International Journal of Molecular Sciences, 2022. 23(21): p. 13385.
- Meier, A.A.; Moon, H.-J.; Sabuncu, S.; Singh, P.; Ronnebaum, T.A.; Ou, S.; Douglas, J.T.; Jackson, T.A.; Moënne-Loccoz, P.; Mure, M. Insight into the Spatial Arrangement of the Lysine Tyrosylquinone and Cu2+ in the Active Site of Lysyl Oxidase-like 2. Int. J. Mol. Sci. 2022, 23, 13966. [Google Scholar] [CrossRef]
- Mure, M. Tyrosine-Derived Quinone Cofactors. Accounts Chem. Res. 2004, 37, 131–139. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Moon, H.-J.; Finney, J.; Meier, A.; Mure, M. Extracellular Processing of Lysyl Oxidase-like 2 and Its Effect on Amine Oxidase Activity. Biochemistry 2018, 57, 6973–6983. [Google Scholar] [CrossRef]
- Xu, L.; Go, E.P.; Finney, J.; Moon, H.; Lantz, M.; Rebecchi, K.; Desaire, H.; Mure, M. Post-translational Modifications of Recombinant Human Lysyl Oxidase-like 2 (rhLOXL2) Secreted from Drosophila S2 Cells*. J. Biol. Chem. 2013, 288, 5357–5363. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Monnier, V.M. Structure Elucidation of a Senescence Cross-Link from Human Extracellular Matrix. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Weis, M.A.; Wu, J.-J. Maturation of Collagen Ketoimine Cross-links by an Alternative Mechanism to Pyridinoline Formation in Cartilage. J. Biol. Chem. 2010, 285, 16675–16682. [Google Scholar] [CrossRef]
- Hanson, D.A.; Eyre, D.R. Molecular Site Specificity of Pyridinoline and Pyrrole Cross-links in Type I Collagen of Human Bone. J. Biol. Chem. 1996, 271, 26508–26516. [Google Scholar] [CrossRef]
- Eyre, D.R.; Weis, M.; Rai, J. Analyses of lysine aldehyde cross-linking in collagen reveal that the mature cross-link histidinohydroxylysinonorleucine is an artifact. J. Biol. Chem. 2019, 294, 6578–6590. [Google Scholar] [CrossRef]
- Teuscher, A.C., et al., Assessing collagen deposition during aging in mammalian tissue and in Caenorhabditis elegans, in Collagen. 2019, Springer. p. 169-188.
- Fenske, N.A.; Lober, C.W. Structural and functional changes of normal aging skin. J. Am. Acad. Dermatol. 1986, 15, 571–585. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Reiser, K.M., Nonenzymatic glycation of collagen in aging and diabetes. Proceedings of the Society for Experimental Biology and Medicine, 1998. 218(1): p. 23-37.
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R. and A. Cerami, Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv pharmacol, 1992. 23(1-34): p. 13.
- Rojas, A.; Morales, M.A. Advanced glycation and endothelial functions: A link towards vascular complications in diabetes. Life Sci. 2004, 76, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Fournet, M., F. Bonté, and A. Desmoulière, Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis, 2018. 9(5): p. 880-900.
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Meade, S.J.; Miller, A.G.; A Gerrard, J. The role of dicarbonyl compounds in non-enzymatic crosslinking: a structure–activity study. Bioorganic Med. Chem. 2003, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R. and V.M. Monnier, Aging of long-lived proteins: Extracellular matrix (collagens, elastins, proteoglycans) and lens crystallins. Comprehensive physiology, 2010: p. 235-305.
- Wautier, J.-L. and P.-J. Guillausseau, Diabetes, advanced glycation endproducts and vascular disease. Vascular Medicine, 1998. 3(2): p. 131-137.
- Nash, A.; Notou, M.; Lopez-Clavijo, A.F.; Bozec, L.; de Leeuw, N.H.; Birch, H.L. Glucosepane is associated with changes to structural and physical properties of collagen fibrils. Matrix Biol. Plus 2019, 4, 100013. [Google Scholar] [CrossRef]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Fessel, G.; Li, Y.; Diederich, V.; Guizar-Sicairos, M.; Schneider, P.; Sell, D.R.; Monnier, V.M.; Snedeker, J.G. Advanced Glycation End-Products Reduce Collagen Molecular Sliding to Affect Collagen Fibril Damage Mechanisms but Not Stiffness. PLOS ONE 2014, 9, e110948. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Añazco, C.; González, I.; Araya, P. Extracellular matrix glycation and receptor for advanced glycation end-products activation: a missing piece in the puzzle of the association between diabetes and cancer. Carcinog. 2018, 39, 515–521. [Google Scholar] [CrossRef]
- Sell, D.R., et al., Glucosepane is a major protein cross-link of the senescent human extracellular matrix: relationship with diabetes. Journal of Biological Chemistry, 2005. 280(13): p. 12310-12315.
- Vaez, M.; Asgari, M.; Hirvonen, L.; Bakir, G.; Khattignavong, E.; Ezzo, M.; Aguayo, S.; Schuh, C.M.; Gough, K.; Bozec, L. Modulation of the biophysical and biochemical properties of collagen by glycation for tissue engineering applications. Acta Biomater. 2023, 155, 182–198. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell, D.R.; Strauch, C.; Sun, W.; Lachin, J.M.; Cleary, P.A.; Genuth, S. The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J. Diabetes its Complicat. 2012, 27, 141–149. [Google Scholar] [CrossRef]
- Bravo, L., Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition reviews, 1998. 56(11): p. 317-333.
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Sciubba, F.; Capitani, D.; Di Cocco, M.E.; D’evoli, L.; Durazzo, A.; Delfini, M.; Boccia, G.L. Role of catechin on collagen type I stability upon oxidation: a NMR approach. Nat. Prod. Res. 2019, 34, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Nascimento, T.G.; Oliveira, J.M.S.; Freitas, P.H.; Haimeur, A.; França, R. Use of polyphenols as a strategy to prevent bond degradation in the dentin-resin interface. Eur. J. Oral Sci. 2018, 126, 146–158. [Google Scholar] [CrossRef]
- Nazaruk, J. and A. Galicka, The influence of selected flavonoids from the leaves of Cirsium palustre (L.) Scop. on collagen expression in human skin fibroblasts. Phytotherapy Research, 2014. 28(9): p. 1399-1405.
- Zhang, Y., et al., Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. European journal of histochemistry: EJH, 2015. 59(2).
- Bae, J.; Lim, S.S.; Kim, S.J.; Choi, J.; Park, J.; Ju, S.M.; Han, S.J.; Kang, I.; Kang, Y. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-B irradiation-induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009, 53, 726–738. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hatasa, Y.; Kawamura, S.; Shibata, T.; Akagawa, M.; Uchida, K. Identification of Polyphenol-Specific Innate Epitopes That Originated from a Resveratrol Analogue. Biochemistry 2017, 56, 4701–4712. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2013, 3, 94–108. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Bierhaus, A., et al., Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl), 2005. 83(11): p. 876-86.
- Verzijl, N.; DeGroot, J.; Ben Zaken, C.; Braun-Benjamin, O.; Maroudas, A.; Bank, R.A.; Mizrahi, J.; Schalkwijk, C.G.; Thorpe, S.R.; Baynes, J.W.; et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002, 46, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, H.Y.; Zuo, G.; Wang, Z.; Lee, J.-Y.; Lim, S.S. Anti-glycation, Carbonyl Trapping and Anti-inflammatory Activities of Chrysin Derivatives. Molecules 2018, 23, 1752. [Google Scholar] [CrossRef]
- Lv, L.; Shao, X.; Chen, H.; Ho, C.-T.; Sang, S. Genistein Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal. Chem. Res. Toxicol. 2011, 24, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.-Y.; Yang, C.S.; Ho, C.-T. Tea Polyphenol (−)-Epigallocatechin-3-Gallate: A New Trapping Agent of Reactive Dicarbonyl Species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In Vitro Antioxidant and Anti-Glycation Activity of Resveratrol and Its Novel Triester with Trolox. Antioxidants 2020, 10, 12. [Google Scholar] [CrossRef]
- Lee, S.M.; Zheng, L.W.; Jung, Y.; Hwang, G.-S.; Kim, Y.-S. Effects of hydroxycinnamic acids on the reduction of furan and α-dicarbonyl compounds. Food Chem. 2019, 312, 126085. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Yang, X.; Zhu, L.; Wu, G.; Qi, X.; Zhang, H. Trapping of reactive carbonyl species by fiber-bound polyphenols from whole grains under simulated physiological conditions. Food Res. Int. 2022, 156, 111142. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J., et al., Basic Structure, Physiology, and Biochemistry of Connective Tissues and Extracellular Matrix Collagens. Adv Exp Med Biol, 2021. 1348: p. 5-43.
- Wu, L.; Shao, H.; Fang, Z.; Zhao, Y.; Cao, C.Y.; Li, Q. Mechanism and Effects of Polyphenol Derivatives for Modifying Collagen. ACS Biomater. Sci. Eng. 2019, 5, 4272–4284. [Google Scholar] [CrossRef] [PubMed]
| Immature cross-links | Mature cross-links | Glycation derived cross-links |
|---|---|---|
|
Aldimine cross-links 1. Dehydro-lysinonorleucine (deH-NL) 2. Dehydro-hydroxylysinonorleucine (deH-HLNL) 3. Dehydro-dihydroxylysinonorleucine (deH-DHLNL) |
Pyrrole containing cross-links 1. Deoxypyrrololine (d-Prl) 2. Pyrrololine (Prl) Pyridinium-salt containing cross-links 1. Pyridinoline (Pyr) 2. Deoxypyridinoline (Dpyr) |
Alpha-dicarbonyl compounds (α-DC) 1. Methylglyoxal (MGO) 2. Glyoxal (GO) |
|
Ketoamine cross-links 1. Lysine-keto-norleucine (LKNL) 2. Hydroxylysine-keto-norleucine (HLKNL) |
Histidine containing cross-links 1. Histidinohydroxylysinonorleucine (HHL) 2. Histidinohydroxymerodesmosine (HHMD) Arginoline Ketoimine cross-link derivate (Eyre et al. 2010) |
Fluorescent cross-linking AGEs 1. Pentosidine (PEN) Non-Fluorescent cross-linking AGEs 1. Carboxymethyl-lysine (CML) 2. Glucosepane (GSP) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





