1. Introduction
The excessive use of pesticides in agro-industrial activities (agriculture, veterinary) has caused them to be increasingly found in wastewater and natural water bodies. The wide variety of pesticides (herbicides, fungicides, acaricides, etc.) found on the market makes it difficult to define a global strategy conducive to their removal from liquid effluents.
Among the most used methods for the removal of pesticides, from the aqueous phase, adsorption on a diversity of adsorbents, particularly on activated carbon (ACs) was highlighted, in reviews made by Kulaishin et. al in 2022 [
1] and Baskar et. al, 2022 [
2]. Some disadvantages concerning other methods currently used were identified, such as time consumption and dependency on environmental conditions for the biodegradation process, energy consumption for electrooxidation, and photocatalytic process [
1].
Adsorption is a process with simple implementation and operation, being therefore widely used, for the removal of inorganic and organic contaminants from gaseous [
3,
4] or liquid effluents, such as drinking and wastewater [
2]. However, adsorption present also has some limitations, such as adsorbent production cost, regeneration, and sustainable management of spent adsorbents [
1,
2,
3,
4,
5].
Commercial ACs have been traditionally obtained from coal, petroleum residues, lignite, coconut shells, and wood, all of which are expensive. To lower the use of nonrenewable precursors and ACs cost, the use of alternative precursors, including agricultural and agro-industrial wastes, was tested. ACs obtained from a diversity of precursors have been performed on pollutant removals from the liquid phase, such as the removal of pesticides (4-chloro-2-methylphenoxyacetic acid-MCPA and diuron) on ACs prepared from Tectona Grandis [
6,
7], dyes on ACs prepared different biomass, activated with KOH, NaOH, and H
2SO
4 [
8,
9], Cu
2+, Zn
2+ and Pb
2+ on ACs prepared from Phaseolus aureus hulls, activated with steam [
10], As
3+ on granulated biochar [
11] and pharmaceutical products, on ACs prepared from waste materials and wood [
12,
13].
Adsorption in ACs is widely applied because of its efficiency, high adsorption capacity, and easy applicability on a large scale. Still, it presents some disadvantages. If not treated adequately, the saturated adsorbents can result in secondary pollution. The adsorbates retained on the ACs surfaces can be leached into the environment where the saturated adsorbents are dumped [
2,
3]. An adequate management policy, of saturated adsorbents, must include their regeneration and reuse in new processes.
Thermal, chemical, and biological methods are most commonly applied for the regeneration of spent adsorbents [
2,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. In the industry, thermal regeneration consists of passing, through the saturated adsorbent, a flow of steam or carbon dioxide, at high temperatures (higher than 1000 K). This procedure allows the desorption of pollutants, but also the unlocking, enlargement, and creation of new pores, favouring an increase in the porous volume. Concerning phenol desorption, thermal regeneration was described as needing an important energy demand and the presence of an oxidative agent to be efficient, which induces a burn-off of the adsorbent varying from 5 to 15% [
21].
Chemical regeneration can be performed using oxidizing chemical agents or through the desorption of adsorbates, by washing the adsorbents, using specific solvents or acid or basic solutions [
16]. The performance of this method depends on the interactions between adsorbate-adsorbent and between adsorbate and the regeneration solvents and solutions used.
Bioregeneration is a simple and eco-friendly process, that needs a low investment to be implemented. For being effective, adsorbates must be biodegradable, which does not happen often, making the process very time-consuming [
2,
19].
Recently, many researchers have investigated the use of microwaves to reduce regeneration time and energy consumption [
4]. A combination of microwave and ultraviolet irradiation was used to regenerate ACs saturated with chloramphenicol. The authors reported that the adsorption performance of granular AC was maintained at a high level, even after five absorption/regeneration cycles [
3]. Nevertheless, it was also noted that the by-products generated during the microwave regeneration process can be more dangerous than the initial pollutants, particularly, if the organic pollutants contain chlorine [
4].
The regeneration of saturated ACs was also tested under a vacuum system, consisting of exposing the spent ACs to a vacuum source. ACs saturated, with toluene, have been regenerated under airflow and the authors reported that after five adsorption/desorption cycles, a performance of 90% was maintained [
22]. Except for the thermal regeneration technique, these techniques are not currently used in the industry [
18].
Regenerated ACs are less expensive when compared with the original ones. However, some characteristics lead to inferior performance when compared to the original ACs. The regenerated ACs present more ash content, some pollutants can be retained, and finally, regenerated ACs become saturated more rapidly.
In this study, the adsorption or desorption kinetics of MCPA will be investigated with an emphasis on the regeneration and reuse of spent ACs. Regeneration will be done through thermal regeneration, washing regeneration, using different solutions and solvents, and a combination of washing and thermal regeneration.
2. Materials and Methods
Two commercial activated carbons were used as received, and after being submitted to different regeneration procedures, on the MCPA removal from the aqueous phase.
2.1. ACs structural and chemical characterization
Both ACs used, one from Merck and the other from Norit (Norit 1240 X, in a powder and granular form), were texturally and chemically characterized, by nitrogen adsorption, at 77 K, elemental analysis, and through the determination of the pH at the point of zero charge. The procedures used for these purposes are described elsewhere [
7]. The surface of the ACs was also analyzed by ATR-FTIR spectroscopy analysis measurements, in the range between 450 and 4000 cm−1, performed on a PerkinElmer Spectrum, Two IR spectrophotometer with an Attenuated Total Reflection (ATR) accessory. Phenom ProX Desktop Scanning Electron Microscope (SEM) instrument was used on the morphology characterization, with an accelerating voltage set to 5 to 15 kV.
2.2. Pesticide adsorption from liquid-phase
The adsorptive used in the adsorption studies was 4-chloro-2-methylphenoxyacetic acid (MCPA) purchased from Sigma - Aldrich with a reported purity of HPLC-Grade higher than 97.4% and used as received. The kinetics studies were carried out at 298 K, through the addition of 10 mg of ACs to 25 mL of an aqueous solution containing 150 mg L-1 of MCPA, at a pH of around 3. At different time intervals, aliquots were removed from the solution and the amount of MCPA present was determined.
After an adequate contact time, the residual concentration, of the pesticide on the solution was assessed by UV/visible spectrophotometry, using a Nicolet Evolution 300 UV-Vis Spectrophotometer. The quantification was done through the absorbance values obtained at the wavelength of 228 and 280 nm for MCPA. The residual pesticide was quantified by employing an external pattern, i.e. using a standard curve prepared for this purpose. The amount of pesticide adsorbed (Qads / mg g −1) on the ACs was assessed using the difference between the initial concentration of pesticide and the remaining concentration in solution, divided by the mass of AC used and considering the volume of solution used.
2.3. ACs regeneration
To perform regeneration studies, the ACs have been first saturated with MCPA. For that purpose, a fixed quantity of ACs (100 mg) was added to 100 mL of an aqueous solution containing 250 mg L-1 of MCPA. After the adsorption equilibrium was reached, the ACs maximum adsorption capacity was evaluated. These assays were done multiple times which allowed the calculation of standard deviation and error. The supernatant was then removed and different desorption experiments were performed. For this purpose, six different treatments were applied: (1) acid aqueous washing with HNO3 – 1 mol dm-3 (2) basic aqueous washing with NaOH (0.01, 0.1, and 1.0 mol dm-3) (3) washing with distillate water (4) washing with ethanol (5) thermal desorption at 873 K, for 30 minutes (6) two-step treatment, washing with aqueous solutions and ethanol and heating at 573 K, for 30 minutes. The chemical characteristics of the spent and the regenerated ACs were obtained and correlated with their performance.
2.3.1. ACs regeneration by washing with solvents
Once dried, the spent ACs were placed in a volume of 100 mL of ethanol or 100 mL of each of the tested washing solutions. The solutions used were the nitric acid solution, (1 mol dm-3 - pH around 1, ethanol, distilled water (pH = 6), and sodium hydroxide solutions, (0.001 0.1 and 1 mol dm-3). The suspensions were then put in a thermostable bath under the agitation of 70 rpm, for 24 h. The supernatant was removed and the desorption washing was repeated three times. After each cycle, the concentration of the pesticide, in the supernatant, was quantified and the total amount of pesticide desorbed was calculated. The regenerated ACs were reused in a new adsorption cycle and their performance in the removal of MCPA from the aqueous phase was evaluated.
2.3.2. Thermal regeneration of spent ACs
About 100 mg of the spent AC were placed in a steel container and put into a furnace, High Temp Technology, TR–334/2018, from Thermolab, where it heated at a rate of 10 K min−1, until 873 K, under a nitrogen flow rate of 85 cm3 min−1, and kept for 30 min. The carbonaceous material was cooled down, always under the same nitrogen flow. The regenerated ACs were reused and their performance was evaluated on MCPA removal from the aqueous phase.
2.3.3. ACs regeneration by basic aqueous washing followed by thermal treatment
The spent AC, after three adsorption-desorption cycles, as described in point 2.3.1, was recovered after the supernatant removal. Then, about 100 mg of the ACs, washed with different solvents were placed in a steel container and put into a furnace, High Temp Technology, TR–334/2018, from Thermolab. ACs were then submitted to a rate of 10 K min−1, until 573 K, under a nitrogen flow rate of 85 cm3 min−1, and kept for 30 min. The carbonaceous material was cooled down, always under the same nitrogen flow. The regenerated ACs were reused and their performance was evaluated on MCPA removal from the aqueous phase.
3. Results
3.1. Characterization of the adsorbent materials
Both commercial ACs, Merck and Norit, were texturally characterized based on N2 adsorption at 77 K, and chemically characterized based on the determination of the point of zero charge, and elemental analysis [
24]. To facilitate the discussion, the results are presented in
Table 1.
Norit 1240 X was characterized through nitrogen adsorption in a granular form. As seen in
Table 1, it presents a slightly high apparent surface area and total porous volume, with a similar micropore volume and means pore size when compared with the AC from Merck. Based on these results, it is possible to predict that textural properties will not have a determinate role in the performance of the ACs against MCPA adsorption.
On the other hand, Norit 1240 X presents a more basic character (pHpzc = 9.73) when compared with Merck AC (pHpzc = 7.27), which may have some influence on the process of removing pollutants, from the aqueous phase. The ACs surface, as received and after being saturated with MCPA and regenerated under different procedures, was analyzed by ATR-FTIR spectroscopy analysis, and by SEM-EDX.
3.2. Chemical characterization of the adsorbent materials
To verify the influence of the MCPA adsorption and regenerations treatments, on the ACs surface, these were characterized by SEM-EDX and FTIR.
Figure 1a,b illustrate the surface morphology of ACs before MCPA adsorption, c) illustrated the AC surface covered by an MCPA white pellicule, and d) show the AC surface after being saturated and regenerated by washing method followed by a thermal treatment.
The presence of an MCPA film covers the ACs surface and hinders their adsorption activity due to the blockage of the pores. Even though it is possible to see that the cavities were not completely blocked,
Figure 1c. The thermal treatment allows the desorption of the MCPA from the AC surface, as shown in
Figure 1d).
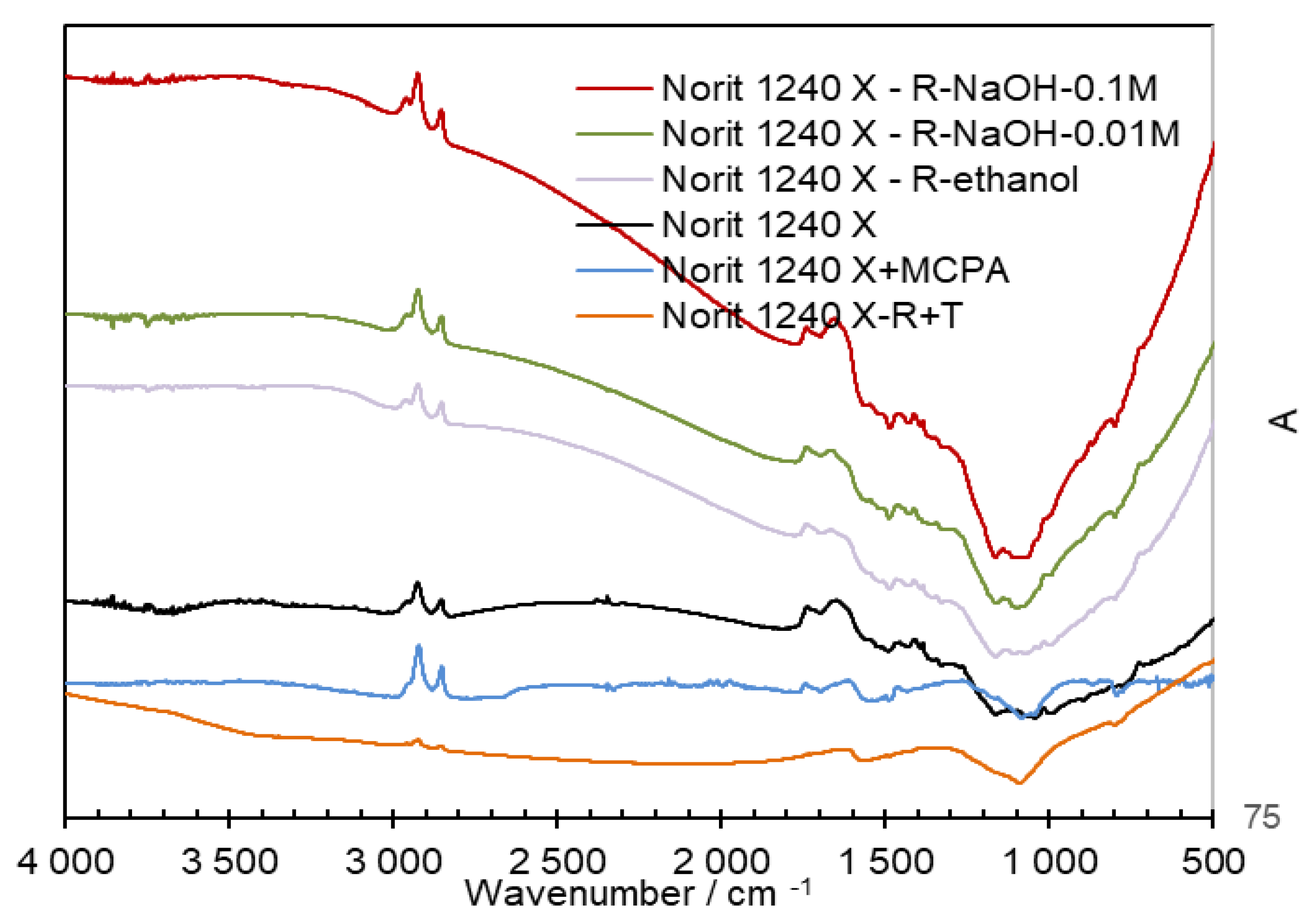
Information about the surface functional groups on different steps in the adsorption-desorption cycle was obtained by FT-IR spectroscopy (as shown in
Figure 2). The spectra presented are from Norit AC, as the Merck samples have more similar spectra. In
Figure 2, the broader band from 3020 to 3300 cm
-1 was related to the O-H group, on an alcohol group. The interferences of the O-H group were identified around 1420 cm
-1 and at 880 cm
-1, which reflected the contribution of O-H in deformation mode. These two bands were less expressed on the AC regenerated with a NaOH solution followed by the thermal regeneration (Norit 1240 X -RT). The decrease in the intensity of the bands can be attributed to the decomposition of the functional groups containing oxygen at high temperatures, such as reported by Liao et. al [
25]. The bands at 2945 and 2889 cm
-1 are characteristics of the alkyl groups, namely CH
3 and CH
2. The band at 790 cm
-1 allows the identification of the C-H groups on the alkenes structures, on a benzene structure. The bands at 1560 and 1680 cm
-1 could be due to the C=C in a cyclic structure. The band at 1760 cm
-1 could be attributed to the C=O bond on a carboxylic or ester group. This band was less present on the AC regenerated with NaOH solution followed by a thermal treatment. The bands present at 1089 and 1200 cm
-1 could be due to the C-O bond on primary and secondary alcohol, respectively. The presence of bands characteristic to the MCPA, on the saturated AC surface, was not identified.
3.3. MCPA kinetic studies
The adsorption capacity of ACs for different pollutants, in the aqueous phase, depends on numerous factors, and that complexity represents a challenge for researchers. Some of the parameters influencing the removal of MCPA, by ACs, from the aqueous phase were previously optimized [
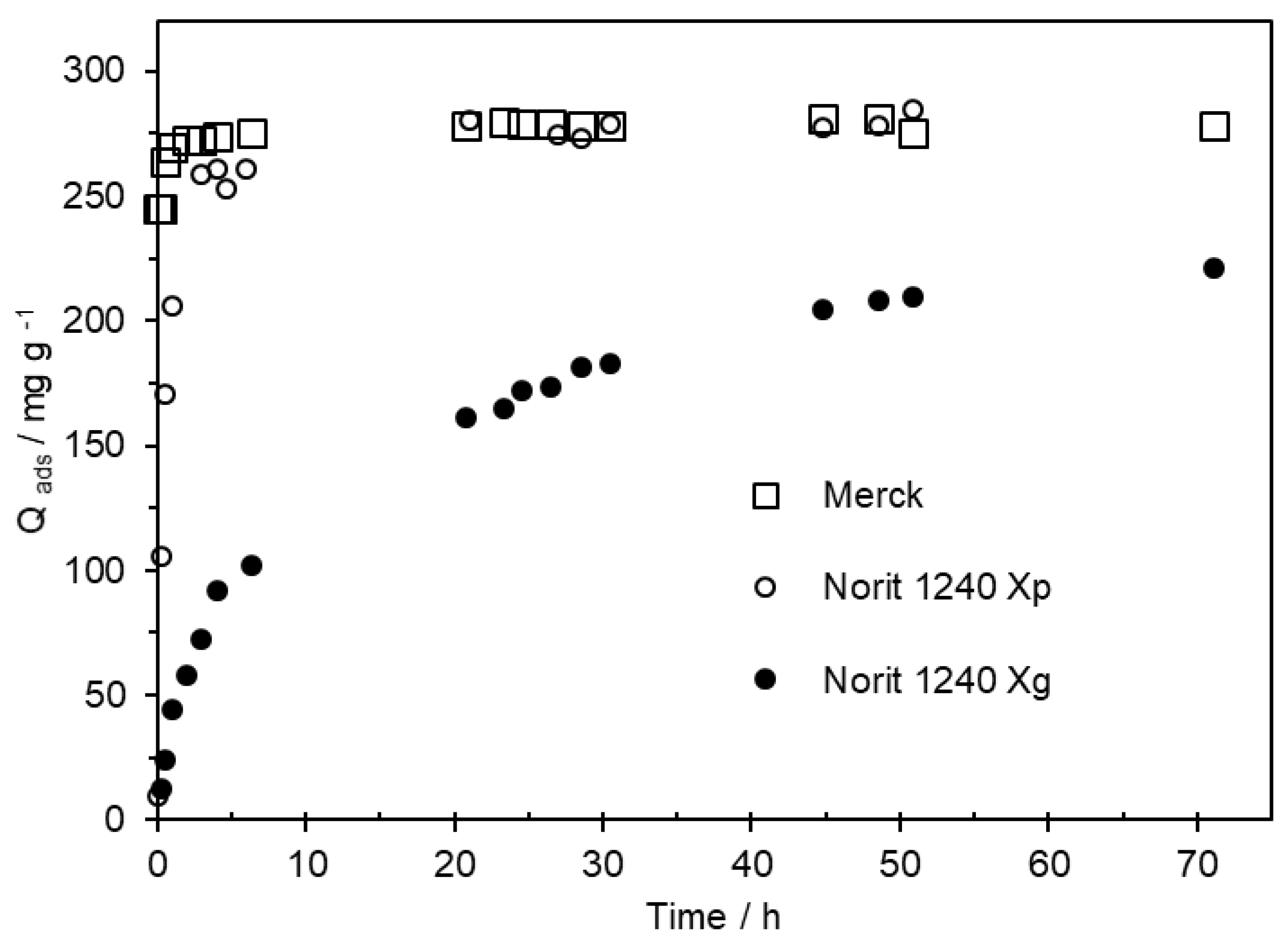
7]. Based on these conditions, the MCPA adsorption was done at pH = 3. The time needed to establish the adsorption equilibrium was evaluated on Merck and Norit 1240 X ACs. The Norit AC was used in a granular (Norit 1240 X
g) and powder form (Norit 1240 X
p), as results shown in
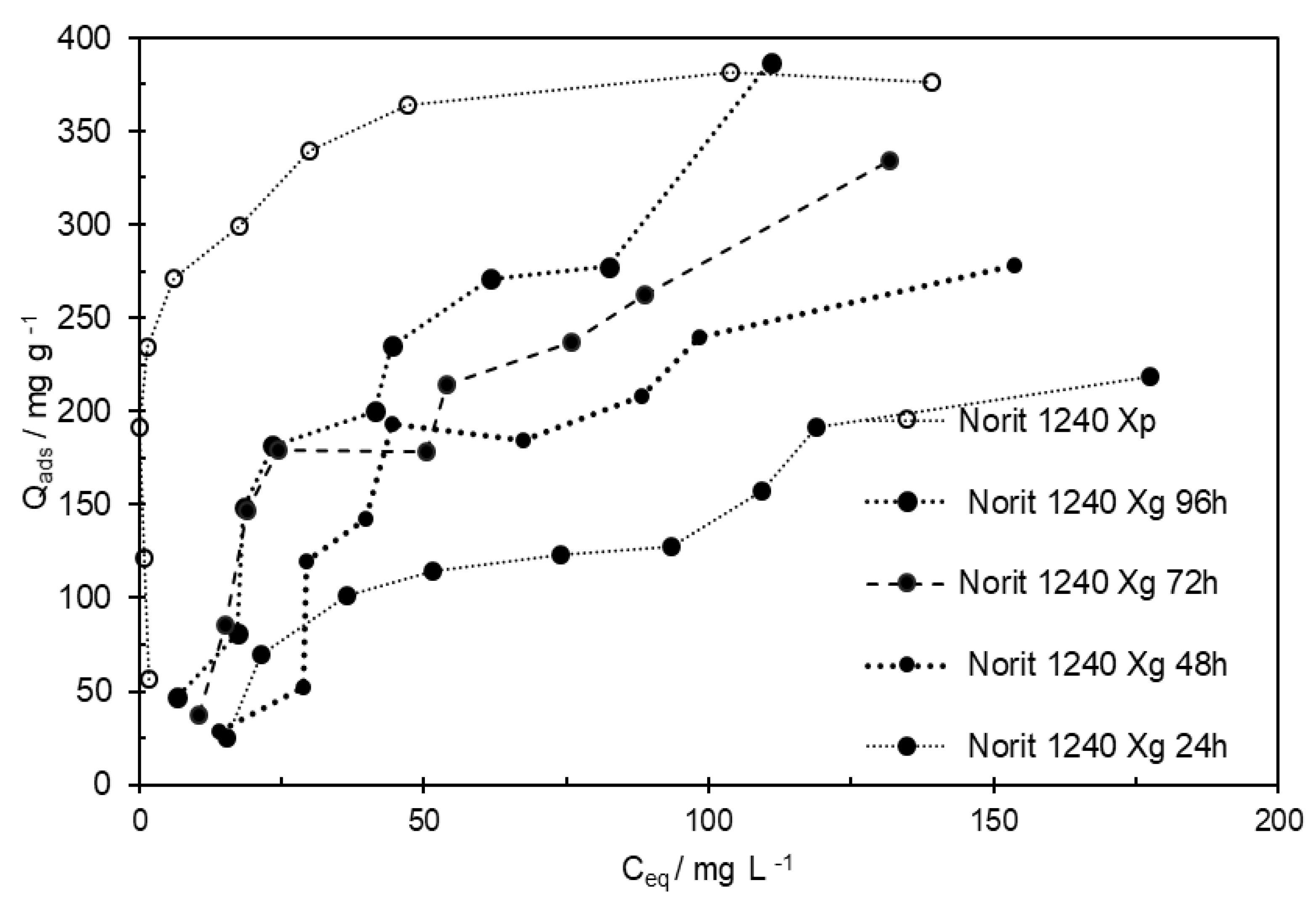
Figure 3.
On Merck and Norit 1240Xp, the equilibrium of the MCPA adsorption process was achieved after a contact time of less than 6 h. But, with Norit 1240Xg, the maximum adsorption capacity was not completely reached, even after a contact time of 72 hours.
To evaluate the mechanisms of the adsorption kinetics, the experimental data were fitted by the pseudo-first-order and pseudo-second-order kinetic models. The pseudo-first-order model can be expressed by equation 1.
The pseudo-second-order model can be expressed by equation 2.
The slope of equation 2 was related to the initial rate of the adsorption using the following expression: 1 / (K2 * (Qmax)2) = 1/V0 ;
On equations 1 and 2, Q
max and Q
ads are the MCPA amounts adsorbed at the equilibrium and at a time (t) (mg g
-1); K
1 – is the equilibrium rate constant of pseudo-first-order reaction (h
– 1); K
2 – is the equilibrium rate constant of pseudo-second-order reaction (h
– 1), and v
0 – is the initial adsorption rate. The data obtained with both equations are included in
Table 2. The R
2 values presented in
Table 2 allow stating that, the pseudo-second-order model fits well the experimental data when compared with the pseudo-first one. The values of the maximum amount of MCPA calculated by the pseudo-second-order model (Q
max,
cal2) are quite similar to the values obtained experimentally (Q
max,
exp), except for the values obtained with Norit 1240 X
g.
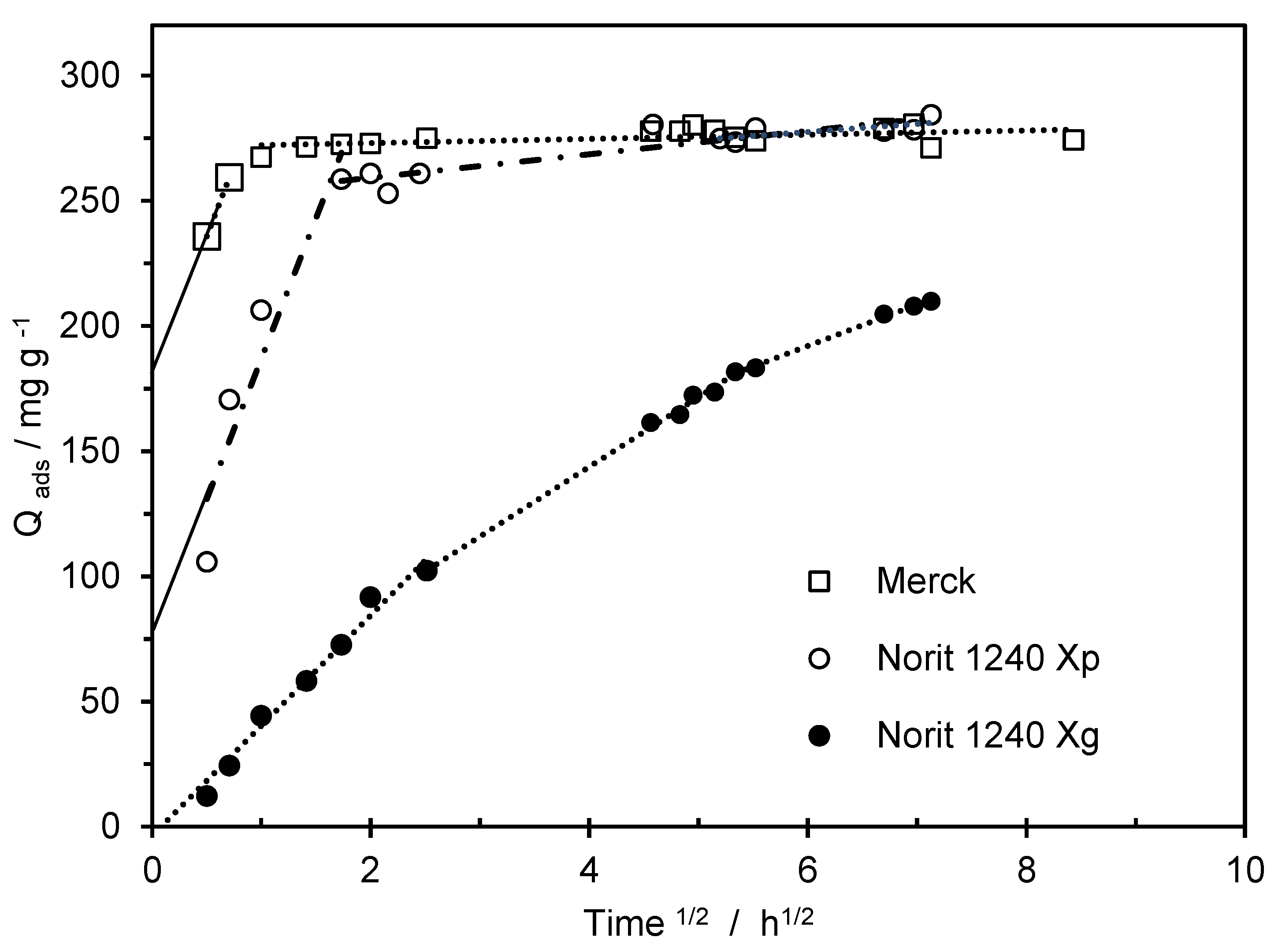
The removal of pollutants, from the aqueous phase, can be controlled by the external transport from the liquid phase to the solid external surface, by the access of the pollutants to the solid pores, and by the diffusion into the micropore. To better understand the limiting step in the MCPA adsorption processes, the data were plotted based on the Weber-Morris equation, as data presented in
Figure 4 and
Table 3. The Weber-Morris model is expressed by equation 3. K
ip is the intra-particule diffusion rate constant (mg g
-1 h
1/2) and C is a constant related to the thickness of the adsorption boundary layer [
25].
For Merck and Norit 1240 X
p ACs, the representation of Q
ads versus t
1/2 presents two and three linear steps, respectively (the second and third steps, on Norit 1240 X
p, have a very similar slope), and all linear regression intercepts the y-axis in a positive value. Based on Liao et. al, 2013, this allows stating that intraparticle diffusion is not the only rate-controlling step for MCPA adsorption [
25]. On both ACs, on the first step, and after a contact time of 1h, the amount adsorbed corresponds to 97.4 and 74%, (Merck and Norit 1240 X
p) of the total amount of MCPA adsorbed. This first step of adsorption was attributed to the occupation of the available surface sites, through electrostatic attraction. The first step adsorption is responsible for the high adsorption rate obtained, from the pseudo-second-order model and for the high K
ip1 values presented in
Table 3.
Merck and Norit 1240 X
p ACs presented very high initial constant rates, which agrees with the kinetic profile presented in
Figure 1. The values of the intraparticle rate diffusion, in the second and third steps, are very small when compared with those obtained in the first step. At this point, the superficial sites and porous volume available for the MCPA adsorption are now residual, and the adsorption rate is almost null.
When the Weber-Morris representation is applied to the Norit 1240 Xg three well-defined linear regions appeared. The interception of the first linear regression with the Y-axis is close to zero, which may indicate that the limiting factors, in the adsorption process, are mainly related to intraparticle diffusion. The values of Kip decreased as the contact time increased in the three MCPA-ACs systems. However, the Kip1 obtained in Norit 1240 Xg is much lower than the values obtained in the other two ACs. Some essays were done on increasing the speed of agitation to promote the contact between Norit 240 Xg and the MCPA present in the solution. However, doubling the agitation speed between tests, no significant changes were observed in the amounts of MCPA adsorbed for different contact times. These results allow confirming, that on Norit 1240 Xg, during the first hour, the vacant adsorption sites on the external surface are quickly occupied, after that, the intraparticle rate diffusion is the main factor, or the limiting step, that controls the MCPA adsorption.
3.4. Pesticide removal from the aqueous phase
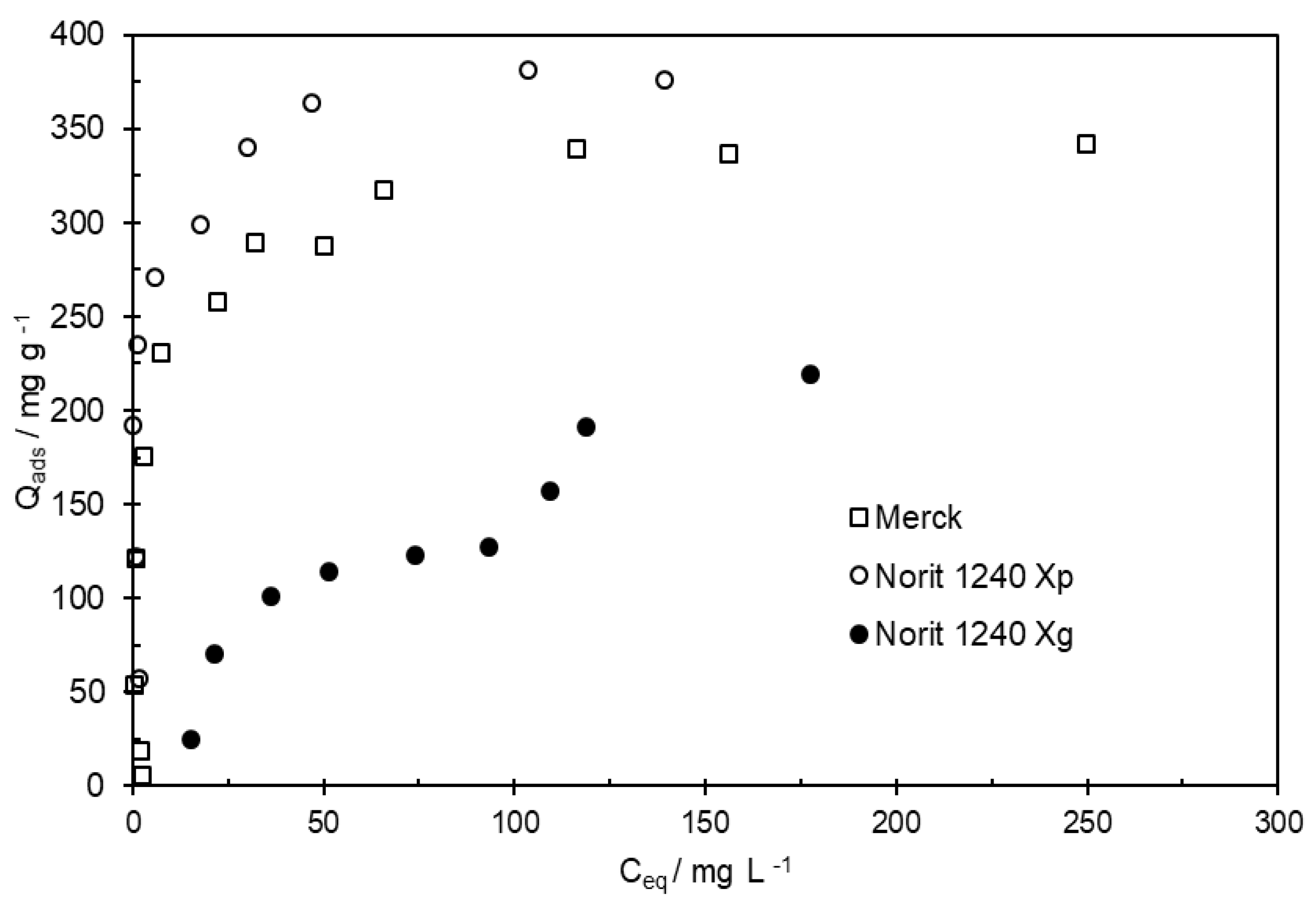
Adsorption isotherms were performed from MCPA solutions with concentrations varying from 5 to 400 mg L
-1, at a pH =3, after an equilibrium time of 24 h. The maximum adsorption capacity attaints 378 mg g
-1 on Norit 1240 X
p, as shown in
Figure 5. Some assays were done in triplicate (or even more) and were very reproducible with a maximum standard deviation of 5 mg g
-1.
After 24 h, the amount of MCPA adsorbed on Norit 1240 X
g was only 54% of the amount adsorbed on Norit 1240 X
p. For the AC in a granular form, the granules stay at the Erlenmeyer bottom, and the contact between adsorbent and adsorbate is less efficient. In this case, the diffusion of the pesticide along the solution to contact with granular AC could be one of the limiting factors on the MCPA adsorption. The contact time between MCPA and Norit 1240 X
g was increased, to 96 h, as shown in
Figure 6. As the contact time increase, the amount of MCPA adsorbed on Norit 1240 X
g increases. To clarify the different contributions of MCPA systems, the agitation of the suspension was duplicated. However, no significant changes were observed. These results corroborate those obtained during the kinetic studies and allow us to rule out the diffusion of MCPA in the solution as a limiting factor.
Previous studies have led to the conclusion that the effect of the entry of adsorbate into the pores occurs only when the average pore diameter was less than 1.5 to 1.7 times the second widest dimension of the adsorbate [
26,
27]. Norit 1240 Xg AC presents a mean pore size of 1.04 nm. MCPA presents a molecular diameter between 7 and 9 Å [
26]. This comparison allows confirming that the pore size exclusion effects seem not to be the predominant factor, in the MCPA – Norit 1240 X
g system.
However, it must be highlighted that the granular form of the Norit 1240 X
g was imposed through the polymeric coating and it can cause reduced pore inlet and hinder access to MCPA. Yet, it is interesting to note, that after a contact time of 96 h, between MCPA and Norit 1240 X
g, the amount of MCPA adsorbed, for higher concentrations, is almost similar to that obtained on Norit 1240 X
p,
Figure 4. This finding allows us to confirm that, on Norit 1240 X
g, the pore volume is available to the pesticide, and the diffusion in the narrow pores or narrow pores entrance are the limiting factor for the slow adsorption rate of MCPA molecules.
3.5. Regeneration of ACs
The regeneration, or desorption of pollutants, from the adsorbent, is an important process since the adsorbent no longer remains effective after saturation. After being saturated with MCPA, the ACs were regenerated with different washing solutions and through thermal treatment. First, the same volume of six different solvents or solutions (ethanol, distilled water, NaOH – 1, 0.1 and 0.01 mol dm-3, and HNO3 – 0.1 mol dm-3) was added to a similar amount of saturated MCPA-ACs. These suspensions were kept under agitation, for 24 h. The MCPA, which was previously retained in the ACs, thus moved on to the solution.
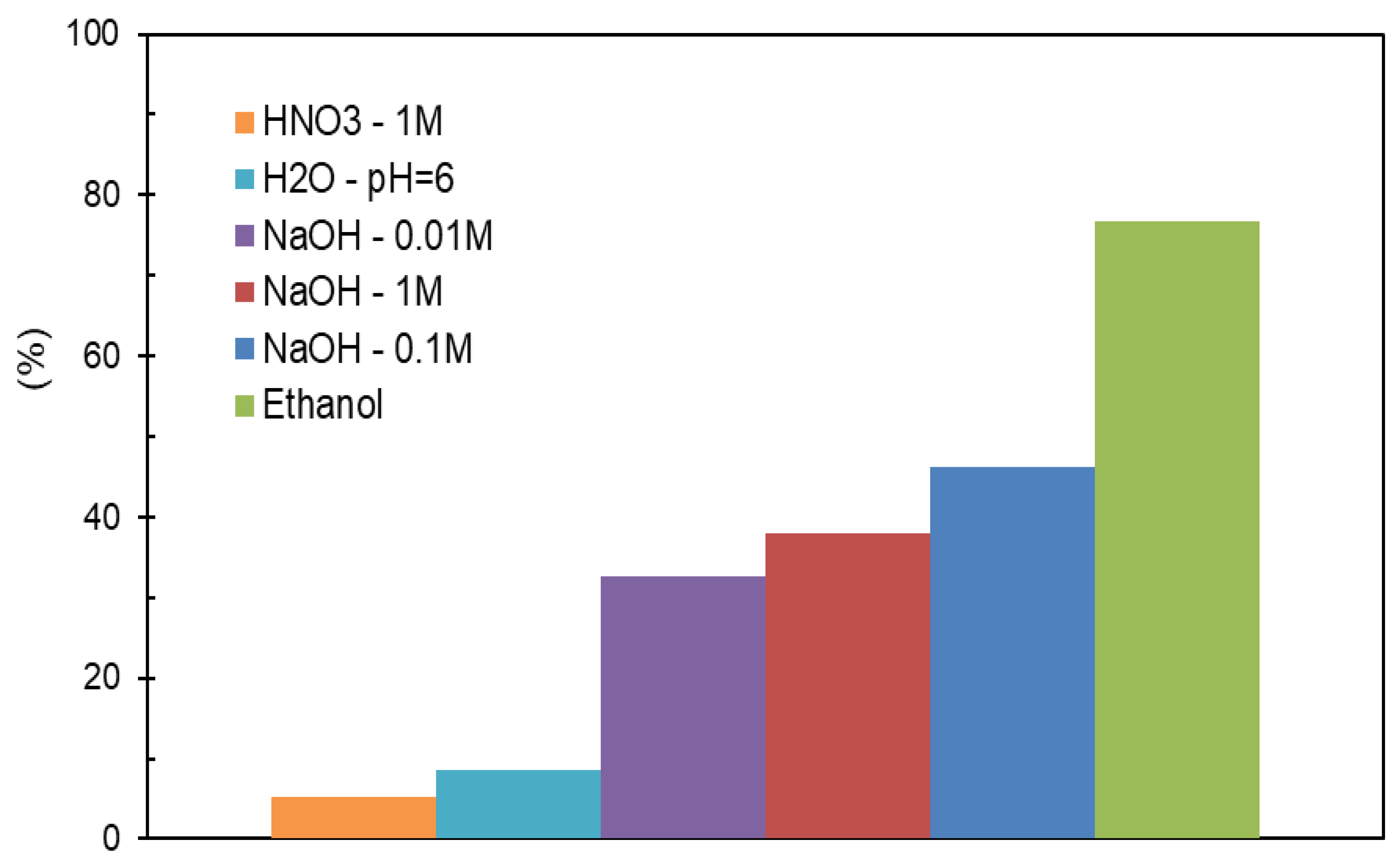
The evaluation of the MCPA amount desorbed, from each AC, was done and the data presented in
Figure 7 allow us to realize that distilled water, as well as, acidic solutions are not suitable for the regeneration of MCPA-saturated ACs.
These results were not surprising, since the adsorption of MCPA, on different ACs, was favoured when performed from acidic solutions. In a review paper, Omorogie et. al, 2016, reported that NaOH solutions were successfully used to desorb tannin and phenol from organoclays adsorbents. The same authors reported that HNO
3 solutions were useful to desorb MB from a composite adsorbent, and methanol was employed to regenerate carbonaceous adsorbents [
14].
Using just one washing step, the basic solutions and ethanol performed well on ACs regeneration. The follow-up of the different assays allowed us to verify that the desorption of the MCPA increases with the contact time between ACs and washing solutions. However, after a contact time of 24 h, the amount of MCPA that passed to the solutions was residual. To increase the percentage of MCPA desorption from the ACs, on each regeneration cycle, mainly with ethanol or basic solutions, the ACs were washed with 3*100 mL, of ethanol or NaOH solutions (0.1 and 0.01 mol dm
-3). The amount of MCPA that passed on to the solution was quantified and the regenerated ACs were used on a new adsorption cycle, such as the results presented in
Table 4 and
Figure 8.
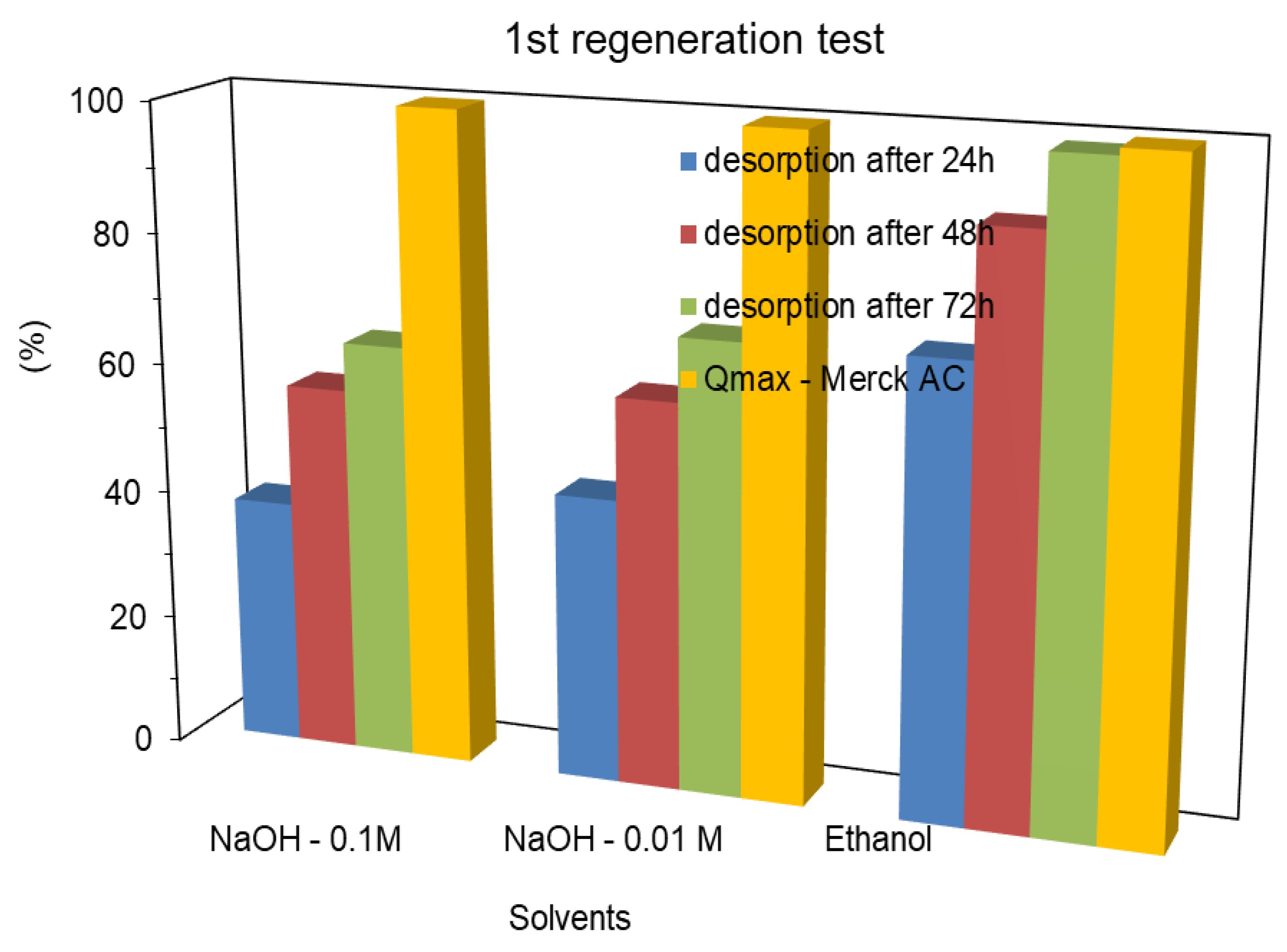
On Merck AC, on the first regeneration cycle after 24 h the desorption percentage of MCPA, with the basic solutions, achieved 37.7 and 43.7% with NaOH – 0.1 and 0.01M, respectively. Ethanol washing allowed to reach a removal percentage of 69.2%, as shown in
Figure 5. After 72 h, (3*100 mL washing steps), the desorption percentage of MCPA, with the basic solutions, reached 63.8 and 69.0%, with NaOH – 0.1 and 0.01M, respectively. Yet, ethanol was allowed to reach a desorption percentage, higher than 99%.
However, the amount of MCPA that passed on the solution was higher than the amount adsorbed in the following step. This statement allows inferring that Merck spent AC is deactivated with various types of physicochemical interactions with the washing solutions, leading to blocking the well-developed pores and eventually degrading the removal abilities concerning MCPA molecules.
The second and third regeneration cycles of Merck AC led to a significative degradation of the adsorbent which resulted in a successive decrease of the MCPA amount adsorbed in the different cycles (R1 to R3,
Table 3). However, when the equilibrium time was prolonged the amount of MCPA adsorbed increased. This finding leads to the conclusion that the different stages of washing the AC promote a reduction of pore intake.
Concerning Norit 1240 Xp, on each adsorption cycle, the MCPA amount desorbed was lower than the amount desorbed on Merck, (54.2, 64.5 and 98.9% on ACs regenerated with NaOH-0.1, 0.01M and ethanol, respectively). Surprisingly, the amount of MCPA adsorbed on the regenerated ACs was similar to or even higher than the amount adsorbed on the Merck AC.
On Norit 1240 X
g, the amount of MCPA adsorbed showed greater deviations between the various assays. The washing procedure used on Norit 1240 X
g was less effective. And the amount of MCPA that passed on the solution, on the first regeneration cycle, was lower (30.3, 45.2 and 84.8% on ACs regenerated with NaOH-0.1, 0.01M, and ethanol, respectively) when compared with those obtained from Merck or Norit 1240 X
p. After being submitted to one regeneration cycle the Norit 1240 X
g was reused, on a new adsorption cycle of MCPA from the aqueous phase. The MCPA amount adsorbed on the second and third cycles was lower than the amount adsorbed in the previous adsorption cycle. The trend was different when the AC was in equilibrium with the MCPA solution for a long time, as shown in
Table 3 (R3*).
In Norit 1240 X
g AC, the maximum amount adsorbed varied between 59 and 69% of the amount adsorbed in the same AC in powder form. In granular form, all porous volume is accessible to the nitrogen molecule, which is a small molecule. However, the restrictions in pore entrance limit access to large molecules, such as MCPA. This restriction in pore access explains the lower performance and slower kinetics, as presented in
Figure 1.
Concerning the washing solutions, such as reported by Alvarez et. al, 2004, desorption is not performed if the concentration of NaOH is not sufficiently high [
21]. However, a very high NaOH concentration can promote the retention of the OH groups on the active sites and delays the subsequent adsorption step [
19]. This phenomenon can explain the reduction of the MCPA amount adsorbed on the regenerated Merck AC.
3.6. Regeneration by basic aqueous washing followed by thermal treatment
Since in the third regeneration cycle, the amount of MCPA desorb decreased drastically, on all ACs, these were then submitted to a thermal regeneration process. After three adsorption-regeneration cycles, the loaded-ACs were regenerated by thermal treatment, under nitrogen flux, at 573 K, for 15 minutes. The regenerated ACs were then used for a fourth cycle of MCPA adsorption, from the aqueous phase.
The Merck AC regenerated was able to adsorb between 54.2% and 78.9% of the MCPA adsorbed on the first cycle. Yet, the amounts adsorbed were 1.63 to 2.65 times higher than the amounts adsorbed in the previous step (R3).
The Norit 1240 X
p and Norit 1240 X
g ACs regenerated were used on a fourth adsorption cycle. Surprisingly, in all trials, the amount of MCPA adsorbed was higher than the amount adsorbed during the first cycle. In Norit 1240 Xp and Norit 1240 Xg, chemical adsorption seems to take place, and, desorption through the washing methods was more difficult to achieve. However, thermal treatment is quite effective for the regeneration of Norit 1240 X AC, in powder or granular form. These results are consistent with the reduction of the acidic functional groups identified in the FTIR spectra, such that the ACs with high basic character present a better performance in the removal of pesticides from the aqueous phase [
26].
4. Conclusions
This work presents the results concerning different ACs regenerating methods, namely ethanol washing, basic aqueous washing, and two-step combined treatment. The results obtained on different steps in the MCPA adsorption – desorption cycles show that the use of ethanol has a high potential for removing MCPA from microporous adsorbents. However, mainly, on the granular AC, the combined treatment of the washing solutions followed by a thermal treatment, at 573 K, was more efficient in recovering the surface properties of the spent AC than the individual basic aqueous washing treatments. It was also clear that Merck saturated AC was easy to regenerate through the washing basic solutions than the Norit 1204 X AC. In Norit 1240 Xp and Norit 1240 Xg, chemical adsorption seems to take place, and, desorption through the washing methods was more difficult to achieve. However, thermal treatment is thus quite effective in the regeneration of Norit 1240 X AC, in powder or granular form, such the chemical bonds can be broken, and the adsorption capacity of the regenerate AC, in particular in Norit 1240 Xg – RT, becomes higher than that achieved by the virgin AC (Norti 1240 Xg).
The adsorption isotherms were well described in the pseudo-second-order model and the Weber-Morris model allows us to state that on Norit 1240 Xg the limiting factor to the MCPA adsorption and kinetic was the pore diffusion.
At present, washing methods for adsorbent regeneration, using different solvents, are not used at an industrial level. However, the investigation of environmentally friendly regeneration methods is necessary to reduce energy consumption in thermal regeneration and achieve the objective of the circular economy.
Author Contributions
Conceptualization, I.C., P.A.M.M.; methodology, J.E.C., P.M; validation, I.C., P.M.; formal analysis, I.C., P.M.; investigation, I.C., P.M.; writing—original draft preparation, P.M., I.C.; writing—review and editing, I.C., J.E.C.; P.M; project administration, I.C., J.E.C; P.M.; funding acquisition, I.C., P.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by FCT (Project UID/QUI/0619/2020) with National (OE) funds.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kulaishin, S.A.; Vedenyapina, M.D.; Kurmysheva, A.Y. Influence of the Surface Characteristics of Activated Carbon on the Adsorption of Herbicides (A Review). Solid Fuel Chem. 2022, 56, 181–198. [Google Scholar] [CrossRef]
- Baskar, A.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; Sarkar, B.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Science of The Total Environment 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.; Zheng, T.; Wang, P. Regeneration of activated carbon saturated with chloramphenicol by microwave and ultraviolet irradiation. Chemical Engineering Journal 2017, 320, 264–270. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Jiang, H.-T.; Ma, C.-Y.; Yong, D. Microwave regeneration characteristics of activated carbon for flue gas desulfurization. J. Fuel Chem. Technol. 2012, 40, 1366–1371. [Google Scholar] [CrossRef]
- González-Poggini, S.; Rosenkranz, A.; Colet-Lagrille, M. Two-Dimensional Nanomaterials for the Removal of Pharmaceuticals from Wastewater: A Critical Review. Processes 2021, 9, 2160. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Belo, C.R.; Mourão, P.A.M. Valorisation of Tectona Grandis tree sawdust through the production of high activated carbon for environmental applications. Bioresour. Technol. 2018, 249, 328–333. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Mourão, P.A.M.; Belo, C.R. Using Tectona Grandis Biomass to Produce Valuable Adsorbents for Pesticide Removal from Liquid Effluent. Materials 2022, 15, 5842–5857. [Google Scholar] [CrossRef]
- Husien, S.; El-Taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Current Research in Green and Sustainable Chemistry 2022, 5, 100325–100340. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Junior, O.P.; Vargas, A.M.M.; Silva, AP.; Zou, A.T.; Almeida, V.C. Thermal regeneration study of high surface area activated carbon obtained from coconut shell: Characterization and application of response surface methodology. Journal of Analytical and Applied Pyrolysis 2013, 101, 53–60. [Google Scholar] [CrossRef]
- Rao, M.M.; Ramana, D.K.; Seshaiah, K.; Wang, M.C.; Chien, S.W. C. Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. Journal of Hazardous Materials 2009, 166, 1006–1013. [Google Scholar] [CrossRef]
- Mourão, P.A.M.; Di Caprio, F.; Cansado, I.P.P.; Castanheiro, J.; Falcone, I.; Astolfi, M.L.; Pagnanelli, F. Granulation and activation of an arsenic adsorbent made of iron oxide doped hydrochar. Chemical Engineering Transaction 2022, 93, 91–96. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.Y.Y.; Chen, W. Biomass-derived activated carbons for the removal of pharmaceutical micropollutants from wastewater: A review. Separation and Purification Technology 2020, 253, 117536. [Google Scholar] [CrossRef]
- Ania, J.O.; Parra, B.; Menéndez, J.A.; Pis, J.J. Microwave-assisted regeneration of activated carbons loaded with pharmaceuticals. Water Research 2007, 41, 3299–3306. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Babalola, J.M.; Unuabonah, E.I. Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: a critical review. Desalination and Water Treatment 2016, 57, 518–544. [Google Scholar] [CrossRef]
- Nahm, S.W.; Shim, W.G.; Park, Y.-K.; Kim, S.C. Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chemical Engineering Journal 2012, 210, 500–509. [Google Scholar] [CrossRef]
- Sang Youp Hwang, S.Y.; Lee, G.B.; Kim, J.H.; Hong, B.U.; Park, J.E. Pre-Treatment Methods for Regeneration of spent Activated Carbon. Molecules 2020, 25, 4561. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Ledesma, B.; González, J.F.; Al-Kassir, A.; Engo, G.; Álvarez-Murillo, A. Two stage thermal regeneration of exhausted activated carbons. Steam gasification of effluents. Journal of Analytical and Applied Pyrolysis 2013, 103, 201–206. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of carbonaceous adsorbents. Part I: Thermal Regeneration. Microporous and Mesoporous Materials 2015, 202, 259–276. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of carbonaceous adsorbents. Part II: Chemical, Microbiological and Vacuum Regeneration. Microporous and Mesoporous Materials 2015, 202, 277–296. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. Journal of Food Engineering 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Álvarez, P.M.; Beltrán, F.J.; Gómez-Serrano, V.; Jaramillo, J.; Rodrı́guez, E.M. Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol. Water Research 2004, 38, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Pack, S.-H.; Jeon, Y.-W. Effect of vacuum regeneration of activated carbon on volatile organic compound adsorption. Environmental Engineering Research 2017, 22, 169–174. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Extending granular activated carbon (GAC) bed life: A column study of in-situ chemical regeneration of pesticide loaded activated carbon for water treatment. Chemosphere 2022, 286, 131888–131998. [Google Scholar] [CrossRef] [PubMed]
- Cansado, I.P.P.; Mourão, P.A.M.; Morais, I.D.; Peniche, V.; Janeirinho, J. Removal of 4-Ethylphenol and 4-Ethylguaiacol, from Wine-like Model Solutions, by Commercial Modified Activated Carbons Produced from Coconut Shell. Appl. Sci. 2022, 12, 11754. [Google Scholar] [CrossRef]
- Liao, P.; Yuan, S.; Xie, W.; Zhang, W.; Tong, M.; Wang, K. Adsorption of nitrogen-heterocyclic compounds on bamboo charcoal: Kinetics, thermodynamics, and microwave regeneration. Journal of Colloid and Interface Science 2012, 39, 189–195. [Google Scholar] [CrossRef]
- Belo, C.R.; Cansado, I.P.P.; Mourão, P.A.M. Synthetic polymers blend used in the production of high activated carbon for pesticide removals from the liquid phase. Environ. Technol. 2017, 38, 285–296. [Google Scholar] [CrossRef]
- Wang, X.; Shu, L.; Wang, S.; et al. Sorption of peat humic acids to multi-walled carbon nanotubes. Environ Sci Technol. 2011, 45, 9276–9283. [Google Scholar] [CrossRef]
Figure 1.
SEM images were obtained at different stages of the MCPA adsorption-desorption cycle. (Norit 1240 Xp + MCPA – RT – means saturated MCPA-AC, after being regenerated by washing with NaOH – 0.01mol dm-3 and thermal temperature at 873 K).
Figure 1.
SEM images were obtained at different stages of the MCPA adsorption-desorption cycle. (Norit 1240 Xp + MCPA – RT – means saturated MCPA-AC, after being regenerated by washing with NaOH – 0.01mol dm-3 and thermal temperature at 873 K).
Figure 2.
FTIR spectra were obtained on the Norit 1240 X AC at different steps on the MCPA adsorption-desorption cycles.
Figure 2.
FTIR spectra were obtained on the Norit 1240 X AC at different steps on the MCPA adsorption-desorption cycles.
Figure 3.
Kinetic study of the MCPA adsorption in Norit 1240 X (in granular -Norit 1240 Xg and powder form -Norit 1240 Xp) and Merck ACs. The kinetic studies were done from a solution containing 250 mg L-1 of MCPA.
Figure 3.
Kinetic study of the MCPA adsorption in Norit 1240 X (in granular -Norit 1240 Xg and powder form -Norit 1240 Xp) and Merck ACs. The kinetic studies were done from a solution containing 250 mg L-1 of MCPA.
Figure 4.
Weber-Morris representation for the experimental kinetic data.
Figure 4.
Weber-Morris representation for the experimental kinetic data.
Figure 5.
MCPA adsorption isotherms obtained on the ACs from Merck, Norit 1240Xp, Norit 1240Xg, obtained after an equilibrium time of 24 h.
Figure 5.
MCPA adsorption isotherms obtained on the ACs from Merck, Norit 1240Xp, Norit 1240Xg, obtained after an equilibrium time of 24 h.
Figure 6.
MCPA adsorption isotherms obtained on Norit 1240Xg, after different equilibrium times.
Figure 6.
MCPA adsorption isotherms obtained on Norit 1240Xg, after different equilibrium times.
Figure 7.
Percentage of MCPA desorbed, from Merck AC, using different washing solutions.
Figure 7.
Percentage of MCPA desorbed, from Merck AC, using different washing solutions.
Figure 8.
Percentage of MCPA desorption, on the first regeneration process, on Merck AC, through washing methods, using a new washing solution, every 24 h.
Figure 8.
Percentage of MCPA desorption, on the first regeneration process, on Merck AC, through washing methods, using a new washing solution, every 24 h.
Table 1.
Chemical and textural characteristics of the Merck and Norit 1240X ACs. On
Table 1, A
BET is the apparent surface area, obtained using the BET method, V
s is the total porous volume, A
s - is the external surface area, (V
s and A
s – were obtained using the alfa-s method), V
0 - is the volume of the micropores and L
0 - is the mean pore size.
Table 1.
Chemical and textural characteristics of the Merck and Norit 1240X ACs. On
Table 1, A
BET is the apparent surface area, obtained using the BET method, V
s is the total porous volume, A
s - is the external surface area, (V
s and A
s – were obtained using the alfa-s method), V
0 - is the volume of the micropores and L
0 - is the mean pore size.
| Activated Carbon |
ABET/ m2 g-1 |
As/
m2 g-1 |
Vs/
cm3 g-1 |
VDR/ cm3 g-1 |
L0 /
nm |
C / % |
H/ % |
S/ % |
pHpzc |
| Norit 1240X |
975 |
60 |
0.43 |
0.23 |
1.04 |
84.4 |
0.19 |
0.49 |
9.73 |
| Merck |
927 |
170 |
0.36 |
0.23 |
1.02 |
85.2 |
0.22 |
0.32 |
7.27 |
Table 2.
Parameters of the application of pseudo-first-order and pseudo-second-order models. The kinetic studies were done from a solution with 250 mg L-1 of MCPA.
Table 2.
Parameters of the application of pseudo-first-order and pseudo-second-order models. The kinetic studies were done from a solution with 250 mg L-1 of MCPA.
| ACs |
Qmax, exp
/ mg g-1
|
Pseudo first-order model |
Pseudo-second order model |
| Qmax1, cal1 / mg g-1
|
K1
/ h-1
|
R2
|
Qmax, cal2
/ mg g-1
|
V0 /
mg g-1 h-1
|
K2
/ h-1
|
R2
|
| Merck |
280.6 |
10.6 |
0.029 |
0.90 |
277.8 |
5000 |
0.13 |
0.98 |
| Norit 1240Xp |
284.3 |
29.4 |
0.018 |
0.72 |
277.9 |
833 |
0.020 |
0.99 |
| Norit 1240Xg
|
220.8 |
195.3 |
0.024 |
0.99 |
142.9 |
60 |
0.028 |
0.95 |
Table 3.
Parameters from the application of the Weber-Morris model. The kinetic studies were done from a solution containing 250 mg L-1 of MCPA. Kip1, Kip2, and Kip3 are the intra-particule diffusion rate constants for the first, second, and third adsorption steps, respectively.
Table 3.
Parameters from the application of the Weber-Morris model. The kinetic studies were done from a solution containing 250 mg L-1 of MCPA. Kip1, Kip2, and Kip3 are the intra-particule diffusion rate constants for the first, second, and third adsorption steps, respectively.
| ACs |
Qads max
mg g-1
|
Kip1
mg g-1 h 1/2
|
C |
Kip2
mg g-1 h 1/2
|
C |
Kip3
mg g-1 h 1/2
|
C |
| Merck |
280.6 |
114.0 |
178.8 |
0.8 |
271.3 |
--- |
--- |
| Norit 1240 Xp |
284.3 |
112.2 |
74.8 |
4.7 |
249.8 |
3.2 |
258.2 |
| Norit 1240 Xg |
220.8 |
44.0 |
0.0 |
27.8 |
32.4 |
16.5 |
92.7 |
Table 4.
Performance of ACs after different regeneration cycles in MCPA removal, from the aqueous phase. R1, R2, and R3 means the first, second, and third regeneration cycles, (* - the adsorption cycle was extended up to 96 h).
Table 4.
Performance of ACs after different regeneration cycles in MCPA removal, from the aqueous phase. R1, R2, and R3 means the first, second, and third regeneration cycles, (* - the adsorption cycle was extended up to 96 h).
| Activated Carbon |
Regeneration cycles |
Qads /
mg g-1
|
Qads /
mg g-1
|
Qads /
mg g-1
|
| |
|
NaOH - 0.1M |
NaOH - 0.01M |
Etanol |
| Merck |
R1 |
229.7 |
230.7 |
231.6 |
| R2 |
169.9 |
176.4 |
197.3 |
| R3 |
76.3 |
55.7 |
96.5 |
| R4 – (R3+T=573K) |
124.6 |
147.4 |
182.7 |
| Norit 1240 Xp (powder) |
R1 |
226.9 |
232.2 |
231.9 |
| R2 |
207.4 |
178.0 |
212.1 |
| R3 |
79.2 |
67.4 |
88.8 |
| R4 - (R3+T=573K) |
230.4 |
221.9 |
239.4 |
| Norit 1240 Xg 8granules) |
R1 |
157.9 |
148.6 |
155.8 |
| R2 |
84.5 |
103.3 |
102.8 |
| R2 + 96h* |
141.3 |
167.8 |
180.4 |
| R3 |
77.5 |
74.3 |
142.7 |
| R4 (R3+T=573K) |
126.1 |
154.3 |
181.8 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).